Investigating the Potential of Propranolol as an Anti-Tumor Agent in Colorectal Cancer Cell Lines
Abstract
1. Introduction
2. Results
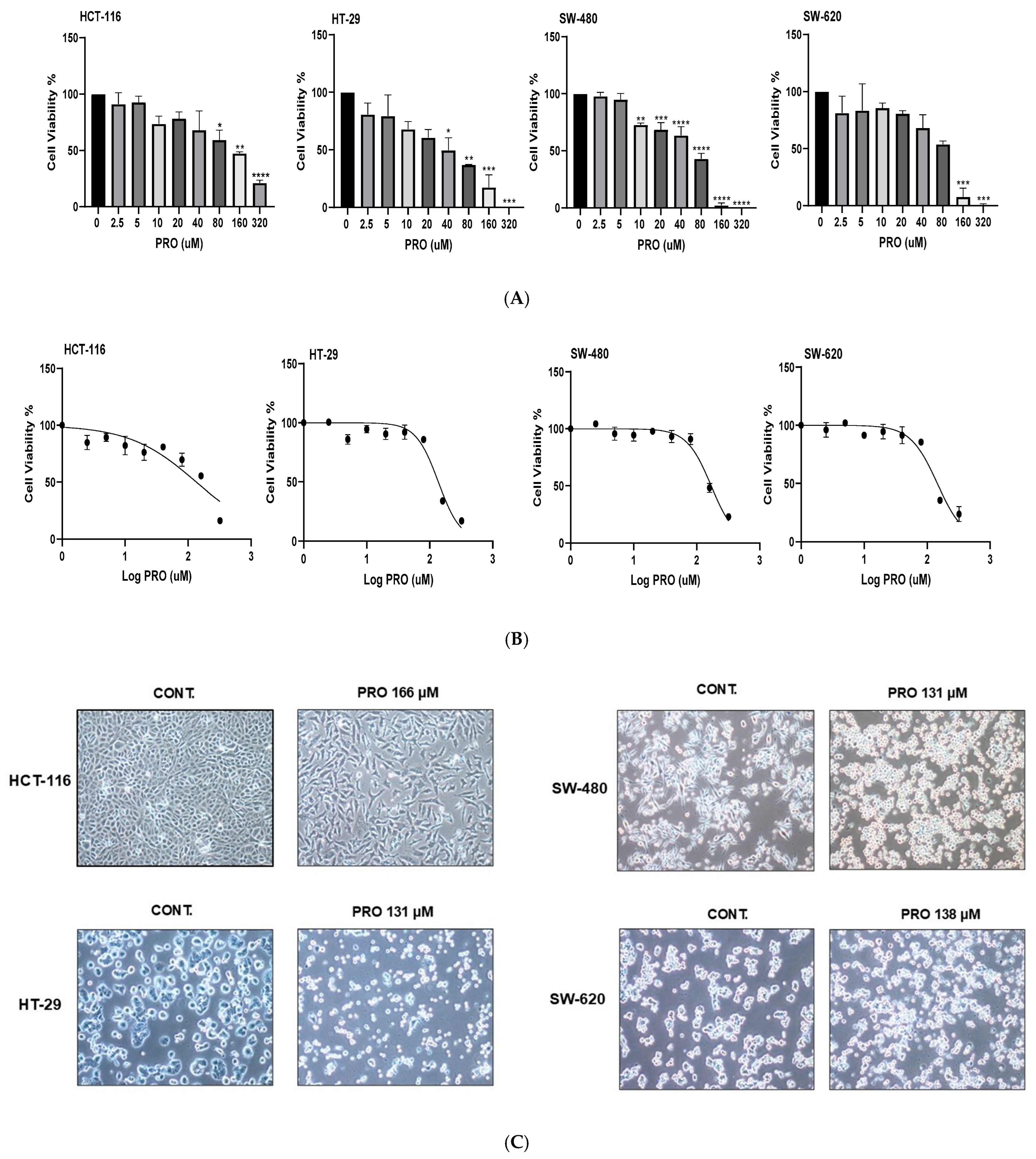
2.1. PRO Induced Cytotoxicity in a Cell-Type-Specific and Dose-Dependent Manner Using Trypan Blue Exclusion Assay
2.2. PRO Induced Cytotoxicity in a Cell-Type-Specific, Dose-Dependent, and Time-Dependent Manner Using the MTT Assay
2.3. PRO Modified Cell Morphology
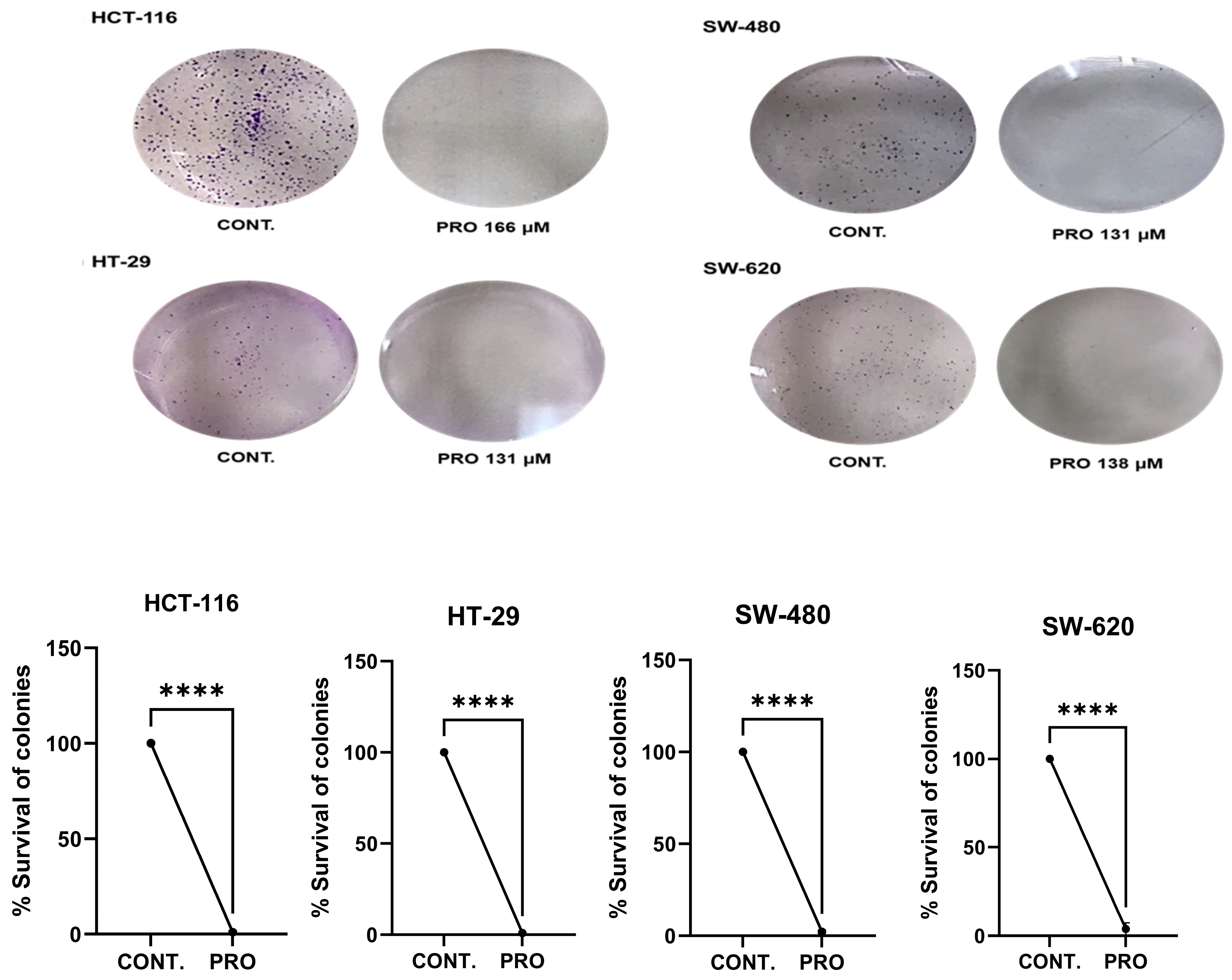
2.4. PRO Inhibited Cell Colony Formation
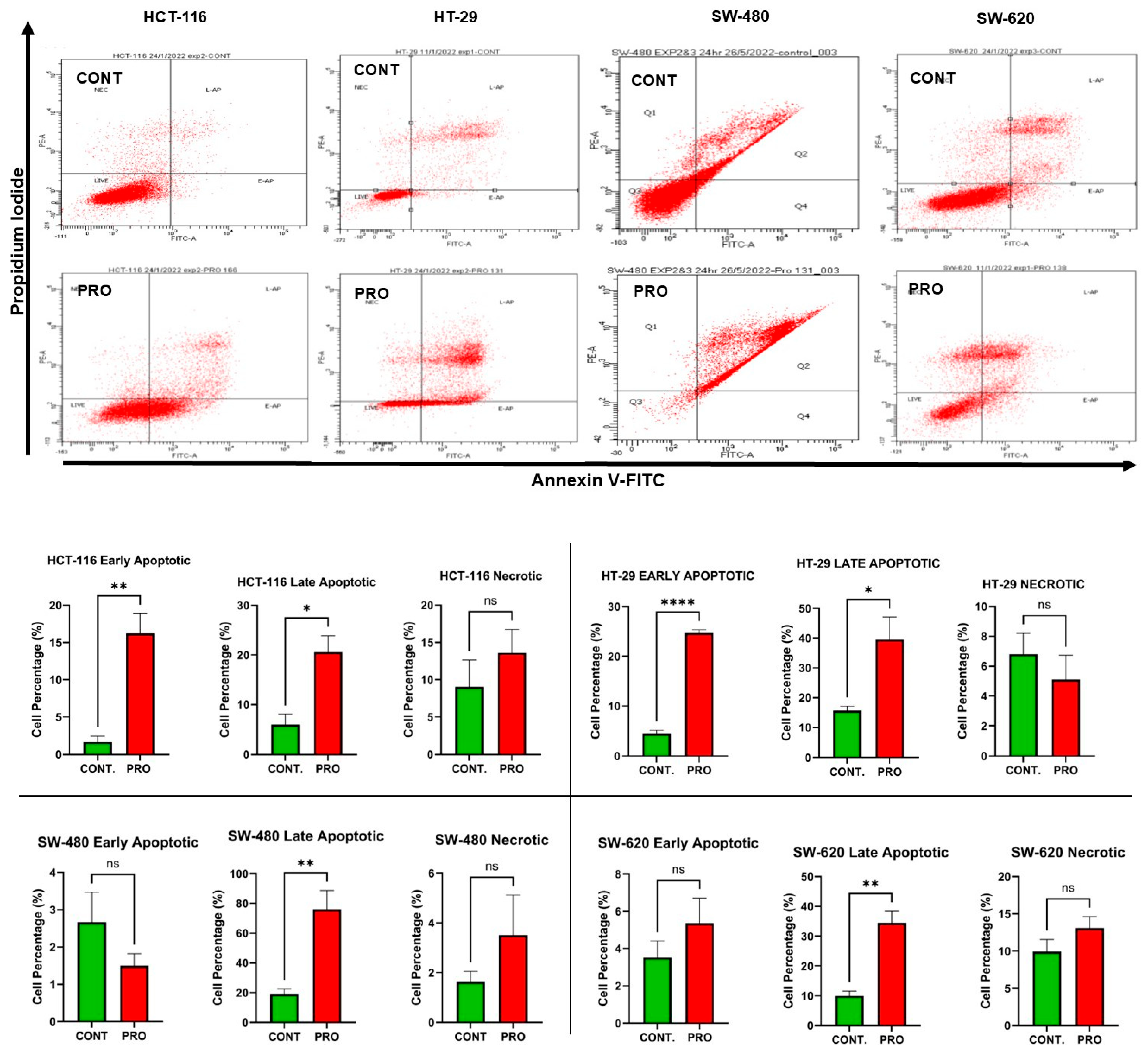
2.5. PRO Induced Apoptosis in CRC Cell Lines
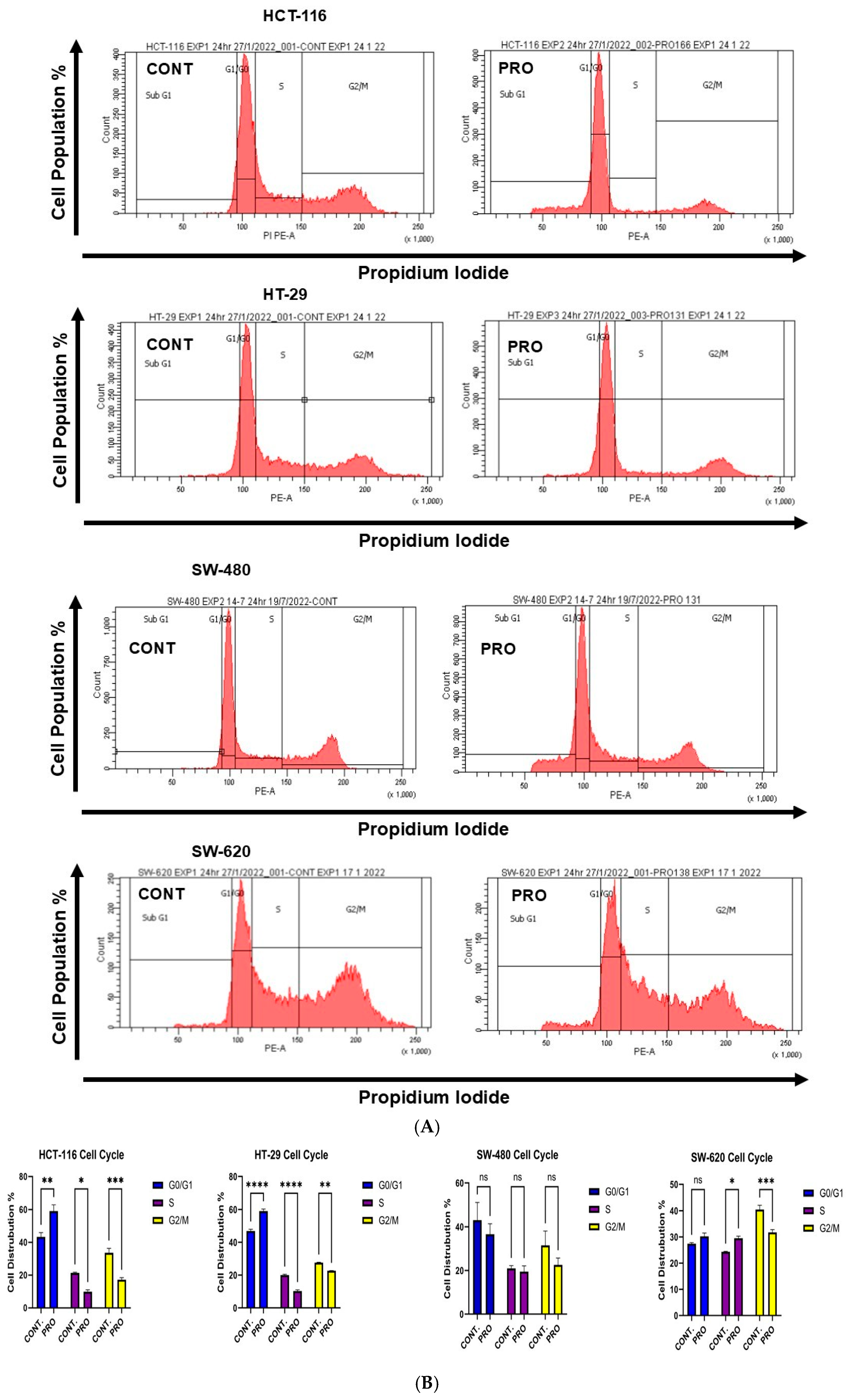
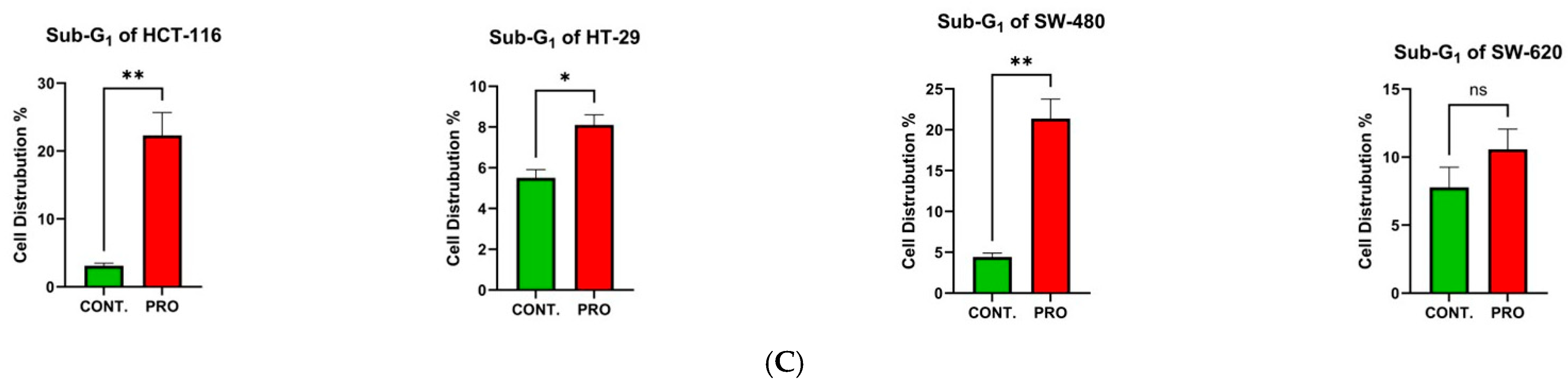
2.6. Effect of PRO on the Distribution of Cell Cycle Phases
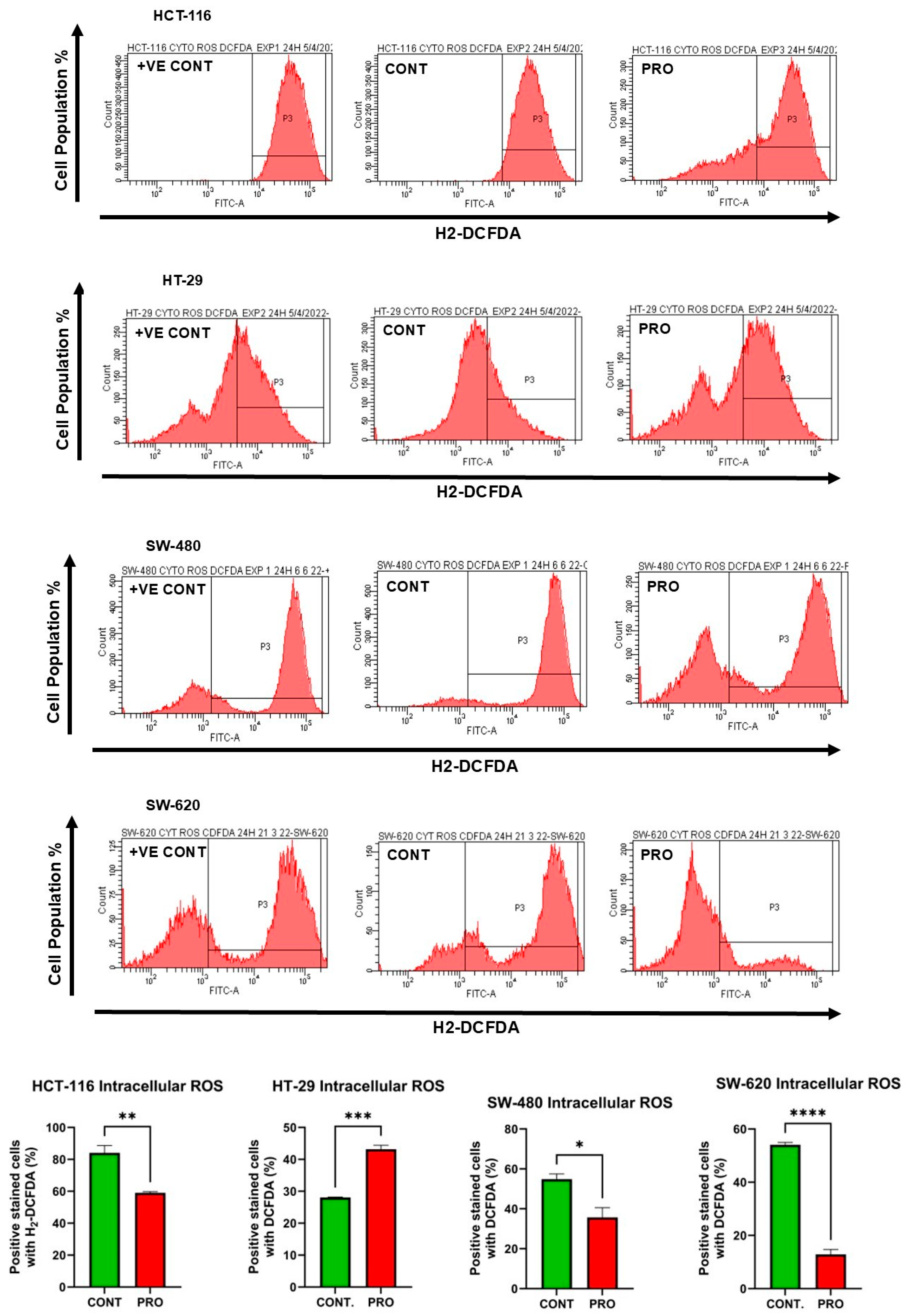
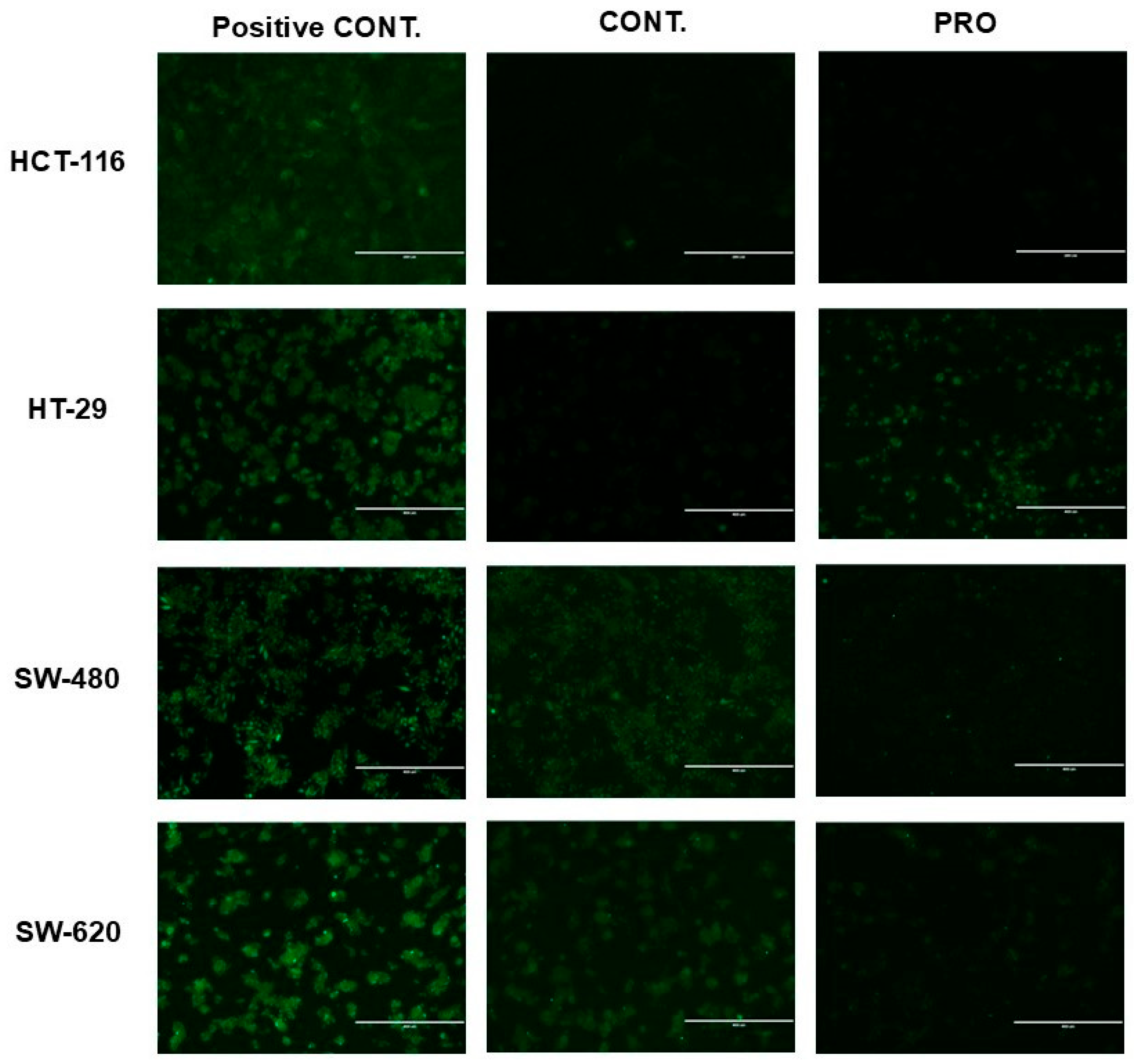
2.7. Effect of PRO on the Generation of Intracellular ROS
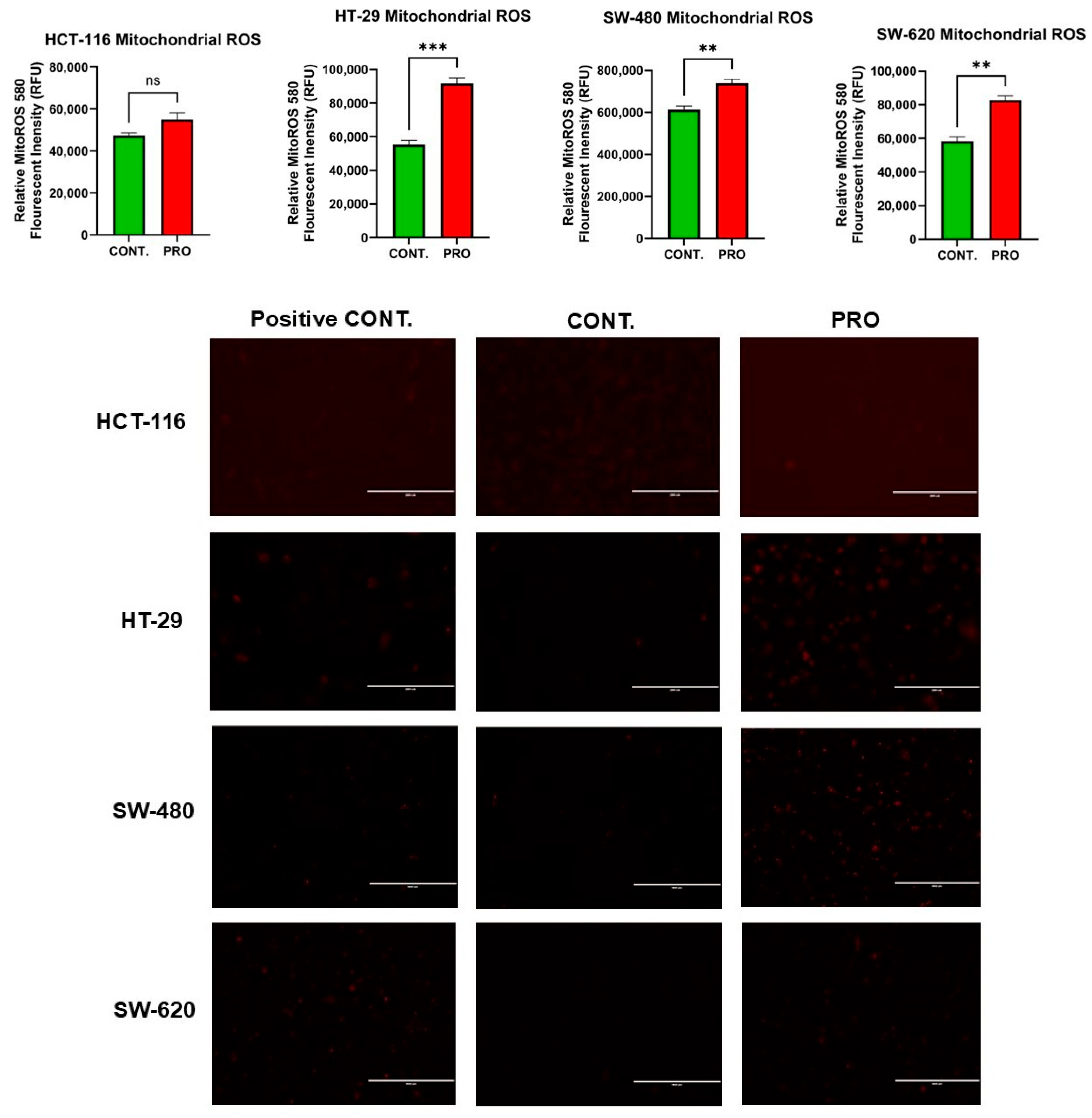
2.8. Effect of PRO on the Generation of Mito ROS
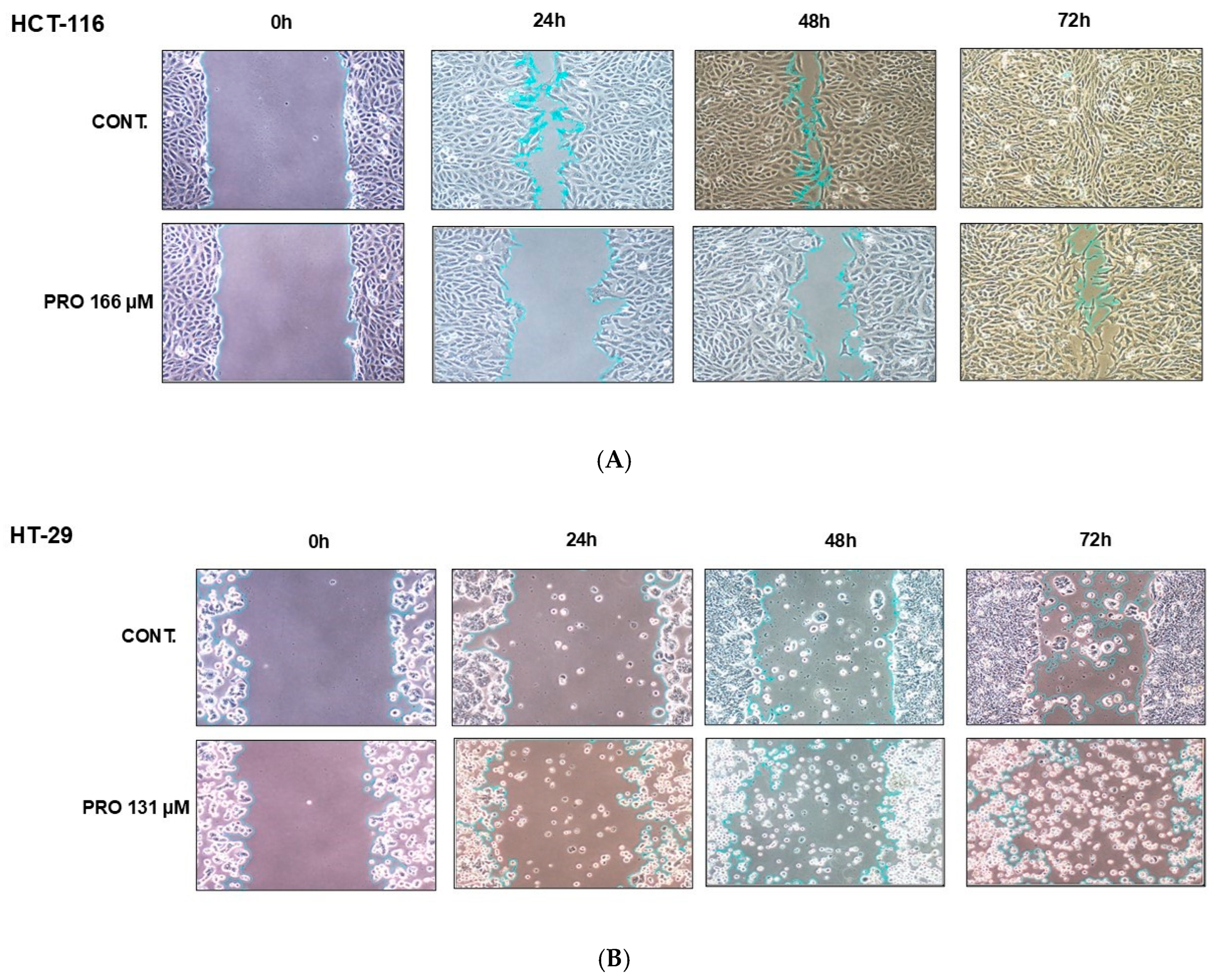
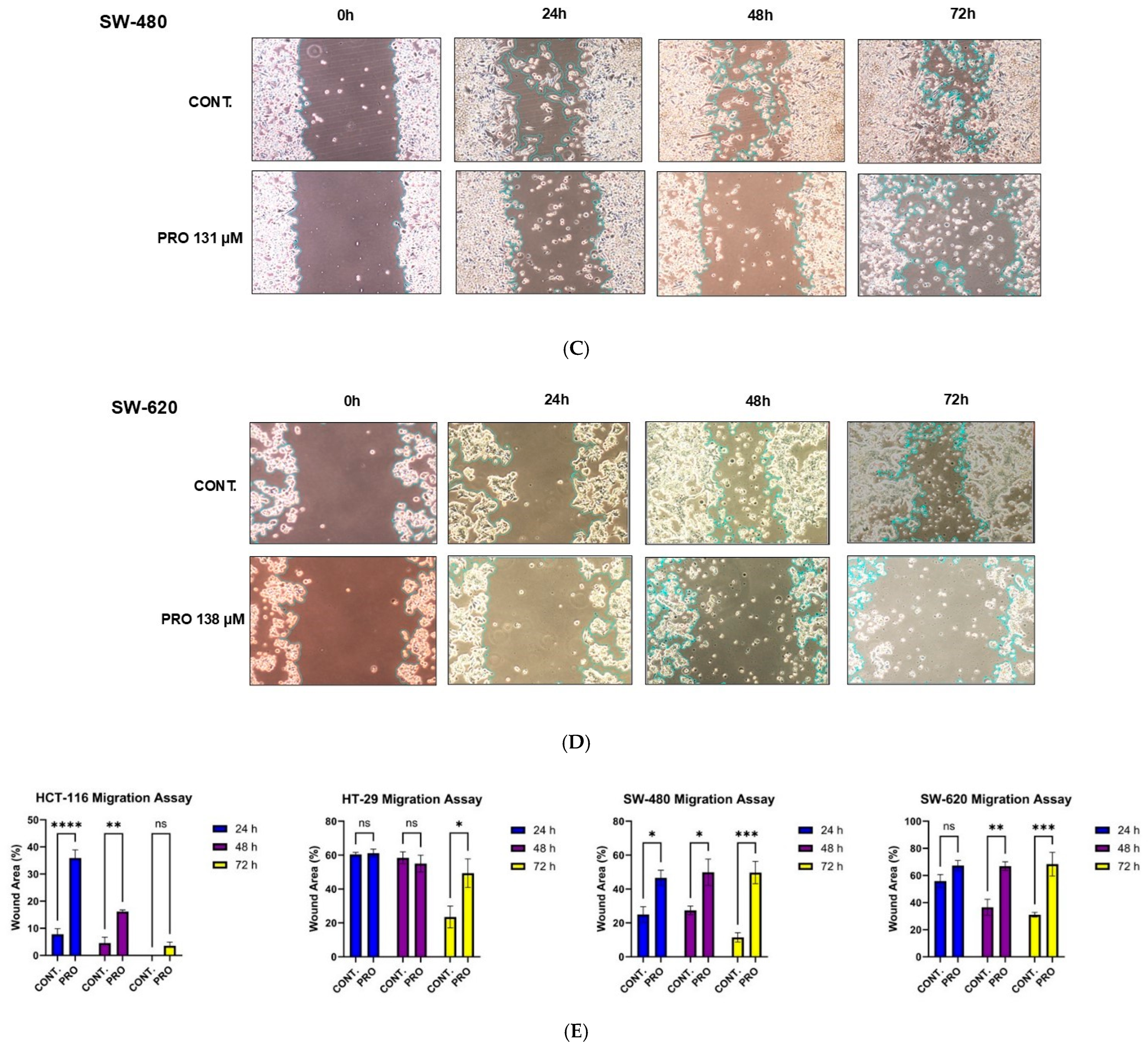
2.9. PRO Inhibited Cell Migration in Cell-Type-Specific and Time-Dependent Manner
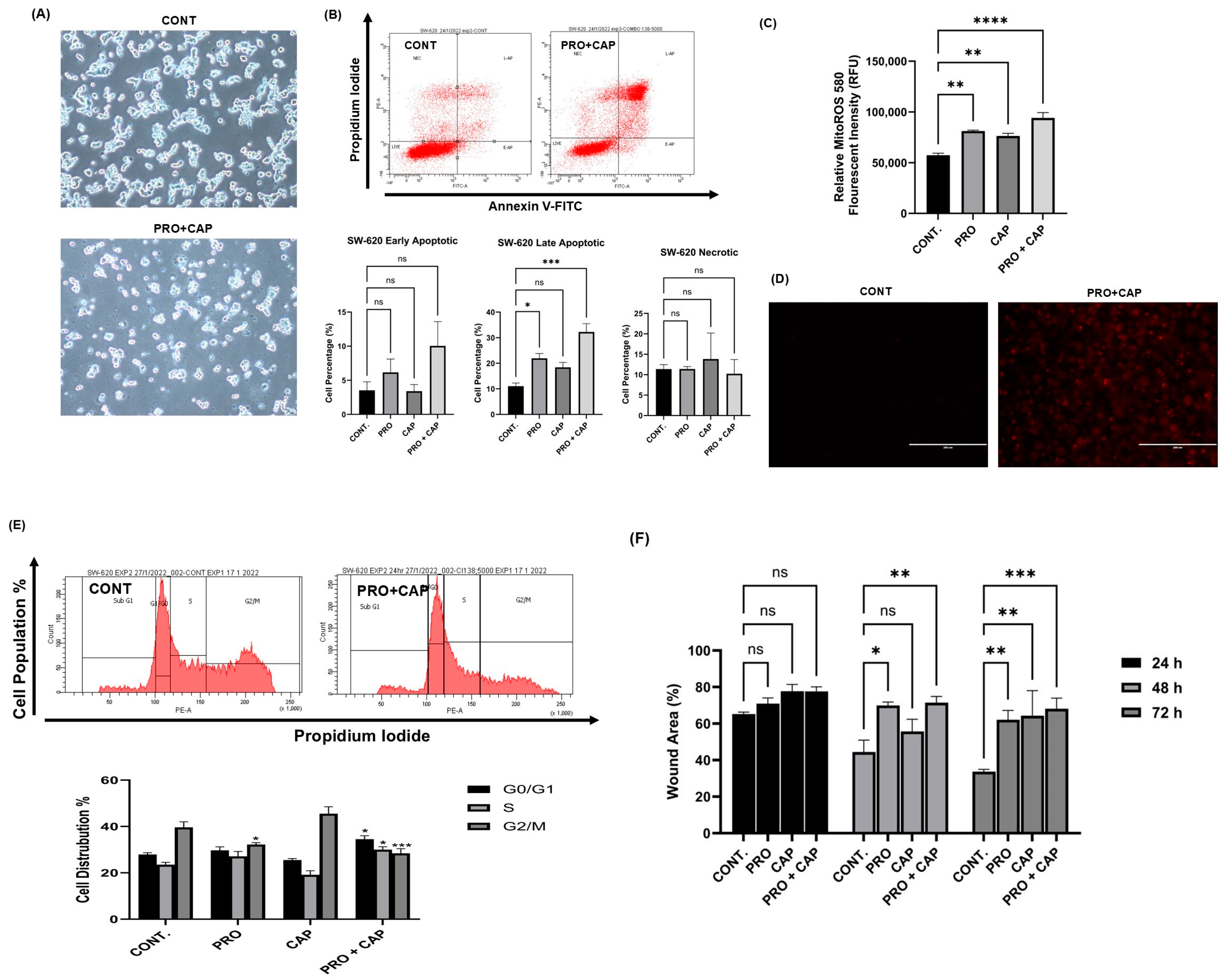
2.10. Propranolol Synergizes the Anti-Tumor Activity of Xeloda (Capecitabine) in the Metastatic CRC Cell Line
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Propranolol Therapy
4.3. Trypan Blue Exclusion Assay
4.4. MTT Assay
4.4.1. Cell Viability at Various Cell Densities
4.4.2. Cell Viability over Time Intervals
4.5. Morphological Analysis
4.6. Colony Formation Assay
4.7. Detection of Cell Death Mechanism
4.8. Cell Cycle Analysis
4.9. Measurement of Intracellular ROS Level
4.10. Measurement of Mitochondrial Reactive Oxygen Species (Mito ROS) Level
4.11. In Vitro Migration Assay
4.12. The Combination Therapy
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pantziarka, P.; Bouche, G.; Sukhatme, V.; Meheus, L.; Rooman, I.; Sukhatme, V.P. Repurposing Drugs in Oncology (ReDO)—Propranolol as an Anti-Cancer Agent. Ecancermedicalscience 2016, 10, 680. [Google Scholar] [CrossRef]
