Murburn Bioenergetics and “Origins–Sustenance–Termination–Evolution of Life”: Emergence of Intelligence from a Network of Molecules, Unbound Ions, Radicals and Radiations
Abstract
1. Introduction
2. Conventional Views of Life-Sustaining Mechanisms and Associated Issues
3. Life’s Origins Must Have Used DRS
4. Murburn Concept: A Constitutive and Integrative Pillar of Life, from Its Very Origins
4.1. Fighting Against Stark Reality?
4.2. Wishful Thinking?
4.3. The Turnaround!
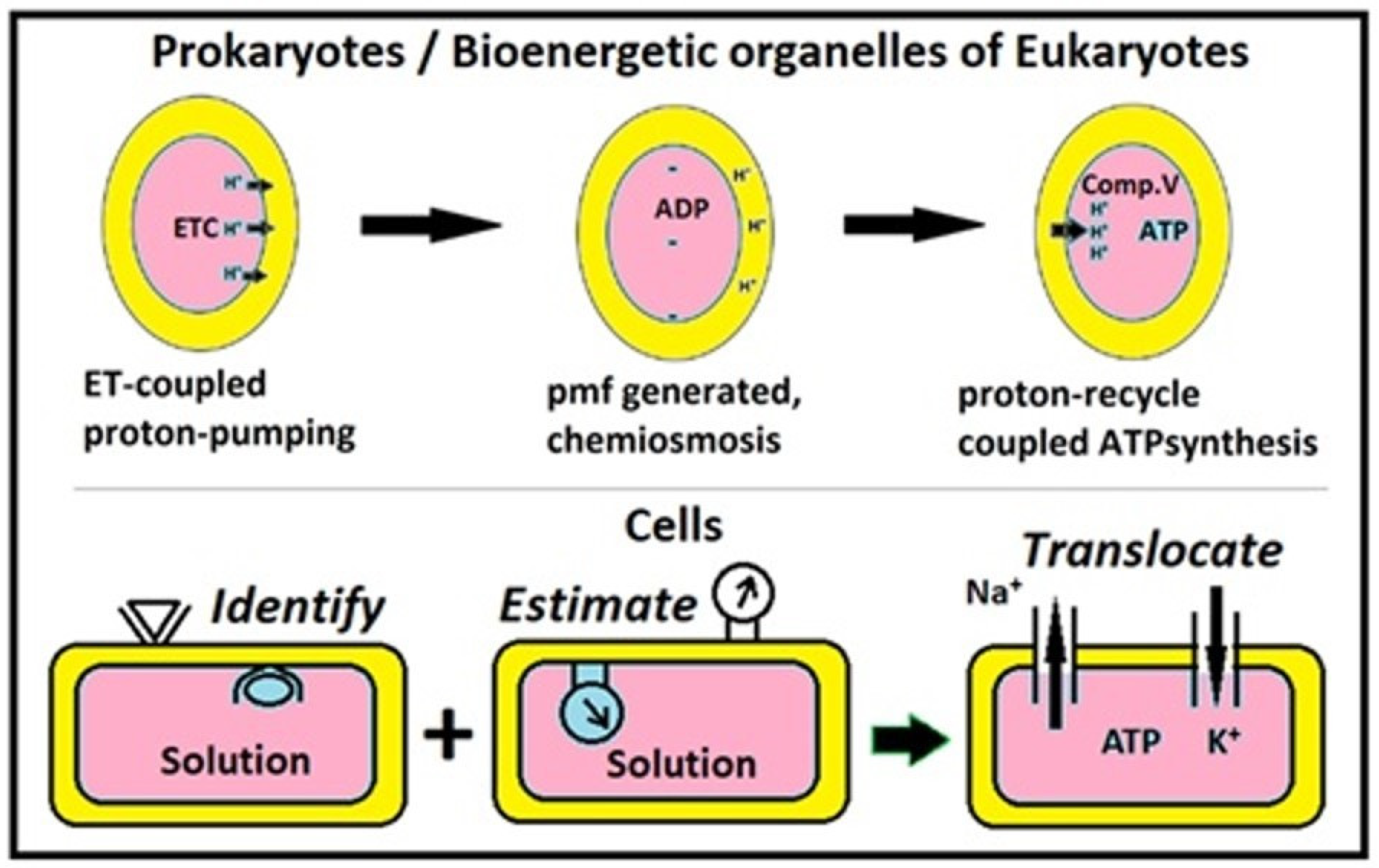
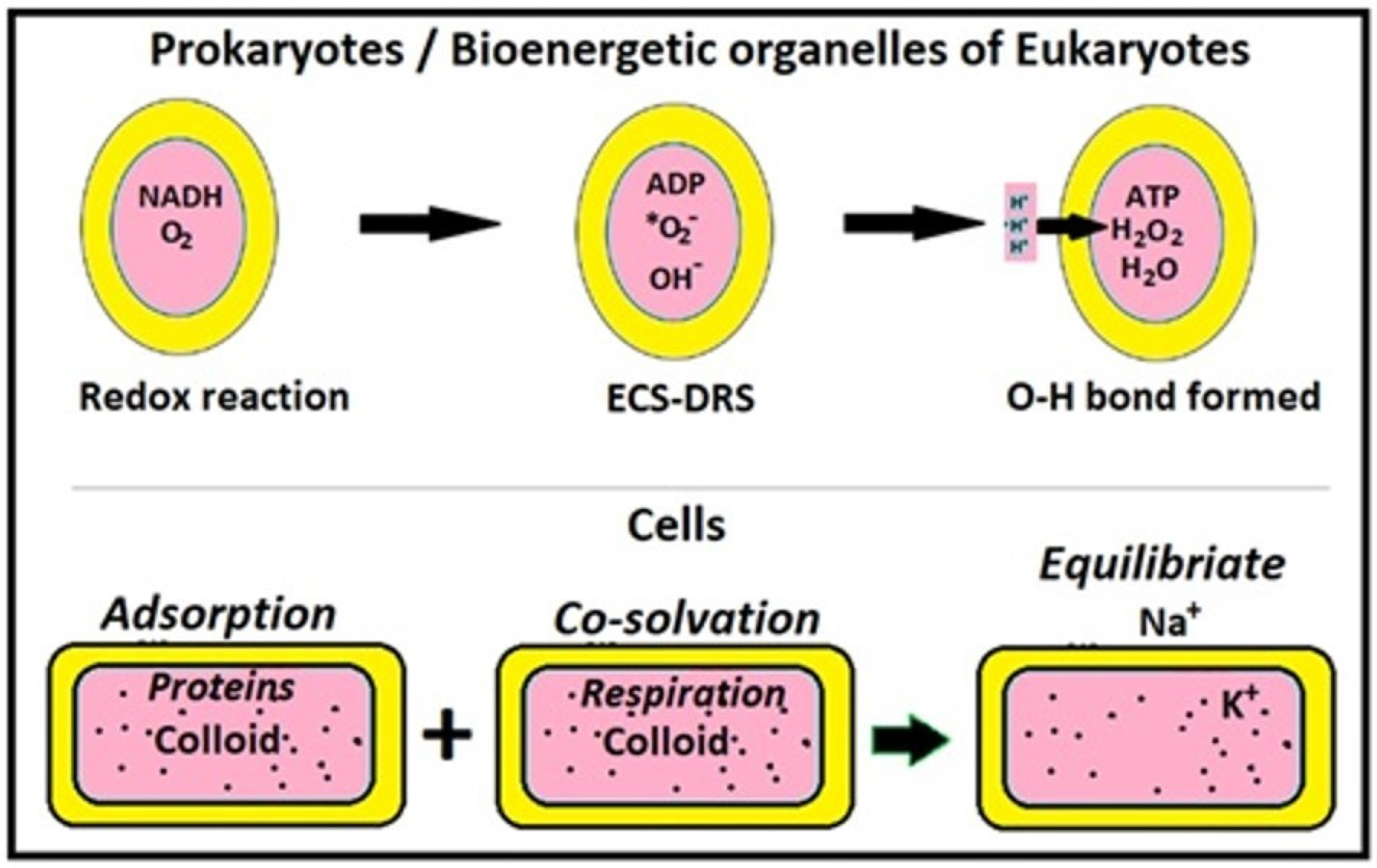
4.4. Elaborate Comparison of ETC-CRAS and Murburn Bioenergetics
4.5. Some Properties of Murburn Mechanisms
- ECS-coupled anionic DRS formation occurs at the redox centers such as d or pi-electron-containing cofactor systems like metals or flavins/retinoids (or both, like hemes) [1,4,18]. Thus, murburn concept is the primary rationale for electrical activity in cells. Even the activity of Na,K-ATPase is explained with DRS [19].
- They essentially abide by simple thermodynamic principles and pose minimal affinity-based requirements, offering a significant evolutionary advantage [1].
- As the presence of substrate exerts a thermodynamic pull, constitutional wastage of redox equivalents and collateral damage are minimized.
