Plasma Lysophosphatidylcholine Levels Correlate with Prognosis and Immunotherapy Response in Squamous Cell Carcinoma
Abstract
1. Introduction
2. Results
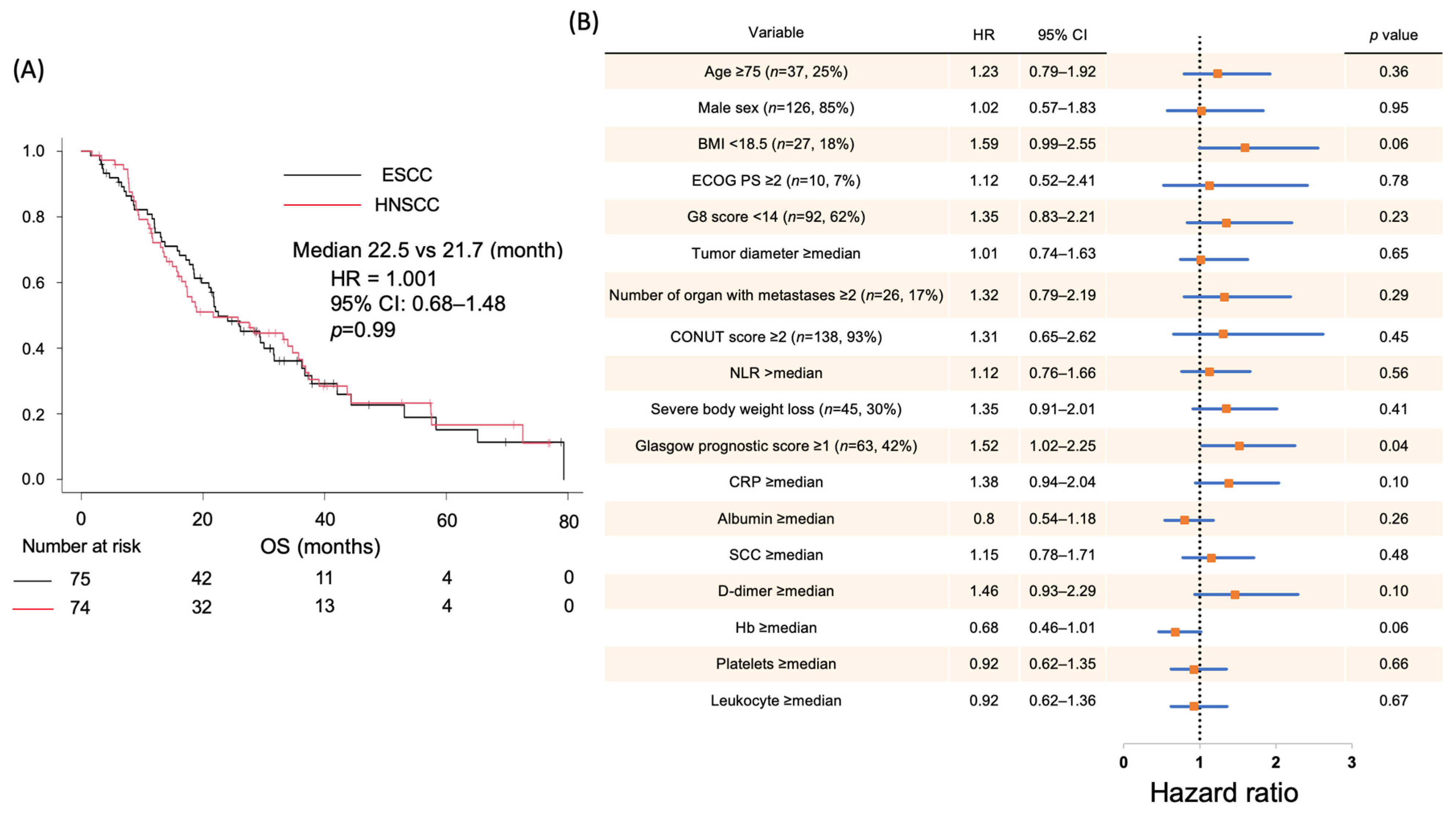
2.1. Correlation Between Clinical Data and Prognosis in Patients with SCC
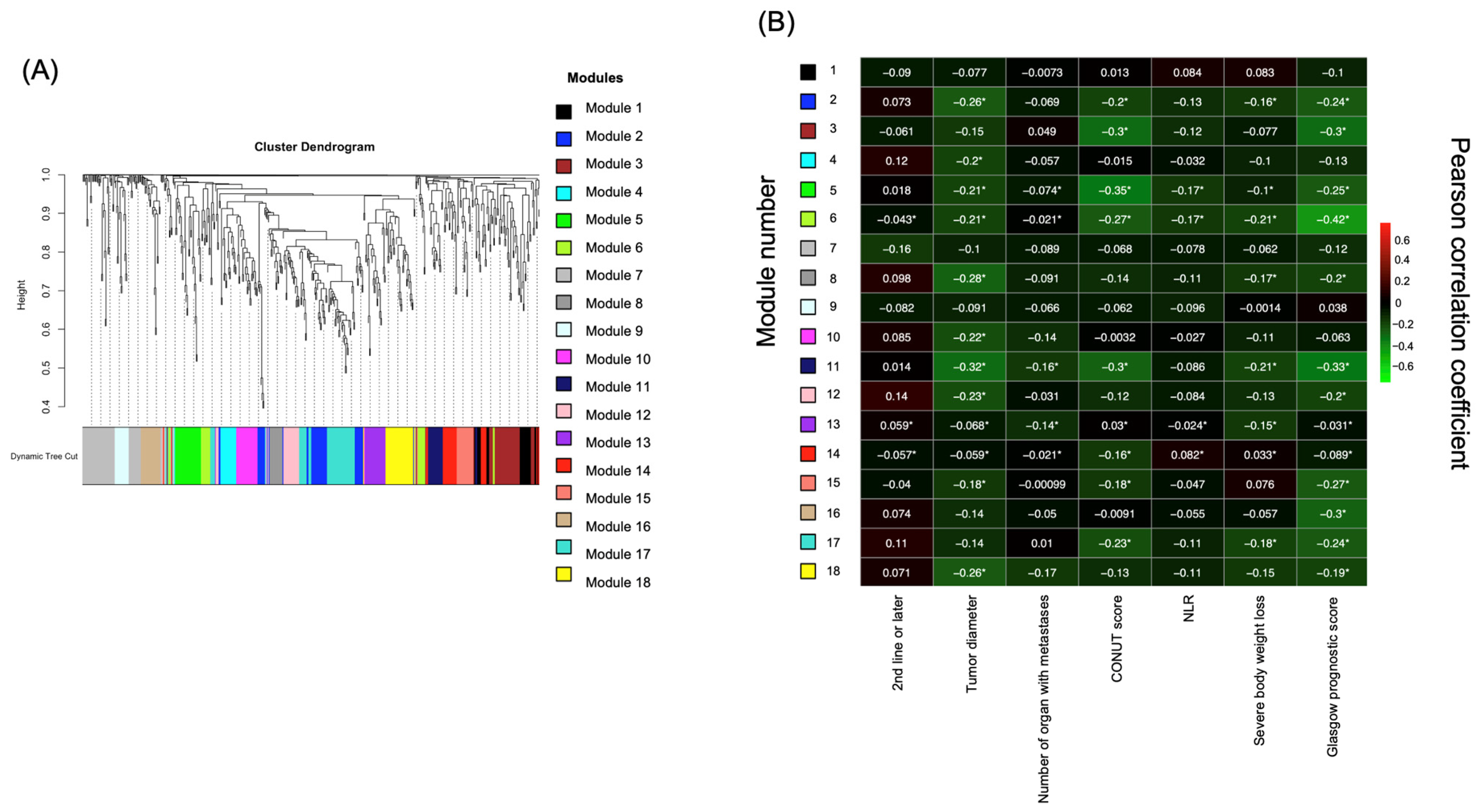
2.2. Identification of Prognostic Factors Based on Targeted Metabolome Data via Weighted Gene Correlation Network Analysis (WGCNA)
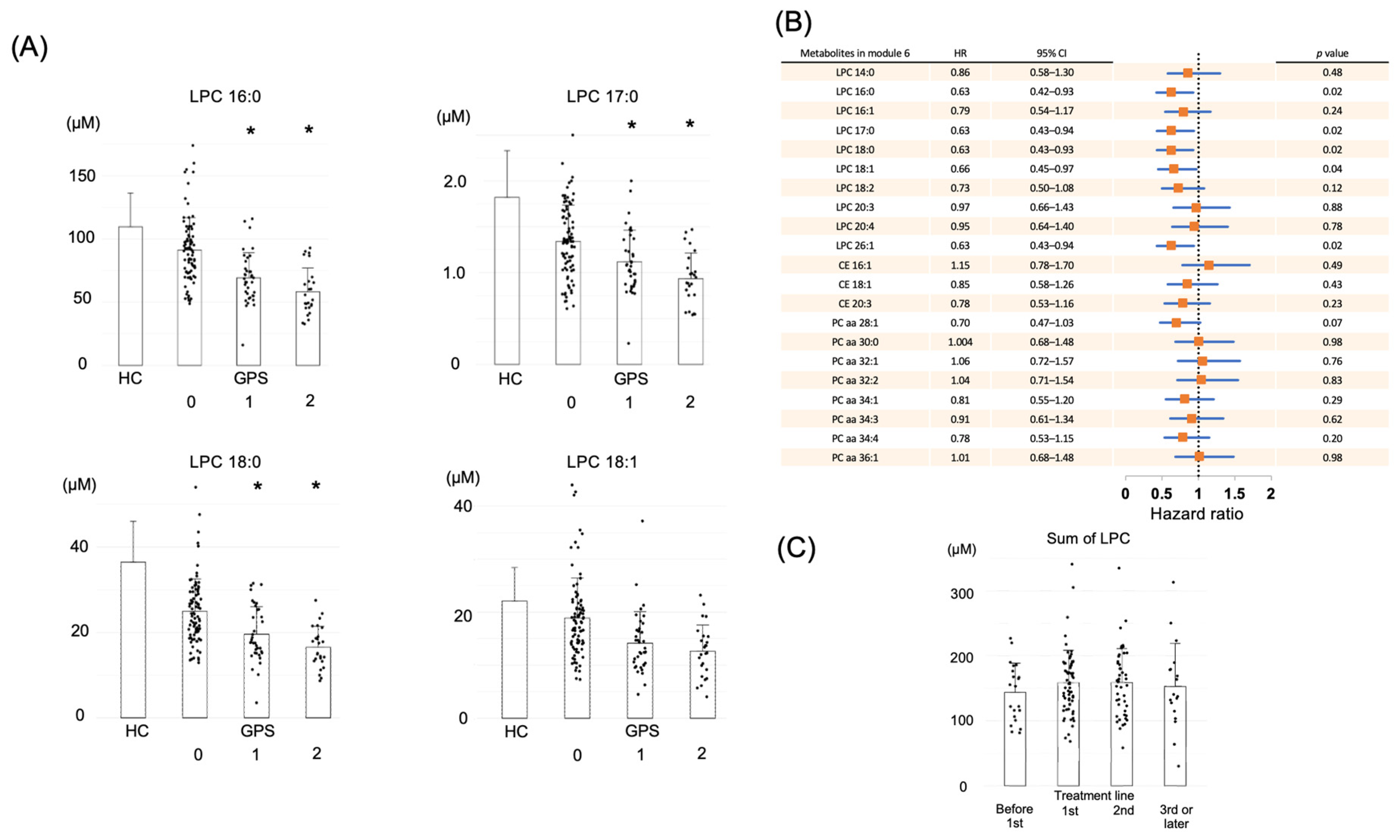
2.3. LPC in Module 6 Shows a Correlation with Prognosis
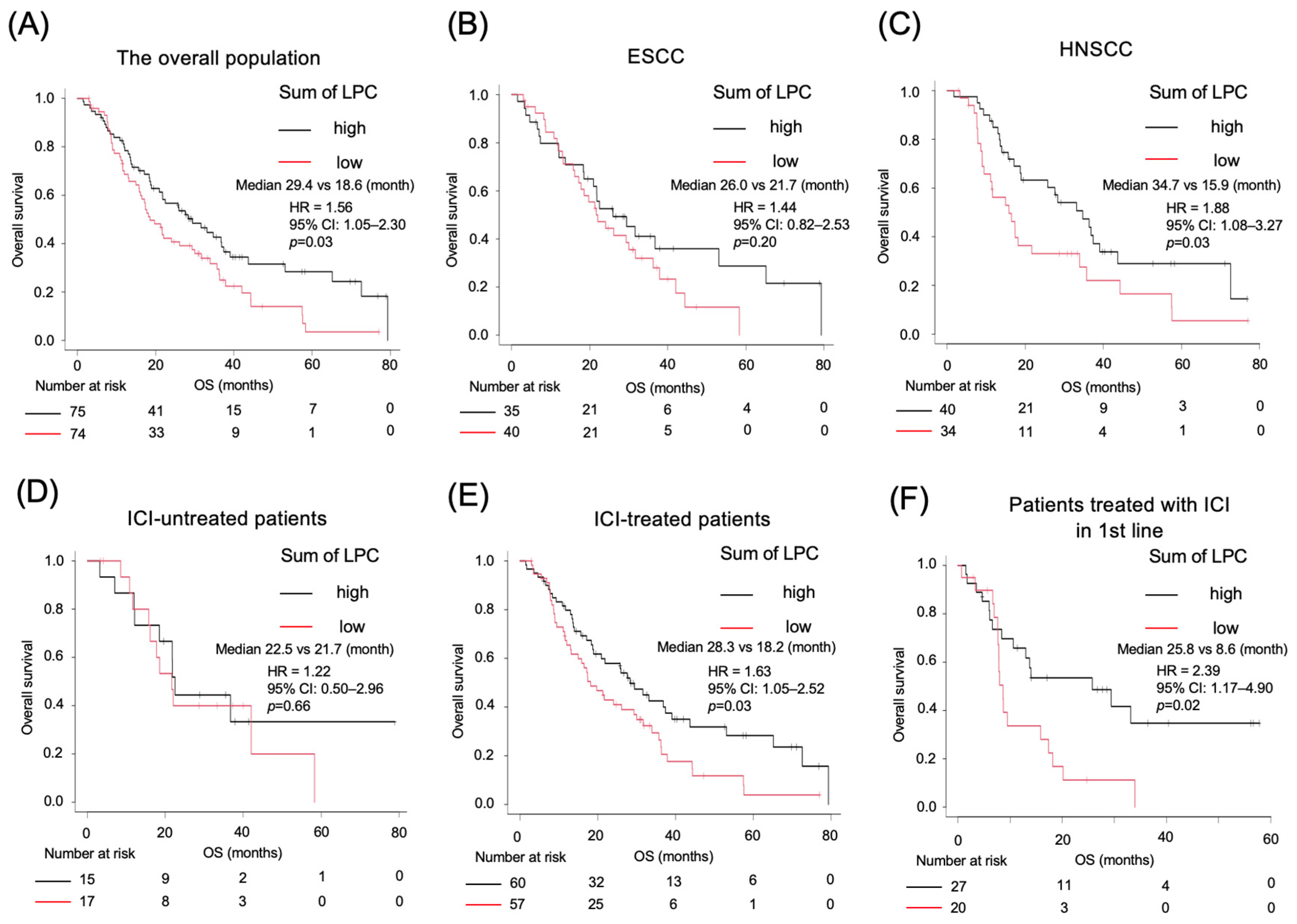
2.4. Kaplan–Meier Survival Analysis Based on LPC Levels
2.5. Association of Decreased LPC with Biological Processes and Inflammatory Pathways
3. Discussion
4. Materials and Methods
4.1. Clinical Samples and Data
4.2. Metabolomics Analysis
4.3. Weighted Gene Correlation Network Analysis (WGCNA)
4.4. Proteomics Analysis
4.5. Cytokine Assay
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCC | Squamous cell carcinoma |
| LPC | Lysophosphatidylcholine |
| WGCNA | Weighted gene correlation network analysis |
| OS | Overall survival |
| GPS | Glasgow prognostic score |
| CONUT | Controlling nutritional status |
| HR | Hazard ratio |
| CI | Confidence interval |
| PS | Performance status |
| PC | Phosphatidylcholine |
| CE | Cholesteryl ester |
| HC | Healthy cohort |
| ICI | Immune checkpoint inhibitor |
| PFS | Progression-free survival |
| CRP | C-reactive protein |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein |
| LPA | Lysophosphatidic acid |
| irAE | Immune-related adverse events |
References
- Martínez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef]
- Kao, K.C.; Vilbois, S.; Tsai, C.H.; Ho, P.C. Metabolic communication in the tumour–immune microenvironment. Nat. Cell Biol. 2022, 24, 1574–1583. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef]
- Broadfield, L.A.; Pane, A.A.; Talebi, A.; Swinnen, J.V.; Fendt, S.M. Lipid metabolism in cancer: New perspectives and emerging mechanisms. Dev. Cell 2021, 56, 1363–1393. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Patel, B.M. The burning furnace: Alteration in lipid metabolism in cancer-associated cachexia. Mol. Cell. Biochem. 2022, 477, 1709–1723. [Google Scholar] [CrossRef] [PubMed]
