Understanding the Molecular Basis of Miller–Dieker Syndrome
Abstract
1. Introduction
2. Overview of Miller–Dieker Syndrome
3. Genetic Basis and Diagnosis of MDS
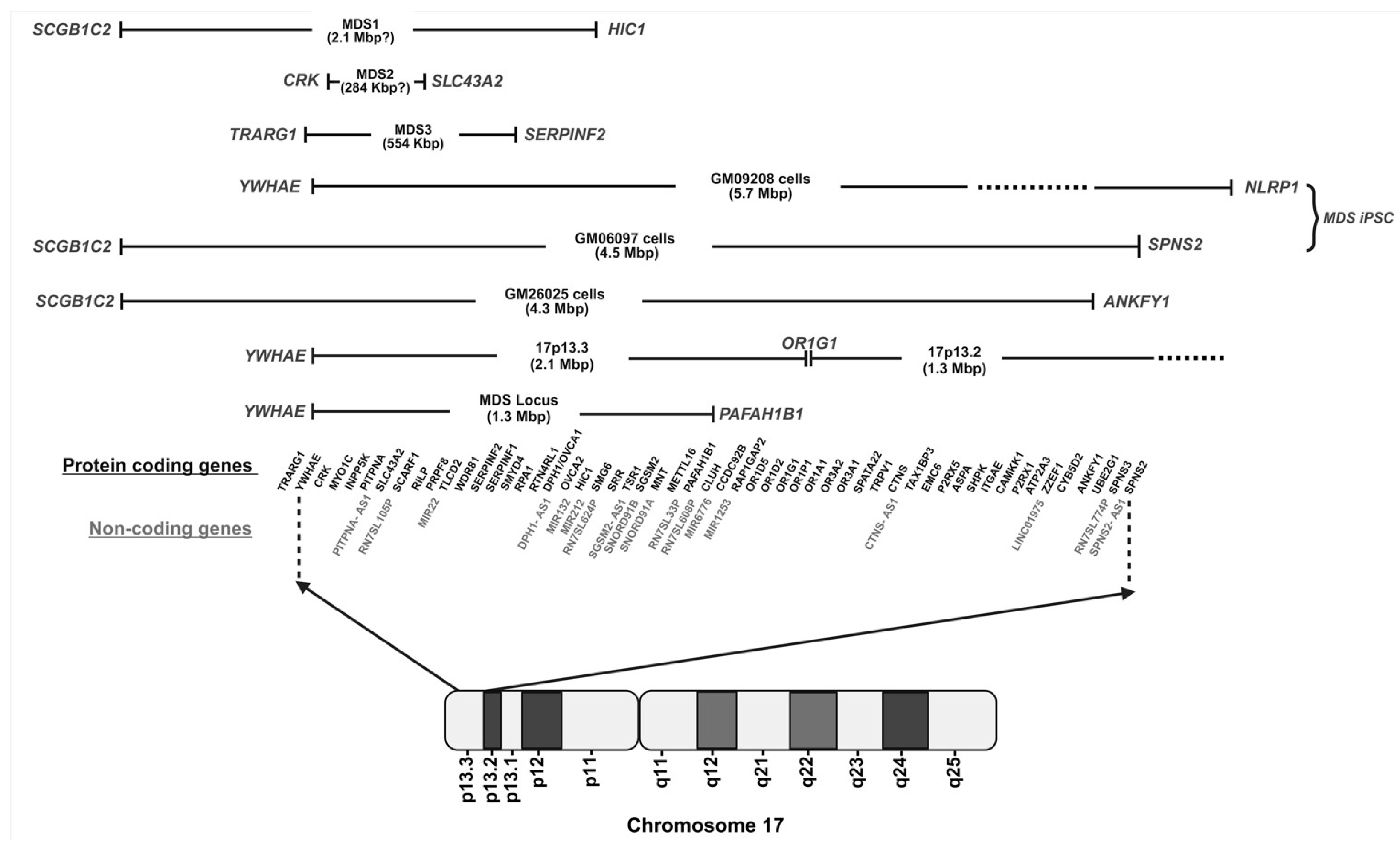
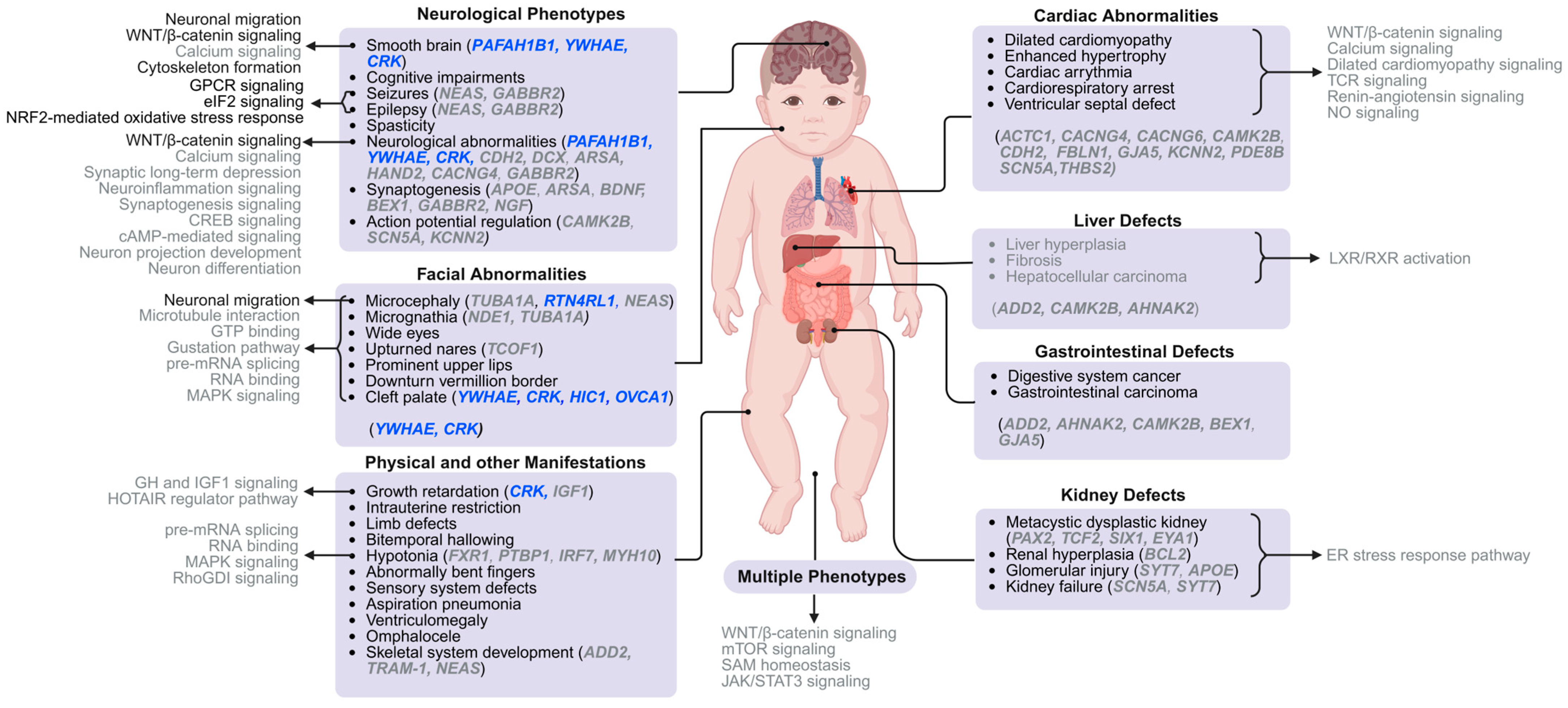
4. Understanding the Molecular Pathways Underlying MDS Phenotypes
4.1. Smooth Brain
4.2. Facial Dysmorphic Features
4.3. Other Characteristics of MDS Patients
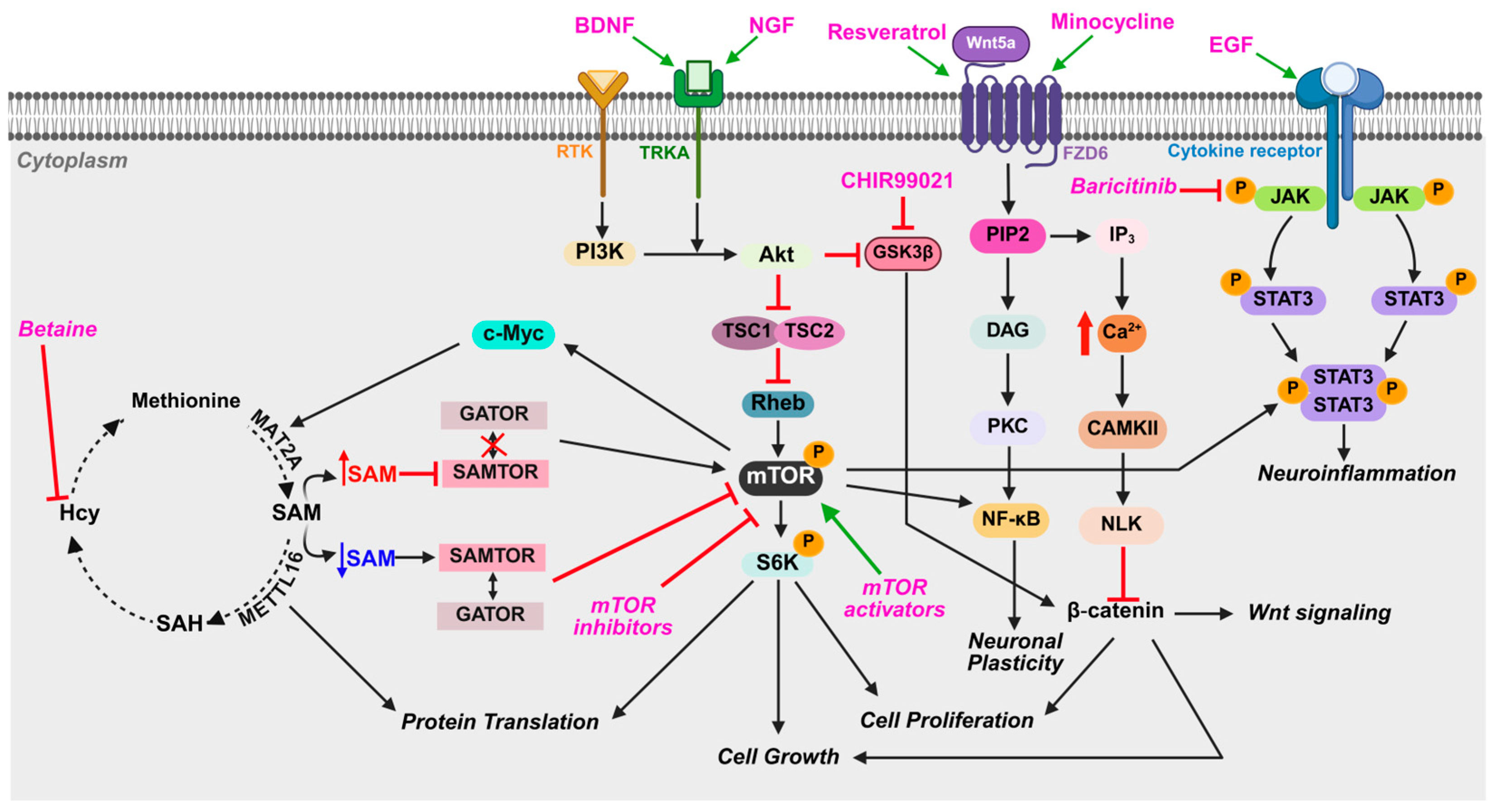
4.4. Notable Molecular Pathways and Mechanisms
5. Current and Potential Treatment Options for MDS
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Case Representation (Additional Notes About Patient) | Age at Diagnosis and Method | Region Deleted | MDS Phenotype/Symptoms Reported | Treatment to Control the Complications |
|---|---|---|---|---|
| Born weighing 2.69 kg (5.93 lbs.) [20] | 5 weeks old using, ultrasound and CT scan | 17p13.3q25.3 deletion | Microcephaly, micrognathia, low set ears, thin upper lips, bilateral clinodactyly and cryptorchidism | Not reported |
| Born weighing 2.308 kg (5.08 lbs.) [70] | 38 weeks of gestation, using FISH, brain ultrasound, CT scan, MRI | 17p13.2 and LIS1 haploinsufficiency in 17p13.3 | Facial dysmorphism, intrauterine growth restriction, paucity of gyral and sulcal development, growth retardation, developmental delay, and seizures. | Not reported |
| Born weighing 975 g (or 2.15 lbs.) [133] | 30 weeks of gestation, using CGH | 17p13.3-p13.2 region deletion (including PAFAH1B1 and YWHAE) | Seizures with apnea and behavioral arrest, epileptic spasms, severe LIS on Day 76 MRI scan | Phenobarbital administration for seizure control, corpus callosotomy (CC) to treat seizures |
| Presented with developmental delays and febrile-induced epileptic seizures [162] | 32 months, using MRI, whole genome sequencing and video electroencephalogram | 17p13.3p13.2 heterozygous inversion spanning 1.02 Mbps | A low hairline at the back, binocular esotropia, widely spaced eyes, a flat nasal bridge, broad gaps between teeth, excessive drooling, delayed psychomotor development, and mild muscle weakness | Not reported |
| Recurrent seizures for 5 days, born with cyanosis and respiratory distress [37] | 6 months, using head CT scan | Not reported | Facial dysmorphism, growth and developmental delays, diffuse agyria, micrognathia | Not reported |
| Reported with pre- and postnatal growth retardation [35] | 15 years, using exome sequencing | De novo 17p13.3 deletion (including CRK) | Intrauterine, growth retardation | Recombinant human growth hormone therapy partially decreased the height deficiency |
| Reported with pre- and postnatal growth retardation [35] | 11 years and 10 months, using exome sequencing | De novo 17p13.3 deletion (including CRK) | Intrauterine, growth retardation | Recombinant human growth hormone therapy induced the growth |
| History of intrauterine growth retardation, short stature, intractable epilepsy, expressive language disorder, clinodactyly, and retinal freckling [38] | 5 years, using aCGH and cytogenetic analysis | 17p13.3 deletion (1759 kbp region including YWHAE and CRK but not PAFAH1B1) and a mosaic r17 | Developmental delays, dysmorphic facial features, other physical abnormalities | Not reported |
| History of developmental delay and recurrent seizures [46] | 3 years, using brain MRI and electroencephalogram | LIS/Subcortical band heterotopia (LIS/SBH) spectrum (deletion not reported) | Delayed motor coordination, pneumonia, acute gastroenteritis, mild ventriculomegaly | Carbamazepine and folic acid to control the focal seizures |
| Diagnosis of intra-uterine growth retardation with fetal distress and no fetal movements [32] | 39 weeks of gestation, using CT scan and chromosomal analysis | Microdeletion of the terminal end of 17p | Epileptic fits and hypotonia | Fits controlled through medication |
| Born weighing 2.52 kg (5.6 lbs.), admitted at 6 weeks of age due to jaundice [51] | 6 weeks, using SNP microarray | De novo 17p13.3 deletion (1.4 Mbp region including YWHAE and CRK but not PAFAH1B1) | Micrognathia, tented upper lip, broad forehead, one febrile seizure, developmental delay | Not reported |
| Born at 37 weeks of pregnancy with birth weight of 1844 g (or 4 lbs.). Admitted for low birth weight and respiratory distress [163] | At birth, using electroencephalography, brain MRI, and FISH | Not reported | Microcephaly, a narrow forehead, small nose and chin, and cardiac malformations, repeated afebrile seizures | Zonisamide and levetiracetam |
| Presented with a worsening of seizures in the setting of a Pseudomonal and Enterococcal urinary tract infection [42] | 6 months, using MRI of neuraxis | LIS1 17p13.3 deletion | Pseudomonal and Enterococcal urinary tract infection, seizures, epilepsy, cerebral palsy, cognitive delays, prominent forehead, upturned short nose | Not reported |
| Born weighing 2.069 kg (4.56 lbs.) with a complicated pregnancy due to intrauterine growth retardation, delivery induced to prevent complications [41] | 33 weeks of gestation, using microarray analysis and later with CT scan, ultrasound and MRI | Sub telomeric region deletion of 17p13.3 (including YWHAE) (see MDS1 in Figure 1) | Developmental delays, macrocephaly, ventriculomegaly, generalized seizures, idiopathic generalized epilepsy | Not reported |
