The Therapeutic Potential of Glymphatic System Activity to Reduce the Pathogenic Accumulation of Cytotoxic Proteins in Alzheimer’s Disease
Abstract
1. Background
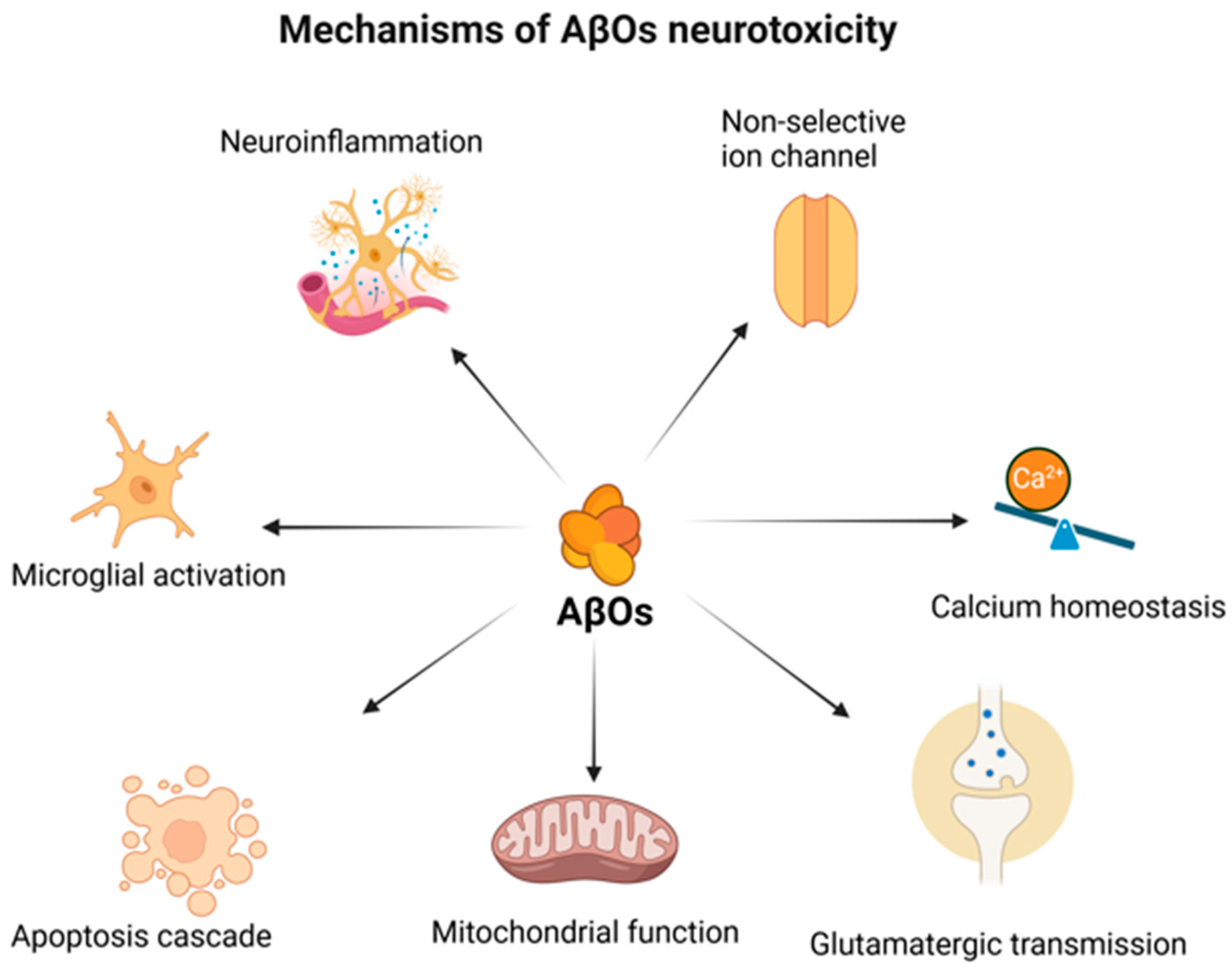
2. The (Un)certain Role of Amyloid-β in Neurodegeneration
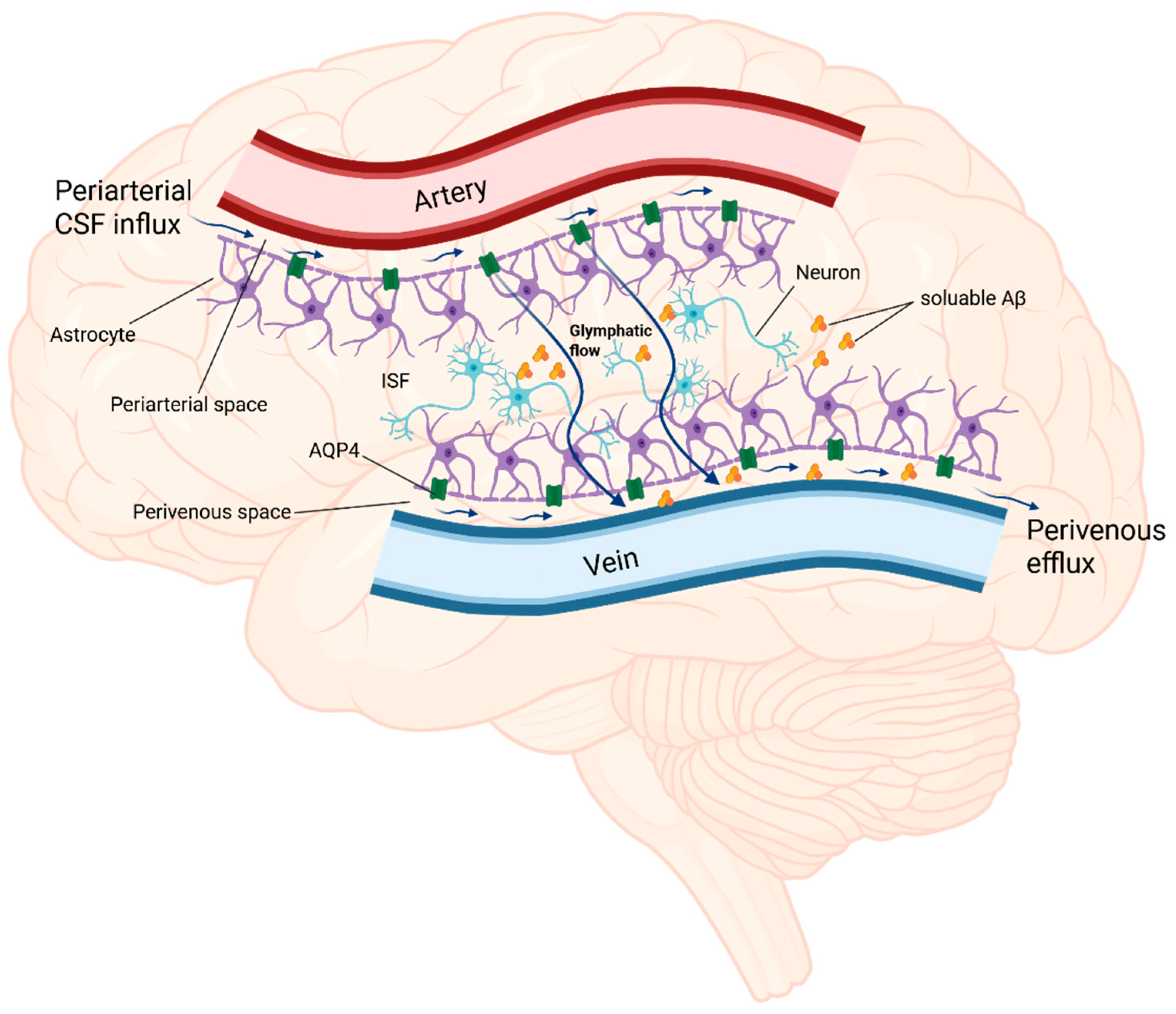
3. The Glymphatic System—Its Role in Pathogenesis of AD
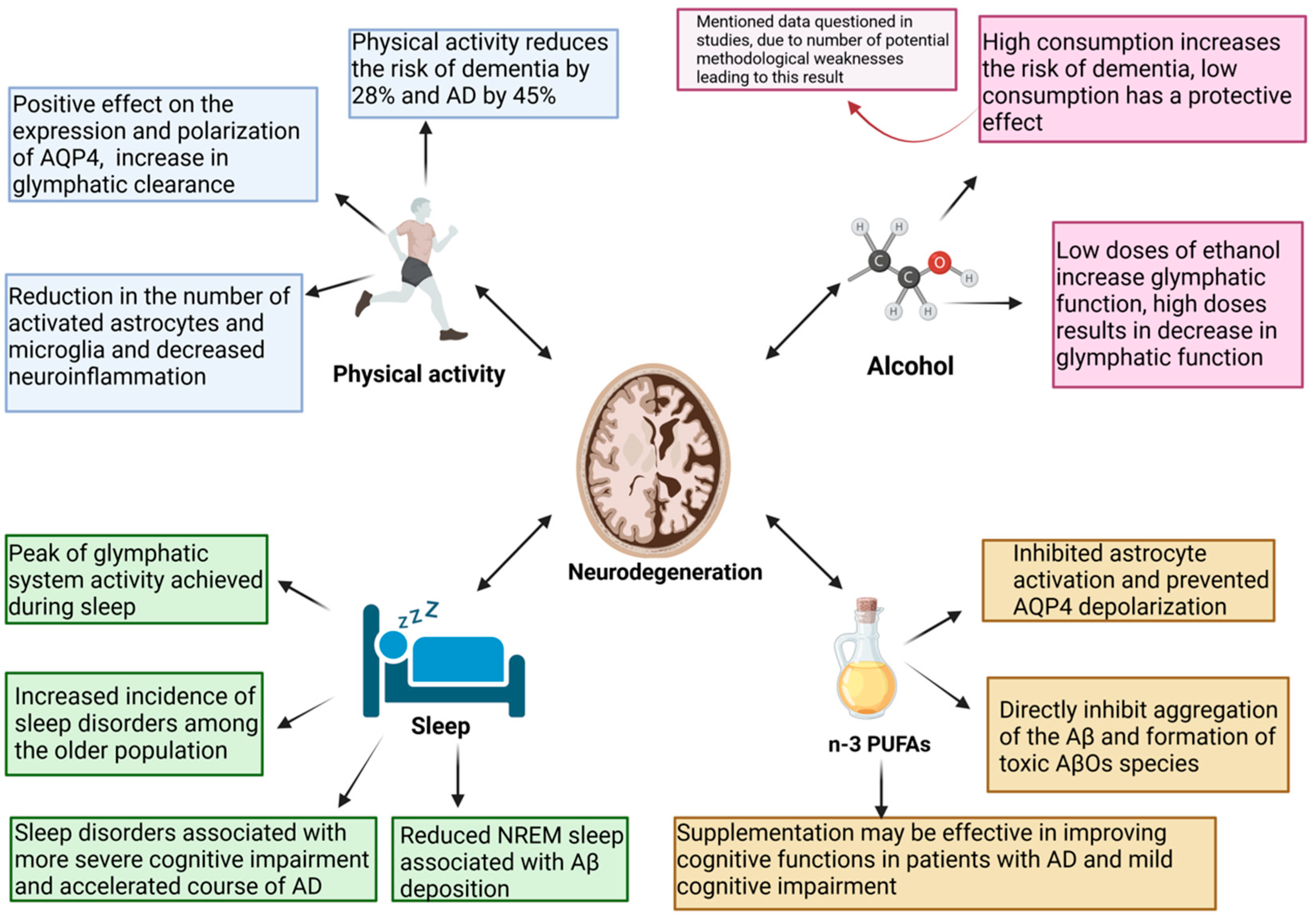
4. The Glymphatic System—A Novel Potential Therapeutic Target
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santiago, J.A.; Potashkin, J.A. The Impact of Disease Comorbidities in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 631770. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Mao, C.; Hu, X.; Zhang, S.; Yang, Z.; Hu, Z.; Sun, H.; Fan, Y.; Dong, Y.; Yang, J.; et al. New Insights Into the Pathogenesis of Alzheimer’s Disease. Front. Neurol. 2019, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, G.; Chakrabarti, S.; Chatterjee, U.; Saso, L. Proteinopathy, oxidative stress and mitochondrial dysfunction: Cross talk in Alzheimer’s disease and Parkinson’s disease. Drug. Des. Devel. Ther. 2017, 11, 797–810. [Google Scholar] [CrossRef]
- Brookmeyer, R.; Corrada, M.M.; Curriero, F.C.; Kawas, C. Survival following a diagnosis of Alzheimer disease. Arch. Neurol. 2002, 59, 1764–1767. [Google Scholar] [CrossRef]
- Vellone, E.; Piras, G.; Talucci, C.; Cohen, M.Z. Quality of life for caregivers of people with Alzheimer’s disease. J. Adv. Nurs. 2008, 61, 222–231. [Google Scholar] [CrossRef]
- Grand, J.H.; Caspar, S.; Macdonald, S.W. Clinical features and multidisciplinary approaches to dementia care. J. Multidiscip. Healthc. 2011, 4, 125–147. [Google Scholar] [CrossRef]
- 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023, 19, 1598–1695. [CrossRef]
- Niu, H.; Alvarez-Alvarez, I.; Guillen-Grima, F.; Aguinaga-Ontoso, I. Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurologia 2017, 32, 523–532. [Google Scholar] [CrossRef]
- Nagy, Z.; Esiri, M.M.; Jobst, K.A.; Morris, J.H.; King, E.M.; McDonald, B.; Litchfield, S.; Smith, A.; Barnetson, L.; Smith, A.D. Relative roles of plaques and tangles in the dementia of Alzheimer’s disease: Correlations using three sets of neuropathological criteria. Dementia 1995, 6, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Price, J.L.; Morris, J.C. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann. Neurol. 1999, 45, 358–368. [Google Scholar] [CrossRef]
- Goedert, M. Tau protein and the neurofibrillary pathology of Alzheimer’s disease. Ann. N. Y. Acad. Sci. 1996, 777, 121–131. [Google Scholar] [CrossRef]
- Kovacs, G.G. Molecular pathology of neurodegenerative diseases: Principles and practice. J. Clin. Pathol. 2019, 72, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Jiang, Z.F. Accumulated amyloid-beta peptide and hyperphosphorylated tau protein: Relationship and links in Alzheimer’s disease. J. Alzheimers. Dis. 2009, 16, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-beta: A crucial factor in Alzheimer’s disease. Med. Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Muller, U.C.; Deller, T.; Korte, M. Not just amyloid: Physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 2017, 18, 281–298. [Google Scholar] [CrossRef]
- Chow, V.W.; Mattson, M.P.; Wong, P.C.; Gleichmann, M. An overview of APP processing enzymes and products. Neuromolecular Med. 2010, 12, 1–12. [Google Scholar] [CrossRef]
- Tolar, M.; Hey, J.; Power, A.; Abushakra, S. Neurotoxic Soluble Amyloid Oligomers Drive Alzheimer’s Pathogenesis and Represent a Clinically Validated Target for Slowing Disease Progression. Int. J. Mol. Sci. 2021, 22, 6355. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Rogers, J.; Morrison, J.H. Quantitative morphology and regional and laminar distributions of senile plaques in Alzheimer’s disease. J. Neurosci. 1985, 5, 2801–2808. [Google Scholar] [CrossRef]
- Permanne, B.; Adessi, C.; Fraga, S.; Frossard, M.J.; Saborio, G.P.; Soto, C. Are beta-sheet breaker peptides dissolving the therapeutic problem of Alzheimer’s disease? J. Neural Transm. 2002, 62, 293–301. [Google Scholar] [CrossRef]
- Novakovic, D.; Feligioni, M.; Scaccianoce, S.; Caruso, A.; Piccinin, S.; Schepisi, C.; Errico, F.; Mercuri, N.B.; Nicoletti, F.; Nistico, R. Profile of gantenerumab and its potential in the treatment of Alzheimer’s disease. Drug Des. Devel. Ther. 2013, 7, 1359–1364. [Google Scholar] [CrossRef]
- Kepp, K.P.; Robakis, N.K.; Hoilund-Carlsen, P.F.; Sensi, S.L.; Vissel, B. The amyloid cascade hypothesis: An updated critical review. Brain 2023, 146, 3969–3990. [Google Scholar] [CrossRef]
