Beyond Cutting: CRISPR-Driven Synthetic Biology Toolkit for Next-Generation Microalgal Metabolic Engineering
Abstract
1. Introduction
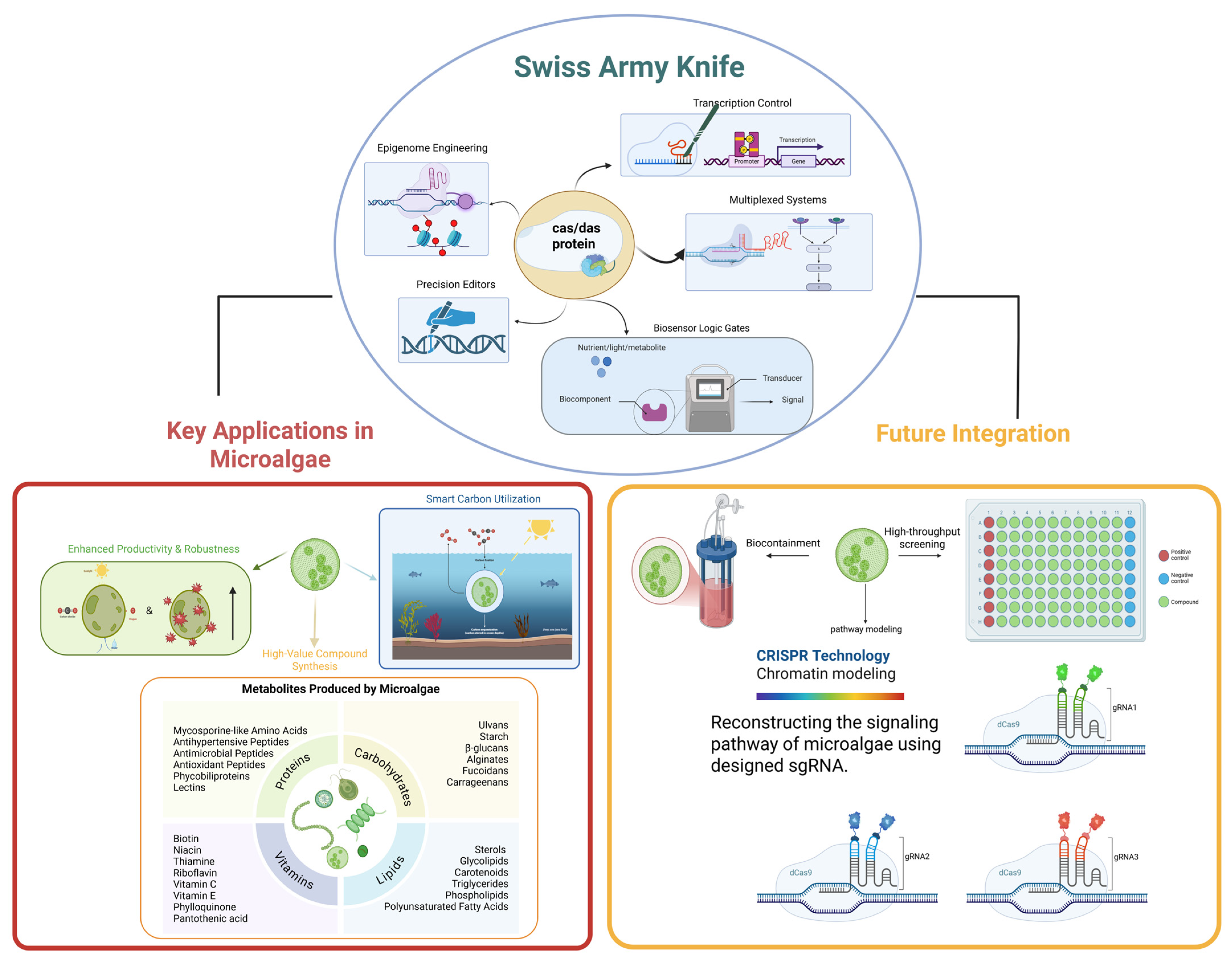
2. The CRISPR Toolkit: Core Components Beyond Cutting
2.1. Foundations for Precision Editing
2.1.1. Cas Protein Variants and Algal Adaptability
2.1.2. Delivery Strategies: Overcoming the Algal Fortress
2.1.3. Harnessing and Optimizing Repair Mechanisms
2.2. Transcriptional Regulation: CRISPR Activation and Interference (CRISPRa/i)
2.2.1. Core Principles
2.2.2. Strategies and Case Studies in Microalgae
2.2.3. Advantages
2.3. Epigenome Engineering
2.3.1. Core Principles of Epigenome Editing
2.3.2. Potential and Applications in Microalgae
2.4. Base Editing
2.4.1. Core Mechanisms of Base Editing
2.4.2. Applications in Microalgae
2.4.3. Advantages and Limitations
2.5. Prime Editing: Expanding the Horizon of Precision
Overcoming Limitations in Microalgae
2.6. Multiplexed Genome Engineering: Rewiring Complex Pathways
2.7. CRISPR Logic Gates and Biosensors: Enabling Smart Control
2.7.1. CRISPR-Based Logic Gates
2.7.2. Biosensor-Integrated Control for Closed-Loop Regulation
2.8. CRISPR-Enabled Directed Evolution: Accelerating Strain Optimization
3. Future-Oriented Applications: Integrated CRISPR Strategies for Next-Generation Metabolic Engineering
3.1. Future Photosynthesis Enhancement: Multi-Layered CRISPR Strategies
3.2. Next-Generation Lipid/Biofuel Optimization: Integrated Control Systems
3.3. Advanced Biosynthesis: Engineering High-Value Compound Pathways
3.4. Prospective Stress Resilience Engineering
3.5. Future Carbon Utilization Strategies
3.6. Forward-Looking Industrial Strain Design
4. Challenges, Limitations, and Future Perspectives
4.1. Microalgae-Specific Challenges
4.1.1. Genetic Diversity and Tool Compatibility
4.1.2. The Formidable Cell Wall Barrier
4.1.3. Transformation and Screening Inefficiency
4.1.4. Endogenous CRISPR System Interference
4.1.5. Inadequate Chassis Knowledge
4.2. Toolkit Refinement
4.2.1. Enhancing Efficiency, Precision, and Specificity
4.2.2. Expanding the Editing Repertoire
4.3. System Integration and Automation
4.4. Scaling up Applications and Industrial Considerations
4.5. Ethical and Biosafety Considerations
4.6. Towards a CRISPR-Engineered Algal Future
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarker, N.K.; Kaparaju, P. Microalgal Bioeconomy: A Green Economy Approach Towards Achieving Sustainable Development Goals. Sustainability 2024, 16, 11218. [Google Scholar] [CrossRef]
- Yadav, D.K.; Singh, A.; Agrawal, V.; Yadav, N. Algal Biomass: A Natural Resource of High-Value Biomolecules. In Bioprospecting of Plant Biodiversity for Industrial Molecules; Wiley: New York, NY, USA, 2021; pp. 303–334. [Google Scholar]
- Santin, A.; Russo, M.T.; Ferrante, M.I.; Balzano, S.; Orefice, I.; Sardo, A. Highly valuable polyunsaturated fatty acids from microalgae: Strategies to improve their yields and their potential exploitation in aquaculture. Molecules 2021, 26, 7697. [Google Scholar] [CrossRef]
- Juneja, C.; Itankar, R.; Pal, S. Biotechnological Techniques for Recovery of Renewable Resources From Municipal Wastewater and Value-Added Products Development. In Biotechnological Applications in Industrial Waste Valorization; Springer: Berlin/Heidelberg, Germany, 2025; pp. 271–321. [Google Scholar]
- Gurreri, L.; Rindina, M.C.; Luciano, A.; Lima, S.; Scargiali, F.; Fino, D.; Mancini, G. Environmental sustainability of microalgae-based production systems: Roadmap and challenges towards the industrial implementation. Sustain. Chem. Pharm. 2023, 35, 101191. [Google Scholar] [CrossRef]
- Sproles, A.E.; Fields, F.J.; Smalley, T.N.; Le, C.H.; Badary, A.; Mayfield, S.P. Recent advancements in the genetic engineering of microalgae. Algal Res. 2021, 53, 102158. [Google Scholar] [CrossRef]
- Naduthodi, M.I.S.; Mohanraju, P.; Südfeld, C.; D’Adamo, S.; Barbosa, M.J.; Van Der Oost, J. CRISPR–Cas ribonucleoprotein mediated homology-directed repair for efficient targeted genome editing in microalgae Nannochloropsis oceanica IMET1. Biotechnol. Biofuels 2019, 12, 66. [Google Scholar] [CrossRef]
- Feng, S.; Xie, X.; Liu, J.; Li, A.; Wang, Q.; Guo, D.; Li, S.; Li, Y.; Wang, Z.; Guo, T. A potential paradigm in CRISPR/Cas systems delivery: At the crossroad of microalgal gene editing and algal-mediated nanoparticles. J. Nanobiotechnol. 2023, 21, 370. [Google Scholar] [CrossRef]
- Ambily, B.; Limna Mol, V.; Sini, H.; Nevin, K. CRISPR-based microalgal genome editing and the potential for sustainable aquaculture: A comprehensive review. J. Appl. Phycol. 2025, 37, 265–285. [Google Scholar] [CrossRef]
- Shin, S.-E.; Lim, J.-M.; Koh, H.G.; Kim, E.K.; Kang, N.K.; Jeon, S.; Kwon, S.; Shin, W.-S.; Lee, B.; Hwangbo, K. CRISPR/Cas9-induced knockout and knock-in mutations in Chlamydomonas reinhardtii. Sci. Rep. 2016, 6, 27810. [Google Scholar] [CrossRef] [PubMed]
- Rau, E.-M.; Ertesvåg, H. Method development progress in genetic engineering of thraustochytrids. Mar. Drugs 2021, 19, 515. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.M.; Hasty, J. Multiplexing with CRISPR-Cas Arrays; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Saber Sichani, A.; Ranjbar, M.; Baneshi, M.; Torabi Zadeh, F.; Fallahi, J. A review on advanced CRISPR-based genome-editing tools: Base editing and prime editing. Mol. Biotechnol. 2023, 65, 849–860. [Google Scholar] [CrossRef]
- Trosset, J.-Y.; Carbonell, P. Synthetic biology for pharmaceutical drug discovery. Drug Des. Dev. Ther. 2015, 6285–6302. [Google Scholar] [CrossRef]
- Arshad, S.; Qadir, M.L.; Hussain, N.; Ali, Q.; Han, S.; Ali, D. Advances in CRISPR/Cas9 technology: Shaping the future of photosynthetic microorganisms for biofuel production. Funct. Plant Biol. 2025, 52, FP24255. [Google Scholar] [CrossRef]
- Gale, G.A.; Schiavon Osorio, A.A.; Mills, L.A.; Wang, B.; Lea-Smith, D.J.; McCormick, A.J. Emerging species and genome editing tools: Future prospects in cyanobacterial synthetic biology. Microorganisms 2019, 7, 409. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Lauersen, K.J. Gene delivery technologies with applications in microalgal genetic engineering. Biology 2021, 10, 265. [Google Scholar] [CrossRef]
- Uranga, M.; Daròs, J.A. Tools and targets: The dual role of plant viruses in CRISPR–Cas genome editing. Plant Genome 2023, 16, e20220. [Google Scholar] [CrossRef] [PubMed]
- Asmamaw Mengstie, M.; Teshome Azezew, M.; Asmamaw Dejenie, T.; Teshome, A.A.; Tadele Admasu, F.; Behaile Teklemariam, A.; Tilahun Mulu, A.; Mekonnen Agidew, M.; Adugna, D.G.; Geremew, H. Recent advancements in reducing the off-target effect of CRISPR-Cas9 genome editing. Biol. Targets Ther. 2024, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ghribi, M.; Nouemssi, S.B.; Meddeb-Mouelhi, F.; Desgagné-Penix, I. Genome editing by CRISPR-Cas: A game change in the genetic manipulation of Chlamydomonas. Life 2020, 10, 295. [Google Scholar] [CrossRef]
- Spicer, A.; Molnar, A. Gene editing of microalgae: Scientific progress and regulatory challenges in Europe. Biology 2018, 7, 21. [Google Scholar] [CrossRef]
- Munawar, N.; Ahmad, A. CRISPR/Cas system: An introduction. In CRISPR Crops: The Future of Food Security; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–35. [Google Scholar]
- Hsieh-Feng, V.; Yang, Y. Efficient expression of multiple guide RNAs for CRISPR/Cas genome editing. Abiotech 2020, 1, 123–134. [Google Scholar] [CrossRef]
- Allshire, R.C.; Madhani, H.D. Ten principles of heterochromatin formation and function. Nat. Rev. Mol. Cell Biol. 2018, 19, 229–244. [Google Scholar] [CrossRef]
- Morrison, O.; Thakur, J. Molecular complexes at euchromatin, heterochromatin and centromeric chromatin. Int. J. Mol. Sci. 2021, 22, 6922. [Google Scholar] [CrossRef]
- Russell, J.J.; Theriot, J.A.; Sood, P.; Marshall, W.F.; Landweber, L.F.; Fritz-Laylin, L.; Polka, J.K.; Oliferenko, S.; Gerbich, T.; Gladfelter, A. Non-model model organisms. BMC Biol. 2017, 15, 55. [Google Scholar] [CrossRef]
- Mosey, M.; Douchi, D.; Knoshaug, E.P.; Laurens, L.M. Methodological review of genetic engineering approaches for non-model algae. Algal Res. 2021, 54, 102221. [Google Scholar] [CrossRef]
- Kumar, G.; Shekh, A.; Jakhu, S.; Sharma, Y.; Kapoor, R.; Sharma, T.R. Bioengineering of microalgae: Recent advances, perspectives, and regulatory challenges for industrial application. Front. Bioeng. Biotechnol. 2020, 8, 914. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.K.; Das, A.; Kumari, R.; Kajla, S. Recent progress and challenges in CRISPR-Cas9 engineered algae and cyanobacteria. Algal Res. 2023, 71, 103068. [Google Scholar] [CrossRef]
- Patel, V.K.; Soni, N.; Prasad, V.; Sapre, A.; Dasgupta, S.; Bhadra, B. CRISPR–Cas9 system for genome engineering of photosynthetic microalgae. Mol. Biotechnol. 2019, 61, 541–561. [Google Scholar] [CrossRef]
