When Bone Forms Where It Shouldn’t: Heterotopic Ossification in Muscle Injury and Disease
Abstract
1. Introduction
2. Heterotopic Ossification
3. Causes of Heterotopic Ossification: Genetic and Trauma-Related Etiologies
3.1. Genetic
3.2. Trauma-Induced HO
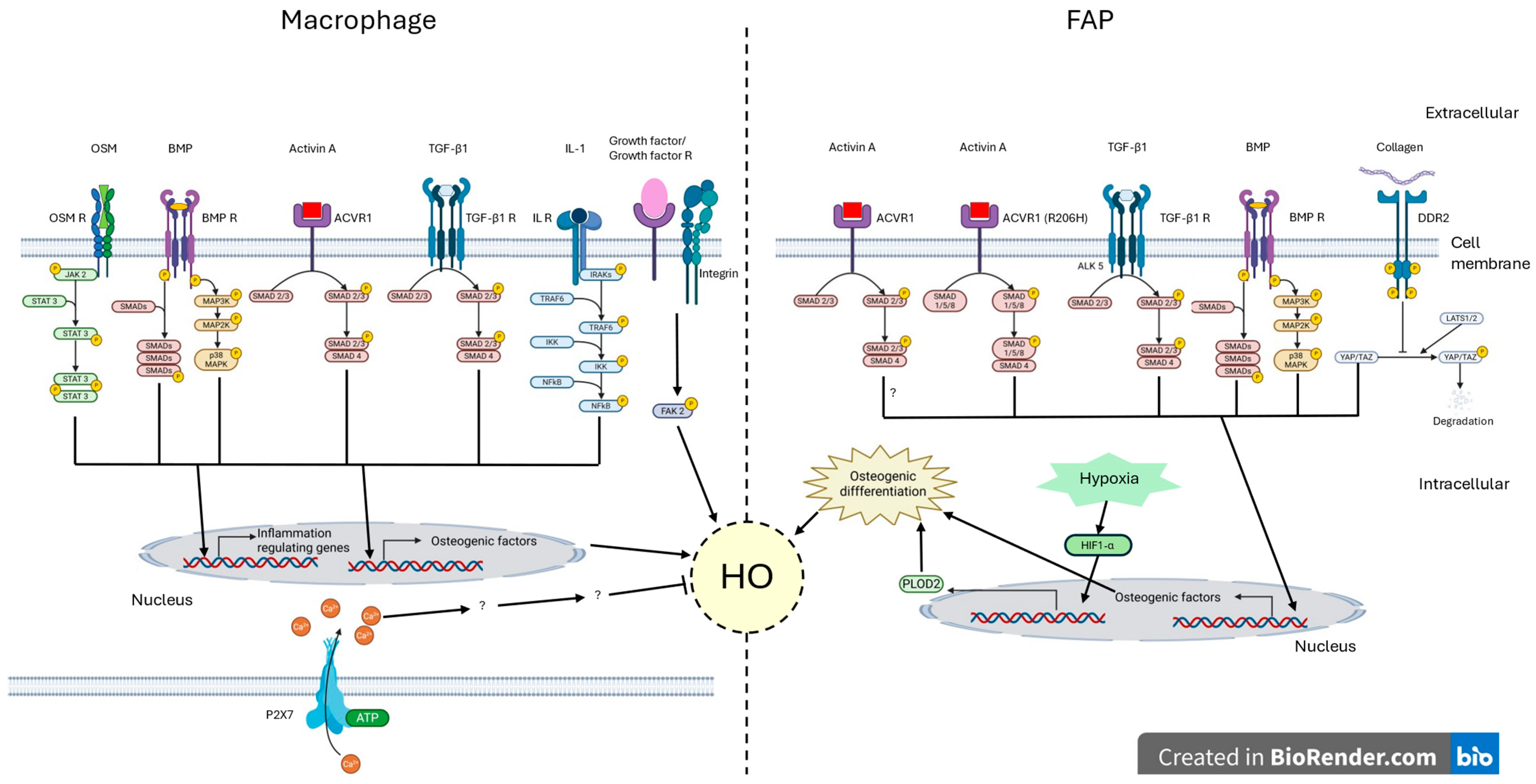
4. Abnormal Inflammation in Heterotopic Ossification
5. An Aberrant New Extracellular Matrix
6. FAP at the Core of Myositis Ossificans
7. Neuroendocrine Signalling in Neuro-Heterotopic Ossification
8. Current and Potential Treatments for Heterotopic Ossification
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACVR1/ALK2 | Activin A Receptor Type I/Activin Receptor-Like Kinase 2 |
| AHO | Albright Hereditary Osteodystrophy |
| ALK | Activin Receptor-Like Kinase |
| BMP | Bone Morphogenetic Protein |
| CCL | Chemokine (C-C motif) Ligand |
| CD | Cluster of Differentiation |
| CGRP | Calcitonin Gene-Related Peptide |
| CNS | Central Nervous System |
| CREB | cAMP Response Element-Binding Protein |
| CTX | Cardiotoxin |
| CXCL8 | C-X-C Motif Chemokine Ligand 8 |
| DDR2 | Discoidin Domain Receptor 2 |
| DNA | Deoxyribonucleic Acid |
| ECM | Extracellular Matrix |
| ED | Ectopic Inorganic Calcium Phosphate Deposit |
| ENPP1 | Ectonucleotide Pyrophosphate/Phosphodiesterase 1 |
| FAK2 | Focal Adhesion Kinase 2 |
| FAPs | Fibro-Adipogenic Progenitor Cells |
| FGFR3 | Fibroblast Growth Factor Receptor 3 |
| FOP | Fibrodysplasia Ossificans Progressiva |
| GR | Glucocorticoid Receptor |
| GNAS | Guanine Nucleotide-Binding Protein, Alpha Stimulating Activity Polypeptide |
| GFP | Green Fluorescent Protein |
| Gαs | α-Subunit of the Stimulatory G Protein |
| HIF1-α | Hypoxia-Inducible Factor 1-alpha |
| HO | Heterotopic Ossification |
| IL | Interleukin |
| JAK | Janus Kinase |
| LATS1/2 | Large Tumour Suppressor Kinases 1 and 2 |
| LECs | Lymphatic Endothelial Cells |
| MAPK | Mitogen-Activated Protein Kinases |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MMP | Metalloproteinases |
| MN | Motoneuron |
| MPC | Myeloid Progenitor Cell |
| MSC | Mesenchymal Stem Cell |
| mTOR | Mechanistic Target of Rapamycin |
| NFkB | Nuclear Factor-Kappa B |
| NGF | Nerve Growth Factor |
| NHO | Neurogenic Heterotopic Ossification |
| NK | Natural Killer Cell |
| NK-1 | Neurokinin-1 |
| NSAID | Nonsteroidal Anti-Inflammatory Drug |
| OPG | Osteoprotegerin |
| OSM | Oncostatin M |
| PKA | Protein Kinase A |
| PLOD2 | Procollagen-Lysine, 2-Oxoglutarate 5-Dioxygenase 2 |
| POH | Progressive Osseous Heteroplasia |
| PTH | Parathyroid Hormone |
| Rag-1 | Recombination-Activating Gene 1 |
| RANTES | Regulated on Activation, Normal T Cell Expressed and Secreted |
| RUNX2 | Runt-Related Transcription Factor 2 |
| SMA | Spinal Muscular Atrophy |