- Herrup, K. The case for rejecting the amyloid cascade hypothesis. Nat. Neurosci. 2015, 18, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Ricciarelli, R.; Fedele, E. The Amyloid Cascade Hypothesis in Alzheimer’s Disease: It’s Time to Change Our Mind. Curr. Neuropharmacol. 2017, 15, 926–935. [Google Scholar] [CrossRef]
- Hoilund-Carlsen, P.F.; Revheim, M.E.; Costa, T.; Alavi, A.; Kepp, K.P.; Sensi, S.L.; Perry, G.; Robakis, N.K.; Barrio, J.R.; Vissel, B. Passive Alzheimer’s immunotherapy: A promising or uncertain option? Ageing Res. Rev. 2023, 90, 101996. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Rudkowska, M.; Orzeł-Sajdłowska, A. Aducanumab—Hope or Disappointment for Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4367. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; De Strooper, B. Alzheimer’s disease: Where next for anti-amyloid therapies? Brain 2017, 140, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Tampi, R.R.; Forester, B.P.; Agronin, M. Aducanumab: Evidence from clinical trial data and controversies. Drugs Context 2021, 10. [Google Scholar] [CrossRef]
- Fedele, E. Anti-Amyloid Therapies for Alzheimer’s Disease and the Amyloid Cascade Hypothesis. Int. J. Mol. Sci. 2023, 24, 14499. [Google Scholar] [CrossRef]
- Mullard, A. FDA approves third anti-amyloid antibody for Alzheimer disease. Nat. Rev. Drug Discov. 2024, 23, 571. [Google Scholar] [CrossRef]
- Lowe, S.L.; Willis, B.A.; Hawdon, A.; Natanegara, F.; Chua, L.; Foster, J.; Shcherbinin, S.; Ardayfio, P.; Sims, J.R. Donanemab (LY3002813) dose-escalation study in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12112. [Google Scholar] [CrossRef]
- Swanson, C.J.; Zhang, Y.; Dhadda, S.; Wang, J.; Kaplow, J.; Lai, R.Y.K.; Lannfelt, L.; Bradley, H.; Rabe, M.; Koyama, A.; et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Abeta protofibril antibody. Alzheimers Res. Ther. 2021, 13, 80. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Espay, A.J.; Kepp, K.P.; Herrup, K. Lecanemab and Donanemab as Therapies for Alzheimer’s Disease: An Illustrated Perspective on the Data. eNeuro 2024, 11. [Google Scholar] [CrossRef]
- Alves, F.; Kalinowski, P.; Ayton, S. Accelerated Brain Volume Loss Caused by Anti-beta-Amyloid Drugs: A Systematic Review and Meta-analysis. Neurology 2023, 100, e2114–e2124. [Google Scholar] [CrossRef]
- Honig, L.S.; Barakos, J.; Dhadda, S.; Kanekiyo, M.; Reyderman, L.; Irizarry, M.; Kramer, L.D.; Swanson, C.J.; Sabbagh, M. ARIA in patients treated with lecanemab (BAN2401) in a phase 2 study in early Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2023, 9, e12377. [Google Scholar] [CrossRef] [PubMed]
- Honig, L.S.; Sabbagh, M.N.; van Dyck, C.H.; Sperling, R.A.; Hersch, S.; Matta, A.; Giorgi, L.; Gee, M.; Kanekiyo, M.; Li, D.; et al. Updated safety results from phase 3 lecanemab study in early Alzheimer’s disease. Alzheimers Res. Ther. 2024, 16, 105. [Google Scholar] [CrossRef]
- Zimmer, J.A.; Ardayfio, P.; Wang, H.; Khanna, R.; Evans, C.D.; Lu, M.; Sparks, J.; Andersen, S.; Lauzon, S.; Nery, E.S.M.; et al. Amyloid-Related Imaging Abnormalities With Donanemab in Early Symptomatic Alzheimer Disease: Secondary Analysis of the TRAILBLAZER-ALZ and ALZ 2 Randomized Clinical Trials. JAMA Neurol. 2025, 82, 461–469. [Google Scholar] [CrossRef]
- Espay, A.J.; Vizcarra, J.A.; Marsili, L.; Lang, A.E.; Simon, D.K.; Merola, A.; Josephs, K.A.; Fasano, A.; Morgante, F.; Savica, R.; et al. Revisiting protein aggregation as pathogenic in sporadic Parkinson and Alzheimer diseases. Neurology 2019, 92, 329–337. [Google Scholar] [CrossRef]
- Delaere, P.; Duyckaerts, C.; Masters, C.; Beyreuther, K.; Piette, F.; Hauw, J.J. Large amounts of neocortical beta A4 deposits without neuritic plaques nor tangles in a psychometrically assessed, non-demented person. Neurosci. Lett. 1990, 116, 87–93. [Google Scholar] [CrossRef]
- Aizenstein, H.J.; Nebes, R.D.; Saxton, J.A.; Price, J.C.; Mathis, C.A.; Tsopelas, N.D.; Ziolko, S.K.; James, J.A.; Snitz, B.E.; Houck, P.R.; et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol. 2008, 65, 1509–1517. [Google Scholar] [CrossRef]
- Polvikoski, T.; Sulkava, R.; Myllykangas, L.; Notkola, I.L.; Niinisto, L.; Verkkoniemi, A.; Kainulainen, K.; Kontula, K.; Perez-Tur, J.; Hardy, J.; et al. Prevalence of Alzheimer’s disease in very elderly people: A prospective neuropathological study. Neurology 2001, 56, 1690–1696. [Google Scholar] [CrossRef]
