Tabernanthalog, a Non-Hallucinogenic Psychedelic, Alleviates Cancer-Induced Cognitive Deficits via Serotonergic Pathways
Abstract
1. Introduction
2. Results
2.1. Lung Cancer Induces Anxiety-like Behavior and Contextual Memory Impairment in Mice
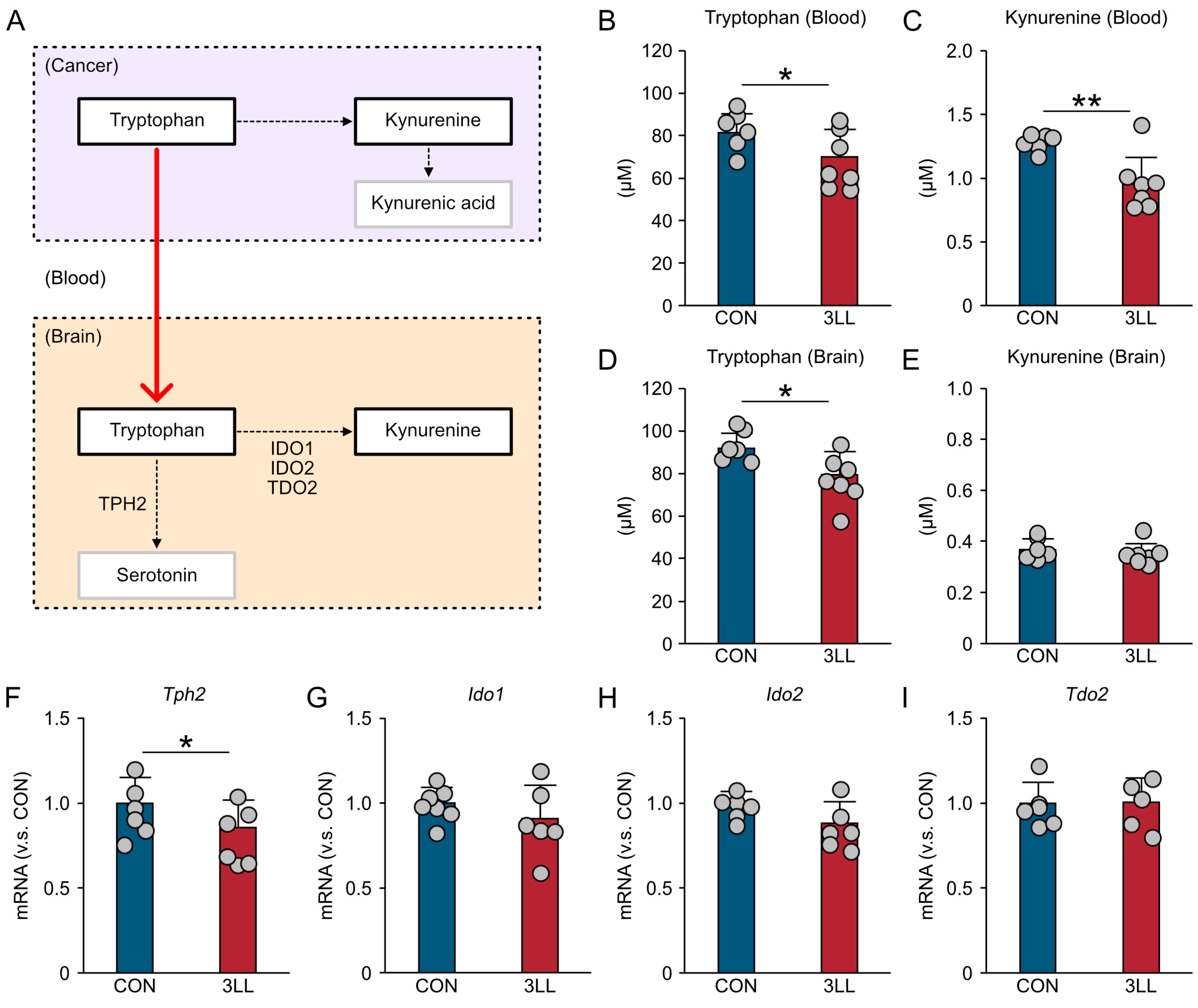
2.2. Reduced Hippocampal Tryptophan Availability and Serotonin Synthesis Gene Expression in 3LL Mice