| SMAD | Small Mother Against Decapentaplegic |
| SP | Substance P |
| SCI | Spinal Cord Injury |
| STAT | Signal Transducer and Activator of Transcription |
| TAZ | Transcriptional Coactivator with PDZ-Binding Motif |
| TBI | Traumatic Brain Injury |
| TGF-β | Transforming Growth Factor Beta |
| TNF-α | Tumour Necrosis Factor Alpha |
| TrkA | Tropomyosin Receptor Kinase A |
| TRPV1 | Transient Receptor Potential Vanilloid-1 |
| YAP | Yes-Associated Protein |
References
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med 2020, 12, e1462. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L.; Plowman, S.A. Understanding Muscle Contraction. In Sports-Specific Rehabilitation; Donatelli, R., Ed.; Churchill Livingstone: Saint Louis, MO, USA, 2007; pp. 15–38. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, L. The Many Roles of Macrophages in Skeletal Muscle Injury and Repair. Front. Cell Dev. Biol. 2022, 10, 952249. [Google Scholar] [CrossRef] [PubMed]
- Molina, T.; Fabre, P.; Dumont, N.A. Fibro-adipogenic progenitors in skeletal muscle homeostasis, regeneration and diseases. Open Biol. 2021, 11, 210110. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interf. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Q.; Yu, C.; Wang, F.; Kong, X. Functional roles of CCL5/RANTES in liver disease. Liver Res. 2020, 4, 28–34. [Google Scholar] [CrossRef]
- Laumonier, T.; Menetrey, J. Muscle injuries and strategies for improving their repair. J. Exp. Orthop. 2016, 3, 15. [Google Scholar] [CrossRef]
- Meyers, C.; Lisiecki, J.; Miller, S.; Levin, A.; Fayad, L.; Ding, C.; Sono, T.; McCarthy, E.; Levi, B.; James, A.W. Heterotopic Ossification: A Comprehensive Review. JBMR Plus 2019, 3, e10172. [Google Scholar] [CrossRef]
- Cholok, D.; Chung, M.T.; Ranganathan, K.; Ucer, S.; Day, D.; Davis, T.A.; Mishina, Y.; Levi, B. Heterotopic ossification and the elucidation of pathologic differentiation. Bone 2018, 109, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sabou, F.L.P.; Necula, R.; Samota, I.; Sabou, A. The presence of hydroxyapatite crystal in heterotopic ossifications analyzed by means of x-ray powder diffraction method. Rom. J. Biophys. 2013, 23, 221–230. [Google Scholar]
- González, M.S. Skeletal Cartilage and Bone Formation, Composition, and Function in Small Mammals, Birds, and Reptiles. Vet. Clin. N. Am. Exot. Anim. Pract. 2019, 22, 123–134. [Google Scholar] [CrossRef]
- Chan, E.D.; Morales, D.V.; Welsh, C.H.; McDermott, M.T.; Schwarz, M.I. Calcium Deposition with or without Bone Formation in the Lung. Am. J. Respir. Crit. Care Med. 2002, 165, 1654–1669. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, U.; Tiwari, V. Fibrodysplasia Ossificans Progressiva. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK576373/ (accessed on 31 January 2025).