- Albiñana, V.; Gallardo-Vara, E.; Casado-Vela, J.; Recio-Poveda, L.; Botella, L.M.; Cuesta, A.M. Propranolol: A “Pick and Roll” Team Player in Benign Tumors and Cancer Therapies. J. Clin. Med. 2022, 11, 4539. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, P.H.; Harper, J.I.; Baselga, E.; Bonnet, D.; Boon, L.M.; Di Chiara Atti, M.; El Hachem, M.; Oranje, A.P.; Rubin, A.T.; Weibel, L.; et al. Treatment of Infantile Haemangiomas: Recommendations of a European Expert Group. Eur. J. Pediatr. 2015, 174, 855–865. [Google Scholar] [CrossRef]
- Verbaanderd, C.; Meheus, L.; Huys, I.; Pantziarka, P. Repurposing Drugs in Oncology: Next Steps. Trends Cancer 2017, 3, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Scapozza, L.; Ruiz i Altaba, A. Drug Repurposing in Oncology: Compounds, Pathways, Phenotypes and Computational Approaches for Colorectal Cancer. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 434–454. [Google Scholar] [CrossRef]
- Coelho, M.; Moz, M.; Correia, G.; Teixeira, A.; Medeiros, R.; Ribeiro, L. Antiproliferative Effects of β-Blockers on Human Colorectal Cancer Cells. Oncol. Rep. 2015, 33, 2513–2520. [Google Scholar] [CrossRef]
- Wolter, J.K.; Wolter, N.E.; Blanch, A.; Partridge, T.; Cheng, L.; Morgenstern, D.A.; Podkowa, M.; Kaplan, D.R.; Irwin, M.S. Anti-Tumor Activity of the Beta-Adrenergic Receptor Antagonist Propranolol in Neuroblastoma. Oncotarget 2014, 5, 161. [Google Scholar] [CrossRef]
- Chang, P.Y.; Huang, W.Y.; Lin, C.L.; Huang, T.C.; Wu, Y.Y.; Chen, J.H.; Kao, C.H. Propranolol Reduces Cancer Risk: A Population-Based Cohort Study. Medicine 2015, 94, e1097. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, H.; Wang, F.; Xu, R.; Wang, P.; Tang, F.; Zhang, X.; Zhu, Z.; Lv, H.; Han, T. Propranolol Suppresses the Proliferation and Induces the Apoptosis of Liver Cancer Cells. Mol. Med. Rep. 2018, 17, 5213–5221. [Google Scholar] [CrossRef]
