Effects of ε-Poly-L-Lysine/Chitosan Composite Coating on the Storage Quality, Reactive Oxygen Species Metabolism, and Membrane Lipid Metabolism of Tremella fuciformis
Abstract
1. Introduction
2. Results
2.1. Effect of ε-PL + CTS Composite Coating on the Quality and Physicochemical Characteristics of T. fuciformis After Harvesting
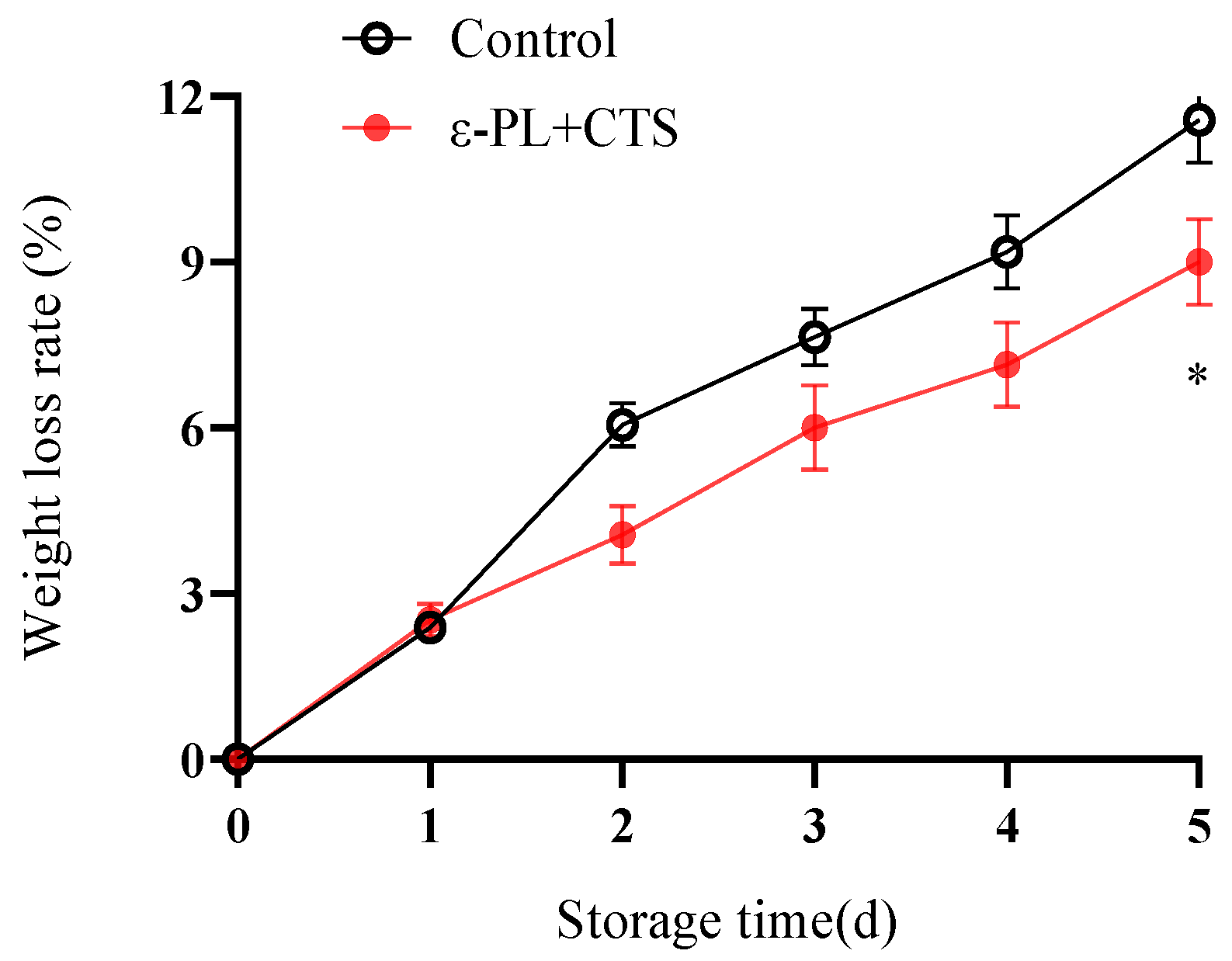
2.1.1. Weight Loss Rate
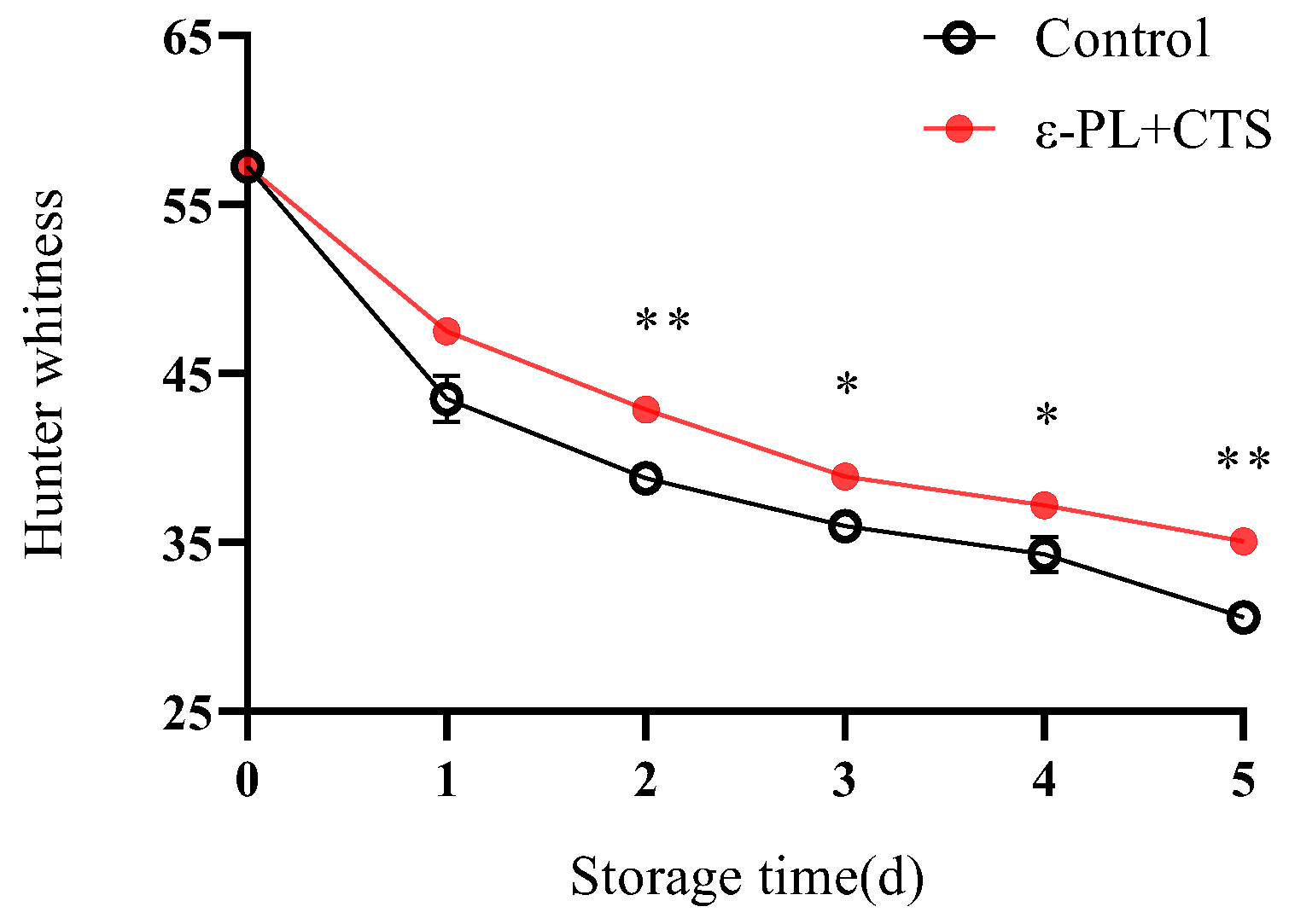
2.1.2. Hunter Whiteness
2.1.3. Browning (b* Value)
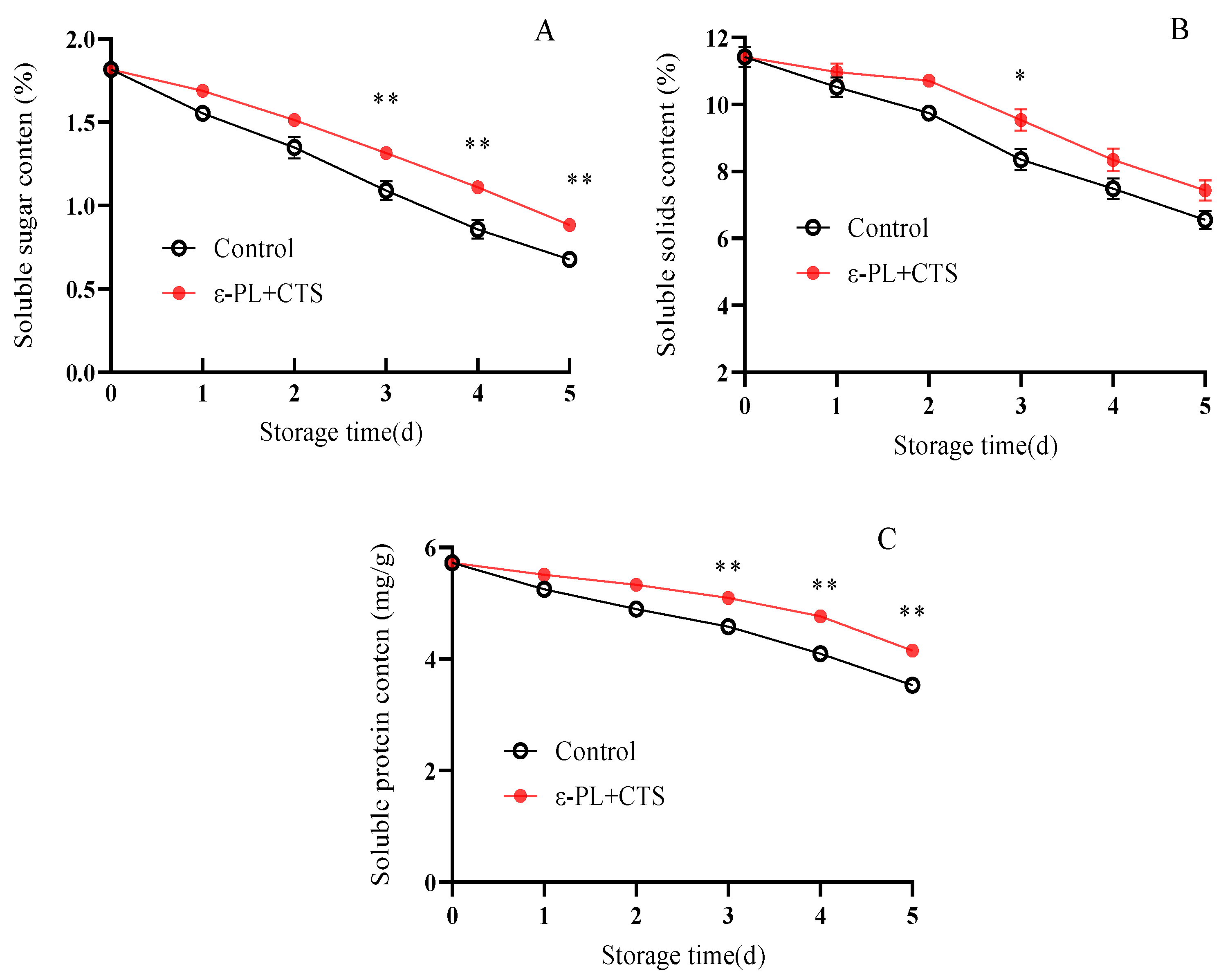
2.1.4. Changes in Soluble Sugars, Soluble Solids (TSSs), and Soluble Protein Content
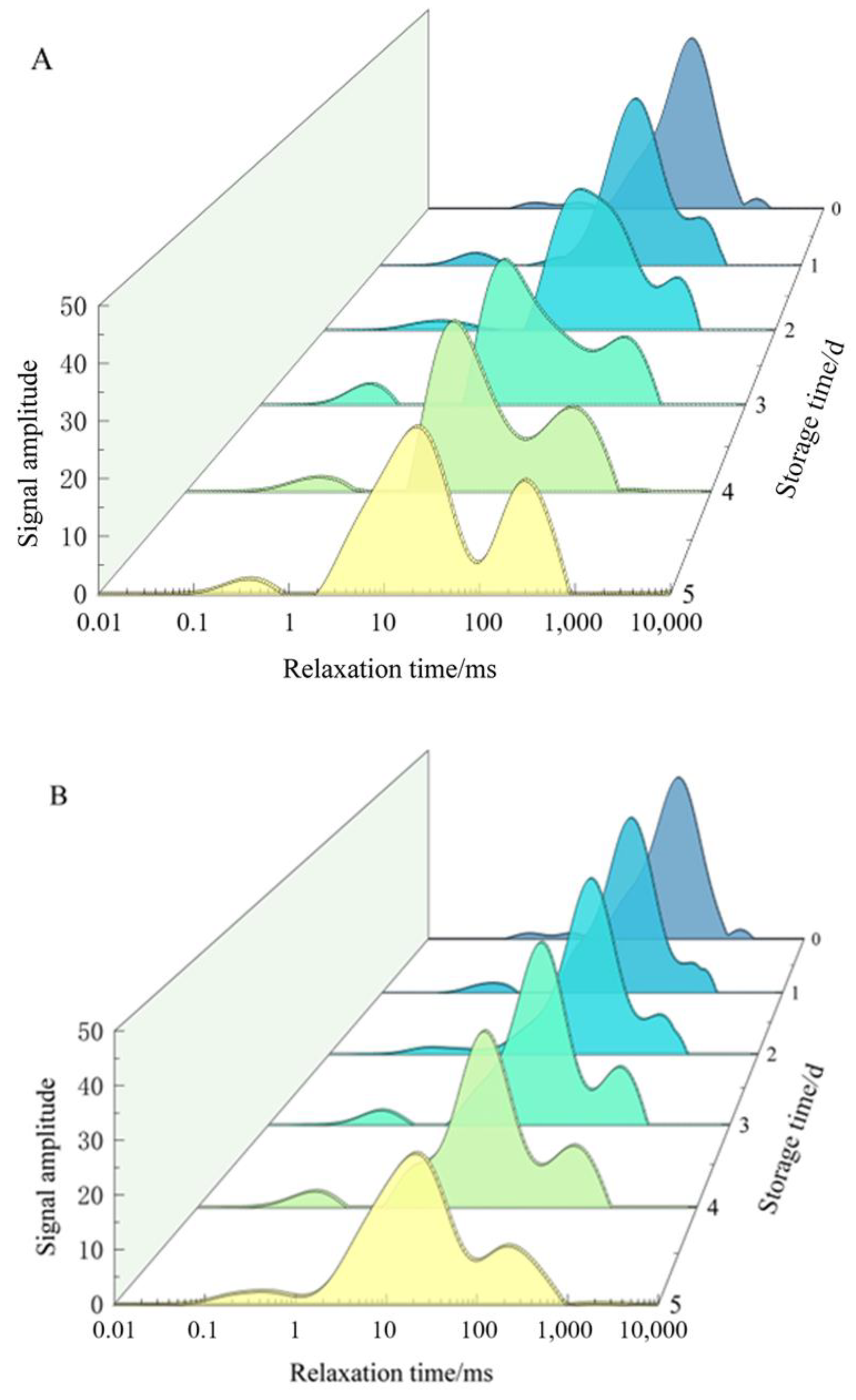
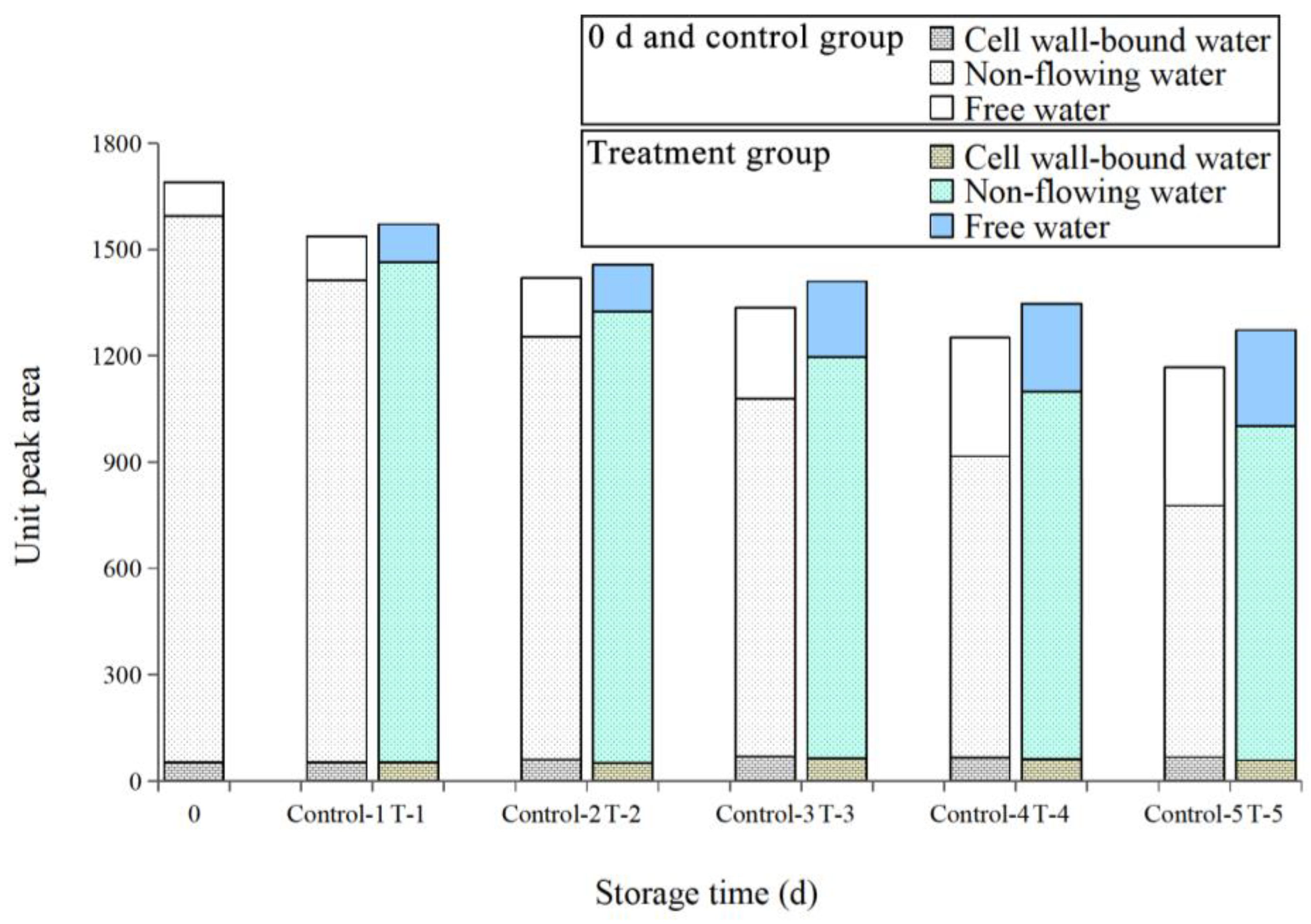
2.1.5. Moisture Migration
Effect of ε-PL + CTS Composite Coating on the Lateral Relaxation Behavior of T. fuciformis Hydrogen Protons
Effect of ε-PL + CTS Composite Coating on T. fuciformis Relaxation Time
Effect of ε-PL + CTS Composite Coating on Unit Peak Area of T. fuciformis
2.1.6. Bongkrekic Acid
2.2. Effect of ε-PL + CTS Composite Coating on the Metabolism of Reactive Oxygen Species of T. fuciformis After Harvesting
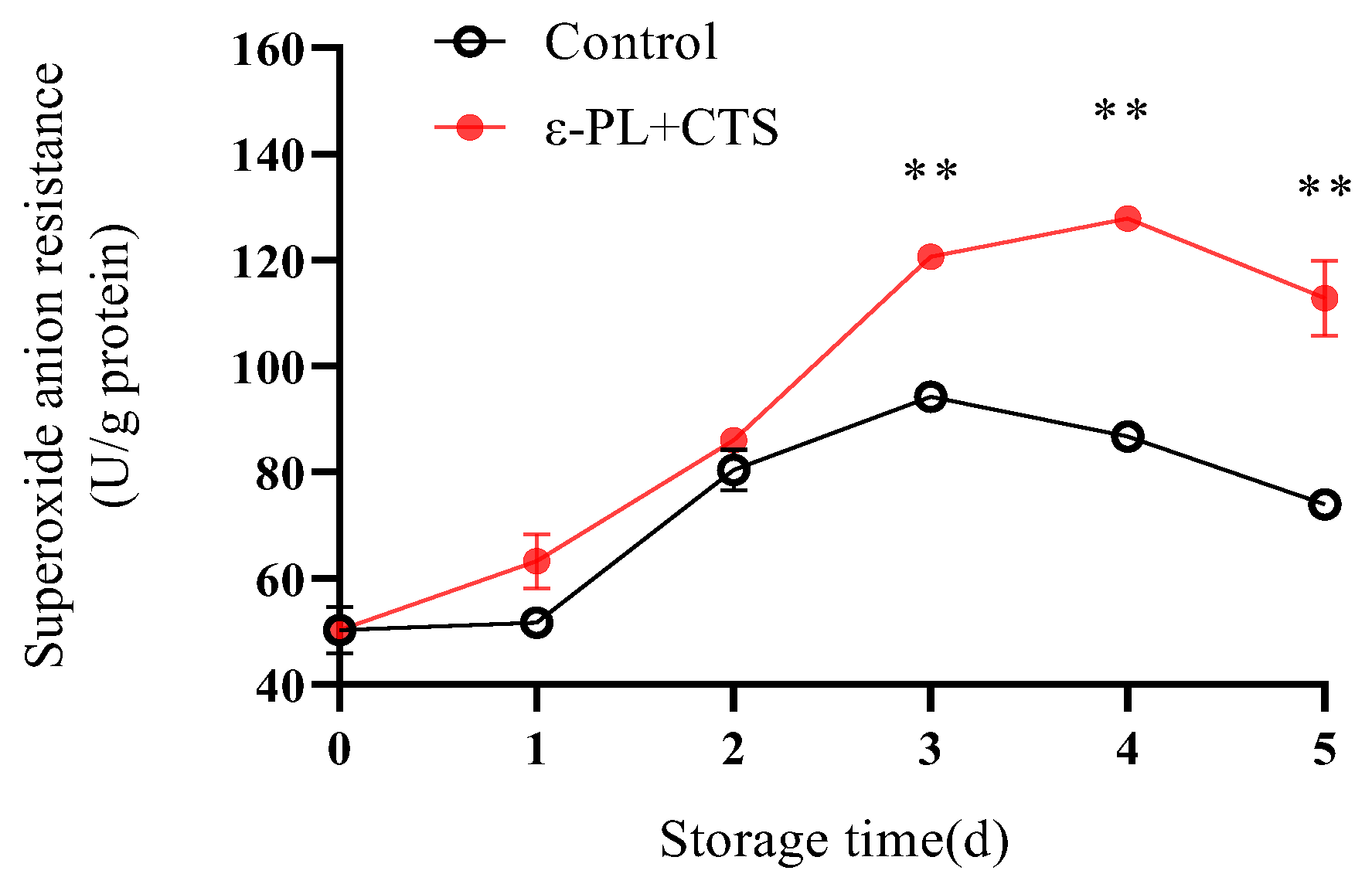
2.2.1. Superoxide Anion (O2−) Resistance Capacity
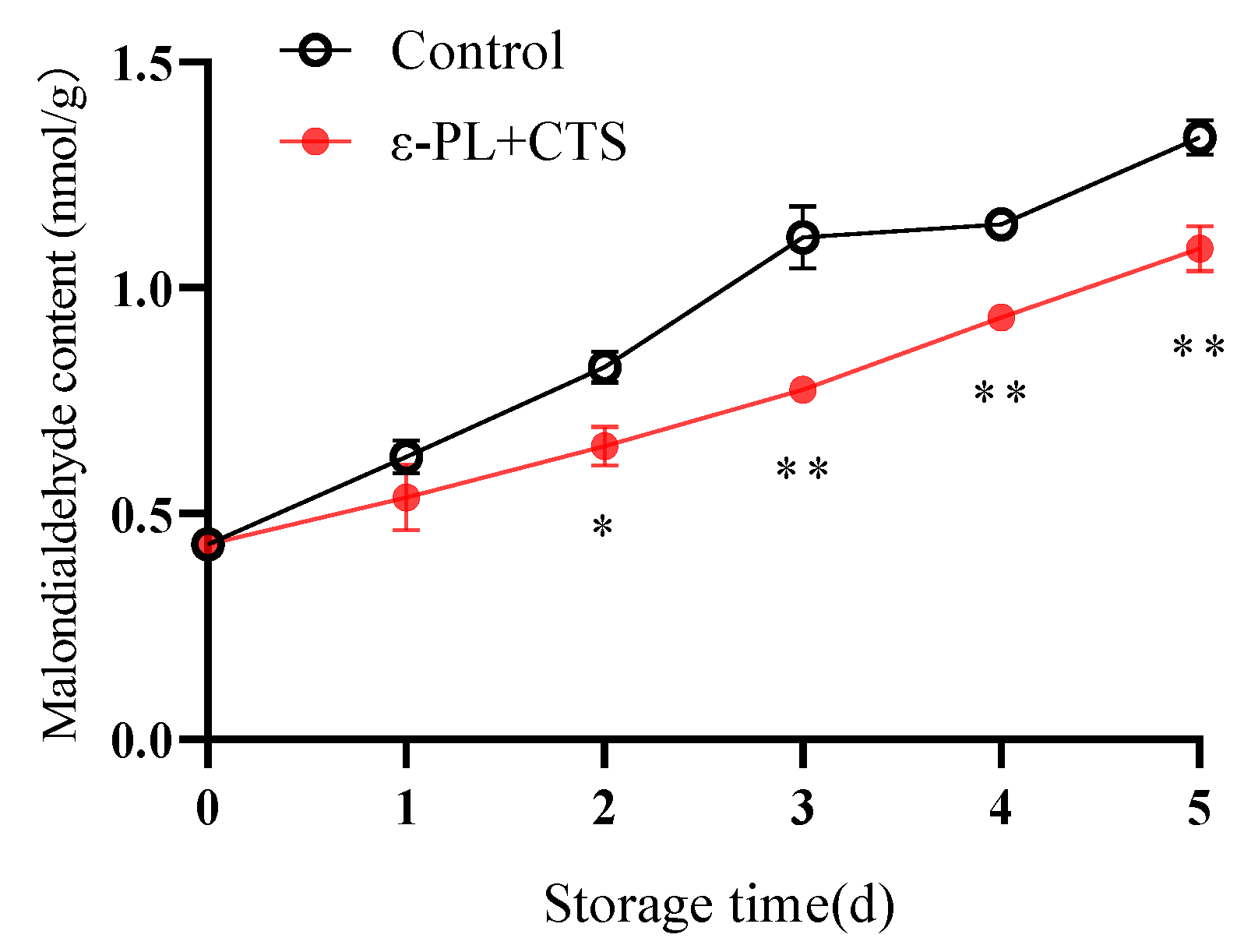
2.2.2. Malondialdehyde (MDA) Content
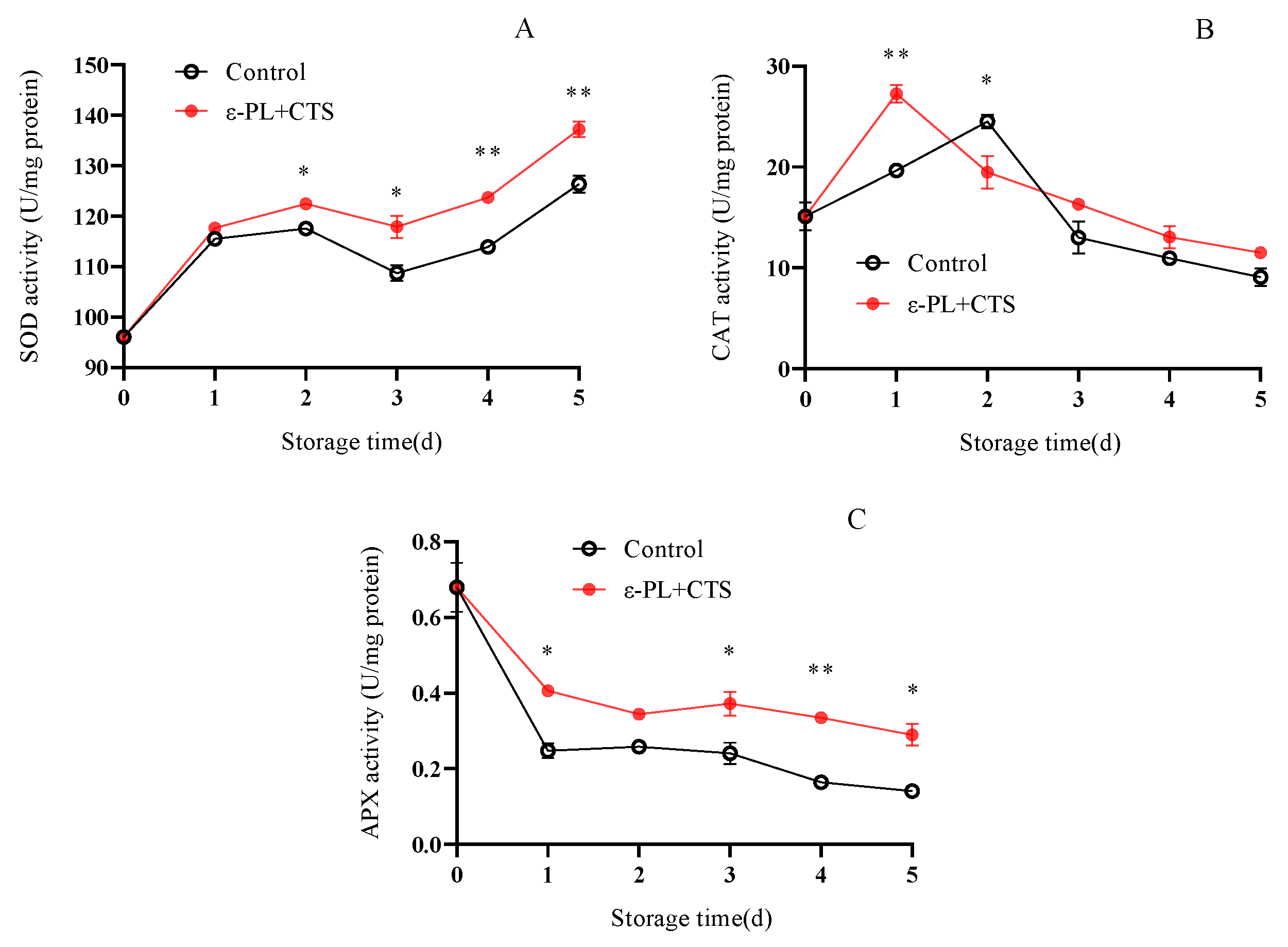
2.2.3. Reactive Oxygen Scavenging Enzyme (SOD, CAT, APX) Activity
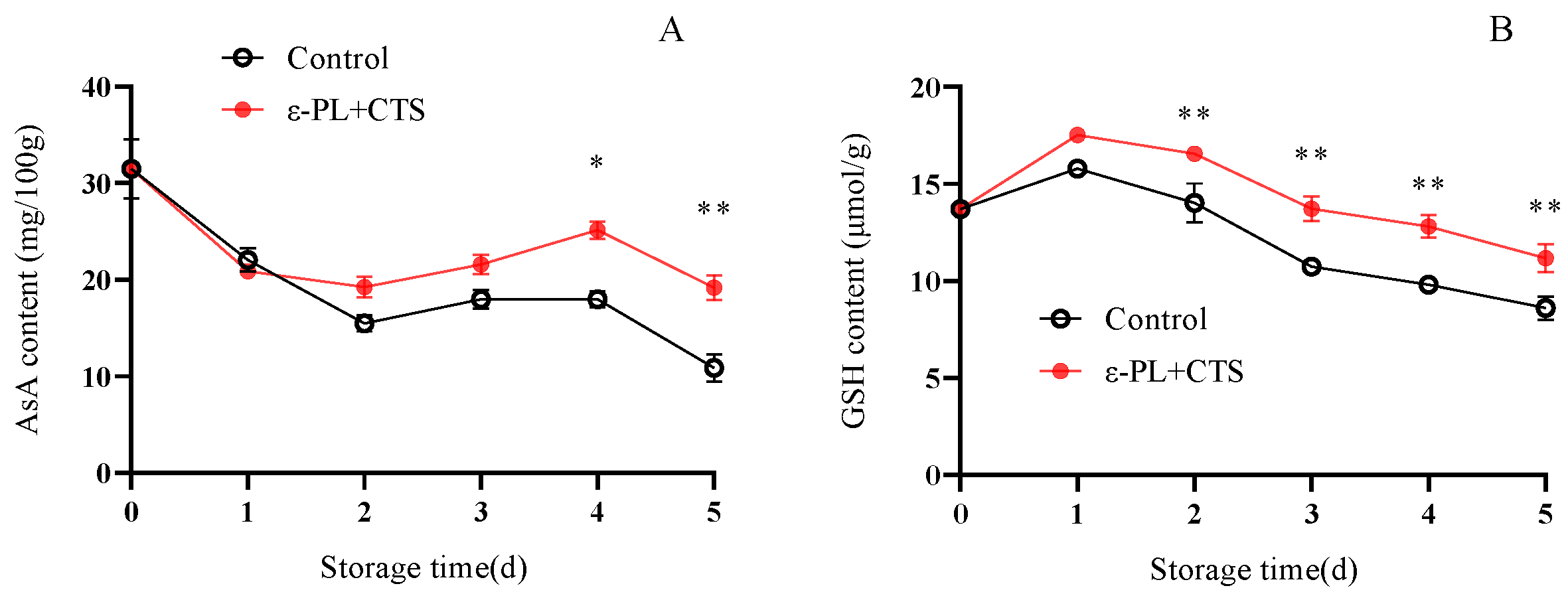
2.2.4. Reactive Oxygen Non-Enzymatic Scavengers (AsA and GSH) Content
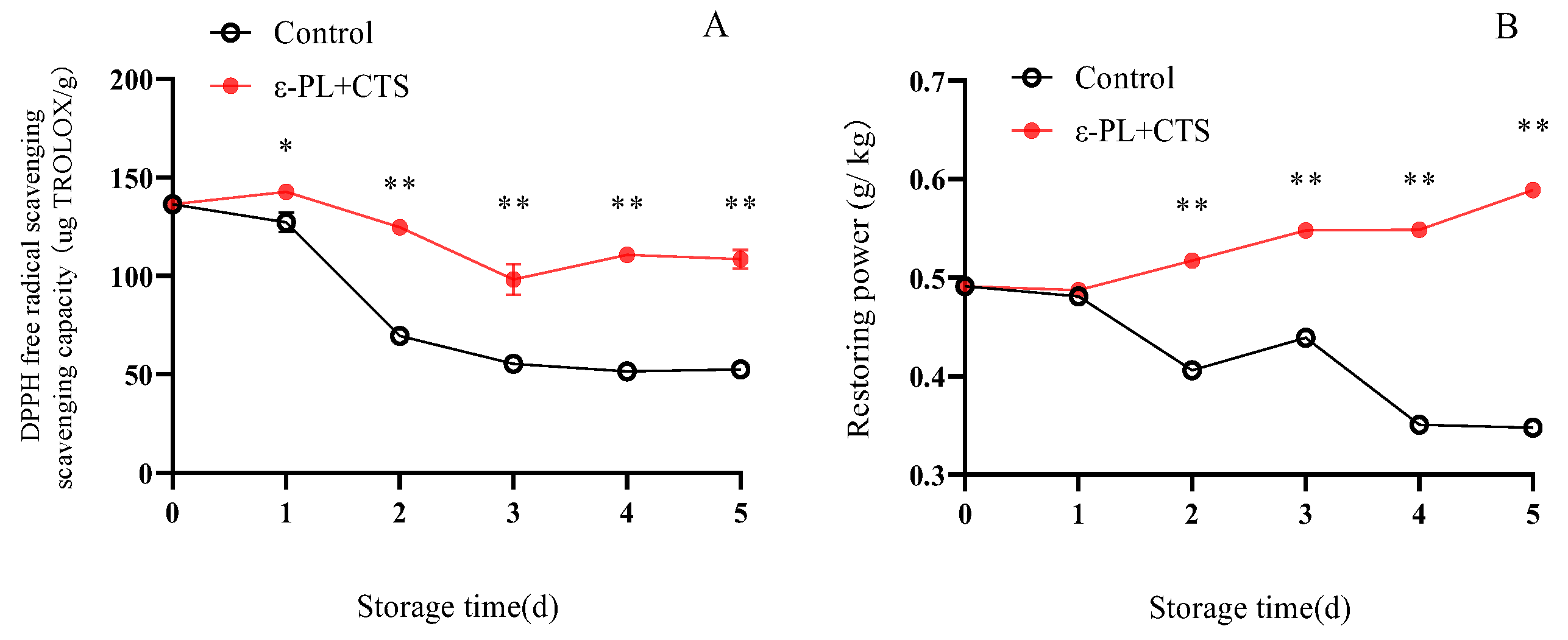
2.2.5. DPPH Radical Scavenging and Reducing Power
2.3. Effect of ε-PL + CTS Composite Coating on Lipid Metabolism of Post-Harvest T. fuciformis Membranes
2.3.1. Cell Membrane Permeability
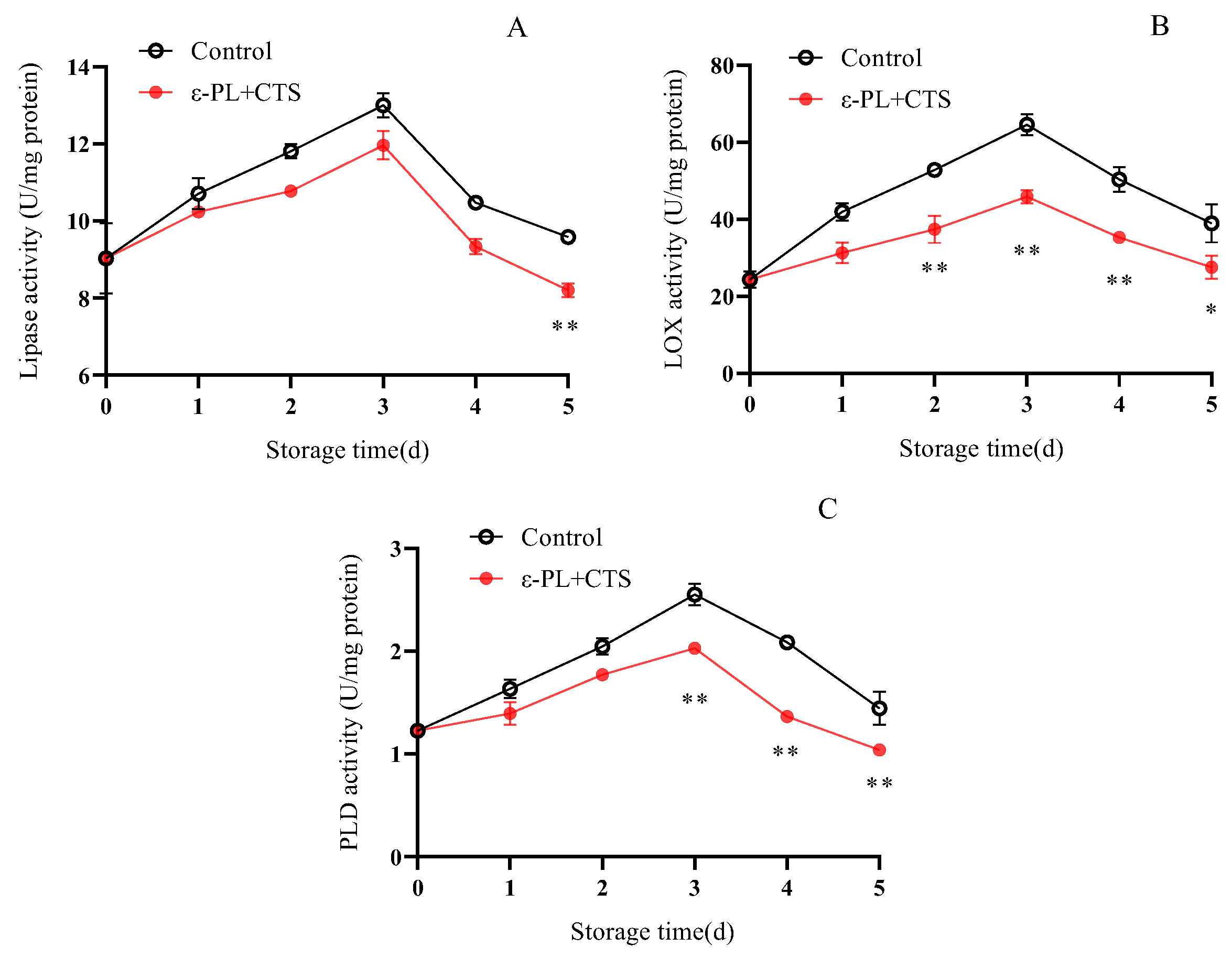
2.3.2. Membrane Lipid Degradation-Related Enzyme (Lipase, LOX, Phospholipase PLD) Activities
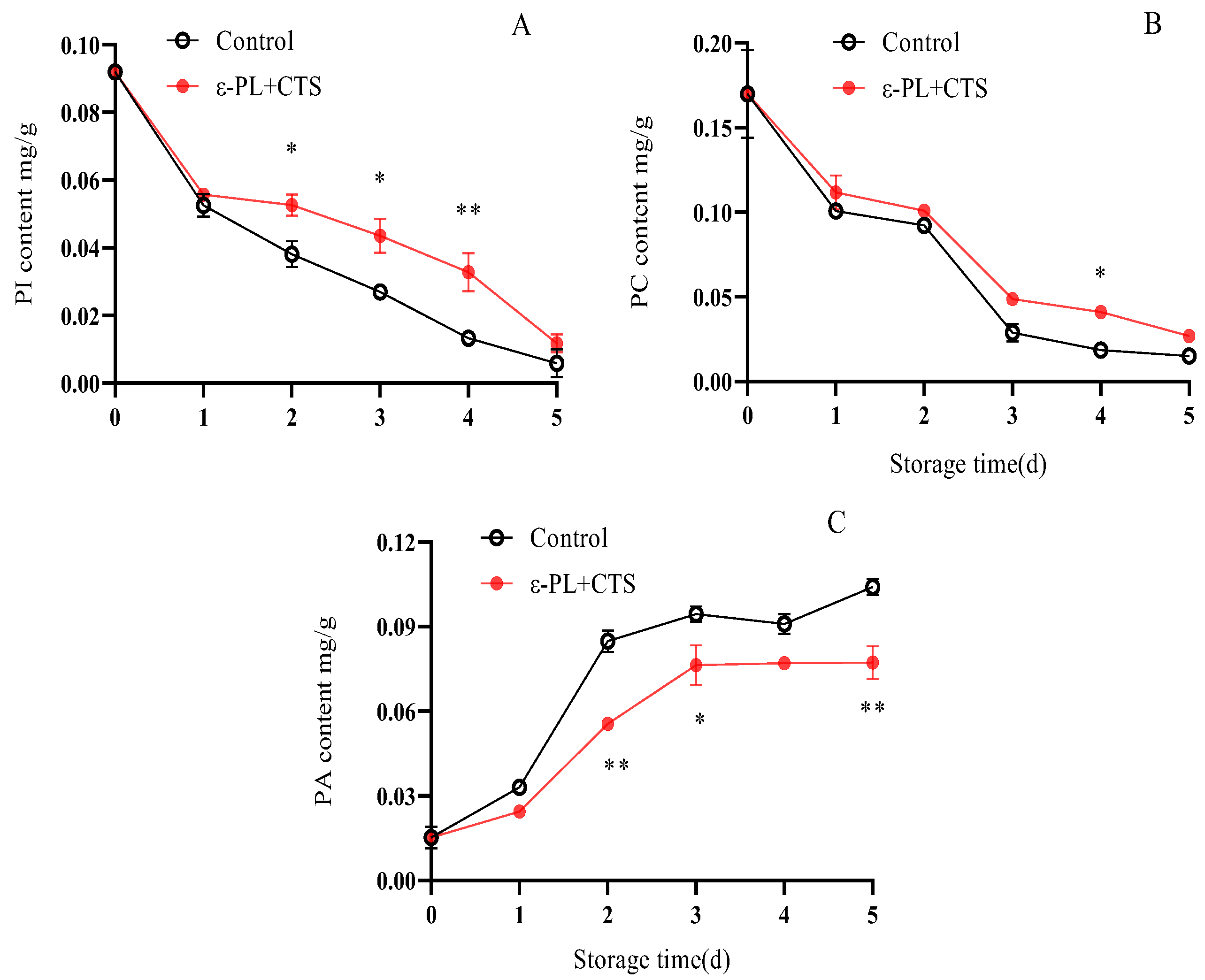
2.3.3. Phospholipid Fraction Content
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Instruments and Equipment
4.3. Determination of Optimal Coating Concentration
4.4. Formal Trials for This Study
4.5. Determination of Physicochemical Properties and Physiological Parameters
4.5.1. Measurement of Weight Loss Rate
4.5.2. Measurement of Hunter Whiteness
4.5.3. Measurement of Browning Condition (b* Value)