- The micro-dimensioned life forms were primordial owing to the necessity to curtail the free availability of protons for oxygenic systems to evolve subsequently.
- The evolution of Complex V is seen primarily as a pH-chemostat regulating murburn reactions at redox centers through proton supply, and an enhancer of ATP yields by serving as auxiliary murzyme (consuming H+; not recycling it!) at high respiratory rates [11].
- If DRS are produced in greater amounts, they could react among themselves, producing non-radical end-products and dissipating energy as heat (thermogenic uncoupling), an important inbuilt safety measure [20].
- Fast DRS-detoxifying enzymes in “solution” and e-buffering molecules like cytochrome c in aqueous milieus or ubiquinone in membrane milieus are yet other agents in the evolutionary repertoire that enable DRS-based physiology [16].
- Therefore, rather than seeing DRS as toxic and wasteful agents, it must be realized that DRS are the elixir of life. Yet, in spite of all the safeguarding measures (listed above) collateral reactions over prolonged periods can produce the deleterious effects of DRS, as chronically reflected in aging and acutely in pathophysiology.
5. The First Cell Was (and Every Cell Now Is!) Essentially a Coacervate
- Accrete/adsorb additional oligomeric material (growth).
- Pinch off buds (physico-chemical basis of division).
- Can swell and shrink by colloid osmosis, without a membrane [6,7,22]. Volume changes effectuate all physicochemical parameters, an overlooked metabolic regulatory mechanism assuring dynamic coherence (cytotonus control) [30]. Accordingly, actin-like proteins became the (overlooked) activator of oxidative phosphorylation [5,30], also influencing (overlooked) metabolic control by cell perfusion [31].
- An exclusion zone at their phase boundary characterized by charge separation and a boundary potential (without pumps), sensitive to environmental influences including IR/VIS/UV radiation [32] and internal-to-external DRS ratios [1], executes some sensory/effector functions. If prepared with added lecithin, the action potential trace obtained is almost indistinguishable from an axonal one [28]!
6. Murburn and Coacervation
7. Electromagnetic Causes/Effects of Murburn–Coacervation
- (i)
- ATP adsorption/hydrolysis indirectly and intricately combined with direct DRS effects on “order disorder cycles”; and
- (ii)
8. DRS–Coacervate Dynamics, CEM, Cancer and Degenerative Diseases
9. Summative Ideas
- Murburn concept constitutes a paradigm shift in bioenergetics.
- It explains why aerobic life needs oxygen for instant functioning.
- The physiological roles of DRS at low concentrations are disclosed.
- Cooperation between coacervation and murburn mechanisms is indicated.
- Dynamics of DRS are crucial for life’s origin, evolution and (patho)physiology.
- Murburn insights suggest new approaches to aging and therapy.
- A.
- Murburn concept (ECS-DRS) forms the primary drive and bioenergetics pillar of cellular life. From this perspective, it can be easily discerned that TMP is a consequential effect of the ECS-DRS murburn process and not the driving force of life. The useful work performed by an automobile is not directly from exhaust or noise (which is analogous to the TMP), but it is because of the “burning of fuel which produces an expansive gas” that the vehicle runs! Murburn is the core logic of how cells automate and integrate to function as “simple chemical engines” (SCEs), a thermodynamically viable system that does useful work, and how “electronic intelligence emerges” (and is not resident in the ion-pumping membrane, as misperceived earlier!) from a discretized distribution and network of molecules and ions.
- B.
- The pre-biotic “burning” activity of iron producing DR(O)S initiated the direction towards life: synthesis of hydroxy-, keto-, carboxylic and amino acids and later ATP by random murburn chemistry. Early coacervations increased complexity from the molecule to the “cell-size” level and from “normal” water to highly ordered water, affording several typical life properties (except those of the central dogma). A significant amount of the emitted electromagnetic energy is re-absorbed by coherent cellular systems (microtubules, centrosomes, centrioles, DNA, etc.) and re-emitted coherently at different wavelengths, which is “healthy dynamics” for the normal functioning of cells. External radiations and select chemicals could perturb DRS metabolism, leading to a disturbed (less coherent) CEM affecting cellular coordination, including chromosome/DNA integrity, explaining why cancer/mutagenesis is preceded by decreased coherence [74]. The non-genetic/hormonal/neuronal modalities of regulations/control via analog–digital logics [14,76] should be investigated in this context. While much remains to be explored, we have conclusively demonstrated that ideas like ETC-CRAS and ion pumping do not abide by the laws of physics, whereas the murburn–coacervate view (and associated CEM view) affords us a tangible/holistic perspective to understand the underlying chemical–physical logic of health and diseases. This was elaborated upon in the two-part review of how murburn concept enables spontaneous and automated operation of multiple systems, starting from a molecular level and seamlessly integrated all the way to the macroscopic levels [77,78]. Many recent investigations demonstrate that DROS play an important, though not understood, adaptive, regulatory role in cell motility [79,80,81,82,83]. DRS (murburn) can also bring about inheritable and epigenetic changes in DNA (and also cross-react with diverse cellular components) and its cumulative effects lead to death. Thus, murburn can drive evolution, generate diversities in species and also explain differences among identical twins.
- C.
- Chemico-physical mechanisms in (biological) living systems are affected and explored/ratified by probing with inhibitory agents. Murburn concept is the only thermodynamic/kinetic explanation available explaining the globally debilitating effect of cyanide on physiology, ascertaining the significance of DRS to life, with full context to the structures of proteins and membrane physiology [84,85,86,87]. While the primary oxygen activation reaction is: O2 + e− → *O2− (−250 kJ/mol) (which explains how the mitochondrial/chloroplast quinones can effectively enhance catalysis via this equilibrium, as its one-electron reduction is comparable with −247 kJ/mol), the cyanide-catalyzed DRS cycling to water is: *OH + *O2− → OH− + O2 (−275 kJ/mol) [87,88]. Thereby, when cyanide is present, DRS are unavailable for essential life-sustaining activities owing to electrons sinking into the life solvent of water, as espoused by the murburn “thermodynamic pull” theory. The stoichiometric heme binding at cytochrome oxidase fails as an explanation in this regard [86]. Further, it is improbable that cells/neurons also evolved highly specific high-throughput ion channels as some currently believe. If so, how could cyanide perturb the cellular functions? Since (i) DRS like NO (and CO too!) are recognized as a molecular messenger [89] and DRS-based signaling is described more and more in the literature [90], (ii) DRS production is also known to be directly correlated to ATP synthesis (and trans-membrane potential) in mitochondria in routine physiology [91] and photodynamic (low-level laser) therapy [92], (iii) DRS production activities are associated with good health measures like exercise [93] and longevity [94], (iv) the perception of DRS is changing [95], and redox activities of small molecules like vitamin C and proteins like cytochrome c have been recharted, our attribution to murburn concept as a founding bioenergetics–coherence principle and seamless integrator of chemico-physical functions of life [1,14,76,77] is validated. Affording such recognition also enables us to specifically explain diverse conundrums like: non-specific post-translational modifications, the unusual hormetic enhancement of heme-enzyme activity by azide (erstwhile presumed to serve as a potent inhibitor!) and idiosyncratic effects of diverse drugs, lack of stereoselectivity in biological halogenation reactions [1,77,78], etc. Recently, researchers found that mitochondria isolated from cancer or non-cancer cells could impact the respiratory activity of each other when they were physically separated from one another [96]. Such non-chemical signaling between disconnected mitochondria can only be explained via electromagnetic radiations, which can be produced by the high-energy-yielding DRS/ROS reactions (some of which are listed in Box 3). Thus, such potent and bewildering findings can also be efficiently reasoned with murburn concept! Quite simply, with murburn concept, we can understand the Janus, i.e., both the Dr. Jekyll and Mr. Hyde, persona of DRS/ROS, reported in diverse contexts [97,98,99]! Slowly and surely, the murburn bioenergetics principles are being cited, discussed and accepted in scientific community [100,101,102,103,104,105,106,107,108], and some textbooks of cell biology [109] and medical biochemistry [110] have incorporated it. It is now imperative to recognize that stochastic intermolecular interactions (and electromagnetic radiations/fields thereof) and collective array of interconnected events mediated via DRS/ROS also contribute towards sustaining life, as is being explored and unraveled constantly [111]! Table 4 presents an appraisal of select systems where we have convincingly demonstrated the applicability of murburn concept.
- D.
