Endothelial Impairment in HIV-Associated Preeclampsia: Roles of Asymmetric Dimethylarginine and Prostacyclin
Abstract
1. Introduction
Rationale of Study
2. Results
2.1. Clinical Findings and Demographics
2.2. Concentration of Plasma ADMA
- Preeclampsia HIV-positive vs. Preeclampsia HIV-negative—A statistically significant reduction in ADMA levels were observed in the PE+ (median = 9504 ng/mL; 95% CI: 8878–10,040) compared to the PE− group (median = 10,500 ng/mL; 95% CI: 9602–14,230), (Mann–Whitney U = 125.5; p = 0.0174 *; Figure 1A).
- Normotensive HIV-positive vs. HIV-negative—No significant difference in ADMA expression was observed between N+ and N− groups (p = 0.3204; Figure 1B).
- Preeclampsia HIV-positive vs. Normotensive HIV-positive—ADMA concentration did not differ significantly between PE+ and N+ groups (p = 0.3718; Figure 1C).
- Preeclampsia HIV-negative vs. Normotensive HIV-negative—Although PE− had a higher median ADMA level (10,500 ng/mL; 95% CI: 9602–14,230) compared to N− (median = 9484 ng/mL; 95% CI: 8711–10,170), the difference was near significant (Mann–Whitney U = 184.5; p = 0.0512; Figure 1D).
- HIV status—No significant difference in ADMA levels was observed between HIV-positive (median = 9784 ng/mL; 95% CI: 8952–11,140) and HIV-negative individuals (median = 9734 ng/mL; 95% CI: 9455–11,900), (Mann–Whitney U = 779.0; p = 0.3591; Figure 1E).
- Pregnancy type—ADMA concentrations did not differ significantly between preeclamptic (median = 9854 ng/mL; 95% CI: 9482–11,890) and normotensive groups (median = 9694 ng/mL; 95% CI: 8923–11,160) (Mann–Whitney U = 802.0; p = 0.4769; Figure 1F).
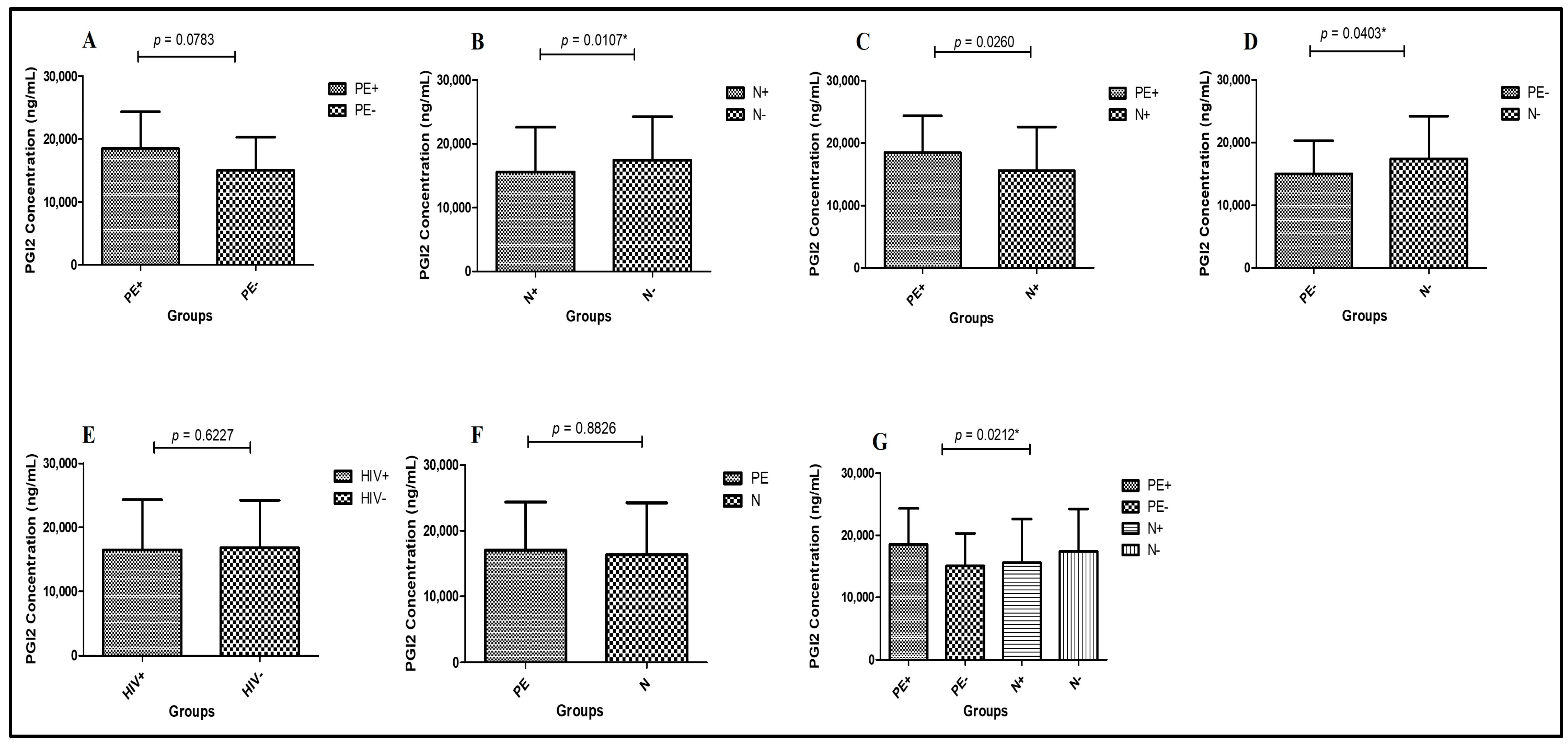
