DHA–Triacylglycerol Accumulation in Tacrolimus-Induced Nephrotoxicity Identified by Lipidomic Profiling
Abstract
1. Introduction
2. Results
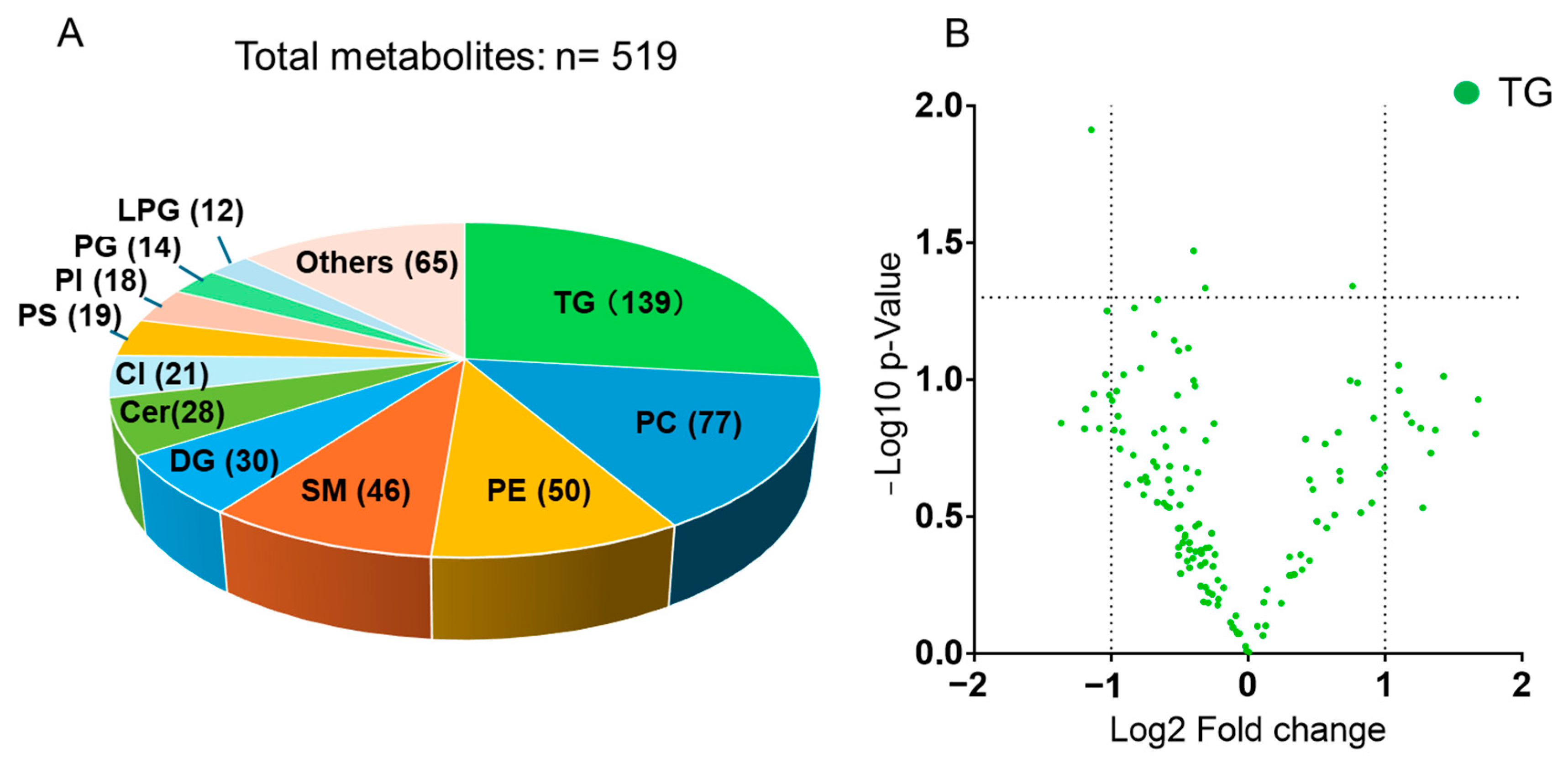
2.1. Summary of Lipidomic Results
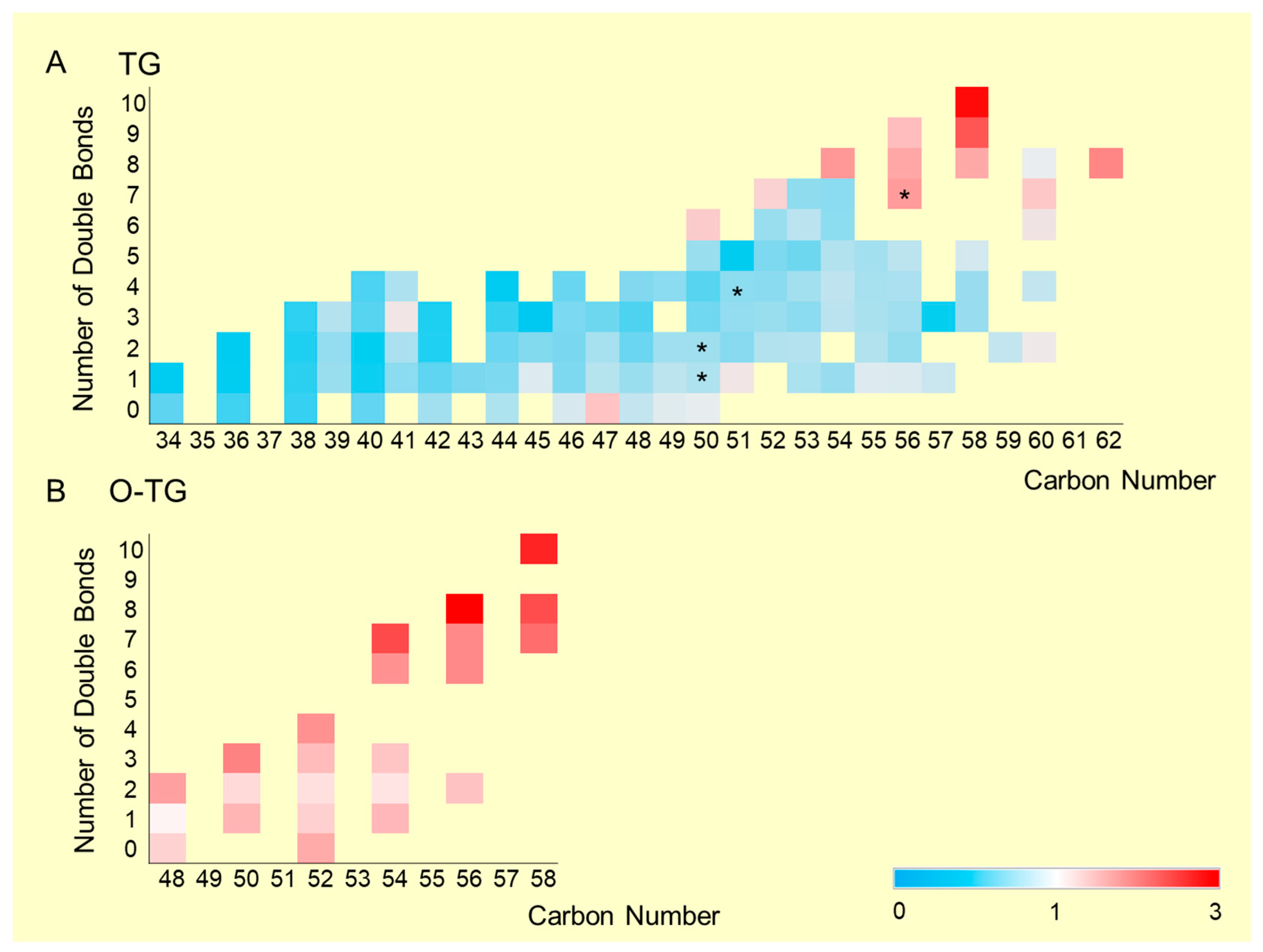
2.2. Distribution and Trends of Detected TGs
2.3. TGs Containing Major Polyunsaturated Fatty Acids (PUFAs)
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Lipidomic Analysis
4.2.1. Materials for Lipid Analysis
4.2.2. Lipid Preparation
4.2.3. Lipidomic Analysis by LC-MS/MS
4.3. Statistical Analysis and Graphic Design
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGC | automatic gain control |
| Cer | ceramide |
| CKD | chronic kidney disease |
| Cl | cardiolipin |
| DHA | docosahexaenoic acid |
| DG | diglyceride |
| DPA | docosapentaenoic acid |
| FFA | free fatty acid |
| HESI | heated electrospray ionization |
| LC | liquid chromatography |
| LPG | Lysophosphatidylglycerol, |
| MS | mass spectrometry |
| O-TG | ether-linked triacylglycerol |
| PC | phosphatidylcholine |
| PE | phosphatidylethanolamine |
| PG | Phosphatidylglycerol |
| PI | phosphatidylinositol |
| PS | phosphatidylserine |
| PUFA | polyunsaturated fatty acid |
| SM | sphingomyelin |
| TAC | tacrolimus |
| TG | triacylglycerol |
References
- Dheer, D.; Jyoti; Gupta, P.N.; Shankar, R. Tacrolimus: An updated review on delivering strategies for multifarious diseases. Eur. J. Pharm. Sci. 2018, 114, 217–227. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Pintoffl, J.P.; Sturm, A.; Wiedenmann, B.; Dignass, A.U. Tacrolimus is safe and effective in patients with severe steroid-refractory or steroid-dependent inflammatory bowel disease--a long-term follow-up. Am. J. Gastroenterol. 2006, 101, 1048–1056. [Google Scholar] [CrossRef]
- Kitahara, K.; Kawai, S. Cyclosporine and tacrolimus for the treatment of rheumatoid arthritis. Curr. Opin. Rheumatol. 2007, 19, 238–245. [Google Scholar] [CrossRef]
