Developments in the Study of Inert Gas Biological Effects and the Underlying Molecular Mechanisms
Abstract
1. Introduction
2. Physical and Chemical Properties of Inert Gas
2.1. Solubility
2.2. Pressure
2.3. Density
2.4. Other Aspects
3. Biological Effects of Inert Gas
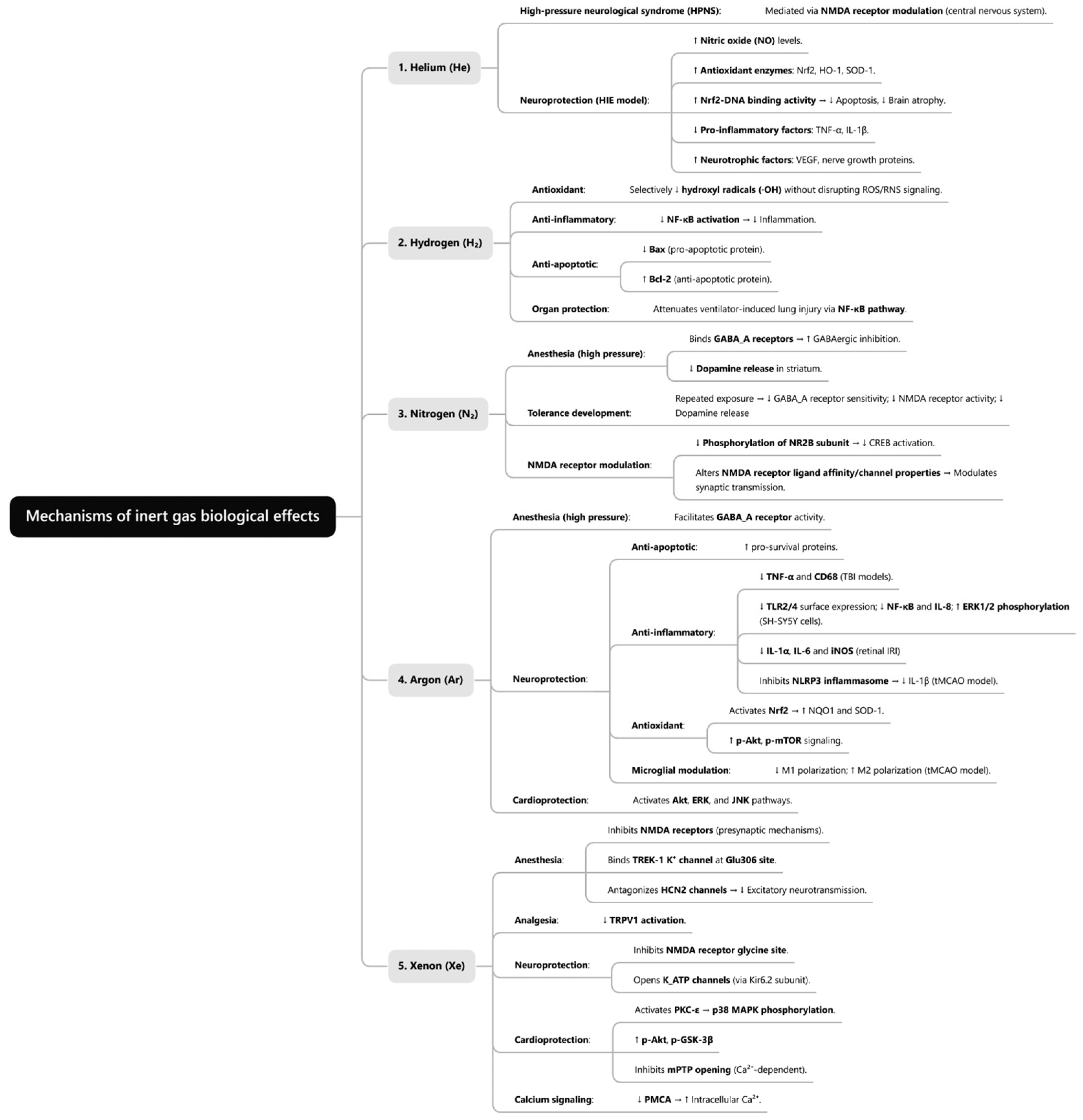
3.1. Biological Effects of Helium
3.2. Biological Effects of Hydrogen
3.3. Biological Effects of Nitrogen
3.4. Biological Effects of Argon
3.5. Biological Effects of Xenon
3.6. Potential Adverse Effects of Inert Gas
4. Mechanisms of Generation of Biological Effects of Inert Gas
4.1. Lipid-Related Theory
4.2. Protein Theory
4.2.1. Helium
4.2.2. Hydrogen
4.2.3. Nitrogen
4.2.4. Argon
4.2.5. Xenon
5. Challenges in Translating Laboratory Discoveries to Clinical Practice
6. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, H.; Chen, Z.; Zhao, H.; Huang, H.; Liu, W. Noble gas and neuroprotection: From bench to bedside. Front. Pharmacol. 2022, 13, 1028688. [Google Scholar] [CrossRef]
- Hancock, J.T. Are protein cavities and pockets commonly used by redox active signalling molecules? Plants 2023, 12, 2594. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Shen, L.; Ge, J.W.; Zhang, R.F. The transfer of hydrogen from inert gas to therapeutic gas. Med. Gas. Res. 2017, 7, 265–272. [Google Scholar]
- Kot, J.; Sobczak, O.; Młynarczyk, B.; Sharma, R.; Lenkiewicz, E.; Sićko, Z. Decompression sickness of medical personnel of a hyperbaric centre: A report of cases during 25 years of activity. Int. Marit. Health 2024, 75, 228–235. [Google Scholar] [CrossRef]
- Battino, R.; Seybold, P.; Campanell, F. Correlations involving the solubility of gases in water at 298.15 K and 101325 Pa. J. Chem. Eng. Data 2011, 56, 727–732. [Google Scholar] [CrossRef]
- Bennett, P.B. Inert Gas Narcosis. In The Physiology and Medicine of Diving, 4th ed.; Bennett, P.B., Elliott, D.H., Eds.; Saunders: London, UK, 1993; pp. 171–193. [Google Scholar]
- Dickinson, R.; Franks, N.P. Bench-to-bedside review: Molecular pharmacology and clinical use of inert gas in anesthesia and neuroprotection. Crit. Care 2010, 14, 229. [Google Scholar] [CrossRef]
- Suresh, K.A. Noble gases: A research study. Int. J. Res. Sci. Technol. 2013, 2, 74–82. [Google Scholar]
- Pagliaro, P.; Weber, N.C.; Femminò, S.; Alloatti, G.; Penna, C. Gasotransmitters and noble gases in cardioprotection: Unraveling molecular pathways for future therapeutic strategies. Basic. Res. Cardiol. 2024, 119, 509–544. [Google Scholar] [CrossRef]
