Pro-Apoptotic Effects of Unsymmetrical Bisacridines in 3D Pancreatic Multicellular Tumor Spheroids
Abstract
1. Introduction
2. Results
2.1. Optimization of 3D Culture Conditions
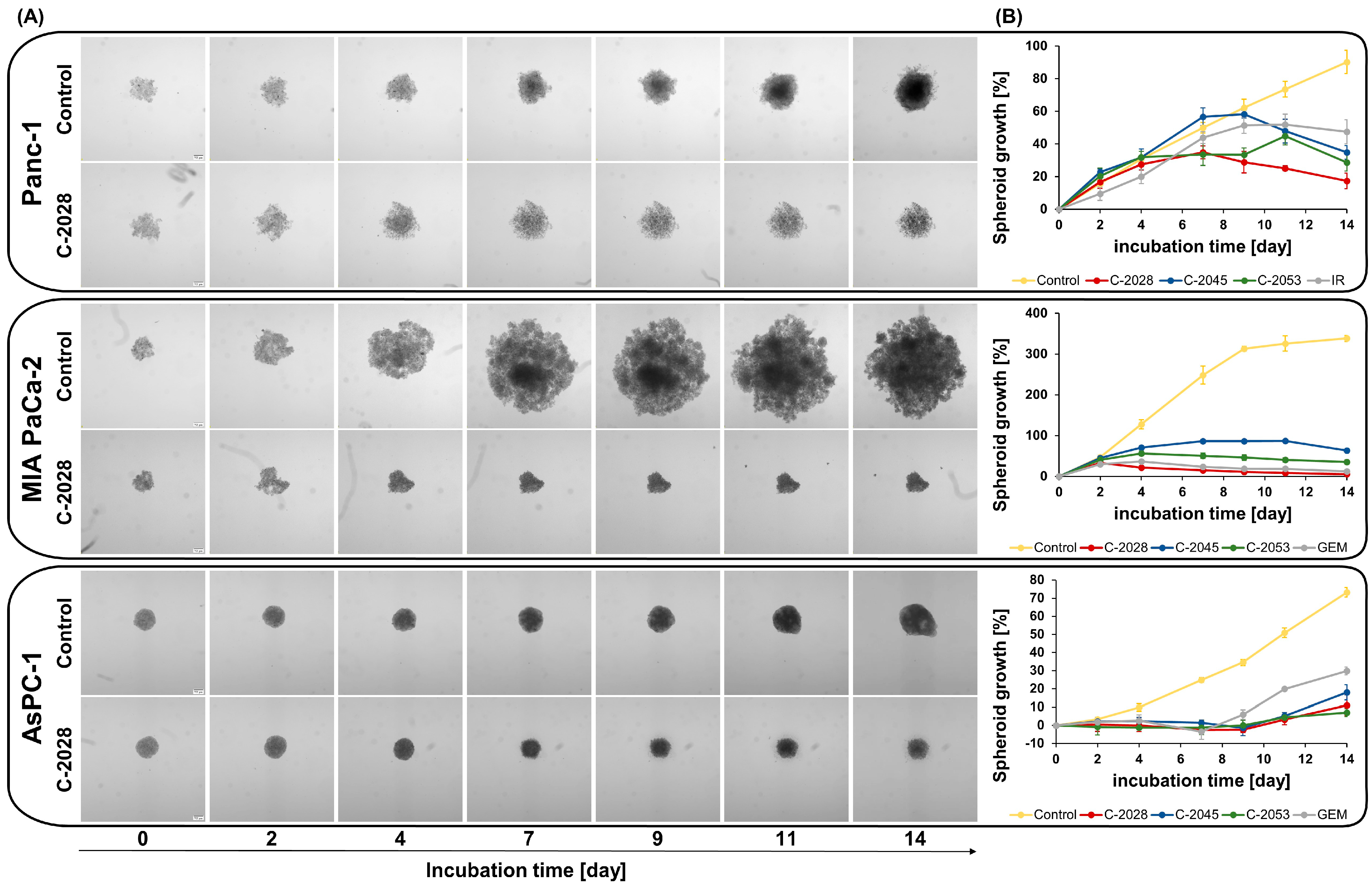
2.2. Spheroid Size and Morphology Assessment After UAs Treatment
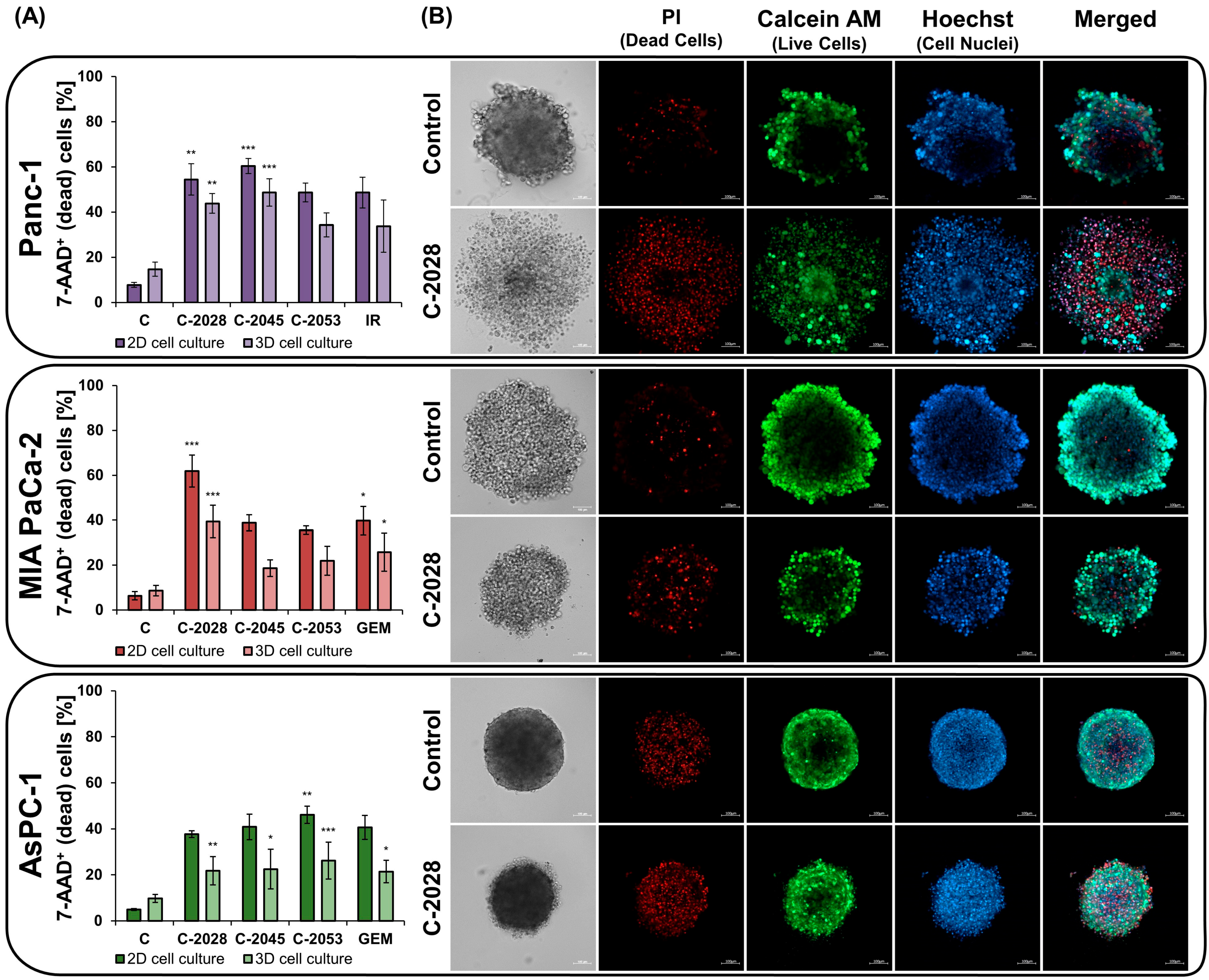
2.3. The Impact of UAs on Cell Viability in 2D and 3D
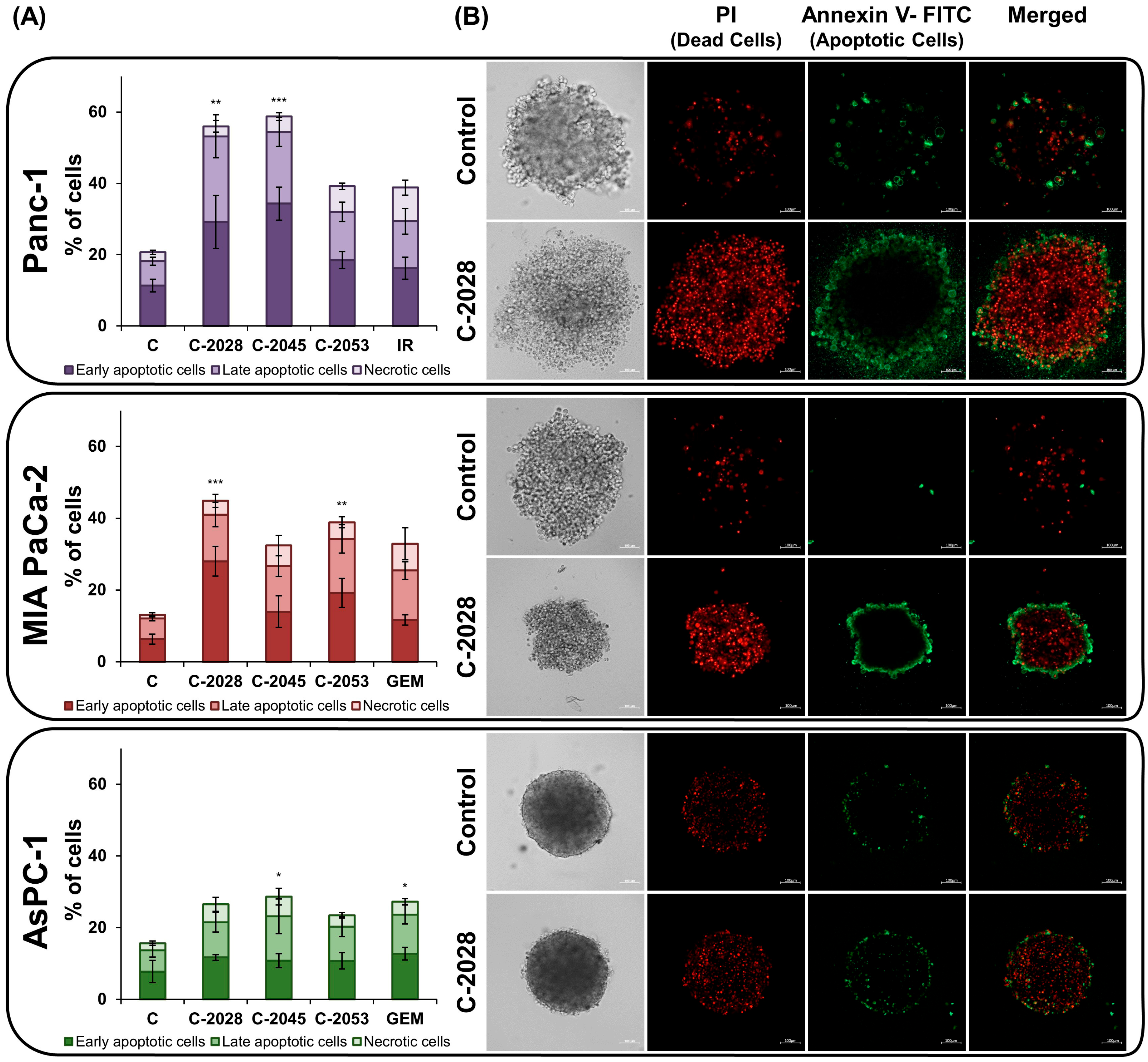
2.4. AnnexinV–FITC/PI Double Staining
3. Discussion
4. Materials and Methods
4.1. Tested Compounds
4.2. Cell Lines and Culture Conditions
4.3. Generation of Multicellular Tumor Spheroids
4.4. Spheroid Size and Morphology Assessment
4.5. Cell Death Assay
4.6. Annexin V/PI Dual Staining
4.7. Spheroid Staining
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| 7-AAD | 7-aminoactionomycin D |
| ABC | ATP-binding cassette |
| DMEM HG | High-glucose Dulbecco’s modified Eagle’s medium |
| DMSO | Dimethylsulfoxide |
| FACS | Fluorescence-Activated Cell Sorting |
| FOLFIRINOX | Multidrug: 5-fluorouracil, leucovorin, irinotecan and oxaliplatin |
| GEM | Gemcitabine |
| IR | Irinotecan |
| KRAS | Kirsten rat sarcoma virus gene |
| MCTS | Multicellular tumor spheroids |
| MDR | Multidrug resistance |
| NALIROX | Multidrug: 5-fluorouracil, leucovorin, liposomal irinotecan, and oxaliplatin |
| PBS | Phosphate-buffer saline |
| PC | Pancreatic cancer |
| PI | Propidium iodide |
| RPMI | Roswell Park Memorial Institute |
| RT | Room temperature |
| SMAD4 | Mothers against decapentaplegic homolog 4 |
| UAs | Unsymmetrical bisacridines |
| ULA | Ultra-low attachment |
References
- Quiñonero, F.; Mesas, C.; Doello, K.; Cabeza, L.; Perazzoli, G.; Jimenez-Luna, C.; Rosa Rama, A.; Melguizo, C.; Prados, J. The Challenge of Drug Resistance in Pancreatic Ductal Adenocarcinoma: A Current Overview. Cancer Biol. Med. 2019, 16, 688–699. [Google Scholar] [CrossRef]
