LIMK2-1 Is a Phosphorylation-Dependent Inhibitor of Protein Phosphatase-1 Catalytic Subunit and Myosin Phosphatase Holoenzyme
Abstract
1. Introduction
2. Results
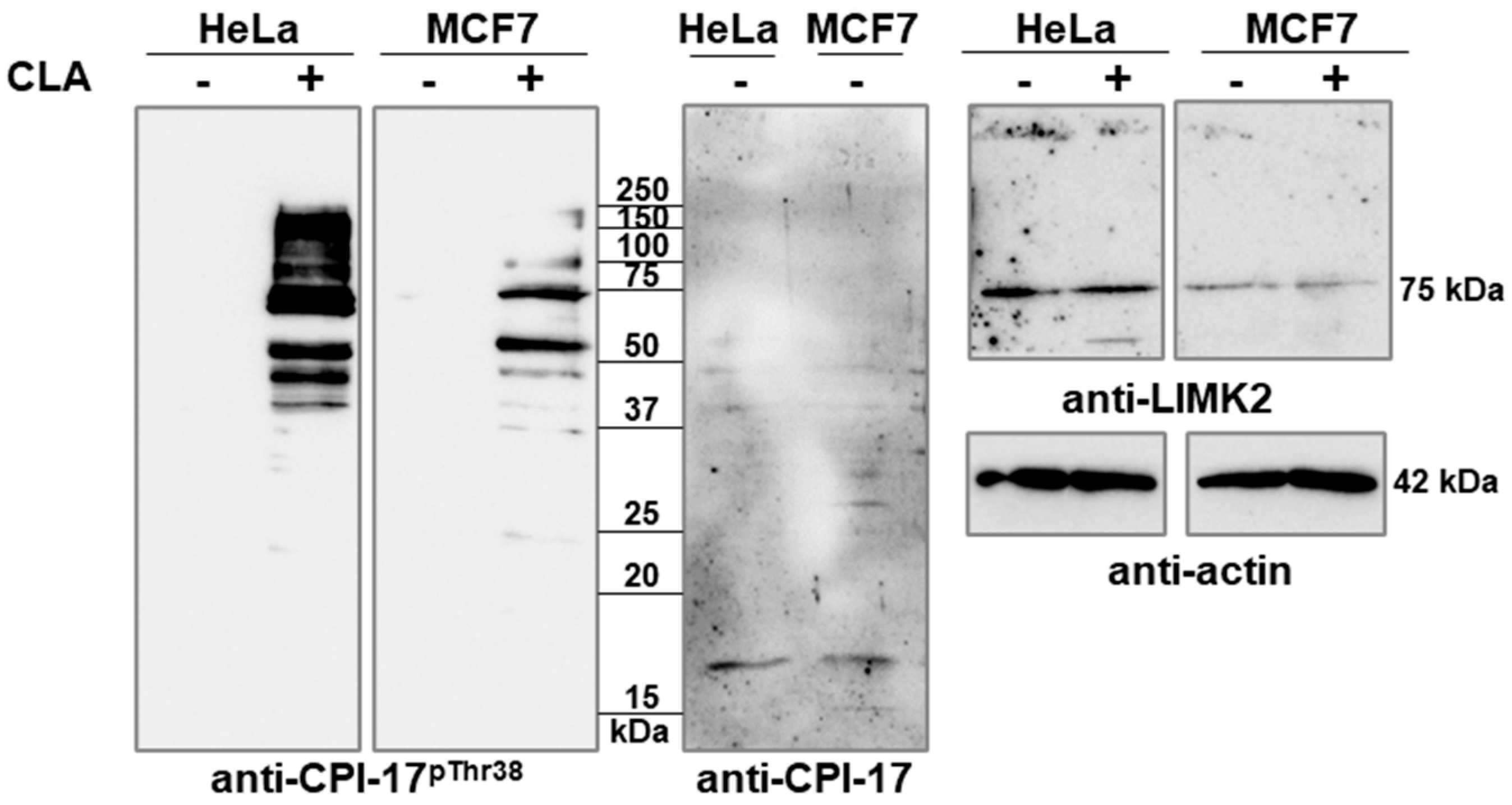
2.1. CLA Induces Phosphorylation of CPI-17-like Sequences of Proteins in HeLa and MCF7 Cells
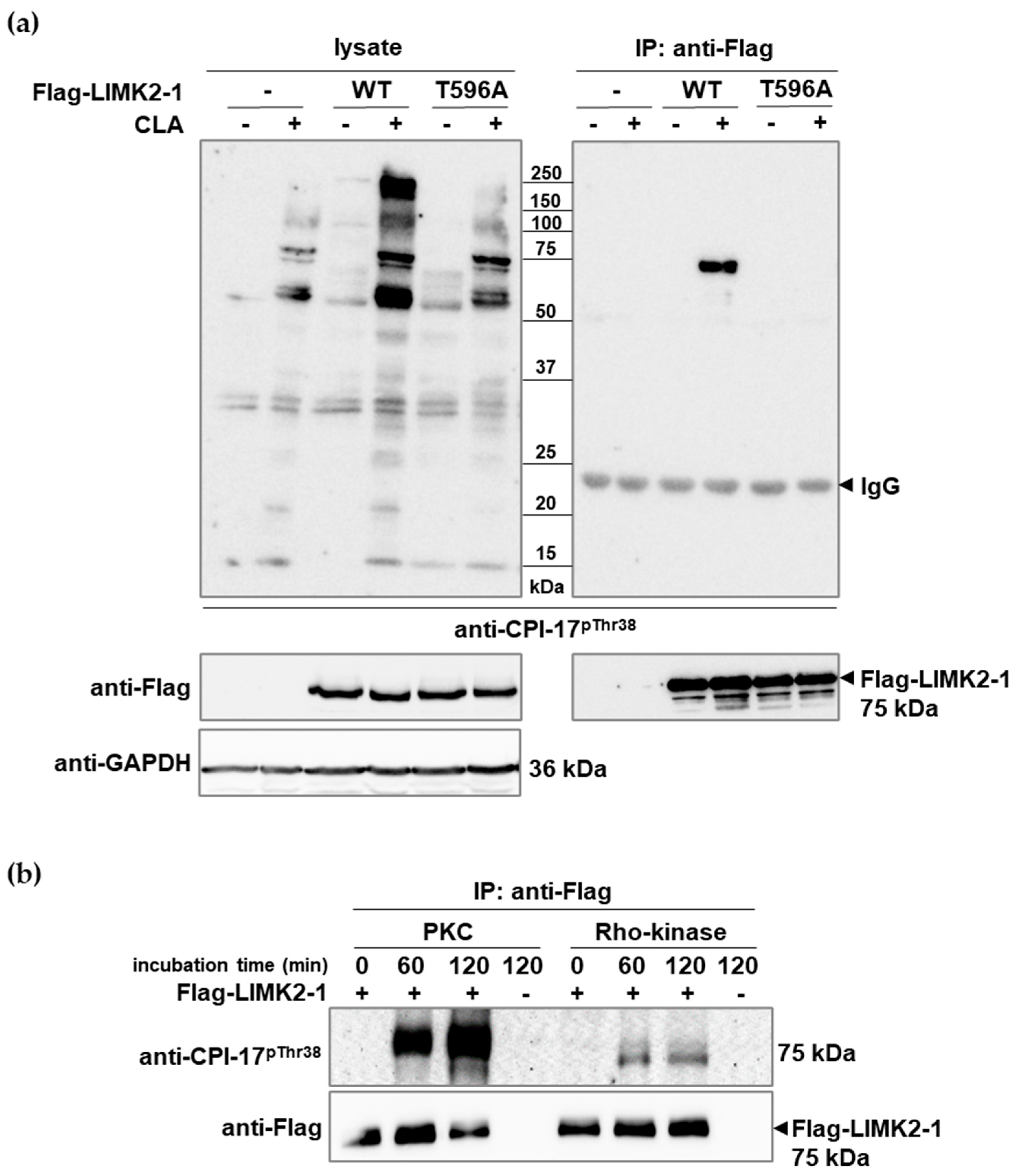
2.2. Phosphorylation of Flag-LIMK2-1 at the CPI-17-like Sequences
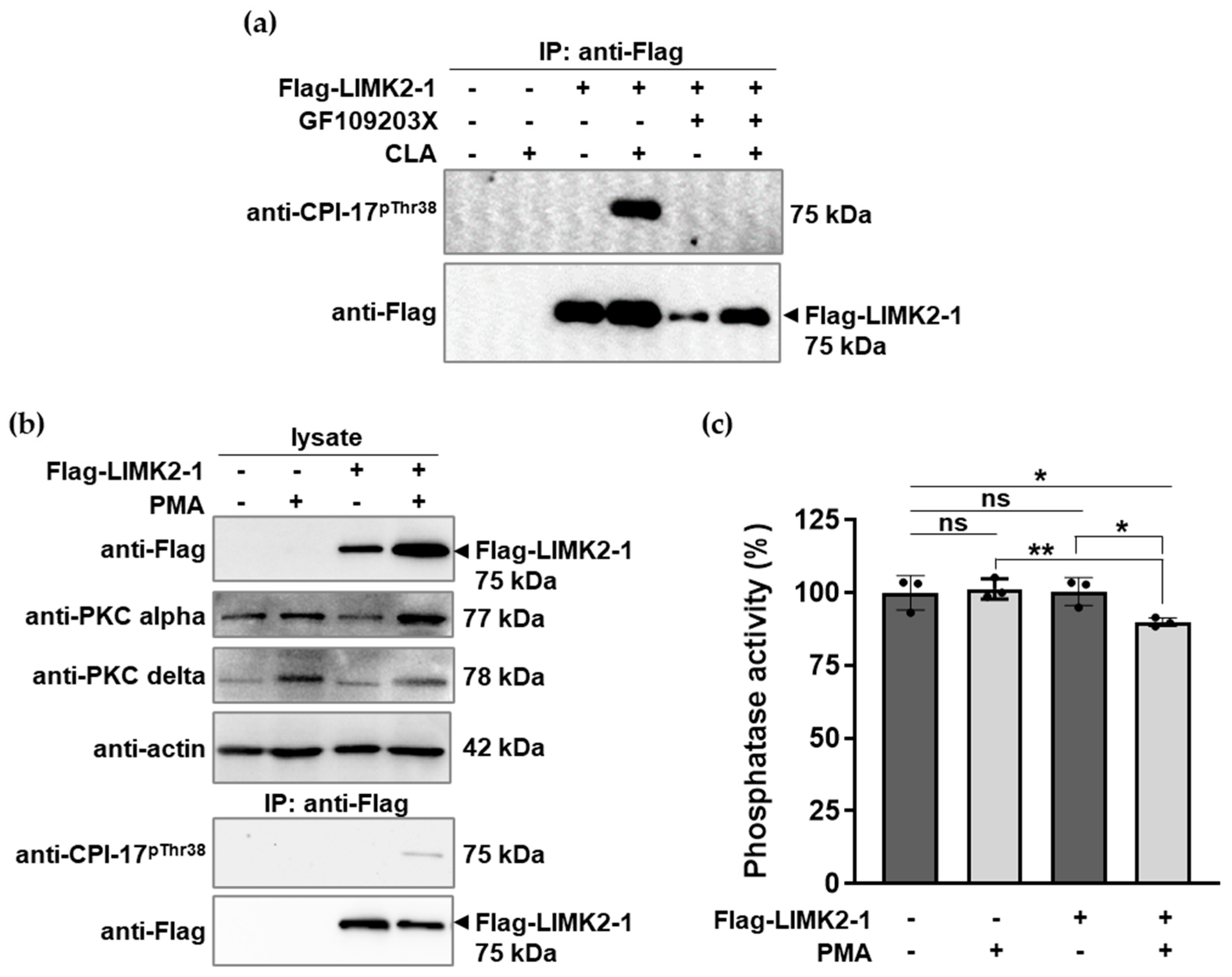
2.3. LIMK2-1 Interacts with and Inhibits PP1c and Reconstituted Myosin Phosphatase
3. Discussion
4. Materials and Methods
4.1. Proteins and Antibodies
4.2. Cell Cultures and Treatments
4.3. Expression Plasmids and Site-Directed Mutagenesis
4.4. Cell Transfections
4.5. Immunoprecipitation and Purification of Flag-LIMK2-1
4.6. Western Blotting
4.7. In Vitro Phosphorylation
4.8. Protein Phosphatase Assay
4.9. Sequence Similarity Search
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CLA | Calyculin A |
| CPI-17 | Protein kinase C-activated protein phosphatase-1 inhibitor of 17 kDa |
| Flag-LIMK2-1 | Flag-tagged LIM kinase 2 isoform 1 |
| GBPI-1 | Gastrointestinal- and brain-specific PP1 inhibitor |
| HA-PP1cα | HA-tagged protein phosphatase 1 catalytic subunit α isoform |
| HA-PP1cδ | HA-tagged protein phosphatase 1 catalytic subunit δ isoform |
| KEPI | Kinase C-enhanced PP1 inhibitor |
| LIMK2 | LIM kinase 2 |
| LIMK2-1 | LIM kinase 2 isoform 1 |
| MLC20 | 20 kDa light chain of myosin |
| 32P-MLC20 | 32P-labeled MLC20 |
| MP | Myosin phosphatase |
| MYPT1 | Myosin phosphatase target subunit-1 |
| PHI-1 | Pphosphatase holoenzyme inhibitor-1 |
| PKC | Protein kinase C |
| PMA | Phorbol 12-myristate 13-acetate |