- Aehling, C.; Weber, N.C.; Zuurbier, C.J.; Preckel, B.; Galmbacher, R.; Stefan, K.; Hollmann, M.W.; Popp, E.; Knapp, J. Effects of combined helium pre/post-conditioning on the brain and heart in a rat resuscitation model. Acta Anaesthesiol. Scand. 2018, 62, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Pai, M.S.; Yeh, K.C.; Hung, C.F.; Wang, S.J. Hydrogen inhalation exerts anti-seizure effects by preventing oxidative stress and inflammation in the hippocampus in a rat model of kainic acid-induced seizures. Neurochem. Int. 2025, 183, 105925. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, J.; Gong, Z.; Wang, Y.; Wang, Q.; Yang, X.; Liu, F.; Liu, T. Hydrogen Protects Mitochondrial function by increasing the expression of PGC-1α and ameliorating myocardial ischaemia-reperfusion injury. J. Cell Mol. Med. 2024, 28, e70236. [Google Scholar] [CrossRef]
- Zheng, C.M.; Hou, Y.C.; Liao, M.T.; Tsai, K.W.; Hu, W.C.; Yeh, C.C.; Lu, K.C. Potential role of molecular hydrogen therapy on oxidative stress and redox signaling in chronic kidney disease. Biomed. Pharmacother. 2024, 176, 116802. [Google Scholar] [CrossRef]
- Case, E.M.; Haldane, J.B.S. Human physiology under high pressure: I. Effects of nitrogen, carbon dioxide, and cold. J. Hyg. 1941, 41, 225–249. [Google Scholar] [CrossRef]
- Bjurstedt, H.; Severin, G. The prevention of decompression sickness and nitrogen narcosis by the use of hydrogen as a substitute for nitrogen, the Arne Zetterstrôm method for deep-sea diving. Mil. Surg. 1948, 103, 107–116. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Ohno, K.; Hancock, J.T. The on/off history of hydrogen in medicine: Will the interest persist this time around? Oxygen 2023, 3, 143–162. [Google Scholar] [CrossRef]
- Harris, R.J.; Challen, C.J.; Mitchell, S.J. The first deep rebreather dive using hydrogen: Case report. Diving Hyperb. Med. 2024, 54, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Behnke, A.R.; Thomson, R.M.; Motley, E.P. The psychologic effects from breathing air at 4 atmospheres pressure. Am. J. Physiol.-Leg. Content 1935, 112, 554–558. [Google Scholar] [CrossRef]
- Behnke, A.R.; Yarbrough, O.D. Respiratory resistance, oil-water solubility, and mental effects of argon, compared with helium and nitrogen. Am. J. Physiol.-Leg. Content 1939, 126, 409–415. [Google Scholar] [CrossRef]
- Rostain, J.C.; Balon, N. Recent neurochemical basis of inert gas narcosis and pressure effects. Undersea Hyperb. Med. 2006, 33, 197–204. [Google Scholar]
- Rostain, J.C.; Lavoute, C. Neurochemistry of pressure-induced nitrogen and metabolically inert gas narcosis in the central nervous system. Compr. Physiol. 2016, 6, 1579–1590. [Google Scholar] [CrossRef]
- Ballentine, C.J. Geochemistry: Earth holds its breath. Nature 2007, 449, 294–296. [Google Scholar] [CrossRef]
- Ulbrich, F.; Schallner, N.; Coburn, M.; Loop, T.; Lagrèze, W.A.; Biermann, J.; Goebel, U. Argon inhalation attenuates retinal apoptosis after ischemia/reperfusion injury in a time- and dose-dependent manner in rats. PLoS ONE 2014, 9, e115984. [Google Scholar] [CrossRef] [PubMed]