- Jain, A.; Bhardwaj, V. Therapeutic Resistance in Pancreatic Ductal Adenocarcinoma: Current Challenges and Future Opportunities. World J. Gastroenterol. 2021, 27, 6527–6550. [Google Scholar] [CrossRef]
- National Cancer Institute Cancer Stat Facts: Pancreatic Cancer. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 1 March 2025).
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Nichetti, F.; Rota, S.; Ambrosini, P.; Pircher, C.; Gusmaroli, E.; Droz Dit Busset, M.; Pusceddu, S.; Sposito, C.; Coppa, J.; Morano, F.; et al. NALIRIFOX, FOLFIRINOX, and Gemcitabine With Nab-Paclitaxel as First-Line Chemotherapy for Metastatic Pancreatic Cancer. JAMA Netw. Open 2024, 7, e2350756. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Xing, C.; Ding, C.; Zhang, H.; Chen, L.; You, L.; Dai, M.; Zhao, Y. Tumor Microenvironment in Chemoresistance, Metastasis and Immunotherapy of Pancreatic Cancer. Am. J. Cancer Res. 2020, 10, 1937–1953. [Google Scholar]
- Marin, J.; Monte, M.; Macias, R.; Romero, M.; Herraez, E.; Asensio, M.; Ortiz-Rivero, S.; Cives-Losada, C.; Di Giacomo, S.; Gonzalez-Gallego, J.; et al. Expression of Chemoresistance-Associated ABC Proteins in Hepatobiliary, Pancreatic and Gastrointestinal Cancers. Cancers 2022, 14, 3524. [Google Scholar] [CrossRef] [PubMed]
- Rajabpour, A.; Rajaei, F.; Teimoori-Toolabi, L. Molecular Alterations Contributing to Pancreatic Cancer Chemoresistance. Pancreatology 2017, 17, 310–320. [Google Scholar] [CrossRef]
- Paluszkiewicz, E.; Horowska, B.; Borowa-Mazgaj, B.; Peszyńska-Sularz, G.; Paradziej-Łukowicz, J.; Augustin, E.; Konopa, J.; Mazerska, Z. Design, Synthesis and High Antitumor Potential of New Unsymmetrical Bisacridine Derivatives towards Human Solid Tumors, Specifically Pancreatic Cancers and Their Unique Ability to Stabilize DNA G-Quadruplexes. Eur. J. Med. Chem. 2020, 204, 112599. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, T.; Kosno, M.; Andrałojć, W.; Pakuła, J.; Stojałowski, R.; Borzyszkowska-Bukowska, J.; Paluszkiewicz, E.; Mazerska, Z. The Interactions of Pu22 G-Quadruplex, Derived from c-MYC Promoter Sequence, with Antitumor Acridine Derivatives—An NMR/MD Combined Study. Mol. Ther. Nucleic Acids 2025, 36, 102513. [Google Scholar] [CrossRef]
- Laskowski, T.; Kosno, M.; Andrałojć, W.; Frackowiak, J.E.; Borzyszkowska-Bukowska, J.; Szczeblewski, P.; Radoń, N.; Świerżewska, M.; Woźny, A.; Paluszkiewicz, E.; et al. The Interactions of Monomeric Acridines and Unsymmetrical Bisacridines (UAs) with DNA Duplexes: An Insight Provided by NMR and MD Studies. Sci. Rep. 2023, 13, 3431. [Google Scholar] [CrossRef]
- Pawłowska, M.; Kulesza, J.; Augustin, E. C-Myc Protein Level Affected by Unsymmetrical Bisacridines Influences Apoptosis and Senescence Induced in HCT116 Colorectal and H460 Lung Cancer Cells. Int. J. Mol. Sci. 2022, 23, 3061. [Google Scholar] [CrossRef]
- Kurdyn, A.; Pawłowska, M.; Paluszkiewicz, E.; Cichorek, M.; Augustin, E. C-Myc Inhibition and P21 Modulation Contribute to Unsymmetrical Bisacridines-Induced Apoptosis and Senescence in Pancreatic Cancer Cells. Pharmacol. Rep. 2025, 77, 182–209. [Google Scholar] [CrossRef]
- Kulesza, J.; Paluszkiewicz, E.; Augustin, E. Cellular Effects of Selected Unsymmetrical Bisacridines on the Multicellular Tumor Spheroids of HCT116 Colon and A549 Lung Cancer Cells in Comparison to Monolayer Cultures. Int. J. Mol. Sci. 2023, 24, 15780. [Google Scholar] [CrossRef]