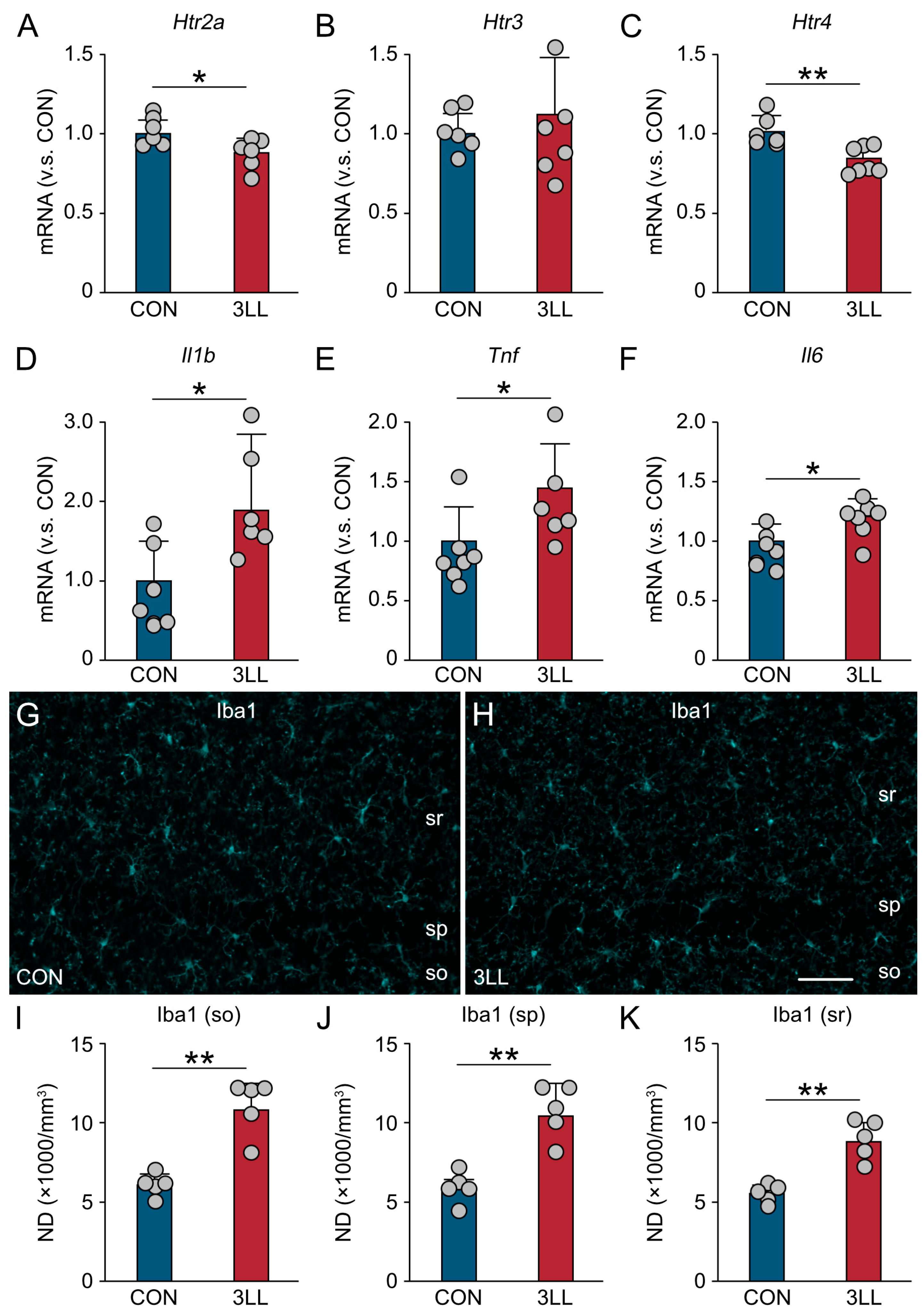
2.3. Downregulation of Serotonergic Receptors and Heightened Neuroinflammation in the Hippocampus of 3LL Mice
2.4. Reduced Serotonergic Terminals and Enhanced Microglial Activation in the Hippocampus of 3LL Mice
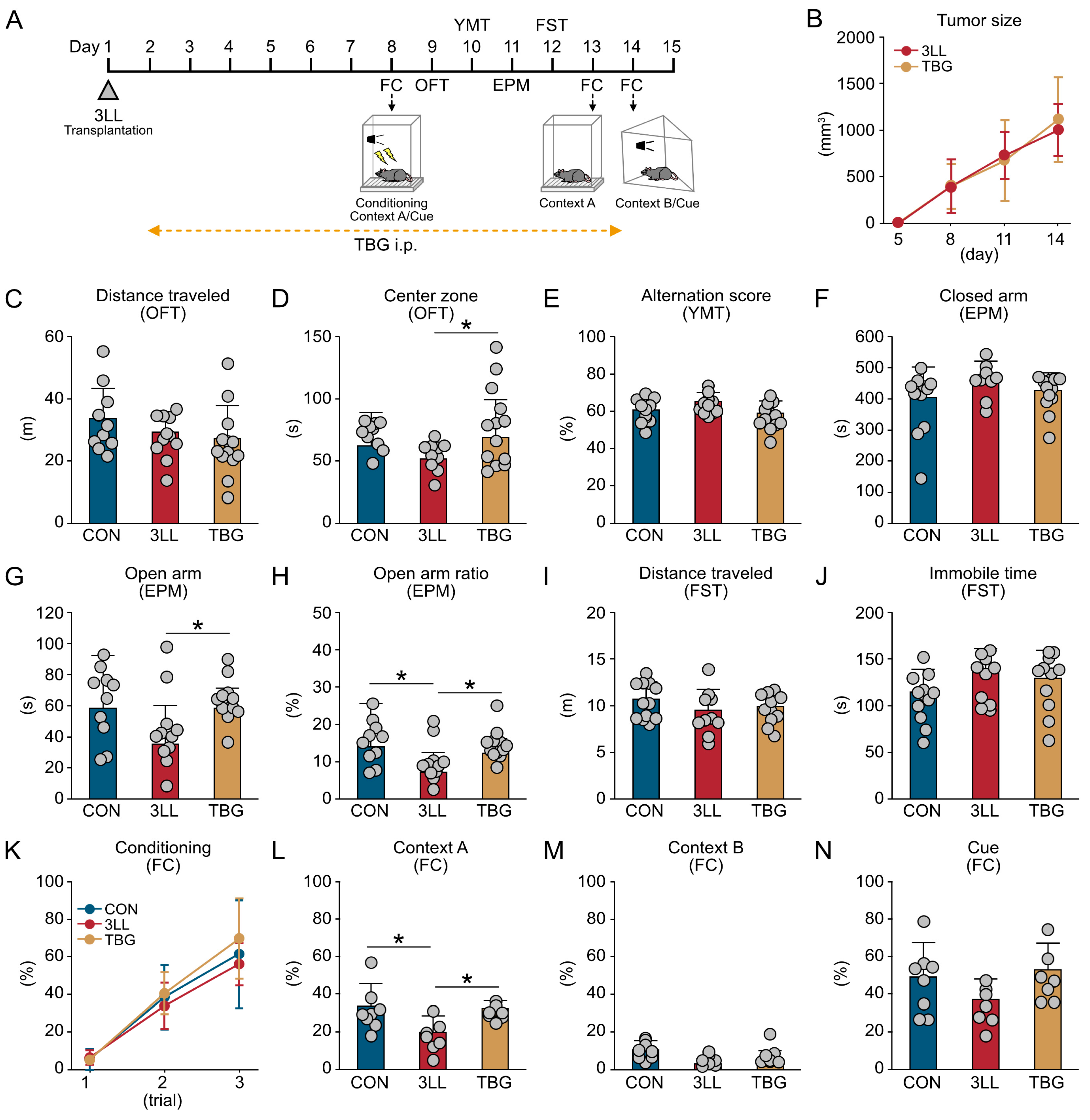
2.5. TBG Alleviates Anxiety-like Behavior and Cognitive Impairment in 3LL Mice
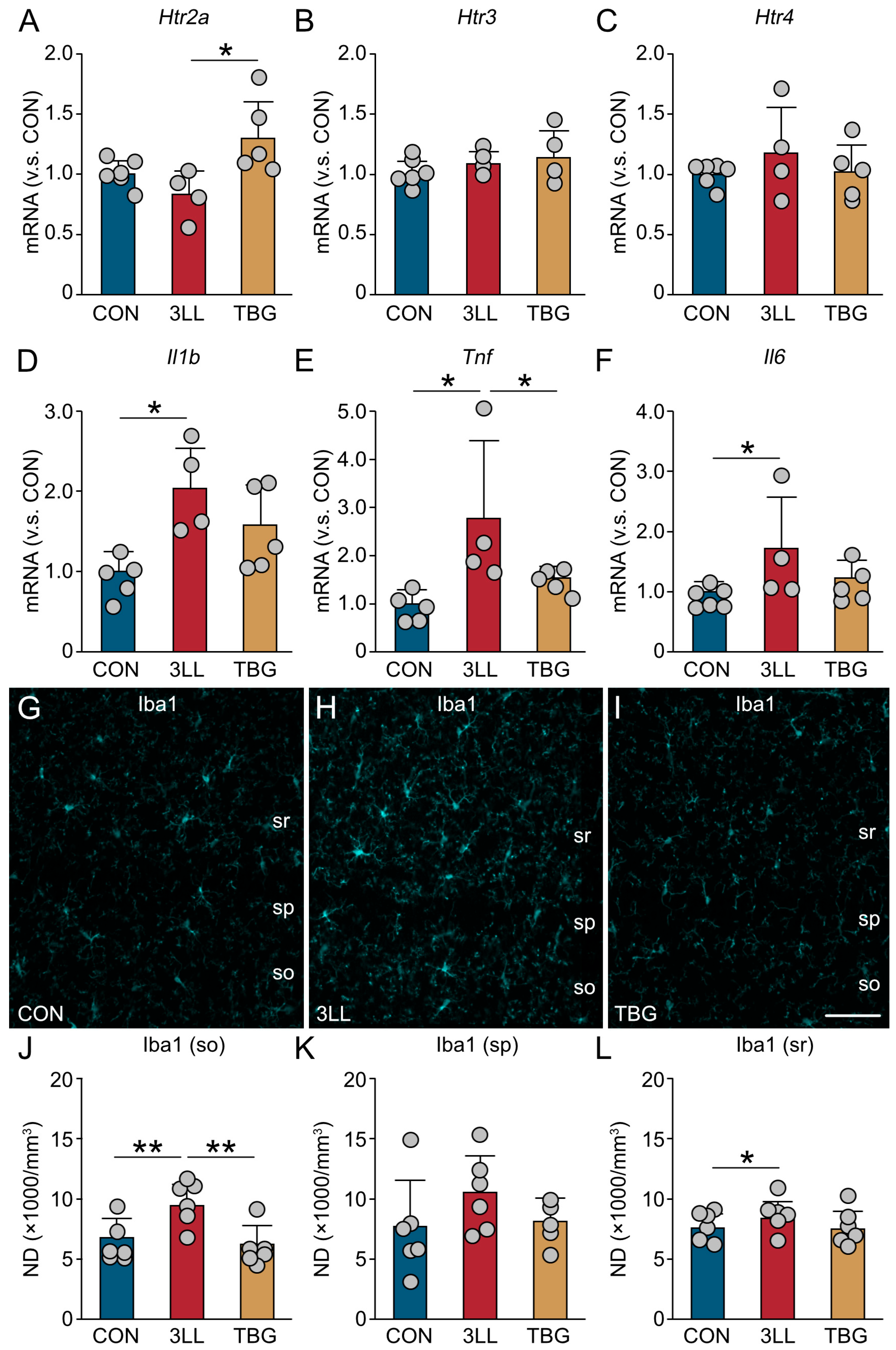