2.3. Concentrations of Plasma PGI2
- Preeclampsia HIV-positive vs. Normotensive HIV-positive—PGI2 concentrations were significantly higher in PE+ (median = 18,480 ng/mL; 95% CI: 19,430–15,720) compared to N+ (median = 15,580 ng/mL; 95% CI: 17,660–15,290) (Mann–Whitney U = 131.5; p = 0.0260 *; Figure 2A).
- Normotensive HIV-positive vs. Normotensive HIV-negative—PGI2 levels were significantly lower in N+ (median = 15,580 ng/mL; 95% CI: 16,660–13,310) compared to N− (median = 17,380 ng/mL; 95% CI: 19,500–16,440) (Mann–Whitney U = 118.5; p = 0.0107 *; Figure 2B).
- Preeclampsia HIV-negative vs. Normotensive HIV-negative—PGI2 concentrations were significantly reduced in PE− (median = 15,030 ng/mL; 95% CI: 17,110–13,180) compared to N− (median = 17,380 ng/mL; 95% CI: 19,500–16,440) groups (Mann–Whitney U = 138.5; p = 0.0403 *; Figure 2C).
- Preeclampsia HIV-positive vs. Preeclampsia HIV-negative—There were no statistically significant differences in PGI2 levels between PE+ (median = 18,480 ng/mL; 95% CI: 19,430–15,720) and PE− (median = 15,030 ng/mL; 95% CI: 17,110–13,180) groups (Mann–Whitney U = 150.0; p = 0.0783; Figure 2D).
- Pregnancy type—Regardless of HIV status, PGI2 concentrations did not differ significantly between PE (median = 17,060 ng/mL; 95% CI: 17,710–15,010) and N groups (median = 16,360 ng/mL; 95% CI: 17,660–15,290) (Mann–Whitney U = 865.0; p = 0.8826; Figure 2E).
- HIV status—PGI2 concentrations were similar, although non-significantly elevated, in HIV+ individuals (median = 16,480 ng/mL; 95% CI: 17,550–15,010) compared to HIV- individuals (median = 16,810 ng/mL; 95% CI: 17,830–15,290) (Mann–Whitney U = 826.5; p = 0.6227; Figure 2F).