- Costelli, C. Genetic and Phylogenetic Characterization of Microalgae Strains in View of Their Exploitation for CO2 Capture and Biofuel Production. Ph.D. Thesis, Università degli Studi di Cagliar, Cagliari, Italy, 2015. [Google Scholar]
- Steadman, C.; Small, E.; Banerjee, S.; Twary, S. Best practices for methylome characterization in novel species: A case study in the microalgae Nannochloropsis. Commun. Biol. 2025, 8, 648. [Google Scholar] [CrossRef]
- Casabianca, S.; Cornetti, L.; Capellacci, S.; Vernesi, C.; Penna, A. Genome complexity of harmful microalgae. Harmful Algae 2017, 63, 7–12. [Google Scholar] [CrossRef]
- Noel, E.A. Characterization of Novel Chlorovirus Glycosyltransferases that Synthesize Atypical Glycans; The University of Nebraska-Lincoln: Lincoln, NE, USA, 2021. [Google Scholar]
- Doron, L.; Segal, N.A.; Shapira, M. Transgene expression in microalgae—From tools to applications. Front. Plant Sci. 2016, 7, 505. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, C.F.; de Jaeger, L.; Sturme, M.H.; Lip, K.Y.; Olijslager, J.W.; Springer, J.; Wolbert, E.J.; Martens, D.E.; Eggink, G.; Weusthuis, R.A. Improved DNA/protein delivery in microalgae–A simple and reliable method for the prediction of optimal electroporation settings. Algal Res. 2018, 33, 448–455. [Google Scholar] [CrossRef]
- Bolaños-Martínez, O.C.; Mahendran, G.; Rosales-Mendoza, S.; Vimolmangkang, S. Current status and perspective on the use of viral-based vectors in eukaryotic microalgae. Mar. Drugs 2022, 20, 434. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Ohnuma, M.; Kuroiwa, T.; Ohbayashi, R.; Hirooka, S.; Miyagishima, S.-Y. Development of a double nuclear gene-targeting method by two-step transformation based on a newly established chloramphenicol-selection system in the red alga Cyanidioschyzon merolae. Front. Plant Sci. 2017, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Yu, J. Comparative Characterization of Isoprene Synthases from Monocot Species for Improved Isoprene Production in Microalgae; Padua Research Archive: Padua, Italy, 2023. [Google Scholar]
- Gu, Y.; Teo, M.Y.M.; In, L.L.A.; Shimizu, K.; Chae, K.-J.; Tran, T.N.T.; Khoo, K.S. Genetic engineering of Haematococcus pluvialis microalgae for the enhancement of astaxanthin production: A review. Biocatal. Agric. Biotechnol. 2024, 60, 103298. [Google Scholar] [CrossRef]
- Yamada, T.; Onimatsu, H.; Van Etten, J.L. Chlorella viruses. Adv. Virus Res. 2006, 66, 293–336. [Google Scholar]
- Yang, B.; Liu, J.; Jiang, Y.; Chen, F. Chlorella species as hosts for genetic engineering and expression of heterologous proteins: Progress, challenge and perspective. Biotechnol. J. 2016, 11, 1244–1261. [Google Scholar] [CrossRef]
- Rocha-Martins, M.; Cavalheiro, G.R.; Matos-Rodrigues, G.E.; Martins, R.A. From gene targeting to genome editing: Transgenic animals applications and beyond. An. Acad. Bras. Ciênc. 2015, 87, 1323–1348. [Google Scholar] [CrossRef]
- Bradshaw, J.E.; Bradshaw, J.E. Gene Editing and Genetic Transformation of Potatoes. In Potato Breeding: Theory and Practice; Springer: Berlin/Heidelberg, Germany, 2021; pp. 505–551. [Google Scholar]
- Jeon, S.; Lim, J.-M.; Lee, H.-G.; Shin, S.-E.; Kang, N.K.; Park, Y.-I.; Oh, H.-M.; Jeong, W.-J.; Jeong, B.-R.; Chang, Y.K. Current status and perspectives of genome editing technology for microalgae. Biotechnol. Biofuels 2017, 10, 267. [Google Scholar] [CrossRef]
- Mohan, C.; Satish, L.; Muthubharathi, B.C.; Selvarajan, D.; Easterling, M.; Yau, Y.-Y. CRISPR-Cas technology: A genome-editing powerhouse for molecular plant breeding. In Biotechnological Innovations for Environmental Bioremediation; Springer: Berlin/Heidelberg, Germany, 2022; pp. 803–879. [Google Scholar]
- Salsman, J.; Dellaire, G. Precision genome editing in the CRISPR era. Biochem. Cell Biol. 2017, 95, 187–201. [Google Scholar] [CrossRef]
- Li, G.; Yang, X.; Luo, X.; Wu, Z.; Yang, H. Modulation of cell cycle increases CRISPR-mediated homology-directed DNA repair. Cell Biosci. 2023, 13, 215. [Google Scholar] [CrossRef]
- Gürel, F.; Zhang, Y.; Sretenovic, S.; Qi, Y. CRISPR-Cas nucleases and base editors for plant genome editing. Abiotech 2020, 1, 74–87. [Google Scholar] [CrossRef]
- Richardson, C.; Kelsh, R.N.; Richardson, J.R. New advances in CRISPR/Cas-mediated precise gene-editing techniques. Dis. Models Mech. 2023, 16, dmm049874. [Google Scholar] [CrossRef] [PubMed]
- Brocken, D.J.; Tark-Dame, M.; Dame, R.T. dCas9: A versatile tool for epigenome editing. Curr. Issues Mol. Biol. 2018, 26, 15–32. [Google Scholar] [CrossRef]
- Chen, S.; Yu, X.; Guo, D. CRISPR-Cas targeting of host genes as an antiviral strategy. Viruses 2018, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.; Qi, L. Genetic and epigenetic control of gene expression by CRISPR–Cas systems. F1000Research 2017, 6, F1000. [Google Scholar] [CrossRef] [PubMed]
- Devillars, A.; Magon, G.; Pirrello, C.; Palumbo, F.; Farinati, S.; Barcaccia, G.; Lucchin, M.; Vannozzi, A. Not only editing: A cas-cade of CRISPR/Cas-based tools for functional genomics in plants and animals. Int. J. Mol. Sci. 2024, 25, 3271. [Google Scholar] [CrossRef] [PubMed]
- Alerasool, N.; Segal, D.; Lee, H.; Taipale, M. An efficient KRAB domain for CRISPRi applications in human cells. Nat. Methods 2020, 17, 1093–1096. [Google Scholar] [CrossRef]
- Kunii, A.; Hara, Y.; Takenaga, M.; Hattori, N.; Fukazawa, T.; Ushijima, T.; Yamamoto, T.; Sakuma, T. Three-component repurposed technology for enhanced expression: Highly accumulable transcriptional activators via branched tag arrays. CRISPR J. 2018, 1, 337–347. [Google Scholar] [CrossRef]
- Dhokane, D.; Shaikh, A.; Yadav, A.; Giri, N.; Bandyopadhyay, A.; Dasgupta, S.; Bhadra, B. CRISPR-based bioengineering in microalgae for production of industrially important biomolecules. Front. Bioeng. Biotechnol. 2023, 11, 1267826. [Google Scholar] [CrossRef]
- Diankristanti, P.A.; Ho, N.H.E.; Chen, J.-H.; Nagarajan, D.; Chen, C.-Y.; Hsieh, Y.-M.; Ng, I.-S.; Chang, J.-S. Unlocking the potential of microalgae as sustainable bioresources from up to downstream processing: A critical review. Chem. Eng. J. 2024, 488, 151124. [Google Scholar] [CrossRef]
- Ciurkot, K. Expanding CRISPR-based toolbox for enhanced production of β-carotene in S. cerevisiae. Curr. Microbiol. 2021, 77, 468–478. [Google Scholar]
- Gomide, M.d.S.; Leitão, M.d.C.; Coelho, C.M. Biocircuits in plants and eukaryotic algae. Front. Plant Sci. 2022, 13, 982959. [Google Scholar] [CrossRef]
- Jagadevan, S.; Banerjee, A.; Banerjee, C.; Guria, C.; Tiwari, R.; Baweja, M.; Shukla, P. Recent developments in synthetic biology and metabolic engineering in microalgae towards biofuel production. Biotechnol. Biofuels 2018, 11, 185. [Google Scholar] [CrossRef]
- Naduthodi, M.I.; Südfeld, C.; Avitzigiannis, E.K.; Trevisan, N.; van Lith, E.; Alcaide Sancho, J.; D’Adamo, S.; Barbosa, M.; van der Oost, J. Comprehensive genome engineering toolbox for microalgae Nannochloropsis oceanica based on CRISPR-Cas systems. ACS Synth. Biol. 2021, 10, 3369–3378. [Google Scholar] [CrossRef] [PubMed]
- Christian, S. Lipids and More: Advancing Nannochloropsis as A Biotechnological Multipurpose Chassis. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2021. [Google Scholar]
- Singh, V.; Dhar, P.K. Genome Engineering via CRISPR-Cas9 System; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Ding, W.; Zhang, Y.; Shi, S. Development and application of CRISPR/Cas in microbial biotechnology. Front. Bioeng. Biotechnol. 2020, 8, 711. [Google Scholar] [CrossRef]
- Vogler, B.W.; Ashford, A.; Posewitz, M.C. CRISPR/Cas9 disruption of glucan synthase in Nannochloropsis gaditana attenuates accumulation of β-1, 3-glucose oligomers. Algal Res. 2021, 58, 102385. [Google Scholar] [CrossRef]
- Vogler, B. Development of CRISPR/Cas9 in Nannochloropsis and Other Algae Toward Understanding and Manipulating Energy Allocation; Colorado School of Mines: Golden, CO, USA, 2018. [Google Scholar]
- Balamurugan, S.; Li, D.-W.; Wang, X.; Li, H.-Y. Unleashing the potential of biotechnological strategies for the sustainable production of microalgal polysaccharides. Crit. Rev. Food Sci. Nutr. 2025, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, F.; Chen, L.; Zhang, W. Engineering fatty acid biosynthesis in microalgae: Recent progress and perspectives. Mar. Drugs 2024, 22, 216. [Google Scholar] [CrossRef]
- Xing, C.; Li, J.; Yuan, H.; Yang, J. Physiological and transcription level responses of microalgae Auxenochlorella protothecoides to cold and heat induced oxidative stress. Environ. Res. 2022, 211, 113023. [Google Scholar] [CrossRef] [PubMed]
- Minami, S. Applications of Protein Engineering in Synthetic Biology and Bioproduction; University of California: Davis, CA, USA, 2023. [Google Scholar]
- Gupta, A.; Kang, K.; Pathania, R.; Saxton, L.; Saucedo, B.; Malik, A.; Torres-Tiji, Y.; Diaz, C.J.; Dutra Molino, J.V.; Mayfield, S.P. Harnessing genetic engineering to drive economic bioproduct production in algae. Front. Bioeng. Biotechnol. 2024, 12, 1350722. [Google Scholar] [CrossRef]