4.5.4. Determination of Soluble Sugars, Soluble Solids, and Soluble Protein Content
Determination of Soluble Sugar Content
Determination of Soluble Solids
Determination of Soluble Protein Content
4.5.5. Determination of Water Status by LF-NMR
4.5.6. Determination of Bongkrekic Acid
4.5.7. Determination of Superoxide Anion (O2−) Resistance Capacity
4.5.8. Determination of Malondialdehyde (MDA) Content
4.5.9. Determination of Reactive Oxygen Scavenging Enzymes (SOD, CAT, APX)
Determination of Superoxide Dismutase (SOD) Activity
Determination of Catalase (CAT) Activity
Determination of Ascorbate Peroxidase (APX) Activity
4.5.10. Determination of Reactive Oxygen Non-Enzymatic Scavenging Substances (AsA, GSH)
Determination of Reduced Ascorbic Acid (AsA) Content
Determination of Reduced Glutathione (GSH) Content
4.5.11. Determination of 2,2-Diphenyl-1-Trinitrohydrazine (DPPH) Radical Scavenging Activity and Reducing Power
4.5.12. Determination of Cell Membrane Permeability
4.5.13. Determination of Membrane Lipid Degrading Enzyme Activities
Determination of Lipase Activity
Determination of Lipoxygenase (LOX) Activity
Phospholipase D (PLD) Activity Assay
4.5.14. Determination of Phospholipid Composition
4.5.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiang, Y.; He, M.; Zhang, S.; Su, Y.; Lin, S.; Guo, Z.; Zeng, S.; Zheng, B. Pressure-controlled steam explosion as pretreatment for efficient extraction of T. fuciformis polysaccharide: Structure and bioactivity. Int. J. Biol. Macromol. 2024, 280 Pt 2, 135766. [Google Scholar] [CrossRef]
- Deng, W.; Wu, L.; Xiao, Z.; Li, Y.; Zheng, Z.; Chen, S. Structural characterization and anti-inflammatory activity of polysaccharides from T. fuciformis on monosodium urate-stimulated RAW264.7 macrophages. Foods 2023, 12, 4398. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Sun, T.; Liu, H.; Wang, D. The effects of T. fuciformis polysaccharide on the physicochemical, multiscale structure and digestive properties of Cyperus esculentus starch. Foods 2024, 13, 1425. [Google Scholar] [CrossRef]
- Lin, Y.; Lai, D.; Wang, D.; Zhou, F.; Tan, B.K.; Zhang, Z.; Hu, J.; Lin, S. Application of curcumin-mediated antibacterial photodynamic technology for preservation of fresh T. fuciformis. LWT-Food Sci. Technol. 2021, 147, 111657. [Google Scholar] [CrossRef]