- Berlau, D.J.; Corrada, M.M.; Head, E.; Kawas, C.H. APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology 2009, 72, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Viola, K.L.; Klein, W.L. Amyloid beta oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015, 129, 183–206. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, X.; Ma, L.; Wei, W.; Li, Z.; Chang, S.; Wen, J.; Sun, J.; Li, H. Role of Abeta in Alzheimer’s-related synaptic dysfunction. Front. Cell Dev. Biol. 2022, 10, 964075. [Google Scholar] [CrossRef]
- Lee, S.J.; Nam, E.; Lee, H.J.; Savelieff, M.G.; Lim, M.H. Towards an understanding of amyloid-beta oligomers: Characterization, toxicity mechanisms, and inhibitors. Chem. Soc. Rev. 2017, 46, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Okamoto, S.; Lipton, S.A.; Xu, H. Oligomeric Abeta-induced synaptic dysfunction in Alzheimer’s disease. Mol. Neurodegener. 2014, 9, 48. [Google Scholar] [CrossRef]
- Bode, D.C.; Baker, M.D.; Viles, J.H. Ion Channel Formation by Amyloid-beta42 Oligomers but Not Amyloid-beta40 in Cellular Membranes. J. Biol. Chem. 2017, 292, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- McDaid, J.; Mustaly-Kalimi, S.; Stutzmann, G.E. Ca2+ Dyshomeostasis Disrupts Neuronal and Synaptic Function in Alzheimer’s Disease. Cells 2020, 9, 2655. [Google Scholar] [CrossRef]
- Bezprozvanny, I.B. Calcium signaling and neurodegeneration. Acta Naturae 2010, 2, 72–82. [Google Scholar] [CrossRef]
- Ferreira, A. Calpain dysregulation in Alzheimer’s disease. ISRN Biochem. 2012, 2012, 728571. [Google Scholar] [CrossRef]
- Bukke, V.N.; Archana, M.; Villani, R.; Romano, A.D.; Wawrzyniak, A.; Balawender, K.; Orkisz, S.; Beggiato, S.; Serviddio, G.; Cassano, T. The Dual Role of Glutamatergic Neurotransmission in Alzheimer’s Disease: From Pathophysiology to Pharmacotherapy. Int. J. Mol. Sci. 2020, 21, 7452. [Google Scholar] [CrossRef]
- Meldrum, B.S. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [CrossRef]
- Danysz, W.; Parsons, C.G. Alzheimer’s disease, beta-amyloid, glutamate, NMDA receptors and memantine--searching for the connections. Br. J. Pharmacol. 2012, 167, 324–352. [Google Scholar] [CrossRef] [PubMed]
- Brito-Moreira, J.; Paula-Lima, A.C.; Bomfim, T.R.; Oliveira, F.B.; Sepulveda, F.J.; De Mello, F.G.; Aguayo, L.G.; Panizzutti, R.; Ferreira, S.T. Abeta oligomers induce glutamate release from hippocampal neurons. Curr. Alzheimer. Res. 2011, 8, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hong, S.; Shepardson, N.E.; Walsh, D.M.; Shankar, G.M.; Selkoe, D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 2009, 62, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, M.; Koeglsperger, T.; Shepardson, N.E.; Shankar, G.M.; Selkoe, D.J. Soluble Abeta oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J. Neurosci. 2011, 31, 6627–6638. [Google Scholar] [CrossRef]
- Dringenberg, H.C. The history of long-term potentiation as a memory mechanism: Controversies, confirmation, and some lessons to remember. Hippocampus 2020, 30, 987–1012. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Mollinari, C.; Garaci, E.; Merlo, D.; Pei, G. Amyloid-beta Oligomers-Induced Mitochondrial DNA Repair Impairment Contributes to Altered Human Neural Stem Cell Differentiation. Curr. Alzheimer. Res. 2019, 16, 934–949. [Google Scholar] [CrossRef]
- Rui, Y.; Zheng, J.Q. Amyloid beta oligomers elicit mitochondrial transport defects and fragmentation in a time-dependent and pathway-specific manner. Mol. Brain 2016, 9, 79. [Google Scholar] [CrossRef]
- Kim, J.; Yang, Y.; Song, S.S.; Na, J.H.; Oh, K.J.; Jeong, C.; Yu, Y.G.; Shin, Y.K. Beta-amyloid oligomers activate apoptotic BAK pore for cytochrome c release. Biophys. J. 2014, 107, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Bachiller, S.; Jimenez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front. Cell Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef]
- Yin, J.; Valin, K.L.; Dixon, M.L.; Leavenworth, J.W. The Role of Microglia and Macrophages in CNS Homeostasis, Autoimmunity, and Cancer. J. Immunol. Res. 2017, 2017, 5150678. [Google Scholar] [CrossRef]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef]
- Balducci, C.; Forloni, G. Doxycycline for Alzheimer’s Disease: Fighting beta-Amyloid Oligomers and Neuroinflammation. Front. Pharmacol. 2019, 10, 738. [Google Scholar] [CrossRef]