- González Castro, N.; Bjelic, J.; Malhotra, G.; Huang, C.; Alsaffar, S.H. Comparison of the feasibility, efficiency, and safety of genome editing technologies. Int. J. Mol. Sci. 2021, 22, 10355. [Google Scholar] [CrossRef]
- Dhokane, D.; Chandrashekharaiah, P.; Kushwaha, S.; Bhadra, B.; Bandyopadhyay, A. Recent Advances in Microalgal Genome Editing with Special Emphasis on CRISPR Mediated Modification Systems. In Microalgae for Sustainable Products; Royal Society of Chemistry: London, UK, 2022. [Google Scholar]
- George, J.; Kahlke, T.; Abbriano, R.M.; Kuzhiumparambil, U.; Ralph, P.J.; Fabris, M. Metabolic engineering strategies in diatoms reveal unique phenotypes and genetic configurations with implications for algal genetics and synthetic biology. Front. Bioeng. Biotechnol. 2020, 8, 513. [Google Scholar] [CrossRef]
- La Russa, M.F.; Qi, L.S. The new state of the art: Cas9 for gene activation and repression. Mol. Cell Biol. 2015, 35, 3800–3809. [Google Scholar] [CrossRef]
- Raicu, A.-M. Regulation of Gene Expression in Drosophila by the Rb and CtBP Transcriptional Corepressors; Michigan State University: East Lansing, MI, USA, 2023. [Google Scholar]
- Chu, L.L. CRISPR-Cas system in microbial hosts for terpenoid production. Crit. Rev. Biotechnol. 2022, 42, 1116–1133. [Google Scholar] [CrossRef]
- Cardiff, R.A.; Carothers, J.M.; Zalatan, J.G.; Sauro, H.M. Systems-Level Modeling for CRISPR-Based Metabolic Engineering. ACS Synth. Biol. 2024, 13, 2643–2652. [Google Scholar] [CrossRef]
- Nishida, K.; Kondo, A. CRISPR-derived genome editing technologies for metabolic engineering. Metab. Eng. 2021, 63, 141–147. [Google Scholar] [CrossRef]
- Bacova, R.; Kolackova, M.; Klejdus, B.; Adam, V.; Huska, D. Epigenetic mechanisms leading to genetic flexibility during abiotic stress responses in microalgae: A review. Algal Res. 2020, 50, 101999. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, G.; Zhang, L. Epigenetic Regulation in Algae: Implications for Growth, Development, and Stress Response. Int. J. Aquac. 2024, 14, 257. [Google Scholar] [CrossRef]
- Tejedor, J.R.; Peñarroya, A.; Gancedo-Verdejo, J.; Santamarina-Ojeda, P.; Pérez, R.F.; López-Tamargo, S.; Díez-Borge, A.; Alba-Linares, J.J.; González-del-Rey, N.; Urdinguio, R.G. CRISPR/dCAS9-mediated DNA demethylation screen identifies functional epigenetic determinants of colorectal cancer. Clin. Epigenet. 2023, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.R.; Cui, Y.; Lubecka, K.; Stefanska, B.; Irudayaraj, J. CRISPR-dCas9 mediated TET1 targeting for selective DNA demethylation at BRCA1 promoter. Oncotarget 2016, 7, 46545. [Google Scholar] [CrossRef] [PubMed]
- Martella, A.; Fisher, D.I. Regulation of gene expression and the elucidative role of CRISPR-based epigenetic modifiers and CRISPR-induced chromosome conformational changes. CRISPR J. 2021, 4, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Einhaus, A.; Krieger, A.; Köhne, L.; Rautengarten, B.; Jacobebbinghaus, N.; Saudhof, M.; Baier, T.; Kruse, O. Genome editing of epigenetic transgene silencing in Chlamydomonas reinhardtii. Trends Biotechnol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Steadman, C.R. Epigenetics in Algae. In Epigenetics in Aquaculture; Wiley: Hoboken, NJ, USA, 2023; pp. 383–411. [Google Scholar]
- Dhaouadi, F.; Awwad, F.; Diamond, A.; Desgagné-Penix, I. Diatoms’ breakthroughs in biotechnology: Phaeodactylum tricornutum as a model for producing high-added value molecules. Am. J. Plant Sci. 2020, 11, 1632. [Google Scholar] [CrossRef]
- Kim, E.-J.; Ma, X.; Cerutti, H. Gene silencing in microalgae: Mechanisms and biological roles. Bioresour. Technol. 2015, 184, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wan, C.; Mehmood, M.A.; Chang, J.-S.; Bai, F.; Zhao, X. Manipulating environmental stresses and stress tolerance of microalgae for enhanced production of lipids and value-added products—A review. Bioresour. Technol. 2017, 244, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, J.; Deng, B.; Jiang, Y.; Zhu, J.; Chen, L.; Li, M.; Li, J. Applications and prospects of CRISPR/Cas9-mediated base editing in plant breeding. Curr. Issues Mol. Biol. 2023, 45, 918–935. [Google Scholar] [CrossRef]
- Hua, K.; Han, P.; Zhu, J.-K. Improvement of base editors and prime editors advances precision genome engineering in plants. Plant Physiol. 2022, 188, 1795–1810. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; He, Y.; Li, S.; Yan, L.; Li, Y.; Zhu, Z.; Xia, L. Plant base editing and prime editing: The current status and future perspectives. J. Integr. Plant Biol. 2023, 65, 444–467. [Google Scholar] [CrossRef]
- Kantor, A.; McClements, M.E.; MacLaren, R.E. CRISPR-Cas9 DNA base-editing and prime-editing. Int. J. Mol. Sci. 2020, 21, 6240. [Google Scholar] [CrossRef]
- Rallapalli, K.L.; Komor, A.C. The design and application of DNA-editing enzymes as base editors. Annu. Rev. Biochem. 2023, 92, 43–79. [Google Scholar] [CrossRef]
- Carlaw, T.M. Therapeutic Genome Editing for the Treatment of Genetic Diseases: Testing the Safety and Effectiveness of CRISPR/Cas9 Therapeutic Base Editing. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2023. [Google Scholar]
- Anzalone, A.V.; Koblan, L.W.; Liu, D.R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 2020, 38, 824–844. [Google Scholar] [CrossRef]
- Ochoa-Sanchez, A.; Perez-Sanchez, G.; Torres-Ledesma, A.M.; Valdez, J.P.R.; Rinaldi, G.; Moguel, B.B.; Molina-Aguilar, C. Prime Editing, a novel genome-editing tool that may surpass conventional CRISPR-Cas9. Re GEN Open 2021, 1, 75–82. [Google Scholar] [CrossRef]
- Averina, O.; Kuznetsova, S.; Permyakov, O.; Sergiev, P. Current knowledge of base editing and prime editing. Mol. Biol. 2024, 58, 571–587. [Google Scholar] [CrossRef]
- Chintagunta, A.D.; Kumar, N.; Kumar, P.N.; Kumar, P.S.; Kumar, S. Metabolic Engineering and Genome Editing Strategies for Enhanced Lipid Production in Oleaginous Microorganisms; Tech Science Press: Henderson, NV, USA, 2024. [Google Scholar]
- Ng, I.S.; Keskin, B.B.; Tan, S.I. A critical review of genome editing and synthetic biology applications in metabolic engineering of microalgae and cyanobacteria. Biotechnol. J. 2020, 15, 1900228. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Z.; Li, G.; Dang, L.; Huang, S.; He, L.; Ma, Y.E.; Li, C.; Liu, M.; Yang, G. Efficient gene silencing by adenine base editor-mediated start codon mutation. Mol. Ther. 2020, 28, 431–440. [Google Scholar] [CrossRef]
- Li, M.; Huo, Y.-X.; Guo, S. CRISPR-mediated base editing: From precise point mutation to genome-wide engineering in nonmodel microbes. Biology 2022, 11, 571. [Google Scholar] [CrossRef]
- Molla, K.A.; Sretenovic, S.; Bansal, K.C.; Qi, Y. Precise plant genome editing using base editors and prime editors. Nat. Plants 2021, 7, 1166–1187. [Google Scholar] [CrossRef]
- Cui, Y.; Cao, Q.; Li, Y.; He, M.; Liu, X. Advances in cis-element-and natural variation-mediated transcriptional regulation and applications in gene editing of major crops. J. Exp. Bot. 2023, 74, 5441–5457. [Google Scholar] [CrossRef]
- Thenge, P.P. Base Editing Enables Single-Step, Highly Multiplex Genome Editing in Human iPSCS with Negligible Genotoxicity; University of Minnesota: Minneapolis, MN, USA, 2024. [Google Scholar]
- Doman, J.L. Development of Enhanced Base Editors and Prime Editors Through Protein Engineering and Directed Evolution. Ph.D. Thesis, Harvard University, Cambridge, MA, USA, 2023. [Google Scholar]
- Molla, K.A.; Yang, Y. CRISPR/Cas-mediated base editing: Technical considerations and practical applications. Trends Biotechnol. 2019, 37, 1121–1142. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Birkenshaw, A.; Thomson, T.; Carlaw, T.; Zhang, L.-H.; Ross, C.J. Increasing the targeting scope of CRISPR base editing system beyond NGG. Cris. J. 2022, 5, 187–202. [Google Scholar] [CrossRef]
- Wang, L.; Han, H. Strategies for improving the genome-editing efficiency of class 2 CRISPR/Cas system. Heliyon 2024, 10, e38588. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.B.; Harrison, P.T.; Scholefield, J. Prime editing: Therapeutic advances and mechanistic insights. Gene Ther. 2025, 32, 83–92. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, S.; Qin, H.; Yao, K. From bench to bedside: Cutting-edge applications of base editing and prime editing in precision medicine. J. Transl. Med. 2024, 22, 1133. [Google Scholar] [CrossRef]
- Doman, J.L.; Sousa, A.A.; Randolph, P.B.; Chen, P.J.; Liu, D.R. Designing and executing prime editing experiments in mammalian cells. Nat. Protoc. 2022, 17, 2431–2468. [Google Scholar] [CrossRef] [PubMed]
- Scholefield, J.; Harrison, P.T. Prime editing—An update on the field. Gene Ther. 2021, 28, 396–401. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, C.; Yang, Y.; Zhao, S.; Kang, G.; He, X.; Song, J.; Yang, J. Versatile nucleotides substitution in plant using an improved prime editing system. Mol. Plant 2020, 13, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cao, D.; Han, R. Recent advances in therapeutic gene editing technologies. Mol. Ther. 2025, 33, 2619–2644. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, V.; Hashemi, A. Challenges of in vitro genome editing with CRISPR/Cas9 and possible solutions: A review. Gene 2020, 753, 144813. [Google Scholar] [CrossRef]