2.6. Upregulation of Serotonergic Receptors and Suppression of Neuroinflammation in the Hippocampus of TBG Mice
2.7. TBG Inhibits Alterations in Microglial Morphology and Gene Expression in the Hippocampus of 3LL Mice
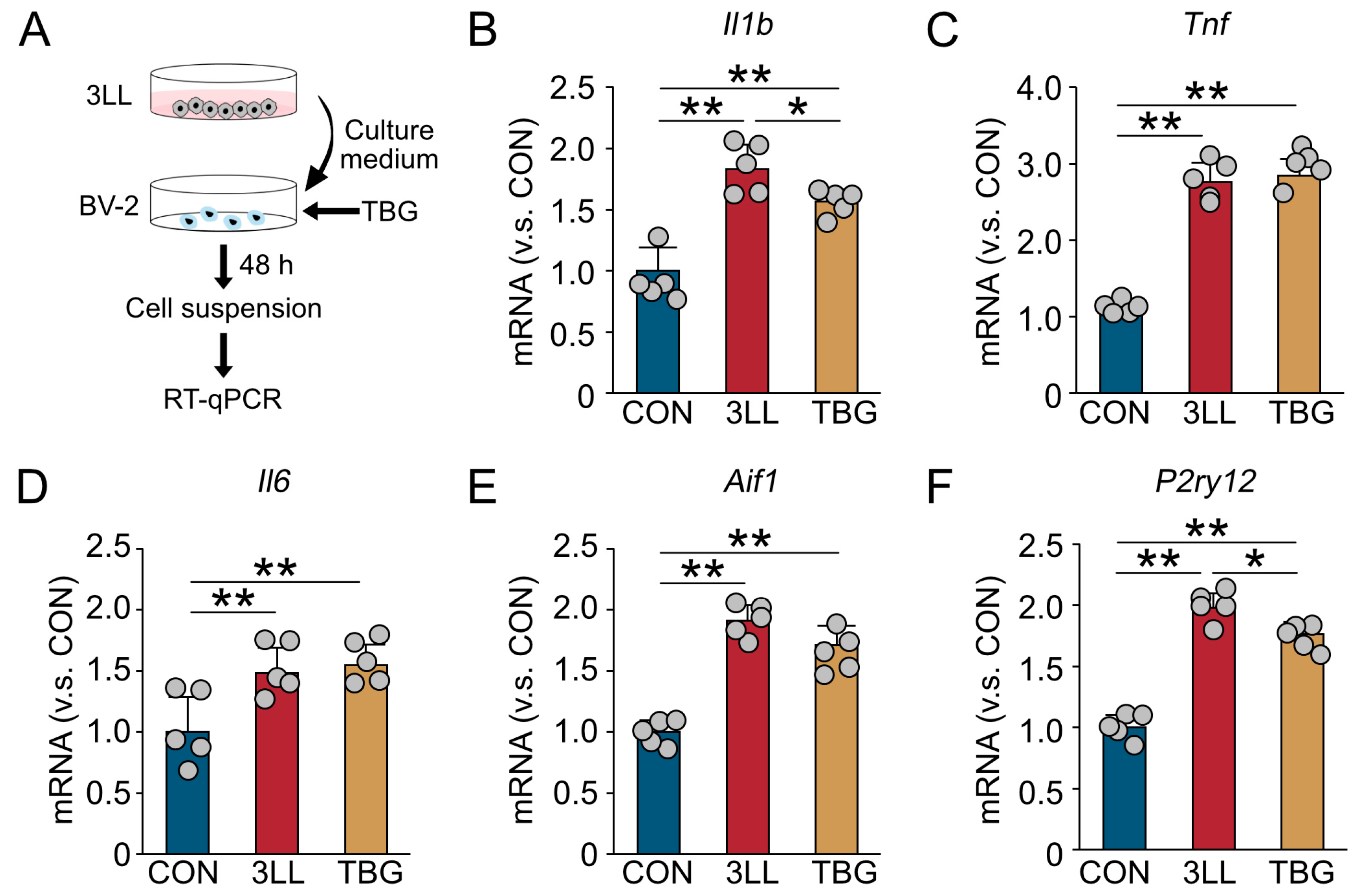
2.8. TBG Suppresses Neuroinflammatory Gene Expression in BV-2 Microglia Exposed to 3LL Cell-Conditioned Medium
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Line
4.3. Cancer Transplantation
4.4. Drug Administration and Animal Groups
4.5. Behavioral Test Battery
4.6. Perfusion Fixation and Sectioning
4.7. Measurement of Tryptophan and Kynurenine Concentrations
4.8. Voxel Counting Analysis of 5-HT+ Terminals
4.9. Optical Disector Analysis
4.10. Morphometric Analysis
- (1)
- Number of primary processes: The total number of primary processes.