- Baazim, H.; Antonio-Herrera, L.; Bergthaler, A. The interplay of immunology and cachexia in infection and cancer. Nat. Rev. Immunol. 2022, 22, 309–321. [Google Scholar] [CrossRef]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-generation sequencing technology: Current trends and advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef] [PubMed]
- Shirota, H.; Komine, K.; Takahashi, M.; Takahashi, S.; Miyauchi, E.; Niizuma, H.; Tada, H.; Shimada, M.; Niihori, T.; Aoki, Y.; et al. Clinical decisions by the molecular tumor board on comprehensive genomic profiling tests in Japan: A retrospective observational study. Cancer Med. 2023, 12, 6170–6181. [Google Scholar] [CrossRef]
- Garg, P.; Singhal, S.; Kulkarni, P.; Horne, D.; Malhotra, J.; Salgia, R.; Singhal, S.S. Advances in Non-Small Cell Lung Cancer: Current Insights and Future Directions. J. Clin. Med. 2024, 13, 4189. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Lu, H.I.; Chien, C.Y.; Lo, C.M.; Wang, Y.M.; Chou, S.Y.; Su, Y.Y.; Shih, L.H.; Li, S.H. Treatment Outcomes of Patients with Locally Advanced Synchronous Esophageal and Head/Neck Squamous Cell Carcinoma Receiving Curative Concurrent Chemoradiotherapy. Sci. Rep. 2017, 7, 41785. [Google Scholar] [CrossRef]
- Peng, H.; Tan, X. The Prognostic Significance of Sarcopenia and the Neutrophil-to-Lymphocyte Ratio in Elderly Patients with Esophageal Squamous Cell Carcinoma. Cancer Manag. Res. 2021, 13, 3209–3218. [Google Scholar] [CrossRef]
- Zhou, D.; Duan, Z.; Li, Z.; Ge, F.; Wei, R.; Kong, L. The significance of glycolysis in tumor progression and its relation-ship with the tumor microenvironment. Front. Pharmacol. 2022, 13, 1091779. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cui, L.; Lu, S.; Xu, S. Amino acid metabolism in tumor biology and therapy. Cell Death Dis. 2024, 15, 42. [Google Scholar] [CrossRef]