- Delle Cave, D.; Rizzo, R.; Sainz, B.; Gigli, G.; del Mercato, L.L.; Lonardo, E. The Revolutionary Roads to Study Cell–Cell Interactions in 3D In Vitro Pancreatic Cancer Models. Cancers 2021, 13, 930. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of Applying Multicellular Tumor Spheroids in Preclinical Phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Mitrakas, A.G.; Tsolou, A.; Didaskalou, S.; Karkaletsou, L.; Efstathiou, C.; Eftalitsidis, E.; Marmanis, K.; Koffa, M. Applications and Advances of Multicellular Tumor Spheroids: Challenges in Their Development and Analysis. Int. J. Mol. Sci. 2023, 24, 6949. [Google Scholar] [CrossRef] [PubMed]
- Minami, F.; Sasaki, N.; Shichi, Y.; Gomi, F.; Michishita, M.; Ohkusu-Tsukada, K.; Toyoda, M.; Takahashi, K.; Ishiwata, T. Morphofunctional Analysis of Human Pancreatic Cancer Cell Lines in 2- and 3-Dimensional Cultures. Sci. Rep. 2021, 11, 6775. [Google Scholar] [CrossRef]
- Truong, L.-H.; Pauklin, S. Pancreatic Cancer Microenvironment and Cellular Composition: Current Understandings and Therapeutic Approaches. Cancers 2021, 13, 5028. [Google Scholar] [CrossRef]
- Deer, E.L.; González-Hernández, J.; Coursen, J.D.; Shea, J.E.; Ngatia, J.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. Phenotype and Genotype of Pancreatic Cancer Cell Lines. Pancreas 2010, 39, 425–435. [Google Scholar] [CrossRef]
- Svirshchevskaya, E.; Doronina, E.; Grechikhina, M.; Matushevskaya, E.; Kotsareva, O.; Fattakhova, G.; Sapozhnikov, A.; Felix, K. Characteristics of Multicellular Tumor Spheroids Formed by Pancreatic Cells Expressing Different Adhesion Molecules. Life Sci. 2019, 219, 343–352. [Google Scholar] [CrossRef]
- Shichi, Y.; Gomi, F.; Sasaki, N.; Nonaka, K.; Arai, T.; Ishiwata, T. Epithelial and Mesenchymal Features of Pancreatic Ductal Adenocarcinoma Cell Lines in Two- and Three-Dimensional Cultures. J. Pers. Med. 2022, 12, 746. [Google Scholar] [CrossRef] [PubMed]
