Stem Cell-Derived Corneal Epithelium: Engineering Barrier Function for Ocular Surface Repair
Abstract
1. Introduction
2. Limbal Stem Cell (LSC) Implantation for Corneal Repair in Clinical Practice
2.1. Autologous Limbal Stem Cell Transplantation (for Unilateral LSCD)
2.2. Allogeneic Limbal Stem Cell Transplantation (for Bilateral LSCD)
2.3. Key Clinical Considerations and Challenges for All LSC Transplantation
3. Limitations of Corneal Transplantation
3.1. Donor Tissue Shortage
3.2. Immune Rejection
4. Advantages of Autologous Stem Cell-Derived Epithelium
4.1. Unlimited Renewal Capacity
4.2. Directed Epithelial Differentiation
4.3. Reduced Immunogenicity and Rejection Risk
4.4. Customization Through Tissue Engineering
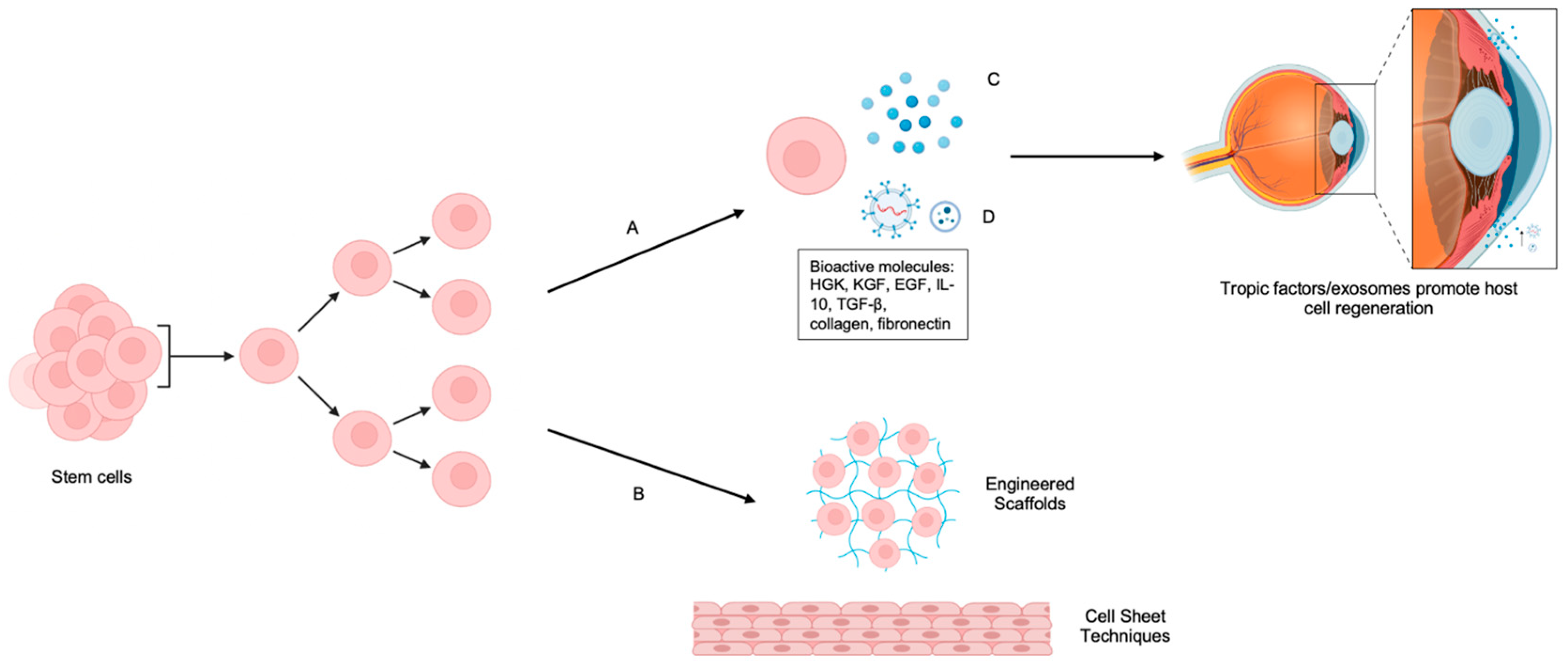
4.5. Trophic Support
5. Progress in the Field
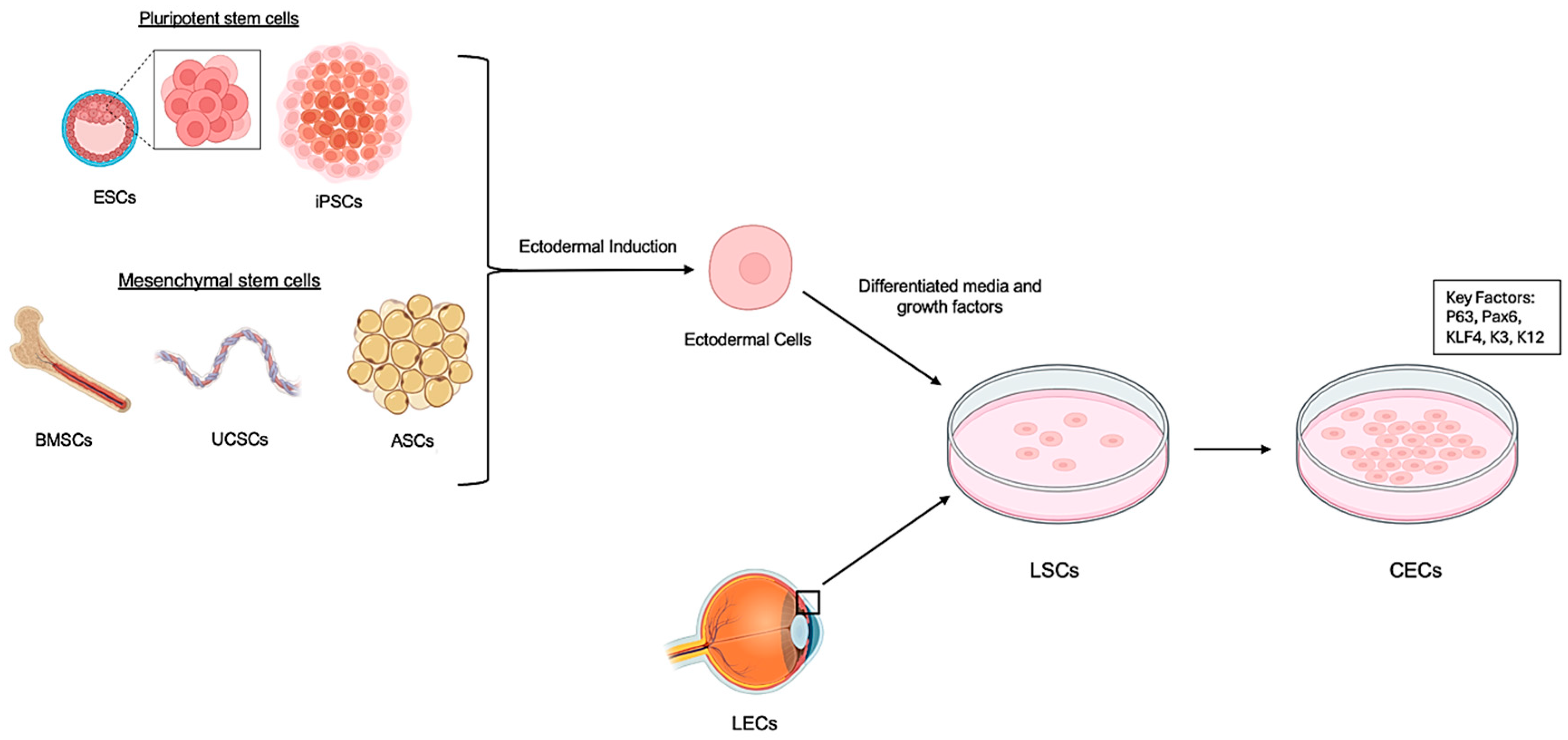
5.1. Multiple Stem Cell Sources
5.2. Optimized Differentiation Protocols