- Long, J.; Zhang, C.J.; Zhu, N.; Du, K.; Yin, Y.F.; Tan, X.; Liao, D.F.; Qin, L. Lipid metabolism and carcinogenesis, cancer development. Am. J. Cancer Res. 2018, 8, 778–791. [Google Scholar]
- Schmidt, D.R.; Patel, R.; Kirsch, D.G.; Lewis, C.A.; Vander Heiden, M.G.; Locasale, J.W. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J. Clin. 2021, 71, 333–358. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, N.; Ji, N.; Chen, Z.S. Proteomics technologies for cancer liquid biopsies. Mol. Cancer 2022, 21, 53. [Google Scholar] [CrossRef]
- Danzi, F.; Pacchiana, R.; Mafficini, A.; Scupoli, M.T.; Scarpa, A.; Donadelli, M.; Fiore, A. To metabolomics and beyond: A technological portfolio to investigate cancer metabolism. Sig. Transduct. Target. Ther. 2023, 8, 137. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef]
- de Ulíbarri, J.I.; González-Madroño, A.; de Villar, N.G.P.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Faria, S.S.; Fernandes PCJr Silva, M.J.B.; Lima, V.C.; Fontes, W.; Freitas-Junior, R.; Eterovic, A.K.; Forget, P. The neutro-phil-to-lymphocyte ratio: A narrative review. Ecancermedicalscience 2016, 10, 702. [Google Scholar]
- Yap, D.W.T.; Leone, A.G.; Wong, N.Z.H.; Zhao, J.J.; Tey, J.C.S.; Sundar, R.; Pietrantonio, F. Effectiveness of immune checkpoint inhibitors in patients with advanced esophageal squamous cell carcinoma: A meta-analysis including low PD-L1 subgroups. JAMA Oncol. 2023, 9, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.P.; Li, Z.; Kim, S.B.; et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEY-NOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 2021, 398, 759–771. [Google Scholar] [CrossRef]
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.Y.; Chin, K.; Kadowaki, S.; Ahn, M.J.; Hamamoto, Y.; Doki, Y.; et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1506–1517. [Google Scholar] [CrossRef]
- Law, S.H.; Chan, M.L.; Marathe, G.K.; Parveen, F.; Chen, C.H.; Ke, L.Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef]