- Ekberg, H.; Tedesco-Silva, H.; Demirbas, A.; Vítko, Š.; Nashan, B.; Gürkan, A.; Margreiter, R.; Hugo, C.; Grinyó, J.M.; Frei, U.; et al. Reduced Exposure to Calcineurin Inhibitors in Renal Transplantation. N. Engl. J. Med. 2007, 357, 2562–2575. [Google Scholar] [CrossRef]
- Hart, A.; Smith, J.M.; Skeans, M.A.; Gustafson, S.K.; Wilk, A.R.; Castro, S.; Robinson, A.; Wainright, J.L.; Snyder, J.J.; Kasiske, B.L.; et al. OPTN/SRTR 2017 Annual Data Report: Kidney. Am. J. Transpl. 2019, 19 (Suppl. S2), 19–123. [Google Scholar] [CrossRef]
- Thomson, A.W.; Bonham, C.A.; Zeevi, A. Mode of action of tacrolimus (FK506): Molecular and cellular mechanisms. Ther. Drug Monit. 1995, 17, 584–591. [Google Scholar] [CrossRef]
- Farouk, S.S.; Rein, J.L. The Many Faces of Calcineurin Inhibitor Toxicity—What the FK? Adv. Chronic Kidney Dis. 2020, 27, 56–66. [Google Scholar] [CrossRef]
- Bentata, Y. Tacrolimus: 20 years of use in adult kidney transplantation. What we should know about its nephrotoxicity. Artif. Organs 2020, 44, 140–152. [Google Scholar] [CrossRef]
- Stegall, M.D.; Cornell, L.D.; Park, W.D.; Smith, B.H.; Cosio, F.G. Renal Allograft Histology at 10 Years After Transplantation in the Tacrolimus Era: Evidence of Pervasive Chronic Injury. Am. J. Transpl. 2018, 18, 180–188. [Google Scholar] [CrossRef]
- Randhawa, P.S.; Shapiro, R.; Jordan, M.L.; Starzl, T.E.; Demetris, A.J. The Histopathological Changes Associated with Allograft Rejection and Drug Toxicity in Renal Transplant Recipients Maintained on FK506. Am. J. Surg. Pathol. 1993, 17, 60–68. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Ma, S.-X.; Chen, Y.-Y.; Chen, L.; Liu, B.-L.; Liu, Q.-Q.; Zhao, Y.-Y. Chronic kidney disease: Biomarker diagnosis to therapeutic targets. Clin. Chim. Acta 2019, 499, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.O.; Townsend, R.R.; Feldman, H.I.; Pappan, K.L.; Kensicki, E.; Vander Jagt, D.L. Plasma metabolomic profiles in different stages of CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 363–370. [Google Scholar] [CrossRef]