- Supposing a murburn reaction releases ~500 kJ/mol (is exergonic!), the outcome could emit: 1–2 UV/Vis photons by electronic transitions (if the states are excited), decades of IR photons by vibrational modes (perhaps the most dominant outcome!), thousands of microwave/radiowave photons (if spins or dipoles are involved, as in radical recombination and ionic current oscillations) and trillions of extremely low-frequency (ELF) quanta (if large-scale macroscopic charge mobilization occurs). The exact distribution depends on the reaction mechanism and the microenvironment (whether in water or at lipid interface). In most cases, IR dominates (explaining the higher temperature of living beings), but specialized reactions can shift into other bands, depending upon the nature of molecules involved. Table 5 presents the panoramic spectrum of interactions that the murburn paradigm could help understand, given that only murburn reactions could emanate higher energy frequency radiations. If we consider that the energy of breaking an O-O bond in a peroxide molecule is around 142–146 kJ/mol (~820 nm photon), then the explanation for photodynamic therapy (observation of increased ROS and ATP production, alteration of mitochondrial TMP, etc.) is easily afforded with the murburn theorization that the hydroxyl radical formed leads to the outcomes. Therefore, it is not anything perverse to imagine that reactions that could give high energy yields can also emit various spectral bandwidth radiations (as shown in Table 5), and all these may bring about an intermolecular or inter-structural coherence that was not deemed possible earlier. Cell biologists and medical professionals should open up their minds to these new possibilities. Most importantly, as seen from all contemporary textbooks, the erstwhile bioenergetic explanations for cellular respiration (or photosynthesis or thermogenesis) afforded low free energy yields in their steps (examples: the highest-energy-yielding reactions of glycolysis or the Krebs cycle provide lower energy than what is afforded by ATP hydrolysis). This write-up provides a tangible connection of the stochastic ECS-DRS fulcrum of life with the impact and/or generation of radiations in (patho)physiology, of both low and high frequencies. Therefore, murburn concept constitutes a holistic perspective of matter–radiation interactions in/among living and ecological systems.
- E.
- The murburn principle predates genetic mechanisms as a foundation of life; therefore, we can clearly envision how crucial it is for understanding life! In spite of having identical genes, the operation of murburn in cells induces chance-based differences between siblings; becoming a primary cause of idiosyncrasies. The classical perspectives in epigenetic and post-translational modifications are purely active-site enzyme-centered highly specific reactions. Murburn insights extend it to have relevance even outside the active site [97], independent of S-adenosyl methionine and ATP. Murburn concept can also help us reason that nucleic acid materials went on to incorporate phosphates and pentose sugar with hydroxyl groups within them to enhance the ruggedness of the molecular sequence to external radical attacks. Murburn reactions in CYP enzymes can generate genotoxic intermediates from procarcinogens. Excessive ROS (due to murburn activity in mitochondria or cytochrome P450 systems) can lead to oxidative stress, causing aberrant epigenetic changes linked to cancer initiation and progression. Increased ROS from murburn activity can mutate DNA (particularly in mitochondria!), activate oncogenes or silence tumor suppressors via epigenetic mechanisms and even affect activities of epigenetic enzymes. Cancer cells often exhibit the Warburg effect (aerobic glycolysis) and mitochondrial dysfunction, which may involve murburn-type redox processes [75]. Murburn concept provides a fresh perspective on how redox reactions influence epigenetics and cancer, particularly through ROS-mediated epigenetic alterations and metabolic rewiring. Posing immense application potentials (refer to Table 4 for a listing of systems already unraveled) in diverse fields and offering a true possibility of understanding aging and several physiological and pathological mechanisms, DRS dynamics and murburn concept are a treasure trove waiting to be opened [115,116,117]! Further research could uncover novel biomarkers or therapies targeting murburn pathways in routine physiology/oncology and mainstream traditional Chinese/Indian ways of medicine.
10. Outstanding Concerns and Questions
- Temporal and spatial landscape of DRS dynamics around proteins.
- DRS–protein (surface)–water, DRS–coacervate and DRS–EM interactions.
- Magnitudes and variations of various EM radiations/fields.
- New thermodynamic foundations on matter–energy exchange in cells.
- Dynamic controls determining various types of chemo/phototaxis and motility.
- DRS-mediated overall digital/analog logic of regulations.
- DRS-mediated one-electron outcomes in cybernetics.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ETC-CRAS | electron transport chain–chemiosmotic rotary ATP synthesis |
| BROS | bound reactive oxygen species |
| CEM | chemo-electromagnetic matrix |
| DR(O)S | diffusible reactive (oxygen) species |
| ECS | effective charge separation |
| ELF | extremely low-frequency waves |
| EoI | emergence of intelligence |
| ETC | electron transport chain |
| Murburn | from “MURed BURNing” (confined and mild unrestricted burning) |
| OSTEoL | origin, sustenance, termination and evolution of life |
| PCHEMS | powering, coherence, homeostasis, electro-mechanical and sensing–response activities (instantaneous signature of life) |
| pmf | proton motive force |
| POM-water | polarized and oriented multi-layer water |
| TEA | terminal electron acceptor |
| TMP | trans-membrane potential |
References
- Manoj, K.M.; Jaeken, L. Synthesis of theories on cellular powering, coherence, homeostasis and electro-mechanics: Murburn concept and evolutionary perspectives. J. Cell. Physiol. 2023, 238, 931–953. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Inhibition of Fe2+- and Fe3+ -induced hydroxyl radical production by the iron-chelating drug deferiprone. Free Rad. Biol. Med. 2015, 78, 118–122. [Google Scholar] [CrossRef]
- De Duve, C. Blueprint for a Cell. The Nature and Origin of Life; Neil Patterson Publ: Burlington, NC, USA, 1991. [Google Scholar]
- Manoj, K.M.; Soman, V.; Jacob, V.D.; Parashar, A.; Gideon, D.A.; Kumar, M.; Manekkathodi, A.; Ramasany, S.; Pakshirajan, K.; Bazhin, N.M. Chemiosmotic and murburn explanations for aerobic respiration. Predictive capabilities, structure-function correlations and chemico-physical logic. Arch. Biochem. Biophys. 2019, 676, 108–128. [Google Scholar] [CrossRef]
- Jaeken, L. The Coacervate-Coherence Nature of Life: Fundamentals of Cell Physiology; BioMedES (Amazon): Inverurie, UK, 2021. [Google Scholar]
- Ling, G.N. Nano-protoplasm: The ultimate unit of life. Physiol. Chem. Phys. Med. NMR 2007, 39, 111–234. [Google Scholar] [PubMed]
- Pollack, G.H. Cells, Cells and the Engines of Life. A New Unifying Approach to Cell Function; Ebner and Sons: Seattle, WA, USA, 2001. [Google Scholar]
- Hill, A.V. The state of water in muscle and blood and the osmotic behaviour of muscle. Proc. Roy. Soc. Lond. B 1930, 106, 477–505. [Google Scholar]
- Matveev, V.V. Cell theory, intrinsically disordered proteins, and the physics of the origin of life. Progr. Biophys. Mol. Biol. 2019, 149, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, E. What is Life? The Physical Aspect of the Living Cell; Cambridge University Press: Cambridge, UK, 1944; (Canto edition, 1992, Cambridge University Press). [Google Scholar]
- Manoj, K.M.; Bazhin, N.M.; Tamagawa, H.; Jaeken, L.; Parashar, A. The physiological role of complex V in ATP synthesis: Murzyme functioning is viable whereas rotary conformation change model is untenable. J. Biomol. Struct. Dyn. 2022, 41, 3993–4012. [Google Scholar] [CrossRef] [PubMed]
- Keilin, D.; Hartree, E.F. Cytochrome and cytochrome oxidase. Proc. Roy. Soc. B 1939, 127, 167–191. [Google Scholar] [CrossRef]
- Manoj, K.M. Aerobic respiration: Criticism of the proton-centric explanation involving rotary adenosine triphosphate synthesis, chemiosmosis principle, proton pumps and electron transport chain. Biochem. Insights 2018, 11, 1178626418818442. [Google Scholar] [CrossRef]
- Manoj, K.M. The ubiquitous biochemical logic of murburn concept. Biomed. Revs. 2018, 29, 89–97. [Google Scholar] [CrossRef]