- Kan, C.; Ding, N.; Yang, J.; Tan, Z.; McGuire, T.L.; Lu, H.; Zhang, K.; Berger, D.M.P.; Kessler, J.A.; Kan, L. BMP-dependent, injury-induced stem cell niche as a mechanism of heterotopic ossification. Stem Cell Res. Ther. 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Zhang, C.; Wu, S.; Peng, Z.; Tania, M. Genetic abnormalities in fibrodysplasia ossificans progressiva. Genes Genet. Syst. 2012, 87, 213–219. [Google Scholar] [CrossRef]
- Huso, D.L.; Edie, S.; Levine, M.A.; Schwindinger, W.; Wang, Y.; Jüppner, H.; Germain-Lee, E.L. Heterotopic Ossifications in a Mouse Model of Albright Hereditary Osteodystrophy. PLoS ONE 2011, 6, e21755. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, R.J.; Ramaswamy, G.; Fong, J.T.; Shore, E.M.; Kaplan, F.S. Progressive osseous heteroplasia: Diagnosis, treatment, and prognosis. Appl. Clin. Genet. 2015, 8, 37–48. [Google Scholar] [CrossRef]
- Bastepe, M.; Turan, S.; He, Q. Heterotrimeric G Proteins in The Control of Parathyroid Hormone Actions. J. Mol. Endocrinol. 2017, 58, R203–R224. [Google Scholar] [CrossRef]
- Toom, A.; Haviko, T.; Rips, L. Heterotopic ossification after total hip arthroplasty. Int. Orthop. 2001, 24, 323–326. [Google Scholar] [CrossRef][Green Version]
- Dragonas, C.G.; Mamarelis, G.; Shahid, S.; Tsekes, D. A Systematic Review of Heterotopic Ossification Following Shoulder Arthroplasty: Is There a Clinical Value? Cureus 2023, 15, e47374. [Google Scholar] [CrossRef] [PubMed]
- Arabi, H.; Sellahi, H. Ossification post-traumatique du ligament collatéral médial du genou: À propos d’un cas. Pan Afr. Med. J. 2016, 24, 254. [Google Scholar] [CrossRef] [PubMed]
- Maradit Kremers, H.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of Total Hip and Knee Replacement in the United States. J. Bone Jt. Surg. Am. 2015, 97, 1386–1397. [Google Scholar] [CrossRef]
- Medina, A.; Shankowsky, H.; Savaryn, B.; Shukalak, B.; Tredget, E.E. Characterization of heterotopic ossification in burn patients. J. Burn Care Res. 2014, 35, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Devilbiss, Z.; Hess, M.; Ho, G.W. Myositis Ossificans in Sport: A Review. Curr. Sports Med. Rep. 2018, 17, 290–295. [Google Scholar] [CrossRef]
- Roger, B.; Guermazi, A.; Skaf, A. (Eds.) Muscle Injuries in Sport Athletes: Clinical Essentials and Imaging Findings. In Sports and Traumatology; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Baskerville, R.; Castell, L.; Bermon, S. Sports and Immunity, from the recreational to the elite athlete. Infect. Dis. Now 2024, 54, 104893. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Wheatley, B.M.; Cholok, D.; Agarwal, S.; Yu, P.B.; Levi, B.; Davis, T.A. The traumatic bone: Trauma-induced heterotopic ossification. Transl. Res. J. Lab. Clin. Med. 2017, 186, 95–111. [Google Scholar] [CrossRef]
- Forsberg, J.A.; Pepek, J.M.; Wagner, S.; Wilson, K.; Flint, J.; Andersen, R.C.; Tadaki, D.; A Gage, F.; Stojadinovic, A.; Elster, E.A. Heterotopic ossification in high-energy wartime extremity injuries: Prevalence and risk factors. J. Bone Jt. Surg. Am. 2009, 91, 1084–1091. [Google Scholar] [CrossRef]
- Wittenberg, R.; Peschke, U.; Botel, U. Heterotopic ossification after spinal cord injury. Epidemiology and risk factors. J. Bone Jt. Surg. Br. 1992, 74-B, 215–218. [Google Scholar] [CrossRef]
- van Kuijk, A.; Geurts, A.; van Kuppevelt, H. Neurogenic heterotopic ossification in spinal cord injury. Spinal Cord 2002, 40, 313–326. [Google Scholar] [CrossRef]
- Sullivan, M.P.; Torres, S.J.; Mehta, S.; Ahn, J. Heterotopic ossification after central nervous system trauma. Bone Jt. Res. 2013, 2, 51–57. [Google Scholar] [CrossRef]
- Garland, D.E.; O′Hollaren, R.M. Fractures and dislocations about the elbow in the head-injured adult. Clin. Orthop. 1982, 168, 38–41. [Google Scholar] [CrossRef]
- Anthonissen, J.; Steffen, C.T.; Alessandri, B.; Baranowski, A.; Rommens, P.M.; Victor, J.; Hofmann, A. Traumatic brain injury enhances the formation of heterotopic ossification around the hip: An animal model study. Arch. Orthop. Trauma Surg. 2019, 140, 1029–1035. [Google Scholar] [CrossRef]