3. Discussion
4. Materials and Methods
4.1. Ethics Approval
4.2. Study Population
4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS. Global HIV & AIDS Statistics-Fact Sheet; UNAIDS: Geneva, Switzerland, 2024. [Google Scholar]
- Statistics South Africa. Mid-Year Population Estimates 2022; Statistics South Africa: Pretoria, South Africa, 2022. [Google Scholar]
- Marinda, E.; Simbayi, L.; Zuma, K.; Zungu, N.; Moyo, S.; Kondlo, L.; Jooste, S.; Nadol, P.; Igumbor, E.; Dietrich, C.; et al. Towards achieving the 90–90–90 HIV targets: Results from the south African 2017 national HIV survey. BMC Public Health 2020, 20, 1375. [Google Scholar] [CrossRef]
- Moosa, A.; Gengiah, T.N.; Lewis, L.; Naidoo, K. Long-term adherence to antiretroviral therapy in a South African adult patient cohort: A retrospective study. BMC Infect. Dis. 2019, 19, 775. [Google Scholar] [CrossRef]
- Ademuyiwa, S.E.A.; Saliu, I.O.; Akinola, B.K.; Akinmoladun, A.C.; Olaleye, M.T.; Ademuyiwa, A.I.; Akindahunsi, A.A. Impact of highly active antiretroviral drug therapy (HAART) on biochemical, hematologic, atherogenic and anthropometric profiles of human immunodeficiency virus patients at a tertiary hospital in Owo, Nigeria. Bull. Nat. Res. Cent. 2022, 46, 263. [Google Scholar] [CrossRef]
- Department of Health, South Africa. 2019 ART Clinical Guidelines for the Management of HIV in Adults, Pregnancy, Adolescents, Children, Infants and Neonates; Republic of South Africa National Department of Health: Pretoria, South Africa, 2019. [Google Scholar]
- WHO. Progress Report 2016: Prevent HIV, Test and Treat All: WHO Support for Country Impact; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Jiang, H.; Zhou, Y.; Tang, W. Maintaining HIV care during the COVID-19 pandemic. Lancet HIV 2020, 7, e308–e309. [Google Scholar] [CrossRef] [PubMed]
- Mhango, M.; Chitungo, I.; Dzinamarira, T. COVID-19 lockdowns: Impact on facility-based HIV testing and the case for the scaling up of home-based testing services in sub-Saharan Africa. AIDS Behav. 2020, 24, 3014–3016. [Google Scholar] [CrossRef]
- Moodley, J. Deaths from hypertensive disorders of pregnancy during 2017–2019: Declining trends in South Africa. Obstet. Gynaecol. Forum 2020, 30, 3–5. [Google Scholar]
- Magee, L.; Singer, J.; Lee, T.; Rey, E.; Asztalos, E.; Hutton, E.; Helewa, M.; Logan, A.; Ganzevoort, W.; Welch, R. The impact of pre-eclampsia definitions on the identification of adverse outcome risk in hypertensive pregnancy–analyses from the CHIPS trial (Control of Hypertension in Pregnancy Study). BJOG Int. J. Obstet. Gynaecol. 2021, 128, 1373–1382. [Google Scholar] [CrossRef]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef]
- George, E.M.; Granger, J.P. Endothelin: Key mediator of hypertension in preeclampsia. Am. J. Hypertens. 2011, 24, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Gathiram, P.; Moodley, J. Pre-eclampsia: Its pathogenesis and pathophysiolgy: Review articles. Cardiol. J. Afr. 2016, 27, 71–78. [Google Scholar] [CrossRef]
- Mannaerts, D.; Faes, E.; Gielis, J.; Van Craenenbroeck, E.; Cos, P.; Spaanderman, M.; Gyselaers, W.; Cornette, J.; Jacquemyn, Y. Oxidative stress and endothelial function in normal pregnancy versus pre-eclampsia, a combined longitudinal and case control study. BMC Preg Child. 2018, 18, 60. [Google Scholar] [CrossRef]
- Kornacki, J.; Wirstlein, P.; Wender-Ozegowska, E. Markers of endothelial injury and dysfunction in early-and late-onset preeclampsia. Life 2020, 10, 239. [Google Scholar] [CrossRef]
