The Persistence of Cross-Reactive Immunity to Influenza B/Yamagata Neuraminidase Despite the Disappearance of the Lineage: Structural and Serological Evidence
Abstract
1. Introduction
2. Results
2.1. Results of Phylogenetic Analysis of Hemagglutinin and Neuraminidase of Influenza B Viruses
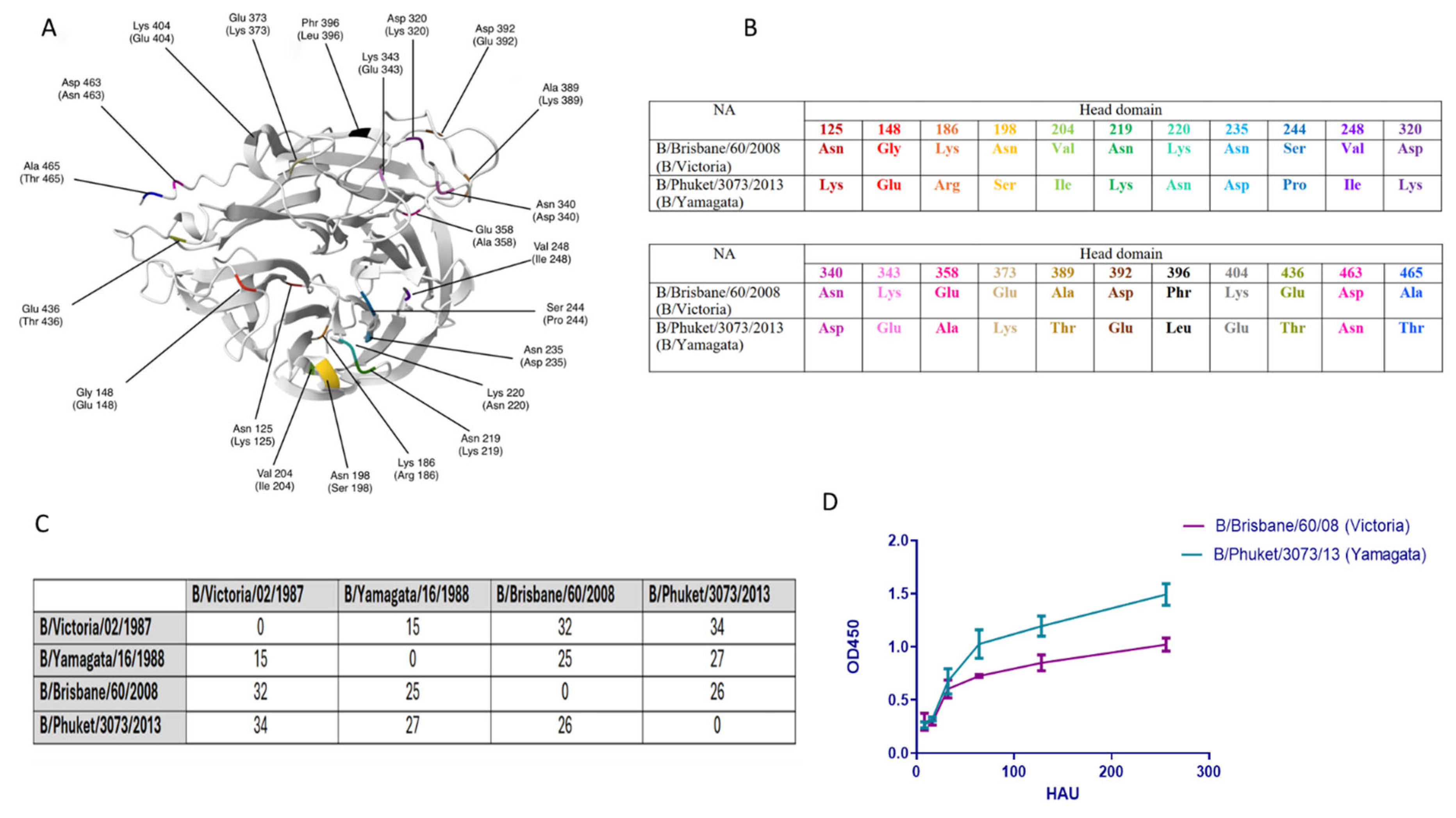
2.2. Structural Changes and Enzymatic Activity of Neuraminidase of Influenza Viruses B/Victoria and B/Yamagata
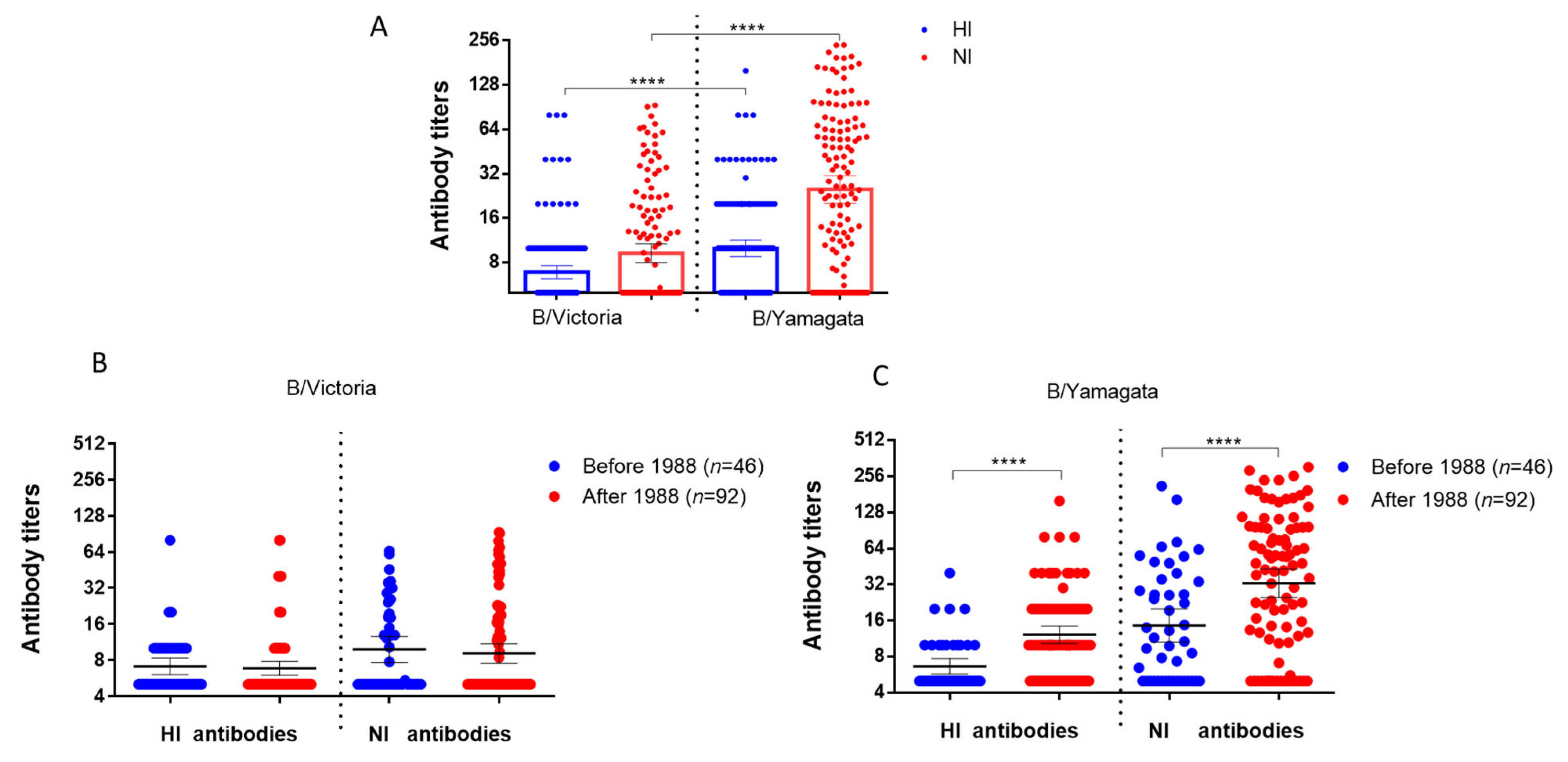
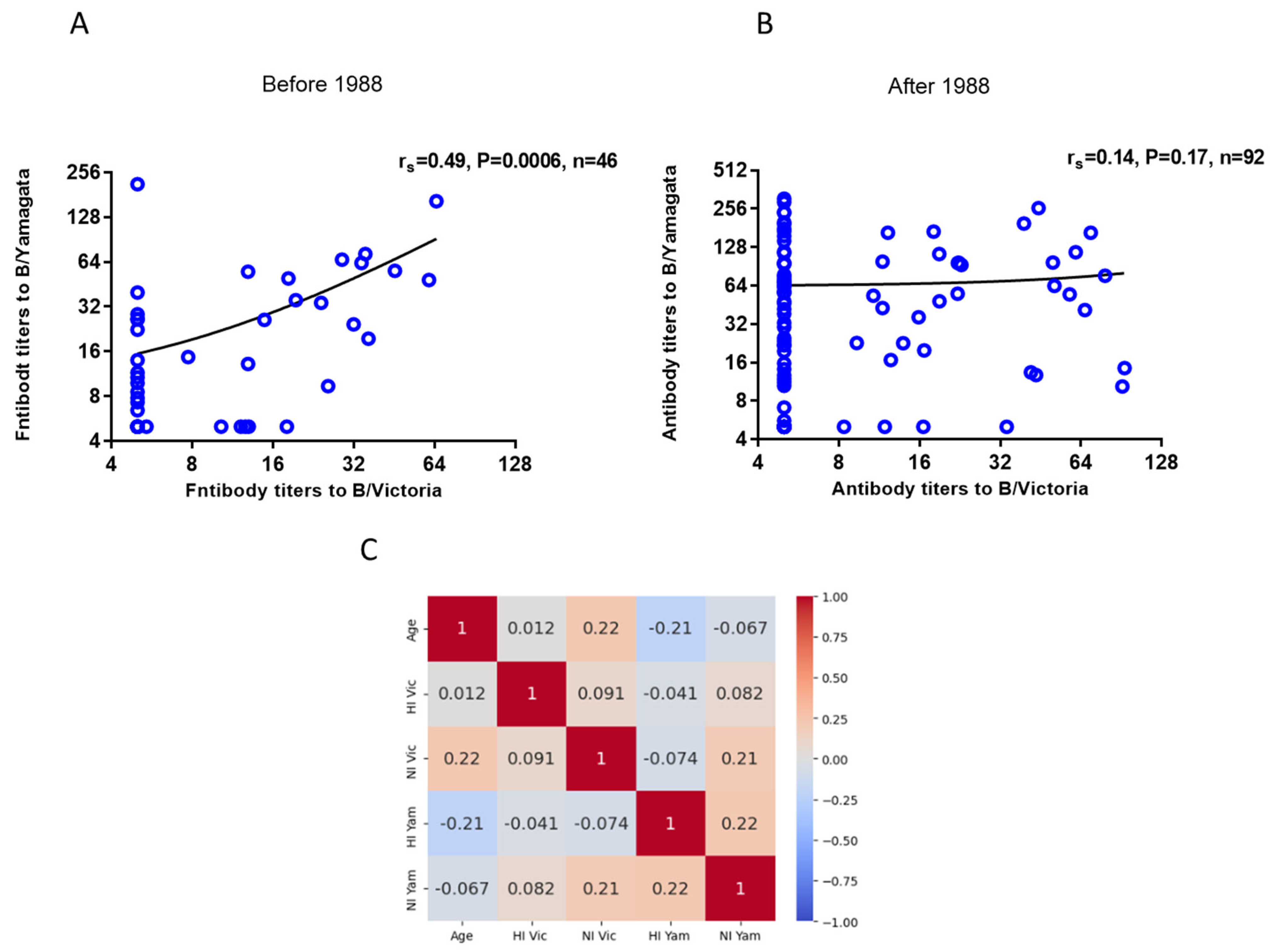
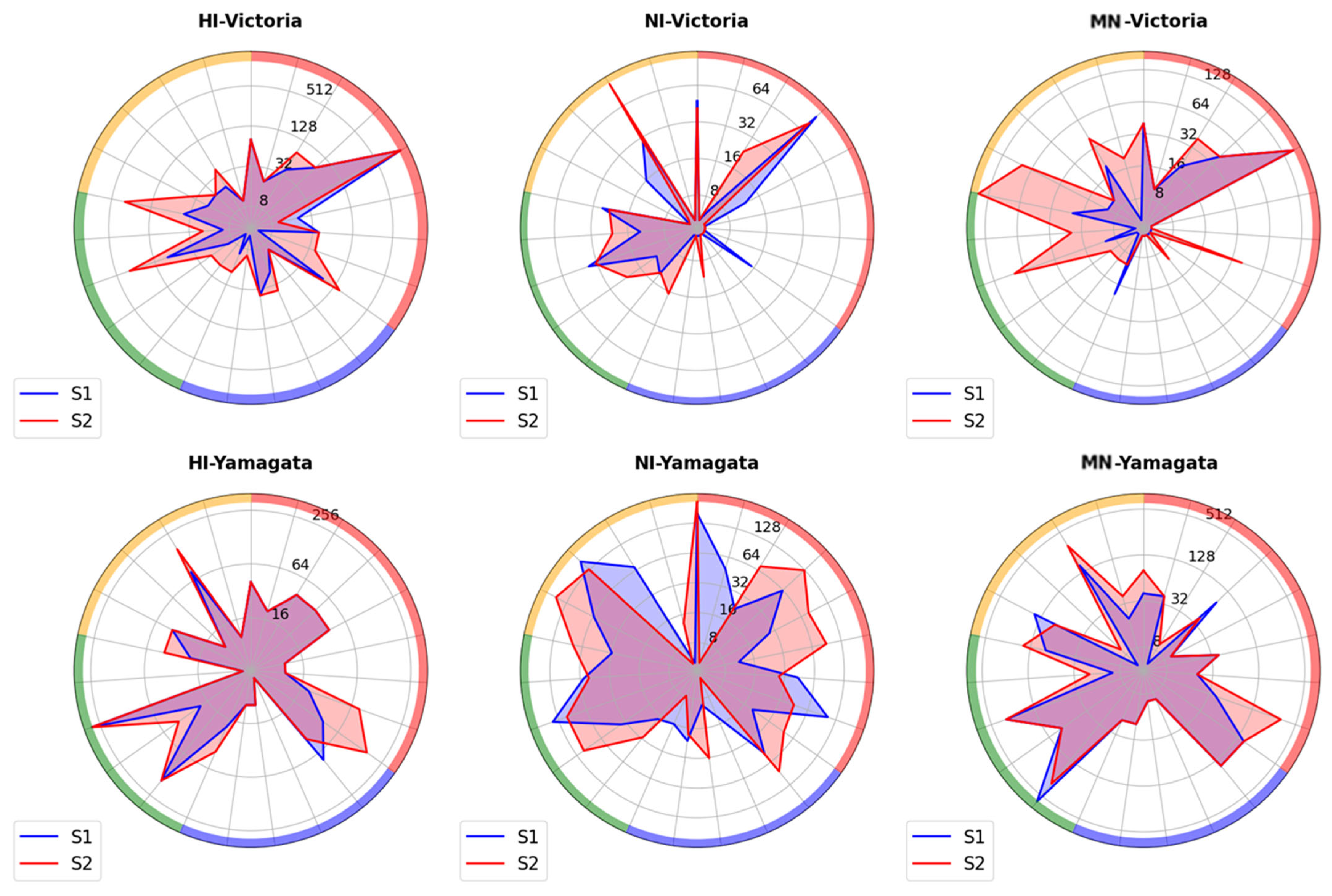
2.3. Investigating the Presence of Antibodies Against Influenza Viruses of the B/Victoria and B/Yamagata Lineages in Archived Blood Samples from Patients Who Were Tested in 2023
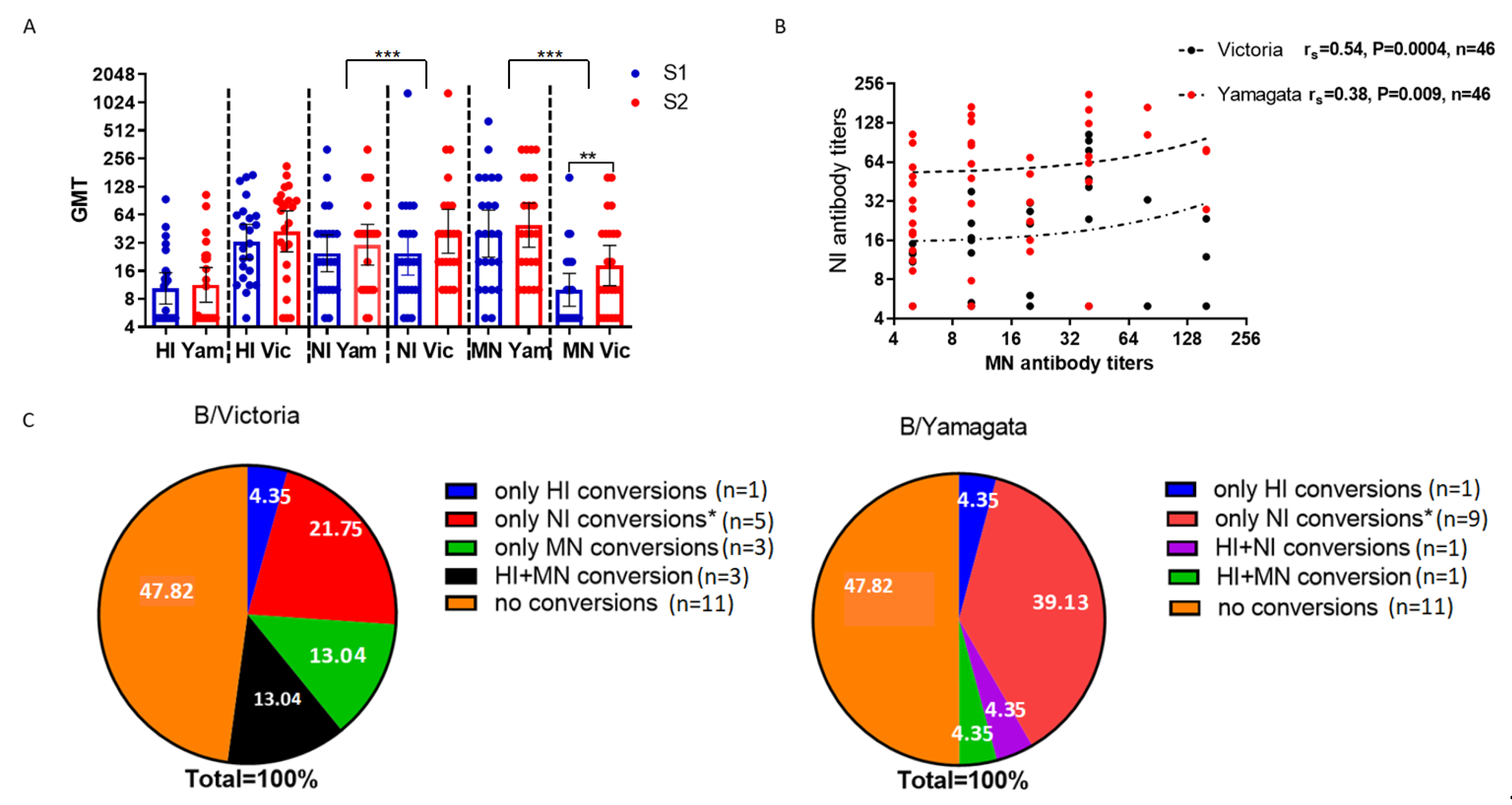
2.4. Study of Antibodies to Influenza B Viruses After Vaccination with Seasonal Influenza Vaccines
3. Discussion
4. Materials and Methods
4.1. In Silico Analysis
4.2. Cultivation of Viruses in Developing Chicken Embryos
4.3. Surveyed Contingents
4.4. Processing of Blood Serum
4.5. Setting up the Hemagglutination Inhibition Reaction (HI)
4.6. The Enzyme-Linked Lectin Assay (ELLA)
4.7. Microneutralization Test (MN)
4.8. Statistical Processing of Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EID50/mL | 50% embryonic infectious dose |
| ELLA | Enzyme-linked lectin assay |
| GMT | The geometric mean titers |
| HA | Hemagglutinin |
| IAV | Influenza A viruses |
| IBV | Influenza B viruses |
| HAU | Hemagglutinating units |
| IIV | Inactivated influenza vaccines |
| LAIV | Live influenza vaccine |
| NA | Neuraminidase |
| NI | Neuraminidase inhibiting |
| MN | Microneutralization |
| OD | Optical density |
| RDE | Receptor-destroying enzyme |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| TCID50 | 50% tissue culture infection dose |