- Shivani; Chauhan, H.; Singh, K. Genome-Editing Advances for Disease Resistance in Plants. In Biotechnological Advances for Disease Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2024; pp. 293–316. [Google Scholar]
- Liao, H.; Wu, J.; VanDusen, N.J.; Li, Y.; Zheng, Y. CRISPR-Cas9-mediated homology-directed repair for precise gene editing. Mol. Ther. Nucleic Acids 2024, 35, 102344. [Google Scholar] [CrossRef]
- Li, M.; Lin, Y.; Cheng, Q.; Wei, T. Prime Editing: A Revolutionary Technology for Precise Treatment of Genetic Disorders. Cell Prolif. 2025, 58, e13808. [Google Scholar] [CrossRef]
- Esland, L.; Larrea-Alvarez, M.; Purton, S. Selectable markers and reporter genes for engineering the chloroplast of Chlamydomonas reinhardtii. Biology 2018, 7, 46. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, S.; Jiang, Y.; Liu, R.; Xiao, Y. Development and optimization of CRISPR prime editing system in photoautotrophic cells. Molecules 2022, 27, 1758. [Google Scholar] [CrossRef]
- Zong, Y.; Liu, Y.; Xue, C.; Li, B.; Li, X.; Wang, Y.; Li, J.; Liu, G.; Huang, X.; Cao, X. An engineered prime editor with enhanced editing efficiency in plants. Nat. Biotechnol. 2022, 40, 1394–1402. [Google Scholar] [CrossRef]
- George, J. Exploring Genetic Engineering Strategies to Enable Heterologous Monoterpenoid Production in Model Microalgae, Chlamydomonas reinhardtii and Phaeodactylum tricornutum. Ph.D. Thesis, University of Technology Sydney, Ultimo, Australia, 2021. [Google Scholar]
- Kurita, T.; Iwai, M.; Ohta, H.; Sakuma, T.; Yamamoto, T. Genome editing for biodiesel production in oleaginous microalga, Nannochloropsis species. Gene Genome Ed. 2023, 6, 100027. [Google Scholar] [CrossRef]
- Gong, C.; Huang, S.; Song, R.; Qi, W. Comparative study between the CRISPR/Cpf1 (Cas12a) and CRISPR/Cas9 systems for multiplex gene editing in maize. Agriculture 2021, 11, 429. [Google Scholar] [CrossRef]
- McCarty, N.S.; Graham, A.E.; Studená, L.; Ledesma-Amaro, R. Multiplexed CRISPR technologies for gene editing and transcriptional regulation. Nat. Commun. 2020, 11, 1281. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, Y.; Wang, Y.; Gao, D.; King, J.; Xu, Y.; Liang, F.-S. Investigating crosstalk between H3K27 acetylation and H3K4 trimethylation in CRISPR/dCas-based epigenome editing and gene activation. Sci. Rep. 2021, 11, 15912. [Google Scholar] [CrossRef]
- Cho, S.; Shin, J.; Cho, B.-K. Applications of CRISPR/Cas system to bacterial metabolic engineering. Int. J. Mol. Sci. 2018, 19, 1089. [Google Scholar] [CrossRef] [PubMed]
- Bu, M.; Fan, W.; Li, R.; He, B.; Cui, P. Lipid metabolism and improvement in oilseed crops: Recent advances in multi-omics studies. Metabolites 2023, 13, 1170. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.; Wei, Z.; Rohila, J.S.; Zhao, K. Multiplex genome-editing technologies for revolutionizing plant biology and crop improvement. Front. Plant Sci. 2021, 12, 721203. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Park, D.; Kim, J. Logical regulation of endogenous gene expression using programmable, multi-input processing CRISPR guide RNAs. Nucleic Acids Res. 2024, 52, 8595–8608. [Google Scholar] [CrossRef]
- Santos-Moreno, J.; Schaerli, Y. CRISPR-based gene expression control for synthetic gene circuits. Biochem. Soc. Trans. 2020, 48, 1979–1993. [Google Scholar] [CrossRef]
- Chung, H.K.; Lin, M.Z. On the cutting edge: Protease-based methods for sensing and controlling cell biology. Nat. Methods 2020, 17, 885–896. [Google Scholar] [CrossRef]
- Teixeira, A.P.; Fussenegger, M. Synthetic Gene Circuits for Regulation of Next-Generation Cell-Based Therapeutics. Adv. Sci. 2024, 11, 2309088. [Google Scholar] [CrossRef]
- Mansouri, M.; Fussenegger, M. Small-Molecule Regulators for Gene Switches to Program Mammalian Cell Behaviour. ChemBioChem 2024, 25, e202300717. [Google Scholar] [CrossRef]
- Khan, Z. Engineering Disease Resistance in Plants Using CRISPR-Cas; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- İbişoğlu, M.; Palabıyık, B.; Yılmaz, S.; Topal Sarıkaya, A. Does transcription factor SPCC320. 03 regulate expression of hexose transporter genes in fission yeast? In Proceedings of the 31st International Conference on Yeast Genetics and Molecular Biology, Florence, Italy, 20–25 August 2023. [Google Scholar]
- Guo, W. Computational Modeling of Planktonic and Biofilm Metabolism; Virginia Tech: Blacksburg, VI, USA, 2017. [Google Scholar]
- Fattore, N.; Bellan, A.; Pedroletti, L.; Vitulo, N.; Morosinotto, T. Acclimation of photosynthesis and lipids biosynthesis to prolonged nitrogen and phosphorus limitation in Nannochloropsis gaditana. Algal Res. 2021, 58, 102368. [Google Scholar] [CrossRef]
- Remmers, I.M. Photosynthetic Efficiency in Microalgal Lipid Production. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2017. [Google Scholar]
- Wei, L.; Jiang, Z.; Liu, B. A CRISPR/dCas9-based transcription activated system developed in marine microalga Nannochloropsis oceanica. Aquaculture 2022, 546, 737064. [Google Scholar] [CrossRef]
- Alboresi, A.; Perin, G.; Vitulo, N.; Diretto, G.; Block, M.; Jouhet, J.; Meneghesso, A.; Valle, G.; Giuliano, G.; Maréchal, E. Light remodels lipid biosynthesis in Nannochloropsis gaditana by modulating carbon partitioning between organelles. Plant Physiol. 2016, 171, 2468–2482. [Google Scholar] [CrossRef] [PubMed]
- Garagounis, C.; Delkis, N.; Papadopoulou, K.K. Unraveling the roles of plant specialized metabolites: Using synthetic biology to design molecular biosensors. New Phytol. 2021, 231, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Hudson, E.P. Synthetic biology in cyanobacteria and applications for biotechnology. In Cyanobacteria Biotechnolgy; Wiley: Hoboken, NJ, USA, 2021; pp. 123–170. [Google Scholar]
- Kant, G.; Pandey, A.; Shekhar, H.; Srivastava, S. Enhanced bio-synthesis of isoprene via modifying mevalonate and methylerythritol phosphate pathways for industrial application: A review. Process Biochem. 2023, 130, 256–271. [Google Scholar] [CrossRef]
- Teng, Y.; Zhang, J.; Jiang, T.; Zou, Y.; Gong, X.; Yan, Y. Biosensor-enabled pathway optimization in metabolic engineering. Curr. Opin. Biotechnol. 2022, 75, 102696. [Google Scholar] [CrossRef]
- Davey, M.P.; Horst, I.; Duong, G.-H.; Tomsett, E.V.; Litvinenko, A.C.; Howe, C.J.; Smith, A.G. Triacylglyceride production and autophagous responses in Chlamydomonas reinhardtii depend on resource allocation and carbon source. Eukaryot. Cell 2014, 13, 392–400. [Google Scholar] [CrossRef]
- Terashima, M. Chlamydomonas: Triacylglycerol accumulation. In Chlamydomonas: Biotechnology and Biomedicine; Springer: Berlin/Heidelberg, Germany, 2017; pp. 193–217. [Google Scholar]
- Rapacz, E. Development of Tomato Genotypes with Enhanced Xanthophyll Content in Ripe Fruit. Ph.D. Thesis, Royal Holloway University, Egham, UK, 2019. [Google Scholar]
- Liu, Y.; Cheng, H.; Li, H.; Zhang, Y.; Wang, M. A programmable CRISPR/Cas9 Toolkit improves Lycopene production in Bacillus subtilis. Appl. Environ. Microbiol. 2023, 89, e00230-23. [Google Scholar] [CrossRef]
- Naseri, G.; Behrend, J.; Rieper, L.; Mueller-Roeber, B. COMPASS for rapid combinatorial optimization of biochemical pathways based on artificial transcription factors. Nat. Commun. 2019, 10, 2615. [Google Scholar] [CrossRef]
- Parveen, A.; Bhatnagar, P.; Gautam, P.; Bisht, B.; Nanda, M.; Kumar, S.; Vlaskin, M.S.; Kumar, V. Enhancing the bio-prospective of microalgae by different light systems and photoperiods. Photochem. Photobiol. Sci. 2023, 22, 2687–2698. [Google Scholar] [CrossRef]
- Schüller, A.; Studt-Reinhold, L.; Strauss, J. How to completely squeeze a fungus—Advanced genome mining tools for novel bioactive substances. Pharmaceutics 2022, 14, 1837. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.-Q.; Wang, L.-R.; Zhang, Z.-X.; Sun, X.-M.; Huang, H. Stresses as first-line tools for enhancing lipid and carotenoid production in microalgae. Front. Bioeng. Biotechnol. 2020, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Trovão, M.; Schüler, L.M.; Machado, A.; Bombo, G.; Navalho, S.; Barros, A.; Pereira, H.; Silva, J.; Freitas, F.; Varela, J. Random mutagenesis as a promising tool for microalgal strain improvement towards industrial production. Mar. Drugs 2022, 20, 440. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, J.; Xing, Y.; Zhao, Y.; Liu, Y. Development of delivery strategies for CRISPR-Cas9 genome editing. BMEMat 2023, 1, e12025. [Google Scholar] [CrossRef]
- Antonacci, A.; Scognamiglio, V. Biotechnological advances in the design of algae-based biosensors. Trends Biotechnol. 2020, 38, 334–347. [Google Scholar] [CrossRef]