- Chasapi, S.A.; Karagkouni, E.; Kalavrizioti, D.; Vamvakas, S.; Zompra, A.; Takis, P.G.; Goumenos, D.S.; Spyroulias, G.A. NMR-Based Metabolomics in Differential Diagnosis of Chronic Kidney Disease (CKD) Subtypes. Metabolites 2022, 12, 490. [Google Scholar] [CrossRef]
- Han, X.; Ye, H. Overview of Lipidomic Analysis of Triglyceride Molecular Species in Biological Lipid Extracts. J. Agric. Food Chem. 2021, 69, 8895–8909. [Google Scholar] [CrossRef]
- Nishida, S.; Ishima, T.; Kimura, N.; Iwami, D.; Nagai, R.; Imai, Y.; Aizawa, K. Metabolomic Profiling of Mice with Tacrolimus-Induced Nephrotoxicity: Carnitine Deficiency in Renal Tissue. Biomedicines 2024, 12, 521. [Google Scholar] [CrossRef]
- Nishida, S.; Ishima, T.; Iwami, D.; Nagai, R.; Aizawa, K. Whole Blood Metabolomic Profiling of Mice with Tacrolimus-Induced Chronic Nephrotoxicity: NAD+ Depletion with Salvage Pathway Impairment. Antioxidants 2025, 14, 62. [Google Scholar] [CrossRef]
- Afshinnia, F.; Rajendiran, T.M.; Wernisch, S.; Soni, T.; Jadoon, A.; Karnovsky, A.; Michailidis, G.; Pennathur, S. Lipidomics and Biomarker Discovery in Kidney Disease. Semin. Nephrol. 2018, 38, 127–141. [Google Scholar] [CrossRef]
- Lidgard, B.; Hoofnagle, A.N.; Zelnick, L.R.; de Boer, I.H.; Fretts, A.M.; Kestenbaum, B.R.; Lemaitre, R.N.; Robinson-Cohen, C.; Bansal, N. High-Density Lipoprotein Lipidomics and Mortality in CKD. Kidney Med. 2023, 5, 100708. [Google Scholar] [CrossRef]
- Baek, J.; He, C.; Afshinnia, F.; Michailidis, G.; Pennathur, S. Lipidomic approaches to dissect dysregulated lipid metabolism in kidney disease. Nat. Rev. Nephrol. 2022, 18, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, J.; Wu, C.; Wang, L.; Liu, P.; Li, P. Lipidomics-based natural products for chronic kidney disease treatment. Heliyon 2025, 11, e41620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Guan, L.; Chen, Y.; Chen, P.; Sun, J.; Gonzalez, F.J.; Huang, M.; Bi, H. Lipidomics reveals carnitine palmitoyltransferase 1C protects cancer cells from lipotoxicity and senescence. J. Pharm. Anal. 2021, 11, 340–350. [Google Scholar] [CrossRef]