References
- Caini, S.; Kusznierz, G.; Garate, V.V.; Wangchuk, S.; Thapa, B.; de Paula, F.J., Jr.; de Almeida, W.A.F.; Njouom, R.; Fasce, R.A.; Bustos, P.; et al. The epidemiological signature of influenza B virus and its B/Victoria and B/Yamagata lineages in the 21st century. PLoS ONE 2019, 14, e0222381. [Google Scholar] [CrossRef]
- Tsybalova, L.M.; Stepanova, L.A.; Ramsay, E.S.; Vasin, A.V. Influenza B: Prospects for the development of cross-protective vaccines. Viruses 2022, 14, 1323. [Google Scholar] [CrossRef]
- Lim, C.M.L.; Komarasamy, T.V.; Adnan, N.A.A.B.; Radhakrishnan, A.K.; Balasubramaniam, V.R.M.T. Recent advances, approaches and challenges in the development of universal influenza vaccines. Influenza Other Respir. Viruses 2024, 18, e13276. [Google Scholar] [CrossRef] [PubMed]
- Karakus, U.; Mena, I.; Kottur, J.; El Zahed, S.S.; Seoane, R.; Yildiz, S.; Chen, L.; Plancarte, M.; Lindsay, L.; Halpin, R.; et al. H19 influenza A virus exhibits species-specific MHC class II receptor usage. Cell Host Microbe 2024, 32, 1089–1102.e10. [Google Scholar] [CrossRef]
- Sharp, D.G.; Taylor, A.R.; McLean, I.W.; Beard, D.; Beard, J.W.; Feller, A.E.; Dingle, J.H. Isolation and Characterization of Influenza Virus B (Lee Strain). Science 1943, 98, 307–308. [Google Scholar] [CrossRef]
- Rota, P.A.; Wallis, T.R.; Harmon, M.W.; Rota, J.S.; Kendal, A.P.; Nerome, K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 1990, 175, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Nerome, R.; Hiromoto, Y.; Sugita, S.; Tanabe, N.; Ishida, M.; Matsumoto, M.; Lindstrom, S.E.; Takahashi, T.; Nerome, K. Evolutionary characteristics of influenza B virus since its first isolation in 1940: Dynamic circulation of deletion and insertion mechanism. Arch. Virol. 