| Prenatal diagnosis of omphalocele with mild ventriculomegaly [49] | 3 years, using FISH, fetal echocardiography, MRI | 17p13.3 deletion | Developmental delays, seizures, no gyral formations | Not reported |
| Born weighing 3.2 kgs (7.05 lbs.) and presented for growth evaluation at the age of 10.8 years [67] | 10.8 years, using aCGH, MRI | 17p13.3 deletion (284 kbp including CRK and MYO1C) (see MDS2 in Figure 1) | Intellectual disability, facial and limb abnormalities, feeding difficulties, delayed psychomotor development | Substantial catch-up response following growth hormone treatment |
| Born weighing 2.4 kg (5.3 lbs.) and presented with ocular malformation and speech delay [36] | 37 weeks of gestation, using aCGH, brain MRI | Distal deletion in 17p13.3 region (554 kbps) (see MDS3 in Figure 1) | Intra-uterine growth retardation, short nose, a pointed chin and an everted inferior lip, low set ears, prominent forehead, no motor delay | Not reported |
| Born weighing 1.8 kg (4 lbs.); brought in due to epilepsy and developmental delays [51] | 9 years, using microarray, brain MRI | 17p13.3 deletion (140 kbp including YWHAE, TRARG1 and BHLHA9, but not CRK or PAFAH1B1) | Prematurity, respiratory distress syndrome, flat mid face, hyperbilirubinemia, seizures | Not reported |
| Born weighing 2.8 kgs (6.2 lbs.); brought in due to new-onset infantile spasms and a history of delay [28] | 5 months, using MRI, electroencephalogram | 17p13.3 deletion (contiguous large heterozygous deletion including PAFAH1B1) | Prominent forehead, cardiac defect, bitemporal hollowing, short nose with upturned nares, mild hypotonia, thickened upper lip, pachygyria | On oral vigabatrin and neurodevelopmental therapy until 9 months old |
| Grade I LIS with midline calcification and aspiration pneumonia [93] | 18 months, using head ultrasound, G-band chromosome analysis, and electroencephalogram | 17p13.3 deletion | Dilated bilateral ventricles, prominent forehead, bitemporal hollowing, short nose with upturned nares, prominent upper lip, micrognathia | Not reported |
| Worsening abnormal movements beginning from 3 months of age [45] | 4 months, using whole exome sequencing and electroencephalogram | Not reported | Developmental delays | Intravenous pyridoxine administration |
| Admitted with increased seizures, urinary tract infection, and a buttock abscess [42] | 6 months, using MRI | 17p13.3 deletion | Agyria, ventriculomegaly, dermal sinus tract | Not reported |
| Born weighing 4 pounds 9 ounces, with pregnancy complications due to intrauterine growth retardation [41] | 38 weeks of gestation, using FISH | Terminal deletion of 17p13.3 distal to MDS locus | Ventriculomegaly, epileptiform discharges, developmental delays, hypotonia, frontal bossing, hypertelorism | Not reported |
References
- Tau, G.Z.; Peterson, B.S. Normal Development of Brain Circuits. Neuropsychopharmacology 2010, 35, 147–168. [Google Scholar] [CrossRef]
- Majolo, F.; Marinowic, D.R.; Palmini, A.L.F.; DaCosta, J.C.; Machado, D.C. Migration and Synaptic Aspects of Neurons Derived from Human Induced Pluripotent Stem Cells from Patients with Focal Cortical Dysplasia II. Neuroscience 2019, 408, 81–90. [Google Scholar] [CrossRef]
- Mariani, J.; Simonini, M.V.; Palejev, D.; Tomasini, L.; Coppola, G.; Szekely, A.M.; Horvath, T.L.; Vaccarino, F.M. Modeling Human Cortical Development in Vitro Using Induced Pluripotent Stem Cells. Proc. Natl. Acad. Sci. USA 2012, 109, 12770–12775. [Google Scholar] [CrossRef]
- Bershteyn, M.; Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Nene, A.; Wynshaw-Boris, A.; Kriegstein, A.R. Human iPSC-Derived Cerebral Organoids Model Cellular Features of Lissencephaly and Reveal Prolonged Mitosis of Outer Radial Glia. Cell Stem Cell 2017, 20, 435–449.e4. [Google Scholar] [CrossRef]
- Lindborg, B.A.; Brekke, J.H.; Vegoe, A.L.; Ulrich, C.B.; Haider, K.T.; Subramaniam, S.; Venhuizen, S.L.; Eide, C.R.; Orchard, P.J.; Chen, W.; et al. Rapid Induction of Cerebral Organoids From Human Induced Pluripotent Stem Cells Using a Chemically Defined Hydrogel and Defined Cell Culture Medium. Stem Cells Transl. Med. 2016, 5, 970–979. [Google Scholar] [CrossRef]
- Renner, M.; Lancaster, M.A.; Bian, S.; Choi, H.; Ku, T.; Peer, A.; Chung, K.; Knoblich, J.A. Self-organized Developmental Patterning and Differentiation in Cerebral Organoids. EMBO J. 2017, 36, 1316–1329. [Google Scholar] [CrossRef]
- Velasco, S.; Kedaigle, A.J.; Simmons, S.K.; Nash, A.; Rocha, M.; Quadrato, G.; Paulsen, B.; Nguyen, L.; Adiconis, X.; Regev, A.; et al. Individual Brain Organoids Reproducibly Form Cell Diversity of the Human Cerebral Cortex. Nature 2019, 570, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Mestres, I.; Chuang, J.-Z.; Calegari, F.; Conde, C.; Sung, C.-H. SARA Regulates Neuronal Migration during Neocortical Development through L1 Trafficking. Development 2016, 143, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Gstrein, T.; Edwards, A.; Přistoupilová, A.; Leca, I.; Breuss, M.; Pilat-Carotta, S.; Hansen, A.H.; Tripathy, R.; Traunbauer, A.K.; Hochstoeger, T.; et al. Mutations in Vps15 Perturb Neuronal Migration in Mice and Are Associated with Neurodevelopmental Disease in Humans. Nat. Neurosci. 2018, 21, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Rahimi-Balaei, M.; Bergen, H.; Kong, J.; Marzban, H. Neuronal Migration During Development of the Cerebellum. Front. Cell. Neurosci. 2018, 12, 484. [Google Scholar] [CrossRef]