- Xie, W.Y.; He, R.H.; Zhang, J.; He, Y.J.; Wan, Z.; Zhou, C.F.; Tang, Y.J.; Li, Z.; McLeod, H.L.; Liu, J. β-Blockers Inhibit the Viability of Breast Cancer Cells by Regulating the ERK/COX-2 Signaling Pathway and the Drug Response Is Affected by ADRB2 Single Nucleotide Polymorphisms. Oncol. Rep. 2019, 41, 341–350. [Google Scholar] [CrossRef]
- Liao, P.; Song, K.; Zhu, Z.; Liu, Z.; Zhang, W.; Li, W.; Hu, J.; Hu, Q.; Chen, C.; Chen, B.; et al. Propranolol suppresses the growth of colorectal cancer through simultaneously activating autologous CD8+ T cells and inhibiting tumor AKT/MAPK pathway. Clin. Pharmacol. Ther. 2020, 108, 606–615. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, F.; Yang, R.; Zheng, X.; Gao, H.; Zhang, P. Effect of chronic restraint stress on human colorectal carcinoma growth in mice. PLoS ONE 2013, 8, e61435. [Google Scholar] [CrossRef]
- Chin, C.C.; Li, J.M.; Lee, K.F.; Huang, Y.C.; Wang, K.C.; Lai, H.C.; Cheng, C.C.; Kuo, Y.H.; Shi, C.S. Selective β2-AR blockage suppresses colorectal cancer growth through regulation of EGFR-Akt/ERK1/2 signaling, G1-phase arrest, and apoptosis. J. Cell Physiol. 2016, 231, 459–472. [Google Scholar] [CrossRef]
- Zhao, S.; Fan, S.; Shi, Y.; Ren, H.; Hong, H.; Gao, X.; Zhang, M.; Qin, Q.; Li, H. Propranolol induced apoptosis and autophagy via the ROS/JNK signaling pathway in human ovarian cancer. J. Cancer 2020, 11, 5900–5910. [Google Scholar] [CrossRef]
- Zhang, L.; Mai, H.M.; Zheng, J.; Zheng, J.W.; Wang, Y.A.; Qin, Z.P.; Li, K.L. Propranolol inhibits angiogenesis via down-regulating the expression of vascular endothelial growth factor in hemangioma derived stem cell. Int. J. Clin. Exp. Pathol. 2014, 7, 48–55. [Google Scholar]
- Shibuya, C.M.; Tjioe, K.C.; Oliveira, S.H.P.; Bernabé, D.G. Propranolol inhibits cell viability and expression of the pro-tumorigenic proteins Akt, NF-ĸB, and VEGF in oral squamous cell carcinoma. Arch. Oral Biol. 2022, 136, 105383. [Google Scholar] [CrossRef] [PubMed]