- Kopantzev, E.P.; Kopantseva, M.R.; Grankina, E.V.; Mikaelyan, A.; Egorov, V.I.; Sverdlov, E.D. Activation of IGF/IGF-IR Signaling Pathway Fails to Induce Epithelial-Mesenchymal Transition in Pancreatic Cancer Cells. Pancreatology 2019, 19, 390–396. [Google Scholar] [CrossRef]
- Ungefroren, H.; Thürling, I.; Färber, B.; Kowalke, T.; Fischer, T.; De Assis, L.V.M.; Braun, R.; Castven, D.; Oster, H.; Konukiewitz, B.; et al. The Quasimesenchymal Pancreatic Ductal Epithelial Cell Line PANC-1—A Useful Model to Study Clonal Heterogeneity and EMT Subtype Shifting. Cancers 2022, 14, 2057. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.J.; Cortes, E.; Lachowski, D.; Cheung, B.C.H.; Karim, S.A.; Morton, J.P.; del Río Hernández, A. Matrix Stiffness Induces Epithelial–Mesenchymal Transition and Promotes Chemoresistance in Pancreatic Cancer Cells. Oncogenesis 2017, 6, e352. [Google Scholar] [CrossRef]
- Liao, Q.; Hu, Y.; Zhao, Y.-P.; Zhou, T.; Zhang, Q. Assessment of Pancreatic Carcinoma Cell Chemosensitivity Using a Three-Dimensional Culture System. Chin. Med. J. (Engl.) 2010, 123, 1871–1877. [Google Scholar] [CrossRef]
- Wen, Z.; Liao, Q.; Hu, Y.; You, L.; Zhou, L.; Zhao, Y. A Spheroid-Based 3-D Culture Model for Pancreatic Cancer Drug Testing, Using the Acid Phosphatase Assay. Braz. J. Med. Biol. Res. 2013, 46, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Abbas, S.; Saxena, A.K.; Tiwari, S.; Sharma, L.K.; Tiwari, M. Critical Role of Three-Dimensional Tumorsphere Size on Experimental Outcome. Biotechniques 2020, 69, 333–338. [Google Scholar] [CrossRef]
- Alwahsh, M.; Al-Doridee, A.; Jasim, S.; Awwad, O.; Hergenröder, R.; Hamadneh, L. Cytotoxic and Molecular Differences of Anticancer Agents on 2D and 3D Cell Culture. Mol. Biol. Rep. 2024, 51, 721. [Google Scholar] [CrossRef]