- Wang, Y.N.; Zhang, Z.H.; Liu, H.J.; Guo, Z.Y.; Zou, L.; Zhang, Y.M.; Zhao, Y.Y. Integrative phosphatidylcholine metabolism through phospholipase A(2) in rats with chronic kidney disease. Acta Pharmacol. Sin. 2023, 44, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Afshinnia, F.; Nair, V.; Lin, J.; Rajendiran, T.M.; Soni, T.; Byun, J.; Sharma, K.; Fort, P.E.; Gardner, T.W.; Looker, H.C.; et al. Increased lipogenesis and impaired β-oxidation predict type 2 diabetic kidney disease progression in American Indians. JCI Insight 2019, 4, e130317. [Google Scholar] [CrossRef]
- Afshinnia, F.; Rajendiran, T.M.; Soni, T.; Byun, J.; Wernisch, S.; Sas, K.M.; Hawkins, J.; Bellovich, K.; Gipson, D.; Michailidis, G.; et al. Impaired β-Oxidation and Altered Complex Lipid Fatty Acid Partitioning with Advancing CKD. J. Am. Soc. Nephrol. 2018, 29, 295–306. [Google Scholar] [CrossRef]
- Sieber, J.; Weins, A.; Kampe, K.; Gruber, S.; Lindenmeyer, M.T.; Cohen, C.D.; Orellana, J.M.; Mundel, P.; Jehle, A.W. Susceptibility of podocytes to palmitic acid is regulated by stearoyl-CoA desaturases 1 and 2. Am. J. Pathol. 2013, 183, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Demirci, H.; Popovic, S.; Dittmayer, C.; Yilmaz, D.E.; El-Shimy, I.A.; Mülleder, M.; Hinze, C.; Su, M.; Mertins, P.; Kirchner, M.; et al. Immunosuppression with cyclosporine versus tacrolimus shows distinctive nephrotoxicity profiles within renal compartments. Acta Physiol. (Oxford) 2024, 240, e14190. [Google Scholar] [CrossRef] [PubMed]
- Oyouni, A.A.A.; Saggu, S.; Tousson, E.; Rehman, H. Immunosuppressant drug tacrolimus induced mitochondrial nephrotoxicity, modified PCNA and Bcl-2 expression attenuated by Ocimum basilicum L. in CD1 mice. Toxicol. Rep. 2018, 5, 687–694. [Google Scholar] [CrossRef]
- Borgonovi, S.M.; Iametti, S.; Di Nunzio, M. Docosahexaenoic Acid as Master Regulator of Cellular Antioxidant Defenses: A Systematic Review. Antioxidants 2023, 12, 1283. [Google Scholar] [CrossRef]
- Koh, H.B.; Kim, H.W.; Joo, Y.S.; Jung, C.Y.; Kim, H.J.; Chang, T.I.; Park, J.T.; Yoo, T.H.; Kang, S.W.; Han, S.H. Plasma Levels of Polyunsaturated Fatty Acids and Adverse Kidney Outcomes. Am. J. Kidney Dis. 2024, 84, 179–194.e171. [Google Scholar] [CrossRef]