- Fjæstad, K.Y.; Rømer, A.M.A.; Goitea, V.; Johansen, A.Z.; Thorseth, M.L.; Carretta, M.; Engelholm, L.H.; Grøntved, L.; Junker, N.; Madsen, D.H. Blockade of beta-adrenergic receptors reduces cancer growth and enhances the response to anti-CTLA4 therapy by modulating the tumor microenvironment. Oncogene 2022, 41, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Anselmino, L.E.; Baglioni, M.V.; Malizia, F.; Laluce, N.C.; Etichetti, C.B.; Marignac, V.L.; Rozados, V.; Scharovsky, O.G.; Girardini, J.; Rico, M.J.; et al. Repositioning metformin and propranolol for colorectal and triple negative breast cancers treatment. Sci. Rep. 2021, 11, 8091. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.M.; Al Doghaither, H.A.; Alkhatabi, H.A.; Basabrain, M.A.; Pushparaj, P.N. Propranolol and Capecitabine Synergy on Inducing Ferroptosis in Human Colorectal Cancer Cells: Potential Implications in Cancer Therapy. Cancers 2025, 17, 1470. [Google Scholar] [CrossRef]
- Mueller, M.; Schneider, M.A.; Deplazes, B.; Cabalzar-Wondberg, D.; Rickenbacher, A.; Turina, M. Colorectal cancer of the young displays distinct features of aggressive tumor biology: A single-center cohort study. World J. Gastrointest. Surg. 2021, 13, 164. [Google Scholar] [CrossRef]
- Alzahrani, S.M.; Al Doghaither, H.A.; Al Ghafari, A.B. General insight into cancer: An overview of colorectal cancer. Mol. Clin. Oncol. 2021, 15, 271. [Google Scholar] [CrossRef]
- Zhang, Z.; Bao, C.; Jiang, L.; Wang, S.; Wang, K.; Lu, C.; Fang, H. When cancer drug resistance meets metabolomics (bulk, single-cell and/or spatial): Progress, potential, and perspective. Front. Oncol. 2023, 12, 1054233. [Google Scholar] [CrossRef]
- Ricon-Becker, I.; Haldar, R.; Shabat Simon, M.; Gutman, M.; Cole, S.W.; Ben-Eliyahu, S.; Zmora, O. Effect of perioperative COX-2 and beta-adrenergic inhibition on 5-year disease-free-survival in colorectal cancer: A pilot randomized controlled Colorectal Metastasis PreventIon Trial (COMPIT). Eur. J. Surg. Oncol. 2023, 49, 655–661. [Google Scholar] [CrossRef]