- Hu, J.; Liang, M.; Xian, Y.; Chen, R.; Wang, L.; Hou, X.; Wu, Y. Development and validation of a multianalyte method for quantification of aflatoxins and bongkrekic acid in rice and noodle products using PRiME-UHPLC-MS/MS method. Food Chem. 2022, 395, 133598. [Google Scholar] [CrossRef]
- Zhong, Y.; Dong, S.; Cui, Y.; Dong, X.; Xu, H.; Li, M. Recent advances in postharvest irradiation preservation technology of edible fungi: A review. Foods 2022, 12, 103. [Google Scholar] [CrossRef]
- NeysariFam, V.; Golmohammadi, A.; Tabatabaekoloor, R.; Tahmasebi, M.; Nematollahzadeh, A.; Razavi, M.S.; Nowacka, M.; Ghaani, M. Preservation of button mushrooms using modified atmosphere and bio-nanocomposite film: A combined approach. Appl. Food Res. 2025, 5, 101043. [Google Scholar] [CrossRef]
- Jin, W.; Su, F.; Zhou, H.; Jiang, Q.; Li, Y.; Zhao, H.; Luo, T. Effects of chlorine dioxide on the postharvest storage quality of fresh-cut button mushrooms (Agaricus bisporus). Horticulturae 2024, 10, 1155. [Google Scholar] [CrossRef]
- Yan, X.; Meng, F.; Van, T.T.; Tanaka, F. Impact of chitosan, trans-cinnamaldehyde, poly (vinyl alcohol) and TiO2 bio-nanocomposites on preservation and flavor of postharvest button mushroom (Agaricus bisporus). LWT-Food Sci. Technol. 2025, 217, 117377. [Google Scholar] [CrossRef]
- Subrahmanyam, K.; Gul, K.; Sehrawat, R.; Allai, F.M. Impact of in-package cold plasma treatment on the physicochemical properties and shelf life of button mushrooms (Agaricus bisporus). Food Biosci. 2023, 52, 102425. [Google Scholar] [CrossRef]
- Liu, F.; Xu, Y.; Zeng, M.; Zhang, Y.; Pan, L.; Wang, J.; Huang, S. A novel physical hurdle technology by combining low voltage electrostatic field and modified atmosphere packaging for long-term stored button mushrooms (Agaricus bisporus). Innov. Food Sci. Emerg. Technol. 2023, 90, 103514. [Google Scholar] [CrossRef]
- Qiu, J.; Yang, H.; Zhang, Y.; Xiao, Y.; Wang, L.; Peng, Y.; Yu, X.; Huang, X.; Zhong, T. Emerging trends in the application of riboflavin-mediated photodynamic inactivation for food preservation. Trends Food Sci. Technol. 2024, 143, 104295. [Google Scholar] [CrossRef]
- Qiu, D.; Zhou, J.; Feng, Q.; Ren, K.; Zhang, H.; He, Y.; Li, C.; Liu, J.; Mai, N.T.T. Functionality, physicochemical properties, and applications of chitosan/nano-hydroxyapatite–tea polyphenol films. Food Chem. X 2024, 24, 101762. [Google Scholar] [CrossRef]