- Manoj, K.M.; Gideon, D.A.; Jaeken, L. Why do cells need oxygen? Insights from mitochondrial composition and function. Cell Biol. Int. 2021, 46, 344–358. [Google Scholar] [CrossRef]
- Manoj, K.M.; Gideon, D.A.; Jaeken, L. Interaction of membrane-embedded cytochrome b-complexes with quinols: Classical Q-cycle and murburn model. Cell Biochem. Funct. 2022, 40, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Voeikov, V.L. Fundamental role of water in bioenergetics. In Biophotonics and Coherent Systems in Biology; Bellousov, L.V., Voeikov, V.L., Martynyuk, V.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 89–104. [Google Scholar]
- Manoj, K.M.; Tamagawa, H.; Bazhin, N.H.; Jaeken, L.; Nirusimhan, V.; Faraci, F.; Gideon, D.A. Murburn model of vision: Precepts and proof of concept. J. Cell Physiol. 2022, 237, 3338–3355. [Google Scholar] [CrossRef] [PubMed]
- Manoj, K.M.; Gideon, D.A.; Bazhin, N.M.; Tamagawa, H.; Nirusimhan, M.; Jaeken, L. Na,K-ATPase: A murzyme facilitating thermodynamic equilibriums at the membrane-interface. J. Cell. Physiol. 2023, 238, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Manoj, K.M.; Gideon, D.A.; Jacob, V.D. Murburn scheme for mitochondrial thermogenesis. Biomed. Revs. 2018, 29, 73–82. [Google Scholar] [CrossRef]
- Fox, S.W. The Origins of Prebiological Systems and of Their Molecular Matrices; Academic Press: Cambridge, MA, USA, 1965. [Google Scholar]
- Jaeken, L. The neglected functions of intrinsically disordered proteins and the origin of life. Progr. Biophys. Mol. Biol. 2017, 126, 31–46. [Google Scholar] [CrossRef]
- Ling, G.N. A new theoretical foundation for the polarized-oriented multilayer theory of cell water and for inanimate systems demonstrating long-range dynamic structuring of water molecules. Physiol. Chem. Phys. Med. NMR 2003, 35, 91–130. [Google Scholar] [PubMed]
- Fröhlich, H. General theory of coherent excitations in biological systems. In Nonlinear Electrodynamics in Biological Systems; Adey, W.R., Lawrence, A.F., Eds.; Plenum Press: New York, NY, USA, 1984; pp. 491–496. [Google Scholar]
- Del Giudice, E.; Voeikov, V.; Tedeschi, A.; Vitiello, G. The origin and the special role of coherent water in living systems. In Fields of the Cell; Fels, D., Cifra, M., Scholkmann, F., Eds.; Research Signpost: Trivandrum, India, 2015; pp. 95–111. [Google Scholar]
- Voeikov, V.L.; Del Giudice, E. Water respiration—The basis of the living state. Water 2009, 1, 52–73. [Google Scholar]
- Jerman, I. The origin of life from quantum vacuum, water and polar molecules. Am. J. Mod. Phys. 2016, 5, 34–43. [Google Scholar] [CrossRef]
- Ishima, Y.; Przybylski, A.T.; Fox, S.W. Spontaneous membrane phenomena in spherules from proteoid and lecithin. Biosystems 1981, 13, 243–251. [Google Scholar] [CrossRef]
- Edelmann, L. Doubts about the sodium-potassium pump are not permissible in modern bioscience. Cell. Mol. Biol. 2005, 51, 725–729. [Google Scholar] [PubMed]
- Minkoff, L.; Damadian, R. Biological ion exchanger resins: X. The cytotonus hypothesis: Biological contractility and the total regulation of cellular physiology through quantitative control of cell water. Physiol. Chem. Phys. 1976, 8, 349–388. [Google Scholar] [PubMed]
- Wheatley, D.N. Diffusion, perfusion and the exclusion principle in the structural and functional organization of the living cell: Reappraisal of the properties of the ground substance. J. Exp. Biol. 2003, 206, 1955–1961. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trevors, J.T.; Pollack, G.H. Origin of microbial life hypothesis: A gel cytoplasm lacking a bilayer membrane, with infrared radiation producing exclusion zone (EZ) water, hydrogen as an energy source and thermosynthesis for bioenergetics. Biochimie 2012, 94, 258–262. [Google Scholar] [CrossRef]
- Jaeken, L. The coherence of life: A new physiology challenging (neo)Darwinism. Chaos Solitons Fractals 2009, 42, 348–351. [Google Scholar] [CrossRef]
- Prokhorenko, D.V.; Matveev, V.V. The significance of non-ergodic property of statistical mechanics systems for understanding resting state of a living cell. Brit. J. Math. Comp. Sci. 2011, 1, 46–86. [Google Scholar] [CrossRef]
- Tuszynski, J.A.; Paul, R.; Chatterjee, R.; Sreenivasan, S.R. Relationship between Fröhlich and Davydov models of biological order. Phys. Rev. A 1984, 30, 2666–2675. [Google Scholar] [CrossRef]
- Kauffman, S.A. The Origins of Order; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Noble, R.; Noble, D. Physiology restores purpose to evolutionary biology. Biol. J. Linnean Soc. 2023, 139, 357–369. [Google Scholar] [CrossRef]
- Bauer, E.S. Principy Theoreticheskoi Biologii; VIEM Publishing House: Moscow, Russia, Republished in: Theoretical Biology; Discussed at Length in Voeikov VL, Del Giudice E, 2009: See Further; Akadémiai Kiadó: Budapest, Hungary; 1984. [Google Scholar]
- Jaeken, L.; Matveev, V.V. Coherent behaviour and the bound state of water and K+ imply another model of bioenergetics: Negative entropy instead of high-energy bonds. Open Biochem. J. 2012, 6, 139–159. [Google Scholar] [CrossRef]
- Ling, G.N. The physical state of water and ions in living cells and a new theory of energization of biological work performance by ATP. Mol. Cell. Biochem. 1977, 15, 159–172. [Google Scholar] [CrossRef]
- Thoke, H.S.; Tobiesen, A.; Brewer, J.; Hansen, P.L.; Stock, R.P.; Olsen, L.F.; Bagatolli, L.A. Tight coupling of metabolic oscillations and intracellular water dynamics in Saccharomyces cerevisiae. PLoS ONE 2015, 10, e0117308. [Google Scholar] [CrossRef]
- Olsen, L.F.; Stock, R.P.; Bagatolli, L.A. Glycolytic oscillations and intracellular K+ concentration are strongly coupled in the yeast Saccharomyces cerevisiae. Arch. Biochem. Biophys. 2020, 681, 108257. [Google Scholar] [CrossRef]
- Bagatolli, L.A.; Mangiarotti, A.; Stock, R.P. Cellular metabolism and colloids: Realistically linking physiology and biological physical chemistry. Progr. Biophys. Mol. Biol. 2021, 162, 79–88. [Google Scholar] [CrossRef]
- Manoj, K.M.; Gideon, D.A.; Parashar, A.; Nirusimhan, V.; Annadurai, P.; Jacob, V.D.; Manekkathodi, A. Validating the predictions of murburn model for oxygenic photosynthesis: Analyses of ligand-binding to protein complexes and cross-system comparisons. J. Biomol. Struct. Dyn. 2021, 40, 11024–11056. [Google Scholar] [CrossRef]
- Szent Györgyi, A. Bioenergetics; Academic Press: Cambridge, MA, USA, 1957. [Google Scholar]
- Popp, F.A.; Chang, J.J. The physical background and the informational character of biophoton emission. In Biophotons; Chang, J.J., Fisch, J., Popp, F.A., Eds.; Kluwer: Alphen aan den Rijn, The Netherlands, 1998; pp. 239–250. [Google Scholar]
- Volodyaev, I.; Beloussov, L.V. Revisiting the mitogenic effect. Front. Physiol. 2015, 6, 241. [Google Scholar] [CrossRef]
- Hameroff, S.R. Ultimate Computing; Elsevier Science: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Pokorný, J.; Jandova, A.; Nedbalova, M.; Jelinek, F.; Chifra, M.; Kučera, O.; Havelka, D.; Vbra, J.; Vbra, J., Jr. Mitochondrial Metabolism—Neglected Link of Cancer Transformation and Treatment. Prague Med. Rep. 2012, 113, 81–94. [Google Scholar] [CrossRef]
- Pokorný, J.; Pokorný, J.; Foletti, A.; Kobilkova, J.; Vbra, J.; Vbra, J., Jr. Mitochondrial dysfunction and disturbed coherence. Pharmaceuticals 2015, 8, 675–695. [Google Scholar] [CrossRef]
- Sahu, S.; Ghosh, S.; Asani, K.; Hirata, K.; Fujita, D.; Bandyopadhyay, A. Multi-level memory switching properties of a single brain microtubule. Appl. Phys. Lett. 2013, 102, 123701. [Google Scholar] [CrossRef]
- Sahu, S.; Ghosh, S.; Ghosh, B.; Asani, K.; Hirata, K.; Fujita, D.; Bandyopadhyay, A. Atomic water channel controlling remarkable properties of a single brain microtubule: Correlating single protein to its supramolecular assembly. Biosens. Bioelectron. 2013, 47, 141–148. [Google Scholar] [CrossRef]
- Singh, P.; Sahoo, P.; Saxzna, K.; Manna, J.S.; Ray, K.; Ghosh, S.; Bandyopadhyay, A. Cytoskeletal filaments deep inside a neuron are not silent: They regulate the precise timing of nerve spikes using a pair of vortices. Symmetry 2021, 13, 821. [Google Scholar] [CrossRef]
- Jaeken, L. A new list of functions of the cytoskeleton. IUBMB-Life 2007, 59, 127–133. [Google Scholar] [CrossRef]
- Gariaev, P.P.; Birshtein, B.I.; Larochenko, A.M.; Marcer, P.J.; Tertishny, G.G.; Leonova, K.A.; Kaemph, U. The DNA-Wave Biocomputer. 2001. Available online: https://userpage.fu-berlin.de/~gerbrehm/nw/wavecomputer.pdf (accessed on 10 June 2025).