- Kano, K.; Aoki, J.; Hla, T. Lysophospholipid Mediators in Health and Disease. Annu. Rev. Pathol. 2022, 17, 459–483. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xiao, Y.; Elson, P.; Tan, H.; Plummer, S.J.; Berk, M.; Aung, P.P.; Lavery, I.C.; Achkar, J.P.; Li, L.; et al. Plasma lysophos-phatidylcholine levels: Potential biomarkers for colorectal cancer. J. Clin. Oncol. 2007, 25, 2696–2701. [Google Scholar] [CrossRef]
- Dong, J.; Cai, X.; Zhao, L.; Xue, X.; Zou, L.; Zhang, X.; Liang, X. Lysophosphatidylcholine profiling of plasma: Discrimina-tion of isomers and discovery of lung cancer biomarkers. Metabolomics 2010, 6, 478–488. [Google Scholar] [CrossRef]
- Yin, M.; Tan, S.; Li, X.; Hou, Y.; Cao, G.; Li, K.; Kou, J.; Lou, G. Identification of phosphatidylcholine and lysophosphati-dylcholine as novel biomarkers for cervical cancers in a prospective cohort study. Tumor Biol. 2016, 37, 5485–5492. [Google Scholar] [CrossRef]
- Xu, X.; Cheng, S.; Ding, C.; Lv, Z.; Chen, D.; Wu, J.; Zheng, S. Identification of bile biomarkers of biliary tract cancer through a liquid chromatography/mass spectrometry-based metabolomic method. Mol. Med. Rep. 2015, 11, 2191–2198. [Google Scholar] [CrossRef]
- Goto, T.; Terada, N.; Inoue, T.; Kobayashi, T.; Nakayama, K.; Okada, Y.; Yoshikawa, T.; Miyazaki, Y.; Uegaki, M.; Utsunomi-ya, N.; et al. Decreased expression of lysophosphatidylcholine (16:0/OH) in high resolution imaging mass spec-trometry independently predicts biochemical recurrence after surgical treatment for prostate cancer. Prostate 2015, 75, 1821–1830. [Google Scholar] [CrossRef]
- Matsuda, A.; Yamada, M.; Matsumoto, S.; Sakurazawa, N.; Yamada, T.; Matsutani, T.; Miyashita, M.; Uchida, E. Lyso-phosphatidylcholine as a predictor of postoperative complications after colorectal cancer surgery. Surg. Today. 2018, 48, 936–943. [Google Scholar] [CrossRef]
- Schmidt, J.; Kajtár, B.; Juhász, K.; Péter, M.; Járai, T.; Burián, A.; Kereskai, L.; Gerlinger, I.; Tornóczki, T.; Balogh, G.; et al. Lipid and protein tumor markers for head and neck squamous cell carcinoma identified by imaging mass spectrometry. Oncotarget 2020, 11, 2702–2717. [Google Scholar] [CrossRef]