- Peixoto, R.; Pereira, M.D.L.; Oliveira, M. Beta-blockers and cancer: Where are we? Pharmaceuticals 2020, 13, 105. [Google Scholar] [CrossRef]
- Dosunmu, G.T.; Shergill, A. Colorectal cancer: Genetic underpinning and molecular therapeutics for precision medicine. Genes 2024, 15, 538. [Google Scholar] [CrossRef]
- Hinoue, T.; Weisenberger, D.J.; Pan, F.; Campan, M.; Kim, M.; Young, J.; Whitehall, V.L.; Leggett, B.A.; Laird, P.W. Analysis of the association between CIMP and BRAF in colorectal cancer by DNA methylation profiling. PLoS ONE 2009, 4, e8357. [Google Scholar] [CrossRef]
- Ahmed, D.; Eide, P.W.; Eilertsen, I.A.; Danielsen, S.A.; Eknæs, M.; Hektoen, M.; Lind, G.E.; Lothe, R.A. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis 2013, 2, e71. [Google Scholar] [CrossRef] [PubMed]
- Schulte Am Esch, J.; Windmöller, B.; Hanewinkel, J.; Storm, J.; Förster, C.; Wilkens, L.; Krüger, M.; Kaltschmidt, B.; Kaltschmidt, C. Isolation and characterization of two novel colorectal cancer cell lines, containing a subpopulation with potential stem-like properties: Treatment options by MYC/NMYC inhibition. Cancers 2020, 12, 2582. [Google Scholar] [CrossRef] [PubMed]
- Forgue-Lafitte, M.E.; Coudray, A.M.; Bréant, B.; Mester, J. Proliferation of the human colon carcinoma cell line HT29: Autocrine growth and deregulated expression of the c-myc oncogene. Cancer Res. 1989, 49, 6566–6571. [Google Scholar]
- Sim, J.J.; Park, M.H.; Baek, J.H.; Lee, H.; Jeong, K.Y.; Kim, H.M. Investigation into enhancing capecitabine efficacy in colorectal cancer by inhibiting focal adhesion kinase signaling. Anticancer Res. 2018, 38, 4667–4676. [Google Scholar] [CrossRef] [PubMed]
- Sidorova, M.; Petrikaitė, V. The effect of beta adrenoreceptor blockers on viability and cell colony formation of non-small cell lung cancer cell lines A549 and H1299. Molecules 2022, 27, 1938. [Google Scholar] [CrossRef]