- Yingling, J.; Toyo-oka, K.; Wynshaw-Boris, A. Miller-Dieker Syndrome: Analysis of a Human Contiguous Gene Syndrome in the Mouse. Am. J. Hum. Genet. 2003, 73, 475–488. [Google Scholar] [CrossRef][Green Version]
- Miller, J.Q. lissencephaly in 2 siblings. Neurology 1963, 13, 841–850. [Google Scholar] [CrossRef]
- Dieker, H. The Lissencephaly Syndrom. Birth Defects 1969, 5, 53–64. [Google Scholar]
- Friocourt, G.; Parnavelas, J. Mutations in ARX Result in Several Defects Involving GABAergic Neurons. Front. Cell. Neurosci. 2010, 4, 1437. [Google Scholar] [CrossRef]
- Haverfield, E.V.; Whited, A.J.; Petras, K.S.; Dobyns, W.B.; Das, S. Intragenic Deletions and Duplications of the LIS1 and DCX Genes: A Major Disease-Causing Mechanism in Lissencephaly and Subcortical Band Heterotopia. Eur. J. Hum. Genet. 2009, 17, 911–918. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bahi-Buisson, N.; Souville, I.; Fourniol, F.J.; Toussaint, A.; Moores, C.A.; Houdusse, A.; Lemaitre, J.Y.; Poirier, K.; Khalaf-Nazzal, R.; Hully, M.; et al. New Insights into Genotype-Phenotype Correlations for the Doublecortin-Related Lissencephaly Spectrum. Brain 2013, 136 Pt 1, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Vega, A.; Gutiérrez-Gil, B.; Cuchillo-Ibáñez, I.; Sáez-Valero, J.; Pérez, V.; García-Gámez, E.; Benavides, J.; Arranz, J.J. Identification of a 31-Bp Deletion in the RELN Gene Causing Lissencephaly with Cerebellar Hypoplasia in Sheep. PLoS ONE 2013, 8, e81072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liang, D.; Ercan-Sencicek, A.G.; Bulut, A.S.; Cortes, J.; Cheng, I.Q.; Henegariu, O.; Nishimura, S.; Wang, X.; Peksen, A.B.; et al. Dysregulation of mTOR Signalling Is a Converging Mechanism in Lissencephaly. Nature 2025, 638, 172–181. [Google Scholar] [CrossRef]
- Leibovitz, Z.; Lerman-Sagie, T.; Haddad, L. Fetal Brain Development: Regulating Processes and Related Malformations. Life 2022, 12, 809. [Google Scholar] [CrossRef]
- Dobyns, W.B.; Stratton, R.F.; Parke, J.T.; Greenberg, F.; Nussbaum, R.L.; Ledbetter, D.H. Miller-Dieker Syndrome: Lissencephaly Andmonosomy 17p. J. Pediatr. 1983, 102, 552–558. [Google Scholar] [CrossRef]
- Brock, S.; Dobyns, W.B.; Jansen, A. PAFAH1B1-Related Lissencephaly/Subcortical Band Heterotopia. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Allanson, J.E.; Ledbetter, D.H.; Dobyns, W.B. Classical Lissencephaly Syndromes: Does the Face Reflect the Brain? J. Med. Genet. 1998, 35, 920–923. [Google Scholar] [CrossRef]
- Cardoso, C.; Leventer, R.J.; Ward, H.L.; Toyo-oka, K.; Chung, J.; Gross, A.; Martin, C.L.; Allanson, J.; Pilz, D.T.; Olney, A.H.; et al. Refinement of a 400-Kb Critical Region Allows Genotypic Differentiation between Isolated Lissencephaly, Miller-Dieker Syndrome, and Other Phenotypes Secondary to Deletions of 17p13.3. Am. J. Hum. Genet. 2003, 72, 918–930. [Google Scholar] [CrossRef]
- Sheen, V.L.; Ferland, R.J.; Neal, J.; Harney, M.; Hill, R.S.; Banham, A.; Brown, P.; Chenn, A.; Corbo, J.; Hecht, J.; et al. Neocortical Neuronal Arrangement in Miller Dieker Syndrome. Acta Neuropathol. 2006, 111, 489–496. [Google Scholar] [CrossRef]
- Nagamani, S.C.S.; Zhang, F.; Shchelochkov, O.A.; Bi, W.; Ou, Z.; Scaglia, F.; Probst, F.J.; Shinawi, M.; Eng, C.; Hunter, J.V.; et al. Microdeletions Including YWHAE in the Miller-Dieker Syndrome Region on Chromosome 17p13.3 Result in Facial Dysmorphisms, Growth Restriction, and Cognitive Impairment. J. Med. Genet. 2009, 46, 825–833. [Google Scholar] [CrossRef]
- Bruno, D.L.; Anderlid, B.-M.; Lindstrand, A.; van Ravenswaaij-Arts, C.; Ganesamoorthy, D.; Lundin, J.; Martin, C.L.; Douglas, J.; Nowak, C.; Adam, M.P.; et al. Further Molecular and Clinical Delineation of Co-Locating 17p13.3 Microdeletions and Microduplications That Show Distinctive Phenotypes. J. Med. Genet. 2010, 47, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Barros Fontes, M.I.; dos Santos, A.P.; Rossi Torres, F.; Lopes-Cendes, I.; Cendes, F.; Appenzeller, S.; Kawasaki de Araujo, T.; Lopes Monlleó, I.; Gil-da-Silva-Lopes, V.L. 17p13.3 Microdeletion: Insights on Genotype-Phenotype Correlation. Mol. Syndromol. 2016, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- George, J.M.; Cherian, C.S.; Thomas, R.; Sunnychan, S. Infantile Spasms and Developmental Delay: A Case of Miller–Dieker Syndrome. Indian Pediatr. Case Rep. 2023, 3, 225. [Google Scholar] [CrossRef]
- Van Allen, M.; Clarren, S.K. A Spectrum of Gyral Anomalies in Miller-Dieker (Lissencephaly) Syndrome. J. Pediatr. 1983, 102, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Izmeth, M.G.; Parameshwar, E. The Miller-Dieker Syndrome: A Case Report and Review of the Literature. J. Ment. Defic. Res. 1989, 33 Pt 3, 267–270. [Google Scholar] [CrossRef]
- Roos, L.; Jønch, A.E.; Kjaergaard, S.; Taudorf, K.; Simonsen, H.; Hamborg-Petersen, B.; Brøndum-Nielsen, K.; Kirchhoff, M. A New Microduplication Syndrome Encompassing the Region of the Miller-Dieker (17p13 Deletion) Syndrome. J. Med. Genet. 2009, 46, 703–710. [Google Scholar] [CrossRef]
- King, A.; Upadhyaya, M.; Penney, C.; Doshi, R. A Case of Miller-Dieker Syndrome in a Family with Neurofibromatosis Type I. Acta Neuropathol. 2000, 99, 425–427. [Google Scholar] [CrossRef]