1998, 143, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Raza, M.A.; Amjad, M.N.; Din, G.U.; Yue, L.; Shen, B.; Chen, L.; Dong, W.; Xu, H.; Hu, Y. A comprehensive review of influenza B virus, its biological and clinical aspects. Front. Microbiol. 2024, 15, 1467029. [Google Scholar] [CrossRef]
- Ambrose, C.S.; Levin, M.J. The rationale for quadrivalent influenza vaccines. Hum. Vaccines Immunother. 2012, 8, 81–88. [Google Scholar] [CrossRef]
- Virk, R.K.; Jayakumar, J.; Mendenhall, I.H.; Moorthy, M.; Lam, P.; Linster, M.; Lim, J.; Lin, C.; Oon, L.L.E.; Lee, H.K.; et al. Divergent evolutionary trajectories of influenza B viruses underlie their contemporaneous epidemic activity. Proc. Natl. Acad. Sci. USA 2020, 117, 619–628. [Google Scholar] [CrossRef]
- Eichelberger, M.C.; Wan, H. Influenza neuraminidase as a vaccine antigen. Curr. Top. Microbiol. Immunol. 2015, 386, 275–299. [Google Scholar] [CrossRef]
- Johansson, B.E.; Brett, I.C. Recombinant influenza B virus HA and NA antigens administered in equivalent amounts are immunogenically equivalent and induce equivalent homotypic and broader heterovariant protection in mice than conventional and live influenza vaccines. Hum. Vaccines 2008, 4, 420–424. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Hamelin, M.E.; Janjua, N.Z.; De Serres, G.; Gardy, J.L.; Rhéaume, C.; Bouhy, X.; Boivin, G. Cross-lineage influenza B and heterologous influenza A antibody responses following quadrivalentvs trivalent influenza vaccination in a nested cohort study. J. Infect. Dis. 2015, 212, 1080–1091. [Google Scholar] [CrossRef]
- Wohlbold, T.J.; Nachbagauer, R.; Xu, H.; Tan, G.S.; Hirsh, A.; Brokstad, K.A.; Cox, R.J.; Palese, P.; Krammer, F.; Griffin, D.E. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. mBio 2015, 6, e02556-14. [Google Scholar] [CrossRef] [PubMed]