- Heurtaux, T.; Michelucci, A.; Losciuto, S.; Gallotti, C.; Felten, P.; Dorban, G.; Grandbarbe, L.; Morga, E.; Heuschling, P. Microglial activation depends on beta-amyloid conformation: Role of the formylpeptide receptor 2. J. Neurochem. 2010, 114, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; Heurtaux, T.; Grandbarbe, L.; Morga, E.; Heuschling, P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J. Neuroimmunol. 2009, 210, 3–12. [Google Scholar] [CrossRef]
- Salvadores, N.; Moreno-Gonzalez, I.; Gamez, N.; Quiroz, G.; Vegas-Gomez, L.; Escandon, M.; Jimenez, S.; Vitorica, J.; Gutierrez, A.; Soto, C.; et al. Abeta oligomers trigger necroptosis-mediated neurodegeneration via microglia activation in Alzheimer’s disease. Acta Neuropathol. Commun. 2022, 10, 31. [Google Scholar] [CrossRef]
- Parajuli, B.; Sonobe, Y.; Horiuchi, H.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Oligomeric amyloid beta induces IL-1beta processing via production of ROS: Implication in Alzheimer’s disease. Cell Death Dis. 2013, 4, e975. [Google Scholar] [CrossRef]
- Nakanishi, A.; Kaneko, N.; Takeda, H.; Sawasaki, T.; Morikawa, S.; Zhou, W.; Kurata, M.; Yamamoto, T.; Akbar, S.M.F.; Zako, T.; et al. Amyloid beta directly interacts with NLRP3 to initiate inflammasome activation: Identification of an intrinsic NLRP3 ligand in a cell-free system. Inflamm. Regen. 2018, 38, 27. [Google Scholar] [CrossRef]
- Di Battista, A.M.; Heinsinger, N.M.; Rebeck, G.W. Alzheimer’s Disease Genetic Risk Factor APOE-epsilon4 Also Affects Normal Brain Function. Curr. Alzheimer Res. 2016, 13, 1200–1207. [Google Scholar] [CrossRef]
- Cerf, E.; Gustot, A.; Goormaghtigh, E.; Ruysschaert, J.M.; Raussens, V. High ability of apolipoprotein E4 to stabilize amyloid-beta peptide oligomers, the pathological entities responsible for Alzheimer’s disease. FASEB J. 2011, 25, 1585–1595. [Google Scholar] [CrossRef]
- Avila, J.; Lucas, J.J.; Perez, M.; Hernandez, F. Role of tau protein in both physiological and pathological conditions. Physiol. Rev. 2004, 84, 361–384. [Google Scholar] [CrossRef]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-beta and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, W.; Zhao, M.; Ma, L.; Jiang, X.; Pei, H.; Cao, Y.; Li, H. Interaction between Abeta and Tau in the Pathogenesis of Alzheimer’s Disease. Int. J. Biol. Sci. 2021, 17, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Rajmohan, R.; Reddy, P.H. Amyloid-Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. J. Alzheimer’s Dis. 2017, 57, 975–999. [Google Scholar] [CrossRef]
- Rapoport, M.; Dawson, H.N.; Binder, L.I.; Vitek, M.P.; Ferreira, A. Tau is essential to beta -amyloid-induced neurotoxicity. Proc. Natl. Acad. Sci. USA 2002, 99, 6364–6369. [Google Scholar] [CrossRef]
- Roberson, E.D.; Scearce-Levie, K.; Palop, J.J.; Yan, F.; Cheng, I.H.; Wu, T.; Gerstein, H.; Yu, G.Q.; Mucke, L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science 2007, 316, 750–754. [Google Scholar] [CrossRef]
- Walker, L.C.; Diamond, M.I.; Duff, K.E.; Hyman, B.T. Mechanisms of protein seeding in neurodegenerative diseases. JAMA Neurol. 2013, 70, 304–310. [Google Scholar] [CrossRef]
- Prusiner, S.B. Cell biology. A unifying role for prions in neurodegenerative diseases. Science 2012, 336, 1511–1513. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Eide, P.K.; Ringstad, G. MRI with intrathecal MRI gadolinium contrast medium administration: A possible method to assess glymphatic function in human brain. Acta Radiol. Open 2015, 4, 2058460115609635. [Google Scholar] [CrossRef] [PubMed]
- Taoka, T.; Ito, R.; Nakamichi, R.; Nakane, T.; Sakai, M.; Ichikawa, K.; Kawai, H.; Naganawa, S. Diffusion-weighted image analysis along the perivascular space (DWI-ALPS) for evaluating interstitial fluid status: Age dependence in normal subjects. Jpn. J. Radiol. 2022, 40, 894–902. [Google Scholar] [CrossRef]
- Eide, P.K.; Vatnehol, S.A.S.; Emblem, K.E.; Ringstad, G. Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Sci. Rep. 2018, 8, 7194. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.; Steinklein, J.M.; Waldman, L.; Zhou, X.; Filippi, C.G. Measuring Glymphatic Flow in Man Using Quantitative Contrast-Enhanced MRI. AJNR Am. J. Neuroradiol. 2019, 40, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Jessen, N.A.; Munk, A.S.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef]
- Silva, I.; Silva, J.; Ferreira, R.; Trigo, D. Glymphatic system, AQP4, and their implications in Alzheimer’s disease. Neurol. Res. Pract. 2021, 3, 5. [Google Scholar] [CrossRef]