| PP1 | Protein phosphatase-1 |
| PP1c | PP1 catalytic subunit |
| PP1cα | PP1 catalytic subunit α isoform |
| PP1cδ | PP1 catalytic subunit δ isoform |
References
- Eto, M.; Ohmori, T.; Suzuki, M.; Furuya, K.; Morita, F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C. Isolation from porcine aorta media and characterization. J. Biochem. 1995, 118, 1104–1107. [Google Scholar] [CrossRef]
- Hartshorne, D.J.; Ito, M.; Erdődi, F. Role of protein phosphatase type 1 in contractile functions: Myosin phosphatase. J. Biol. Chem. 2004, 279, 37211–37214. [Google Scholar] [CrossRef] [PubMed]
- Erdődi, F.; Kiss, E.; Walsh, M.P.; Stefansson, B.; Deng, J.T.; Eto, M.; Brautigan, D.L.; Hartshorne, D.J. Phosphorylation of protein phosphatase type-1 inhibitory proteins by integrin-linked kinase and cyclic nucleotide-dependent protein kinases. Biochem. Biophys. Res. Commun. 2003, 306, 382–387. [Google Scholar] [CrossRef]
- Eto, M. Regulation of cellular protein phosphatase-1 (PP1) by phosphorylation of the CPI-17 family, C-kinase-activated PP1 inhibitors. J. Biol. Chem. 2009, 284, 35273–35277. [Google Scholar] [CrossRef]
- Eto, M.; Senba, S.; Morita, F.; Yazawa, M. Molecular cloning of a novel phosphorylation-dependent inhibitory protein of protein phosphatase-1 (CPI17) in smooth muscle: Its specific localization in smooth muscle. FEBS Lett. 1997, 410, 356–360. [Google Scholar] [CrossRef]
- Hayashi, Y.; Senba, S.; Yazawa, M.; Brautigan, D.L.; Eto, M. Defining the structural determinants and a potential mechanism for inhibition of myosin phosphatase by the protein kinase C-potentiated inhibitor protein of 17 kDa. J. Biol. Chem. 2001, 276, 39858–39863. [Google Scholar] [CrossRef]