- Paškevičiūtė, M.; Petrikaitė, V. Differences of Statin Activity in 2D and 3D Pancreatic Cancer Cell Cultures. Drug Des. Devel. Ther. 2017, 11, 3273–3280. [Google Scholar] [CrossRef]
- Tidwell, T.R.; Røsland, G.; Tronstad, K.J.; Søreide, K.; Hagland, H.R. Comparing in Vitro Cytotoxic Drug Sensitivity in Colon Andpancreatic Cancer Using 2D and 3D Cell Models: Contrastingviability and Growth Inhibition in Clinically Relevant Doseand Repeated Drug Cycles. Cancer Med. 2024, 13, e7318. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. The Relevance of Using 3D Cell Cultures, in Addition to 2D Monolayer Cultures, When Evaluating Breast Cancer Drug Sensitivity and Resistance. Oncotarget 2016, 7, 45745–45756. [Google Scholar] [CrossRef]
- Ascione, F.; Ferraro, R.; Dogra, P.; Cristini, V.; Guido, S.; Caserta, S. Gradient-Induced Instability in Tumour Spheroids Unveils the Impact of Microenvironmental Nutrient Changes. Sci. Rep. 2024, 14, 20837. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, S.-K.; Khawar, I.A.; Jeong, S.-Y.; Chung, S.; Kuh, H.-J. Microfluidic Co-Culture of Pancreatic Tumor Spheroids with Stellate Cells as a Novel 3D Model for Investigation of Stroma-Mediated Cell Motility and Drug Resistance. J. Exp. Clin. Cancer Res. 2018, 37, 4. [Google Scholar] [CrossRef]
- Jaros, S.W.; Komarnicka, U.K.; Kyzioł, A.; Pucelik, B.; Nesterov, D.S.; Kirillov, A.M.; Smoleński, P. Therapeutic Potential of a Water-Soluble Silver-Diclofenac Coordination Polymer on 3D Pancreatic Cancer Spheroids. J. Med. Chem. 2022, 65, 11100–11110. [Google Scholar] [CrossRef] [PubMed]
- Achilli, T.-M.; McCalla, S.; Meyer, J.; Tripathi, A.; Morgan, J.R. Multilayer Spheroids To Quantify Drug Uptake and Diffusion in 3D. Mol. Pharm. 2014, 11, 2071–2081. [Google Scholar] [CrossRef]