- (2)
- Number of nodes: The total number of process nodes.
- (3)
- Number of ends: The total number of process ends.
- (4)
- Total process length: The sum of the lengths of all process branches.
- (5)
- Mean process length: The average length of all process branches.
- (6)
- Process volume: The total volume of all process branches.
- (7)
- Convex hull: The area of the convex polygon formed by connecting the tips of the most distal processes.
- (8)
- Cell body: the area of the cell body.
- (9)
- Length of 1st/2nd/3rd processes: The total length of the 1st, 2nd, and 3rd process branches, respectively.
- (10)
- Number of nodes in 1st/2nd/3rd processes: The total number of nodes in the 1st, 2nd, and 3rd processes, respectively.
- (11)
- Number of intersections (Sholl analysis): The number of intersections between processes and concentric circles at increasing distances from the cell body [22].
- (12)
- Process length (Sholl analysis): The cumulative length of processes intersecting concentric circles at increasing distances from the cell body.
4.11. Culture of BV-2 Microglia
4.12. RT–qPCR
- (1)
- Tph2.
- (2)
- Ido1.
- (3)
- Ido2.
- (4)
- Tdo2.
- (5)
- Htr2a.
- (6)
- Htr3.
- (7)
- Htr4.
- (8)
- Il1b.
- (9)
- Tnf.
- (10)
- Il6.
- (11)
- Aif1.
- (12)
- Tmem119.
- (13)
- P2ry12.
- (14)
- P2ry13.
- (15)
- Cd68.
- (16)
- Cd33.
- (17)
- Cx3cr1.
- (18)
- C1q.
- (19)
- Gapdh.
4.13. Statistical Analysis and Illustration Preparations
| Gene | Forward | Reverse |
| Tph2 | CCACCATTGTGACCCTGAATCC | ATGAGGACTCGGTGAGAGCATC |
| Ido1 | GCAGACTGTGTCCTGGCAAACT | AGAGACGAGGAAGAAGCCCTTG |
| Ido2 | GACAGTCTTGGTGGAGAAGGCA | ATCCTGGATGGAGAGTCTCAGC |
| Tdo2 | CATGCTCAAGGTGATAGCTCGG | GGAAGCCTGATGCTGGAGACAG |
| Htr2a | CCTGATGTCACTTGCCATAGCTG | CAGGTAAATCCAGACGGCACAG |
| Htr3 | CACACTCCTTCTGGGATACTCAG | GATGGTCTCAGCGAGGCTTATC |
| Htr4 | TGCTCACGTTCCTTGCAGTGGT | GTCAGCAAAGGCGAGAGACACA |
| Il1b | TGGACCTTCCAGGATGAGGACA | GTTCATCTCGGAGCCTGTAGTG |
| Tnf | GGTGCCTATGTCTCAGCCTCTT | GCCATAGAACTGATGAGAGGGAG |
| Il6 | TACCACTTCACAAGTCGGAGGC | CTGCAAGTGCATCATCGTTGTTC |
| Aif1 | TCTGCCGTCCAAACTTGAAGCC | CTCTTCAGCTCTAGGTGGGTCT |
| Tmem119 | ACTACCCATCCTCGTTCCCTGA | TAGCAGCCAGAATGTCAGCCTG |
| P2ry12 | CATTGACCGCTACCTGAAGACC | GCCTCCTGTTGGTGAGAATCATG |
| P2ry13 | TGGCATCAGGTGGTCAGTCACA | TTGTGCCTGCTGTCCTTACTCC |
| Cd68 | GGCGGTGGAATACAATGTGTCC | AGCAGGTCAAGGTGAACAGCTG |
| Cd33 | TGCAGAACATCACAATGAGAAAC | GAAAGGACCATCCAGCTCAA |
| Cx3cr1 | TCTGGACTCACTACCTCATCAG | TCCGGTTGTTCATGGAGTTGG |
| C1q | ATGGAGACCTCTCAGGGATG | ATACCAGTCCGGATGCCAGC |
| Gapdh | CATCACTGCCACCCAGAAGACTG | ATGCCAGTGAGCTTCCCGTTCAG |
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, Q.; Wang, L.; King, T.Z.; Chen, H.; Zhang, L.; Ni, J.; Mao, H. Seeing through “brain fog”: Neuroimaging assessment and imaging biomarkers for cancer-related cognitive impairments. Cancer Imaging 2024, 24, 158. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, B.; Zhang, P.; Yuan, Y.; Yue, S.; Chen, X.; Liang, J.; Tang, Z.; Zhang, B. Neuroscience of cancer: Unraveling the complex interplay between the nervous system, the tumor and the tumor immune microenvironment. Mol. Cancer 2025, 24, 24. [Google Scholar] [CrossRef]
- Oppegaard, K.; Harris, C.S.; Shin, J.; Paul, S.M.; Cooper, B.A.; Chan, A.; Anguera, J.A.; Levine, J.; Conley, Y.; Hammer, M.; et al. Cancer-related cognitive impairment is associated with perturbations in inflammatory pathways. Cytokine 2021, 148, 155653. [Google Scholar] [CrossRef]
- Hoogland, I.C.; Houbolt, C.; van Westerloo, D.J.; van Gool, W.A.; van de Beek, D. Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflamm. 2015, 12, 114. [Google Scholar] [CrossRef]
- Slominski, R.M.; Raman, C.; Chen, J.Y.; Slominski, A.T. How cancer hijacks the body’s homeostasis through the neuroendocrine system. Trends Neurosci. 2023, 46, 263–275. [Google Scholar] [CrossRef]
- Vita, G.; Compri, B.; Matcham, F.; Barbui, C.; Ostuzzi, G. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst. Rev. 2023, 3, Cd011006. [Google Scholar]