- Committee on Diagnostic Error in Health Care; Board on Health Care Services; Institute of Medicine; The National Academies of Sciences, Engineering, and Medicine. Improving Diagnosis in Health Care; Balogh, E.P., Miller, B.T., Ball, J.R., Eds.; National Academies Press: Washington, DC, USA, 2015; p. 21794. [Google Scholar] [CrossRef]
- Dobyns, W.B. The Neurogenetics of Lissencephaly. Neurol. Clin. 1989, 7, 89–105. [Google Scholar] [CrossRef]
- Deodati, A.; Inzaghi, E.; Germani, D.; Fausti, F.; Cianfarani, S. Crk Haploinsufficiency Is Associated with Intrauterine Growth Retardation and Severe Postnatal Growth Failure. Horm. Res. Paediatr. 2022, 94, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Schiff, M.; Delahaye, A.; Andrieux, J.; Sanlaville, D.; Vincent-Delorme, C.; Aboura, A.; Benzacken, B.; Bouquillon, S.; Elmaleh-Berges, M.; Labalme, A.; et al. Further Delineation of the 17p13.3 Microdeletion Involving YWHAE but Distal to PAFAH1B1: Four Additional Patients. Eur. J. Med. Genet. 2010, 53, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Asih, D.; Ernes, A. 6-month-old infant with lissencephaly type i associated with miller dieker syndrome: A case report. Ibnu Sina J. Kedokt. Dan Kesehat.-Fak. Kedokt. Univ. Islam Sumat. Utara 2024, 23, 252–257. [Google Scholar] [CrossRef]
- Kim, S.Y.; Wohler, E.; Gutierrez, M.J.; Sadreameli, C.; Kossoff, E.; Sobreira, N.L. Ring Chromosome 17 Syndrome-A Case Report and Discussion of Diagnostic Methods. Am. J. Med. Genet. A 2024, 197, e63925. [Google Scholar] [CrossRef]
- Mahgoub, L.; Aziz, K.; Davies, D.; Leonard, N. Miller–Dieker Syndrome Associated with Congenital Lobar Emphysema. AJP Rep. 2014, 4, 13–16. [Google Scholar] [CrossRef]
- Bellucco, F.T.; Nunes, N.; Colovati, M.E.S.; Malinverni, A.C.M.; Caneloi, T.P.; Soares, M.F.; Perez, A.B.A.; Melaragno, M.I. Miller-Dieker Syndrome Due to a 5.5-Mb 17p Deletion in a 17;Y Pseudodicentric Chromosome. Cytogenet. Genome Res. 2017, 152, 29–32. [Google Scholar] [CrossRef]
- Tenney, J.R.; Hopkin, R.J.; Schapiro, M.B. Deletion of 14-3-3{varepsilon} and CRK: A Clinical Syndrome with Macrocephaly, Developmental Delay, and Generalized Epilepsy. J. Child Neurol. 2011, 26, 223–227. [Google Scholar] [CrossRef]
- Hsieh, D.T.; Jennesson, M.M.; Thiele, E.A.; Caruso, P.A.; Masiakos, P.T.; Duhaime, A.-C. Brain and Spinal Manifestations of Miller-Dieker Syndrome. Neurol. Clin. Pract. 2013, 3, 82–83. [Google Scholar] [CrossRef]
- Köhler, A.; Hain, J.; Müller, U. Clinical and Molecular Genetic Findings in Five Patients with Miller-Dieker Syndrome. Clin. Genet. 1995, 47, 161–164. [Google Scholar] [CrossRef]
- Matarese, C.A.; Renaud, D.L. Classical (Type I) Lissencephaly and Miller-Dieker Syndrome. Pediatr. Neurol. 2009, 40, 324–325. [Google Scholar] [CrossRef]
- Ying Eng, N.; Nie, D.A. Infantile Epileptic Spasms Syndrome in a Child with Lissencephaly Associated with de Novo PAFAH1B1 vAriant and Coincidental CMV Infection. Epilepsy Behav. Rep. 2024, 26, 100664. [Google Scholar] [CrossRef] [PubMed]
- Ngowi, E.; Datoo, A.; Ally, P.; Salum, H.; Edward, K. Lissencephaly with Subcortical Band Heterotopia in an East African Child: A Case Report. Radiol. Case Rep. 2025, 20, 480–483. [Google Scholar] [CrossRef] [PubMed]
- De Wit, M.-C.Y.; De Rijk-Van Andel, J.; Halley, D.J.; Poddighe, P.J.; Arts, W.F.M.; De Coo, I.F.; Mancini, G.M. Long-Term Follow-up of Type 1 Lissencephaly: Survival Is Related to Neuroimaging Abnormalities. Dev. Med. Child Neurol. 2011, 53, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Pilz, D.T.; Quarrell, O.W. Syndromes with Lissencephaly. J. Med. Genet. 1996, 33, 319–323. [Google Scholar] [CrossRef]
- Chitayat, D.; Toi, A.; Babul, R.; Blaser, S.; Moola, S.; Yarkoni, D.; Sermer, M.; Johnson, J.A.; Vasjar, J.; Teshima, I. Omphalocele in Miller-Dieker Syndrome: Expanding the Phenotype. Am. J. Med. Genet. 1997, 69, 293–298. [Google Scholar] [CrossRef]
- Rahnemai-Azar, A.A.; Rahnemaiazar, A.A.; Naghshizadian, R.; Kurtz, A.; Farkas, D.T. Percutaneous Endoscopic Gastrostomy: Indications, Technique, Complications and Management. World J. Gastroenterol. 2014, 20, 7739–7751. [Google Scholar] [CrossRef]
- Baker, E.K.; Brewer, C.J.; Ferreira, L.; Schapiro, M.; Tenney, J.; Wied, H.M.; Kline-Fath, B.M.; Smolarek, T.A.; Weaver, K.N.; Hopkin, R.J. Further Expansion and Confirmation of Phenotype in Rare Loss of YWHAE Gene Distinct from Miller–Dieker Syndrome. Am. J. Med. Genet. A 2023, 191, 526–539. [Google Scholar] [CrossRef]
- Mishima, T.; Watari, M.; Iwaki, Y.; Nagai, T.; Kawamata-Nakamura, M.; Kobayashi, Y.; Fujieda, S.; Oikawa, M.; Takahashi, N.; Keira, M.; et al. Miller-Dieker Syndrome with Unbalanced Translocation 45, X, Psu Dic(17;Y)(P13;P11.32) Detected by Fluorescence in Situ Hybridization and G-Banding Analysis Using High Resolution Banding Technique. Congenit. Anom. 2017, 57, 61–63. [Google Scholar] [CrossRef]
- Ensembl Genome Browser 113. Available online: https://useast.ensembl.org/index.html (accessed on 8 January 2025).
- UCSC Genome Browser Home. Available online: https://genome.ucsc.edu/index.html (accessed on 14 January 2025).
- BioRender. Available online: https://app.biorender.com/gallery/illustrations/folder/679577296b485b59c62e700b (accessed on 22 March 2025).