5.3. Stratified Epithelium Derived from Stem Cells for Corneal Regeneration
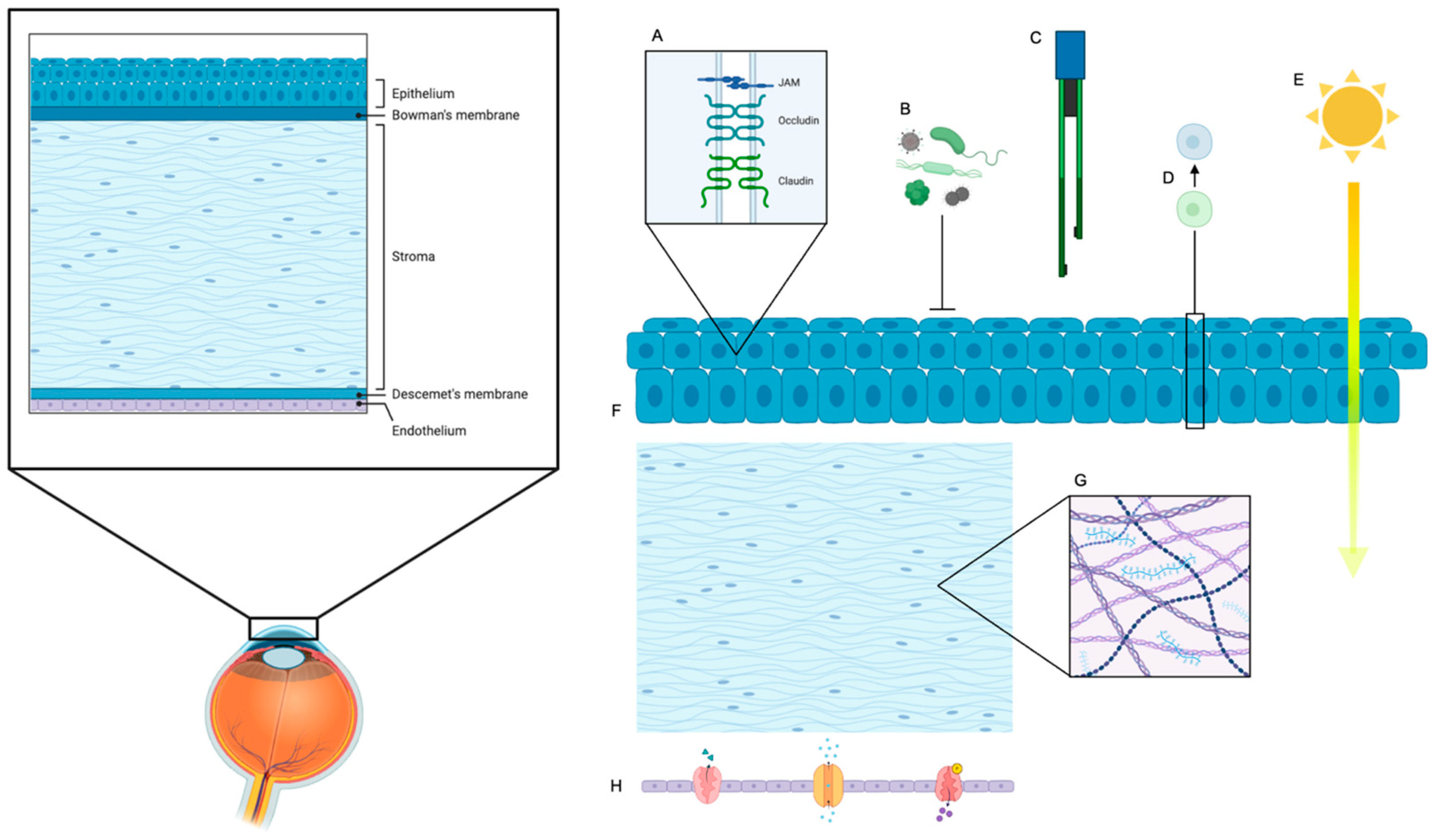
6. Key Functional Parameters of Barrier Function
6.1. Transepithelial Electrical Resistance (TEER)
6.2. Molecular Permeability
6.3. Tight Junctions
6.4. Fluid Regulation and Drug Penetration
6.5. Crucial Role of Underlying Corneal Stroma and Endothelium in Ocular Surface Reconstruction
6.6. The Emerging Role of Extracellular Vesicles in Corneal Reconstruction
7. Development of Biocompatible Scaffolds for Corneal Tissue Engineering
7.1. Natural Materials
7.2. Synthetic Polymers
7.3. Emerging Scaffold Technologies
- Porous hyaluronic acid hydrogels: The porous structure of these hydrogels significantly enhances nutrient diffusion throughout the scaffold, which is crucial for cell viability and metabolism, and improves compatibility with endothelial cells, which are highly sensitive to their microenvironment [67,68].