- Wojtowicz, A.; Zembala-Szczerba, M.; Babczyk, D.; Kołodziejczyk-Pietruszka, M.; Lewaczyńska, O.; Huras, H. Early-and late-onset preeclampsia: A comprehensive cohort study of laboratory and clinical findings according to the new ISHHP criteria. Int. J. Hypertens. 2019, 2019, 4108271. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Chaemsaithong, P.; Yeo, L.; Romero, R. Pre-eclampsia part 1: Current understanding of its pathophysiology. Nat. Rev. Nephrol. 2014, 10, 466–480. [Google Scholar] [CrossRef]
- Laresgoiti-Servitje, E. A leading role for the immune system in the pathophysiology of preeclampsia. J. Leukoc. Biol. 2013, 94, 247–257. [Google Scholar] [CrossRef]
- McElwain, C.J.; Tuboly, E.; McCarthy, F.P.; McCarthy, C.M. Mechanisms of endothelial dysfunction in pre-eclampsia and gestational diabetes mellitus: Windows into future cardiometabolic health? Front. Endocrinol. 2020, 11, 655. [Google Scholar] [CrossRef] [PubMed]
- Kornacki, J.; Gutaj, P.; Kalantarova, A.; Sibiak, R.; Jankowski, M.; Wender-Ozegowska, E. Endothelial Dysfunction in Pregnancy Complications. Biomedicines 2021, 9, 1756. [Google Scholar] [CrossRef] [PubMed]
- Afrose, D.; Chen, H.; Ranashinghe, A.; Liu, C.-c.; Henessy, A.; Hansbro, P.M.; McClements, L. The diagnostic potential of oxidative stress biomarkers for preeclampsia: Systematic review and meta-analysis. Biol. Sex Differ. 2022, 13, 26. [Google Scholar] [CrossRef]
- Maynard, S.E.; Karumanchi, S.A. Angiogenic factors and preeclampsia. Sem. Nephrol. 2011, 31, 33–46. [Google Scholar] [CrossRef]
- Roberts, J.; Cooper, D.W. Pathogenesis and genetics of pre-eclampsia. Lancet 2001, 357, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Addis, D.R.; Lambert, J.A.; Ren, C.; Doran, S.; Aggarwal, S.; Jilling, T.; Matalon, S. Vascular Endothelial Growth Factor-121 Administration Mitigates Halogen Inhalation-Induced Pulmonary Injury and Fetal Growth Restriction in Pregnant Mice. J. Am. Heart Assoc. 2020, 9, e013238. [Google Scholar] [CrossRef]
- Yu, W.; Gao, W.; Rong, D.; Wu, Z.; Khalil, R.A. Molecular determinants of microvascular dysfunction in hypertensive pregnancy and preeclampsia. Microcirculation 2019, 26, e12508. [Google Scholar] [CrossRef]
- Oliveira, L.G.d.; Karumanchi, A.; Sass, N. Preeclampsia: Oxidative stress, inflammation and endothelial dysfunction. Rev. Bras. Ginecol. Obstet. 2010, 32, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Prefumo, F.; Sebire, N.; Thilaganathan, B. Decreased endovascular trophoblast invasion in first trimester pregnancies with high-resistance uterine artery Doppler indices. Hum. Reprod. 2004, 19, 206–209. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D. Predictive value of the sFlt-1: PlGF ratio in women with suspected preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Cooke, J.P. Asymmetrical dimethylarginine: The Uber marker? Circulation 2004, 109, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Birdsey, G.M.; Anthony, S.; Arrigoni, F.I.; Leiper, J.M.; Vallance, P. Dimethylarginine dimethylaminohydrolase activity modulates ADMA levels, VEGF expression, and cell phenotype. Biochem. Biophys. Res. Commun. 2003, 308, 984–989. [Google Scholar] [CrossRef]
- Noorbakhsh, M.; Kianpour, M.; Nematbakhsh, M. Serum levels of asymmetric dimethylarginine, vascular endothelial growth factor, and nitric oxide metabolite levels in preeclampsia patients. ISRN Obstet. Gynecol. 2013, 2013, 104213. [Google Scholar] [CrossRef]
- Holden, D.P.; Fickling, S.A.; Whitley, G.S.J.; Nussey, S.S. Plasma concentrations of asymmetric dimethylarginine, a natural inhibitor of nitric oxide synthase, in normal pregnancy and preeclampsia. Am. J. Obstet. Gynecol. 1998, 178, 551–556. [Google Scholar] [CrossRef]