- Wang, H.; Tang, R.; Jiang, L.; Jia, Y. The role of PIK3CA gene mutations in colorectal cancer and the selection of treatment strategies. Front. Pharmacol. 2024, 15, 1494802. [Google Scholar] [CrossRef] [PubMed]
- Barathova, M.; Grossmannova, K.; Belvoncikova, P.; Kubasova, V.; Simko, V.; Skubla, R.; Csaderova, L.; Pastorek, J. Impairment of hypoxia-induced CA IX by beta-blocker propranolol—Impact on progression and metastatic potential of colorectal cancer cells. Int. J. Mol. Sci. 2020, 21, 8760. [Google Scholar] [CrossRef] [PubMed]
- Işeri, O.D.; Sahin, F.I.; Terzi, Y.K.; Yurtcu, E.; Erdem, S.R.; Sarialioglu, F. Beta-adrenoreceptor antagonists reduce cancer cell proliferation, invasion, and migration. Pharm. Biol. 2014, 52, 1374–1381. [Google Scholar] [CrossRef]
- Zhang, T.; Qian, Y.; Yuan, C.; Wu, Y.; Qian, H.; Lu, H.; Hu, C.; Li, W. Propranolol suppresses proliferation and migration of HUVECs through regulation of the miR-206/VEGFA axis. BioMed Res. Int. 2021, 2021, 7629176. [Google Scholar] [CrossRef]
- Shah, M.A.; Rogoff, H.A. Implications of reactive oxygen species on cancer formation and its treatment. Semin. Oncol. 2021, 48, 238–245. [Google Scholar] [CrossRef]
- Liao, X.; Che, X.; Zhao, W.; Zhang, D.; Long, H.; Chaudhary, P.; Li, H. Effects of propranolol in combination with radiation on apoptosis and survival of gastric cancer cells in vitro. Radiat. Oncol. 2010, 5, 98. [Google Scholar] [CrossRef]
- Bustamante, P.; Miyamoto, D.; Goyeneche, A.; de Alba Graue, P.G.; Jin, E.; Tsering, T.; Dias, A.B.; Burnier, M.N.; Burnier, J.V. Beta-blockers exert potent anti-tumor effects in cutaneous and uveal melanoma. Cancer Med. 2019, 8, 7265–7277. [Google Scholar] [CrossRef]
- Seydi, E.; Tabbati, Y.; Pourahmad, J. Toxicity of atenolol and propranolol on rat heart mitochondria. Drug Res. 2020, 70, 151–157. [Google Scholar] [CrossRef]