- Mogensen, F.L.-H.; Delle, C.; Nedergaard, M. The Glymphatic System (En)during Inflammation. Int. J. Mol. Sci. 2021, 22, 7491. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. Fluid transport in the brain. Physiol. Rev. 2022, 102, 1025–1151. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Achariyar, T.M.; Li, B.; Liao, Y.; Mestre, H.; Hitomi, E.; Regan, S.; Kasper, T.; Peng, S.; Ding, F.; et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2016, 93, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, K.; Zhu, J. Glymphatic system: An emerging therapeutic approach for neurological disorders. Front. Mol. Neurosci. 2023, 16, 1138769. [Google Scholar] [CrossRef]
- Xu, Z.; Xiao, N.; Chen, Y.; Huang, H.; Marshall, C.; Gao, J.; Cai, Z.; Wu, T.; Hu, G.; Xiao, M. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Abeta accumulation and memory deficits. Mol. Neurodegener. 2015, 10, 58. [Google Scholar] [CrossRef]
- Hsu, J.L.; Wei, Y.C.; Toh, C.H.; Hsiao, I.T.; Lin, K.J.; Yen, T.C.; Liao, M.F.; Ro, L.S. Magnetic Resonance Images Implicate That Glymphatic Alterations Mediate Cognitive Dysfunction in Alzheimer Disease. Ann. Neurol. 2023, 93, 164–174. [Google Scholar] [CrossRef]
- Mattsson, N.; Insel, P.S.; Landau, S.; Jagust, W.; Donohue, M.; Shaw, L.M.; Trojanowski, J.Q.; Zetterberg, H.; Blennow, K.; Weiner, M.; et al. Diagnostic accuracy of CSF Ab42 and florbetapir PET for Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2014, 1, 534–543. [Google Scholar] [CrossRef]
- Kamagata, K.; Andica, C.; Takabayashi, K.; Saito, Y.; Taoka, T.; Nozaki, H.; Kikuta, J.; Fujita, S.; Hagiwara, A.; Kamiya, K.; et al. Association of MRI Indices of Glymphatic System With Amyloid Deposition and Cognition in Mild Cognitive Impairment and Alzheimer Disease. Neurology 2022, 99, e2648–e2660. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.E.; Lim, M.M.; Bateman, R.J.; Lee, J.J.; Smyth, L.P.; Cirrito, J.R.; Fujiki, N.; Nishino, S.; Holtzman, D.M. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 2009, 326, 1005–1007. [Google Scholar] [CrossRef]
- Lucey, B.P.; Mawuenyega, K.G.; Patterson, B.W.; Elbert, D.L.; Ovod, V.; Kasten, T.; Morris, J.C.; Bateman, R.J. Associations Between beta-Amyloid Kinetics and the beta-Amyloid Diurnal Pattern in the Central Nervous System. JAMA Neurol. 2017, 74, 207–215. [Google Scholar] [CrossRef]
- Boespflug, E.L.; Iliff, J.J. The Emerging Relationship Between Interstitial Fluid-Cerebrospinal Fluid Exchange, Amyloid-beta, and Sleep. Biol. Psychiatry 2018, 83, 328–336. [Google Scholar] [CrossRef]
- Roh, J.H.; Huang, Y.; Bero, A.W.; Kasten, T.; Stewart, F.R.; Bateman, R.J.; Holtzman, D.M. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer’s disease pathology. Sci. Transl. Med. 2012, 4, 150ra122. [Google Scholar] [CrossRef]
- Rainey-Smith, S.R.; Mazzucchelli, G.N.; Villemagne, V.L.; Brown, B.M.; Porter, T.; Weinborn, M.; Bucks, R.S.; Milicic, L.; Sohrabi, H.R.; Taddei, K.; et al. Genetic variation in Aquaporin-4 moderates the relationship between sleep and brain Abeta-amyloid burden. Transl. Psychiatry 2018, 8, 47. [Google Scholar] [CrossRef]
- Chandra, A.; Farrell, C.; Wilson, H.; Dervenoulas, G.; De Natale, E.R.; Politis, M.; Alzheimer’s Disease Neuroimaging, I. Aquaporin-4 polymorphisms predict amyloid burden and clinical outcome in the Alzheimer’s disease spectrum. Neurobiol. Aging 2021, 97, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Verheggen, I.C.M.; Van Boxtel, M.P.J.; Verhey, F.R.J.; Jansen, J.F.A.; Backes, W.H. Interaction between blood-brain barrier and glymphatic system in solute clearance. Neurosci. Biobehav. Rev. 2018, 90, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 2015, 11, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Harrison, I.F.; Ismail, O.; Machhada, A.; Colgan, N.; Ohene, Y.; Nahavandi, P.; Ahmed, Z.; Fisher, A.; Meftah, S.; Murray, T.K.; et al. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain 2020, 143, 2576–2593. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Yamada, K.; Nishiyama, R.; Hashimoto, T.; Nishida, I.; Abe, Y.; Yasui, M.; Iwatsubo, T. Glymphatic system clears extracellular tau and protects from tau aggregation and neurodegeneration. J. Exp. Med. 2022, 219, e20211275. [Google Scholar] [CrossRef]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Zhou, Y.; Zhang, K.; Jiao, B.; Hu, J.; Zhang, Y.; Wang, Z.; Lou, M.; Bai, R. Age- and time-of-day dependence of glymphatic function in the human brain measured via two diffusion MRI methods. Front. Aging Neurosci. 2023, 15, 1173221. [Google Scholar] [CrossRef]