- 3D Bioprinting: This cutting-edge technology allows for the precise deposition of cells and biomaterials layer by layer, enabling the creation of highly complex and anatomically accurate corneal constructs with defined cellular arrangements and spatial control over growth factor delivery. This offers unprecedented control over scaffold architecture, moving towards truly personalized corneal grafts [11,73].
8. Translational Research
9. Challenges in Corneal Regeneration
9.1. Overcoming Translational Hurdles
9.2. Future Directions in Corneal Regeneration
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masters, B.R. Kanski’s Clinical Ophthalmology, A Systematic Approach, 8th ed.; Brad Bowling, Ed.; 970p., 2600 illustrations; Elsevier: Amsterdam, The Netherlands, 2017; Volume 255, pp. 1867–1868. ISBN 9780702055720. [Google Scholar] [CrossRef]
- Flynn, J.T. Adler’s Physiology of the Eye: Clinical Application, 10th ed. Arch. Ophthalmol. (1960) 2003, 121, 1667. [Google Scholar] [CrossRef]
- Dua, H.S.; Azuara-Blanco, A. Limbal Stem Cells of the Corneal Epithelium. Surv. Ophthalmol. 2000, 44, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef]
- Alfonso, E.C. Treatment of Severe Ocular-Surface Disorders With Corneal Epithelial Stem Cell Transplantation. Arch. Ophthalmol. (1960) 2000, 118, 123–124. [Google Scholar] [CrossRef]
- Tseng, S.C. Concept and application of limbal stem cells. Eye 1989, 3 Pt 2, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, B.; Czyz, C.N.; Mahabadi, N.; Havens, S.J. Corneal Graft Rejection. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Tan, D.T.; Dart, J.K.; Holland, E.J.; Kinoshita, S. Corneal transplantation. Lancet 2012, 379, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Jurkunas, U.V.; Kaufman, A.R.; Yin, J.; Ayala, A.; Maguire, M.; Samarakoon, L.; Johns, L.K.; Parekh, M.; Li, S.; Gauthier, A.; et al. Cultivated autologous limbal epithelial cell (CALEC) transplantation for limbal tem cell deficiency: A phase I/II clinical trial of the first xenobiotic-free, serum-free, antibiotic-free manufacturing protocol developed in the US. Nat. Commun. 2025, 16, 1607. [Google Scholar] [CrossRef]
- Nosrati, H.; Alizadeh, Z.; Nosrati, A.; Ashrafi-Dehkordi, K.; Banitalebi-Dehkordi, M.; Sanami, S.; Khodaei, M. Stem cell-based therapeutic strategies for corneal epithelium regeneration. Tissue Cell 2021, 68, 101470. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Wang, H.; Li, M.; Wang, X.; Liu, R.; Zhang, D.; Xu, W. Corneal regeneration strategies: From stem cell therapy to tissue engineered stem cell scaffolds. Biomed. Pharmacother. 2023, 165, 115206. [Google Scholar] [CrossRef]
- Ghiasi, M.; Jadidi, K.; Hashemi, M.; Zare, H.; Salimi, A.; Aghamollaei, H. Application of mesenchymal stem cells in corneal regeneration. Tissue Cell 2021, 73, 101600. [Google Scholar] [CrossRef]
- Hayashi, R.; Ishikawa, Y.; Ito, M.; Kageyama, T.; Takashiba, K.; Fujioka, T.; Tsujikawa, M.; Miyoshi, H.; Yamato, M.; Nakamura, Y.; et al. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. PLoS ONE 2012, 7, e45435. [Google Scholar] [CrossRef]
- Soma, T.; Oie, Y.; Takayanagi, H.; Matsubara, S.; Yamada, T.; Nomura, M.; Yoshinaga, Y.; Maruyama, K.; Watanabe, A.; Takashima, K.; et al. Induced pluripotent stem-cell-derived corneal epithelium for transplant surgery: A single-arm, open-label, first-in-human interventional study in Japan. Lancet 2024, 404, 1929–1939. [Google Scholar] [CrossRef]
- Tonti, E.; Manco, G.A.; Spadea, L.; Zeppieri, M. Focus on limbal stem cell deficiency and limbal cell transplantation. World J. Transpl. 2023, 13, 321–330. [Google Scholar] [CrossRef]
- Hayashi, R.; Ishikawa, Y.; Katori, R.; Sasamoto, Y.; Taniwaki, Y.; Takayanagi, H.; Tsujikawa, M.; Sekiguchi, K.; Quantock, A.J.; Nishida, K. Coordinated generation of multiple ocular-like cell lineages and fabrication of functional corneal epithelial cell sheets from human iPS cells. Nat. Protoc. 2017, 12, 683–696. [Google Scholar] [CrossRef]
- Gu, S.; Xing, C.; Han, J.; Tso, M.O.M.; Hong, J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol. Vis. 2009, 15, 99–107. [Google Scholar] [PubMed]
- Reza, H.M.; Ng, B.-Y.; Gimeno, F.L.; Phan, T.T.; Ang, L.P.-K. Umbilical Cord Lining Stem Cells as a Novel and Promising Source for Ocular Surface Regeneration. Stem Cell Rev. Rep. 2011, 7, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, B.; Yang, Y.; Jiang, Q.; Li, T.; Gong, J.; Tang, H.; Zhang, Q. Stem cell-derived exosomes: Emerging therapeutic opportunities for wound healing. Stem Cell Res. Ther. 2023, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.S.; Nikpoor, N.; Rao Donthineni, P.; Singh, V.; Chodosh, J.; Basu, S. Autologous limbal stem cell transplantation: A systematic review of clinical outcomes with different surgical techniques. Br. J. Ophthalmol. 2020, 104, 247–253. [Google Scholar] [CrossRef]
- Ozer, M.D.; Altınkurt, E.; Yilmaz, Y.C.; Gedik, A.C.; Alparslan, N. The Surgical Outcomes of Limbal Allograft Transplantation in Eyes Having Limbal Stem Cell Deficiency. J. Curr. Ophthalmol. 2020, 32, 132–141. [Google Scholar] [CrossRef]
- Sasamoto, Y.; Yeung, P.C.; Tran, J.; Frank, M.H.; Frank, N.Y. Protocol for isolating human BCAM-positive corneal progenitor cells by flow cytometry and cell sorting. STAR Protoc. 2023, 4, 102503. [Google Scholar] [CrossRef]