- Page, C.K.; Shepard, J.D.; Ray, S.D.; Ferguson, J.A.; Rodriguez, A.J.; Han, J.; Jacob, J.C.; Rowe-Haas, D.K.; Akinpelu, J.Y.; Friedman, L.M.; et al. Neuraminidase-specific antibodies drive differential cross-protection between contemporary FLUBV lineages. Sci. Adv. 2015, 11, eadu3344. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.; Dai, Y.-N.; McMahon, M.; Schmitz, A.J.; Turner, J.S.; Tan, J.; Lei, T.; Alsoussi, W.B.; Strohmeier, S.; Amor, M.; et al. Human antibodies targeting influenza B virus neuraminidase active site are broadly protective. Immunity 2020, 53, 852–863.e7. [Google Scholar] [CrossRef]
- Belshe, R.B. The need for quadrivalent vaccine against seasonal influenza. Vaccine 2010, 28, D45–D53. [Google Scholar] [CrossRef]
- Desheva, Y.A.; Smolonogina, T.A.; Doroshenko, E.M.; Rudenko, L.G. Development of the quadrivalent live attenuated influenza vaccine including two influenza B lineages–Victoria and Yamagata. Probl. Virol. 2016, 61, 16–20. [Google Scholar] [CrossRef]
- Do, T.H.T.; Wheatley, A.K.; Kent, S.J.; Koutsakos, M. Influenza B virus neuraminidase: A potential target for next-generation vaccines? Expert Rev. Vaccines 2024, 23, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, S.E.; Hiromoto, Y.; Nishimura, H.; Saito, T.; Nerome, R.; Nerome, K. Comparative analysis of evolutionary mechanisms of the hemagglutinin and three internal protein genes of influenza B virus: Multiple cocirculating lineages and frequent reassortment of the NP, M, and NS genes. J. Virol. 1999, 73, 4413–4426. [Google Scholar] [CrossRef]
- Reina, J. The Victoria and Yamagata lineages of influenza B viruses, unknown and undervalued. Rev. Esp. Quimioter. 2022, 35, 231–235. [Google Scholar] [CrossRef]