- Patwari, P.; Pruckner, F.; Fabris, M. Biosensors in microalgae: A roadmap for new opportunities in synthetic biology and biotechnology. Biotechnol. Adv. 2023, 68, 108221. [Google Scholar] [CrossRef]
- Kuo, E.Y.; Yang, R.Y.; Chin, Y.Y.; Chien, Y.L.; Chen, Y.C.; Wei, C.Y.; Kao, L.J.; Chang, Y.H.; Li, Y.J.; Chen, T.Y. Multiomics approaches and genetic engineering of metabolism for improved biorefinery and wastewater treatment in microalgae. Biotechnol. J. 2022, 17, 2100603. [Google Scholar] [CrossRef]
- Huang, M.; Wang, K.; Li, A.; Zhu, X.; Zhou, Z.; Yang, C.; Bi, C.; Zhang, X. Mini and enhanced CRISPR activators for cancer therapies. J. Adv. Res. 2024. [Google Scholar] [CrossRef]
- Turner, T.J.; Zourray, C.; Schorge, S.; Lignani, G. Recent advances in gene therapy for neurodevelopmental disorders with epilepsy. J. Neurochem. 2021, 157, 229–262. [Google Scholar] [CrossRef]
- O’Geen, H.; Ren, C.; Nicolet, C.M.; Perez, A.A.; Halmai, J.; Le, V.M.; Mackay, J.P.; Farnham, P.J.; Segal, D.J. dCas9-based epigenome editing suggests acquisition of histone methylation is not sufficient for target gene repression. Nucleic Acids Res. 2017, 45, 9901–9916. [Google Scholar] [CrossRef]
- Thakore, P.I.; Black, J.B.; Hilton, I.B.; Gersbach, C.A. Editing the epigenome: Technologies for programmable transcription and epigenetic modulation. Nat. Methods 2016, 13, 127–137. [Google Scholar] [CrossRef]
- Fayyaz, M.; Chew, K.W.; Show, P.L.; Ling, T.C.; Ng, I.-S.; Chang, J.-S. Genetic engineering of microalgae for enhanced biorefinery capabilities. Biotechnol. Adv. 2020, 43, 107554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bamidele, N.; Liu, P.; Ojelabi, O.; Gao, X.D.; Rodriguez, T.; Cheng, H.; Kelly, K.; Watts, J.K.; Xie, J. Adenine base editing in vivo with a single adeno-associated virus vector. GEN Biotechnol. 2022, 1, 285–299. [Google Scholar] [CrossRef]
- Koodamvetty, A.; Thangavel, S. Advancing Precision Medicine: Recent Innovations in Gene Editing Technologies. Adv. Sci. 2025, 12, 2410237. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zeng, Y.; Wang, Y.; Niu, Y. The development and application of a base editor in biomedicine. BioMed Res. Int. 2020, 2020, 2907623. [Google Scholar] [CrossRef] [PubMed]
- Bharath, S.; Hemanth, S.; Ragi, S. Genomic Sculptors: Carving Perfection with Base Editing Tools in the Field of Crop Improvement. In Multidisciplinary Research in Agriculture and Allied Sciences; Dr. Yuvraj Arjun Shinde: Indore, India, 2023; p. 169. [Google Scholar]
- Matsoukas, I.G. Prime editing: Genome editing for rare genetic diseases without double-strand breaks or donor DNA. Front. Genet. 2020, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Petrova, I.O.; Smirnikhina, S.A. The development, optimization and future of prime editing. Int. J. Mol. Sci. 2023, 24, 17045. [Google Scholar] [CrossRef]
- Shah, K.; Kaur, A.; Saxena, S.; Arora, S. CRISPR-Based Approach: A Way Forward to Sustainable Development Goals (SDGs). In Gene Editing in Plants: CRISPR-Cas and Its Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 709–733. [Google Scholar]
- Hu, Y.; Li, W. Development and application of CRISPR-cas based tools. Front. Cell Dev. Biol. 2022, 10, 834646. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Zhang, R.; Qin, X.; Shen, X.; You, C. Morphological Structure Identification, Comparative Mitochondrial Genomics and Population Genetic Analysis toward Exploring Interspecific Variations and Phylogenetic Implications of Malus baccata ‘ZA’and Other Species. Biomolecules 2024, 14, 912. [Google Scholar] [CrossRef]
- Jiang, C.; Geng, L.; Wang, J.; Liang, Y.; Guo, X.; Liu, C.; Zhao, Y.; Jin, J.; Liu, Z.; Mu, Y. Multiplexed gene engineering based on dCas9 and gRNA-tRNA array encoded on single transcript. Int. J. Mol. Sci. 2023, 24, 8535. [Google Scholar] [CrossRef] [PubMed]
- Munir, A.; Amin, I.; Zahoor, M.K.; Majeed, H.N.; Almoammar, H.; Ghaffar, A.; Ahmad, A. Multiplex genome editing in plants through CRISPR-Cas. In CRISPRized Horticulture Crops; Elsevier: Amsterdam, The Netherlands, 2024; pp. 127–142. [Google Scholar]
- Malcı, K.; Walls, L.E.; Rios-Solis, L. Multiplex genome engineering methods for yeast cell factory development. Front. Bioeng. Biotechnol. 2020, 8, 589468. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Hu, T.; Venkatesan, M.; Allam, M.; Schneider, F.; Ramalingam, S.S.; Sun, S.-Y.; Coskun, A.F. Multiplexed protein profiling reveals spatial subcellular signaling networks. Iscience 2022, 25, 104980. [Google Scholar] [CrossRef] [PubMed]
- Montecillo, J.A.V.; Chu, L.L.; Bae, H. CRISPR-Cas9 system for plant genome editing: Current approaches and emerging developments. Agronomy 2020, 10, 1033. [Google Scholar] [CrossRef]
- Ma, Z.; Cheah, W.Y.; Ng, I.-S.; Chang, J.-S.; Zhao, M.; Show, P.L. Microalgae-based biotechnological sequestration of carbon dioxide for net zero emissions. Trends Biotechnol. 2022, 40, 1439–1453. [Google Scholar] [CrossRef]
- Lin, J.Y.; Lin, W.R.; Ng, I.S. CRISPRa/i with A daptive S ingle G uide A ssisted R egulation D NA (ASGARD) mediated control of Chlorella sorokiniana to enhance lipid and protein production. Biotechnol. J. 2022, 17, 2100514. [Google Scholar] [CrossRef]
- Du, P.; Lou, C.; Zhao, X.; Wang, Q.; Ji, X.; Wei, W. CRISPR-based genetic switches and other complex circuits: Research and application. Life 2021, 11, 1255. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Du, Y.-C.; Wang, D.-X.; Ma, J.-Y.; Tang, A.-N.; Kong, D.-M. Signal amplification and output of CRISPR/Cas-based biosensing systems: A review. Anal. Chim. Acta 2021, 1185, 338882. [Google Scholar] [CrossRef]
- Ream, M.; Prather, K.L. Engineered autonomous dynamic regulation of metabolic flux. Nat. Rev. Bioeng. 2024, 2, 233–243. [Google Scholar] [CrossRef]
- Goold, H.D.; Wright, P.; Hailstones, D. Emerging opportunities for synthetic biology in agriculture. Genes 2018, 9, 341. [Google Scholar] [CrossRef]
- Babu, S.S.; Gondi, R.; Vincent, G.S.; JohnSamuel, G.C.; Jeyakumar, R.B. Microalgae biomass and lipids as feedstock for biofuels: Sustainable biotechnology strategies. Sustainability 2022, 14, 15070. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of light conditions on microalgae growth and content of lipids, carotenoids, and fatty acid composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, A.; Muthukaliannan, G.K. Genetic manipulation for carotenoid production in microalgae an overview. Curr. Res. Biotechnol. 2022, 4, 221–228. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhong, S.; Li, Q. The research progress and frontier studies on soybean production technology: Genetic breeding, pest and disease control, and precision agriculture applications. Adv. Resour. Res. 2025, 5, 1065–1082. [Google Scholar]
- Mutale-Joan, C.; El Arroussi, H. Biotechnological strategies overcoming limitations to H. pluvialis-derived astaxanthin production and Morocco’s potential. Crit. Rev. Food Sci. Nutr. 2025, 65, 1404–1419. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Q.; Wang, N.; Ye, S.; Wang, Y.; Zhang, J.; Lin, C.; Zhu, Q. Advances in bamboo genomics: Growth and development, stress tolerance, and genetic engineering. J. Integr. Plant Biol. 2025, 67, 1725–1755. [Google Scholar] [CrossRef]
- Li, G.; Xiao, W.; Yang, T.; Lyu, T. Optimization and process effect for microalgae carbon dioxide fixation technology applications based on carbon capture: A comprehensive review. C 2023, 9, 35. [Google Scholar] [CrossRef]
- Hu, J.; Wang, D.; Chen, H.; Wang, Q. Advances in genetic engineering in improving photosynthesis and microalgal productivity. Int. J. Mol. Sci. 2023, 24, 1898. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.K.; Agrawal, K.; Upadhyaya, H.M.; Gupta, V.K.; Verma, P. Microalgae conversion to alternative energy, operating environment and economic footprint: An influential approach towards energy conversion, and management. Energy Convers. Manag. 2022, 269, 116118. [Google Scholar] [CrossRef]
- Dawiec-Liśniewska, A.; Podstawczyk, D.; Bastrzyk, A.; Czuba, K.; Pacyna-Iwanicka, K.; Okoro, O.V.; Shavandi, A. New trends in biotechnological applications of photosynthetic microorganisms. Biotechnol. Adv. 2022, 59, 107988. [Google Scholar] [CrossRef]
- Chintagunta, A.; Prashant, S.; Satya, N. Metabolic engineering and genome editing strategies for enhanced lipid production in microalgae. Biocell 2024, 48, 1181. [Google Scholar] [CrossRef]
- Diao, J.; Song, X.; Guo, T.; Wang, F.; Chen, L.; Zhang, W. Cellular engineering strategies toward sustainable omega-3 long chain polyunsaturated fatty acids production: State of the art and perspectives. Biotechnol. Adv. 2020, 40, 107497. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Khan, A.; Tran, L.-S.P. Genetic engineering: A promising tool to engender physiological, biochemical, and molecular stress resilience in green microalgae. Front. Plant Sci. 2016, 7, 400. [Google Scholar] [CrossRef]
- Tamaki, S.; Mochida, K.; Suzuki, K. Diverse biosynthetic pathways and protective functions against environmental stress of antioxidants in microalgae. Plants 2021, 10, 1250. [Google Scholar] [CrossRef]