- Eto, M.; Karginov, A.; Brautigan, D.L. A novel phosphoprotein inhibitor of protein type-1 phosphatase holoenzymes. Biochemistry 1999, 38, 16952–16957. [Google Scholar] [CrossRef]
- Liu, Q.-R.; Zhang, P.-W.; Zhen, Q.; Walther, D.; Wang, X.-B.; Uhl, G.R. KEPI, a PKC-dependent Protein Phosphatase 1 Inhibitor Regulated by Morphine∗. J. Biol. Chem. 2002, 277, 13312–13320. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.R.; Zhang, P.W.; Lin, Z.; Li, Q.F.; Woods, A.S.; Troncoso, J.; Uhl, G.R. GBPI, a novel gastrointestinal- and brain-specific PP1-inhibitory protein, is activated by PKC and inactivated by PKA. Biochem. J. 2004, 377, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Eto, M.; Kitazawa, T.; Matsuzawa, F.; Aikawa, S.-I.; Kirkbride, J.A.; Isozumi, N.; Nishimura, Y.; Brautigan, D.L.; Ohki, S.-Y. Phosphorylation-induced conformational switching of CPI-17 produces a potent myosin phosphatase inhibitor. Structure 2007, 15, 1591–1602. [Google Scholar] [CrossRef]
- Somlyo, A.P.; Somlyo, A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003, 83, 1325–1358. [Google Scholar] [CrossRef]
- Jin, H.; Sperka, T.; Herrlich, P.; Morrison, H. Tumorigenic transformation by CPI-17 through inhibition of a merlin phosphatase. Nature 2006, 442, 576–579. [Google Scholar] [CrossRef]
- Riecken, L.B.; Zoch, A.; Wiehl, U.; Reichert, S.; Scholl, I.; Cui, Y.; Ziemer, M.; Anderegg, U.; Hagel, C.; Morrison, H. CPI-17 drives oncogenic Ras signaling in human melanomas via Ezrin-Radixin-Moesin family proteins. Oncotarget 2016, 7, 78242–78254. [Google Scholar] [CrossRef] [PubMed]