- Fong, K.W.; Ghai, S.; Toi, A.; Blaser, S.; Winsor, E.J.T.; Chitayat, D. Prenatal Ultrasound Findings of Lissencephaly Associated with Miller–Dieker Syndrome and Comparison with Pre- and Postnatal Magnetic Resonance Imaging. Ultrasound Obstet. Gynecol. 2004, 24, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.W.; Ghai, S.; Toi, A.; Chitayat, D.; Blaser, S. OC029: Lissencephaly: Prenatal Ultrasound Findings in a Review of 16 Cases. Ultrasound Obstet. Gynecol. 2003, 22, 10. [Google Scholar] [CrossRef]
- Bowman, P.; Grimes, H.; Dallosso, A.R.; Berry, I.; Mullin, S.; Rankin, J.; Low, K.J. Whole Genome Sequencing for Copy Number Variant Detection to Improve Diagnosis and Management of Rare Diseases. Dev. Med. Child. Neurol. 2025, 67, 126–131. [Google Scholar] [CrossRef]
- Alvarado, M.; Bass, H.N.; Caldwell, S.; Jamehdor, M.; Miller, A.A.; Jacob, P. Miller-Dieker Syndrome: Detection of a Cryptic Chromosome Translocation Using In Situ Hybridization in a Family With Multiple Affected Offspring. Am. J. Dis. Child. 1993, 147, 1291–1294. [Google Scholar] [CrossRef]
- Kato, M.; Dobyns, W.B. Lissencephaly and the Molecular Basis of Neuronal Migration. Hum. Mol. Genet. 2003, 12 (Suppl. S1), R89–R96. [Google Scholar] [CrossRef]
- Mahendran, G.; Breger, K.; McCown, P.J.; Hulewicz, J.P.; Bhandari, T.; Addepalli, B.; Brown, J.A. Multi-Omics Approach Reveals Genes and Pathways Affected in Miller-Dieker Syndrome. Mol. Neurobiol. 2024, 62, 5073–5094. [Google Scholar] [CrossRef]
- Ingenuity Pathways Analysis (IPA)|NIH Library. Available online: https://www.nihlibrary.nih.gov/resources/tools/ingenuity-pathways-analysis-ipa (accessed on 25 December 2024).
- Liu, X.; Bennison, S.A.; Robinson, L.; Toyo-oka, K. Responsible Genes for Neuronal Migration in the Chromosome 17p13.3: Beyond Pafah1b1(Lis1), Crk and Ywhae(14-3-3ε). Brain Sci. 2021, 12, 56. [Google Scholar] [CrossRef]
- GO Enrichment Analysis. Gene Ontology Resource. Available online: http://geneontology.org/docs/go-enrichment-analysis/ (accessed on 25 December 2024).
- Toyo-oka, K.; Shionoya, A.; Gambello, M.J.; Cardoso, C.; Leventer, R.; Ward, H.L.; Ayala, R.; Tsai, L.-H.; Dobyns, W.; Ledbetter, D.; et al. 14-3-3epsilon Is Important for Neuronal Migration by Binding to NUDEL: A Molecular Explanation for Miller-Dieker Syndrome. Nat. Genet. 2003, 34, 274–285. [Google Scholar] [CrossRef]
- Yu, Y.-R.; You, L.-R.; Yan, Y.-T.; Chen, C.-M. Role of OVCA1/DPH1 in Craniofacial Abnormalities of Miller–Dieker Syndrome. Hum. Mol. Genet. 2014, 23, 5579–5596. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, J.R.; Graakjær, J.; Brandt, C.; Birkebæk, N.H. Further Delineation of 17p13.3 Microdeletion Involving CRK. The Effect of Growth Hormone Treatment. Eur. J. Med. Genet. 2012, 55, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, B.; Luginbuehl, I.; Marciniak, B.; Dalens, B.J. Miller-Dieker Lissencephaly Syndrome. In Syndromes: Rapid Recognition and Perioperative Implications; The McGraw-Hill Companies: New York, NY, USA, 2006. [Google Scholar]
- Blazejewski, S.M.; Bennison, S.A.; Smith, T.H.; Toyo-Oka, K. Neurodevelopmental Genetic Diseases Associated With Microdeletions and Microduplications of Chromosome 17p13.3. Front. Genet. 2018, 9, 80. [Google Scholar] [CrossRef]
- Chen, C.-P.; Liu, Y.-P.; Lin, S.-P.; Chen, M.; Tsai, F.-J.; Chen, Y.-T.; Chen, L.-F.; Hwang, J.K.; Wang, W. Ventriculomegaly, Intrauterine Growth Restriction, and Congenital Heart Defects as Salient Prenatal Sonographic Findings of Miller-Dieker Lissencephaly Syndrome Associated with Monosomy 17p (17p13.2 --> Pter) in a Fetus. Taiwan J. Obstet. Gynecol. 2010, 49, 81–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, Y.J.; Byun, S.Y.; Jo, S.A.; Shin, Y.B.; Cho, E.H.; Lee, E.Y.; Hwang, S.-H. Miller-Dieker Syndrome with Der(17)t(12;17)(Q24.33;P13.3)Pat Presenting with a Potential Risk of Mis-Identification as a de Novo Submicroscopic Deletion of 17p13.3. Korean J. Lab. Med. 2011, 31, 49–53. [Google Scholar] [CrossRef]
- Hadj Amor, M.; Dimassi, S.; Taj, A.; Slimani, W.; Hannachi, H.; Mlika, A.; Ben Helel, K.; Saad, A.; Mougou-Zerelli, S. Neuronal Migration Genes and a Familial Translocation t (3;17): Candidate Genes Implicated in the Phenotype. BMC Med. Genet. 2020, 21, 26. [Google Scholar] [CrossRef]
- Shi, X.; Huang, W.; Lu, J.; He, W.; Liu, Q.; Wu, J. Prenatal Diagnosis of Miller–Dieker Syndrome by Chromosomal Microarray. Ann. Hum. Genet. 2021, 85, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Zillich, L.; Gasparotto, M.; Rossetti, A.C.; Fechtner, O.; Maillard, C.; Hoffrichter, A.; Zillich, E.; Jabali, A.; Marsoner, F.; Artioli, A.; et al. Capturing the Biology of Disease Severity in an Organoid Model of LIS1-Lissencephaly. bioRxiv 2025. [Google Scholar] [CrossRef]
- Iefremova, V.; Manikakis, G.; Krefft, O.; Jabali, A.; Weynans, K.; Wilkens, R.; Marsoner, F.; Brändl, B.; Müller, F.-J.; Koch, P.; et al. An Organoid-Based Model of Cortical Development Identifies Non-Cell-Autonomous Defects in Wnt Signaling Contributing to Miller-Dieker Syndrome. Cell Rep. 2017, 19, 50–59. [Google Scholar] [CrossRef]
- Keays, D.A.; Tian, G.; Poirier, K.; Huang, G.-J.; Siebold, C.; Cleak, J.; Oliver, P.L.; Fray, M.; Harvey, R.J.; Molnár, Z.; et al. Mutations in Alpha-Tubulin Cause Abnormal Neuronal Migration in Mice and Lissencephaly in Humans. Cell 2007, 128, 45–57. [Google Scholar] [CrossRef]
- Buchsbaum, I.Y.; Cappello, S. Neuronal Migration in the CNS during Development and Disease: Insights from in Vivo and in Vitro Models. Development 2019, 146, dev163766. [Google Scholar] [CrossRef]
- Wynshaw-Boris, A. Lissencephaly and LIS1: Insights into the Molecular Mechanisms of Neuronal Migration and Development. Clin. Genet. 2007, 72, 296–304. [Google Scholar] [CrossRef]
- Moon, H.M.; Wynshaw-Boris, A. Cytoskeleton in Action: Lissencephaly, a Neuronal Migration Disorder. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.M.; Youn, Y.H.; Pemble, H.; Yingling, J.; Wittmann, T.; Wynshaw-Boris, A. LIS1 Controls Mitosis and Mitotic Spindle Organization via the LIS1–NDEL1–Dynein Complex. Hum. Mol. Genet. 2014, 23, 449–466. [Google Scholar] [CrossRef]
- Hirotsune, S.; Fleck, M.W.; Gambello, M.J.; Bix, G.J.; Chen, A.; Clark, G.D.; Ledbetter, D.H.; McBain, C.J.; Wynshaw-Boris, A. Graded Reduction of Pafah1b1 (Lis1) Activity Results in Neuronal Migration Defects and Early Embryonic Lethality. Nat. Genet. 1998, 19, 333–339. [Google Scholar] [CrossRef]
- Cornell, B.; Wachi, T.; Zhukarev, V.; Toyo-Oka, K. Regulation of Neuronal Morphogenesis by 14-3-3epsilon (Ywhae) via the Microtubule Binding Protein, Doublecortin. Hum. Mol. Genet. 2016, 25, 4405–4418. [Google Scholar] [CrossRef]
- Dix, C.I.; Soundararajan, H.C.; Dzhindzhev, N.S.; Begum, F.; Suter, B.; Ohkura, H.; Stephens, E.; Bullock, S.L. Lissencephaly-1 Promotes the Recruitment of Dynein and Dynactin to Transported mRNAs. J. Cell Biol. 2013, 202, 479–494. [Google Scholar] [CrossRef]
- Elshenawy, M.M.; Kusakci, E.; Volz, S.; Baumbach, J.; Bullock, S.L.; Yildiz, A. Lis1 Activates Dynein Motility by Modulating Its Pairing with Dynactin. Nat. Cell Biol. 2020, 22, 570–578. [Google Scholar] [CrossRef]