- Del Riccio, M.; Nunes, M.C.; Cowling, B.J.; Lina, B.; McCauley, J.W.; Meijer, A.; Nohynek, H.; Boudewijns, B.; Caini, S. Post-disappearance scenarios: Policy implications following the potential disappearance of B/Yamagata lineage influenza viruses. Eurosurveillance 2024, 29, 2400196. [Google Scholar] [CrossRef]
- Xu, Q.; Wei, H.; Wen, S.; Chen, J.; Lei, Y.; Cheng, Y.; Huang, W.; Wang, D.; Shu, Y. Factors affecting the immunogenicity of influenza vaccines in humans. BMC Infect. Dis. 2023, 23, 211. [Google Scholar] [CrossRef]
- Bragstad, K.; Paulsen, T.H.; Tønnessen, R.; Klüwer, B.; Rydland, K.; Aune, T.; Hungnes, O. Influenza Virological and Epidemiological Season Report Prepared for the WHO Consultation on the Composition of Influenza Virus Vaccines for the Southern Hemisphere 2022. Norwegian Institute of Public Health. 2021. Available online: https://www.nb.no/maken/item/URN:NBN:no-nb_pliktmonografi_000028523 (accessed on 2 July 2025).
- Rosu, M.E.; Lexmond, P.; Bestebroer, T.M.; Hauser, B.M.; Smith, D.J.; Herfst, S.; Fouchier, R.A.M. Substitutions near the HA receptor binding site explain the origin and major antigenic change of the B/Victoria and B/Yamagata lineages. Proc. Natl. Acad. Sci. USA 2022, 119, e2211616119. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.C.; Donato, C.M.; Arevalo, P.; Rimmelzwaan, G.F.; Wood, T.; Lopez, L.; Huang, Q.S.; Dhanasekaran, V.; Koelle, K.; Cobey, S. Lineage-specific protection and immune imprinting shape the age distributions of influenza B cases. Nat. Commun. 2021, 12, 4313. [Google Scholar] [CrossRef] [PubMed]
- Jumat, M.R.; Sugrue, R.J.; Tan, B.H. Genetic characterization of influenza B viruses detected in Singapore, 2004 to 2009. BMC Res. Notes 2014, 7, 863. [Google Scholar] [CrossRef]