- Teerlink, T.; Luo, Z.; Palm, F.; Wilcox, C.S. Cellular ADMA: Regulation and action. Pharmacol. Res. 2009, 60, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Böger, R.H.; Diemert, A.; Schwedhelm, E.; Lüneburg, N.; Maas, R.; Hecher, K. The role of nitric oxide synthase inhibition by asymmetric dimethylarginine in the pathophysiology of preeclampsia. Gynecol. Obstet. Investig. 2010, 69, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, A.; Hedner, T.; Milsom, I. Increased circulating concentrations of asymmetric dimethyl arginine (ADMA), an endogenous inhibitor of nitric oxide synthesis, in preeclampsia. Acta Obstet. Gynecol. Scand. 1998, 77, 808–813. [Google Scholar] [CrossRef]
- Kim, Y.; Park, H.; Lee, H.; Ha, E.; Suh, S.; Oh, S.; Yoo, H.-S. Reduced l-arginine Level and Decreased Placental eNOS Activity in Preeclampsia. Placenta 2006, 4, 438–444. [Google Scholar] [CrossRef]
- Maas, R.; Böger, R.H.; Schwedhelm, E.; Casas, J.P.; López-Jaramillo, P.; Serrano, N.; Díaz, L.A. Plasma concentrations of asymmetric dimethylarginine (ADMA) in Colombian women with pre-eclampsia. JAMA 2004, 291, 823–824. [Google Scholar] [CrossRef]
- Braekke, K.; Ueland, P.M.; Harsem, N.K.; Staff, A.C. Asymmetric dimethylarginine in the maternal and fetal circulation in preeclampsia. Pediatr. Res. 2009, 66, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Shixia, C.; Duan, T. First-trimester maternal serum levels of sFLT1, PGF and ADMA predict preeclampsia. PLoS ONE 2015, 10, e0124684. [Google Scholar] [CrossRef] [PubMed]
- Chedraui, P.; Solis, E.J.; Bocci, G.; Gopal, S.; Russo, E.; Escobar, G.S.; Hidalgo, L.; Pérez-López, F.R.; Genazzani, A.R.; Mannella, P. Feto-placental nitric oxide, asymmetric dimethylarginine and vascular endothelial growth factor (VEGF) levels and VEGF gene polymorphisms in severe preeclampsia. J. Matern.-Fetal Neonatal Med. 2013, 26, 226–232. [Google Scholar] [CrossRef]
- Demir, B.; Demir, S.; Pasa, S.; Guven, S.; Atamer, Y.; Atamer, A.; Kocyigit, Y. The role of homocysteine, asymmetric dimethylarginine and nitric oxide in pre-eclampsia. J. Obstet. Gynaecol. 2012, 32, 525–528. [Google Scholar] [CrossRef]
- Sandrim, V.C.; Palei, A.C.; Metzger, I.F.; Cavalli, R.C.; Duarte, G.; Tanus-Santos, J.E. Interethnic differences in ADMA concentrations and negative association with nitric oxide formation in preeclampsia. Clin. Chim. Acta 2010, 411, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Xue, M.; Qin, D.; Zhu, X.; Wang, C.; Zhu, J.; Hao, T.; Cheng, L.; Chen, X.; Bai, Z. HIV-1 Tat promotes Kaposi’s sarcoma-associated herpesvirus (KSHV) vIL-6-induced angiogenesis and tumorigenesis by regulating PI3K/PTEN/AKT/GSK-3β signaling pathway. PLoS ONE 2013, 8, e53145. [Google Scholar] [CrossRef]
- Paladugu, R.; Fu, W.; Conklin, B.S.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Hiv Tat protein causes endothelial dysfunction in porcine coronary arteries. J. Vasc. Surg. 2003, 38, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, L.; Bruder-Nascimento, T.; Greene, L.; Kennard, S.; Belin de Chantemèle, E.J. Chronic exposure to HIV-derived protein tat impairs endothelial function via indirect alteration in fat mass and nox1-mediated mechanisms in mice. Int. J. Mol. Sci. 2021, 22, 10977. [Google Scholar] [CrossRef] [PubMed]
- Naicker, T.; Govender, N.; Abel, T.; Naidoo, N.; Moodley, M.; Pillay, Y.; Singh, S.; Khaliq, O.P.; Moodley, J. HIV associated preeclampsia: A multifactorial appraisal. Int. J. Mol. Sci. 2021, 22, 9157. [Google Scholar] [CrossRef]