- Fernandez-Buenaga, R.; Aiello, F.; Zaher, S.S.; Grixti, A.; Ahmad, S. Twenty years of limbal epithelial therapy: An update on managing limbal stem cell deficiency. BMJ Open Ophthalmol. 2018, 3, e000164. [Google Scholar] [CrossRef]
- Nakajima, R.; Takeda, S. Fabrication of corneal epithelial cell sheets maintaining colony-forming cells without feeder cells by oxygen-controlled method. Exp. Eye Res. 2014, 118, 53–60. [Google Scholar] [CrossRef]
- Galindo, S.; de la Mata, A.; López-Paniagua, M.; Herreras, J.M.; Pérez, I.; Calonge, M.; Nieto-Miguel, T. Subconjunctival injection of mesenchymal stem cells for corneal failure due to limbal stem cell deficiency: State of the art. Stem Cell Res. Ther. 2021, 12, 60. [Google Scholar] [CrossRef]
- Sotozono, C.; Inatomi, T.; Nakamura, T.; Koizumi, N.; Yokoi, N.; Ueta, M.; Matsuyama, K.; Kaneda, H.; Fukushima, M.; Kinoshita, S. Cultivated oral mucosal epithelial transplantation for persistent epithelial defect in severe ocular surface diseases with acute inflammatory activity. Acta Ophthalmol. 2014, 92, e447–e453. [Google Scholar] [CrossRef]
- Català, P.; Thuret, G.; Skottman, H.; Mehta, J.S.; Parekh, M.; Ní Dhubhghaill, S.; Collin, R.W.J.; Nuijts, R.M.M.A.; Ferrari, S.; LaPointe, V.L.S.; et al. Approaches for corneal endothelium regenerative medicine. Prog. Retin. Eye Res. 2022, 87, 100987. [Google Scholar] [CrossRef] [PubMed]
- Haagdorens, M.; Van Acker, S.I.; Van Gerwen, V.; Ní Dhubhghaill, S.; Koppen, C.; Tassignon, M.J.; Zakaria, N. Limbal Stem Cell Deficiency: Current Treatment Options and Emerging Therapies. Stem Cells Int. 2016, 2016, 9798374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, W.; Ren, X.; Yin, Y.; Chen, W.; Chen, F.; Zhu, J.; Shen, L. Autologous glueless simple oral mucosal transplantation for the repair of limbal stem cell deficiency ocular surface in a rabbit model. Sci. Rep. 2025, 15, 10855. [Google Scholar] [CrossRef]
- Kinoshita, S.; Nakamura, T.; Morser, J.; Nishikawa, S.I. Corneal Cells for Regeneration. Promises Chall. Regen. Med. 2005, 54, 63–83. [Google Scholar] [CrossRef]
- Jeang, L.J.; Margo, C.E.; Espana, E.M. Diseases of the corneal endothelium. Exp. Eye Res. 2021, 205, 108495. [Google Scholar] [CrossRef]
- Thuret, G.; Courrier, E.; Poinard, S.; Gain, P.; Baud’Huin, M.; Martinache, I.; Cursiefen, C.; Maier, P.; Hjortdal, J.; Sanchez Ibanez, J.; et al. One threat, different answers: The impact of COVID-19 pandemic on cornea donation and donor selection across Europe. Br. J. Ophthalmol. 2022, 106, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Kobal, N.; Marzidovšek, M.; Schollmayer, P.; Maličev, E.; Hawlina, M.; Marzidovšek, Z.L. Molecular and Cellular Mechanisms of the Therapeutic Effect of Mesenchymal Stem Cells and Extracellular Vesicles in Corneal Regeneration. Int. J. Mol. Sci. 2024, 25, 11121. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.S.; Riau, A.K.; Yam, G.H.-F.; Yusoff, N.Z.B.M.; Han, E.J.Y.; Goh, T.-W.; Lai, R.C.; Lim, S.K.; Mehta, J.S. Mesenchymal Stem Cell Exosomes as Immunomodulatory Therapy for Corneal Scarring. Int. J. Mol. Sci. 2023, 24, 7456. [Google Scholar] [CrossRef]
- Robbins, B.T.; Montreuil, K.A.; Kundu, N.; Kumar, P.; Agrahari, V. Corneal Treatment, Repair, and Regeneration: Exosomes at Rescue. Pharmaceutics 2024, 16, 1424. [Google Scholar] [CrossRef]
- Fagerholm, P.; Lagali, N.S.; Merrett, K.; Jackson, W.B.; Munger, R.; Liu, Y.; Polarek, J.W.; Söderqvist, M.; Griffith, M. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci. Transl. Med. 2010, 2, 46ra61. [Google Scholar] [CrossRef]
- Joyce, N.C. Proliferative capacity of corneal endothelial cells. Exp. Eye Res. 2012, 95, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Kawakita, T.; Shimmura, S.; Hornia, A.; Higa, K.; Tseng, S.C.G. Stratified epithelial sheets engineered from a single adult murine corneal/limbal progenitor cell. J. Cell. Mol. Med. 2008, 12, 1303–1316. [Google Scholar] [CrossRef]
- Nieto-Miguel, T.; Galindo, S.; Reinoso, R.; Corell, A.; Martino, M.; Pérez-Simón, J.A.; Calonge, M. In vitro simulation of corneal epithelium microenvironment induces a corneal epithelial-like cell phenotype from human adipose tissue mesenchymal stem cells. Curr. Eye Res. 2013, 38, 933–944. [Google Scholar] [CrossRef]
- Mavilio, F.; Pellegrini, G.; Ferrari, S.; Di Nunzio, F.; Di Iorio, E.; Recchia, A.; Maruggi, G.; Ferrari, G.; Provasi, E.; Bonini, C.; et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat. Med. 2006, 12, 1397–1402. [Google Scholar] [CrossRef]
- Li, Y.; Inoue, T.; Takamatsu, F.; Maeda, N.; Ohashi, Y.; Nishida, K. Development of genetically modified eliminable human dermal fibroblast feeder cells for ocular surface regeneration medicine. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7522–7531. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.V.; Jackson, C.J.; Utheim, T.P.; Jirsova, K. Ex vivo cultivated oral mucosal epithelial cell transplantation for limbal stem cell deficiency: A review. Stem Cell Res. Ther. 2020, 11, 301. [Google Scholar] [CrossRef]
- Yamane, S.; Higa, K.; Umezawa, T.; Serikawa, M.; Shimazaki, J.; Abe, S. Engineered three-dimensional rabbit oral epithelial–mesenchymal–muscular hybrid sheets. Int. J. Oral Sci. 2016, 8, 145–154. [Google Scholar] [CrossRef]
- McKay, T.B.; Schlötzer-Schrehardt, U.; Pal-Ghosh, S.; Stepp, M.A. Integrin: Basement membrane adhesion by corneal epithelial and endothelial cells. Exp. Eye Res. 2020, 198, 108138. [Google Scholar] [CrossRef]