- Kamphorst, J.J.; Cross, J.R.; Fan, J.; de Stanchina, E.; Mathew, R.; White, E.P.; Thompson, C.B.; Rabinowiz, J.D. Hypoxic and Ras-trans formed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc. Natl. Acad. Sci. USA 2013, 110, 8882–8887. [Google Scholar] [CrossRef]
- Kamangar, F.; Chow, W.H.; Abnet, C.; Dawsey, S. Environmental causes of esophageal cancer. Gastroenterol. Clin. N. Am. 2009, 38, 27–57. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, W.; Chen, C.; Yan, B.; Zhu, L.; Chen, X.; Peng, C. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 2020, 247, 117443. [Google Scholar] [CrossRef]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging roles of lysophospholipids in health and disease. Prog. Lipid Res. 2020, 80, 101068. [Google Scholar] [CrossRef]
- Jin, H.R.; Wang, J.; Wang, Z.J.; Xi, M.J.; Xia, B.H.; Deng, K.; Yang, J.L. Lipid metabolic reprogramming in tumor microenvironment: From mechanisms to therapeutics. J. Hematol. Oncol. 2023, 16, 103. [Google Scholar] [CrossRef]
- Sevastou, I.; Kaffe, E.; Mouratis, M.A.; Aidinis, V. Lysoglycerophospholipids in chronic inflammatory disorders: The PLA2/LPC and ATX/LPA axes. Biochim. Biophys. Acta 2013, 1831, 42–60. [Google Scholar] [CrossRef]
- Saito, K.; Gemma, A.; Tatsumi, K.; Hattori, N.; Ushiki, A.; Tsushima, K.; Saito, Y.; Abe, M.; Horimasu, Y.; Kashiwada, T.; et al. Identification and characterization of lysophosphatidylcholine 14:0 as a biomarker for drug-induced lung disease. Sci. Rep. 2022, 12, 19819. [Google Scholar] [CrossRef]
- Yan, J.J.; Jung, J.S.; Kee, J.E.; Lee, J.; Huh, S.O.; Kim, H.S.; Jung, K.C.; Cho, J.Y.; Nam, J.S.; Suh, H.W.; et al. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat. Med. 2002, 10, 161–167. [Google Scholar] [CrossRef]
- Saito, R.F.; Andrade, L.N.S.; Bustos, S.O.; Chammas, R. Phosphatidylcholine-derived lipid mediators: The crosstalk be-tween cancer cells and immune cells. Front. Immunol. 2022, 13, 768606. [Google Scholar] [CrossRef]