- Mihelic, F.M. Experimental evidence supportive of the quantum DNA model. In Proceedings of the Proc. SPIE 10984, Quantum Information Science, Sensing and Computation XL, Baltimore, MD, USA, 14–18 April 2019; p. 1098404. [Google Scholar] [CrossRef]
- Sun, G.; Li, J.; Zhou, W.; Hoyle, R.G.; Zhao, Y. Electromagnetic interactions in regulation of cell behaviour and morphogenesis. Front. Cell Dev. Biol. 2022, 10, 1014030. [Google Scholar] [CrossRef]
- Levin, M. Bioelectric signalling. Reprogrammable circuits underlying embryogenesis, regeneration and cancer. Cell 2021, 184, 1971–1989. [Google Scholar] [CrossRef]
- Cosic, I. Macromolecular bioactivity: Is it resonant interaction between molecules? Theory and applications. IEEE Trans. Biomed. Eng. 1994, 41, 1101–1114. [Google Scholar] [CrossRef]
- Gurwitsch, A.G. Über den Begriff des embryonalen Felden. Biol. Zentralblatt 1912, 32, 458–486. [Google Scholar]
- Fels, D. Physical non-contact communication between microscopic aquatic species: Novel experimental evidences for an interspecies information exchange. J. Biophys. 2016, 2016, 7406356. [Google Scholar] [CrossRef]
- Sonnenschein, C.; Soto, A.M. Cancer Genes: The Vestigial Remains of a Fallen Theory. In Genetic Explanations: Sense and Nonsense; Krimsky, S., Gruber, J., Eds.; Harvard University Press: Cambridge, MA, USA, 2013; pp. 81–93. [Google Scholar]
- McCullough, A.R.; Coleman, W.B.; Smith, G.J.; Grisham, J.W. Age-dependent induction of hepatic tumor regression by the tissue microenvironment after transplantation of neoplastically transformed liver epithelial cells into the liver. Cancer Res. 1997, 57, 1807–1873. [Google Scholar] [PubMed]
- Maffini, M.V.; Calabro, J.M.; Soto, A.M.; Sonnenschein, C. Stromal regulation of neoplastic development: Age-dependent normalization of neoplastic mammary cells by mammary stroma. Am. J. Pathol. 2005, 167, 1405–1410. [Google Scholar] [CrossRef]
- Bussard, K.M.; Boulanger, C.A.; Booth, B.W.; Bruno, R.D.; Smith, G.H. Reprogramming human cancer cells in the mouse mammary gland. Cancer Res. 2010, 70, 6336–6343. [Google Scholar] [CrossRef]
- Damadian, R. Biological ion exchange resins: II. QUERP water and ion exchange selectivity. Biophys. J. 1971, 11, 761–772. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fröhlich, H. Coherent electric vibrations in biological systems and the cancer problem. IEEE Trans. Microw. Theory Tech. 1978, 26, 613–617. [Google Scholar] [CrossRef]
- Webb, S.J. Nonlinear phenomena in bioenergetics and oncology as seen in 25 years of research with millimeter microwaves and Raman spectroscopy. In Nonlinear Electrodynamics in Biological Systems; Adey, W.R., Lawrence, A.F., Eds.; Plenum: Boston, MA, USA, 1984; pp. 548–566. [Google Scholar]
- Zimmerman, J.W.; Jimenez, H.; Pennison, M.J.; Brezovich, I.; Morgan, D.; Mudry, A.; Costa, F.P.; Barbault, A.; Pasche, B. Targeted treatment of cancer with radiofrequency electromagnetic fields amplitude-modulated at tumor-specific frequencies. Chin. J. Cancer 2013, 32, 573–581. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Acebo, P.; Ginez, D.; Calvo, P.; Bianco-Rivero, A.; Ortega, A.D.; Fernandez, P.L.; Roncador, G. Fernandez-Malavé E, Chamorro M, Cuezva JM, Cancer abolishes the tissue type-specific differences in the phenotype of energetic metabolism. Transl. Oncol. 2009, 2, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Patti, G.H. The Warburg effect: A signature of mitochondrial overload. Trends Cell Biol. 2023, 33, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Geesink, J.H.; Schmieke, M. Organizing and disorganizing resonances of microtubules, stem cells, and proteins calculated by a quantum equation of coherence. J. Modern Phys. 2022, 13, 1530–1580. [Google Scholar] [CrossRef]
- Plankar, M.; Jerman, I.; Kraṧovec, R. On the origin of cancer: Can we ignore coherence? Progr. Biophys. Mol. Biol. 2021, 106, 380–390. [Google Scholar] [CrossRef]
- Manoj, K.M.; Nirusimhan, V.; Parashar, A.; Jesucastin, E.; Gideon, D.A. Murburn precepts for lactic-acidosis, Cori cycle and Warburg effect: Interactive dynamics of dehydrogenases, protons, and oxygen. J. Cell. Physiol. 2022, 237, 1903–1922. [Google Scholar] [CrossRef]
- Penketh, P.G. Is DNA repair controlled by a biological logic circuit? Theor. Biosci. 2022, 141, 41–47. [Google Scholar] [CrossRef]
- Manoj, K.M.; Jaeken, L.; Bazhin, N.M.; Tamagawa, H.; Kavdia, M.; Manekkathodi, A. Murburn concept in cellular function and bioenergetics, Part 1: Understanding murzymes at the cellular level. AIP Adv. 2023, 13, 120702. [Google Scholar] [CrossRef]
- Manoj, K.M.; Jaeken, L.; Bazhin, N.M.; Tamagawa, H.; Gideon, D.A.; Kavdia, M. Murburn concept in cellular function and bioenergetics, Part 2: Understanding integrations-translations from molecular to macroscopic levels. AIP Adv. 2023, 13, 120701. [Google Scholar] [CrossRef]
- Ishimoto, T.; Mori, H. Control of actin-polymerization via reactive oxygen species using light or radiation. Front. Cell Dev. Biol. 2022, 10, 1014008. [Google Scholar] [CrossRef]
- Balta, E.; Kramer, J.; Samstag, Y. Redox regulation of the actin skeleton in cell migration and adhesion: On the way to a spatiotemporal view. Front. Cell Dev. Biol. 2021, 8, 618261. [Google Scholar] [CrossRef]
- Passarolli, C.; Petrini, S.; Pastore, A.; Bonetto, V.; Sale, P.; Gaeta, L.M.; Tozzi, G.; Bertini, E.; Canepari, M.; Rossi, R.; et al. Myosin as a potential redox-sensor: An invitro study. J. Muscle Res. Cell Motil. 2008, 29, 119–126. [Google Scholar] [CrossRef]
- Rashdan, N.A.; Shrestha, B.; Pattillo, C.B. S-glutathionylation: Friend or foe in cardiovascular health and disease. Redox Biol. 2020, 37, 101693. [Google Scholar] [CrossRef]
- Elkrief, D.; Matusovsky, O.; Cheng, Y.S.; Rassier, D.E. From amino-acid to disease: The effects of oxidation on actin-myosin interactions in muscle. J. Muscle Res. Cell Motil. 2023, 44, 225–254. [Google Scholar] [CrossRef]
- Parashar, A.; Venkatachalam, A.; Gideon, D.A.; Manoj, K.M. Cyanide does more to inhibit heme enzymes, than merely serving as an active-site ligand. Biochem. Biophys. Res. Commun. 2014, 455, 190–193. [Google Scholar] [CrossRef]
- Manoj, K.M.; Parashar, A.; Venkatachalam, A.; Goyal, S.; Satyalipsu; Singh, P.G.; Gade, S.K.; Periyasami, K.; Jacob, R.S.; Sardar, D.; et al. Atypical profiles and modulations of heme-enzymes catalyzed outcomes by low amounts of diverse additives suggest diffusible radicals‘ obligatory involvement in such redox reactions. Biochimie 2016, 125, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Manoj, K.M.; Gideon, D.A. Structural foundations for explaining the physiological roles of murzymes embedded in diverse phos-pholipid membranes. Biochim Biophys Acta Biomembr. 2022, 1864, 183981. [Google Scholar] [CrossRef] [PubMed]
- Manoj, K.M.; Ramasamy, S.; Parashar, A.; Gideon, D.A.; Soman, V.; Jacob, V.D.; Pakshirajan, K. Acute toxicity of cyanide in aerobic respiration: Theoretical and experimental support for murburn explanation. Biomol. Concepts. 2020, 11, 32–56. [Google Scholar] [CrossRef] [PubMed]
- Manoj, K.M.; Bazhin, N.M. The murburn precepts for aerobic respiration and redox homeostasis. Prog. Biophys. Mol. Biol. 2021, 167, 104–120. [Google Scholar] [CrossRef]
- Prabhakar, N.R. NO and CO as second messengers in oxygen sensing in the carotid body. Respir. Physiol. 1999, 115, 161–168. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G. Mitochondrial membrane potential and aging. Aging Cell. 2004, 3, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-Y.; Lee, M.Y.; Chung, P.-S.; Kim, S.; Choi, B.; Suh, M.-W.; Rhee, C.-K.; Jung, J.Y. Enhanced mitochondrial membrane potential and ATP synthesis by photobiomodulation increases viability of the auditory cell line after gentamicin-induced intrinsic apoptosis. Sci. Rep. 2019, 9, 19248. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Ristow, M.; Schmeisser, S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011, 51, 327–336. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Mould, R.R.; Kalampouka, I.; Thomas, E.L.; Guy, G.W.; Nunn, A.V.W.; Bell, J.D. Non-chemical signaling between mitochondria. Front. Physiol. 2023, 14, 1268075. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Mitran, S.; Sivanesan, S.; Chang, E.; Buga, A.M. ROS and brain diseases: The good, the bad, and the ugly. Oxid. Med. Cell Longev. 2013, 2013, 963520. [Google Scholar] [CrossRef]