- Burmeister, W.P.; Ruigrok, R.W.; Cusack, S. The 2.2 A resolution crystal structure of influenza B neuraminidase and its complex with sialic acid. EMBO J. 1992, 11, 49–56. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, H.-X.; Koutsakos, M.; Jegaskanda, S.; Esterbauer, R.; Tilmanis, D.; Aban, M.; Kedzierska, K.; Hurt, A.C.; Kent, S.J.; et al. Cross-lineage protection by human antibodies binding the influenza B hemagglutinin. Nat. Commun. 2019, 10, 324. [Google Scholar] [CrossRef]
- Waldock, J.; Remarque, E.J.; Zheng, L.; Ho, S.; Hoschler, K.; Neumann, B.; Sediri-Schön, H.; Trombetta, C.M.; Montomoli, E.; Marchi, S.; et al. Haemagglutination inhibition and virus microneutralisation serology assays: Use of harmonised protocols and biological standards in seasonal influenza serology testing and their impact on inter-laboratory variation and assay correlation: A FLUCOP collaborative study. Front. Immunol. 2023, 14, 1155552. [Google Scholar] [CrossRef]
- Verschoor, C.P.; Singh, P.; Russell, M.L.; Bowdish, D.M.E.; Brewer, A.; Cyr, L.; Ward, B.J.; Loeb, M.; Cowling, B.J. Microneutralization assay titres correlate with protection against seasonal influenza H1N1 and H3N2 in children. PLoS ONE 2015, 10, e0131531. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.K.; Cauchemez, S.; Perera, R.A.P.M.; Freeman, G.; Fang, V.J.; Ip, D.K.M.; Leung, G.M.; Peiris, J.S.M.; Cowling, B.J. Association between antibody titers and protection against influenza virus infection within households. J. Infect. Dis. 2014, 210, 684–692. [Google Scholar] [CrossRef]
- Monto, A.S.; Petrie, J.G.; Cross, R.T.; Johnson, E.; Liu, M.; Zhong, W.; Levine, M.; Katz, J.M.; Ohmit, S.E. Antibody to influenza virus neuraminidase: An independent correlate of protection. J. Infect. Dis. 2015, 212, 1191–1199. [Google Scholar] [CrossRef]
