Sulforaphane-Rich Broccoli Sprout Extract Promotes Hair Regrowth in an Androgenetic Alopecia Mouse Model via Enhanced Dihydrotestosterone Metabolism
Abstract
1. Introduction
2. Results
2.1. BSE Characterization
2.1.1. BSE Chemical Profile
2.1.2. Feature-Based Molecular Networking (FBMN) Analysis
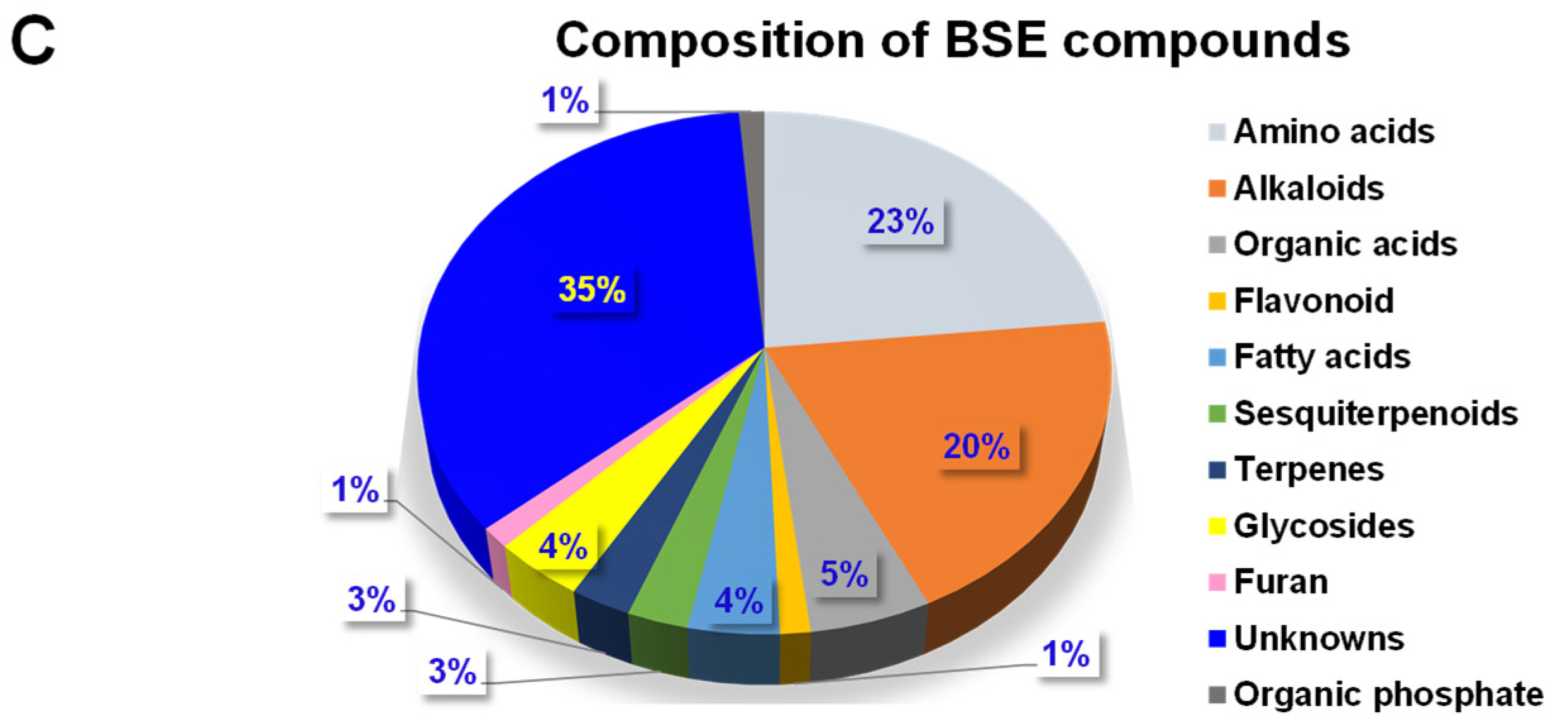
2.1.3. Chemical Composition and Classification
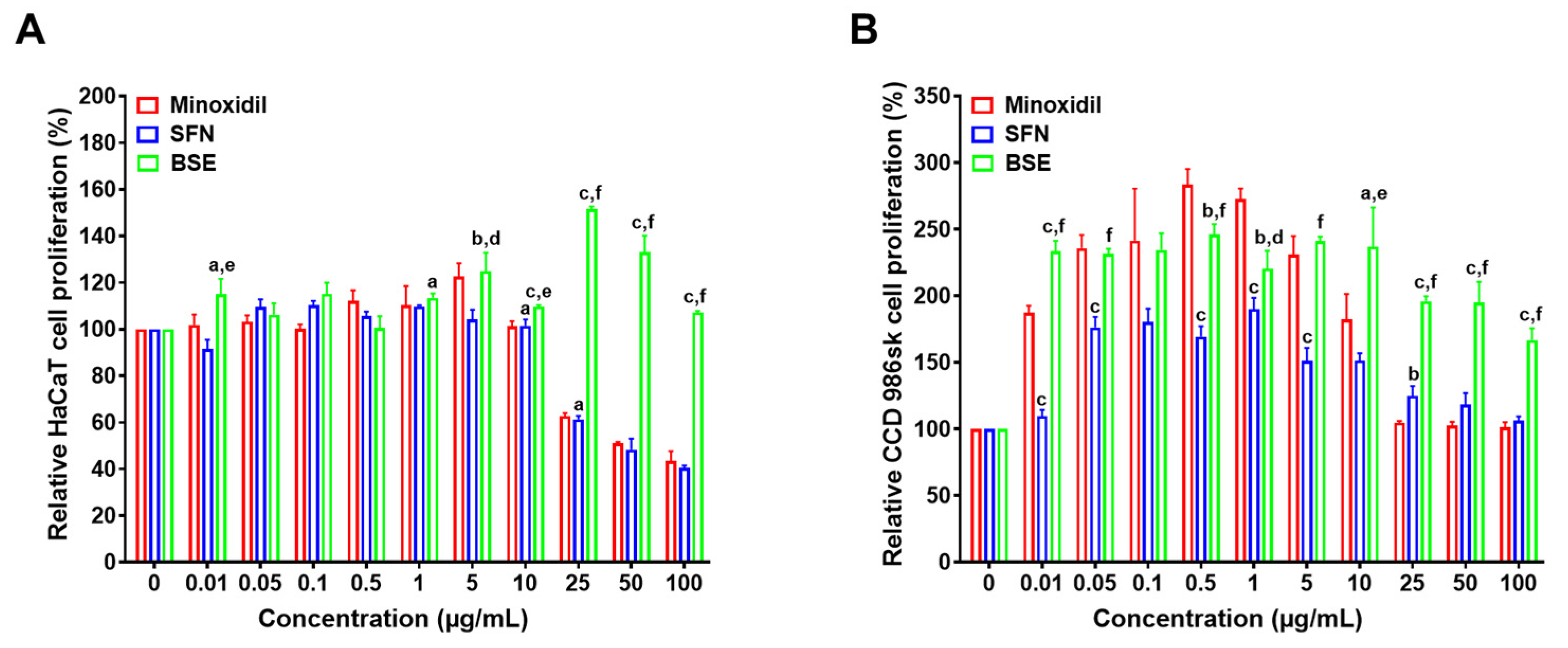
2.2. In Vitro Cell Proliferation Assay
2.3. In Vitro Scratch Wound Assay
2.4. In Vivo Oral Absorption in Rats
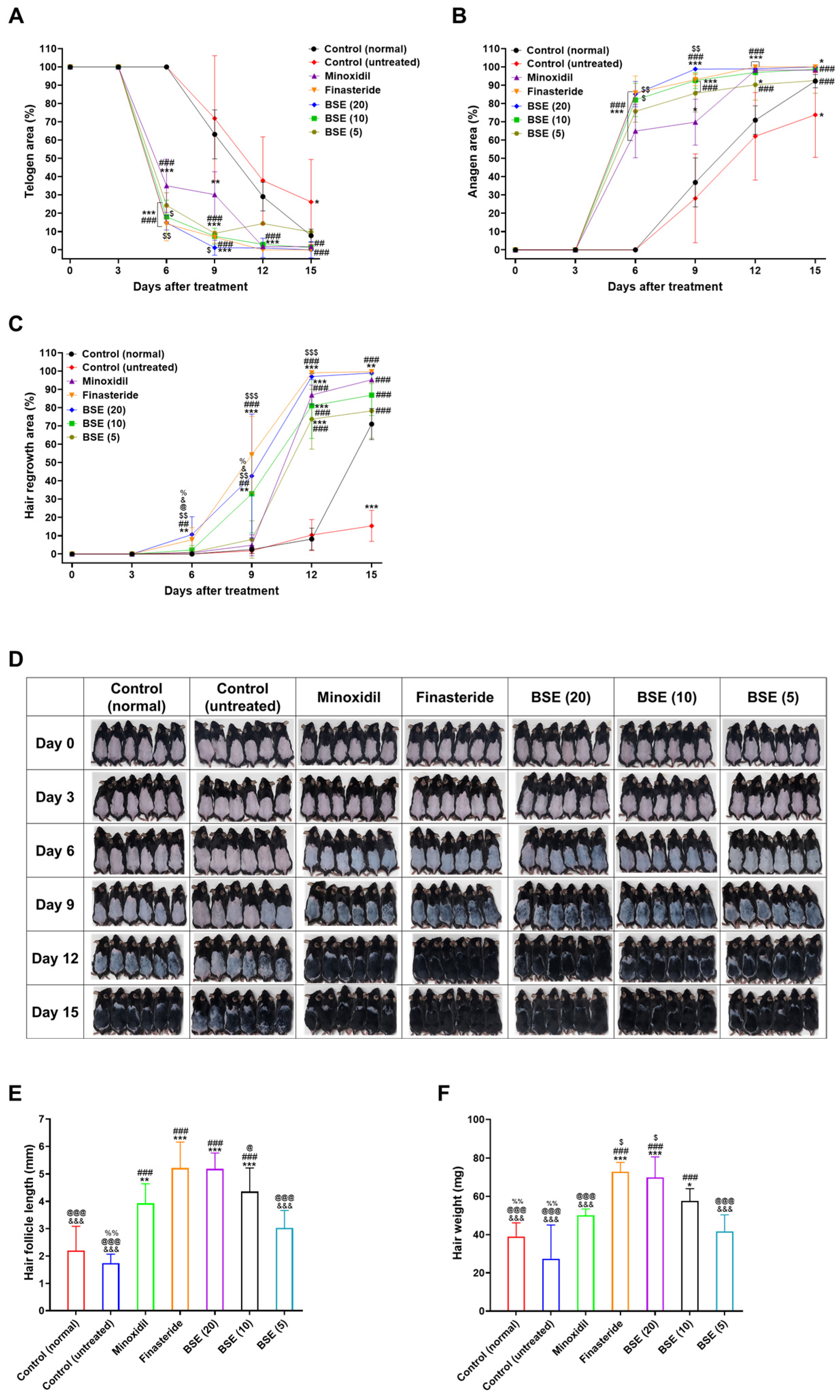
2.5. In Vivo Hair Regrowth Efficacy
2.6. Hair Follicle Length and Hair Weight
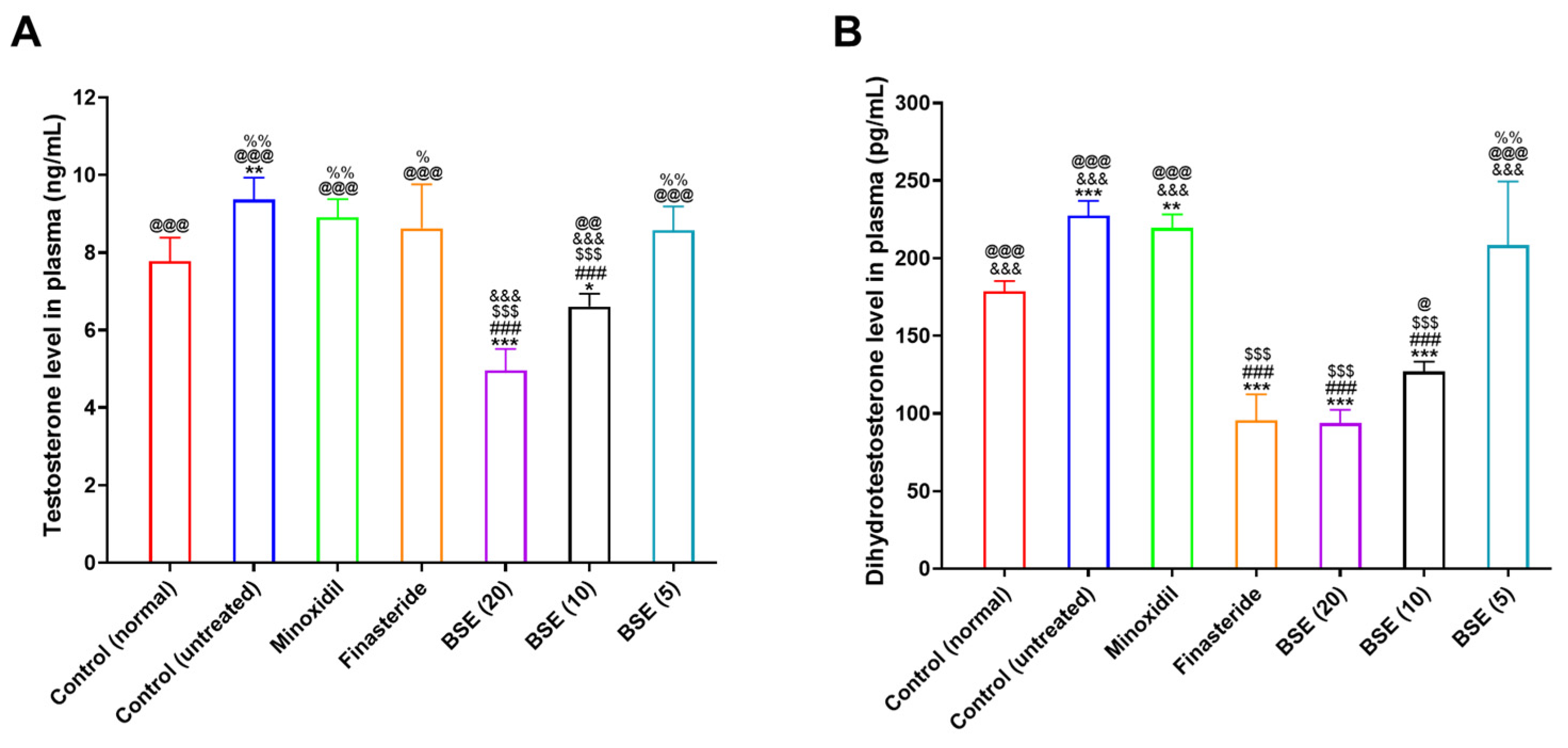
2.7. Plasma Testosterone and DHT Levels
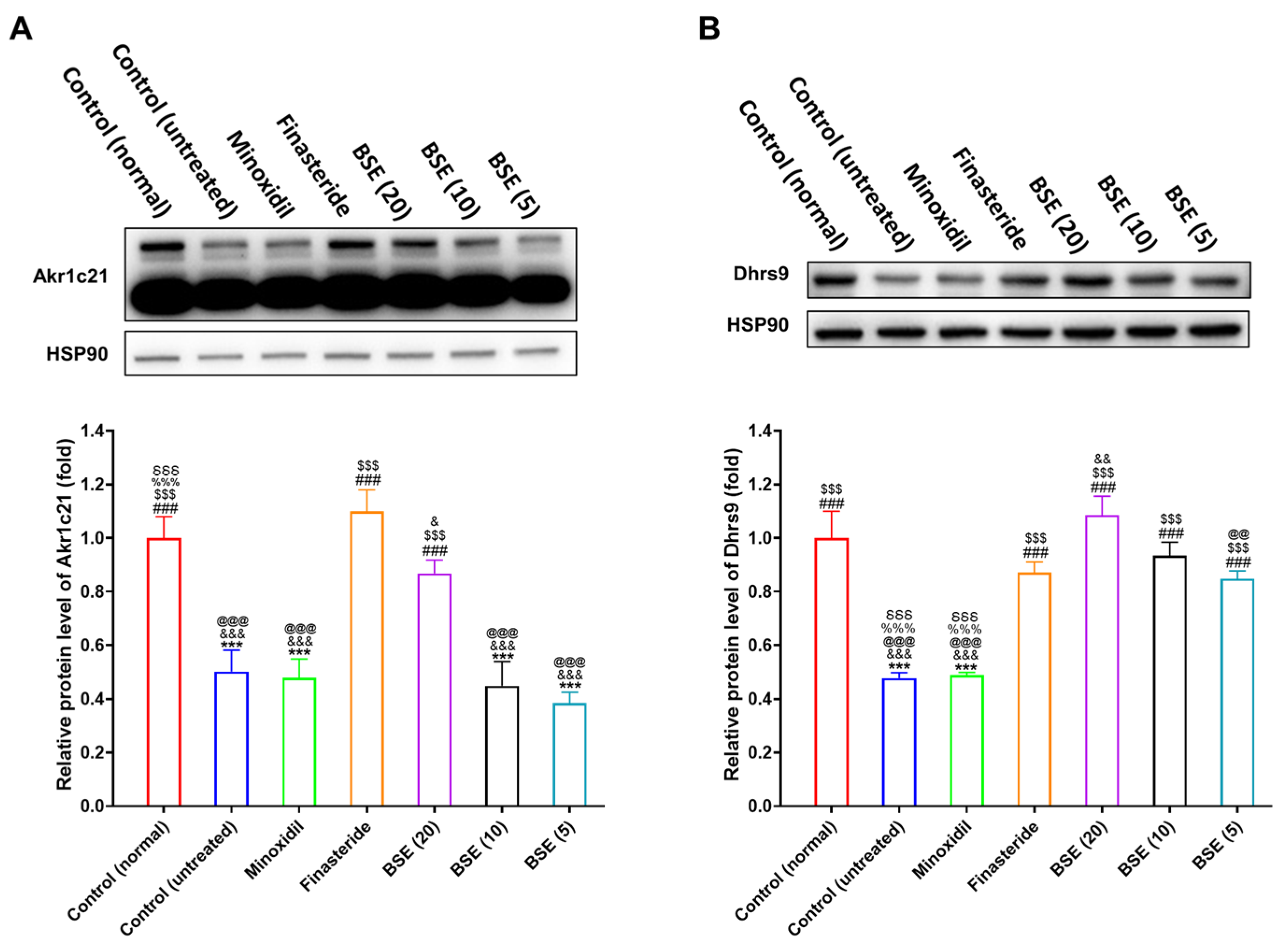
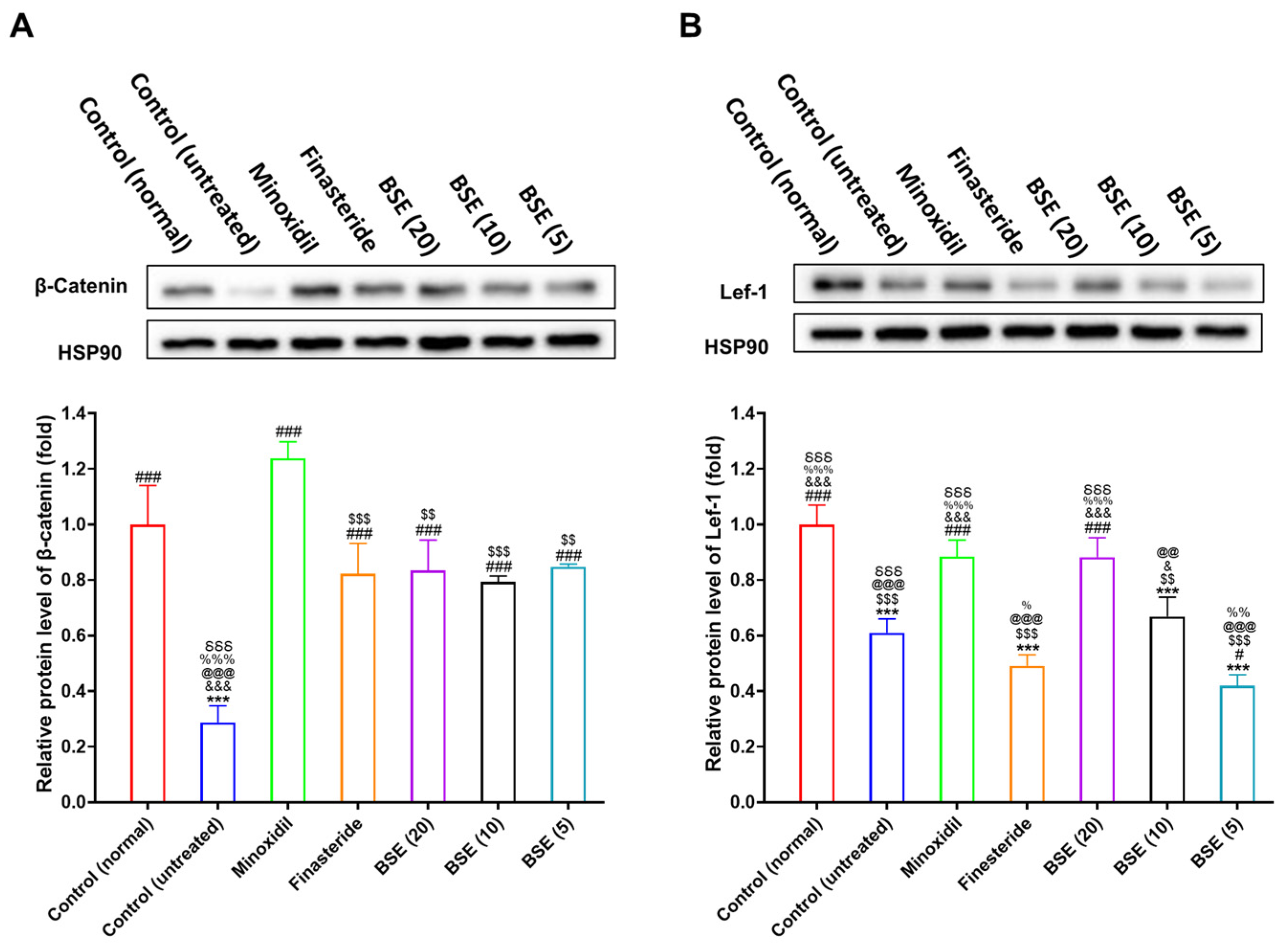
2.8. Restoration of DHT Metabolism and Wnt Signaling by BSE Treatment
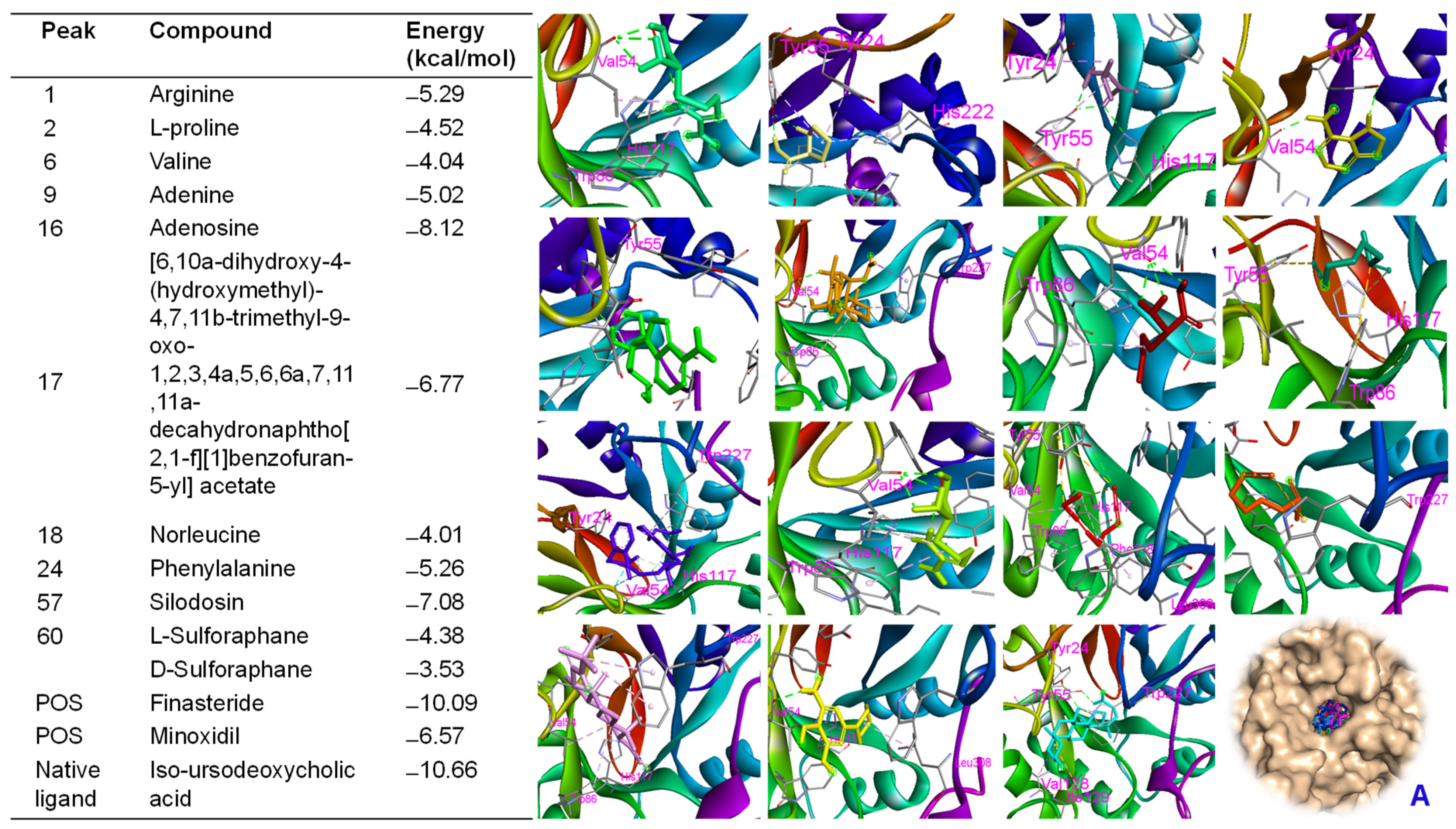
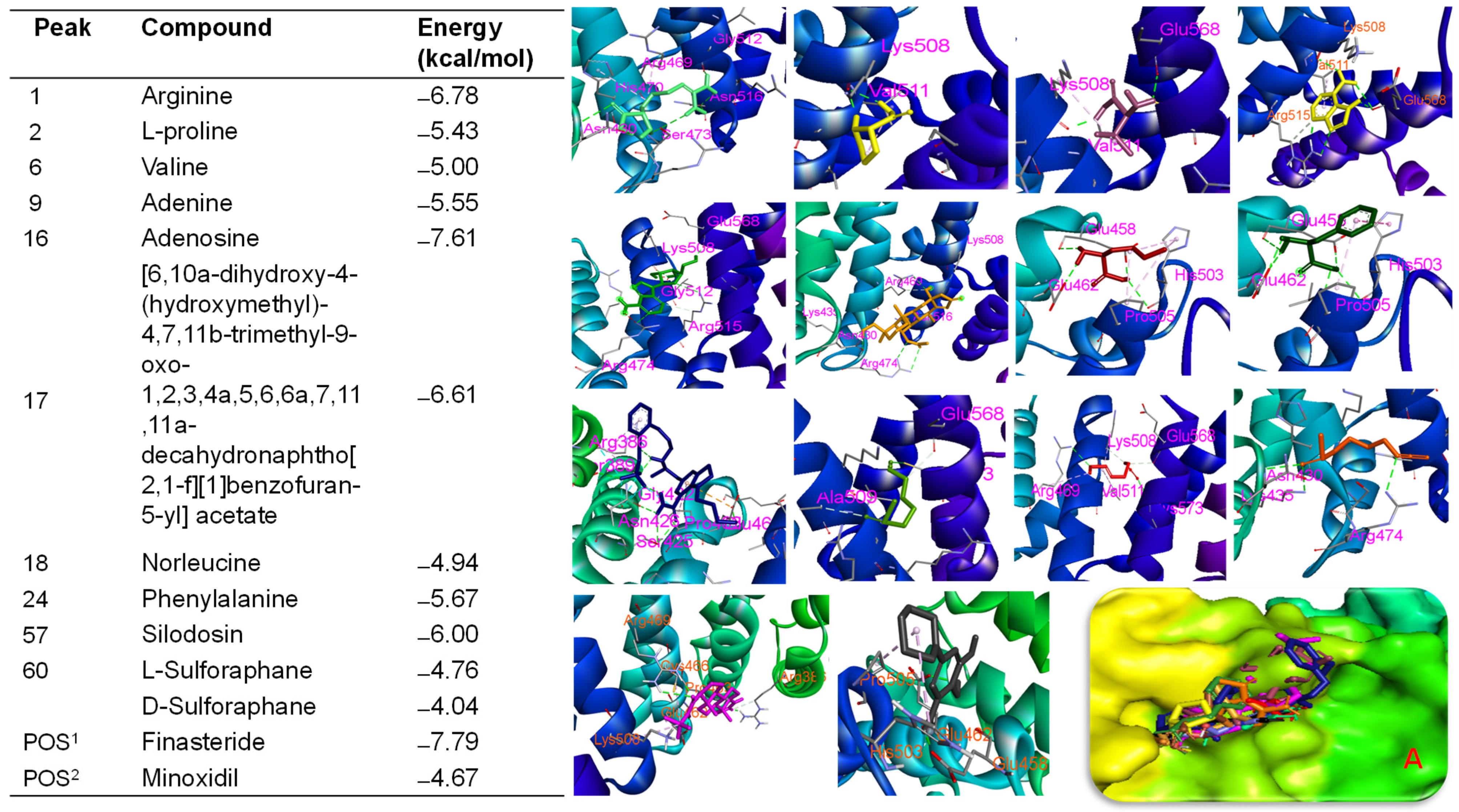
2.9. Molecular Docking Approach