- Yu, Z.; Hao, R.; Chen, X.; Ma, L.; Zhang, Y.; Yang, H. Protocol to develop a microfluidic human corneal barrier-on-a-chip to evaluate the corneal epithelial wound repair process. STAR Protoc. 2023, 4, 102122. [Google Scholar] [CrossRef]
- Ban, Y.; Dota, A.; Cooper, L.J.; Fullwood, N.J.; Nakamura, T.; Tsuzuki, M.; Mochida, C.; Kinoshita, S. Tight junction-related protein expression and distribution in human corneal epithelium. Exp. Eye Res. 2003, 76, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-Y.; Cheng, H.-Y.; Ma, D.H.-K. Investigation of Overrun-Processed Porous Hyaluronic Acid Carriers in Corneal Endothelial Tissue Engineering. PLoS ONE 2015, 10, e0136067. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Blazejewska, E.A.; Call, M.K.; Yamanaka, O.; Liu, H.; Schlötzer-Schrehardt, U.; Kruse, F.E.; Kao, W.W. From hair to cornea: Toward the therapeutic use of hair follicle-derived stem cells in the treatment of limbal stem cell deficiency. Stem Cells 2011, 29, 57–66. [Google Scholar] [CrossRef]
- Melnyk, S.; Bollag, W.B. Aquaporins in the Cornea. Int. J. Mol. Sci. 2024, 25, 3748. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-L.; Lin, C.-T.; Li, J.-W.; Hu, F.-R.; Chen, C.-C. ERK1/2 Activation Regulates the Wound Healing Process of Rabbit Corneal Endothelial Cells. Curr. Eye Res. 2009, 34, 103–111. [Google Scholar] [CrossRef]
- Yin, J.; Liu, W.; Liu, C.; Zhao, G.; Zhang, Y.; Liu, W.; Hua, J.; Dou, Z.; Lei, A. Establishment of goat limbal stem cell strain expressing Venus fluorescent protein and construction of limbal epithelial sheets. Shengwu Gongcheng Xuebao (Chin. J. Biotechnol.) 2010, 26, 1636–1644. [Google Scholar]
- Guo, W.; Wang, P.; Liu, Z.-H.; Ye, P. Analysis of differential expression of tight junction proteins in cultured oral epithelial cells altered by Porphyromonas gingivalis, Porphyromonas gingivalis lipopolysaccharide, and extracellular adenosine triphosphate. Int. J. Oral Sci. 2018, 10, e8. [Google Scholar] [CrossRef]
- Nakamura, T.; Takeda, K.; Inatomi, T.; Sotozono, C.; Kinoshita, S. Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br. J. Ophthalmol. 2011, 95, 942–946. [Google Scholar] [CrossRef]
- He, J.; Ou, S.; Ren, J.; Sun, H.; He, X.; Zhao, Z.; Wu, H.; Qu, Y.; Liu, T.; Jeyalatha, V.; et al. Tissue engineered corneal epithelium derived from clinical-grade human embryonic stem cells. Ocul. Surf. 2020, 18, 672–680. [Google Scholar] [CrossRef]
- Subramaniam, S.; Sejpal, K.; Fatima, A.; Gaddipati, S.; Vemuganti, G.; Sangwan, V. Coculture of autologous limbal and conjunctival epithelial cells to treat severe ocular surface disorders: Long-term survival analysis. Indian. J. Ophthalmol. 2013, 61, 202–207. [Google Scholar] [CrossRef]
- Soma, T.; Hayashi, R.; Sugiyama, H.; Tsujikawa, M.; Kanayama, S.; Oie, Y.; Nishida, K. Maintenance and distribution of epithelial stem/progenitor cells after corneal reconstruction using oral mucosal epithelial cell sheets. PLoS ONE 2014, 9, e110987. [Google Scholar] [CrossRef]
- Susaimanickam, P.J.; Maddileti, S.; Pulimamidi, V.K.; Boyinpally, S.R.; Naik, R.R.; Naik, M.N.; Reddy, G.B.; Sangwan, V.S.; Mariappan, I. Generating minicorneal organoids from human induced pluripotent stem cells. Development 2017, 144, 2338–2351. [Google Scholar] [CrossRef] [PubMed]
- Soleimanifar, F.; Mortazavi, Y.; Nadri, S.; Soleimani, M. Conjunctiva derived mesenchymal stem cell (CJMSCs) as a potential platform for differentiation into corneal epithelial cells on bioengineered electrospun scaffolds. J. Biomed. Mater. Res. Part A 2017, 105, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Visalli, F.; Fava, F.; Capobianco, M.; Musa, M.; D’Esposito, F.; Russo, A.; Scollo, D.; Longo, A.; Gagliano, C.; Zeppieri, M. Innovative Bioscaffolds in Stem Cell and Regenerative Therapies for Corneal Pathologies. Bioengineering 2024, 11, 859. [Google Scholar] [CrossRef]
- Nguyen, K.N.; Bobba, S.; Richardson, A.; Park, M.; Watson, S.L.; Wakefield, D.; Di Girolamo, N. Native and synthetic scaffolds for limbal epithelial stem cell transplantation. Acta Biomater. 2018, 65, 21–35. [Google Scholar] [CrossRef]
- Tran, L.T.; Li, J.Y. The role of eye banking with cell-based therapies. Curr. Opin. Ophthalmol. 2023, 34, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.M.D.F.F.M.; Vanathi, M.M.; Kumar, A.D.T.; Dash, Y.M.S.; Priya, S.M. Corneal Graft Rejection. Surv. Ophthalmol. 2007, 52, 375–396. [Google Scholar] [CrossRef]
- Koizumi, N.; Nishida, K.; Amano, S.; Kinoshita, S. Progress in the development of tissue engineering of the cornea in Japan. Nippon Ganka Gakkai Zasshi 2007, 111, 493–503. [Google Scholar]
- Chung, E.H.; DeGregorio, P.G.; Wasson, M.; Zieske, J.D. Epithelial regeneration after limbus-to-limbus debridement. Expression of alpha-enolase in stem and transient amplifying cells. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1336–1343. [Google Scholar]
- Hill, J.C. Immunosuppression in corneal transplantation. Eye 1995, 9, 247–253. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, D.H.; Shin, E.J.; Lee, H.J.; Wee, W.R.; Jeon, S.; Kim, M.K. Comparative Analysis of Substrate-Free Cultured Oral Mucosal Epithelial Cell Sheets from Cells of Subjects with and without Stevens-Johnson Syndrome for Use in Ocular Surface Reconstruction. PLoS ONE 2016, 11, e0147548. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Choi, S.H.; Wolosin, J.M.; Chung, S.-H.; Joo, C.-K. Regeneration of the corneal epithelium with conjunctival epithelial equivalents generated in serum- and feeder-cell-free media. Mol. Vis. 2013, 19, 2542–2550. [Google Scholar] [PubMed]