- Seo, S.K.; Kwon, B. Immune regulation through tryptophan metabolism. Exp. Mol. Med. 2023, 55, 1371–1379. [Google Scholar] [CrossRef]
- Li, C.; Zhao, H. Tryptophan and Its Metabolites in Lung Cancer: Basic Functions and Clinical Significance. Front. Oncol. 2021, 11, 707277. [Google Scholar] [CrossRef]
- Lanser, L.; Kink, P.; Egger, E.M.; Willenbacher, W.; Fuchs, D.; Weiss, G.; Kurz, K. Inflammation-Induced Tryptophan Breakdown is Related With Anemia, Fatigue, and Depression in Cancer. Front. Immunol. 2020, 11, 249. [Google Scholar] [CrossRef]
- Sălcudean, A.; Popovici, R.A.; Pitic, D.E.; Sârbu, D.; Boroghina, A.; Jomaa, M.; Salehi, M.A.; Kher, A.A.M.; Lica, M.M.; Bodo, C.R.; et al. Unraveling the Complex Interplay Between Neuroinflammation and Depression: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 1645. [Google Scholar] [CrossRef]
- Pienaar, L.; Baijnath, S.; Millen, A.M.E. Unravelling the neuroinflammatory links in depression: The potential of a lipopolysaccharide preclinical model. Discov. Med. 2024, 1, 93. [Google Scholar] [CrossRef]
- Wan, M.; Ding, L.; Wang, D.; Han, J.; Gao, P. Serotonin: A Potent Immune Cell Modulator in Autoimmune Diseases. Front. Immunol. 2020, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinflammation, homeostasis, and stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Sun, Z.; Ren, S.; Liu, M.; Wang, G.; Yang, J. Microglia in depression: An overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflamm. 2022, 19, 132. [Google Scholar] [CrossRef]
- Mariani, N.; Everson, J.; Pariante, C.M.; Borsini, A. Modulation of microglial activation by antidepressants. J. Psychopharmacol. 2022, 36, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, N.D.; Cikrikcili, U.; Akan, M.; Yucesan, E. Psychedelic therapy in depression and substance use disorders. Eur. J. Neurosci. 2024, 60, 4063–4077. [Google Scholar] [CrossRef]
- Marchi, M.; Farina, R.; Rachedi, K.; Laonigro, F.; Žuljević, M.F.; Pingani, L.; Ferrari, S.; Somers, M.; Boks, M.P.M.; Galeazzi, G.M. Psychedelics as an intervention for psychological, existential distress in terminally ill patients: A systematic review and network meta-analysis. J. Psychopharmacol. 2024, 10, 2698811241303594. [Google Scholar] [CrossRef]
- Holze, F.; Gasser, P.; Müller, F.; Strebel, M.; Liechti, M.E. LSD-assisted therapy in patients with anxiety: Open-label prospective 12-month follow-up. Br. J. Psychiatry 2024, 225, 362–370. [Google Scholar] [CrossRef]
- Cameron, L.P.; Tombari, R.J.; Lu, J.; Pell, A.J.; Hurley, Z.Q.; Ehinger, Y.; Vargas, M.V.; McCarroll, M.N.; Taylor, J.C.; Myers-Turnbull, D.; et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 2021, 589, 474–479. [Google Scholar] [CrossRef]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan Metabolic Pathways and Brain Serotonergic Activity: A Comparative Review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Green, T.R.F.; Rowe, R.K. Quantifying microglial morphology: An insight into function. Clin. Exp. Immunol. 2024, 216, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Sholl, D.A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 1953, 87, 387–406. [Google Scholar]
- Országhová, Z.; Mego, M.; Chovanec, M. Long-Term Cognitive Dysfunction in Cancer Survivors. Front. Mol. Biosci. 2021, 8, 770413. [Google Scholar] [CrossRef]
- Bray, V.J.; Dhillon, H.M.; Vardy, J.L. Systematic review of self-reported cognitive function in cancer patients following chemotherapy treatment. J. Cancer Surviv. 2018, 12, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.; Marks, D.L. Pretreatment Cancer-Related Cognitive Impairment-Mechanisms and Outlook. Cancers 2019, 11, 687. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.M.; Nagaraja, S.; Ocampo, A.; Tam, L.T.; Wood, L.S.; Pallegar, P.N.; Greene, J.J.; Geraghty, A.C.; Goldstein, A.K.; Ni, L.; et al. Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell 2019, 176, 43–55.e13. [Google Scholar] [CrossRef]