- Toyo-oka, K.; Wachi, T.; Hunt, R.F.; Baraban, S.C.; Taya, S.; Ramshaw, H.; Kaibuchi, K.; Schwarz, Q.P.; Lopez, A.F.; Wynshaw-Boris, A. 14-3-3ε and ζ Regulate Neurogenesis and Differentiation of Neuronal Progenitor Cells in the Developing Brain. J. Neurosci. 2014, 34, 12168–12181. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Ayala, R.; Nguyen, M.-D.; Xie, Z.; Gleeson, J.G.; Tsai, L.-H. Ndel1 Operates in a Common Pathway with LIS1 and Cytoplasmic Dynein to Regulate Cortical Neuronal Positioning. Neuron 2004, 44, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Hines, T.J.; Gao, X.; Sahu, S.; Lange, M.M.; Turner, J.R.; Twiss, J.L.; Smith, D.S. An Essential Postdevelopmental Role for Lis1 in Mice. eNeuro 2018, 5, e0350-17.2018. [Google Scholar] [CrossRef]
- Denommé-Pichon, A.-S.; Collins, S.C.; Bruel, A.-L.; Mikhaleva, A.; Wagner, C.; Vancollie, V.E.; Thomas, Q.; Chevarin, M.; Weber, M.; Prada, C.E.; et al. YWHAE Loss of Function Causes a Rare Neurodevelopmental Disease with Brain Abnormalities in Human and Mouse. Genet. Med. 2023, 25, 100835. [Google Scholar] [CrossRef]
- Park, T.-J.; Curran, T. Crk and Crk-like Play Essential Overlapping Roles Downstream of Disabled-1 in the Reelin Pathway. J. Neurosci. 2008, 28, 13551–13562. [Google Scholar] [CrossRef]
- Feng, W.-X.; Wang, X.-F.; Wu, Y.; Li, X.-M.; Chen, S.-H.; Wang, X.-H.; Wang, Z.-H.; Fang, F.; Chen, C.-H. Clinical Analysis of PAFAH1B1 Gene Variants in Pediatric Patients with Epilepsy. Seizure Eur. J. Epilepsy 2024, 117, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Dong, L.; Li, Y.; Gao, M.; He, P.C.; Liu, W.; Wei, J.; Zhao, Z.; Gao, L.; Han, L.; et al. METTL16 Exerts an m6A-Independent Function to Facilitate Translation and Tumorigenesis. Nat. Cell Biol. 2022, 24, 205–216. [Google Scholar] [CrossRef]
- Jones, K.L.; Gilbert, E.F.; Kaveggia, E.G.; Opitz, J.M. The MIller-Dieker Syndrome. Pediatrics 1980, 66, 277–281. [Google Scholar] [CrossRef]
- Wakiguchi, C.; Godai, K.; Mukaihara, K.; Ohnou, T.; Kuniyoshi, T.; Masuda, M.; Kanmura, Y. Management of General Anesthesia in a Child with Miller–Dieker Syndrome: A Case Report. JA Clin. Rep. 2015, 1, 14. [Google Scholar] [CrossRef]
- Mignon-Ravix, C.; Cacciagli, P.; El-Waly, B.; Moncla, A.; Milh, M.; Girard, N.; Chabrol, B.; Philip, N.; Villard, L. Deletion of YWHAE in a Patient with Periventricular Heterotopias and Pronounced Corpus Callosum Hypoplasia. J. Med. Genet. 2010, 47, 132–136. [Google Scholar] [CrossRef]
- Jugessur, A.; Shi, M.; Gjessing, H.K.; Lie, R.T.; Wilcox, A.J.; Weinberg, C.R.; Christensen, K.; Boyles, A.L.; Daack-Hirsch, S.; Nguyen, T.T.; et al. Maternal Genes and Facial Clefts in Offspring: A Comprehensive Search for Genetic Associations in Two Population-Based Cleft Studies from Scandinavia. PLoS ONE 2010, 5, e11493. [Google Scholar] [CrossRef]
- Syrbe, S.; Harms, F.L.; Parrini, E.; Montomoli, M.; Mütze, U.; Helbig, K.L.; Polster, T.; Albrecht, B.; Bernbeck, U.; van Binsbergen, E.; et al. Delineating SPTAN1 Associated Phenotypes: From Isolated Epilepsy to Encephalopathy with Progressive Brain Atrophy. Brain 2017, 140, 2322–2336. [Google Scholar] [CrossRef] [PubMed]
- Cakan, D.G.; Ulkur, F.; Taner, T.U. The Genetic Basis of Facial Skeletal Characteristics and Its Relation with Orthodontics. Eur. J. Dent. 2012, 6, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Aiken, J.; Moore, J.K.; Bates, E.A. TUBA1A Mutations Identified in Lissencephaly Patients Dominantly Disrupt Neuronal Migration and Impair Dynein Activity. Hum. Mol. Genet. 2019, 28, 1227–1243. [Google Scholar] [CrossRef]
- Kumar, R.A.; Pilz, D.T.; Babatz, T.D.; Cushion, T.D.; Harvey, K.; Topf, M.; Yates, L.; Robb, S.; Uyanik, G.; Mancini, G.M.S.; et al. TUBA1A Mutations Cause Wide Spectrum Lissencephaly (Smooth Brain) and Suggest That Multiple Neuronal Migration Pathways Converge on Alpha Tubulins. Hum. Mol. Genet. 2010, 19, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Alkuraya, F.S.; Cai, X.; Emery, C.; Mochida, G.H.; Al-Dosari, M.S.; Felie, J.M.; Hill, R.S.; Barry, B.J.; Partlow, J.N.; Gascon, G.G.; et al. Human Mutations in NDE1 Cause Extreme Microcephaly with Lissencephaly. Am. J. Hum. Genet. 2011, 88, 536–547. [Google Scholar] [CrossRef]
- Trainor, P.A.; Dixon, J.; Dixon, M.J. Treacher Collins Syndrome: Etiology, Pathogenesis and Prevention. Eur. J. Hum. Genet. 2009, 17, 275–283. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D.; Reutiman, T.J.; Thuras, P.D. Expression of GABAB Receptors Is Altered in Brains of Subjects with Autism. Cerebellum 2009, 8, 64–69. [Google Scholar] [CrossRef]
- Zhang, M.-W.; Liang, X.-Y.; Wang, J.; Gao, L.-D.; Liao, H.-J.; He, Y.-H.; Yi, Y.-H.; He, N.; Liao, W.-P. Epilepsy-Associated Genes: An Update. Seizure—Eur. J. Epilepsy 2024, 116, 4–13. [Google Scholar] [CrossRef]
- Petrov, T.; Rafols, J.A.; Alousi, S.S.; Kupsky, W.J.; Johnson, R.; Shah, J.; Shah, A.; Watson, C. Cellular Compartmentalization of Phosphorylated eIF2alpha and Neuronal NOS in Human Temporal Lobe Epilepsy with Hippocampal Sclerosis. J. Neurol. Sci. 2003, 209, 31–39. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, H.; Hu, Y.; Zhou, C.; Wu, J.; Wu, Y.; Wang, H.; Lenahan, C.; Huang, L.; Nie, S.; et al. Puerarin Attenuates Oxidative Stress and Ferroptosis via AMPK/PGC1α/Nrf2 Pathway after Subarachnoid Hemorrhage in Rats. Antioxidants 2022, 11, 1259. [Google Scholar] [CrossRef]
- Cheah, C.S.; Yu, F.H.; Westenbroek, R.E.; Kalume, F.K.; Oakley, J.C.; Potter, G.B.; Rubenstein, J.L.; Catterall, W.A. Specific Deletion of NaV1.1 Sodium Channels in Inhibitory Interneurons Causes Seizures and Premature Death in a Mouse Model of Dravet Syndrome. Proc. Natl. Acad. Sci. USA 2012, 109, 14646–14651. [Google Scholar] [CrossRef]
- Martin, M.S.; Dutt, K.; Papale, L.A.; Dubé, C.M.; Dutton, S.B.; de Haan, G.; Shankar, A.; Tufik, S.; Meisler, M.H.; Baram, T.Z.; et al. Altered Function of the SCN1A Voltage-Gated Sodium Channel Leads to γ-Aminobutyric Acid-Ergic (GABAergic) Interneuron Abnormalities. J. Biol. Chem. 2010, 285, 9823–9834. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, G.; Martinello, K.; Labate, A.; Cifelli, P.; Fucile, S.; Di Gennaro, G.; Quattrone, A.; Esposito, V.; Limatola, C.; Giangaspero, F.; et al. Modulation of GABAergic Dysfunction Due to SCN1A Mutation Linked to Hippocampal Sclerosis. Ann. Clin. Transl. Neurol. 2020, 7, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, N.R.; Bae, M.H.; Han, Y.M.; Byun, S.-Y.; Park, K.H. Miller-Dieker Syndrome in an Extremely Low Birth Weight Infant. Perinatology 2017, 28, 162–165. [Google Scholar] [CrossRef]
- Park, S.J.; Baek, J.; Chun, S.; Choi, E.K. Anesthetic Management and Bispectral Index in a Child with Miller–Dieker Syndrome: A Case Report. Children 2023, 10, 631. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, A.G.; Gunasekaran, S.; Jacob, R.S.; Omkumar, R.V. Role of Ca2+/Calmodulin-Dependent Protein Kinase Type II in Mediating Function and Dysfunction at Glutamatergic Synapses. Front. Mol. Neurosci. 2022, 15, 855752. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, J.; Lin, X.; Yang, L.; Zhou, Q.; Mi, X.; Li, Q.; Wang, S.; Li, D.; Liu, X.-M.; et al. METTL16 Promotes Translation and Lung Tumorigenesis by Sequestering Cytoplasmic eIF4E2. Cell Rep. 2023, 42, 112150. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, J.N.; Sivasudhan, E.; Tegowski, M.; Xing, Z.; McGinnis, M.M.; Hunter, O.V.; Featherston, K.M.; Sethia, K.; Tu, B.P.; Meyer, K.D.; et al. The Catalytic Efficiency of METTL16 Affects Cellular Processes by Governing the Intracellular S-Adenosylmethionine Setpoint. Cell Rep. 2025, 44, 115966. [Google Scholar] [CrossRef]