- Yang, H.; Reinach, P.S.; Koniarek, J.P.; Wang, Z.; Iserovich, P.; Fischbarg, J. Fluid transport by cultured corneal epithelial cell layers. Br. J. Ophthalmol. 2000, 84, 199–204. [Google Scholar] [CrossRef]
- Di, G.; Qi, X.; Zhao, X.; Zhang, S.; Danielson, P.; Zhou, Q. Corneal Epithelium-Derived Neurotrophic Factors Promote Nerve Regeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4695–4702. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, Y.; Soma, T.; Azuma, S.; Maruyama, K.; Hashikawa, Y.; Katayama, T.; Sasamoto, Y.; Takayanagi, H.; Hosen, N.; Shiina, T.; et al. Long-term survival in non-human primates of stem cell-derived, MHC-unmatched corneal epithelial cell sheets. Stem Cell Rep. 2022, 17, 1714–1729. [Google Scholar] [CrossRef]
- Joyce, N.C.; Harris, D.L.; Markov, V.; Zhang, Z.; Saitta, B. Potential of human umbilical cord blood mesenchymal stem cells to heal damaged corneal endothelium. Mol. Vis. 2012, 18, 547–564. [Google Scholar]
- Yu, Z.; Hao, R.; Du, J.; Wu, X.; Chen, X.; Zhang, Y.; Li, W.; Gu, Z.; Yang, H. A Human Cornea-on-A-Chip for the Study of Epithelial Wound Healing by Extracellular Vesicles. SSRN Electron. J. 2021, 25, 104200. [Google Scholar] [CrossRef]
- Pınarlı, F.A.; Okten, G.; Beden, U.; Fışgın, T.; Kefeli, M.; Kara, N.; Duru, F.; Tomak, L. Keratinocyte growth factor-2 and autologous serum potentiate the regenerative effect of mesenchymal stem cells in cornea damage in rats. Int. J. Ophthalmol. 2014, 7, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, J.A. Molecular mechanisms underlying the corneal endothelial pump. Exp. Eye Res. 2012, 95, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kan, K.; Nishida, K.; Yamato, M.; Okano, T. Corneal regeneration by transplantation of corneal epithelial cell sheets fabricated with automated cell culture system in rabbit model. Biomaterials 2013, 34, 9010–9017. [Google Scholar] [CrossRef]
- Charron, D.; Suberbielle-Boissel, C.; Tamouza, R.; Al-Daccak, R. Anti-HLA antibodies in regenerative medicine stem cell therapy. Hum. Immunol. 2012, 73, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, F. Primary Culture of Cornea-Limbal Epithelial Cells In Vitro. In Corneal Regeneration: Methods and Protocols; Ahearne, M., Ed.; Springer: New York, NY, USA, 2020; pp. 29–37. [Google Scholar]
- Mofidfar, M.; Abdi, B.; Ahadian, S.; Mostafavi, E.; Desai, T.A.; Abbasi, F.; Sun, Y.; Manche, E.E.; Ta, C.N.; Flowers, C.W. Drug delivery to the anterior segment of the eye: A review of current and future treatment strategies. Int. J. Pharm. 2021, 607, 120924. [Google Scholar] [CrossRef]
- Zavala, J.; López Jaime, G.R.; Rodríguez Barrientos, C.A.; Valdez-Garcia, J. Corneal endothelium: Developmental strategies for regeneration. Eye 2013, 27, 579–588. [Google Scholar] [CrossRef]
- Calonge, M.; Pérez, I.; Galindo, S.; Nieto-Miguel, T.; López-Paniagua, M.; Fernández, I.; Alberca, M.; García-Sancho, J.; Sánchez, A.; Herreras, J.M. A proof-of-concept clinical trial using mesenchymal stem cells for the treatment of corneal epithelial stem cell deficiency. Transl. Res. 2019, 206, 18–40. [Google Scholar] [CrossRef]
- Yang, X.; Moldovan, N.I.; Zhao, Q.; Mi, S.; Zhou, Z.; Chen, D.; Gao, Z.; Tong, D.; Dou, Z. Reconstruction of damaged cornea by autologous transplantation of epidermal adult stem cells. Mol. Vis. 2008, 14, 1064–1070. [Google Scholar]
- Venkatakrishnan, J.; Saeed, Y.; Kao, W.W.Y. Trends in using mesenchymal stromal/stem cells (MSCs) in treating corneal diseases. Ocul. Surf. 2022, 26, 255–267. [Google Scholar] [CrossRef]
- Venugopal, B.; Mohan, S.; Kumary, T.V.; Anil Kumar, P.R. Peripheral Blood As a Source of Stem Cells for Regenerative Medicine: Emphasis Towards Corneal Epithelial Reconstruction—An In Vitro Study. Tissue Eng. Regen. Med. 2020, 17, 495–510. [Google Scholar] [CrossRef]
- Sangwan, V.S.; Vemuganti, G.K.; Singh, S.; Balasubramanian, D. Successful reconstruction of damaged ocular outer surface in humans using limbal and conjuctival stem cell culture methods. Biosci. Rep. 2003, 23, 169–174. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. Immunology of Corneal Allografts: Insights from Animal Models. J. Clin. Exp. Ophthalmol. 2015, 6, 429. [Google Scholar] [CrossRef] [PubMed]
- Omoto, M.; Miyashita, H.; Shimmura, S.; Higa, K.; Kawakita, T.; Yoshida, S.; McGrogan, M.; Shimazaki, J.; Tsubota, K. The Use of Human Mesenchymal Stem Cell-Derived Feeder Cells for the Cultivation of Transplantable Epithelial Sheets. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.; Wanczyk, H.; Jensen, T.; Finck, C. Assessment of iPSC teratogenicity throughout directed differentiation toward an alveolar-like phenotype. Differentiation 2019, 105, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Güell, J.L.; El Husseiny, M.A.; Manero, F.; Gris, O.; Elies, D. Historical Review and Update of Surgical Treatment for Corneal Endothelial Diseases. Ophthalmol. Ther. 2014, 3, 1–15. [Google Scholar] [CrossRef]
- Bertolin, M.; Breda, C.; Ferrari, S.; Van Acker, S.I.; Zakaria, N.; Di Iorio, E.; Migliorati, A.; Ponzin, D.; Ferrari, B.; Lužnik, Z.; et al. Optimized Protocol for Regeneration of the Conjunctival Epithelium Using the Cell Suspension Technique. Cornea 2019, 38, 469–479. [Google Scholar] [CrossRef]