- Ranjbar, S.; Malcata, F.X. Is genetic engineering a route to enhance microalgae-mediated bioremediation of heavy metal-containing effluents? Molecules 2022, 27, 1473. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.-G.; Le, T.T.; Choi, D.Y.; Cho, D.H.; Yun, J.-H.; Choi, H.I.; Kim, H.-S.; Lee, Y.J. Progress and challenges in CRISPR/Cas applications in microalgae. J. Microbiol. 2025, 63, e2501028. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, I.; Ansari, M.R.; Bari, M.N.; Khan, M.S.; Kamal, M.A.; Anwar, M. Enhancing Food Safety: Adapting to Microbial Responses Under Diverse Environmental Stressors. Preprints 2025. [Google Scholar] [CrossRef]
- Priyadharsini, P.; Nirmala, N.; Dawn, S.; Baskaran, A.; SundarRajan, P.; Gopinath, K.; Arun, J. Genetic improvement of microalgae for enhanced carbon dioxide sequestration and enriched biomass productivity: Review on CO2 bio-fixation pathways modifications. Algal Res. 2022, 66, 102810. [Google Scholar] [CrossRef]
- Hu, J.; Meng, W.; Su, Y.; Qian, C.; Fu, W. Emerging technologies for advancing microalgal photosynthesis and metabolism toward sustainable production. Front. Mar. Sci. 2023, 10, 1260709. [Google Scholar] [CrossRef]
- Shanmugam, S.; Ngo, H.-H.; Wu, Y.-R. Advanced CRISPR/Cas-based genome editing tools for microbial biofuels production: A review. Renew. Energy 2020, 149, 1107–1119. [Google Scholar] [CrossRef]
- Rock, A.; Novoveská, L.; Green, D. Synthetic biology is essential to unlock commercial biofuel production through hyper lipid-producing microalgae: A review. Appl. Phycol. 2021, 2, 41–59. [Google Scholar] [CrossRef]
- Joshi, S.; Pandey, A.; Sirohi, R.; Park, S.H. Current Developments in Biotechnology and Bioengineering: Designer Microbial Cell Factories: Metabolic Engineering and Applications; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Chen, Z.; Zhu, J.; Du, M.; Chen, Z.; Liu, Q.; Zhu, H.; Lei, A.; Wang, J. A synthetic biology perspective on the bioengineering tools for an industrial microalga: Euglena gracilis. Front. Bioeng. Biotechnol. 2022, 10, 882391. [Google Scholar] [CrossRef]
- Barbosa, M.J.; Janssen, M.; Südfeld, C.; D’Adamo, S.; Wijffels, R.H. Hypes, hopes, and the way forward for microalgal biotechnology. Trends Biotechnol. 2023, 41, 452–471. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Jiang, J.-Y.; Shi, T.-Q.; Sun, X.-M.; Zhao, Q.-Y.; Huang, H.; Ren, L.-J. Application of the CRISPR/Cas system for genome editing in microalgae. Appl. Microbiol. Biotechnol. 2019, 103, 3239–3248. [Google Scholar] [CrossRef]
- Hao, X.; Chen, W.; Amato, A.; Jouhet, J.; Maréchal, E.; Moog, D.; Hu, H.; Jin, H.; You, L.; Huang, F. Multiplexed CRISPR/Cas9 editing of the long-chain acyl-CoA synthetase family in the diatom Phaeodactylum tricornutum reveals that mitochondrial ptACSL3 is involved in the synthesis of storage lipids. New Phytol. 2022, 233, 1797–1812. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, S.; Malcata, F.X. Challenges and prospects for sustainable microalga-based oil: A comprehensive review, with a focus on metabolic and genetic engineering. Fuel 2022, 324, 124567. [Google Scholar] [CrossRef]
- Ahmad Kamal, A.H.; Mohd Hamidi, N.F.; Zakaria, M.F.; Ahmad, A.; Harun, M.R.; Chandra Segaran, T.; Jusoh, M. Genetically engineered microalgae for enhanced bioactive compounds. Discov. Appl. Sci. 2024, 6, 482. [Google Scholar] [CrossRef]
- Kumari, R.; Patel, V.K.; Kumari, P.; Kajla, S. Yield Enhancement in Algal Production and Genetic Engineering Tools for Microalgal Biorefinery. In Value Added Products from Bioalgae Based Biorefineries: Opportunities and Challenges; Springer: Berlin/Heidelberg, Germany, 2024; pp. 489–522. [Google Scholar]
- Garg, D.; Samota, M.K.; Kontis, N.; Patel, N.; Bala, S.; Rosado, A.S. Revolutionizing biofuel generation: Unleashing the power of CRISPR-Cas mediated gene editing of extremophiles. Microbiol. Res. 2023, 274, 127443. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Jaiswal, K.K.; Vlaskin, M.S.; Nanda, M.; Tripathi, M.K.; Kumar, S. Microalgae with a truncated light-harvesting antenna to maximize photosynthetic efficiency and biomass productivity: Recent advances and current challenges. Process Biochem. 2021, 104, 83–91. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Ho, S.-H. How to enhance carbon capture by evolution of microalgal photosynthesis? Sep. Purif. Technol. 2022, 291, 120951. [Google Scholar] [CrossRef]
- Yang, B.; Liu, J.; Ma, X.; Guo, B.; Liu, B.; Wu, T.; Jiang, Y.; Chen, F. Genetic engineering of the Calvin cycle toward enhanced photosynthetic CO2 fixation in microalgae. Biotechnol. Biofuels 2017, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lu, Y.; Xin, Y.; Wei, L.; Huang, S.; Xu, J. Genome editing of model oleaginous microalgae Nannochloropsis spp. by CRISPR/Cas9. Plant J. 2016, 88, 1071–1081. [Google Scholar] [CrossRef]
- Han, X.; Song, X.; Li, F.; Lu, Y. Improving lipid productivity by engineering a control-knob gene in the oleaginous microalga Nannochloropsis oceanica. Metab. Eng. Commun. 2020, 11, e00142. [Google Scholar] [CrossRef] [PubMed]
- Shaigani, P.; Fuchs, T.; Graban, P.; Prem, S.; Haack, M.; Masri, M.; Mehlmer, N.; Brueck, T. Mastering targeted genome engineering of GC-rich oleaginous yeast for tailored plant oil alternatives for the food and chemical sector. Microb. Cell Factories 2023, 22, 25. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, L.; Ma, L.; Zhai, Y.; Hu, Y.; Li, G.; Chen, G.; Li, D. CRISPR/Cas9-mediated metabolic engineering for enhanced PUFA production in Schizochytrium limacinum. Chem. Eng. J. 2025, 517, 164320. [Google Scholar] [CrossRef]
- Wan, C.; Chen, B.-L.; Zhao, X.-Q.; Bai, F.-W. Stress response of microalgae and its manipulation for development of robust strains. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2019; pp. 95–113. [Google Scholar]
- Kneip, J.S.; Kniepkamp, N.; Jang, J.; Mortaro, M.G.; Jin, E.; Kruse, O.; Baier, T. CRISPR/Cas9-Mediated knockout of the Lycopene ε-Cyclase for efficient astaxanthin production in the Green Microalga Chlamydomonas reinhardtii. Plants 2024, 13, 1393. [Google Scholar] [CrossRef]
- Patel, A.K.; Tambat, V.S.; Chen, C.-W.; Chauhan, A.S.; Kumar, P.; Vadrale, A.P.; Huang, C.-Y.; Dong, C.-D.; Singhania, R.R. Recent advancements in astaxanthin production from microalgae: A review. Bioresour. Technol. 2022, 364, 128030. [Google Scholar] [CrossRef]
- Kumari, A.; Pabbi, S.; Tyagi, A. Recent advances in enhancing the production of long chain omega-3 fatty acids in microalgae. Crit. Rev. Food Sci. Nutr. 2024, 64, 10564–10582. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; She, Y.; Chen, X.; Huang, X.; Xu, T.; Miao, C.; Wu, Q.; Li, Z.; Li, C.; Cheng, Y. Enhanced thermotolerance via overexpression of a stromal ascorbate peroxidase in Nannochloropsis oceanica. Plant Cell Rep. 2025, 44, 139. [Google Scholar] [CrossRef]
- Xu, J.; Soni, V.; Chopra, M.; Chan, O. Genetic modification of the HSP90 gene using CRISPR-Cas9 to enhance thermotolerance in T. suecica. Undergrad. Res. Nat. Clin. Sci. Technol. J. 2020, 4, 1–6. [Google Scholar] [CrossRef]
- Chankova, S.G.; Yurina, N.P.; Todorova, T.I.; Parvanova, P.N. Does overproduction of chaperone proteins favour the repair of DNA injuries induced by oxidative stress? (Mini review). BioRisk 2023, 20, 7–22. [Google Scholar] [CrossRef]
- Zhu, J.; Li, S.; Chen, W.; Xu, X.; Wang, X.; Wang, X.; Han, J.; Jouhet, J.; Amato, A.; Maréchal, E. Delta-5 elongase knockout reduces docosahexaenoic acid and lipid synthesis and increases heat sensitivity in a diatom. Plant Physiol. 2024, 196, 1356–1373. [Google Scholar] [CrossRef]
- Barati, B.; Gan, S.-Y.; Lim, P.-E.; Beardall, J.; Phang, S.-M. Green algal molecular responses to temperature stress. Acta Physiol. Plant. 2019, 41, 26. [Google Scholar] [CrossRef]
- Sun, L.; Zheng, P.; Sun, J.; Wendisch, V.F.; Wang, Y. Genome-scale CRISPRi screening: A powerful tool in engineering microbiology. Eng. Microbiol. 2023, 3, 100089. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Diankristanti, P.A.; Chen, P.-Y.; Yi, Y.-C.; Ng, I.-S. Sustainable Polyhydroxybutyrate Production via Metabolic Flux Redirection Using Clustered Regularly Interspaced Short Palindromic Repeats Interference in Escherichia coli and Carbon Capture with Microalgae. ACS Sustain. Chem. Eng. 2025, 13, 4418–4426. [Google Scholar] [CrossRef]
- Hassanien, A.; Saadaoui, I.; Schipper, K.; Al-Marri, S.; Dalgamouni, T.; Aouida, M.; Saeed, S.; Al-Jabri, H.M. Genetic engineering to enhance microalgal-based produced water treatment with emphasis on CRISPR/Cas9: A review. Front. Bioeng. Biotechnol. 2023, 10, 1104914. [Google Scholar] [CrossRef]
- Maltsev, Y.; Kulikovskiy, M.; Maltseva, S. Nitrogen and phosphorus stress as a tool to induce lipid production in microalgae. Microb. Cell Factories 2023, 22, 239. [Google Scholar] [CrossRef]
- Al-Hoqani, U.; Young, R.; Purton, S. The biotechnological potential of Nannochloropsis. Perspect. Phycol. 2017, 4, 1–15. [Google Scholar] [CrossRef]