- Xiong, Y.; Kamboj, M.; Ajlouni, S.; Fang, Z. Incorporation of salmon bone gelatine with chitosan, gallic acid and clove oil as edible coating for the cold storage of fresh salmon fillet. Food Control 2021, 125, 107994. [Google Scholar] [CrossRef]
- Bahmani, R.; Razavi, F.; Mortazavi, S.N.; Gohari, G.; Juárez-Maldonado, A. Enhancing postharvest quality and shelf life of strawberries through advanced coating technologies: A comprehensive investigation of chitosan and glycine betaine nanoparticle treatments. Plants 2024, 13, 1136. [Google Scholar] [CrossRef]
- Gui, N.; Zhang, X.; Yang, C.; Ran, R.; Yang, C.; Zeng, X.; Li, G. A high-strength collagen-based antimicrobial film grafted with ε-polylysine fabrication by riboflavin-mediated ultraviolet irradiation for pork preservation. Food Chem. 2024, 461, 140889. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Han, C.; Chen, Q.; Sun, F.; Fu, M.; Zhao, H.; Xiao, Z.; Tang, Z. ε-Polylysine treatment maintains sensory quality, texture, flavor, and inhibits aerobic bacteria growth in vacuum-packed fresh-cut lettuce. Postharvest Biol. Technol. 2024, 214, 113011. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X. Changes in color, antioxidant, and free radical scavenging enzyme activity of mushrooms under high oxygen modified atmospheres. Postharvest Biol. Technol. 2012, 69, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, Y.; Li, D.; Xiang, C.; Jiang, Z.; Wang, J. The main factors inducing postharvest lignification in king oyster mushrooms (Pleurotus eryngii): Wounding and ROS-mediated senescence. Food Chem. 2019, 301, 125224. [Google Scholar] [CrossRef]
- Wang, X.; Huang, X.; Zhang, F.; Hou, F.; Yi, F.; Sun, X.; Yang, Q.; Han, X.; Liu, Z. Characterization of chitosan/zein composite film combined with tea polyphenol and its application on postharvest quality improvement of mushroom (Lyophyllum decastes Sing.). Food Packag. Shelf Life 2022, 33, 100869. [Google Scholar] [CrossRef]
- Asheri, M.; Farokhzad, A.; Naghavi, M.R.; Ghasemzadeh, R.; Azadi, P.; Zargar, M. Methyl jasmonate effects on Lactuca serriola L.: Antioxidant defense and bioactive compound changes. Cell. Mol. Biol. 2024, 70, 170–175. [Google Scholar] [CrossRef]