- Rao, V.; Bhushan, R.; Kumari, P.; Cheruku, S.P.; Ravichandiran, V.; Kumar, N. Chemobrain: A review on mechanistic insight, targets and treatments. Adv. Cancer Res. 2022, 155, 29–76. [Google Scholar]
- Yang, M.; Kim, J.; Kim, J.S.; Kim, S.H.; Kim, J.C.; Kang, M.J.; Jung, U.; Shin, T.; Wang, H.; Moon, C. Hippocampal dysfunctions in tumor-bearing mice. Brain Behav. Immun. 2014, 36, 147–155. [Google Scholar] [CrossRef]
- Walker, A.K.; Chang, A.; Ziegler, A.I.; Dhillon, H.M.; Vardy, J.L.; Sloan, E.K. Low dose aspirin blocks breast cancer-induced cognitive impairment in mice. PLoS ONE 2018, 13, e0208593. [Google Scholar] [CrossRef]
- Kurz, K.; Fiegl, M.; Holzner, B.; Giesinger, J.; Pircher, M.; Weiss, G.; Denz, H.A.; Fuchs, D. Fatigue in patients with lung cancer is related with accelerated tryptophan breakdown. PLoS ONE 2012, 7, e36956. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.W.; Jin, Y.; Shih, A.; Arazi, A.; Goodwin, S.; Roeser, J.; Furie, R.A.; Aranow, C.; Volpe, B.; Diamond, B.; et al. Associations between circulating interferon and kynurenine/tryptophan pathway metabolites: Support for a novel potential mechanism for cognitive dysfunction in SLE. Lupus Sci. Med. 2022, 9, e000808. [Google Scholar] [CrossRef]
- Dorcas Aremu, T.; Ramírez Ortega, D.; Blanco Ayala, T.; González Esquivel, D.F.; Pineda, B.; Pérez de la Cruz, G.; Salazar, A.; Flores, I.; Meza-Sosa, K.F.; Sánchez Chapul, L.; et al. Modulation of Brain Kynurenic Acid by N-Acetylcysteine Prevents Cognitive Impairment and Muscular Weakness Induced by Cisplatin in Female Rats. Cells 2024, 13, 1989. [Google Scholar] [CrossRef]
- Bell, C.; Abrams, J.; Nutt, D. Tryptophan depletion and its implications for psychiatry. Br. J. Psychiatry 2001, 178, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Pantovic-Stefanovic, M.; Karanovic, J.; Jurisic, V.; Dunjic-Kostic, B.; Nesic, M.; Dodic, S.; Gostiljac, M.; Puric, M.; Savic Pavicevic, D.; Ivkovic, M. Mood disorders and 5-HTR2A genetic variants-the moderator effect of inflammation on expression of affective polarity phenotype. BMC Psychiatry 2024, 24, 747. [Google Scholar] [CrossRef]
- Ledo, J.H.; Azevedo, E.P.; Beckman, D.; Ribeiro, F.C.; Santos, L.E.; Razolli, D.S.; Kincheski, G.C.; Melo, H.M.; Bellio, M.; Teixeira, A.L.; et al. Cross Talk Between Brain Innate Immunity and Serotonin Signaling Underlies Depressive-Like Behavior Induced by Alzheimer’s Amyloid-β Oligomers in Mice. J. Neurosci. 2016, 36, 12106–12116. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.I.; Ledwos, N.; Fellows, E.; Baer, J.; Rosenblat, J.D.; Blumberger, D.M.; Mulsant, B.H.; Castle, D.J. Serotonergic psychedelics for depression: What do we know about neurobiological mechanisms of action? Front. Psychiatry 2022, 13, 1076459. [Google Scholar] [CrossRef]
- Arias, H.R.; Micheli, L.; Rudin, D.; Bento, O.; Borsdorf, S.; Ciampi, C.; Marin, P.; Ponimaskin, E.; Manetti, D.; Romanelli, M.N.; et al. Non-hallucinogenic compounds derived from iboga alkaloids alleviate neuropathic and visceral pain in mice through a mechanism involving 5-HT. Biomed. Pharmacother. 2024, 177, 116867. [Google Scholar] [CrossRef] [PubMed]
- Catlow, B.J.; Song, S.; Paredes, D.A.; Kirstein, C.L.; Sanchez-Ramos, J. Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp. Brain Res. 2013, 228, 481–491. [Google Scholar] [CrossRef]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
- Grob, C.S.; Danforth, A.L.; Chopra, G.S.; Hagerty, M.; McKay, C.R.; Halberstadt, A.L.; Greer, G.R. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch. Gen. Psychiatry 2011, 68, 71–78. [Google Scholar] [CrossRef]
- Mampay, M.; Flint, M.S.; Sheridan, G.K. Tumour brain: Pretreatment cognitive and affective disorders caused by peripheral cancers. Br. J. Pharmacol. 2021, 178, 3977–3996. [Google Scholar] [CrossRef] [PubMed]
- de Deus, J.L.; Maia, J.M.; Soriano, R.N.; Amorim, M.R.; Branco, L.G.S. Psychedelics in neuroinflammation: Mechanisms and therapeutic potential. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 137, 111278. [Google Scholar] [CrossRef]