- Faure, A.; Bruzzese, L.; Steinberg, J.G.; Jammes, Y.; Torrents, J.; Berdah, S.V.; Garnier, E.; Legris, T.; Loundou, A.; Chalopin, M.; et al. Effectiveness of pure argon for renal transplant preservation in a preclinical pig model of heterotopic autotransplantation. J. Transl. Med. 2016, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.H.; Loomis, W.F.; Tobias, C.A.; Turpin, F.H. Preliminary observations on the narcotic effect of xenon with a review of values for solubilities of gases in water and oils. J. Physiol. 1946, 105, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Wu, S.H.; Chen, W.C.; Pei, M.Q.; Liu, Y.B.; Liu, C.Y.; Lin, S.; He, H.F. Effects of xenon anesthesia on postoperative neurocognitive disorders: A systematic review and meta-analysis. BMC Anesthesiol. 2023, 23, 366. [Google Scholar] [CrossRef]
- McGuigan, S.; Pelentritou, A.; Scott, D.A.; Sleigh, J. Xenon anaesthesia is associated with a reduction in frontal electroencephalogram peak alpha frequency. BJA Open 2024, 12, 100358. [Google Scholar] [CrossRef]
- Laaksonen, M.; Rinne, J.; Rahi, M.; Posti, J.P.; Laitio, R.; Kivelev, J.; Saarenpää, I.; Laukka, D.; Frösen, J.; Ronkainen, A.; et al. Effect of xenon on brain injury, neurological outcome, and survival in patients after aneurysmal subarachnoid hemorrhage—Study protocol for a randomized clinical trial. Trial 2023, 24, 417. [Google Scholar] [CrossRef]
- Sherstyukova, E.; Sergunova, V.; Kandrashina, S.; Chernysh, A.; Inozemtsev, V.; Lomakina, G.; Kozlova, E. Red blood cell storage with Xenon: Safe or disruption? Cells 2024, 13, 411. [Google Scholar] [CrossRef]
- Borowska-Solonynko, A.; Dąbkowska, A. Gas embolism as a potential cause of death by helium poisoning—Postmortem computed tomography changes in two cases of suicidal helium inhalation. Leg. Med. 2018, 31, 59–65. [Google Scholar] [CrossRef]
- Overton, E. Studien über die Narkose, Zugleich ein Beitrag zur allgemeinen Pharmakologie; Gustav Fischer: Jena, Germany, 1901; p. 195. [Google Scholar]
- Pavel, M.A.; Petersen, E.N.; Wang, H.; Lerner, R.A.; Hansen, S.B. Studies on the mechanism of general anesthesia. Proc. Natl. Acad. Sci. USA 2020, 117, 13757–13766. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, N.; Gu, J.; Li, H.F.; Qiu, Y.; Liao, D.F.; Qin, L. Crosstalk between lipid rafts and aging: New frontiers for delaying aging. Aging Dis. 2022, 13, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Figueroa, A.D.; Karttunen, M.; Ruiz-Suárez, J.C. Cholesterol sequestration by xenon nano bubbles leads to lipid raft destabilization. Soft Matter 2020, 16, 9655–9661. [Google Scholar] [CrossRef] [PubMed]
- Franks, N.P.; Lieb, W.R. Where do general anaesthetics act? Nature 1978, 274, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Eger, E.I., 2nd; Saidman, L.J.; Brandstater, B. Temperature dependence of halothane and cyclopropane anesthesia in dogs: Correlation with some theories of anesthetic action. Anesthesiology 1965, 26, 764–770. [Google Scholar] [CrossRef]
- Franks, N.P.; Lieb, W.R. Temperature dependence of the potency of volatile general anesthetics: Implications for in vitro experiments. Anesthesiology 1996, 84, 716–720. [Google Scholar] [CrossRef]