- Hagmann, W.; Faissner, R.; Schnölzer, M.; Löhr, M.; Jesnowski, R. Membrane Drug Transporters and Chemoresistance in Human Pancreatic Carcinoma. Cancers 2010, 3, 106–125. [Google Scholar] [CrossRef]
- Harpstrite, S.E.; Gu, H.; Natarajan, R.; Sharma, V. Interrogation of Multidrug Resistance (MDR1) P-Glycoprotein (ABCB1) Expression in Human Pancreatic Carcinoma Cells. Nucl. Med. Commun. 2014, 35, 1067–1070. [Google Scholar] [CrossRef]
- Pawłowska, M.; Kulesza, J.; Paluszkiewicz, E.; Augustin, E.; Mazerska, Z. Unsymmetrical Bisacridines’ Interactions with ABC Transporters and Their Cellular Impact on Colon LS 174T and Prostate DU 145 Cancer Cells. Molecules 2024, 29, 5582. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Peluso, J.; Chalhoub, S.; Idoux Gillet, Y.; Benkirane-Jessel, N.; Rochel, N.; Fuhrmann, G.; Ubeaud-Sequier, G. Quercetin Potentializes the Respective Cytotoxic Activity of Gemcitabine or Doxorubicin on 3D Culture of AsPC-1 or HepG2 cells, through the inhibition of HIF-1α and MDR1. PLoS ONE 2020, 15, e0240676. [Google Scholar] [CrossRef]
- Tidwell, T.R.; Røsland, G.V.; Tronstad, K.J.; Søreide, K.; Hagland, H.R. Metabolic Flux Analysis of 3D Spheroids Reveals Significant Differences in Glucose Metabolism from Matched 2D Cultures of Colorectal Cancer and Pancreatic Ductal Adenocarcinoma Cell Lines. Cancer Metab. 2022, 10, 9. [Google Scholar] [CrossRef]
- Pilch, J.; Potęga, A.; Kowalczyk, A.; Kasprzak, A.; Kowalik, P.; Bujak, P.; Paluszkiewicz, E.; Augustin, E.; Nowicka, A.M. PH-Responsive Drug Delivery Nanoplatforms as Smart Carriers of Unsymmetrical Bisacridines for Targeted Cancer Therapy. Pharmaceutics 2023, 15, 201. [Google Scholar] [CrossRef] [PubMed]
- Patki, M.; Saraswat, A.; Bhutkar, S.; Dukhande, V.; Patel, K. In Vitro Assessment of a Synergistic Combination of Gemcitabine and Zebularine in Pancreatic Cancer Cells. Exp. Cell Res. 2021, 405, 112660. [Google Scholar] [CrossRef] [PubMed]