- Zeppenfeld, D.M.; Simon, M.; Haswell, J.D.; D’Abreo, D.; Murchison, C.; Quinn, J.F.; Grafe, M.R.; Woltjer, R.L.; Kaye, J.; Iliff, J.J. Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol. 2017, 74, 91–99. [Google Scholar] [CrossRef]
- He, X.F.; Liu, D.X.; Zhang, Q.; Liang, F.Y.; Dai, G.Y.; Zeng, J.S.; Pei, Z.; Xu, G.Q.; Lan, Y. Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front. Mol. Neurosci. 2017, 10, 144. [Google Scholar] [CrossRef]
- Shokri-Kojori, E.; Wang, G.J.; Wiers, C.E.; Demiral, S.B.; Guo, M.; Kim, S.W.; Lindgren, E.; Ramirez, V.; Zehra, A.; Freeman, C.; et al. beta-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. USA 2018, 115, 4483–4488. [Google Scholar] [CrossRef]
- Reddy, O.C.; van der Werf, Y.D. The Sleeping Brain: Harnessing the Power of the Glymphatic System through Lifestyle Choices. Brain Sci. 2020, 10, 868. [Google Scholar] [CrossRef]
- Li, J.; Vitiello, M.V.; Gooneratne, N.S. Sleep in Normal Aging. Sleep Med. Clin. 2018, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hablitz, L.M.; Vinitsky, H.S.; Sun, Q.; Staeger, F.F.; Sigurdsson, B.; Mortensen, K.N.; Lilius, T.O.; Nedergaard, M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 2019, 5, eaav5447. [Google Scholar] [CrossRef]
- Landolt, H.P.; Borbely, A.A. Age-dependent changes in sleep EEG topography. Clin. Neurophysiol. 2001, 112, 369–377. [Google Scholar] [CrossRef]
- Nedergaard, M.; Goldman, S.A. Glymphatic failure as a final common pathway to dementia. Science 2020, 370, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Gordon, N.P.; Yao, J.H.; Brickner, L.A.; Lo, J.C. Prevalence of sleep-related problems and risks in a community-dwelling older adult population: A cross-sectional survey-based study. BMC Public Health 2022, 22, 2045. [Google Scholar] [CrossRef] [PubMed]
- Tatineny, P.; Shafi, F.; Gohar, A.; Bhat, A. Sleep in the Elderly. Mo. Med. 2020, 117, 490–495. [Google Scholar]
- Miner, B.; Kryger, M.H. Sleep in the Aging Population. Sleep Med. Clin. 2017, 12, 31–38. [Google Scholar] [CrossRef]
- Vitiello, M.V.; Borson, S. Sleep disturbances in patients with Alzheimer’s disease: Epidemiology, pathophysiology and treatment. CNS Drugs 2001, 15, 777–796. [Google Scholar] [CrossRef]
- Wennberg, A.M.V.; Wu, M.N.; Rosenberg, P.B.; Spira, A.P. Sleep Disturbance, Cognitive Decline, and Dementia: A Review. Semin. Neurol. 2017, 37, 395–406. [Google Scholar] [CrossRef]
- Moe, K.E.; Vitiello, M.V.; Larsen, L.H.; Prinz, P.N. Symposium: Cognitive processes and sleep disturbances: Sleep/wake patterns in Alzheimer’s disease: Relationships with cognition and function. J. Sleep Res. 1995, 4, 15–20. [Google Scholar] [CrossRef]
- Guarnieri, B.; Sorbi, S. Sleep and Cognitive Decline: A Strong Bidirectional Relationship. It Is Time for Specific Recommendations on Routine Assessment and the Management of Sleep Disorders in Patients with Mild Cognitive Impairment and Dementia. Eur. Neurol. 2015, 74, 43–48. [Google Scholar] [CrossRef]
- Kuang, H.; Zhu, Y.G.; Zhou, Z.F.; Yang, M.W.; Hong, F.F.; Yang, S.L. Sleep disorders in Alzheimer’s disease: The predictive roles and potential mechanisms. Neural Regen. Res. 2021, 16, 1965–1972. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, M.V.; Prinz, P.N.; Williams, D.E.; Frommlet, M.S.; Ries, R.K. Sleep disturbances in patients with mild-stage Alzheimer’s disease. J. Gerontol. 1990, 45, M131–M138. [Google Scholar] [CrossRef] [PubMed]
- Parhizkar, S.; Holtzman, D.M. APOE mediated neuroinflammation and neurodegeneration in Alzheimer’s disease. Semin. Immunol. 2022, 59, 101594. [Google Scholar] [CrossRef]
- Eisenbaum, M.; Pearson, A.; Ortiz, C.; Koprivica, M.; Cembran, A.; Mullan, M.; Crawford, F.; Ojo, J.; Bachmeier, C. Repetitive head trauma and apoE4 induce chronic cerebrovascular alterations that impair tau elimination from the brain. Exp. Neurol. 2024, 374, 114702. [Google Scholar] [CrossRef]
- Castellano, J.M.; Kim, J.; Stewart, F.R.; Jiang, H.; DeMattos, R.B.; Patterson, B.W.; Fagan, A.M.; Morris, J.C.; Mawuenyega, K.G.; Cruchaga, C.; et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci. Transl. Med. 2011, 3, 89ra57. [Google Scholar] [CrossRef]
- Alata, W.; Ye, Y.; St-Amour, I.; Vandal, M.; Calon, F. Human apolipoprotein E varepsilon4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J. Cereb. Blood Flow. Metab. 2015, 35, 86–94. [Google Scholar] [CrossRef]
- Wang, X.; Lou, N.; Eberhardt, A.; Yang, Y.; Kusk, P.; Xu, Q.; Forstera, B.; Peng, S.; Shi, M.; Ladron-de-Guevara, A.; et al. An ocular glymphatic clearance system removes beta-amyloid from the rodent eye. Sci. Transl. Med. 2020, 12, eaaw3210. [Google Scholar] [CrossRef]