- Hagel, C.; Dornblut, C.; Schulz, A.; Wiehl, U.; Friedrich, R.; Huckhagel, T.; Mautner, V.F.; Morrison, H. The putative oncogene CPI-17 is up-regulated in schwannoma. Neuropathol. Appl. Neurobiol. 2016, 42, 664–668. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Shi, Y.; Yin, D.; Dai, P.; Zhao, W.; Zhang, T. CPI-17 overexpression and its correlation with the NF2 mutation spectrum in sporadic vestibular schwannomas. Otol. Neurotol. 2020, 41, e94–e102. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.E.; Mitchell, H.D.; Gralinski, L.E.; Eisfeld, A.J.; Josset, L.; Bankhead, A.; Neumann, G.; Tilton, S.C.; Schäfer, A.; Li, C. The effect of inhibition of PP1 and TNFα signaling on pathogenesis of SARS coronavirus. BMC Syst. Biol. 2016, 10, 93. [Google Scholar] [CrossRef]
- Wenzel, K.; Daskalow, K.; Herse, F.; Seitz, S.; Zacharias, U.; Schenk, J.A.; Schulz, H.; Hubner, N.; Micheel, B.; Schlag, P.M. Expression of the protein phosphatase 1 inhibitor KEPI is downregulated in breast cancer cell lines and tissues and involved in the regulation of the tumor suppressor EGR1 via the MEK-ERK pathway. Biol. Chem. 2007, 388, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Kirkbride, J.A.; Nilsson, G.Y.; Kim, J.I.; Takeya, K.; Tanaka, Y.; Tokumitsu, H.; Suizu, F.; Eto, M. PHI-1, an Endogenous Inhibitor Protein for Protein Phosphatase-1 and a Pan-Cancer Marker, Regulates Raf-1 Proteostasis. Biomolecules 2023, 13, 1741. [Google Scholar] [CrossRef]
- Eto, M. Rediscovery of PHI-1/PPP1R14B: Emerging Roles of Cellular PP1 Signaling Mediated by the PPP1R14B Gene Product in Multiple Cancers and Beyond. Biomolecules 2025, 15, 344. [Google Scholar] [CrossRef]
- Takizawa, N.; Niiro, N.; Ikebe, M. Dephosphorylation of the two regulatory components of myosin phosphatase, MBS and CPI17. FEBS Lett. 2002, 515, 127–132. [Google Scholar] [CrossRef]