2.10. Molecular Dynamic Simulation Analysis of Ligand–Protein Complexes
2.10.1. β-Catenin (1JDH) Complexes
RMSD and Structural Stability
Rg and Compactness
Solvent Accessibility and Flexibility
H-Bond
RMSF and Residue-Level Flexibility
2.10.2. Akr1c2 (1IHI) Complexes
RMSD and Stability
Rg and Structural Compactness
SASA
Hydrogen Bonding
RMSF
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. BSE Preparation and Quantification
4.3.1. Sample Preparation and Analysis
4.3.2. LC-MS/MS and Compound Annotation
4.4. In Vitro Cell Proliferation Assay
4.5. In Vitro Scratch Wound Recovery Assay
4.6. In Vivo Oral Absorption in Rats
4.7. In Vivo Hair Regrowth Efficacy
4.8. Plasma Testosterone and DHT Levels
4.9. Western Blot Assay
4.10. Molecular Docking
4.11. Molecular Dynamic Simulation
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGA | Androgenetic alopecia |
| FDA | Food drug administration |
| Akr1c2 | Aldo-keto reductase family 1 member C2 |
| AUCinf | Area under the curve to infinity |
| AUClast | Area under the curve at the last time point |
| BSE | Broccoli sprout extract |
| CCD-986sk | Dermal fibroblast |
| Cmax | Maximum plasma concentration |
| Dhrs9 | Dehydrogenase/reductase 9 |
| DHT | Dihydrotestosterone |
| FBMN | Feature-based molecular networking |
| GNPS | Global natural products social molecular networking |
| HaCaT | Keratinocytes |
| HDP | Human dermal papilla |
| HSD | Hepatic hydroxysteroid dehydrogenase |
| IV | Intravenous |
| Lef-1 | Lymphoid enhancer-binding factor-1 |
| Rg | Radius of gyration |
| RMSD | Root mean square deviation |
| SASA | Solvent-accessible surface area |
| SFN | Sulforaphane |
| SMSF | Root mean square fluctuation |
| T1/2 | Half-life |
| Tmax | Time to maximum peak drug concentration |
References
- Islam, N.; Leung, P.S.; Huntley, A.C.; Gershwin, M.E. The autoimmune basis of alopecia areata: A comprehensive review. Autoimmun. Rev. 2015, 14, 81–89. [Google Scholar] [CrossRef]
- Dhami, L. Psychology of Hair Loss Patients and Importance of Counseling. Indian J. Plast. Surg. 2021, 54, 411–415. [Google Scholar] [CrossRef]