- Li, J.-X.; Dang, Y.-M.; Liu, M.-C.; Gao, L.-Q.; Lin, H. Fibroblasts in heterotopic ossification: Mechanisms and therapeutic targets. Int. J. Biol. Sci. 2025, 21, 544–564. [Google Scholar] [CrossRef] [PubMed]
- Loder, S.J.; Agarwal, S.; Chung, M.T.; Cholok, D.; Hwang, C.; Visser, N.; Vasquez, K.; Sorkin, M.; Habbouche, J.; Sung, H.H.; et al. Characterizing the Circulating Cell Populations in Traumatic Heterotopic Ossification. Am. J. Pathol. 2018, 188, 2464–2473. [Google Scholar] [CrossRef]
- Kan, C.; Tan, Z.; Wang, H.; Wang, W.; Yang, J.; Zhang, Y.; Lu, X.; Cheng, Q.; Chai, L.; Peng, C.; et al. Spatiotemporal Analysis of Mesenchymal Stem Cells Fate Determination by Inflammatory Niche Following Soft Tissue Injury at a Single-Cell Level. Adv. Sci. 2024, 11, e2310282. [Google Scholar] [CrossRef]
- Nunez, J.H.; Juan, C.B.; Sun, Y.; Hong, J.B.; Bancroft, A.C.B.; Hwang, C.; Medrano, J.M.; Huber, A.K.; Tower, R.J.; Levi, B. Neutrophil and NETosis Modulation in Traumatic Heterotopic Ossification. Ann. Surg. 2023, 278, e1289–e1298. [Google Scholar] [CrossRef]
- Sorkin, M.; Huber, A.K.; Hwang, C.; Carson, W.F.; Menon, R.; Li, J.; Vasquez, K.; Pagani, C.; Patel, N.; Li, S.; et al. Regulation of heterotopic ossification by monocytes in a mouse model of aberrant wound healing. Nat. Commun. 2020, 11, 722. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Chu, J.; Ao, X.; Jiang, T.; Yan, B.; Huang, M.; Zhang, Z. Macrophage-derived neurotrophin-3 promotes heterotopic ossification in rats. Lab. Invest. 2020, 100, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, X.; Wu, Y.; Xu, G.; Qu, X. Oncostatin M promotes osteogenic differentiation of tendon-derived stem cells through the JAK2/STAT3 signalling pathway. J. Orthop. Surg. Res. 2024, 19, 407. [Google Scholar] [CrossRef]
- Torossian, F.; Guerton, B.; Anginot, A.; Alexander, K.A.; Desterke, C.; Soave, S.; Tseng, H.-W.; Arouche, N.; Boutin, L.; Kulina, I.; et al. Macrophage-derived oncostatin M contributes to human and mouse neurogenic heterotopic ossifications. JCI Insight 2017, 2, e96034. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, Y.; Pei, Y.; Lian, F.; Lin, H. Role of the NF-kB signalling pathway in heterotopic ossification: Biological and therapeutic significance. Cell Commun. Signal. 2024, 22, 159. [Google Scholar] [CrossRef] [PubMed]
- Prados, B.; del Toro, R.; MacGrogan, D.; Gómez-Apiñániz, P.; Papoutsi, T.; Muñoz-Cánoves, P.; Méndez-Ferrer, S.; de la Pompa, J.L. Heterotopic ossification in mice overexpressing Bmp2 in Tie2+ lineages. Cell Death Dis. 2021, 12, 729. [Google Scholar] [CrossRef]
- Tseng, H.-W.; Kulina, I.; Girard, D.; Gueguen, J.; Vaquette, C.; Salga, M.; Fleming, W.; Jose, B.; Millard, S.M.; Pettit, A.R.; et al. Interleukin-1 Is Overexpressed in Injured Muscles Following Spinal Cord Injury and Promotes Neurogenic Heterotopic Ossification. J. Bone Miner. Res. 2022, 37, 531–546. [Google Scholar] [CrossRef]
- Han, X.; Gao, C.; Lu, W.; Yan, J.; Xu, H.; Guo, Z.; Qin, W.; Lu, N.; Gao, J.; Zhu, W.; et al. Macrophage-Derived Extracellular DNA Initiates Heterotopic Ossification. Inflammation 2023, 46, 2225–2240. [Google Scholar] [CrossRef]
- Tu, B.; Li, J.; Sun, Z.; Zhang, T.; Liu, H.; Yuan, F.; Fan, C. Macrophage-Derived TGF-β and VEGF Promote the Progression of Trauma-Induced Heterotopic Ossification. Inflammation 2023, 46, 202–216. [Google Scholar] [CrossRef]
- Rowe, C.J.; Nwaolu, U.; Salinas, D.; Hong, J.; Nunez, J.; Lansford, J.L.; McCarthy, C.F.; Potter, B.K.; Levi, B.H.; Davis, T.A. Inhibition of focal adhesion kinase 2 results in a macrophage polarization shift to M2 which attenuates local and systemic inflammation and reduces heterotopic ossification after polysystem extremity trauma. Front. Immunol. 2023, 14, 1280884. [Google Scholar] [CrossRef]