- Yu, J.; Xiong, F.; Xu, Y.; Xu, H.; Zhang, X.; Gao, H.; Li, Y. Lipidomics reveals immune-related adverse events in NSCLC patients receiving immune checkpoint inhibitor. Int. Immunopharmacol. 2024, 127, 111412. [Google Scholar] [CrossRef]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Ishihara, R.; Ishikawa, H.; Ito, Y.; Oyama, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawachi, H.; et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: Part 1. Esophagus 2023, 20, 343–372. [Google Scholar] [CrossRef]
- Homma, A.; Ando, M.; Hanai, N.; Harada, H.; Honma, Y.; Kanda, T.; Kano, S.; Kawakita, D.; Kiyota, N.; Kizawa, Y.; et al. Summary of Japanese clinical practice guidelines for head and neck cancer-2022 update edited by the Japan society for head and neck cancer. Auris Nasus Larynx 2024, 51, 174–188. [Google Scholar] [CrossRef]
- Hishinuma, E.; Shimada, M.; Matsukawa, N.; Saigusa, D.; Li, B.; Kudo, K.; Tsuji, K.; Shigeta, S.; Tokunaga, H.; Kumada, K.; et al. Wide-targeted metabolome analysis identifies potential biomarkers for prognosis prediction of epithelial ovarian cancer. Toxins 2021, 13, 461. [Google Scholar] [CrossRef]
- jMorp: Japanese Multi-Omics Reference Panel update report 2023. Nucleic Acids Res. 2024, 52, D622–D632. [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, K.; Hishinuma, E.; Matsukawa, N.; Shirasago, Y.; Watanabe, M.; Sato, T.; Sato, Y.; Kumondai, M.; Kikuchi, M.; Ko-shiba, S.; et al. Global Proteomics for Identifying the Alteration Pathway of Niemann–Pick Disease Type C Using Hepatic Cell Models. Int. J. Mol. Sci. 2023, 24, 15642. [Google Scholar] [CrossRef] [PubMed]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Overall No. | 149 |
|---|---|---|
| Age | Median (range) | 69 (31–88) |
| Sex | Male | 126 (85%) |
| Female | 23 (15%) | |
| BMI, kg/m2 | Median (range) | 21.3 (14.4–34.9) |
| Body weight loss in past 6 months, n | ≥5% | 42 (28%) |
| ≥2% and BMI < 18.5 | 4 (3%) | |
| Others | 103 (69%) | |
| ECOG PS, n | 0 | 63 (42%) |
| 1 | 74 (50%) | |
| 2, 3 | 10 (7%) | |
| Unknown | 2 (1%) | |
| Primary lesion, n | Head and neck | 74 (50%) |
| Nasopharynx | 7 (5%) | |
| Oropharynx | 8 (5%) | |
| Hypopharynx | 20 (13%) | |
| Larynx | 5 (3%) | |
| Oral cavity | 32 (21%) | |
| Paranasal and nasal cavity | 1 (1%) | |