- Kurz, K.; Teerlink, T.; Sarcletti, M.; Weiss, G.; Zangerle, R.; Fuchs, D. Plasma concentrations of the cardiovascular risk factor asymmetric dimethylarginine (ADMA) are increased in patients with HIV-1 infection and correlate with immune activation markers. Pharmacol. Res. 2009, 60, 508–514. [Google Scholar] [CrossRef]
- Haissman, J.M.; Haugaard, A.K.; Knudsen, A.; Kristoffersen, U.S.; Seljeflot, I.; Pedersen, K.K.; Lebech, A.-M.; Hasbak, P.; Kjær, A.; Ostrowski, S.R. Marker of endothelial dysfunction asymmetric dimethylarginine is elevated in HIV infection but not associated with subclinical atherosclerosis. J. Acquir. Immune Defic Syndr. 2016, 73, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.V.; Neuhaus, J.; Duprez, D.; Freiberg, M.; Bernardino, J.I.; Badley, A.D.; Nixon, D.E.; Lundgren, J.D.; Tracy, R.P.; Neaton, J.D. HIV replication, inflammation, and the effect of starting antiretroviral therapy on plasma asymmetric dimethylarginine, a novel marker of endothelial dysfunction. J. Acquir. Immune Defic. Syndr. 2012, 60, 128–134. [Google Scholar] [CrossRef]
- Savvidou, M.D.; Hingorani, A.D.; Tsikas, D.; Frölich, J.C.; Vallance, P.; Nicolaides, K.H. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet 2003, 361, 1511–1517. [Google Scholar] [CrossRef]
- Siroen, M.P.; Teerlink, T.; Nijveldt, R.J.; Prins, H.A.; Richir, M.C.; Leeuwen, P.A.v. The clinical significance of asymmetric dimethylarginine. Annu. Rev. Nutr. 2006, 26, 203–228. [Google Scholar] [CrossRef][Green Version]
- Parikh, R.V.; Scherzer, R.; Grunfeld, C.; Nitta, E.M.; Leone, A.; Martin, J.N.; Deeks, S.G.; Ganz, P.; Hsue, P.Y. Elevated Levels of Asymmetric Dimethylarginine are Associated With Lower CD4+ Count and Higher Viral Load in HIV-Infected Individuals. Atherosclerosis 2013, 229, 246–252. [Google Scholar] [CrossRef]
- Leone, A.; Moncada, S.; Vallance, P.; Calver, A.; Collier, J. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992, 339, 572–575. [Google Scholar] [CrossRef]
- MacAllister, R.J.; Fickling, S.A.; Whitley, G.S.; Vallance, P. Metabolism of methylarginines by human vasculature; implications for the regulation of nitric oxide synthesis. Br. J. Pharmacol. 1994, 112, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Fickling, S.; Williams, D.; Vallance, P.; Nussey, S.; Whitley, G.S.J. Plasma concentrations of endogenous inhibitor of nitric oxide synthesis in normal pregnancy and pre-eclampsia. Lancet 1993, 342, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Ehsanipoor, R.M.; Fortson, W.; Fitzmaurice, L.E.; Liao, W.-X.; Wing, D.A.; Chen, D.-B.; Chan, K. Nitric oxide and carbon monoxide production and metabolism in preeclampsia. Reprod. Sci. 2013, 20, 542–548. [Google Scholar] [CrossRef][Green Version]
- López-Alarcón, M.; Montalvo-Velarde, I.; Vital-Reyes, V.; Hinojosa-Cruz, J.; Leaños-Miranda, A.; Martínez-Basila, A. Serial determinations of asymmetric dimethylarginine and homocysteine during pregnancy to predict pre-eclampsia: A longitudinal study. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1586–1592. [Google Scholar] [CrossRef]
- Zheng, J.-J.; Wang, H.-O.; Huang, M.; Zheng, F.-Y. Assessment of ADMA, estradiol, and progesterone in severe preeclampsia. Clin. Exp. Hypertens. 2016, 38, 347–351. [Google Scholar] [CrossRef]
- Krzyzanowska, K.; Mittermayer, F.; Wolzt, M.; Schernthaner, G. Asymmetric dimethylarginine predicts cardiovascular events in patients with type 2 diabetes. Diabetes Care 2007, 30, 1834–1839. [Google Scholar] [CrossRef]
- Hudson, C.L.; Zemlin, A.E.; Ipp, H. The cardiovascular risk marker asymmetric dimethylarginine is elevated in asymptomatic, untreated HIV-1 infection and correlates with markers of immune activation and disease progression. Ann. Clin. Biochem. 2014, 51, 568–575. [Google Scholar] [CrossRef]