- Stavridou, E.; Karapetsi, L.; Nteve, G.M.; Tsintzou, G.; Chatzikonstantinou, M.; Tsaousi, M.; Martinez, A.; Flores, P.; Merino, M.; Dobrovic, L. Landscape of microalgae omics and metabolic engineering research for strain improvement: An overview. Aquaculture 2024, 587, 740803. [Google Scholar] [CrossRef]
- Mubarik, M.S.; Khan, Z.; Ahmad, M.Q.; Sajjad, M. Applications of CRISPR/Cas Beyond Simple. In CRISPR Crops: The Future of Food Security; Springer: Berlin/Heidelberg, Germany, 2021; p. 231. [Google Scholar]
- Kilaru, A.; Xie, L.H.; Zhang, Q.; Zhang, Y. Tree Peony Species Are a Novel Resource for Production of α-Linolenic Acid; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Khan, A.K.; Kausar, H.; Jaferi, S.S.; Drouet, S.; Hano, C.; Abbasi, B.H.; Anjum, S. An insight into the algal evolution and genomics. Biomolecules 2020, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Specht, E.A.; Karunanithi, P.S.; Gimpel, J.A.; Ansari, W.S.; Mayfield, S.P. Host organisms: Algae. Ind. Biotechnol. Microorg. 2017, 2, 605–641. [Google Scholar]
- van den Hoogen, J. Dissecting Cellular Signaling in Phytophthora. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2018. [Google Scholar]
- Durairaj, S.; Srikanth, P.; Durairaj, S. Genetic Engineering of Algae. In Phycoremediation Processes in Industrial Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2023; pp. 127–145. [Google Scholar]
- Haider, S.; Mussolino, C. Fine-Tuning Homology-Directed Repair (HDR) for Precision Genome Editing: Current Strategies and Future Directions. Int. J. Mol. Sci. 2025, 26, 4067. [Google Scholar] [CrossRef]
- Ramarajan, M. Development of a Molecular Toolkit to Genetically Engineer The Microalga Nannochloropsis gaditana CCMP526 for Biotechnology Applications. Ph.D. Thesis, University of Technology Sydney, Ultimo, Australia, 2020. [Google Scholar]
- Li, F. Cloning and Expression of Virus-Like Particles (VLPs) in Microalgal Expression Systems. Ph.D. Thesis, University College London, London, UK, 2019. [Google Scholar]
- Mattoon, E.M.; McHargue, W.E.; Bailey, C.E.; Zhang, N.; Chen, C.; Eckhardt, J.; Daum, C.G.; Zane, M.; Pennacchio, C.; Schmutz, J. High-throughput identification of novel heat tolerance genes via genome-wide pooled mutant screens in the model green alga Chlamydomonas reinhardtii. Plant Cell Environ. 2023, 46, 865–888. [Google Scholar] [CrossRef]
- Zadabbas Shahabadi, H.; Akbarzadeh, A.; Ofoghi, H.; Kadkhodaei, S. Site-specific gene knock-in and bacterial phytase gene expression in Chlamydomonas reinhardtii via Cas9 RNP-mediated HDR. Front. Plant Sci. 2023, 14, 1150436. [Google Scholar] [CrossRef]
- Tran, N.T.; Kaldenhoff, R. Achievements and challenges of genetic engineering of the model green alga Chlamydomonas reinhardtii. Algal Res. 2020, 50, 101986. [Google Scholar] [CrossRef]
- Naduthodi, M.I.S. A “CRISPY” Tale on Microalgal Genome Editing: Featuring Nannochloropsis oceanica IMET1. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2021. [Google Scholar]
- Ye, Y.; Liu, M.; Yu, L.; Sun, H.; Liu, J. Nannochloropsis as an emerging algal chassis for light-driven synthesis of lipids and high-value products. Mar. Drugs 2024, 22, 54. [Google Scholar] [CrossRef]
- Kilian, O.; Benemann, C.S.; Niyogi, K.K.; Vick, B. High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp. Proc. Natl. Acad. Sci. USA 2011, 108, 21265–21269. [Google Scholar] [CrossRef]
- Chernyavskaya, O. Developing Molecular Tools for the Genetic Manipulation of Nannochloropsis; Institutt for bioteknologi: Trondheim, Norway, 2014. [Google Scholar]
- Serif, M.; Lepetit, B.; Weißert, K.; Kroth, P.G.; Bartulos, C.R. A fast and reliable strategy to generate TALEN-mediated gene knockouts in the diatom Phaeodactylum tricornutum. Algal Res. 2017, 23, 186–195. [Google Scholar] [CrossRef]
- Butler, T.; Kapoore, R.V.; Vaidyanathan, S. Phaeodactylum tricornutum: A diatom cell factory. Trends Biotechnol. 2020, 38, 606–622. [Google Scholar] [CrossRef]
- Ding, W.; Ye, Y.; Yu, L.; Liu, M.; Liu, J. Physiochemical and molecular responses of the diatom Phaeodactylum tricornutum to illumination transitions. Biotechnol. Biofuels Bioprod. 2023, 16, 103. [Google Scholar] [CrossRef]
- Nymark, M.; Sharma, A.K.; Hafskjold, M.C.; Sparstad, T.; Bones, A.M.; Winge, P. CRISPR/Cas9 gene editing in the marine diatom Phaeodactylum tricornutum. Bio-Protoc. 2017, 7, e2442. [Google Scholar] [CrossRef]
- Faessler, A.C. Optimising Tools for Metabolic Engineering in the Marine Diatom Phaeodactylum tricornutum. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2024. [Google Scholar]
- Ren, Q.; Wang, Y.-c.; Lin, Y.; Zhen, Z.; Cui, Y.; Qin, S. The extremely large chloroplast genome of the green alga Haematococcus pluvialis: Genome structure, and comparative analysis. Algal Res. 2021, 56, 102308. [Google Scholar] [CrossRef]
- Kathiresan, S. Genetic Transformation of Green Alga Haematococcus pluvialis for the Regulation of Carotenoid Biosynthesis. Ph.D. Thesis, University of Mysore, Mysore, India, 2009. [Google Scholar]
- Steinbrenner, J.; Sandmann, G. Transformation of the green alga Haematococcus pluvialis with a phytoene desaturase for accelerated astaxanthin biosynthesis. Appl. Environ. Microbiol. 2006, 72, 7477–7484. [Google Scholar] [CrossRef]
- Le-Feuvre, R.; Moraga-Suazo, P.; Gonzalez, J.; Martin, S.S.; Henríquez, V.; Donoso, A.; Agurto-Muñoz, C. Biotechnology applied to Haematococcus pluvialis Fotow: Challenges and prospects for the enhancement of astaxanthin accumulation. J. Appl. Phycol. 2020, 32, 3831–3852. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Zeng, X.-A.; Cheng, J.-H.; Liu, D.-B.; Aadil, R.M. The efficiency and comparison of novel techniques for cell wall disruption in astaxanthin extraction from Haematococcus pluvialis. Int. J. Food Sci. Technol. 2018, 53, 2212–2219. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Shivakumar, S.; Serlini, N.; Esteves, S.M.; Miros, S.; Halim, R. Cell Walls of Lipid-Rich Microalgae: A Comprehensive Review on Characterisation, Ultrastructure, and Enzymatic Disruption. Fermentation 2024, 10, 608. [Google Scholar] [CrossRef]
- Jia, Y.-L.; Li, J.; Nong, F.-T.; Yan, C.-X.; Ma, W.; Zhu, X.-F.; Zhang, L.-H.; Sun, X.-M. Application of adaptive laboratory evolution in lipid and terpenoid production in yeast and microalgae. ACS Synth. Biol. 2023, 12, 1396–1407. [Google Scholar] [CrossRef]
- Sarkar, D. Integrated Modeling of Phototrophic Metabolism Leveraging Multi-Omics Datasets. Ph.D. Thesis, The Pennsylvania State University, Pennsylvania, PA, USA, 2022. [Google Scholar]
- Gong, Z.; Chen, J.; Jiao, X.; Gong, H.; Pan, D.; Liu, L.; Zhang, Y.; Tan, T. Genome-scale metabolic network models for industrial microorganisms metabolic engineering: Current advances and future prospects. Biotechnol. Adv. 2024, 72, 108319. [Google Scholar] [CrossRef] [PubMed]
- Gerken-Starepravo, L.; Zhu, X.; Cho, B.A.; Vega-Ramon, F.; Pennington, O.; del Río-Chanona, E.A.; Jing, K.; Zhang, D. An MIQP framework for metabolic pathways optimisation and dynamic flux analysis. Digit. Chem. Eng. 2022, 100011. [Google Scholar] [CrossRef]
- Simeonidis, E.; Price, N.D. Genome-scale modeling for metabolic engineering. J. Ind. Microbiol. Biotechnol. 2015, 42, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.; Nielsen, J. Genome scale metabolic modeling of cancer. Metab. Eng. 2017, 43, 103–112. [Google Scholar] [CrossRef]
- Panahi, B.; Khalilpour Shadbad, R. Navigating the microalgal maze: A comprehensive review of recent advances and future perspectives in biological networks. Planta 2024, 260, 114. [Google Scholar] [CrossRef]
- Parray, J.A.; Yaseen Mir, M.; Shameem, N.; Parray, J.A.; Yaseen Mir, M.; Shameem, N. Advancement in sustainable agriculture: Computational and bioinformatics tools. In Sustainable Agriculture: Biotechniques in Plant Biology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 465–547. [Google Scholar]
- Greiner, A.; Kelterborn, S.; Evers, H.; Kreimer, G.; Sizova, I.; Hegemann, P. Targeting of photoreceptor genes in Chlamydomonas reinhardtii via zinc-finger nucleases and CRISPR/Cas9. Plant Cell 2017, 29, 2498–2518. [Google Scholar] [CrossRef]
- Javaid, D.; Ganie, S.Y.; Hajam, Y.A.; Reshi, M.S. CRISPR/Cas9 system: A reliable and facile genome editing tool in modern biology. Mol. Biol. Rep. 2022, 49, 12133–12150. [Google Scholar] [CrossRef]
- Selle, K.; Klaenhammer, T.R.; Barrangou, R. CRISPR-based screening of genomic island excision events in bacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 8076–8081. [Google Scholar] [CrossRef]
- Dziubańska-Kusibab, P.J.; Nevedomskaya, E.; Haendler, B. Preclinical anticipation of on-and off-target resistance mechanisms to anti-cancer drugs: A systematic review. Int. J. Mol. Sci. 2024, 25, 705. [Google Scholar] [CrossRef]
- Hosseini, S.Y.; Mallick, R.; Mäkinen, P.; Ylä-Herttuala, S. Insights into Prime Editing Technology: A Deep Dive into Fundamentals, Potentials, and Challenges. Hum. Gene Ther. 2024, 35, 649–668. [Google Scholar] [CrossRef]