- Eto, M.; Kitazawa, T.; Brautigan, D.L. Phosphoprotein inhibitor CPI-17 specificity depends on allosteric regulation of protein phosphatase-1 by regulatory subunits. Proc. Natl. Acad. Sci. USA 2004, 101, 8888–8893. [Google Scholar] [CrossRef] [PubMed]
- Dedinszki, D.; Kiss, A.; Márkász, L.; Márton, A.; Tóth, E.; Székely, L.; Erdődi, F. Inhibition of protein phosphatase-1 and -2A decreases the chemosensitivity of leukemic cells to chemotherapeutic drugs. Cell Signal. 2015, 27, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Dubois, T.; Howell, S.; Zemlickova, E.; Learmonth, M.; Cronshaw, A.; Aitken, A. Novel in vitro and in vivo phosphorylation sites on protein phosphatase 1 inhibitor CPI-17. Biochem. Biophys. Res. Commun. 2003, 302, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Vallée, B.; Cuberos, H.; Doudeau, M.; Godin, F.; Gosset, D.; Vourc’h, P.; Andres, C.R.; Bénédetti, H. LIMK2-1, a new isoform of human LIMK2, regulates actin cytoskeleton remodeling via a different signaling pathway than that of its two homologs, LIMK2a and LIMK2b. Biochem. J. 2018, 475, 3745–3761. [Google Scholar] [CrossRef]
- Kiss, A.; Erdodi, F.; Lontay, B. Myosin phosphatase: Unexpected functions of a long-known enzyme. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2019, 1866, 2–15. [Google Scholar] [CrossRef]
- Kiss, A.; Mahfood, M.; Bodogán, Z.; Kónya, Z.; Bécsi, B.; Erdődi, F. Cell-Penetrating Peptide Based on Myosin Phosphatase Target Subunit Sequence Mediates Myosin Phosphatase Activity. Biomolecules 2025, 15, 705. [Google Scholar] [CrossRef]