- Alpoim, P.N.; Godoi, L.C.; Freitas, L.G.; Gomes, K.B.; Dusse, L.M. Assessment of L-arginine asymmetric 1 dimethyl (ADMA) in early-onset and late-onset (severe) preeclampsia. Nitric Oxide 2013, 33, 81–82. [Google Scholar] [CrossRef]
- Laskowska, M.; Laskowska, K.; Oleszczuk, J. Differences in the association between maternal serum homocysteine and ADMA levels in women with pregnancies complicated by preeclampsia and/or intrauterine growth restriction. Hypertens. Pregnancy 2013, 32, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.S.; Zhang, Y.; Gu, Y.; Lewis, D.F.; Wang, Y. Increased phospholipase A2 and thromboxane but not prostacyclin production by placental trophoblast cells from normal and preeclamptic pregnancies cultured under hypoxia condition. Placenta 2005, 26, 402–409. [Google Scholar] [CrossRef]
- Suzuki, Y.; Hattori, T.; Kajikuri, J.; Yamamoto, T.; Suzumori, K.; Itoh, T. Reduced function of endothelial prostacyclin in human omental resistance arteries in pre-eclampsia. J. Physiol. 2002, 545, 269–277. [Google Scholar] [CrossRef]
- Crews, J.K.; Herrington, J.N.; Granger, J.P.; Khalil, R.A. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension 2000, 35, 367–372. [Google Scholar] [CrossRef]
- Yamamoto, T.; Suzuki, Y.; Kojima, K.; Suzumori, N.; Suzuki, T. The biological investigation of prostacyclin in preeclamptic women seen reduced endothelial function. Hypertens. Pregnancy 2010, 29, 484–491. [Google Scholar] [CrossRef]
- Drenjančević, I.; Jukić, I.; Mihaljević, Z.; Ćosić, A.; Kibel, A. The Metabolites of Arachidonic Acid in Microvascular Function; IntechOpen: London, UK, 2016; Volume 26, pp. 101–133. [Google Scholar]
- Zhao, S.; Gu, Y.; Lewis, D.F.; Wang, Y. Predominant basal directional release of thromboxane, but not prostacyclin, by placental trophoblasts from normal and preeclamptic pregnancies. Placenta 2008, 29, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Walsh, S.W.; Guo, J.; Zhang, J. The imbalance between thromboxane and prostacyclin in preeclampsia is associated with an imbalance between lipid peroxides and vitamin E in maternal blood. Am. J. Obstet. Gynecol. 1991, 165, 1695–1700. [Google Scholar] [CrossRef]
- Fitzgerald, D.; Entman, S.; Mulloy, K.; FitzGerald, G. Decreased prostacyclin biosynthesis preceding the clinical manifestation of pregnancy-induced hypertension. Circulation 1987, 75, 956–963. [Google Scholar] [CrossRef]
- Walsh, S.W. Eicosanoids in preeclampsia. Prostaglandins, Leukot. Essent. Fat. Acids 2004, 70, 223–232. [Google Scholar] [CrossRef]
- Chavarría, M.E.; Lara-González, L.; González-Gleason, A.; García-Paleta, Y.; Vital-Reyes, V.S.; Reyes, A. Prostacyclin/thromboxane early changes in pregnancies that are complicated by preeclampsia. Am. J. Obstet. Gynaecol. 2003, 188, 986–992. [Google Scholar] [CrossRef]
- Brockelsby, J.C.; Anthony, F.W.; Johnson, I.R.; Baker, P.N. The effects of vascular endothelial growth factor on endothelial cells: A potential role in preeclampsia. Am. J. Obstet. Gynecol. 2000, 182, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Padayachee, S.; Moodley, J.; Naicker, T. A review of angiogenic imbalance in HIV-infected hypertensive disorders of pregnancy. Curr. Hypertens. Rep. 2019, 21, 69. [Google Scholar] [CrossRef] [PubMed]
- Nyagol, J.; De Falco, G.; Lazzi, S.; Luzzi, A.; Cerino, G.; Shaheen, S.; Palummo, N.; Bellan, C.; Spina, D.; Leoncini, L. HIV-1 Tat mimetic of VEGF correlates with increased microvessels density in AIDS-related diffuse large B-cell and Burkitt lymphomas. J. Hematop. 2008, 1, 3–10. [Google Scholar] [CrossRef]