- van der Kraan, P.M.; Blaney Davidson, E.N.; Blom, A.; van den Berg, W.B. TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis: Modulation and integration of signaling pathways through receptor-Smads. Osteoarthr. Cartil. 2009, 17, 1539–1545. [Google Scholar] [CrossRef]
- Patel, N.K.; Nunez, J.H.; Sorkin, M.; Marini, S.; Pagani, C.A.; Strong, A.L.; Hwang, C.D.; Li, S.; Padmanabhan, K.R.; Kumar, R.; et al. Macrophage TGF-β signaling is critical for wound healing with heterotopic ossification after trauma. JCI Insight 2022, 7, e144925. [Google Scholar] [CrossRef]
- Mundy, C.; Yao, L.; Sinha, S.; Chung, J.; Rux, D.; Catheline, S.E.; Koyama, E.; Qin, L.; Pacifici, M. Activin A promotes the development of acquired heterotopic ossification and is an effective target for disease attenuation in mice. Sci. Signal. 2021, 14, eabd0536. [Google Scholar] [CrossRef] [PubMed]
- Kan, L.; Hu, M.; Gomes, W.A.; Kessler, J.A. Transgenic mice overexpressing BMP4 develop a fibrodysplasia ossificans progressiva (FOP)-like phenotype. Am. J. Pathol. 2004, 165, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Papanagiotou, M.; Dailiana, Z.H.; Karachalios, T.; Varitimidis, S.; Hantes, M.; Dimakopoulos, G.; Vlychou, M.; Malizos, K.N. Heterotopic ossification after the use of recombinant human bone morphogenetic protein-7. World J. Orthop. 2017, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; de Gorter, D.J.J.; Hoogaars, W.M.H.; Hoen, P.A.C.; Dijke, P.T. Overactive bone morphogenetic protein signaling in heterotopic ossification and Duchenne muscular dystrophy. Cell. Mol. Life Sci. 2013, 70, 407–423. [Google Scholar] [CrossRef]
- Chenard, K.E.; Teven, C.M.; He, T.-C.; Reid, R.R. Bone morphogenetic proteins in craniofacial surgery: Current techniques, clinical experiences, and the future of personalized stem cell therapy. J. Biomed. Biotechnol. 2012, 2012, 601549. [Google Scholar] [CrossRef]
- Phimphilai, M.; Zhao, Z.; Boules, H.; Roca, H.; Franceschi, R.T. BMP Signaling Is Required for RUNX2-Dependent Induction of the Osteoblast Phenotype. J. Bone Miner. Res. 2006, 21, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Ulsamer, A.; Ortuño, M.J.; Ruiz, S.; Susperregui, A.R.; Osses, N.; Rosa, J.L.; Ventura, F. BMP-2 induces Osterix expression through up-regulation of Dlx5 and its phosphorylation by p38. J. Biol. Chem. 2008, 283, 3816–3826. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, F.; Pei, Y.; Lian, F.; Lin, H. Involvement of the Wnt/β-catenin signalling pathway in heterotopic ossification and ossification-related diseases. J. Cell. Mol. Med. 2024, 28, e70113. [Google Scholar] [CrossRef]
- Hong, J.H.; Lee, G.T.; Lee, J.H.; Kwon, S.J.; Park, S.H.; Kim, S.J.; Kim, I.Y. Effect of bone morphogenetic protein-6 on macrophages. Immunology 2009, 128, e442–e450. [Google Scholar] [CrossRef]
- Cheng, H.; Jiang, W.; Phillips, F.M.; Haydon, R.C.; Peng, Y.; Zhou, L.; Luu, H.H.; An, N.; Breyer, B.; Vanichakarn, P.; et al. Osteogenic Activity of the Fourteen Types of Human Bone Morphogenetic Proteins (BMPs). J. Bone Jt. Surg. 2003, 85, 1544–1552. Available online: https://journals.lww.com/jbjsjournal/fulltext/2003/08000/osteogenic_activity_of_the_fourteen_types_of_human.17.aspx (accessed on 27 February 2025). [CrossRef]
- Kang, Q.; Sun, M.H.; Cheng, H.; Peng, Y.; Montag, A.G.; Deyrup, A.T.; Jiang, W.; Luu, H.H.; Luo, J.; Szatkowski, J.P.; et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004, 11, 1312–1320. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, S.; Shan, C.; Zhu, Q.; Xue, Y.; Zhang, K. The serum levels of activin A and bone morphogenetic protein-4 and -6 in patients with fibrodysplasia ossificans progressiva. Orphanet J. Rare Dis. 2023, 18, 111. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, K.; Agarwal, S.; Cholok, D.; Loder, S.; Li, J.; Hsieh, H.H.S.; Wang, S.C.; Buchman, S.R.; Levi, B. The role of the adaptive immune system in burn-induced heterotopic ossification and mesenchymal cell osteogenic differentiation. J. Surg. Res. 2016, 206, 53–61. [Google Scholar] [CrossRef]