- Franks, N.P.; Lieb, W.R. Do general anaesthetics act by competitive binding to specific receptors? Nature 1984, 310, 599–601. [Google Scholar] [CrossRef]
- Winkler, D.A. Noble gases in medicine: Current status and future prospects. Oxygen 2024, 4, 421–431. [Google Scholar] [CrossRef]
- Jain, K.K. High-pressure neurological syndrome (HPNS). Acta Neurol. Scand. 1994, 90, 45–50. [Google Scholar] [CrossRef]
- Bliznyuk, A.; Grossman, Y.; Moskovitz, Y. The effect of high pressure on the NMDA receptor: Molecular dynamics simulations. Sci. Rep. 2019, 9, 10814. [Google Scholar] [CrossRef]
- Bliznyuk, A.; Hollmann, M.; Grossman, Y. High pressure stress response: Involvement of NMDA receptor subtypes and molecular markers. Front. Physiol. 2019, 10, 1234. [Google Scholar] [CrossRef]
- Li, Y.; Liu, K.; Kang, Z.M.; Sun, X.J.; Liu, W.W.; Mao, Y.F. Helium preconditioning protects against neonatal hypoxia-ischemia via nitric oxide mediated up-regulation of antioxidases in a rat model. Behav. Brain Res. 2016, 300, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, P.; Liu, Y.; Liu, W.; Yin, N. Helium preconditioning protects the brain against hypoxia/ischemia injury via improving the neurovascular niche in a neonatal rat model. Behav. Brain Res. 2016, 314, 165–172. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Huang, C.S.; Kawamura, T.; Peng, X.; Tochigi, N.; Shigemura, N.; Billiar, T.R.; Nakao, A.; Toyoda, Y. Hydrogen inhalation reduced epithelial apoptosis in ventilator-induced lung injury via a mechanism involving nuclear factor-kappa B activation. Biochem. Biophys. Res. Commun. 2011, 408, 253–258. [Google Scholar] [CrossRef]
- Grover, C.A.; Grover, D.H. Albert Behnke: Nitrogen narcosis. J. Emerg. Med. 2014, 46, 225–227. [Google Scholar] [CrossRef]
- Abraini, J.H.; Kriem, B.; Balon, N.; Rostain, J.C.; Risso, J.J. Gamma-aminobutyric acid neuropharmacological investigations on narcosis produced by nitrogen, argon, or nitrous oxide. Anesth. Analg. 2003, 96, 746–749. [Google Scholar] [CrossRef]
- Peng, B.; Hao, D.D.; Li, X.; Wang, G.H.; Guan, Z.Y.; Jiang, Z.L. Inhibition of NR2B-containing NMDA receptors during nitrogen narcosis. Diving Hyperb. Med. 2019, 49, 276–282. [Google Scholar] [CrossRef]
- Antonova, V.V.; Silachev, D.N.; Plotnikov, E.Y.; Pevzner, I.B.; Ivanov, M.E.; Boeva, E.A.; Kalabushev, S.N.; Yadgarov, M.Y.; Cherpakov, R.A.; Grebenchikov, O.A.; et al. Positive effects of argon inhalation after traumatic brain injury in rats. Int. J. Mol. Sci. 2024, 25, 12673. [Google Scholar] [CrossRef]
- Scheid, S.; Lejarre, A.; Wollborn, J.; Buerkle, H.; Goebel, U.; Ulbrich, F. Argon preconditioning protects neuronal cells with a Toll-like receptor-mediated effect. Neural Regen. Res. 2023, 18, 1371–1377. [Google Scholar]