- Aukerman, E.L.; Jafferany, M. The psychological consequences of androgenetic alopecia: A systematic review. J. Cosmet. Dermatol. 2023, 22, 89–95. [Google Scholar] [CrossRef]
- Pozo-Pérez, L.; Tornero-Esteban, P.; López-Bran, E. Clinical and preclinical approach in AGA treatment: A review of current and new therapies in the regenerative field. Stem Cell Res. Ther. 2024, 15, 260. [Google Scholar] [CrossRef] [PubMed]
- Ntshingila, S.; Oputu, O.; Arowolo, A.T.; Khumalo, N.P. Androgenetic alopecia: An update. JAAD Int. 2023, 13, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Natarelli, N.; Gahoonia, N.; Sivamani, R.K. Integrative and Mechanistic Approach to the Hair Growth Cycle and Hair Loss. J. Clin. Med. 2023, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Molecular mechanisms of androgenetic alopecia. Exp. Gerontol. 2002, 37, 981–990. [Google Scholar] [CrossRef]
- Kidangazhiathmana, A.; Santhosh, P. Pathogenesis of Androgenetic Alopecia. Clin. Dermatol. Rev. 2022, 6, 69–74. [Google Scholar] [CrossRef]
- Fuxjager, M.J.; Schuppe, E.R.; Hoang, J.; Chew, J.; Shah, M.; Schlinger, B.A. Expression of 5α- and 5β-reductase in spinal cord and muscle of birds with different courtship repertoires. Front. Zool. 2016, 13, 25. [Google Scholar] [CrossRef]
- Ishizaki, F.; Nishiyama, T.; Kawasaki, T.; Miyashiro, Y.; Hara, N.; Takizawa, I.; Naito, M.; Takahashi, K. Androgen deprivation promotes intratumoral synthesis of dihydrotestosterone from androgen metabolites in prostate cancer. Sci. Rep. 2013, 3, 1528. [Google Scholar] [CrossRef]
- Sawaya, M.E.; Honig, L.S.; Garland, L.D.; Hsia, S.L. delta 5-3 beta-hydroxysteroid dehydrogenase activity in sebaceous glands of scalp in male-pattern baldness. J. Investig. Dermatol. 1988, 91, 101–105. [Google Scholar] [CrossRef]
- Kinter, J.K.; Amraei, R.; Anekar, A.A. Biochemistry, Dihydrotestosterone. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557634/ (accessed on 12 April 2025).