- Ma, J.; Yu, W.; Li, S.; Zheng, B.; Liu, Y.; Jiang, J.; Kou, X.; Xue, Z. Transcriptome analysis reveals the mechanism of polyethylene atmosphere packaging delaying browning of postharvest Pleurotus eryngii. Sci. Hortic. 2024, 323, 112531. [Google Scholar] [CrossRef]
- Yu, Z.; Kang, W.; Zhang, Z.; Yang, Z.; Jiang, Y.; Pan, Y.; Yang, J. Tert-Butylhydroquinone alleviates postharvest pericarp browning of longan fruit by regulating antioxidant metabolism. J. Food Sci. 2024, 89, 7577–7588. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Cui, J.; Zhu, S. Nitric oxide made a major contribution to the improvement of quality in button mushrooms (Agaricus bisporus) by the combined treatment of nitric oxide with 1-MCP. Foods 2022, 11, 3147. [Google Scholar] [CrossRef]
- Sudha, G.; Vadivukkarasi, S.; Shree, R.B.I.; Lakshmanan, P. Antioxidant activity of various extracts from an edible mushroom Pleurotus eous. Food Sci. Biotechnol. 2012, 21, 661–668. [Google Scholar] [CrossRef]
- Dokhanieh, A.Y.; Aghdam, M.S. Postharvest browning alleviation of Agaricus bisporus using salicylic acid treatment. Sci. Hortic. 2016, 207, 146–151. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Du, M.; Zhang, X.; Tian, Y.; Wang, J. 1-Methylcyclopropene (1-MCP) retards the senescence of Pteridium aquilinum var. latiusculum by regulating the cellular energy status and membrane lipid metabolism. Food Sci. Nutr. 2021, 9, 4349–4363. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, J.; Lin, H.; Lin, M.; Lin, Y.; Zheng, Y.; Lin, Y. Membrane lipids metabolism participates in the pulp breakdown of fresh longan caused by Phomopsis longanae Chi. Postharvest Biol. Technol. 2022, 193, 112049. [Google Scholar] [CrossRef]
- Ma, N.; Wang, C.; Pei, F.; Han, P.; Su, A.; Ma, G.; Kimatu, B.M.; Hu, Q.; Fang, D. Polyethylene-based packaging material loaded with nano-Ag/TiO2 delays quality deterioration of Agaricus bisporus via membrane lipid metabolism regulation. Postharvest Biol. Technol. 2022, 183, 111747. [Google Scholar] [CrossRef]
- Wang, T.; Hu, M.; Yuan, D.; Yun, Z.; Gao, Z.; Su, Z.; Zhang, Z. Melatonin alleviates pericarp browning in litchi fruit by regulating membrane lipid and energy metabolisms. Postharvest Biol. Technol. 2020, 160, 111066. [Google Scholar] [CrossRef]
- Huang, Q.; Qian, X.; Jiang, T.; Zheng, X. Effect of chitosan and guar gum based composite edible coating on quality of mushroom (Lentinus edodes) during postharvest storage. Sci. Hortic. 2019, 253, 382–389. [Google Scholar] [CrossRef]