- Goebel, U.; Scheid, S.; Spassov, S.; Schallner, N.; Wollborn, J.; Buerkle, H.; Ulbrich, F. Argon reduces microglial activation and inflammatory cytokine expression in retinal ischemia/reperfusion injury. Neural Regen. Res. 2021, 16, 192–198. [Google Scholar] [CrossRef]
- Xue, K.; Qi, M.; She, T.; Jiang, Z.; Zhang, Y.; Wang, X.; Wang, G.; Xu, L.; Peng, B.; Liu, J.; et al. Argon mitigates post-stroke neuroinflammation by regulating M1/M2 polarization and inhibiting NF-κB/NLRP3 inflammasome signaling. J. Mol. Cell Biol. 2023, 14, mjac077. [Google Scholar] [CrossRef]
- Zhao, H.; Mitchell, S.; Ciechanowicz, S.; Savage, S.; Wang, T.; Ji, X.; Ma, D. Argon protects against hypoxic-ischemic brain injury in neonatal rats through activation of nuclear factor (erythroid-derived 2)-like 2. Oncotarget 2016, 7, 25640. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Yang, T.; Zhao, H.; Fidalgo, A.R.; Vizcaychipi, M.P.; Sanders, R.D.; Yu, B.; Takata, M.; Johnson, M.R.; Ma, D. The protective profile of argon, helium, and xenon in a model of neonatal asphyxia in rats. Crit. Care Med. 2012, 40, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Soto-Gonzalez, L.; Krychtiuk, K.A.; Ruhittel, S.; Kaun, C.; Speidl, W.S.; Kiss, A.; Podesser, B.K.; Yao, S.; Markstaller, K.; et al. Pretreatment with argon protects human cardiac myocyte-like progenitor cells from oxygen glucose deprivation-induced cell death by activation of Akt and differential regulation of MAPkinases. Shock 2018, 49, 556–563. [Google Scholar] [CrossRef]
- Nonaka, K.; Kotani, N.; Akaike, H.; Shin, M.C.; Yamaga, T.; Nagami, H.; Akaike, N. Xenon modulates synaptic transmission to rat hippocampal CA3 neurons at both pre- and postsynaptic sites. J. Physiol. 2019, 597, 5915–5933. [Google Scholar] [CrossRef] [PubMed]
- Kotani, N.; Jang, I.S.; Nakamura, M.; Nonaka, K.; Nagami, H.; Akaike, N. Depression of synaptic N-methyl-D-aspartate responses by xenon and nitrous oxide. J. Pharmacol. Exp. Ther. 2023, 384, 187–196. [Google Scholar] [CrossRef]
- Gruss, M.; Bushell, T.J.; Bright, D.P.; Lieb, W.R.; Mathie, A.; Franks, N.P. Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol. Pharmacol. 2004, 65, 443–452. [Google Scholar] [CrossRef]
- White, J.P.M.; Calcott, G.; Jenes, A.; Hossein, M.; Paule, C.C.; Santha, P.; Davis, J.B.; Ma, D.; Rice, A.S.C.; Nagy, I. Xenon reduces activation of transient receptor potential vanilloid type 1 (TRPV1) in rat dorsal root ganglion cells and in human TRPV1-expressing HEK293 cells. Life Sci. 2011, 88, 141–149. [Google Scholar] [CrossRef]
- Harris, K.; Armstrong, S.P.; Campos-Pires, R.; Kiru, L.; Franks, N.P.; Dickinson, R. Neuroprotection against traumatic brain injury by xenon, but not argon, is mediated by inhibition at the N-methyl-D-aspartate receptor glycine site. Anesthesiology 2013, 119, 1137–1148. [Google Scholar] [CrossRef]
- Bantel, C.; Maze, M.; Trapp, S. Noble gas xenon is a novel adenosine triphosphate-sensitive potassium channel opener. Anesthesiology 2010, 112, 623–630. [Google Scholar] [CrossRef]