- Kimura, S.; Minami, M.; Saito, H.; Kobayashi, T.; Okuyama, H. Dietary docosahexaenoic acid (22: 6n-3) prevents the development of hypertension in SHRSP. Clin. Exp. Pharmacol. Physiol. Suppl. 1995, 22, S308–S309. [Google Scholar] [CrossRef]
- Liu, L.; Cai, H.; Yang, H.; Wang, S.; Li, Y.; Huang, Y.; Gao, M.; Zhang, X.; Zhang, X.; Wang, H.; et al. Targeted metabolomics identified novel metabolites, predominantly phosphatidylcholines and docosahexaenoic acid-containing lipids, predictive of incident chronic kidney disease in middle-to-elderly-aged Chinese adults. Metabolism 2025, 163, 156085. [Google Scholar] [CrossRef]
- Drouin, G.; Rioux, V.; Legrand, P. The n-3 docosapentaenoic acid (DPA): A new player in the n-3 long chain polyunsaturated fatty acid family. Biochimie 2019, 159, 36–48. [Google Scholar] [CrossRef]
- Vandenbussche, C.; Van der Hauwaert, C.; Dewaeles, E.; Franczak, J.; Hennino, M.F.; Gnemmi, V.; Savary, G.; Tavernier, Q.; Nottet, N.; Paquet, A.; et al. Tacrolimus-induced nephrotoxicity in mice is associated with microRNA deregulation. Arch. Toxicol. 2018, 92, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Isogai, N.; Shiono, Y.; Kuramoto, T.; Yoshioka, K.; Ishihama, H.; Funao, H.; Nakamura, M.; Matsumoto, M.; Ishii, K. Potential osteomyelitis biomarkers identified by plasma metabolome analysis in mice. Sci. Rep. 2020, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Khattri, R.B.; Thome, T.; Ryan, T.E. Tissue-Specific 1H-NMR Metabolomic Profiling in Mice with Adenine-Induced Chronic Kidney Disease. Metabolites 2021, 11, 45. [Google Scholar] [CrossRef]
- Zhou, Q.; Kerbl-Knapp, J.; Zhang, F.; Korbelius, M.; Kuentzel, K.B.; Vujić, N.; Akhmetshina, A.; Hörl, G.; Paar, M.; Steyrer, E.; et al. Metabolomic Profiles of Mouse Tissues Reveal an Interplay between Aging and Energy Metabolism. Metabolites 2021, 12, 17. [Google Scholar] [CrossRef]