- McMichael, A.J.; Borrow, P.; Tomaras, G.D.; Goonetilleke, N.; Haynes, B.F. The immune response during acute HIV-1 infection: Clues for vaccine development. Nat. Rev. Immunol. 2010, 10, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Govender, N.; Naicker, T.; Moodley, J. Maternal imbalance between pro-angiogenic and anti-angiogenic factors in HIV-infected women with pre-eclampsia: Cardiovascular topics. Cardiovasc. J. Afr. 2013, 24, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, H.N.P.; Mesquita, E.T.; Ribeiro, M.L.; Bazin, A.R.; Mesquita, C.T.; Teixeira, M.P.; Pellegrini, R.D.C.; Nóbrega, A.C.L.D. Study of vascular reactivity in HIV patients whether or not receiving protease inhibitor. Arq. Bras. Cardiol. 2009, 93, 367–373. [Google Scholar] [CrossRef] [PubMed]

| Pre-Eclamptic HIV-Positive (PE+) (n = 21) | Pre-Eclamptic HIV-Negative (PE−) (n = 21) | Normotensive HIV-Positive (N+) (n = 21) | Normotensive HIV-Negative (N−) (n = 21) | p Value | |
|---|---|---|---|---|---|
| Maternal age (years) | 29 (30.50–26.00) | 28 (37.00–23.00) | 28 (35.00–23.00) | 23 (27.00–19.00) | 0.229 |
| Parity | 1 (2–0) | 2 (3–0) | 2 (2.5–1) | 1 (1–0) | 0.0823 |
| Gravidity | 2 (3–1.5) | 3 (4–1) | 3 (3–2) | 2 (2–1) | 0.0979 |
| Gestational age (weeks) | 23 (26.50–23.00) | 24 (29.00–23.00) | 25 (31.00–23.00) | 27 (32.00–26.00) | <0.0001 *** |
| Systolic blood pressure (mmHg) | 165 (177.0–155.5) | 162 (173.5–123.0) | 115 (121.0–106.5) | 124 (127.5–114.5) | <0.0001 *** |
| Diastolic blood pressure (mmHg) | 106 (117.5–96.50) | 98 (110.0–78.00) | 70 (75.00–67.50) | 75 (86.00–65.50) | <0.0001 *** |
| Maternal weight (kg) (sampling weight) | 72 (85.00–65.55) | 68.50 (83.55–63.35) | 75 (80.25–68.00) | 75 (86.90–70.50) | 0.3433 |
| Baby weight (kg) | 2.54 (1.37–3.05) | 2.59 (1.20–2.90) | 3.20 (2.88–3.38) | 3.33 (3.14–3.65) | <0.0001 *** |
| Preeclamptic Pregnancies | Normotensive Pregnancies | ||||
|---|---|---|---|---|---|
| Preeclamptic HIV-Positive (n = 21) | Preeclamptic HIV-Negative (n = 21) | Normotensive HIV-Positive (n = 21) | Normotensive HIV-Negative (n = 21) | p-Value | |
| ADMA (ng/mL) | 9504 (10,464–8394) | 10,504 (11,804–9214) | 10,224 (11,014–8454) | 9484 (10,424–8814) | p = 0.0969 |
| PGI2 (ng/mL) | 18,480 (20,880–13,555) | 15,030 (19,155–12,305) | 15,580 (18,130–11,355) | 17,380 (20,880–14,855) | p = 0.0212 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mthembu, M.H.; Sibiya, S.; Moodley, J.; Mkhwanazi, N.P.; Naicker, T. Endothelial Impairment in HIV-Associated Preeclampsia: Roles of Asymmetric Dimethylarginine and Prostacyclin. Int. J. Mol. Sci. 2025, 26, 7451. https://doi.org/10.3390/ijms26157451
Mthembu MH, Sibiya S, Moodley J, Mkhwanazi NP, Naicker T. Endothelial Impairment in HIV-Associated Preeclampsia: Roles of Asymmetric Dimethylarginine and Prostacyclin. International Journal of Molecular Sciences. 2025; 26(15):7451. https://doi.org/10.3390/ijms26157451
Chicago/Turabian StyleMthembu, Mbuso Herald, Samukelisiwe Sibiya, Jagidesa Moodley, Nompumelelo P. Mkhwanazi, and Thajasvarie Naicker. 2025. "Endothelial Impairment in HIV-Associated Preeclampsia: Roles of Asymmetric Dimethylarginine and Prostacyclin" International Journal of Molecular Sciences 26, no. 15: 7451. https://doi.org/10.3390/ijms26157451
APA StyleMthembu, M. H., Sibiya, S., Moodley, J., Mkhwanazi, N. P., & Naicker, T. (2025). Endothelial Impairment in HIV-Associated Preeclampsia: Roles of Asymmetric Dimethylarginine and Prostacyclin. International Journal of Molecular Sciences, 26(15), 7451. https://doi.org/10.3390/ijms26157451