- Alexander, K.A.; Tseng, H.-W.; Kulina, I.; Fleming, W.; Vaquette, C.; Genêt, F.; Ruitenberg, M.J.; Lévesque, J.-P. Lymphocytes Are Not Required for Neurogenic Heterotopic Ossification Development after Spinal Cord Injury. Neurotrauma Rep. 2022, 3, 87–96. [Google Scholar] [CrossRef]
- Loder, S.B.; Agarwal, S.; Sorkin, M.; Breuler, C.B.; Li, J.; Peterson, J.B.; Gardenier, J.; Hsieh, H.H.S.D.; Wang, S.C.; Mehrara, B.J.; et al. Lymphatic Contribution to the Cellular Niche in Heterotopic Ossification. Ann. Surg. 2016, 264, 1174–1180. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, J.; Sun, X.; Chen, H.; Huang, S.; Yang, J.; Du, X.; Tan, Q.; Luo, F.; Zhang, R.; et al. Targeting local lymphatics to ameliorate heterotopic ossification via FGFR3-BMPR1a pathway. Nat. Commun. 2021, 12, 4391. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.K.; Donoghue, D.J. FGFR activation in skeletal disorders: Too much of a good thing. Trends Genet. 1997, 13, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Song, Q.; Li, Z.; Wu, X.; Chen, Y.; Lin, H. Targeting fibroblasts in pathological bone formation: Mechanisms and treatments. Front. Cell Dev. Biol. 2025, 13, 1612950. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Strong, A.L.; Sun, Y.; Guo, L.; Juan, C.; Bancroft, A.C.; Choi, J.H.; Pagani, C.A.; Fernandes, A.A.; Woodard, M.; et al. The HIF-1α/PLOD2 axis integrates extracellular matrix organization and cell metabolism leading to aberrant musculoskeletal repair. Bone Res. 2024, 12, 17. [Google Scholar] [CrossRef]
- Lees-Shepard, J.B.; Yamamoto, M.; Biswas, A.A.; Stoessel, S.J.; Nicholas, S.-A.E.; Cogswell, C.A.; Devarakonda, P.M.; Schneider, M.J.; Cummins, S.M.; Legendre, N.P.; et al. Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva. Nat. Commun. 2018, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Assis-Ribas, T.; Forni, M.F.; Winnischofer, S.M.B.; Sogayar, M.; Trombetta-Lima, M. Extracellular matrix dynamics during mesenchymal stem cells differentiation. Dev. Biol. 2018, 437, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Larochette, N. Evaluation de matrices extracellulaires décellularisées comme support de BMP-2 pour l’ingénierie tissulaire osseuse. 2017. Available online: https://ephe.hal.science/hal-01668934 (accessed on 28 March 2025).
- Rodenberg, E.; Azhdarinia, A.; Lazard, Z.W.; Hall, M.; Kwon, S.K.; Wilganowski, N.; Salisbury, E.A.; Merched-Sauvage, M.; Olmsted-Davis, E.A.; Sevick-Muraca, E.M.; et al. Matrix metalloproteinase-9 is a diagnostic marker of heterotopic ossification in a murine model. Tissue Eng. Part A 2011, 17, 2487–2496. [Google Scholar] [CrossRef]
- Reznikov, N.; Bilton, M.; Lari, L.; Stevens, M.M.; Kröger, R. Fractal-like hierarchical organization of bone begins at the nanoscale. Science 2018, 360, eaao2189. [Google Scholar] [CrossRef]
- Huber, A.K.; Patel, N.; Pagani, C.A.; Marini, S.; Padmanabhan, K.R.; Matera, D.L.; Said, M.; Hwang, C.; Hsu, G.C.-Y.; Poli, A.A.; et al. Immobilization after injury alters extracellular matrix and stem cell fate. J. Clin. Investg. 2020, 130, 5444–5460. [Google Scholar] [CrossRef] [PubMed]
- Lodberg, A. Principles of the activin receptor signaling pathway and its inhibition. Cytokine Growth Factor Rev. 2021, 60, 1–17. [Google Scholar] [CrossRef]
- Hatsell, S.J.; Idone, V.; Wolken, D.M.A.; Huang, L.; Kim, H.J.; Wang, L.; Wen, X.; Nannuru, K.C.; Jimenez, J.; Xie, L.; et al. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci. Transl. Med. 2015, 7, 303ra137. [Google Scholar] [CrossRef]
- Wei, E.; Hu, M.; Wu, L.; Pan, X.; Zhu, Q.; Liu, H.; Liu, Y. TGF-β signaling regulates differentiation of MSCs in bone metabolism: Disputes among viewpoints. Stem Cell Res. Ther. 2024, 15, 156. [Google Scholar] [CrossRef]
- Eisner, C.; Cummings, M.; Johnston, G.; Tung, L.W.; Groppa, E.; Chang, C.; Rossi, F.M. Murine Tissue-Resident PDGFRα+ Fibro-Adipogenic Progenitors Spontaneously Acquire Osteogenic Phenotype in an Altered Inflammatory Environment. J. Bone Miner. Res. 2020, 35, 1525–1534. [Google Scholar] [CrossRef]