- McMahon, M.; Tan, J.; O’DEll, G.; Roubidoux, E.K.; Strohmeier, S.; Krammer, F.; Heise, M.T. Immunity induced by vaccination with recombinant influenza B virus neuraminidase protein breaks viral transmission chains in guinea pigs in an exposure intensity-dependent manner. J. Virol. 2023, 97, e01057-23. [Google Scholar] [CrossRef] [PubMed]
- Catani, J.P.P.; Ysenbaert, T.; Smet, A.; Vuylsteke, M.; Vogel, T.U.; Saelens, X.; Huber, V.C. Anti-neuraminidase and anti-hemagglutinin immune serum can confer inter-lineage cross protection against recent influenza B. PLoS ONE 2023, 18, e0280825. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Golosova, O.; Fursov, M. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Sergeeva, M.V.; Romanovskaya-Romanko, E.A.; Krivitskaya, V.Z.; Kudar, P.A.; Petkova, N.N.; Kudria, K.S.; Lioznov, D.A.; Stukova, M.A.; Desheva, Y.A. Longitudinal analysis of neuraminidase and hemagglutinin antibodies to influenza a viruses after immunization with seasonal inactivated influenza vaccines. Vaccines 2023, 11, 1731. [Google Scholar] [CrossRef]
- Isakova-Sivak, I.; Chen, L.-M.; Matsuoka, Y.; Voeten, J.T.M.; Kiseleva, I.; Heldens, J.G.; van den Bosch, H.; Klimov, A.; Rudenko, L.; Cox, N.J.; et al. Genetic bases of the temperature-sensitive phenotype of a master donor virus used in live attenuated influenza vaccines: A/Leningrad/134/17/57 (H2N2). Virology 2011, 412, 297–305. [Google Scholar] [CrossRef]

| No. | Name | Origin of HA | Origin of NA |
|---|---|---|---|
| 1 | H2NB-Brisbane | A/Leningrad/134/17/57 (H2N2) | B/Brisbane/60/2008 (B/Victoria) |