- Bayer, A.U.; Ferrari, F.; Erb, C. High occurrence rate of glaucoma among patients with Alzheimer’s disease. Eur. Neurol. 2002, 47, 165–168. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, S.J. Glaucoma: Ocular Alzheimer’s disease? Front. Biosci. 2003, 8, s1140–s1156. [Google Scholar] [CrossRef]
- Sen, S.; Saxena, R.; Tripathi, M.; Vibha, D.; Dhiman, R. Neurodegeneration in Alzheimer’s disease and glaucoma: Overlaps and missing links. Eye 2020, 34, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Gedek, A.; Koziorowski, D.; Szlufik, S. Assessment of factors influencing glymphatic activity and implications for clinical medicine. Front. Neurol. 2023, 14, 1232304. [Google Scholar] [CrossRef]
- Hamer, M.; Chida, Y. Physical activity and risk of neurodegenerative disease: A systematic review of prospective evidence. Psychol. Med. 2009, 39, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Lin, M.S.; Tzeng, I.S. Relationship Between Exercise and Alzheimer’s Disease: A Narrative Literature Review. Front. Neurosci. 2020, 14, 131. [Google Scholar] [CrossRef]
- Yin, M.; Pu, T.; Wang, L.; Marshall, C.; Wu, T.; Xiao, M. Astroglial water channel aquaporin 4-mediated glymphatic clearance function: A determined factor for time-sensitive treatment of aerobic exercise in patients with Alzheimer’s disease. Med. Hypotheses 2018, 119, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.V.; Steinberg, R.A.; Han, D.; Sumbria, R.K. Alcohol as a Modifiable Risk Factor for Alzheimer’s Disease—Evidence from Experimental Studies. Int. J. Mol. Sci. 2023, 24, 9492. [Google Scholar] [CrossRef] [PubMed]
- Lundgaard, I.; Wang, W.; Eberhardt, A.; Vinitsky, H.S.; Reeves, B.C.; Peng, S.; Lou, N.; Hussain, R.; Nedergaard, M. Beneficial effects of low alcohol exposure, but adverse effects of high alcohol intake on glymphatic function. Sci. Rep. 2018, 8, 2246. [Google Scholar] [CrossRef]
- Xie, C.; Feng, Y. Alcohol consumption and risk of Alzheimer’s disease: A dose-response meta-analysis. Geriatr. Gerontol. Int. 2022, 22, 278–285. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Wan, Y.; Tan, C.; Li, J.; Tan, L.; Yu, J.T. Alcohol consumption and dementia risk: A dose-response meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Tyas, S.L. Alcohol use and the risk of developing Alzheimer’s disease. Alcohol. Res. Health 2001, 25, 299–306. [Google Scholar]
- Rehm, J.; Hasan, O.S.M.; Black, S.E.; Shield, K.D.; Schwarzinger, M. Alcohol use and dementia: A systematic scoping review. Alzheimers. Res. Ther. 2019, 11, 1. [Google Scholar] [CrossRef]
- Canhada, S.; Castro, K.; Perry, I.S.; Luft, V.C. Omega-3 fatty acids’ supplementation in Alzheimer’s disease: A systematic review. Nutr. Neurosci. 2018, 21, 529–538. [Google Scholar] [CrossRef]
- Bo, Y.; Zhang, X.; Wang, Y.; You, J.; Cui, H.; Zhu, Y.; Pang, W.; Liu, W.; Jiang, Y.; Lu, Q. The n-3 Polyunsaturated Fatty Acids Supplementation Improved the Cognitive Function in the Chinese Elderly with Mild Cognitive Impairment: A Double-Blind Randomized Controlled Trial. Nutrients 2017, 9, 54. [Google Scholar] [CrossRef]
- Fotuhi, M.; Mohassel, P.; Yaffe, K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: A complex association. Nat. Clin. Pract. Neurol. 2009, 5, 140–152. [Google Scholar] [CrossRef]
- Wu, S.; Ding, Y.; Wu, F.; Li, R.; Hou, J.; Mao, P. Omega-3 fatty acids intake and risks of dementia and Alzheimer’s disease: A meta-analysis. Neurosci Biobehav Rev 2015, 48, 1–9. [Google Scholar] [CrossRef]
- Li, L.; Xu, W.; Tan, C.C.; Cao, X.P.; Wei, B.Z.; Dong, C.W.; Tan, L.; Alzheimer’s Disease Neuroimaging, I. A gene-environment interplay between omega-3 supplementation and APOE epsilon4 provides insights for Alzheimer’s disease precise prevention amongst high-genetic-risk population. Eur. J. Neurol. 2022, 29, 422–431. [Google Scholar] [CrossRef]
- Ren, H.; Luo, C.; Feng, Y.; Yao, X.; Shi, Z.; Liang, F.; Kang, J.X.; Wan, J.B.; Pei, Z.; Su, H. Omega-3 polyunsaturated fatty acids promote amyloid-beta clearance from the brain through mediating the function of the glymphatic system. FASEB J. 2017, 31, 282–293. [Google Scholar] [CrossRef]
- Jicha, G.A.; Markesbery, W.R. Omega-3 fatty acids: Potential role in the management of early Alzheimer’s disease. Clin. Interv. Aging 2010, 5, 45–61. [Google Scholar] [CrossRef]
- Kopeć, K.; Szleszkowski, S.; Koziorowski, D.; Szlufik, S. Glymphatic System and Mitochondrial Dysfunction as Two Crucial Players in Pathophysiology of Neurodegenerative Disorders. Int. J. Mol. Sci. 2023, 24, 10366. [Google Scholar] [CrossRef]