- Zarewa, S.A.; Binobaid, L.; Sulaiman, A.A.A.; Sobeai, H.M.A.; Alotaibi, M.; Alhoshani, A.; Isab, A.A. Synthesis, characterization, and anticancer activity of phosphanegold(I) complexes of 3-thiosemicarbano-butan-2-one oxime. Biomedicines 2023, 11, 2512. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, P.; Chen, Y.; Zhang, S.; Zhang, Z.; Zhang, Z.; Wang, Y.; Jiang, X.; Lin, K.; Wu, W.; et al. Isoalantolactone induces cell cycle arrest, apoptosis and autophagy in colorectal cancer cells. Front. Pharmacol. 2022, 13, 903599. [Google Scholar] [CrossRef]
- Kumar, R.; Saneja, A.; Panda, A.K. An Annexin V-FITC-Propidium Iodide-Based method for detecting apoptosis in a non-small cell lung cancer cell line. Methods Mol. Biol. 2021, 2279, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Bode, K.; Link, C.; Krammer, P.H.; Weyd, H. Flow-cytometric detection of low-level reactive oxygen species in cell lines and primary immune cells. Bio Protoc. 2020, 10, e3737. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Chun, J.H.; Lin, Y.L.; Liang, Y.C.; Jackson, T.L.; Huang, R.C.C. Tetra-O-Methyl-Nordihydroguaiaretic acid inhibits energy metabolism and synergistically induces anticancer effects with temozolomide on LN229 glioblastoma tumors implanted in mice while preventing obesity in normal mice that consume high-fat diets. PLoS ONE 2023, 18, e0285536. [Google Scholar] [CrossRef] [PubMed]
- Miere, F.; Teuşdea, A.C.; Laslo, V.; Cavalu, S.; Fritea, L.; Dobjanschi, L.; Zdrinca, M.; Ganea, M.; Paşca, C.; Pașc, P.; et al. Evaluation of in vitro wound-healing potential, antioxidant capacity, and antimicrobial activity of Stellaria media (L.) Vill. Appl. Sci. 2021, 11, 11526. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Quantitative Analysis of Dose-Effect Relationships: The Combined Effects of Multiple Drugs or Enzyme Inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
| Cell Line | Cell Densities | ||||
|---|---|---|---|---|---|
| 2 × 103 | 5 × 103 | 10 × 103 | 15 × 103 | 20 × 103 | |
| HCT-116 | 44.73 ± 7.37 | 79.49 ± 11.26 | 166.08 ± 1.31 | 203.80 ± 47.57 | 270.86 ± 42.27 |
| HT-29 | 28.14 ± 4.73 | 43.79 ± 12.22 | 131.16 ± 6.70 | 157.73 ± 7.92 | 161.16 ± 5.60 |