- Sun, J.; Ren, R.; Yao, L.; Li, J.; Tong, L.; Yuan, J.; Wang, D. Effect of combined chitosan and hyperbranched poly-L-lysine based coating on prolonging the shelf life of oyster mushroom (Pleurotus ostreatus). Foods 2023, 13, 77. [Google Scholar] [CrossRef]
- Luo, S.; Sun, Y.; Yuan, X.; Pan, L.; Zheng, Z.; Zhao, Y.; Zhong, X. Infrared radiation blanching–inhibited browning and extended shelf life of pecan kernels. J. Food Sci. 2023, 88, 1566–1579. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Y.; Zhang, S.; Niu, J.; Xu, Z.; Yang, Y.; Lu, Y. Nanoselenium foliar application boosts antioxidant capacity and soluble solids in kiwifruit. Sci. Hortic. 2024, 337, 113521. [Google Scholar] [CrossRef]
- Ma, C.; Wan, Q.; Song, J.; Hao, T.; Xia, S.; Shen, S.; Li, K.; Xue, C.; Jiang, X. Ultrasound-assisted pH shift-induced interfacial remodeling for enhancing soluble yeast protein content: Effects on structure and interfacial properties of proteins under different treatment conditions. Food Hydrocoll. 2024, 149, 109521. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Nong, C.; Lin, T.; Fang, L.; Feng, X.; Chen, Y.; Lin, Y.; Lai, Z.; Miao, L. Piriformospora indica enhances resistance to Fusarium wilt in strawberry by increasing the activity of superoxide dismutase, peroxidase, and catalase, while reducing the content of malondialdehyde in the roots. Horticulturae 2024, 10, 240. [Google Scholar] [CrossRef]
- He, X.; Zhong, J.; Wei, R.; Li, H.; Li, J.; Ren, Y.; Zhai, X.; Hu, W.; Guan, W. Enhancement of quality and self-defense capacity of Agaricus bisporus by UV-C treatment. J. Sci. Food Agric. 2023, 104, 400–408. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, M.; Zhang, B.; Zhang, L.; Wang, D.; Sun, Y. Exogenous nitric oxide treatment delays the senescence of postharvest mung bean sprouts by regulating ascorbic acid metabolism. J. Sci. Food Agric. 2024, 105, 839–849. [Google Scholar] [CrossRef]
- Manda-Hakki, K.; Hassanpour, H. Effect of L-glutathione treatment on biochemical properties, antioxidant capacity and antioxidant enzymes activity in strawberry fruits during storage. Heliyon 2024, 10, e38046. [Google Scholar] [CrossRef]
- Yin, J.; Wang, K.; Zhang, H.; Zhao, Z.; Li, D.; Liu, D.; Xu, X.; Zhao, T. Analysis of the nutritional composition, biological activities, and phenolic metabolic bioactive module of cherry tomatoes. LWT-Food Sci. Technol. 2024, 209, 116762. [Google Scholar] [CrossRef]