- D’Gama, A.M.; Woodworth, M.B.; Hossain, A.A.; Bizzotto, S.; Hatem, N.E.; LaCoursiere, C.M.; Najm, I.; Ying, Z.; Yang, E.; Barkovich, A.J.; et al. Somatic Mutations Activating the mTOR Pathway in Dorsal Telencephalic Progenitors Cause a Continuum of Cortical Dysplasias. Cell Rep. 2017, 21, 3754–3766. [Google Scholar] [CrossRef]
- Andrews, M.G.; Subramanian, L.; Kriegstein, A.R. mTOR Signaling Regulates the Morphology and Migration of Outer Radial Glia in Developing Human Cortex. Elife 2020, 9, e58737. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, T.J.; Pollen, A.A.; Sandoval-Espinosa, C.; Kriegstein, A.R. Transformation of the Radial Glia Scaffold Demarcates Two Stages of Human Cerebral Cortex Development. Neuron 2016, 91, 1219–1227. [Google Scholar] [CrossRef]
- Girodengo, M.; Ultanir, S.K.; Bateman, J.M. Mechanistic Target of Rapamycin Signaling in Human Nervous System Development and Disease. Front. Mol. Neurosci. 2022, 15, 1005631. [Google Scholar] [CrossRef]
- Han, J.; Wang, B.; Xiao, Z.; Gao, Y.; Zhao, Y.; Zhang, J.; Chen, B.; Wang, X.; Dai, J. Mammalian Target of Rapamycin (mTOR) Is Involved in the Neuronal Differentiation of Neural Progenitors Induced by Insulin. Mol. Cell. Neurosci. 2008, 39, 118–124. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Yue, M.; Wang, J.; Kumar, S.; Wechsler-Reya, R.J.; Zhang, Z.; Ogawa, Y.; Kellis, M.; Duester, G.; et al. N6-Methyladenosine RNA Modification Regulates Embryonic Neural Stem Cell Self-Renewal through Histone Modifications. Nat. Neurosci. 2018, 21, 195–206. [Google Scholar] [CrossRef]
- Zhang, F.; Kang, Y.; Wang, M.; Li, Y.; Xu, T.; Yang, W.; Song, H.; Wu, H.; Shu, Q.; Jin, P. Fragile X Mental Retardation Protein Modulates the Stability of Its m6A-Marked Messenger RNA Targets. Hum. Mol. Genet. 2018, 27, 3936–3950. [Google Scholar] [CrossRef]
- Jan, S.M.; Fahira, A.; Hassan, E.S.G.; Abdelhameed, A.S.; Wei, D.; Wadood, A. Integrative Approaches to m6A and m5C RNA Modifications in Autism Spectrum Disorder Revealing Potential Causal Variants. Mamm. Genome 2025, 36, 280–292. [Google Scholar] [CrossRef]
- Orji, O.C.; Stones, J.; Rajani, S.; Markus, R.; öz, M.D.; Knight, H.M. Global Co-Regulatory Cross Talk Between m6A and m5C RNA Methylation Systems Coordinate Cellular Responses and Brain Disease Pathways. Mol. Neurobiol. 2025, 62, 5006–5021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Xu, Y.; Gao, S.; Qin, L.; Austria, Q.; Siedlak, S.L.; Pajdzik, K.; Dai, Q.; He, C.; Wang, W.; et al. METTL3-Dependent RNA m6A Dysregulation Contributes to Neurodegeneration in Alzheimer’s Disease through Aberrant Cell Cycle Events. Mol. Neurodegener. 2021, 16, 70. [Google Scholar] [CrossRef]
- Yu, Z.; Huang, L.; Xia, Y.; Cheng, S.; Yang, C.; Chen, C.; Zou, Z.; Wang, X.; Tian, X.; Jiang, X.; et al. Analysis of m6A Modification Regulators in the Substantia Nigra and Striatum of MPTP-Induced Parkinson’s Disease Mice. Neurosci. Lett. 2022, 791, 136907. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Skuland, T.; Zhou, C.; Li, A.; Hashim, A.; Jermstad, I.; Khan, S.; Dalen, K.T.; Greggains, G.D.; et al. The RNA m6A Landscape of Mouse Oocytes and Preimplantation Embryos. Nat. Struct. Mol. Biol. 2023, 30, 703–709. [Google Scholar] [CrossRef]
- Wang, J.; Sha, Y.; Sun, T. m6A Modifications Play Crucial Roles in Glial Cell Development and Brain Tumorigenesis. Front. Oncol. 2021, 11, 611660. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Guo, F.; Huang, G.; Zhao, Y.; Chen, B.; Wang, C.; Cui, C.; Shi, Y.; Li, S.; et al. Knockdown of METTL16 Disrupts Learning and Memory by Reducing the Stability of MAT2A mRNA. Cell Death Discov. 2022, 8, 432. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, K.E.; Chen, B.; Liu, K.; Hunter, O.V.; Xie, Y.; Tu, B.P.; Conrad, N.K. The U6 snRNA m6A Methyltransferase METTL16 Regulates SAM Synthetase Intron Retention. Cell 2017, 169, 824–835.e14. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.W.Q.; Goh, Y.T.; Goh, W.S.S. Atlas of Quantitative Single-Base-Resolution N6-Methyl-Adenine Methylomes. Nat. Commun. 2019, 10, 5636. [Google Scholar] [CrossRef] [PubMed]
- Mikutis, S.; Gu, M.; Sendinc, E.; Hazemi, M.E.; Kiely-Collins, H.; Aspris, D.; Vassiliou, G.S.; Shi, Y.; Tzelepis, K.; Bernardes, G.J.L. meCLICK-Seq, a Substrate-Hijacking and RNA Degradation Strategy for the Study of RNA Methylation. ACS Cent. Sci. 2020, 6, 2196–2208. [Google Scholar] [CrossRef]
- Ikemoto, S.; Hamano, S.-I.; Hirata, Y.; Matsuura, R.; Koichihara, R. Perampanel in Lissencephaly-Associated Epilepsy. Epilepsy Behav. Case Rep. 2019, 11, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Elmardenly, A.; Aljehani, Z.; Tamim, A.; Alyazidi, A.; Muthaffar, O. Efficacy and Safety of Perampanel in Children with Drug-Resistant Focal-Onset Seizures: A Retrospective Review. Children 2023, 10, 1071. [Google Scholar] [CrossRef]
- Fukuoka, M.; Kuki, I.; Hattori, Y.; Tsuji, H.; Horino, A.; Nukui, M.; Inoue, T.; Okazaki, S.; Kunihiro, N.; Uda, T. Total Callosotomy Ameliorates Epileptic Activity and Improves Cognitive Function in a Patient with Miller-Dieker Syndrome. Epilepsy Behav. Rep. 2024, 26, 100670. [Google Scholar] [CrossRef]
- Inaba, Y.; D’Antuono, M.; Bertazzoni, G.; Biagini, G.; Avoli, M. Diminished Presynaptic GABAB Receptor Function in the Neocortex of a Genetic Model of Absence Epilepsy. Neurosignals 2009, 17, 121–131. [Google Scholar] [CrossRef]
- Mengesdorf, T.; Proud, C.G.; Mies, G.; Paschen, W. Mechanisms Underlying Suppression of Protein Synthesis Induced by Transient Focal Cerebral Ischemia in Mouse Brain. Exp. Neurol. 2002, 177, 538–546. [Google Scholar] [CrossRef]
- Coulson, R.L.; Frattini, V.; Moyer, C.E.; Hodges, J.; Walter, P.; Mourrain, P.; Zuo, Y.; Wang, G.X. Translational Modulator ISRIB Alleviates Synaptic and Behavioral Phenotypes in Fragile X Syndrome. iScience 2024, 27, 109259. [Google Scholar] [CrossRef]
- Sandouka, S.; Shekh-Ahmad, T. Induction of the Nrf2 Pathway by Sulforaphane Is Neuroprotective in a Rat Temporal Lobe Epilepsy Model. Antioxidants 2021, 10, 1702. [Google Scholar] [CrossRef]
- Carmona-Aparicio, L.; Pérez-Cruz, C.; Zavala-Tecuapetla, C.; Granados-Rojas, L.; Rivera-Espinosa, L.; Montesinos-Correa, H.; Hernández-Damián, J.; Pedraza-Chaverri, J.; Sampieri, A.I.; Coballase-Urrutia, E.; et al. Overview of Nrf2 as Therapeutic Target in Epilepsy. Int. J. Mol. Sci. 2015, 16, 18348–18367. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Buckley, J.A.; Li, X.; Liu, Y.; Fox, T.H.; Meares, G.P.; Yu, H.; Yan, Z.; Harms, A.S.; Li, Y.; et al. Inhibition of the JAK/STAT Pathway Protects Against α-Synuclein-Induced Neuroinflammation and Dopaminergic Neurodegeneration. J. Neurosci. 2016, 36, 5144–5159. [Google Scholar] [CrossRef]
- Rodriguez, S.; Hug, C.; Todorov, P.; Moret, N.; Boswell, S.A.; Evans, K.; Zhou, G.; Johnson, N.T.; Hyman, B.T.; Sorger, P.K.; et al. Machine Learning Identifies Candidates for Drug Repurposing in Alzheimer’s Disease. Nat. Commun. 2021, 12, 1033. [Google Scholar] [CrossRef]