- Bátori, R.; Bécsi, B.; Nagy, D.; Kónya, Z.; Hegedűs, C.; Bordán, Z.; Verin, A.; Lontay, B.; Erdődi, F. Interplay of myosin phosphatase and protein phosphatase-2A in the regulation of endothelial nitric-oxide synthase phosphorylation and nitric oxide production. Sci. Rep. 2017, 7, 44698. [Google Scholar] [CrossRef]

| Gene Name | Protein Name | Accession Number | Amino Acids | MW (Da) | Identities | Sequence | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | PPP1R14A | Protein phosphatase 1 regulatory subunit 14A (CPI-17) | NP_150281.1 NP_001230876.1 | 147 120 | 16,693 13,480 | 12/12 | A | R | V | T | V | K | Y | D | R | R | E | L |
| 2. | PPP1R14B | Protein phosphatase 1 regulatory subunit 14B (PHI-1) | NP_619634.1 | 147 | 15,911 | 9/12 (75%) | G | K | V | T | V | K | Y | D | R | K | E | L |
| 3. | PPP1R14C | Protein phosphatase 1 regulatory subunit 14C (KEPI) | NP_112211.1 | 165 | 17,843 | 9/12 (75%) | G | K | V | T | V | K | Y | D | R | K | E | L |
| 4. | PPP1R14D | Protein phosphatase 1 regulatory subunit 14D isoform (GBPI-1) | NP_060196.1 NP_001123615.1 | 145 200 | 16,508 22,430 | 7/12 (58%) | S | R | L | T | V | K | Y | D | R | G | Q | L |
| 5. | LIMK2 | LIM kinase 2 | NP_001026971.1 AAB54055.1 KAI2597385.1 | 686 733 629 | 77,886 no data 71,193 | 7/12 (58%) | G | K | V | T | I | K | Y | D | P | K | E | L |
| 6. | PSKH1 | Serine/threonine-protein kinase H1 | NP_006733.1 | 424 | 48,035 | 7/12 (58%) | P | R | V | T | A | K | Y | D | I | K | A | L |
| 7. | TIGAR | Fructose-2,6-bisphosphatase TIGAR | NP_065108 | 270 | 30,063 | 6/12 (50%) | K | D | M | T | V | K | Y | D | S | R | L | R |
| 8. | BACE2 | Beta-secretase 2 (memapsin 1) | NP_036237.2 | 518 | 56,180 | 5/12 (42%) | F | D | V | T | V | K | Y | T | Q | G | S | W |