- Diviccaro, S.; Melcangi, R.C.; Giatti, S. Post-finasteride syndrome: An emerging clinical problem. Neurobiol. Stress 2020, 12, 100209. [Google Scholar] [CrossRef]
- Kaufman, K.D.; Olsen, E.A.; Whiting, D.; Savin, R.; DeVillez, R.; Bergfeld, W.; Price, V.H.; Van Neste, D.; Roberts, J.L.; Hordinsky, M.; et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J. Am. Acad. Dermatol. 1998, 39, 578–589. [Google Scholar] [CrossRef]
- Park, S.; Lee, J. Modulation of Hair Growth Promoting Effect by Natural Products. Pharmaceutics 2021, 13, 2163. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Kou, X.; Niu, Y.; Liu, Y.; Zheng, B.; Ma, J.; Liu, M.; Xue, Z. Progress on the mechanism of natural products alleviating androgenetic alopecia. Eur. J. Med. Chem. 2024, 264, 116022. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, L.; Arlt, W.; Storbeck, K.H. Intracrine androgen biosynthesis, metabolism and action revisited. Mol. Cell. Endocrinol. 2018, 465, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.E.; Baniasadi, M.; Giansiracusa, D.; Giansiracusa, M.; Garcia, M.; Fryda, Z.; Wong, T.L.; Bishayee, A. Sulforaphane: A Broccoli Bioactive Phytocompound with Cancer Preventive Potential. Cancers 2021, 13, 4796. [Google Scholar] [CrossRef]
- Haslam, I.S.; Jadkauskaite, L.; Szabó, I.L.; Staege, S.; Hesebeck-Brinckmann, J.; Jenkins, G.; Bhogal, R.K.; Lim, F.L.; Farjo, N.; Farjo, B.; et al. Oxidative Damage Control in a Human (Mini-) Organ: Nrf2 Activation Protects against Oxidative Stress-Induced Hair Growth Inhibition. J. Investig. Dermatol. 2017, 137, 295–304. [Google Scholar] [CrossRef]
- Sasaki, M.; Shinozaki, S.; Shimokado, K. Sulforaphane promotes murine hair growth by accelerating the degradation of dihydrotestosterone. Biochem. Biophys. Res. Commun. 2016, 472, 250–254. [Google Scholar] [CrossRef]
- Wu, H.; Liang, H.; Yuan, Q.; Wang, T.; Yan, X. Preparation and stability investigation of the inclusion complex of sulforaphane with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2010, 82, 613–617. [Google Scholar] [CrossRef]
- Tian, G.; Li, Y.; Cheng, L.; Yuan, Q.; Tang, P.; Kuang, P.; Hu, J. The mechanism of sulforaphene degradation to different water contents. Food Chem. 2016, 194, 1022–1027. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.; Chen, Q.S.; Chai, Y.F.; Sun, D.X.; Zhang, G.Q. Analysis of Sulforaphane by HPLC-MS/MS in vitro and in vivo: Chemical Stability, Metabolic Rate and Metabolites. Asian J. Chem. 2015, 27, 1045–1048. [Google Scholar] [CrossRef]
- Son, E.S.; Fei, X.; Yoon, J.H.; Seo, S.Y.; Maeng, H.J.; Jeong, S.H.; Kim, Y.C. Comparison of Pharmacokinetics and Anti-Pulmonary Fibrosis-Related Effects of Sulforaphane and Sulforaphane N-acetylcysteine. Pharmaceutics 2021, 13, 958. [Google Scholar] [CrossRef]
- Fahey, J.W.; Kensler, T.W. The Challenges of Designing and Implementing Clinical Trials with Broccoli Sprouts… and Turning Evidence into Public Health Action. Front. Nutr. 2021, 8, 648788. [Google Scholar] [CrossRef]
- Fahey, J.W.; Wade, K.L.; Wehage, S.L.; Holtzclaw, W.D.; Liu, H.; Talalay, P.; Fuchs, E.; Stephenson, K.K. Stabilized sulforaphane for clinical use: Phytochemical delivery efficiency. Mol. Nutr. Food. Res. 2017, 61, 1600766. [Google Scholar] [CrossRef]
- Dwibedi, C.; Axelsson, A.S.; Abrahamsson, B.; Fahey, J.W.; Asplund, O.; Hansson, O.; Ahlqvist, E.; Tremaroli, V.; Bäckhed, F.; Rosengren, A.H. Effect of broccoli sprout extract and baseline gut microbiota on fasting blood glucose in prediabetes: A randomized, placebo-controlled trial. Nat. Microbiol. 2025, 10, 681–693. [Google Scholar] [CrossRef]
- Atwell, L.L.; Hsu, A.; Wong, C.P.; Stevens, J.F.; Bella, D.; Yu, T.W.; Pereira, C.B.; Löhr, C.V.; Christensen, J.M.; Dashwood, R.H.; et al. Absorption and chemopreventive targets of sulforaphane in humans following consumption of broccoli sprouts or a myrosinase-treated broccoli sprout extract. Mol. Nutr. Food Res. 2015, 59, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Uthaman, S.K.; Kang, W.S.; Park, J.Y.; Kim, S.; Le, D.D.; Oh, S.J.; Murugesh, K.; Oh, L.M.; Lee, M.; Park, J.W. Endogenous extraction yielded high quality sulforaphane from broccoli sprouts unveils potent antioxidant and anti-Alzheimer’s activities. Heliyon 2025, 11, e42673. [Google Scholar] [CrossRef] [PubMed]
- Mamede, R.; Pereira, F.; Aires-de-Sousa, J. Machine learning prediction of UV-Vis spectra features of organic compounds related to photoreactive potential. Sci. Rep. 2021, 11, 23720. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Xia, H.; Xia, G.; Wei, X.; Li, J.; Wu, Y.; Lin, S. Feature-based molecular networking (FBMN): An efficient booster for discovering novel natural products or metabolites. Acta Pharm. Sin. B 2025, 15, 2283–2286. [Google Scholar] [CrossRef]