- Pesta, D.; Roden, M. The Janus Head of Oxidative Stress in Metabolic Diseases and During Physical Exercise. Curr. Diab. Rep. 2017, 17, 41. [Google Scholar] [CrossRef]
- Boonla, C. Oxidative stress in urolithiasis. In Reactive Oxygen Species (ROS) in Living Cells, Edited by Cristiana Filip and Elena Albu; Intech Open: London, UK, 2018. [Google Scholar] [CrossRef]
- Abadie, J.M. A Two-Genome Portrayal of Mitochondrial Disorders: A Review with Clinical Presentations. Front. Biosci. (Schol. Ed.) 2024, 16, 7. [Google Scholar] [CrossRef]
- Billah, M.; Aktar, S.; Sikder, R.K.; Ahammed, G.J.; Hu, W.; Li, F.; Yang, Z. Exploring Regulatory Roles of Plant Thylakoid-Bound Proteins Involved in Abiotic Stress Responses. J. Plant Growth Regul. 2024, 43, 1570–1591. [Google Scholar] [CrossRef]
- Francati, S.; Fiore, M.; Ferraguti, G. The janus face of oxidative stress in health and disease: The cause or the cure? Biomed. Rev. 2023, 34, 13–24. [Google Scholar] [CrossRef]
- Chaldakov, G.N.; Aloe, L.; Yanev, S.G.; Fiore, M.; Tonchev, A.B.; Vinciguerra, M.; Evtimov, N.T.; Ghenev, P.; Dikranian, K. Trackins (Trk-Targeting Drugs): A Novel Therapy for Different Diseases. Pharmaceuticals 2024, 17, 961. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, J.; Xu, R.; Yang, K.; Qu, R.; Liu, S.; Zhang, Y.; Xiang, M. The spatiotemporal heterogeneity of reactive oxygen species in the malignant transformation of viral hepatitis to hepatocellular carcinoma: A new insight. Cell Mol. Biol. Lett. 2025, 30, 70. [Google Scholar] [CrossRef]
- Isahak, W.N.R.W.; Al-Amiery, A. Catalysts driving efficiency and innovation in thermal reactions: A comprehensive review. Green Technol. Sustain. 2024, 2, 100078. [Google Scholar] [CrossRef]
- Zhu, D.; Pham, Q.M.; Wang, C.; Colonnello, E.; Yannas, D.; Nguyen, B.H.; Zhang, Y.; Jannini, E.A.; Sansone, A. Erectile Dysfunction and Oxidative Stress: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 3073. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Anastasiadi, A.T.; Tzounakas, V.L.; Nemkov, T.; Reisz, J.A.; Kriebardis, A.G.; Zimring, J.C.; Spitalnik, S.L.; Busch, M.P. Red Blood Cell Metabolism In Vivo and In Vitro. Metabolites 2023, 13, 793. [Google Scholar] [CrossRef]
- Chirumbolo, S.; Bjørklund, G. PERM Hypothesis: The Fundamental Machinery Able to Elucidate the Role of Xenobiotics and Hormesis in Cell Survival and Homeostasis. Int. J. Mol. Sci. 2017, 18, 165. [Google Scholar] [CrossRef]
- Chaldakov, G.N. Principles of Cell Biology; BioMedES, BioMedES (Amazon): Inverurie, UK, 2022. [Google Scholar]
- Vasudevan, D.M.; Sreekumari, S.; Vaidyanathan, K. Textbook of Biochemistry for Medical Students, 11th ed.; Jaypee Medical Publishers: New Delhi, India, 2025. [Google Scholar]
- Manoj, K.M. Murburn concept and biological intelligence at the cusp of 2025: Sensitivity to sensibility, sensuality to sexuality, cybernetics to cyborg-bionics, and more! Biomed Rev. 2024, 35, 1–27. [Google Scholar] [CrossRef]
- Glenn, E. The Electromagnetic Spectrum. The Physics Hypertextbook. Available online: https://physics.info/em-spectrum/ (accessed on 10 June 2025).
- Tomislav, S. Definition of Frequency Bands (VLF, ELF… etc.). Available online: http://www.vlf.it/frequency/bands.html (accessed on 10 June 2025).
- Alpen, E.L. Radiation Biophysics, 2nd ed.; Academic Press: Cambridge, MA, USA, 1998; Available online: https://phyusdb.wordpress.com/wp-content/uploads/2013/03/radiation_biophysics__second_edition.pdf (accessed on 12 June 2025).
- Kietzmann, T.; Petry, A.; Shvetsova, A.; Gerhold, J.M.; Görlach, A. The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Guillaumet-Adkins, A.; Yañez, Y.; Peris-Diaz, M.D.; Calabria, I.; Palanca-Ballester, C.; Sandoval, J. Epigenetics and Oxidative Stress in Aging. Oxid. Med. Cell. Longev. 2017, 2017, 9175806. [Google Scholar] [CrossRef] [PubMed]
- Shriti, S.; Bhar, A.; Roy, A. Unveiling the role of epigenetic mechanisms and redox signaling in alleviating multiple abiotic stress in plants. Front. Plant Sci. 2024, 15, 1456414. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Zhang, L.; Jin, Z.; Kasumov, T.; Chen, Y.R. Mitochondrial redox regulation and myocardial ischemia-reperfusion injury. Am. J. Physiol Cell Physiol. 2022, 322, C12–C23. [Google Scholar] [CrossRef]
- Junge, W.; Nelson, N. ATP synthase. Annu. Rev. Biochem. 2015, 84, 631–657. [Google Scholar] [CrossRef]
- Lai, Y.W.; Ridone, P.; Peralta, G.; Tanaka, M.M.; Baker, M.A.B. Evolution of the Stator Elements of Rotary Prokaryote Motors. J. Bacteriol. 2020, 202, e00557-19. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Govindjee, G.; Shevela, D.; Björn, L.O. Evolution of the Z-scheme of photosynthesis: A perspective. Photosynth Res. 2017, 133, 5–15. [Google Scholar] [CrossRef]
- Guo, Y.; He, L.; Ding, Y.; Kloo, L.; Pantazis, D.A.; Messinger, J.; Sun, L. Closing Kok’s cycle of nature’s water oxidation catalysis. Nat. Commun. 2024, 15, 5982. [Google Scholar] [CrossRef]
- Crofts, A.R. The modified Q-cycle: A look back at its development and forward to a functional model. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148417. [Google Scholar] [CrossRef]
- Dyla, M.; Kjærgaard, M.; Poulsen, H.; Nissen, P. Structure and Mechanism of P-Type ATPase Ion Pumps. Annu. Rev. Biochem. 2020, 89, 583–603. [Google Scholar] [CrossRef]
- Gouaux, E.; Mackinnon, R. Principles of selective ion transport in channels and pumps. Science. 2005, 310, 1461–1465. [Google Scholar] [CrossRef]

| No. | Criterion/Observation | ETC + CRAS (X) | Murburn (Y) | Analytical Comments |
|---|---|---|---|---|
| 1 | Oxygen’s role and formation of DROS in mitochondria and chloroplasts | Final e-acceptor; stays bound at Complex IV to form water; DRS seen as wasteful/toxic byproduct. | O2 forms DRS which interact with ADP/Pi to make ATP; DRS are essential intermediates. | Y (not X) gives direct redox phosphorylation. Y (not X) explains DRS production. |
| 2 | Proton gradient (ΔpH) and membrane potential (Δψ); bioenergetic membrane must be intact | ETC’s H+ pumping must generate ΔpH of 3 units (1000-fold H+ gradient or Δψ of ~ −200 mV). Cannot explain how ATP synthesis goes on with permeabilized or leaky membrane. | Small ΔpH/Δψ is an epiphenomenon due to ECS-DRS. Not pmf, but ECS-DRS mechanism by O2 and murzymes forms the phosphorylation drive. Practically aprotic environment makes O2-centered DRS more stable. Intact membrane prevents loss of DRS; but is not a must! | No protons to pump in micro-dimensioned organelles! (How can proteins be selective for 0.02 pm H+?); and little pH gradient present! ATP synthesis observed with DRS (even in systems sans ΔpH or Δψ). DRS directly correlates with ΔpH and Δψ in closed organelles. Supports Y, discounts X. |
| 3 | Stoichiometry (component:ATP ratios; H+:P, NADH:P, O:P) | Requires tight stoichiometry: Originally 6 ATP per NADH or per O2. (Modified to <5 later.) | Loose coupling via stochastic interactions of ROS with ADP/Pi near inner membrane Complexes I to V. Ratios can be fractional due to competing reactions. | Data from diverse labs show variable ratios (even >6). Data spectrum supports Y (not X). |
| 4 | Heat generation in brown fat by thermogenin (UCP) | Uncoupling proteins dissipate pmf by tranlocating protons with fatty acids to give heat. (Shaky physics?) Requires fatty acids. | Outward translocation of DRS or inward proton movement facilitated by lys-arg of UCP gives water and heat at interface. Does not require fatty acids. | Structure of UCP and fundamental thermodynamic considerations support Y (not X). |
| 5 | Enhancement of ATP synthesis by xanthine/xanthine oxidase (XO) | Fails to explain the phenomenon for mitochondria and chloroplasts. | Xanthine/XO gives superoxide, which aids ATP synthesis. | Supports Y, not X. |