- Xiang, Z.; Zhang, S.; Yao, X.; Xu, L.; Hu, J.; Yin, C.; Chen, J.; Xu, H. Resveratrol Promotes Axonal Regeneration after Spinal Cord Injury through Activating Wnt/β-Catenin Signaling Pathway. Aging 2021, 13, 23603–23619. [Google Scholar] [CrossRef]
- Wang, G.; Li, Z.; Li, S.; Ren, J.; Suresh, V.; Xu, D.; Zang, W.; Liu, X.; Li, W.; Wang, H.; et al. Minocycline Preserves the Integrity and Permeability of BBB by Altering the Activity of DKK1–Wnt Signaling in ICH Model. Neuroscience 2019, 415, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Surya, K.; Manickam, N.; Jayachandran, K.S.; Kandasamy, M.; Anusuyadevi, M. Resveratrol Mediated Regulation of Hippocampal Neuroregenerative Plasticity via SIRT1 Pathway in Synergy with Wnt Signaling: Neurotherapeutic Implications to Mitigate Memory Loss in Alzheimer’s Disease. J. Alzheimers Dis. 2023, 94, S125–S140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Yu, R.; Zhang, Q.; Zhang, Z.; Li, H.; Ren, C.; Yang, R.; Niu, H. Minocycline Inhibition of Microglial Rescues Nigrostriatal Dopaminergic Neurodegeneration Caused by Mutant Alpha-Synuclein Overexpression. Aging 2020, 12, 14232–14243. [Google Scholar] [CrossRef]
- Plane, J.M.; Shen, Y.; Pleasure, D.E.; Deng, W. Prospects for Minocycline Neuroprotection. Arch. Neurol. 2010, 67, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Zhang, G.; Yan, R.; Zhou, D.; Huang, L.; Zhang, Q.; Li, W.; Huang, G.; Li, Z.; Yan, J. SAM/SAH Mediates Parental Folate Deficiency-Induced Neural Cell Apoptosis in Neonatal Rat Offspring: The Expression of Bcl-2, Bax, and Caspase-3. Int. J. Mol. Sci. 2023, 24, 14508. [Google Scholar] [CrossRef]
- Caudill, M.A.; Wang, J.C.; Melnyk, S.; Pogribny, I.P.; Collins, M.D.; Santos-Guzman, J.; Swendseid, M.E.; Cogger, E.A.; James, S.J.; Jernigan, S. Intracellular S-Adenosylhomocysteine Concentrations Predict Global DNA Hypomethylation in Tissues of Methyl-Deficient Cystathionine β-Synthase Heterozygous Mice. J. Nutr. 2001, 131, 2811–2818. [Google Scholar] [CrossRef]
- Barak, A.J.; Beckenhauer, H.C.; Mailliard, M.E.; Kharbanda, K.K.; Tuma, D.J. Betaine Lowers Elevated S-Adenosylhomocysteine Levels in Hepatocytes from Ethanol-Fed Rats. J. Nutr. 2003, 133, 2845–2848. [Google Scholar] [CrossRef]
- Kato, T.; Pothula, S.; Liu, R.-J.; Duman, C.H.; Terwilliger, R.; Vlasuk, G.P.; Saiah, E.; Hahm, S.; Duman, R.S. Sestrin Modulator NV-5138 Produces Rapid Antidepressant Effects via Direct mTORC1 Activation. J. Clin. Investig. 2019, 129, 2542–2554. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-T.; Lin, Y.-C.; Ho, W.-H.; Liu, C.-L.; Lee, W.-T. Everolimus Is Better than Rapamycin in Attenuating Neuroinflammation in Kainic Acid-Induced Seizures. J. Neuroinflamm. 2017, 14, 15. [Google Scholar] [CrossRef]
- Mühlebner, A.; Bongaarts, A.; Sarnat, H.B.; Scholl, T.; Aronica, E. New Insights into a Spectrum of Developmental Malformations Related to mTOR Dysregulations: Challenges and Perspectives. J. Anat. 2019, 235, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Kleijn, M.; Welsh, G.I.; Scheper, G.C.; Voorma, H.O.; Proud, C.G.; Thomas, A.A. Nerve and Epidermal Growth Factor Induce Protein Synthesis and eIF2B Activation in PC12 Cells. J. Biol. Chem. 1998, 273, 5536–5541. [Google Scholar] [CrossRef]
- Fu, X.; Zhu, J.; Duan, Y.; Lu, P.; Zhang, K. CRISPR/Cas9 Mediated Somatic Gene Therapy for Insertional Mutations: The Vibrator Mouse Model. Precis. Clin. Med. 2021, 4, 168–175. [Google Scholar] [CrossRef]
- Lo Nigro, C.; Chong, S.S.; Smith, A.C.M.; Dobyns, W.B.; Carrozzo, R.; Ledbetter, D.H. Point Mutations and an Intragenic Deletion in LIS1, the Lissencephaly Causative Gene in Isolated Lissencephaly Sequence and Miller-Dieker Syndrome. Human Mol. Genet. 1997, 6, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Hulton, C.H.; Costa, E.A.; Shah, N.S.; Quintanal-Villalonga, A.; Heller, G.; de Stanchina, E.; Rudin, C.M.; Poirier, J.T. Direct Genome Editing of Patient-Derived Xenografts Using CRISPR-Cas9 Enables Rapid in Vivo Functional Genomics. Nat. Cancer 2020, 1, 359–369. [Google Scholar] [CrossRef]
- Musunuru, K.; Grandinette, S.A.; Wang, X.; Hudson, T.R.; Briseno, K.; Berry, A.M.; Hacker, J.L.; Hsu, A.; Silverstein, R.A.; Hille, L.T.; et al. Patient-Specific In Vivo Gene Editing to Treat a Rare Genetic Disease. N. Engl. J. Med. 2025, 392, 2235–2243. [Google Scholar] [CrossRef]
- McCarron, A.; Ling, K.-M.; Montgomery, S.T.; Martinovich, K.M.; Cmielewski, P.; Rout-Pitt, N.; Kicic, A.; Parsons, D.; Donnelley, M. Lentiviral Vector Gene Therapy and CFTR Modulators Show Comparable Effectiveness in Cystic Fibrosis Rat Airway Models. Gene Ther. 2024, 31, 553–559. [Google Scholar] [CrossRef]
- Smith, P.Y.; Hernandez-Rapp, J.; Jolivette, F.; Lecours, C.; Bisht, K.; Goupil, C.; Dorval, V.; Parsi, S.; Morin, F.; Planel, E.; et al. miR-132/212 Deficiency Impairs Tau Metabolism and Promotes Pathological Aggregation in Vivo. Hum. Mol. Genet. 2015, 24, 6721–6735. [Google Scholar] [CrossRef]
- El Fatimy, R.; Li, S.; Chen, Z.; Mushannen, T.; Gongala, S.; Wei, Z.; Balu, D.T.; Rabinovsky, R.; Cantlon, A.; Elkhal, A.; et al. MicroRNA-132 Provides Neuroprotection for Tauopathies via Multiple Signaling Pathways. Acta Neuropathol. 2018, 136, 537–555. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Zuccato, C.; Belyaev, N.D.; Guest, D.J.; Cattaneo, E.; Buckley, N.J. A microRNA-Based Gene Dysregulation Pathway in Huntington’s Disease. Neurobiol. Dis. 2008, 29, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Jovicic, A.; Zaldivar Jolissaint, J.F.; Moser, R.; Silva Santos, M.d.F.; Luthi-Carter, R. MicroRNA-22 (miR-22) Overexpression Is Neuroprotective via General Anti-Apoptotic Effects and May Also Target Specific Huntington’s Disease-Related Mechanisms. PLoS ONE 2013, 8, e54222. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Luo, G.; Yu, X.; Liu, Q.; Peng, M.; Hou, M. Heterozygous Inversion on Chromosome 17 Involving PAFAH1B1 Detected by Whole Genome Sequencing in a Patient Suffering from Pachygyria. Eur. J. Med. Genet. 2025, 73, 104991. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kamishima, M.; Yokoi, K.; Suzuki, S. Unusual Presentation of Acute Encephalopathy with Biphasic Seizures and Late Reduced Diffusion in Miller–Dieker Syndrome. BMJ Case Rep. 2022, 15, e248190. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahendran, G.; Brown, J.A. Understanding the Molecular Basis of Miller–Dieker Syndrome. Int. J. Mol. Sci. 2025, 26, 7375. https://doi.org/10.3390/ijms26157375
Mahendran G, Brown JA. Understanding the Molecular Basis of Miller–Dieker Syndrome. International Journal of Molecular Sciences. 2025; 26(15):7375. https://doi.org/10.3390/ijms26157375
Chicago/Turabian StyleMahendran, Gowthami, and Jessica A. Brown. 2025. "Understanding the Molecular Basis of Miller–Dieker Syndrome" International Journal of Molecular Sciences 26, no. 15: 7375. https://doi.org/10.3390/ijms26157375
APA StyleMahendran, G., & Brown, J. A. (2025). Understanding the Molecular Basis of Miller–Dieker Syndrome. International Journal of Molecular Sciences, 26(15), 7375. https://doi.org/10.3390/ijms26157375