- Weber, N.C.; Toma, O.; Wolter, J.I.; Obal, D.; Müllenheim, J.; Preckel, B.; Schlack, W. The noble gas xenon induces pharmacological preconditioning in the rat heart in vivo via induction of PKC-epsilon and p38 MAPK. Br. J. Pharmacol. 2005, 144, 123–132. [Google Scholar] [CrossRef]
- Mio, Y.; Shim, Y.H.; Richards, E.; Bosnjak, Z.J.; Pagel, P.S.; Bienengraeber, M. Xenon preconditioning: The role of prosurvival signaling, mitochondrial permeability transition and bioenergetics in rats. Anesth. Analg. 2009, 108, 858–866. [Google Scholar] [CrossRef]
- Singh, G.; Janicki, P.K.; Horn, J.L.; Janson, V.E.; Franks, J.J. Inhibition of plasma membrane Ca2+-ATPase pump activity in cultured C6 glioma cells by halothane and xenon. Life Sci. 1995, 56, PL219–PL224. [Google Scholar] [CrossRef]
| Gas | Molecular Weight | Diffusion Coefficient | Solubility in Lipid | Oil–Water Solubility Ratio | Density at STP * | Specific Heat Capacity at Constant Pressure | Thermal Conductivity at 0 °C |
|---|---|---|---|---|---|---|---|
| H2 | 2 | 0.41 | 0.036 | 2.1 | 0.0899 | 14.31 | 0.180 |
| He | 4 | 0.35 | 0.015 | 1.7 | 0.1785 | 5.19 | 0.144 |
| Ne | 20 | 0.32 | 0.019 | 2.07 | 0.9002 | 1.03 | 0.049 |
| N2 | 28 | 0.20 | 0.067 | 5.2 | 1.2506 | 1.04 | 0.024 |
| Ar | 40 | 0.16 | 0.14 | 5.3 | 1.7804 | 0.52 | 0.017 |
| Kr | 83.7 | 0.11 | 0.43 | 9.6 | 3.7360 | 0.25 | 0.009 |
| Xe | 131.3 | 0.08 | 1.7 | 20.0 | 5.8870 | 0.16 | 0.005 |
| Gas | Molecular Weight | Solubility in Lipid | Oil–Water Solubility Ratio | Relative Narcotic Potency |
|---|---|---|---|---|
| He | 4 | 0.015 | 1.7 | 0.23 |
| Ne | 20 | 0.019 | 2.07 | 0.28 |
| H2 | 2 | 0.036 | 2.1 | 0.55 |
| N2 | 28 | 0.067 | 5.2 | 1.00 |
| Ar | 40 | 0.14 | 5.3 | 2.33 |
| Kr | 83.7 | 0.43 | 9.6 | 7.14 |
| Xe | 131.3 | 1.7 | 20.0 | 25.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, M.-N.; Li, X.; Cheng, J.; Jiang, Z.-L. Developments in the Study of Inert Gas Biological Effects and the Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2025, 26, 7551. https://doi.org/10.3390/ijms26157551
Tong M-N, Li X, Cheng J, Jiang Z-L. Developments in the Study of Inert Gas Biological Effects and the Underlying Molecular Mechanisms. International Journal of Molecular Sciences. 2025; 26(15):7551. https://doi.org/10.3390/ijms26157551
Chicago/Turabian StyleTong, Mei-Ning, Xia Li, Jie Cheng, and Zheng-Lin Jiang. 2025. "Developments in the Study of Inert Gas Biological Effects and the Underlying Molecular Mechanisms" International Journal of Molecular Sciences 26, no. 15: 7551. https://doi.org/10.3390/ijms26157551
APA StyleTong, M.-N., Li, X., Cheng, J., & Jiang, Z.-L. (2025). Developments in the Study of Inert Gas Biological Effects and the Underlying Molecular Mechanisms. International Journal of Molecular Sciences, 26(15), 7551. https://doi.org/10.3390/ijms26157551