| 6 | No DRS, no ATP! Provision of higher NADH/(ADP+Pi)/O2 increases both DRS and ATP in milieu. | Cannot explain. | Organellar system works to make ATP via the intermediacy of DRS. | Supports Y, not X. |
| 7 | Provision of NADH progressively reduces Comp I, III and IV (Kaelin) | ETC concept! Electrons serially relayed in ETC from Comp I by CoQ (to Comp III) and Cyt. c (to Comp IV), while O2 stays stuck at Comp IV. | NADH reaction with Comp I produces DRS, which reduce CoQ in membrane, Cyt. c in milieu and form peroxide. All these reduce Comp III and Comp IV in parallel ways. | Q-cycle is impossible. Oxygen does not and cannot stay bound to Comp IV. These discount X and known features support Y. |
| 8 | ATP formation with induced pH gradient (sans light) in chloroplasts. (Jaegendorf–Uribe) | pH gradient drives ATP synthesis in bioenergetic organelles by H+ cycling! | External H+ as a reactant drives many photosynthetic reactions to the right. Supports the murburn thermodynamic role of H+ consumption in the bioenergetic process. | Three-unit pH gradient is non-physiological (unseen in chloroplasts!). The experiment was ill-constructed and the observation was misinterpreted in favor of X. |
| 9 | Light-induced synthesis of ATP in vesicles with membrane-embedded Comp V + bacteriorhodopsin (Racker–Stoeckenius) | Light triggers rhodopsin to pump protons out from vesicles, which return via Comp V to make ATP inside. | Rhodopsin Schiff’s base pKa > 13; cannot pump protons but is a photoactive DRS generator, and the DRS makes ATP. | The experimental control of rhodopsin alone gave 20% ATP formation with light. Does not agree with X, but supports Y. |
| 10 | Effect of uncouplers like dinitrophenol or DNP (Mitchell) | Disrupts pmf needed for ATP synthesis. So oxyen used wastefully; uncoupler shuttles protons inward. | DNP impacts interfacial DRS dynamics and makes water. | If the minuscule protons cannot move across the membrane, how can DNP (which is more massive and has more charges!) flip-flop? Mitchellian explanation fails! Findings support Y, not X. |
| 11 | Effect of oligomycin binding to Comp V (Lardy–Racker) | Blocks F0 and stops ATP synthesis even with Δψ; because protons cannot come in via Comp V’s Fo. | Fo of Comp V serves as an ATPase and pH stat. F1 of Comp V serves as a proton-consuming ATP synthase. | Findings misinterpreted in favor of X; observation actually supports Y. |
| 12 | Effect of rotenone, antimycin B and cyanide (CN) on Comps I, III and IV (respectively) | Bind to Comps I, III and IV, respectively, to disrupt proton pumping by ETC, thus lowering ATP synthesis by definite and proportionate ratios. | The toxins disrupt DRS dynamics in murzone; either preventing DRS formation and interaction or subverting DRS to form water. DRS quenching by CN is very facile. | O2 can outcompete CN to bind Comp IV and toxic dosage of CN is too low (orders lower than hemeFe). Inhibition is too fast to be explained by binding mechanisms. Negates X; fully supports Y. |
| No. | Component(s) | ETC + CRAS (X) | Murburn (Y) | Analytical Comments |
|---|---|---|---|---|
| 1 | NADH and O2 | No particular significance attributed to structure of NADH; O2 binds and reacts only at Comp IV. | 2e, 1H in closed aprotic ambiance allows O-centered 1e chemistry (H2O2 and H2O formation). Mobile O2/DRS interact with all components! | In the reducing ambiance, the highly mobile O2 cannot stay stuck to Comp IV. Indeed, the formation of DRS has been documented for Comp I through Comp IV. Discounts X; supports Y. |
| 2 | Comp I | Collects electrons from NADH and relays to CoQ, pumping out H+ in the process. | Receives electrons from NADH, binds ADP and generates DRS in the murzone for direct phosphorylation. Many FeS centers in the matrix-subtending arm (with many ADP-binding sites) make it a redox ADP-phosphorylating murzyme. | Some Fe-S non-route and some of unfavorable potential. ET along FeS in the matrix arm cannot be spatio-temporally coupled to any H+ pumping by membrane foot. CoQ cannot squirm into the protein to receive electrons. Discounts X; clearly supports Y. |
| 3 | Comp II | Collects electrons from succinate to pass on to CoQ in the membrane. Cannot explain the presence of multiple redox cofactors. | Highly localized DRS with FAD; a membrane-embedded redox phosphorylating murzyme. | Hardly any purpose for this enzyme with X! Presence of multiple redox cofactors (flavin, FeS, heme) and an ADP site supports Y. |
| 4 | Comp III | A complex enzyme that binds many molecules at the same time to convert CoQH2 to CoQ, reduce Cyt. c and pump H+ out. | A murzyme that recycles electrons lost in the membrane phase by making DRS from CoQH2 and enabling bound ADP’s phosphorylation. | Structure, kinetics and thermodynamics do not support X. Simultaneous bindings of many molecules at the lipid phase is not solicited by Y. The solvent-accessible heme and ADP-binding site on apoprotein support Y. |
| 5 | Comp IV | Oxidase binding O2 to receive 4 electrons from Cyt. c and availing 4 H+ to make 2 H2O molecules to pump out H+. | The peroxides formed in phosphorylation and DRS reactions are completely oxidized to water, making ATP in the process. | The terminal e-acceptor role attributed to O2 is a “meaningless role” in X. Peroxidative roles of Comp IV are known, supporting Y. |
| 6 | Comp V | Reversible rotary enzyme that dynamically harvests proton gradient via electro-mechanical forces to make ATP. | An ATPase at Fo (chemostat) and a DRS/H+-dependent ATP synthase at F1 (using incoming H+ as a reactant). | The presence of multiple ADP sites on F1 of Comp V discounts Boyer model and the fact that pure Comp V is an ATPase negates X. The above facts and in vitro H+-driven ATP synthesis support Y. |
| 7 | Respirasomes | Cannot explain. | Structures evolving to minimize murzone and DRS wastage. | Support Y (not X). |
| 8 | CoQ | An “intelligent” and mobile e-relay agent, ferrying electrons and protons from Comp I and Comp II to Comp III, where it has both e-donating at accepting roles. | E-sponging roles in membrane to prevent the escape and loss of DRS; different lengths to enable better stacking in the lipid. | Dual role at Comp III unviable in X. The larger CoQ10 (relatively immobile) is abundant, discounting transport role attributed in X; Quinones’ diversity supports the e-sponging role advocated in Y. |
| 9 | Cyt. c | “Intelligent” mobile electron-relaying agent from Comp III to Comp IV in the intermembrane space. | E-sponging roles in matrix and intermembrane space. Heme’s solvent accessibility supports murburn model. | Cyt. c is present in both phases of mitochondria. Initiation of apoptosis with the leaching of Cyt. c is an evolutionary strategy justifying Y. |
| 10 | Inner membrane | Impermeable to H+ and intelligently regulates pmf. Seeks placement of ETC in specific order and ratios on the cristae. | Packed with redox proteins to enhance ECS-DRS at the interphase. Cardiolipin increases anionic density, preventing DRS escape. | X does not explain preponderance of cardiolipin. |
| 11 | Outer membrane | Needed to form an intermembrane space capable of containing pmf. | A second membrane is present to minimize DRS escape. (No specialty required and none present!) | The fact that intermembrane space and periplasm equilibrate with cytoplasm negates X. |
| 12 | Overall architecture and distribution | The deterministic model seeks the orchestration of a highly complex mechanism across three phases, with hundreds of gene products, all for making one molecule of ATP! | The stochastic model works as a soup of chemicals, in various architectures and arrangements. Each component can work independently; and the system could easily evolve incrementally for the optimization of ECS-DRS mechanism. (Murburn model viable in erythrocytes, which lack mitochondria!) | X is discounted as it does not address the micro-dimensioned aprotic nature of bioenergetic organelles and bacterial cells. The diverse types of architectures found in mitochondria and the scattered and randomized distribution of proteins therein support Y. DRS-mediated ATP synthesis shown in vitro and in vivo. X requires too many components and unheard of modalities! |