- Iuchi, Y.; Okada, F.; Takamiya, R.; Kibe, N.; Tsunoda, S.; Nakajima, O.; Toyoda, K.; Nagae, R.; Suematsu, M.; Soga, T.; et al. Rescue of anaemia and autoimmune responses in SOD1-deficient mice by transgenic expression of human SOD1 in erythrocytes. Biochem. J. 2009, 422, 313–320. [Google Scholar] [CrossRef]
- Nishiumi, S.; Izumi, Y.; Hirayama, A.; Takahashi, M.; Nakao, M.; Hata, K.; Saigusa, D.; Hishinuma, E.; Matsukawa, N.; Tokuoka, S.M.; et al. Comparative Evaluation of Plasma Metabolomic Data from Multiple Laboratories. Metabolites 2022, 12, 135. [Google Scholar] [CrossRef]
- Takumi, H.; Kato, K.; Nakanishi, H.; Tamura, M.; Ohto, N.T.; Nagao, S.; Hirose, J. Comprehensive Analysis of Lipid Composition in Human Foremilk and Hindmilk. J. Oleo Sci. 2022, 71, 947–957. [Google Scholar] [CrossRef]
- Liebisch, G.; Ahrends, R.; Arita, M.; Arita, M.; Bowden, J.A.; Ejsing, C.S.; Griffiths, W.J.; Holčapek, M.; Köfeler, H.; Mitchell, T.W.; et al. Lipidomics needs more standardization. Nat. Metab. 2019, 1, 745–747. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishida, S.; Ishima, T.; Iwami, D.; Nagai, R.; Aizawa, K. DHA–Triacylglycerol Accumulation in Tacrolimus-Induced Nephrotoxicity Identified by Lipidomic Profiling. Int. J. Mol. Sci. 2025, 26, 7549. https://doi.org/10.3390/ijms26157549
Nishida S, Ishima T, Iwami D, Nagai R, Aizawa K. DHA–Triacylglycerol Accumulation in Tacrolimus-Induced Nephrotoxicity Identified by Lipidomic Profiling. International Journal of Molecular Sciences. 2025; 26(15):7549. https://doi.org/10.3390/ijms26157549
Chicago/Turabian StyleNishida, Sho, Tamaki Ishima, Daiki Iwami, Ryozo Nagai, and Kenichi Aizawa. 2025. "DHA–Triacylglycerol Accumulation in Tacrolimus-Induced Nephrotoxicity Identified by Lipidomic Profiling" International Journal of Molecular Sciences 26, no. 15: 7549. https://doi.org/10.3390/ijms26157549
APA StyleNishida, S., Ishima, T., Iwami, D., Nagai, R., & Aizawa, K. (2025). DHA–Triacylglycerol Accumulation in Tacrolimus-Induced Nephrotoxicity Identified by Lipidomic Profiling. International Journal of Molecular Sciences, 26(15), 7549. https://doi.org/10.3390/ijms26157549