| Ear canal | 1 (1%) | |
| Esophagus | 75 (50%) | |
| Treatment line at time of blood collection, n | Before 1st line administration | 21 (14%) |
| 1st line | 64 (43%) | |
| 2nd line | 46 (31%) | |
| 3rd line or later | 18 (12%) | |
| Tumor diameter, mm | Median (range) | 36 (11–143) |
| Number of organs with metastases | ≤1 | 123 (83%) |
| ≥2 | 26 (17%) | |
| CONUT score | Median (range) | 4 (0–9) |
| NLR | Median (range) | 3.9 (0.995–66.6) |
| Glasgow prognostic score | 0 | 86 (58%) |
| 1 | 31 (21%) | |
| 2 | 32 (21%) | |
| CRP, mg/dL | Median (range) | 0.46 (0.02–13.95) |
| Albumin, g/dL | Median (range) | 3.8 (2.2–4.8) |
| SCC, ng/mL | Median (range) | 1.80 (0.40–87.3) |
| D-dimer, μg/mL | Median (range) | 1.3 (0.5–23.2) |
| Hemoglobin, g/dL | Median (range) | 12.1 (7.8–17.0) |
| Platelet, 103/μL | Median (range) | 251 (44–800) |
| Leukocyte, /μL | Median (range) | 6400 (1900–15,500) |
| HDL cholesterol, mg/dL | Median (range) | 17.8 (3.4–140.1) |
| LDL/VLDL cholesterol, mg/dL | Median (range) | 91.8 (34.0–329.8) |
| Total cholesterol, mg/dL | Median (range) | 113.0 (49.0–351.7) |
| Mean Concentration (SD) | ||||
|---|---|---|---|---|
| Metabolites in Module 6 | Healthy Cohort | Patients | ||
| GPS0 | GPS1 | GPS2 | ||
| LPC 14:0 | 1.7 (0.6) | 1.4 (0.5) | 1.0 * (0.3) | 1.0 * (0.5) |
| LPC 16:0 | 113.0 (29.4) | 91.3 (25.7) | 69.3 * (19.9) | 58.3 * (18.7) |
| LPC 16:1 | 2.9 (1.0) | 2.9 (1.3) | 2.0 * (0.8) | 1.73 * (0.7) |
| LPC 17:0 | 1.8 (0.5) | 1.3 (0.4) | 1.1 * (0.3) | 0.94 * (0.3) |
| LPC 18:0 | 37.1 (0.2) | 25.0 (7.6) | 19.6 * (6.5) | 16.5 * (4.9) |
| LPC 18:1 | 23.0 (7.1) | 18.9 (7.5) | 14.2 * (5.9) | 12.6 * (5.0) |
| LPC 18:2 | 33.5 (13.5) | 29.4 (12.2) | 20.9 * (7.7) | 19.0 * (7.7) |
| LPC 20:3 | 1.9 (0.7) | 1.5 (0.6) | 1.1 * (0.5) | 1.0 * (0.4) |
| LPC 20:4 | 5.9 (2.0) | 4.8 (1.9) | 4.0 * (1.6) | 3.2 * (1.2) |
| LPC 26:1 | 0.58 (0.7) | 0.32 (0.1) | 0.27 * (0.1) | 0.25 * (0.1) |
| CE 16:1 | 21.1 (17.0) | 22.1 (16.0) | 15.1 * (9.0) | 12.6 * (6.8) |
| CE 18:1 | 89.0 (64.9) | 108.3 (49.6) | 94.2 (56.8) | 76.1 * (33.7) |
| CE 20:3 | 6.2 (3.9) | 5.3 (2.8) | 4.8 (2.9) | 4.4 (3.0) |