| No. | Aspect | ETC + CRAS (X) | Murburn (Y) | Analytical Comments |
|---|---|---|---|---|
| 1 | Thermodynamics | Endergonic. | Exergonic. | Supports Y, negates X. |
| 2 | Kinetics (e.g., for Comp IV: unusual aspects like KM < Kd; anaerobic ET orders slower than aerobic ET) | Slow, binding-based; anomalous kinetics cannot be explained. | Fast, collision-based; DRS-based mechanism explains atypical kinetics. | Supports Y, negates X. |
| 3 | Pathway/steps | Serial + sequential. | Parallel + unordered. | Y more probable. |
| 4 | Coupling | Indirect, electro-mechanical to chemical (?) | Direct redox phosphorylation; a simple chemical reaction; explains Cohn’s data of fast/multiple labeled O-atoms’ insertion in ATP. | X is unheard of elsewhere! Reaction logic supports Y. |
| 5 | Molecularity of intermediates | Multimolecular (e.g., Q-cycle). | Bimolecular (or trimolecular). | Y is more probable. |
| 6 | Stoichiometry | Fixed and whole number due to binding-based logic. | Variable and non-integral due to uncertainty of DRS function. | Data supports Y. X negated. |
| 7 | Binding of molecules to proteins | High-affinity interactions solicited with long-lived intermediates. | Not fastidious about binding affinity, as the reaction is delocalized. | Data supports Y, as diverse molecules donate and receive electrons to/from the system. |
| 8 | Reversibility | Yes. | No. | Y is more sensible than X. |
| 9 | Conformation change needed? | Obligatory. | Optional. | Y more likely than X. |
| 10 | Long-distance ET at unfavorable redox potential | Required! | This does not seek such seemingly improbable events. | X is more unlikely and unsupported than Y. Promiscuity of ET supports Y. |
| 11 | Schema | Deterministic. | Stochastic. | Y is more likely than X. |
| 12 | Probability | “Irreducibly complex”. | Simple, tangible, viable and probable. | Ockham’s razor prefers Y over X. |
| No. | System/Field | Classical Model | Advantage(s) of Murburn Model | References * |
|---|---|---|---|---|
| 1 | Peroxidase/Catalase (peroxisomes, ecological cycling of lignocellulosics and halogenated organics) | BROS-Compound I | Explains substrate diversity and inhibition; modulation by diverse molecules, practically diffusion-limited catalysis. | [1,4,14,77,84,85,87] |
| 2 | Cyclooxygenase & antibody function (inflammation; immunology) | BROS-Compound I | Explains substrate diversity and variable products; modulation by diverse molecules. | [1,111] |
| 3 | Lactate dehydrogenase (how the reverse reaction is viable for lactate to pyruvate!) | Reversible function | Reasons why lactate has to go into mitochondria or be taken to liver to use the energy equivalents. | [1,75,77] |
| 4 | Hemoglobin structure-function correlation (erythrocyte viability) | No equivalent! | Proposes the first rationale for prolonged viability of erythrocytes in the absence of nucleus and mitochondria. | [1,77,111] |
| 5 | Drug/Xenobiotic metabolism (how cells deal with “chemically unknown” molecules) | P450cam | Explains diversity of CYPs (cytochrome P450), promiscuity of CPR (CYP reductase) and Cyt. b5 and diversity of substrates. | [1,14,15,77,86] |
| 6 | Mitochondrial respiration (ATP synthesis, cyanide toxicity & thermogenesis) | ETC + CRAS | Thermodynamically viable rationale for oxidative phosphorylation and heat production in cells. Primary drive for life! | [1,4,5,11,13,14,15,16,20,77,78,84,85,86,87,88] |
| 7 | Light reaction of photosynthesis (radiation to reaction logic) | Z-scheme and Kok–Joliot cycle + CRAS | Discrediting the Z-scheme model, explained the roles of photosystems and antenna complexes. | [1,11,16,18,44,77,78,86] |
| 8 | Retinal phototransduction (radiation to electrical signal) | Retinal cycle (11cis to all-trans model) | Details direct role for oxygen in photoreception, explaining eye structure. | [1,18,78,86] |
| 9 | Na+/K+ ion-differential and electrosignaling physiology | Classical membrane pump theory (CMPT) | Brings the first electronic model for electrophysiology; more viable/tangible than a merely ion-centric view. | [1,19,78] |
| 10 | Rotary mechanoproteins & bacterial flagella-aided motility (mechanical work) | Berg’s & Boyer’s rotary model | Disclaiming the rotary model for flagellar motility, a facile water-ejection based propulsion was proposed; agreeing with Type 3 secretion system structure. | [1,4,11,13,14,15,78,86] |
| 11 | Unusual dose responses (idiosyncrasies and hormesis), photodynamic therapy, affects in chemically disconnected system, etc. | No explanation! | Reasons out non-genetic interpersonal variabilities, enhanced activity at lower doses in some cases. Could offer novel ways to approach the unusual effects of temperature, electrostatics, etc. on life. | [1,5,14,44,77,111] & this work! |
| 12 | PCHEMS to PTMs (post-translational modfications; cancer, biological intelligence and OSTEoL) | No equivalent! | In conjunction with the central dogma, brings key aspects of molecular and macroscopic cellular activities under a unified perspective. | [1,5,11,14,15,19,20,75,77,78,111] |
| Radiation Type * | Wavelength * | Frequency * | Energy * kJ/mol | Known/Projected Cellular Effects |
|---|---|---|---|---|
| Near UV (UV-A) | 315–400 nm | 750–950 THz | 302–382 | Activates flavins, induces DROS productions, affects mitochondria |
| Visible Light | 400–700 nm | 430–750 THz | 170–300 | Photoreception (opsins), chlorophyll activation, circadian rhythms, ROS modulation, optogenetics, photodynamic therapy, mitochondrial stimulation |
| Near Infrared (NIR) | 700–1400 nm | 214–430 THz | 85–170 | Mitochondrial cytochrome c oxidase activation, alters mitochondrial TMP, photobiomodulation, wound healing, ATP production |
| Mid-Infrared (MIR) | 1400–3000 nm | 100–214 THz | 40–85 | Vibration excitation of bonds, localized heating, influence on protein folding and conformational changes |
| Far Infrared (FIR) | 3–1000 µm | 0.3–100 THz | 1.2–40 | Low-level thermal effects, water absorption influencing its structuring and hydrogen bonds, modulation of membrane fluidity |
| Microwaves | 1 mm–30 cm | 1–300 GHz | 0.004–1.2 | Membrane potential alteration, Ca2+ channel effects, dielectric heating, signal modulation, membrane polarization |
| Radiofrequency (RF) | 30 cm–10 km | 30 kHz–1 GHz | <0.004 | Potential long-term effects on signaling, orientations, etc. |
| Extremely Low Frequency (ELF) | >10 km | 3–30 kHz | <<0.001 | Effects on ion channels, circadian rhythms, potential stress response triggers, EM field and electroreception |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaeken, L.; Manoj, K.M. Murburn Bioenergetics and “Origins–Sustenance–Termination–Evolution of Life”: Emergence of Intelligence from a Network of Molecules, Unbound Ions, Radicals and Radiations. Int. J. Mol. Sci. 2025, 26, 7542. https://doi.org/10.3390/ijms26157542
Jaeken L, Manoj KM. Murburn Bioenergetics and “Origins–Sustenance–Termination–Evolution of Life”: Emergence of Intelligence from a Network of Molecules, Unbound Ions, Radicals and Radiations. International Journal of Molecular Sciences. 2025; 26(15):7542. https://doi.org/10.3390/ijms26157542
Chicago/Turabian StyleJaeken, Laurent, and Kelath Murali Manoj. 2025. "Murburn Bioenergetics and “Origins–Sustenance–Termination–Evolution of Life”: Emergence of Intelligence from a Network of Molecules, Unbound Ions, Radicals and Radiations" International Journal of Molecular Sciences 26, no. 15: 7542. https://doi.org/10.3390/ijms26157542
APA StyleJaeken, L., & Manoj, K. M. (2025). Murburn Bioenergetics and “Origins–Sustenance–Termination–Evolution of Life”: Emergence of Intelligence from a Network of Molecules, Unbound Ions, Radicals and Radiations. International Journal of Molecular Sciences, 26(15), 7542. https://doi.org/10.3390/ijms26157542