- Samanta, K.; Setua, S.; Kumari, S.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Gemcitabine Combination Nano Therapies for Pancreatic Cancer. Pharmaceutics 2019, 11, 574. [Google Scholar] [CrossRef] [PubMed]
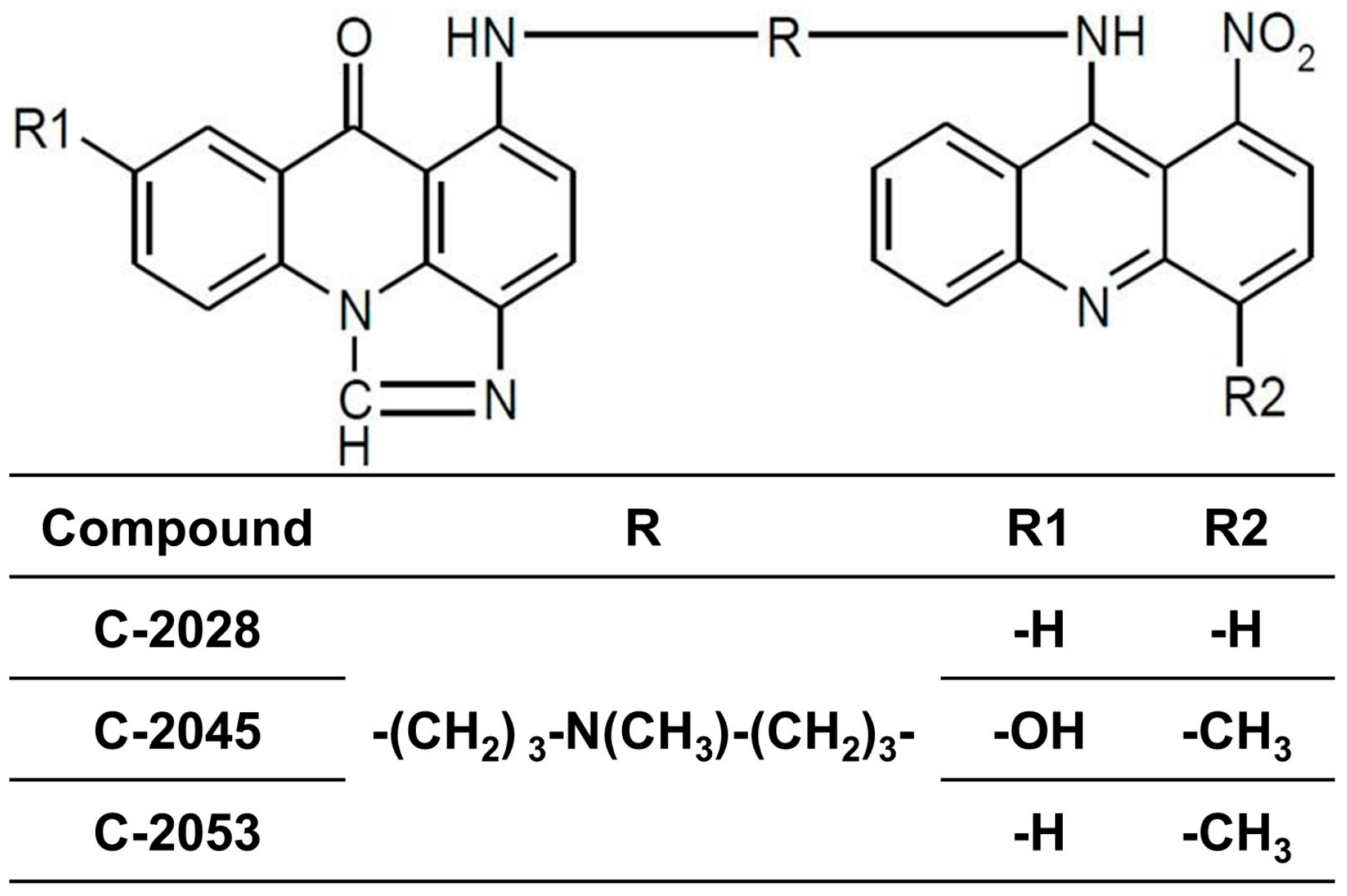
| Compound | Compound Dose [µM] | Cell Line | ||
|---|---|---|---|---|
| Panc-1 | MIA PaCa-2 | AsPC-1 | ||
| C-2028 | IC80 | 0.043 ± 0.002 | 0.042 ± 0.002 | 0.102 ± 0.007 |
| C-2045 | IC80 | 0.323 ± 0.043 | 0.086 ± 0.001 | 0.463 ± 0.011 |
| C-2053 | IC80 | 0.080 ± 0.012 | 0.072 ± 0.004 | 0.486 ± 0.005 |
| GEM | IC50 | not applicable | 0.062 ± 0.010 | 0.026 ± 0.003 |
| IR | IC50 | 8.5 ± 0.13 | not applicable | not applicable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurdyn, A.; Paluszkiewicz, E.; Augustin, E. Pro-Apoptotic Effects of Unsymmetrical Bisacridines in 3D Pancreatic Multicellular Tumor Spheroids. Int. J. Mol. Sci. 2025, 26, 7557. https://doi.org/10.3390/ijms26157557
Kurdyn A, Paluszkiewicz E, Augustin E. Pro-Apoptotic Effects of Unsymmetrical Bisacridines in 3D Pancreatic Multicellular Tumor Spheroids. International Journal of Molecular Sciences. 2025; 26(15):7557. https://doi.org/10.3390/ijms26157557
Chicago/Turabian StyleKurdyn, Agnieszka, Ewa Paluszkiewicz, and Ewa Augustin. 2025. "Pro-Apoptotic Effects of Unsymmetrical Bisacridines in 3D Pancreatic Multicellular Tumor Spheroids" International Journal of Molecular Sciences 26, no. 15: 7557. https://doi.org/10.3390/ijms26157557
APA StyleKurdyn, A., Paluszkiewicz, E., & Augustin, E. (2025). Pro-Apoptotic Effects of Unsymmetrical Bisacridines in 3D Pancreatic Multicellular Tumor Spheroids. International Journal of Molecular Sciences, 26(15), 7557. https://doi.org/10.3390/ijms26157557