| PC aa 28:1 | 3.4 (0.9) | 3.4 (1.1) | 3.0 * (0.9) | 2.9 * (0.9) |
| PC aa 30:0 | 3.8 (1.2) | 3.8 (1.7) | 2.8 * (1.0) | 3.1 * (1.5) |
| PC aa 32:1 | 13.4 (6.6) | 17.8 (15.9) | 10.9 * (5.5) | 11.4 * (7.3) |
| PC aa 32:2 | 3.1 (1.1) | 2.8 (1.6) | 2.0 * (1.2) | 2.0 * (1.6) |
| PC aa 34:1 | 199.6 (53.0) | 207.2 (82.1) | 166.0 * (48.4) | 166.8 * (52.7) |
| PC aa 34:3 | 13.2 (3.9) | 14.1 (6.3) | 10.7 * (4.3) | 10.3 * (3.7) |
| PC aa 34:4 | 1.4 (0.5) | 1.2 (0.6) | 0.87 * (0.4) | 0.79 * (0.4) |
| PC aa 36:1 | 45.4 (11.2) | 39.4 (13.0) | 32.7 * (10.5) | 33.2 * (10.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, T.; Shirota, H.; Hishinuma, E.; Kawaoka, S.; Matsukawa, N.; Kasahara, Y.; Ouchi, K.; Imai, H.; Saijo, K.; Komine, K.; et al. Plasma Lysophosphatidylcholine Levels Correlate with Prognosis and Immunotherapy Response in Squamous Cell Carcinoma. Int. J. Mol. Sci. 2025, 26, 7528. https://doi.org/10.3390/ijms26157528
Iwasaki T, Shirota H, Hishinuma E, Kawaoka S, Matsukawa N, Kasahara Y, Ouchi K, Imai H, Saijo K, Komine K, et al. Plasma Lysophosphatidylcholine Levels Correlate with Prognosis and Immunotherapy Response in Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2025; 26(15):7528. https://doi.org/10.3390/ijms26157528
Chicago/Turabian StyleIwasaki, Tomoyuki, Hidekazu Shirota, Eiji Hishinuma, Shinpei Kawaoka, Naomi Matsukawa, Yuki Kasahara, Kota Ouchi, Hiroo Imai, Ken Saijo, Keigo Komine, and et al. 2025. "Plasma Lysophosphatidylcholine Levels Correlate with Prognosis and Immunotherapy Response in Squamous Cell Carcinoma" International Journal of Molecular Sciences 26, no. 15: 7528. https://doi.org/10.3390/ijms26157528
APA StyleIwasaki, T., Shirota, H., Hishinuma, E., Kawaoka, S., Matsukawa, N., Kasahara, Y., Ouchi, K., Imai, H., Saijo, K., Komine, K., Takahashi, M., Ishioka, C., Koshiba, S., & Kawakami, H. (2025). Plasma Lysophosphatidylcholine Levels Correlate with Prognosis and Immunotherapy Response in Squamous Cell Carcinoma. International Journal of Molecular Sciences, 26(15), 7528. https://doi.org/10.3390/ijms26157528