- Manghwar, H.; Li, B.; Ding, X.; Hussain, A.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas systems in genome editing: Methodologies and tools for sgRNA design, off-target evaluation, and strategies to mitigate off-target effects. Adv. Sci. 2020, 7, 1902312. [Google Scholar] [CrossRef]
- Medford, J.I.; Shih, P.M.; De Paoli, H.C.; Trinh, C.T.; McCormick, A.J.; Hussey, S.G.; Myburg, A.A.; Erik, J.; Mahmudul, H.; Kalluri, U.C. Plant biosystems design research roadmap 1.0. In BioDesign Research; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Wu, J.-L.; Zheng, S.-S.; Wang, L.; Xiang, X.; Li, F.; Lv, M.-L.; Wu, Q.; Huang, Z.-X.; Miao, H.-B. CRISPR-Cas System-Mediated Genetic Modification in Bacillus spp.: Current Status and Future. J. Agric. Food Chem. 2025, 73, 14760–14775. [Google Scholar] [CrossRef]
- Li, C. Modelling Liver Disease in Vitro and in Silico. Ph.D. Thesis, King’s College London, London, UK, 2022. [Google Scholar]
- Kim, T.; Ko, M.; Rha, E.; Kim, H.; Lee, H. Microfluidics-driven high-throughput phenotyping and screening in synthetic biology: From single cells to cell-free systems. Biotechnol. Bioprocess Eng. 2024, 29, 25–33. [Google Scholar] [CrossRef]
- Bjork, S.M.; Joensson, H.N. Microfluidics for cell factory and bioprocess development. Curr. Opin. Biotechnol. 2019, 55, 95–102. [Google Scholar] [CrossRef]
- Brookes, G.; Smyth, S.J. Risk-appropriate regulations for gene-editing technologies. GM Crops Food 2024, 15, 1–14. [Google Scholar] [CrossRef]
- Giacomella, L. Techno-economic assessment (TEA) and life cycle costing analysis (LCCA): Discussing methodological steps and integrability. Insights Into Reg. Dev. 2021, 3, 176–197. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.M. Geographically-Resolved Life Cycle Assessment and Techno-Economic Analysis of Engineered Climate Solutions With an Innovative Framework for Decision Support. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2024. [Google Scholar]
- Piergentili, R.; Del Rio, A.; Signore, F.; Umani Ronchi, F.; Marinelli, E.; Zaami, S. CRISPR-Cas and its wide-ranging applications: From human genome editing to environmental implications, technical limitations, hazards and bioethical issues. Cells 2021, 10, 969. [Google Scholar] [CrossRef]
- Beacham, T.A.; Sweet, J.B.; Allen, M.J. Large scale cultivation of genetically modified microalgae: A new era for environmental risk assessment. Algal Res. 2017, 25, 90–100. [Google Scholar] [CrossRef]
- Sebesta, J.; Xiong, W.; Guarnieri, M.T.; Yu, J. Biocontainment of genetically engineered algae. Front. Plant Sci. 2022, 13, 839446. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y. An engineered bacterium auxotrophic for an unnatural amino acid: A novel biological containment system. PeerJ 2015, 3, e1247. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garrido, I. Microalgae immobilization: Current techniques and uses. Bioresour. Technol. 2008, 99, 3949–3964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, Y.; Wang, C.; Liu, M.; Li, H.; Fu, Y.; Wang, Y.; Nie, Y.; Liu, X.; Ji, W. Large-scale transcriptome comparison reveals distinct gene activations in wheat responding to stripe rust and powdery mildew. BMC Genom. 2014, 15, 898. [Google Scholar] [CrossRef]
- De Monerri, N.C.S.; Kim, K. Pathogens hijack the epigenome: A new twist on host-pathogen interactions. Am. J. Pathol. 2014, 184, 897–911. [Google Scholar] [CrossRef]
- Patra, D. CRISPR-engineered microalgae: A promising approach for the production of therapeutic proteins. Innov. Mol. Biotechnol. 2023, 11, 1. [Google Scholar] [CrossRef]
- Minhas, W.R.; Saleemi, H.; Raheem, M.F.; Khalid, S.S.; Aslam, H.; Ahmad, W. Crispr-Engineered Microbes For Enhanced Biodegradation of Recalcitrant Pollutants a Genomic Approach to Environmental Remediation. Front. Health Inform. 2024, 13. [Google Scholar] [CrossRef]
- Mikhaylova, E.V. Crop genome editing–regulations and policies. In Genome Editing and Global Food Security; Routledge: Oxfordshire, UK, 2023; pp. 212–235. [Google Scholar]
- Winberg, P. Scaling up for New Opportunities in the Practical Use of Algae (Applied Phycology); Rural Industries Research and Development Corporation: Wagga, Australia, 2011. [Google Scholar]
- McHenry, M. A rural bioeconomic strategy to redefine primary production systems within the Australian innovation system: Productivity, management, and impact of climate change. In Agriculture Management for Climate Change; Nova Science Publishers: Hauppauge, NY, USA, 2015; pp. 55–70. [Google Scholar]
- Salehipour-Bavarsad, F.; Nematollahi, M.A.; Pistocchi, R.; Pezzolesi, L. Algal food safety: Possible contaminations, challenges of harmonized quality assessments, and suggested recommendations for the nascent industry of microalgae-based products. Algal Res. 2024, 81, 103579. [Google Scholar] [CrossRef]
- Lowe, C.; Minssen, T.; Skentelbery, C. Emerging Biotechnologies in Europe: Foresight for Policy; Joint Research Centre: Brussels, Belgium, 2024. [Google Scholar]
| Tool Category | Core Mechanism | Key Applications | Key Advantages | Main Limitations | Ref. |
|---|---|---|---|---|---|
| CRISPRa/i | dCas9 fused to activators/repressors |
|
|
| [69,133,161,162] |
| Epigenome Editing | dCas9 fused to epigenetic modifiers |
|
|
| [27,87,89,163,164,165] |
| Base Editors | nCas9 fused to deaminase |
|
|
| [166,167,168,169,170,171,172] |
| Prime Editors | nCas9-RT + pegRNA |
|
|
| [6,45,170,171,172,173,174] |
| Multiplexed Systems | tRNA-gRNA arrays; Orthogonal Cas |
|
|
| [90,175,176,177,178,179,180,181] |
| CRISPR Biosensors/Logic Gates | gRNA switches; dCas-split effectors |
|
|
| [69,159,182,183,184,185,186,187,188] |
| Engineering Goal | Potential CRISPR Tool(s) Applied | Strategy | Key Outcomes | Ref. |
|---|---|---|---|---|
| Enhanced Photosynthesis and Biomass | CRISPRi + Base editing + Multiplexing |
| Improved photon penetration; 10% ↑ CO2 fixation; reduced carbon loss. | [28,101,156,216,217,218] |
| Lipid/Biofuel Optimization | Multiplexed CRISPRi + CRISPRa + Base editing + Biosensors |
| >25% lipid yield ↑ in Nannochloropsis; dynamic flux control; high-oleic acid profiles. | [160,204,219,220,221,222,223] |
| High-Value Compound Synthesis | CRISPRa + Base editing + Epigenome editing + PE |
| Stable astaxanthin flux; enhanced EPA/DHA; scarless protein expression. | [6,9,101,188,197,224,225,226] |
| Stress Resilience | CRISPRa + Base editing + Screening |
| Improved thermotolerance; reduced ROS; identification of novel resilience genes. | [62,198,227,228,229,230,231,232] |
| Carbon Capture and Utilization | Base editing + Multiplexed CRISPRi + Logic gates |
| Improved carbon flux to products; broadened substrate utilization. | [9,101,180,193,215,233,234,235] |
| Integrated Industrial Strain | All tools (epigenome + PE + CRISPRa/i + Base editing) |
| Multi-trait industrial strain: high lipid yield, stable expression, stress resilience. | [28,30,57,122,193,228,236,237,238,239] |
| Species | Model Status | Preferred Cas Variant(s) | Typical Editing Efficiency (KO) | HDR Efficiency | Major Species-Specific Challenges | Ref. |
|---|---|---|---|---|---|---|
| Chlamydomonas reinhardtii (Green Alga) | Model | SpCas9, FnCas12a | Moderate–High (10–50%) | Low (1–5%) | Low HDR, silencing, need for cell wall-deficient strains | [62,247,248,249] |
| Nannochloropsis spp. (Eustigmatophyte) | Non-model | FnCas12a, LbCas12a | High (20–80%) | Very Low (<1%) | Very low HDR, rigid cell wall, silencing, PEG sensitivity | [219,245,250,251,252,253] |
| Phaeodactylum tricornutum (Diatom) | Non-model | FnCas12a, LbCas12a | High (30–70%) | Very Low (<1%) | Very low HDR, silica cell wall, epigenetic silencing | [62,88,250,254,255,256,257,258] |
| Haematococcus pluvialis (Green Alga) | Non-model | SpCas9 (optimized) | Moderate (reported) | Very Low (<1%) | Extremely thick cell wall, low transformation efficiency, astaxanthin interference | [28,259,260,261,262,263,264] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Lu, Q. Beyond Cutting: CRISPR-Driven Synthetic Biology Toolkit for Next-Generation Microalgal Metabolic Engineering. Int. J. Mol. Sci. 2025, 26, 7470. https://doi.org/10.3390/ijms26157470
Yang L, Lu Q. Beyond Cutting: CRISPR-Driven Synthetic Biology Toolkit for Next-Generation Microalgal Metabolic Engineering. International Journal of Molecular Sciences. 2025; 26(15):7470. https://doi.org/10.3390/ijms26157470
Chicago/Turabian StyleYang, Limin, and Qian Lu. 2025. "Beyond Cutting: CRISPR-Driven Synthetic Biology Toolkit for Next-Generation Microalgal Metabolic Engineering" International Journal of Molecular Sciences 26, no. 15: 7470. https://doi.org/10.3390/ijms26157470
APA StyleYang, L., & Lu, Q. (2025). Beyond Cutting: CRISPR-Driven Synthetic Biology Toolkit for Next-Generation Microalgal Metabolic Engineering. International Journal of Molecular Sciences, 26(15), 7470. https://doi.org/10.3390/ijms26157470