- Yang, Q.; Hou, J.; Wang, F.; Qi, Y.; Zhao, Q. Cold shock treatment alleviates pitting in sweet cherry fruit by enhancing antioxidant enzyme activity and regulating membrane lipid metabolism. J. Sci. Food Agric. 2024, 105, 54–64. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, B.; Lin, Y.; Sang, Y.; Lin, M.; Fan, Z.; Chen, Y.; Wang, H.; Lin, H. Involvements of membrane lipid and phenolic metabolism in reducing browning and chilling injury of cold-stored Chinese olive by γ-aminobutyric acid treatment. Postharvest Biol. Technol. 2024, 209, 112664. [Google Scholar] [CrossRef]
- Hao, X.; Feng, Y.; Li, S.; Jiang, Y.; Lu, Y.; Zhou, Q.; Hao, Y. Regulation of plum fruit cracking process during cold storage period by treatment of preharvest chitosan. LWT-Food Sci. Technol. 2024, 203, 116399. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, P.; Bai, S.; Ma, N.; Fang, D.; Yang, W.; Hu, Q.; Pei, F. Caffeic acid-grafted-chitosan/polylactic acid film packaging enhances the postharvest quality of Agaricus bisporus by regulating membrane lipid metabolism. Food Res. Int. 2022, 158, 111557. [Google Scholar] [CrossRef]
- Yu, Z.; Tang, D.; Zhang, Z.; Jiang, Y.; Yang, J.; Pan, Y. Tert-Butylhydroquinone retards longan fruit deterioration by regulating membrane lipid and energy metabolisms. Food Chem. 2024, 457, 140041. [Google Scholar] [CrossRef]
- Li, L.; Yi, P.; Huang, F.; Tang, J.; Sun, J.; Duan, X.; Li, J.; Su, Z.; Ling, D.; Tang, Y.; et al. Effects of phospholipase D inhibitors treatment on membrane lipid metabolism of postharvest banana fruit in response to mechanical wounding stress. Horticulturae 2022, 8, 901. [Google Scholar] [CrossRef]
- Niu, B.; Fei, Y.; Liu, R.; Chen, H.; Fang, X.; Wu, W.; Mu, H.; Gao, H. Effect of oxyresveratrol on the quality and membrane lipid metabolism of shiitake mushroom (Lentinus edodes) during storage. Food Chem. 2023, 427, 136700. [Google Scholar] [CrossRef]


| Treatment | Storage Time /d | Cell Wall-Bound Water T21/ms | Non-Flowing Water T22/ms | Free Water T23/ms |
|---|---|---|---|---|
| Control | 0 | 0.767 ± 0.027 a | 61.720 ± 10.606 a | 414.227 ± 57.148 a |
| 1 | 0.341 ± 0.114 b | 41.604 ± 2.884 b | 383.673 ± 26.594 a | |