- Li, Q.; Wong, H.L.; Ip, Y.L.; Chu, W.Y.; Li, M.S.; Saha, C.; Shih, K.C.; Chan, Y.K. Current microfluidic platforms for reverse engineering of cornea. Mater. Today Bio 2023, 20, 100634. [Google Scholar] [CrossRef]
- Kaluzhny, Y.; Kinuthia, M.W.; Lapointe, A.M.; Truong, T.; Klausner, M.; Hayden, P. Oxidative stress in corneal injuries of different origin: Utilization of 3D human corneal epithelial tissue model. Exp. Eye Res. 2020, 190, 107867. [Google Scholar] [CrossRef]
- Lu, R. Advances in Ophthalmic Organ-on-a-Chip Models: Bridging Translational Gaps in Disease Modeling and Drug Screening. Int. J. Transl. Med. 2024, 4, 710–725. [Google Scholar] [CrossRef]
- Reichl, S. Cell culture models of the human cornea—A comparative evaluation of their usefulness to determine ocular drug absorption in-vitro. J. Pharm. Pharmacol. 2008, 60, 299–307. [Google Scholar] [CrossRef]
- Kaluzhny, Y.; Kinuthia, M.W.; Truong, T.; Lapointe, A.M.; Hayden, P.; Klausner, M. New Human Organotypic Corneal Tissue Model for Ophthalmic Drug Delivery Studies. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2880–2898. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Irani, Y.D. Gene Therapy and Gene Editing for the Corneal Dystrophies. Asia-Pac. J. Ophthalmol. 2016, 5, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Corradini, F.; Venturi, B.; Pellegrini, G.; De Luca, M.; Mace, K.A.; Braun, K.M. Methods for Characterization/Manipulation of Human Corneal Stem Cells and their Applications in Regenerative Medicine. Methods Mol. Biol. 2012, 916, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, A.; Jylhä, A.; Rieck, J.; Nättinen, J.; Ilmarinen, T.; Veréb, Z.; Aapola, U.; Beuerman, R.; Petrovski, G.; Uusitalo, H.; et al. Comparative proteomics reveals human pluripotent stem cell-derived limbal epithelial stem cells are similar to native ocular surface epithelial cells. Sci. Rep. 2015, 5, 14684. [Google Scholar] [CrossRef]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef]
| Method | Indication | Donor Source | Tissue Requirement | Procedure Type | Success Rates | Advantages | Limitations |
|---|---|---|---|---|---|---|---|
| CLAU [3,15] | Unilateral LSCD | Autologous (healthy contralateral eye) | 2–4 clock hours of limbus + conjunctiva | Single stage | Up to 83.2% anatomical success | No immunosuppression, long-term efficacy | Risk of iatrogenic LSCD in donor eye |
| CLET [4,9] | Unilateral LSCD | Autologous (small limbal biopsy) | 1–2 mm2 biopsy | Two-stage, ex vivo expansion on amniotic membrane (GMP) | 60–80% | Minimal donor tissue needed, quality control of cells | Requires cell culture facilities, increased cost |
| SLET [15,20] | Unilateral LSCD | Autologous (small limbal biopsy) | 2–3 mm2 biopsy, minced | Single stage | 75.2–83.8% at 1 year | Combines low donor morbidity with procedural simplicity | Long-term outcomes still under study |
| KLAL [15,21] | Bilateral LSCD | Allogeneic (cadaveric donor) | Large limbal segment | Single stage | 67% at 12 months, 53% at 18 months | Access to LSCs when autologous source unavailable | Lifelong systemic immunosuppression, rejection risk |
| lr-CLAL [15] | Bilateral LSCD | Allogeneic (living relative) | Large limbal segment | Single stage | Limited data, similar to KLAL | Potential for better donor matching | Immunosuppression required, rejection risk |
| Allogeneic CLET [15,22] | Bilateral LSCD | Allogeneic (donor-derived cells) | Small limbal biopsy, expanded ex vivo | Two-stage, ex vivo expansion | 61.4% anatomical, 53% functional success | Less immunogenic than full tissue grafts | Immunosuppression needed, limited long-term survival |
| Classification | Cell Type | Cell Origin | Key Markers | Advantages and Outcomes | Limitations | Clinical Application |
|---|---|---|---|---|---|---|
| PSCs | ESCs (embryonic) | In vitro [57] | CK3, CK12, ABCG2, PAX6, p63, CD44, E-cadherin, CD271, CD29 [4,13,16,57,58] | Strong differentiation potential, full cornea formation potential [5] | Risk of uncontrolled proliferation, immune rejection tumorigenesis, ethical concerns [6] | |
| iPSCs | In vitro [38] | CK3, CK12, ABCG2, PAX6, p63, CD271, CD29 [4] | Patient-specific, capacity for infinite expansion [8] | Immunological compatibility, tumorigenesis, differentiation variability [10] | Clinical study of iPSC derived corneal epithelium for transplant surgery [14] | |
| MSCs | BMSCs | Rabbit, rat, nude rat [17] | CK3, CK12, CD271, CD29 [4,17,33] | Promotes healing, low inflammation [16] | Limited differentiation [16] | MSCs for treatment of corneal stem cell deficiency, subconjunctival injection of MSCs for LSCD [25] |
| ADSCs | In vitro [18] | CK12, TGF-β, CD29 [4,40,59] | Easily accessible, low immunogenicity [16] | Limited differentiation, difficult to ensure purity [16] | ||
| AMSCs | Human [5] | CK3, CK12, CK18, CK19, β1-integrin, CD29 [4,25,60] | Anti-inflammatory, anti-microbial, enhances wound healing [4] | Limited source, susceptible to contamination [19] | ||
| UCSCs | Rabbit [18] | CK15, ABCG2, BMI1, α6 integrin, α9 integrin, β1 integrin, collagen IV, laminin, CD29 [4,18,33] | High differentiation potential, anti-inflammatory properties, low immunogenicity [5] | Limited source, difficult to culture | ||
| ESCs (epithelial) | OMSCs | Rat, human [21] | p63, β1 integrin, collagen VII, laminin, E-cadherin, CD29 [4,43,56] | Strong regenerative potential, good epithelial integration [5] | Not cornea-specific [20] | |
| ESCs (epidermal) | Goat [23] | CK3, CK12, PAX-6, CD271, CD29 [4,49] | Re-epithelialization capacity, enhanced transparency [5] | Not commonly used in ocular applications | ||