| SW-480 | 54.50 ± 15.48 | 104 ± 1.15 | 130.87 ± 8.75 | 135.40 ± 3.26 | 160.96 ± 12.35 |
| SW-620 | 20.75 ± 5.71 | 21.55 ± 3.49 | 138.03 ± 22.35 | 234.50 ± 8.38 | 219.66 ± 26.50 |
| Cell Line | 6 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| HCT-116 | 303.95 ± 3.49 | 163.80 ± 14.78 | 129.26 ± 5.45 | 95.86 ± 13.11 |
| HT-29 | 225.60 ± 4.85 | 135.60 ± 2.40 | 75.71 ± 12.58 | 53.53 ± 6.79 |
| SW-480 | 176.60 ± 37.35 | 131.24 ± 0.66 | 88.66 ± 14.14 | 67.16 ± 12.86 |
| SW-620 | 208.06 ± 10.67 | 150.53 ± 11.54 | 122.10 ± 2.85 | 94.67 ± 11.16 |
| The Median Effective Doses (ED) | CI (ED50) | CI (ED75) | CI (ED90) | CI (ED95) | Type of Interaction |
|---|---|---|---|---|---|
| SW-620 | 0.73 | 0.75 | 0.77 | 0.79 | Synergism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzahrani, S.M.; Al Doghaither, H.A.; Alkhatabi, H.A.; Basabrain, M.A.; Pushparaj, P.N. Investigating the Potential of Propranolol as an Anti-Tumor Agent in Colorectal Cancer Cell Lines. Int. J. Mol. Sci. 2025, 26, 7513. https://doi.org/10.3390/ijms26157513
Alzahrani SM, Al Doghaither HA, Alkhatabi HA, Basabrain MA, Pushparaj PN. Investigating the Potential of Propranolol as an Anti-Tumor Agent in Colorectal Cancer Cell Lines. International Journal of Molecular Sciences. 2025; 26(15):7513. https://doi.org/10.3390/ijms26157513
Chicago/Turabian StyleAlzahrani, Shiekhah Mohammad, Huda Abdulaziz Al Doghaither, Hind Ali Alkhatabi, Mohammad Abdullah Basabrain, and Peter Natesan Pushparaj. 2025. "Investigating the Potential of Propranolol as an Anti-Tumor Agent in Colorectal Cancer Cell Lines" International Journal of Molecular Sciences 26, no. 15: 7513. https://doi.org/10.3390/ijms26157513
APA StyleAlzahrani, S. M., Al Doghaither, H. A., Alkhatabi, H. A., Basabrain, M. A., & Pushparaj, P. N. (2025). Investigating the Potential of Propranolol as an Anti-Tumor Agent in Colorectal Cancer Cell Lines. International Journal of Molecular Sciences, 26(15), 7513. https://doi.org/10.3390/ijms26157513