| 2 | 0.279 ± 0.067 b | 27.431 ± 1.902 c | 431.171 ± 74.092 a | |
| 3 | 0.254 ± 0.027 b | 17.941 ± 7.588 c | 333.933 ± 23.146 ab | |
| 4 | 0.322 ± 0.068 b | 10.777 ± 1.118 bc | 209.368 ± 42.986 bc | |
| 5 | 0.2945 ± 0.051 b | 8.709 ± 0.302 c | 126.038 ± 42.986 c | |
| ε-PL + CTS treatment | 0 | 0.767 ± 0.027 a | 61.720 ± 10.606 a | 414.227 ± 57.148 a |
| 1 | 0.391 ± 0.064 b | 57.960 ± 3.171 a | 398.673 ± 11.594 a | |
| 2 | 0.301 ± 0.021 b | 38.509 ± 9.177 ab | 360.527 ± 49.740 a | |
| 3 | 0.290 ± 0.010 b | 29.970 ± 6.153 b | 346.768 ± 35.981 a | |
| 4 | 0.303 ± 0.042 b | 22.703 ± 4.661 b | 301.813 ± 31.317 ab | |
| 5 | 0.389 ± 0.067 b | 17.690 ± 3.040 b | 212.273 ± 7.366 b |
| Treatment | Storage Time/d | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Control | ND | ND | ND | ND | ND | ND |
| ε-PL + CTS treatment | ND | ND | ND | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Wei, Y.; Li, L.; Yang, M.; Liu, Y.; Li, Q.; Zhou, S.; Lai, C.; Chen, J.; Lai, P. Effects of ε-Poly-L-Lysine/Chitosan Composite Coating on the Storage Quality, Reactive Oxygen Species Metabolism, and Membrane Lipid Metabolism of Tremella fuciformis. Int. J. Mol. Sci. 2025, 26, 7497. https://doi.org/10.3390/ijms26157497
Sun J, Wei Y, Li L, Yang M, Liu Y, Li Q, Zhou S, Lai C, Chen J, Lai P. Effects of ε-Poly-L-Lysine/Chitosan Composite Coating on the Storage Quality, Reactive Oxygen Species Metabolism, and Membrane Lipid Metabolism of Tremella fuciformis. International Journal of Molecular Sciences. 2025; 26(15):7497. https://doi.org/10.3390/ijms26157497
Chicago/Turabian StyleSun, Junzheng, Yingying Wei, Longxiang Li, Mengjie Yang, Yusha Liu, Qiting Li, Shaoxiong Zhou, Chunmei Lai, Junchen Chen, and Pufu Lai. 2025. "Effects of ε-Poly-L-Lysine/Chitosan Composite Coating on the Storage Quality, Reactive Oxygen Species Metabolism, and Membrane Lipid Metabolism of Tremella fuciformis" International Journal of Molecular Sciences 26, no. 15: 7497. https://doi.org/10.3390/ijms26157497
APA StyleSun, J., Wei, Y., Li, L., Yang, M., Liu, Y., Li, Q., Zhou, S., Lai, C., Chen, J., & Lai, P. (2025). Effects of ε-Poly-L-Lysine/Chitosan Composite Coating on the Storage Quality, Reactive Oxygen Species Metabolism, and Membrane Lipid Metabolism of Tremella fuciformis. International Journal of Molecular Sciences, 26(15), 7497. https://doi.org/10.3390/ijms26157497