- Wosczyna, M.N.; Biswas, A.A.; Cogswell, C.A.; Goldhamer, D.J. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J. Bone Miner. Res. 2012, 27, 1004–1017. [Google Scholar] [CrossRef]
- Pagani, C.A.; Bancroft, A.C.; Tower, R.J.; Livingston, N.; Sun, Y.; Hong, J.Y.; Kent, R.N.; Strong, A.L.; Nunez, J.H.; Medrano, J.M.R.; et al. Discoidin domain receptor 2 regulates aberrant mesenchymal lineage cell fate and matrix organization. Sci. Adv. 2022, 8, eabq6152. [Google Scholar] [CrossRef]
- Totaro, A.; Panciera, T.; Piccolo, S. YAP/TAZ upstream signals and downstream responses. Nat. Cell Biol. 2018, 20, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Weiss, S.J. Snail/Slug-YAP/TAZ complexes cooperatively regulate mesenchymal stem cell function and bone formation. Cell Cycle 2017, 16, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, M.; Song, N.-J.; Kim, J.-H.; Seo, D.; Lee, J.-H.; Jung, S.M.; Lee, J.Y.; Lee, J.; Lee, Y.S.; et al. A Reciprocal Role of the Smad4-Taz Axis in Osteogenesis and Adipogenesis of Mesenchymal Stem Cells. Stem Cells 2019, 37, 368–381. [Google Scholar] [CrossRef]
- Li, J.; Yan, J.-F.; Wan, Q.-Q.; Shen, M.-J.; Ma, Y.-X.; Gu, J.-T.; Gao, P.; Tang, X.-Y.; Yu, F.; Chen, J.-H.; et al. Matrix stiffening by self-mineralizable guided bone regeneration. Acta Biomater. 2021, 125, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Ge, C.; Pan, H.; Han, Y.; Mishina, Y.; Kaartinen, V.; Franceschi, R.T. Discoidin domain receptor 2 is an important modulator of BMP signaling during heterotopic bone formation. Bone Res. 2025, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, J.; McNaughton, P.A. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005, 24, 4211–4223. [Google Scholar] [CrossRef]
- Pereira, C.T.; Adams, S.H.; Lloyd, K.C.K.; Knotts, T.A.; James, A.W.; Price, T.J.; Levi, B. Exploring the role of peripheral nerves in trauma-induced heterotopic ossification. JBMR Plus 2025, 9, ziae155. [Google Scholar] [CrossRef] [PubMed]
- Suvas, S. Role of Substance P neuropeptide in inflammation, wound healing and tissue homeostasis. J. Immunol. 2017, 199, 1543–1552. [Google Scholar] [CrossRef]
- Genêt, F.; Kulina, I.; Vaquette, C.; Torossian, F.; Millard, S.; Pettit, A.R.; Sims, N.A.; Anginot, A.; Guerton, B.; Winkler, I.G.; et al. Neurological heterotopic ossification following spinal cord injury is triggered by macrophage-mediated inflammation in muscle. J. Pathol. 2015, 236, 229–240. [Google Scholar] [CrossRef]
- Sang, X.; Wang, Z.; Shi, P.; Li, Y.; Cheng, L. CGRP accelerates the pathogenesis of neurological heterotopic ossification following spinal cord injury. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2569–2574. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.P.-Y.; Chan, L.-S.; Fu, S.-C.; Chan, K.-M. Expression of sensory neuropeptides in tendon is associated with failed healing and activity-related tendon pain in collagenase-induced tendon injury. Am. J. Sports Med. 2010, 38, 757–764. [Google Scholar] [CrossRef]
- Slaets, H.; Nelissen, S.; Janssens, K.; Vidal, P.M.; Lemmens, E.; Stinissen, P.; Hendrix, S.; Hellings, N. Oncostatin M reduces lesion size and promotes functional recovery and neurite outgrowth after spinal cord injury. Mol. Neurobiol. 2014, 50, 1142–1151. [Google Scholar] [CrossRef]
- Juarez, J.K.; Wenke, J.C.; Rivera, J.C. Treatments and Preventative Measures for Trauma-Induced Heterotopic Ossification: A Review. Clin. Transl. Sci. 2018, 11, 365–370. [Google Scholar] [CrossRef]
- Marcum, Z.A.; Hanlon, J.T. Recognizing the Risks of Chronic Nonsteroidal Anti-Inflammatory Drug Use in Older Adults. Ann. Long-Term Care 2010, 18, 24–27. [Google Scholar]