- Nothias, L.F.; Nothias-Esposito, M.; da Silva, R.; Wang, M.; Protsyuk, I.; Zhang, Z.; Sarvepalli, A.; Leyssen, P.; Touboul, D.; Costa, J.; et al. Bioactivity-Based Molecular Networking for the Discovery of Drug Leads in Natural Product Bioassay-Guided Fractionation. J. Nat. Prod. 2018, 81, 758–767. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Penning, T.M. Molecular docking simulations of steroid substrates into human cytosolic hydroxysteroid dehydrogenases (AKR1C1 and AKR1C2): Insights into positional and stereochemical preferences. Steroids 2006, 71, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Ru, Z.Q.; Peng, Y.; Fu, Z.; Jia, Q.Y.; Kang, Z.J.; Li, Y.S.; Huang, Y.B.; Yin, S.G.; Guo, K.; et al. Peptide Cy (RL-QN15) accelerates hair regeneration in diabetic mice by binding to the Frizzled-7 receptor. Zool. Res. 2024, 45, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Xu, Q.; Li, C.; Zhao, H. Effects of sulforaphane on neural stem cell proliferation and differentiation. Genesis 2017, 55, e23022. [Google Scholar] [CrossRef]
- Rossi, A.; Magri, F.; di Fraia, M.; Caro, G.; Fortuna, M.C.; Piacentini, M.; Celleno, L. A new combination of molecules for the treatment of androgenetic alopecia and telogen effluvium: A double-blind randomized, monocentric, placebo-controlled study. Ital. J. Dermatol. Venerol. 2022, 157, 78–83. [Google Scholar] [CrossRef]
- Reygagne, P.; Mandel, V.D.; Delva, C.; Havlíčková, M.; Padlewska, K.; Khalil, R.; Meuleman, V.; Miranda, G.A.; Nevskaya, M.; Michelet, J.F.; et al. An anti-hair loss treatment in the management of mild androgenetic alopecia: Results from a large, international observational study. Dermatol. Ther. 2021, 34, e15134. [Google Scholar] [CrossRef]
- Pode-Shakked, B.; Shemer-Meiri, L.; Harmelin, A.; Stettner, N.; Brenner, O.; Abraham, S.; Schwartz, G.; Anikster, Y. Man made disease: Clinical manifestations of low phenylalanine levels in an inadequately treated phenylketonuria patient and mouse study. Mol. Genet. Metab. 2013, 110, S66–S70. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Le, D.D.; Jang, Y.S.; Truong, V.; Dinh, T.; Dang, T.; Yu, S.; Lee, M. Anti-Inflammatory Effects and Metabolomic Analysis of Ilex Rotunda Extracted by Supercritical Fluid Extraction. Int. J. Mol. Sci. 2024, 25, 11965. [Google Scholar] [CrossRef]
- Katajamaa, M.; Miettinen, J.; Oresic, M. MZmine: Toolbox for processing and visualization of mass spectrometry based molecular profile data. Bioinformatics 2006, 22, 634–636. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- Le, D.D.; Kim, Y.R.; Yu, S.; Dang, T.; Lee, K.T.; Lee, M. Phytochemical and bioactivities of promising flavonoid glycosides and their content from unmatured fruits of Vicia bungei with their bioactivity. Food Chem. 2024, 460, 140541. [Google Scholar] [CrossRef]
- Subedi, L.; Pandey, P.; Shim, J.H.; Kim, K.T.; Cho, S.S.; Koo, K.T.; Kim, B.J.; Park, J.W. Preparation of topical bimatoprost with enhanced skin infiltration and in vivo hair regrowth efficacy in androgenic alopecia. Drug Deliv. 2022, 29, 328–341. [Google Scholar] [CrossRef]
- Pulat, S.; Subedi, L.; Pandey, P.; Bhosle, S.R.; Hur, J.-S.; Shim, J.-H.; Cho, S.-S.; Kim, K.-T.; Ha, H.-H.; Kim, H.; et al. Topical Delivery of Atraric Acid Derived from Stereocaulon japonicum with Enhanced Skin Permeation and Hair Regrowth Activity for Androgenic Alopecia. Pharmaceutics 2023, 15, 340. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Han, Q.; Ma, Z.; Yan, Q.; Pei, Y.; Shi, P.; Zhang, J.; Rong, K.; Ma, K.; Li, P.; et al. Injectable platelet rich fibrin facilitates hair follicle regeneration by promoting human dermal papilla cell proliferation, migration, and trichogenic inductivity. Exp. Cell Res. 2021, 409, 112888. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Pandey, P.; Khadka, B.; Shim, J.H.; Cho, S.S.; Kweon, S.; Byun, Y.; Kim, K.T.; Park, J.W. Enhancement of the anticancer effect of atorvastatin-loaded nanoemulsions by improving oral absorption via multivalent intestinal transporter-targeting lipids. Drug Deliv. 2022, 29, 3397–3413. [Google Scholar] [CrossRef]
- Kim, D.; Lee, E.; Choi, P.G.; Kim, H.S.; Park, S.H.; Seo, H.D.; Hahm, J.H.; Ahn, J.; Jung, C.H. Justicia procumbens prevents hair loss in androgenic alopecia mice. Biomed. Pharmacother. 2024, 170, 115913. [Google Scholar] [CrossRef]
- Tawanwongsri, W.; Desai, D.D.; Nohria, A.; Shapiro, J.; Lo Sicco, K.I. Hair loss in athletic testosterone use in males: A narrative review. Int. J. Dermatol. 2025, 64, 654–658. [Google Scholar] [CrossRef]
- Fu, D.; Huang, J.; Li, K.; Chen, Y.; He, Y.; Sun, Y.; Guo, Y.; Du, L.; Qu, Q.; Miao, Y.; et al. Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 mice simulates androgenetic alopecia. Biomed. Pharmacother. 2021, 137, 111247. [Google Scholar] [CrossRef]