| 2 | H2NB-Phuket | A/Leningrad/134/17/57 (H2N2) | B/Phuket/3073/2013 (B/Yamagata) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desheva, Y.; Kudar, P.; Sergeeva, M.; Wong, P.-F.; Shvedova, T.; Bazhenova, E.; Krylova, E.; Kurpiaeva, M.; Romanovskaya-Romanko, E.; Krivitskaya, V.; et al. The Persistence of Cross-Reactive Immunity to Influenza B/Yamagata Neuraminidase Despite the Disappearance of the Lineage: Structural and Serological Evidence. Int. J. Mol. Sci. 2025, 26, 7476. https://doi.org/10.3390/ijms26157476
Desheva Y, Kudar P, Sergeeva M, Wong P-F, Shvedova T, Bazhenova E, Krylova E, Kurpiaeva M, Romanovskaya-Romanko E, Krivitskaya V, et al. The Persistence of Cross-Reactive Immunity to Influenza B/Yamagata Neuraminidase Despite the Disappearance of the Lineage: Structural and Serological Evidence. International Journal of Molecular Sciences. 2025; 26(15):7476. https://doi.org/10.3390/ijms26157476
Chicago/Turabian StyleDesheva, Yulia, Polina Kudar, Maria Sergeeva, Pei-Fong Wong, Tamara Shvedova, Ekaterina Bazhenova, Evelyna Krylova, Maria Kurpiaeva, Ekaterina Romanovskaya-Romanko, Vera Krivitskaya, and et al. 2025. "The Persistence of Cross-Reactive Immunity to Influenza B/Yamagata Neuraminidase Despite the Disappearance of the Lineage: Structural and Serological Evidence" International Journal of Molecular Sciences 26, no. 15: 7476. https://doi.org/10.3390/ijms26157476
APA StyleDesheva, Y., Kudar, P., Sergeeva, M., Wong, P.-F., Shvedova, T., Bazhenova, E., Krylova, E., Kurpiaeva, M., Romanovskaya-Romanko, E., Krivitskaya, V., Kudria, K., Isakova-Sivak, I., & Stukova, M. (2025). The Persistence of Cross-Reactive Immunity to Influenza B/Yamagata Neuraminidase Despite the Disappearance of the Lineage: Structural and Serological Evidence. International Journal of Molecular Sciences, 26(15), 7476. https://doi.org/10.3390/ijms26157476