- Siegel, P.; Smith, S.; Stark, E.; Burns, C.; Dionne, T.P. A scoping review on active vs. passive range of motion approaches to treat heterotopic ossification at the elbow. Front. Rehabil. Sci. 2024, 5, 1327417. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.A.; Tseng, H.-W.; Lao, H.W.; Girard, D.; Barbier, V.; Ungerer, J.P.; McWhinney, B.C.; Samuel, S.G.; Fleming, W.; Winkler, I.G.; et al. A glucocorticoid spike derails muscle repair to heterotopic ossification after spinal cord injury. Cell Rep. Med. 2024, 5, 101849. [Google Scholar] [CrossRef]
- Yamamura-Idei, Y.; Kitazawa, S.; Kitazawa, R.; Fujimori, T.; Chiba, T.; Maeda, S. Parathyroid hormone-related protein in gastric cancers with heterotopic ossification. Cancer 1993, 72, 1849–1852. [Google Scholar] [CrossRef] [PubMed]
- Wootton, E.; Balcerek, M.; Lazarus, S.; Duncan, E.L. Post-Traumatic Heterotopic Ossification with Incidental Hyperparathyroidism. J. Endocr. Soc. 2021, 5, A212. [Google Scholar] [CrossRef]
- Dufresne, S.S.; Dumont, N.A.; Boulanger-Piette, A.; Fajardo, V.A.; Gamu, D.; Kake-Guena, S.-A.; David, R.O.; Bouchard, P.; Lavergne, É.; Penninger, J.M.; et al. Muscle RANK is a key regulator of Ca2+ storage, SERCA activity, and function of fast-twitch skeletal muscles. Am. J. Physiol.-Cell Physiol. 2016, 310, C663–C672. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, S.S.; Boulanger-Piette, A.; Bossé, S.; Argaw, A.; Hamoudi, D.; Marcadet, L.; Gamu, D.; Fajardo, V.A.; Yagita, H.; Penninger, J.M.; et al. Genetic deletion of muscle RANK or selective inhibition of RANKL is not as effective as full-length OPG-fc in mitigating muscular dystrophy. Acta Neuropathol. Commun. 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Reichenberger, E.J.; O’bRien, K.; Hatori, A.; Carpenter, T.O.; van de Wetering, K.; Flaman, L.; Howe, J.; Ortiz, D.; Sabbagh, Y.; Chen, I.-P. ENPP1 enzyme replacement therapy improves ectopic calcification but does not rescue skeletal phenotype in a mouse model for craniometaphyseal dysplasia. JBMR Plus 2024, 8, ziae103. [Google Scholar] [CrossRef]
- He, Z.; Zhu, Z.; Tang, T.; Wang, F.; Guo, P.; Li, J.; Tung, N.T.C.; Liang, Q.; Liu, S.; Gao, M.; et al. Enpp1 mutations promote upregulation of hedgehog signaling in heterotopic ossification with aging. J. Bone Miner. Metab. 2024, 42, 681–698. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Sarti, A.C.; Coutinho-Silva, R. Purinergic signaling, DAMPs, and inflammation. Am. J. Physiol.-Cell Physiol. 2020, 318, C832–C835. [Google Scholar] [CrossRef] [PubMed]
- Górecki, D.C. P2X7 purinoceptor as a therapeutic target in muscular dystrophies. Curr. Opin. Pharmacol. 2019, 47, 40–45. Available online: https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S1471489218301401?returnurl=null&referrer=null (accessed on 21 March 2025). [CrossRef] [PubMed]
- Rumney, R.M.H.; Róg, J.; Chira, N.; Kao, A.P.; Al-Khalidi, R.; Górecki, D.C. P2X7 Purinoceptor Affects Ectopic Calcification of Dystrophic Muscles. Front. Pharmacol. 2022, 13, 935804. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facchin, A.; Lemaire, S.; Toner, L.G.; Argaw, A.; Frenette, J. When Bone Forms Where It Shouldn’t: Heterotopic Ossification in Muscle Injury and Disease. Int. J. Mol. Sci. 2025, 26, 7516. https://doi.org/10.3390/ijms26157516
Facchin A, Lemaire S, Toner LG, Argaw A, Frenette J. When Bone Forms Where It Shouldn’t: Heterotopic Ossification in Muscle Injury and Disease. International Journal of Molecular Sciences. 2025; 26(15):7516. https://doi.org/10.3390/ijms26157516
Chicago/Turabian StyleFacchin, Anthony, Sophie Lemaire, Li Gang Toner, Anteneh Argaw, and Jérôme Frenette. 2025. "When Bone Forms Where It Shouldn’t: Heterotopic Ossification in Muscle Injury and Disease" International Journal of Molecular Sciences 26, no. 15: 7516. https://doi.org/10.3390/ijms26157516
APA StyleFacchin, A., Lemaire, S., Toner, L. G., Argaw, A., & Frenette, J. (2025). When Bone Forms Where It Shouldn’t: Heterotopic Ossification in Muscle Injury and Disease. International Journal of Molecular Sciences, 26(15), 7516. https://doi.org/10.3390/ijms26157516