- Zheng, Y.; Hsieh, J.C.; Escandon, J.; Cotsarelis, G. Isolation of Mouse Hair Follicle Bulge Stem Cells and Their Functional Analysis in a Reconstitution Assay. Methods Mol. Biol. 2016, 1453, 57–69. [Google Scholar]
- Wang, W.-H.; Ramos, R.; Tai, K.-Y.; Wu, Y.-S.; Chang, T.-Y.; Yan, J.-Y.; Plikus, M.V.; Oh, J.W.; Lin, S.-J. Studying Hair Growth Cycle and its Effects on Mouse Skin. J. Investig. Dermatol. 2023, 143, 1638–1645. [Google Scholar] [CrossRef]
- Heo, Y.S.; Song, H.J. Characterizing cutaneous elastic fibers by eosin fluorescence detected by fluorescence microscopy. Ann. Dermatol. 2011, 23, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Le, D.; Truong, V.; Dang, T.; Yu, S.; Dinh, T.; Lee, M. Phenolics from Ilex rotunda Possess Antioxidative Effects and Block Activation of MAPK and NF-κB Signaling by Inhibiting IL-2 Production in CD3/CD8 Activated Jurkat T Cells. Antioxidants 2025, 14, 281. [Google Scholar] [CrossRef] [PubMed]
- Snyder, H.D.; Kucukkal, T.G. Computational Chemistry Activities with Avogadro and ORCA. J. Chem. Educ. 2021, 98, 1335–1341. [Google Scholar] [CrossRef]
- Le, D.D.; Jang, Y.S.; Truong, V.; Yu, S.; Dinh, T.; Lee, M. Bioactivities of Quinic Acids from Vitex rotundifolia Obtained by Supercritical Fluid Extraction. Antioxidants 2024, 13, 1235. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Yu, J.; Le, D.; Han, S.; Yu, S.; Lee, M. Anti-inflammatory activity of caffeic acid derivatives from Ilex rotunda. Int. Immunopharmacol. 2023, 115, 109610. [Google Scholar] [CrossRef]
- Lemkul, J.A. Introductory Tutorials for Simulating Protein Dynamics with GROMACS. J. Phys. Chem. B 2024, 128, 9418–9435. [Google Scholar] [CrossRef]
- Showalter, S.A.; Brüschweiler, R. Validation of Molecular Dynamics Simulations of Biomolecules Using NMR Spin Relaxation as Benchmarks: Application to the AMBER99SB Force Field. J. Chem. Theory Comput. 2007, 3, 961–975. [Google Scholar] [CrossRef]
- Boonstra, S.; Onck, P.R.; Giessen, E. CHARMM TIP3P Water Model Suppresses Peptide Folding by Solvating the Unfolded State. J. Phys. Chem. B 2016, 120, 3692–3698. [Google Scholar] [CrossRef]
- Kashefolgheta, S.; Wang, S.; Acree, W.E.; Hünenberger, P.H. Evaluation of nine condensed-phase force fields of the GROMOS, CHARMM, OPLS, AMBER, and OpenFF families against experimental cross-solvation free energies. Phys. Chem. Chem. Phys. 2021, 23, 13055–13074. [Google Scholar] [CrossRef]
- Singh, H.; Raja, A.; Prakash, A.; Medhi, B. Gmx_qk: An Automated Protein/Protein-Ligand Complex Simulation Workflow Bridged to MM/PBSA, Based on Gromacs and Zenity-Dependent GUI for Beginners in MD Simulation Study. J. Chem. Inf. Model. 2023, 63, 2603–2608. [Google Scholar] [CrossRef]


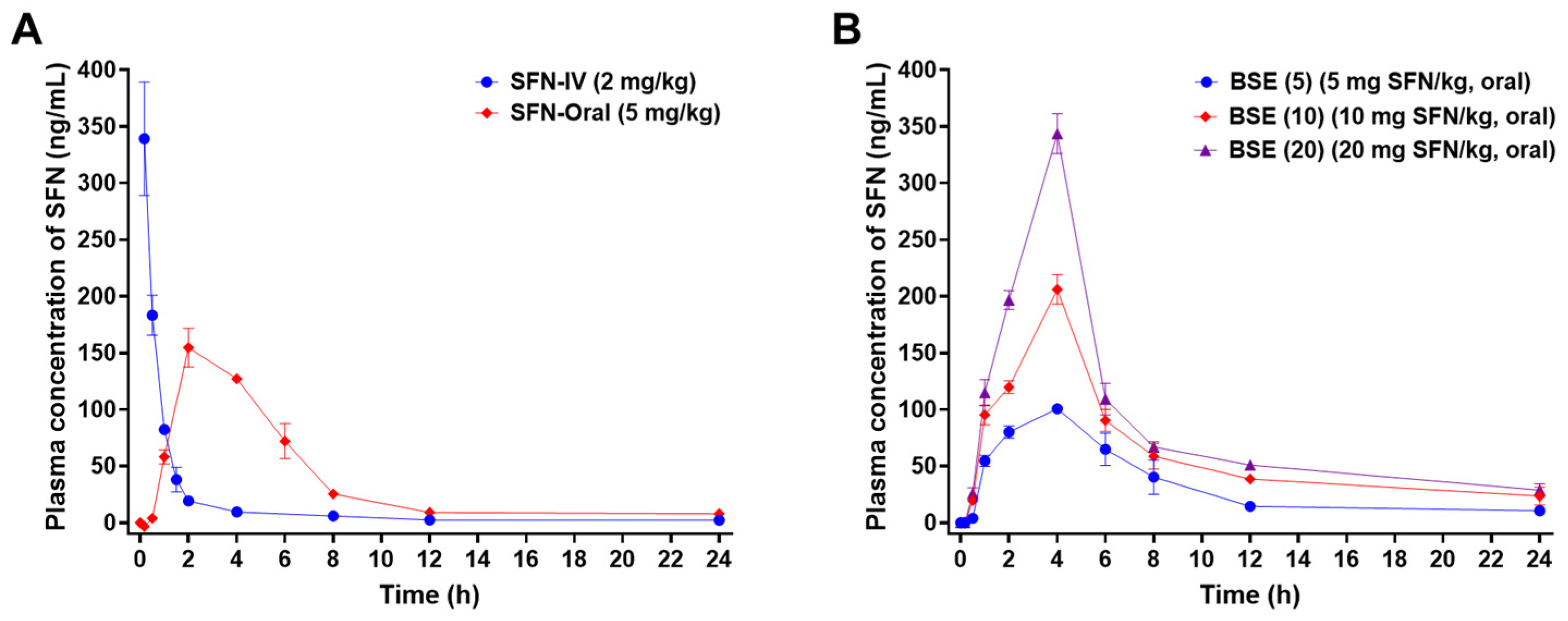


| Test Material | SFN-IV | SFN-Oral | BSE (5) | BSE (10) | BSE (20) |
|---|---|---|---|---|---|
| Administration route | IV | Oral | Oral | Oral | Oral |
| SFN dose (mg/kg) | 2 | 5 | 5 | 10 | 20 |
| Tmax (h) | 2 ± 0 | 4 ± 0 | 4 ± 0 | 4 ± 0 | |
| T1/2 (h) | 7 ± 3 | 5 ± 0 | 7 ± 1 | 14 ± 8 | 13 ± 2 |
| Cmax (ng/mL) | 339 ± 50 | 155 ± 17 | 101 ± 3 | 206 ± 13 | 344 ± 18 |
| AUClast (ng·h/mL) | 403 ± 52 | 874 ± 46 | 794 ± 73 | 1475 ± 17 | 2082 ± 27 |
| AUCinf (ng·h/mL) | 421 ± 67 | 934 ± 41 | 897 ± 71 | 1997 ± 403 | 2650 ± 125 |
| Bioavailability (%) | 100 | 87 ± 5 | 79 ± 7 | 73 ± 1 | 52 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subedi, L.; Le, D.D.; Kim, E.; Phuyal, S.; Bamjan, A.D.; Truong, V.; Kim, N.A.; Shim, J.-H.; Seo, J.B.; Oh, S.-J.; et al. Sulforaphane-Rich Broccoli Sprout Extract Promotes Hair Regrowth in an Androgenetic Alopecia Mouse Model via Enhanced Dihydrotestosterone Metabolism. Int. J. Mol. Sci. 2025, 26, 7467. https://doi.org/10.3390/ijms26157467
Subedi L, Le DD, Kim E, Phuyal S, Bamjan AD, Truong V, Kim NA, Shim J-H, Seo JB, Oh S-J, et al. Sulforaphane-Rich Broccoli Sprout Extract Promotes Hair Regrowth in an Androgenetic Alopecia Mouse Model via Enhanced Dihydrotestosterone Metabolism. International Journal of Molecular Sciences. 2025; 26(15):7467. https://doi.org/10.3390/ijms26157467
Chicago/Turabian StyleSubedi, Laxman, Duc Dat Le, Eunbin Kim, Susmita Phuyal, Arjun Dhwoj Bamjan, Vinhquang Truong, Nam Ah Kim, Jung-Hyun Shim, Jong Bae Seo, Suk-Jung Oh, and et al. 2025. "Sulforaphane-Rich Broccoli Sprout Extract Promotes Hair Regrowth in an Androgenetic Alopecia Mouse Model via Enhanced Dihydrotestosterone Metabolism" International Journal of Molecular Sciences 26, no. 15: 7467. https://doi.org/10.3390/ijms26157467
APA StyleSubedi, L., Le, D. D., Kim, E., Phuyal, S., Bamjan, A. D., Truong, V., Kim, N. A., Shim, J.-H., Seo, J. B., Oh, S.-J., Lee, M., & Park, J. W. (2025). Sulforaphane-Rich Broccoli Sprout Extract Promotes Hair Regrowth in an Androgenetic Alopecia Mouse Model via Enhanced Dihydrotestosterone Metabolism. International Journal of Molecular Sciences, 26(15), 7467. https://doi.org/10.3390/ijms26157467