| HFSCs | Mouse [24] | CK12, CK15, α6 integrin, CD271, CD29 [4,49] | Proper differentiation, suppression of neovascularization | Difficult to isolate | ||
| LESCs | Mouse [4] | Direct differentiation into corneal epithelial cells | Native to cornea, good regenerative capacity [5] | Autologous transport is difficult [26] | Phase I/II clinical trial of cultivated autologous limbal epithelial cell [27] transplantation for LSCD | |
| CSCs | In vitro, human [27] | CK3, CK19, MUC5AC, Ki-67, p63, ABCG2, CD29 [4] | Proper differentiation, potential for epithelial repair [5] | LSCD patients do not have a healthy conjunctiva to harvest from [34] |
| Synthetic Biomaterials | Natural Biomaterials | |
|---|---|---|
| Representative Materials | Polyethylene glycol (PEG), polylactic acid (PLA), polycaprolactone (PCL), hydrogels (e.g., PEG-based or PVA-based hydrogels) [11,27,61] | Amniotic membrane (AM), collagen, chitosan, gelatin [11,27,61] |
| Molecular Composition | Synthetic polymers with repeating chemical units and cross-linking agents to stabilize [11,61] | Derived from extracellular matrix proteins, glycosaminoglycans, and endogenous growth factors [27,60] |
| Biocompatibility | Moderate; requires chemical surface modifications (e.g., plasma treatment or coating with fibronectin/collagen) to improve cell adhesion [11] | Excellent biocompatibility due to natural ECM and growth factor composition, such as AM containing pigment epithelial derived-factor (PEDF) [27,33,34] |
| Transparency | High transparency, especially in hydrogels (e.g., PEG hydrogels) optimized for corneal repair [11,36] | High optical transparency; suitable for corneal epithelial healing [27] |
| Mechanical Properties | Tunable elasticity and strength via polymer chemistry [11,61] | Moderate mechanical strength [27,60] |
| Degradation Profile | Controlled degradation through tailoring polymer composition and cross-link density [27,60] | Biodegrades naturally; degradation rate may not always align with tissue healing rates [27] |
| Cell Adhesion | Requires bioactive coatings (e.g., RGD peptides, collagen, or fibronectin) to enhance cell attachment [11,27] | Naturally promotes corneal epithelial cell adhesion and proliferation [33,61] |
| Immune Response | Potential for immune response due to synthetic nature; requires biocompatible coatings [11] | Low immune response; AM contains IL-10 and TGF-β and other suppressive factors [33,34,35] |
| Anti-inflammatory Properties | Lacks inherent anti-inflammatory properties; external agents (e.g., corticosteroids or antimicrobial peptides) may be incorporated [11] | Possesses natural anti-inflammatory properties, reducing scarring and promoting healing (e.g., PEDF, IL-10) [33,34,35] |
| Antimicrobial Properties | Synthetic polymers lack inherent antimicrobial activity, but antimicrobial agents (e.g., silver nanoparticles or antibiotics) can be added [11] | Natural antimicrobial properties due to lysozyme and other bioactive molecules [27,61] |
| Customization | Highly customizable for mechanical strength, degradation rate, and transparency [11,60,61] | Limited customization; properties depend on donor tissue and processing methods [60] |
| Growth Factor Content | None; requires incorporation of exogenous growth factors (e.g., EGF) for enhanced healing [11] | AM contains intrinsic growth factors like PEDF and epidermal growth factor (EGF) |
| Scalability | Easily scalable; synthetic polymers can be produced in large quantities with reproducible properties [11,60] | Limited scalability; dependent on donors and tissue availability [62] |
| Cost | Cost-effective for large-scale production [60] | High cost due to processing, storage, and donor limitations [62] |
| Applications | Tissue scaffolds, drug delivery systems [11,27] | Ocular surface repair, epithelial defect healing [33,34] |
| Limitations | Lack native bioactivity; potential for cytotoxicity; requires external incorporation of bioactive molecules to mimic natural ECM [11,61] | Donor-dependent variability; limited shelf life; risk of disease transmission [33,62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fresenko, E.E.; Ma, J.-X.; Giegengack, M.; Thompson, A.C.; Atala, A.; Huang, A.J.W.; Zhang, Y. Stem Cell-Derived Corneal Epithelium: Engineering Barrier Function for Ocular Surface Repair. Int. J. Mol. Sci. 2025, 26, 7501. https://doi.org/10.3390/ijms26157501
Fresenko EE, Ma J-X, Giegengack M, Thompson AC, Atala A, Huang AJW, Zhang Y. Stem Cell-Derived Corneal Epithelium: Engineering Barrier Function for Ocular Surface Repair. International Journal of Molecular Sciences. 2025; 26(15):7501. https://doi.org/10.3390/ijms26157501
Chicago/Turabian StyleFresenko, Emily Elizabeth, Jian-Xing Ma, Matthew Giegengack, Atalie Carina Thompson, Anthony Atala, Andrew J. W. Huang, and Yuanyuan Zhang. 2025. "Stem Cell-Derived Corneal Epithelium: Engineering Barrier Function for Ocular Surface Repair" International Journal of Molecular Sciences 26, no. 15: 7501. https://doi.org/10.3390/ijms26157501
APA StyleFresenko, E. E., Ma, J.-X., Giegengack, M., Thompson, A. C., Atala, A., Huang, A. J. W., & Zhang, Y. (2025). Stem Cell-Derived Corneal Epithelium: Engineering Barrier Function for Ocular Surface Repair. International Journal of Molecular Sciences, 26(15), 7501. https://doi.org/10.3390/ijms26157501