- Wiens, K.R.; Brooks, N.A.H.; Riar, I.; Greuel, B.K.; Lindhout, I.A.; Klegeris, A. Psilocin, the Psychoactive Metabolite of Psilocybin, Modulates Select Neuroimmune Functions of Microglial Cells in a 5-HT. Molecules 2024, 29, 5084. [Google Scholar] [CrossRef]
- Floris, G.; Dabrowski, K.R.; Zanda, M.T.; Daws, S.E. Psilocybin reduces heroin seeking behavior and modulates inflammatory gene expression in the nucleus accumbens and prefrontal cortex of male rats. Mol. Psychiatry 2024, 30, 1801–1816. [Google Scholar] [CrossRef]
- Szabo, A.; Frecska, E. Dimethyltryptamine (DMT): A biochemical Swiss Army knife in neuroinflammation and neuroprotection? Neural Regen. Res. 2016, 11, 396–397. [Google Scholar] [CrossRef]
- Zanikov, T.; Gerasymchuk, M.; Ghasemi Gojani, E.; Robinson, G.I.; Asghari, S.; Groves, A.; Haselhorst, L.; Nandakumar, S.; Stahl, C.; Cameron, M.; et al. The Effect of Combined Treatment of Psilocybin and Eugenol on Lipopolysaccharide-Induced Brain Inflammation in Mice. Molecules 2023, 28, 2624. [Google Scholar] [CrossRef]
- Timmermann, C.; Roseman, L.; Haridas, S.; Rosas, F.E.; Luan, L.; Kettner, H.; Martell, J.; Erritzoe, D.; Tagliazucchi, E.; Pallavicini, C.; et al. Human brain effects of DMT assessed via EEG-fMRI. Proc. Natl. Acad. Sci. USA 2023, 120, e2218949120. [Google Scholar] [CrossRef]
- Flanagan, T.W.; Foster, T.P.; Galbato, T.E.; Lum, P.Y.; Louie, B.; Song, G.; Halberstadt, A.L.; Billac, G.B.; Nichols, C.D. Serotonin-2 Receptor Agonists Produce Anti-inflammatory Effects through Functionally Selective Mechanisms That Involve the Suppression of Disease-Induced Arginase 1 Expression. ACS Pharmacol. Transl. Sci. 2024, 7, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Rocks, D.; Cham, H.; Kundakovic, M. Why the estrous cycle matters for neuroscience. Biol. Sex. Differ. 2022, 13, 62. [Google Scholar] [CrossRef]
- Percie Du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Physiol. 2020, 598, 3793–3801. [Google Scholar] [CrossRef] [PubMed]
- West, M.J.; Gundersen, H.J. Unbiased stereological estimation of the number of neurons in the human hippocampus. J. Comp. Neurol. 1990, 296, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hedreen, J.C. Lost caps in histological counting methods. Anat. Rec. 1998, 250, 366–372. [Google Scholar]
- Gundersen, H.J.; Jensen, E.B. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 1987, 147 Pt 3, 229–263. [Google Scholar] [CrossRef]
- Jinno, S.; Aika, Y.; Fukuda, T.; Kosaka, T. Quantitative analysis of GABAergic neurons in the mouse hippocampus, with optical disector using confocal laser scanning microscope. Brain Res. 1998, 814, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arinaga, M.; Yamada, J.; Maeda, S.; Okamura, A.; Oshima, Y.; Zhang, L.; Han, Y.; Iinuma, K.M.; Jinno, S. Tabernanthalog, a Non-Hallucinogenic Psychedelic, Alleviates Cancer-Induced Cognitive Deficits via Serotonergic Pathways. Int. J. Mol. Sci. 2025, 26, 7519. https://doi.org/10.3390/ijms26157519
Arinaga M, Yamada J, Maeda S, Okamura A, Oshima Y, Zhang L, Han Y, Iinuma KM, Jinno S. Tabernanthalog, a Non-Hallucinogenic Psychedelic, Alleviates Cancer-Induced Cognitive Deficits via Serotonergic Pathways. International Journal of Molecular Sciences. 2025; 26(15):7519. https://doi.org/10.3390/ijms26157519
Chicago/Turabian StyleArinaga, Masahide, Jun Yamada, Shoichiro Maeda, Ayumi Okamura, Yuto Oshima, Liye Zhang, Yiying Han, Kyoko M. Iinuma, and Shozo Jinno. 2025. "Tabernanthalog, a Non-Hallucinogenic Psychedelic, Alleviates Cancer-Induced Cognitive Deficits via Serotonergic Pathways" International Journal of Molecular Sciences 26, no. 15: 7519. https://doi.org/10.3390/ijms26157519
APA StyleArinaga, M., Yamada, J., Maeda, S., Okamura, A., Oshima, Y., Zhang, L., Han, Y., Iinuma, K. M., & Jinno, S. (2025). Tabernanthalog, a Non-Hallucinogenic Psychedelic, Alleviates Cancer-Induced Cognitive Deficits via Serotonergic Pathways. International Journal of Molecular Sciences, 26(15), 7519. https://doi.org/10.3390/ijms26157519