- Yi, T.; Gao, P.; Zhu, T.; Yin, H.; Jin, S. Glymphatic System Dysfunction: A Novel Mediator of Sleep Disorders and Headaches. Front. Neurol. 2022, 13, 885020. [Google Scholar] [CrossRef]
- Astara, K.; Tsimpolis, A.; Kalafatakis, K.; Vavougios, G.D.; Xiromerisiou, G.; Dardiotis, E.; Christodoulou, N.G.; Samara, M.T.; Lappas, A.S. Sleep disorders and Alzheimer’s disease pathophysiology: The role of the Glymphatic System. A scoping review. Mech. Ageing Dev. 2023, 217, 111899. [Google Scholar] [CrossRef] [PubMed]
- Lucey, B.P.; McCullough, A.; Landsness, E.C.; Toedebusch, C.D.; McLeland, J.S.; Zaza, A.M.; Fagan, A.M.; McCue, L.; Xiong, C.; Morris, J.C.; et al. Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Sci. Transl. Med. 2019, 11, eaau6550. [Google Scholar] [CrossRef] [PubMed]
- Turton, S.M.; Padgett, S.; Maisel, M.T.; Johnson, C.E.; Buzinova, V.A.; Barth, S.E.; Kohler, K.; Spearman, H.M.; Macheda, T.; Manauis, E.C.; et al. Interactions between daily sleep-wake rhythms, gamma-secretase, and amyloid-beta peptide pathology point to complex underlying relationships. Biochim. Biophys. Acta Mol. Basis. Dis. 2025, 1871, 167840. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, M.; Zhang, M.; Qiu, J.; Cheng, H.; Mou, X.; Chen, Q.; Li, T.; Peng, J.; Li, B. Sleep Quality Improvement Enhances Neuropsychological Recovery and Reduces Blood Aβ42/40 Ratio in Patients with Mild–Moderate Cognitive Impairment. Medicina 2021, 57, 1366. [Google Scholar] [CrossRef]
- Ju, Y.E.; Lucey, B.P.; Holtzman, D.M. Sleep and Alzheimer disease pathology—A bidirectional relationship. Nat. Rev. Neurol. 2014, 10, 115–119. [Google Scholar] [CrossRef] [PubMed]
- David, R.; Zeitzer, J.; Friedman, L.; Noda, A.; O’Hara, R.; Robert, P.; Yesavage, J.A. Non-pharmacologic management of sleep disturbance in Alzheimer’s disease. J. Nutr. Health Aging 2010, 14, 203–206. [Google Scholar] [CrossRef]
- Javed, B.; Javed, A.; Kow, C.S.; Hasan, S.S. Pharmacological and non-pharmacological treatment options for sleep disturbances in Alzheimer’s disease. Expert Rev. Neurother. 2023, 23, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Youn, J.; Lee, H.J.; Lee, S.Y.; Kim, T.G.; Jung, Y.J.; Shin, Y.I.; Choi, B.T.; Jeong, J.; Shin, H.K. Hybrid Electro-optical Stimulation Improves Ischemic Brain Damage by Augmenting the Glymphatic System. Adv. Sci. 2025, 12, e2417449. [Google Scholar] [CrossRef]
- Salehpour, F.; Khademi, M.; Bragin, D.E.; DiDuro, J.O. Photobiomodulation Therapy and the Glymphatic System: Promising Applications for Augmenting the Brain Lymphatic Drainage System. Int. J. Mol. Sci. 2022, 23, 2975. [Google Scholar] [CrossRef]
- Valverde, A.; Hamilton, C.; Moro, C.; Billeres, M.; Magistretti, P.; Mitrofanis, J. Lights at night: Does photobiomodulation improve sleep? Neural Regen. Res. 2023, 18, 474–477. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Penzel, T.; Blokhina, I.; Khorovodov, A.; Fedosov, I.; Yu, T.; Karandin, G.; Evsukova, A.; Elovenko, D.; Adushkina, V.; et al. Night Photostimulation of Clearance of Beta-Amyloid from Mouse Brain: New Strategies in Preventing Alzheimer’s Disease. Cells 2021, 10, 3289. [Google Scholar] [CrossRef] [PubMed]
- Hippius, H.; Neundorfer, G. The discovery of Alzheimer’s disease. Dialogues Clin. Neurosci. 2003, 5, 101–108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopeć, K.; Koziorowski, D.; Szlufik, S. The Therapeutic Potential of Glymphatic System Activity to Reduce the Pathogenic Accumulation of Cytotoxic Proteins in Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 7552. https://doi.org/10.3390/ijms26157552
Kopeć K, Koziorowski D, Szlufik S. The Therapeutic Potential of Glymphatic System Activity to Reduce the Pathogenic Accumulation of Cytotoxic Proteins in Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(15):7552. https://doi.org/10.3390/ijms26157552
Chicago/Turabian StyleKopeć, Kamila, Dariusz Koziorowski, and Stanisław Szlufik. 2025. "The Therapeutic Potential of Glymphatic System Activity to Reduce the Pathogenic Accumulation of Cytotoxic Proteins in Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 15: 7552. https://doi.org/10.3390/ijms26157552
APA StyleKopeć, K., Koziorowski, D., & Szlufik, S. (2025). The Therapeutic Potential of Glymphatic System Activity to Reduce the Pathogenic Accumulation of Cytotoxic Proteins in Alzheimer’s Disease. International Journal of Molecular Sciences, 26(15), 7552. https://doi.org/10.3390/ijms26157552