| 9. | LIPK | Lipase family member K | NP_001073987.1 | 399 | 45,563 | 5/12 (42%) | P | V | V | T | V | K | Y | T | Q | S | P | M |
| 10. | PM20D2 | Xaa-Arg dipeptidase | NP_001010853.1 | 436 | 47,776 | 5/12 (42%) | H | D | V | T | V | K | Y | Y | G | K | A | S |
| 11. | FAM210A | Protein FAM210A | NP_001092271.1 | 272 | 30,777 | 6/12 (50%) | T | S | V | T | V | K | Y | L | R | S | H | G |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, A.; Tóth, E.; Bodogán, Z.; Mahfood, M.; Kónya, Z.; Erdődi, F. LIMK2-1 Is a Phosphorylation-Dependent Inhibitor of Protein Phosphatase-1 Catalytic Subunit and Myosin Phosphatase Holoenzyme. Int. J. Mol. Sci. 2025, 26, 7347. https://doi.org/10.3390/ijms26157347
Kiss A, Tóth E, Bodogán Z, Mahfood M, Kónya Z, Erdődi F. LIMK2-1 Is a Phosphorylation-Dependent Inhibitor of Protein Phosphatase-1 Catalytic Subunit and Myosin Phosphatase Holoenzyme. International Journal of Molecular Sciences. 2025; 26(15):7347. https://doi.org/10.3390/ijms26157347
Chicago/Turabian StyleKiss, Andrea, Emese Tóth, Zsófia Bodogán, Mohamad Mahfood, Zoltán Kónya, and Ferenc Erdődi. 2025. "LIMK2-1 Is a Phosphorylation-Dependent Inhibitor of Protein Phosphatase-1 Catalytic Subunit and Myosin Phosphatase Holoenzyme" International Journal of Molecular Sciences 26, no. 15: 7347. https://doi.org/10.3390/ijms26157347
APA StyleKiss, A., Tóth, E., Bodogán, Z., Mahfood, M., Kónya, Z., & Erdődi, F. (2025). LIMK2-1 Is a Phosphorylation-Dependent Inhibitor of Protein Phosphatase-1 Catalytic Subunit and Myosin Phosphatase Holoenzyme. International Journal of Molecular Sciences, 26(15), 7347. https://doi.org/10.3390/ijms26157347









