MicroRNA528 and Its Regulatory Roles in Monocotyledonous Plants
Abstract
1. Overview of Role of miRNAs in Plant Development and Stress Responses
2. miR528 and Its Targets
2.1. MIR528 Genes in Monocots
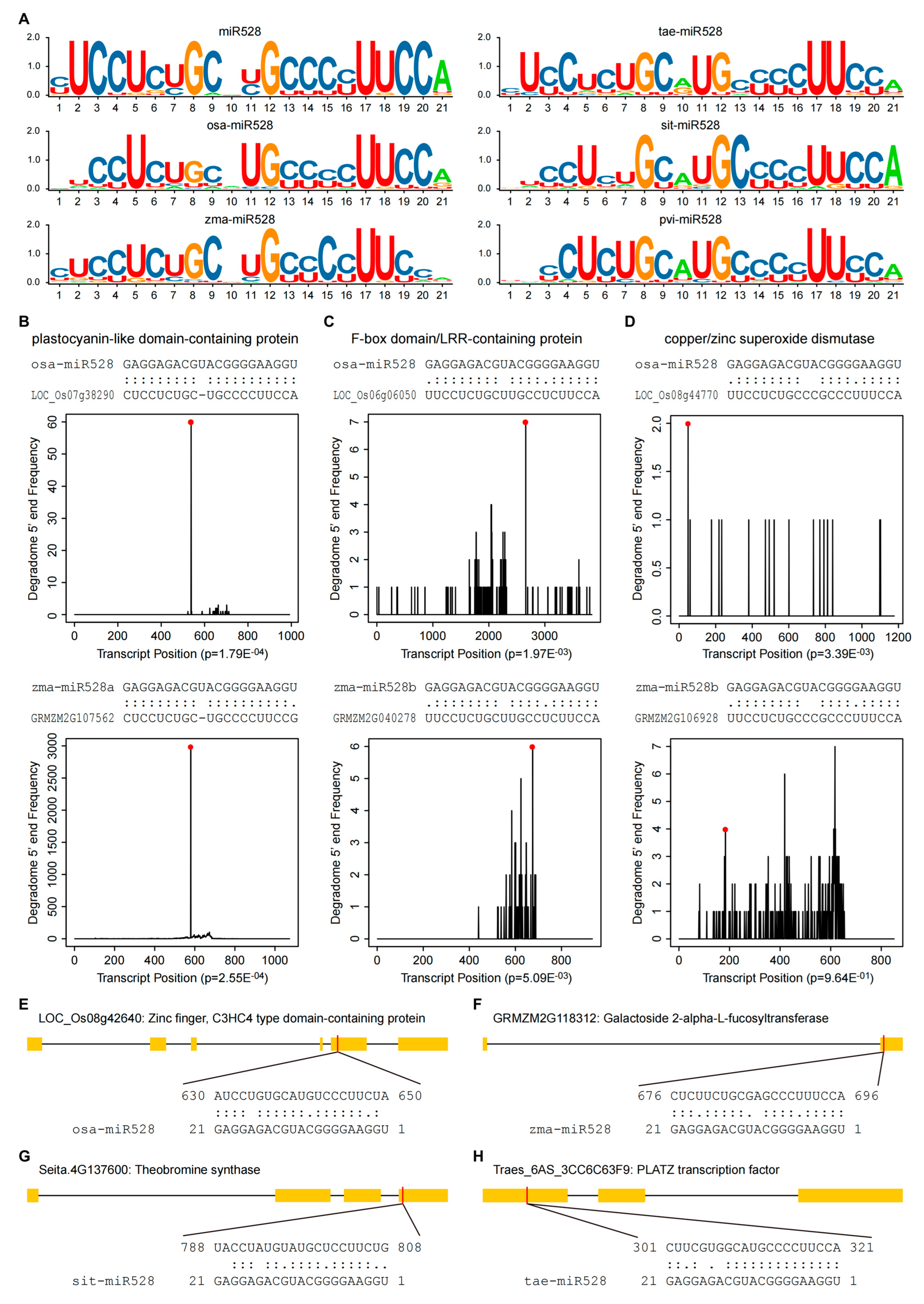
2.2. Targets of miR528
3. Role of miR528 in Plant Growth and Development
3.1. Flowering Time
3.2. Plant Architecture
3.3. Seed Development
3.4. Embryogenic Development
4. Role of miR528 in Plant Stress Responses
4.1. Biotic Stress
4.2. Salt Stress
4.3. Temperature Stress
4.4. Water Stress
4.5. Arsenic (As) Stress
4.6. Heavy-Metal Stress
4.7. Nitrogen Homeostasis
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Yu, Y.; Jia, T.; Chen, X. The “how” and “Where” of Plant microRNAs. New Phytol. 2017, 216, 1002–1017. [Google Scholar] [CrossRef]
- Shahid, S.; Kim, G.; Johnson, N.R.; Wafula, E.; Wang, F.; Coruh, C.; Bernal-Galeano, V.; Phifer, T.; dePamphilis, C.W.; Westwood, J.H.; et al. MicroRNAs from the Parasitic Plant Cuscuta campestris Target Host Messenger RNAs. Nature 2018, 553, 82–85. [Google Scholar] [CrossRef]
- Wang, W.; Liu, D.; Zhang, X.; Chen, D.; Cheng, Y.; Shen, F. Plant MicroRNAs in Cross-Kingdom Regulation of Gene Expression. Int. J. Mol. Sci. 2018, 19, 2007. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Wang, H.; Hu, P.; Hamby, R.; Jin, H. Small RNAs—Big Players in Plant-Microbe Interactions. Cell Host Microbe 2019, 26, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Jones-Rhoades, M.W.; Bartel, D.P. Computational Identification of Plant microRNAs and Their Targets, Including a Stress-Induced miRNA. Mol. Cell 2004, 14, 787–799. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Cao, X.; Qi, Y. MicroRNAs and Their Regulatory Roles in Plant-Environment Interactions. Annu. Rev. Plant Biol. 2019, 70, 489–525. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Hong, P.; Wu, J.Y.; Chen, X.B.; Ye, X.G.; Pan, Y.Y.; Wang, J.; Zhang, X.S. The Tae-miR408-Mediated Control of TaTOC1 Genes Transcription Is Required for the Regulation of Heading Time in Wheat. Plant Physiol. 2016, 170, 1578–1594. [Google Scholar] [CrossRef]
- Bai, Q.; Wang, X.; Chen, X.; Shi, G.; Liu, Z.; Guo, C.; Xiao, K. Wheat miRNA TaemiR408 Acts as an Essential Mediator in Plant Tolerance to Pi Deprivation and Salt Stress via Modulating Stress-Associated Physiological Processes. Front. Plant Sci. 2018, 9, 499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Yan, J.; Gou, F.; Mao, Y.; Tang, G.; Botella, J.R.; Zhu, J.-K. Short Tandem Target Mimic Rice Lines Uncover Functions of miRNAs in Regulating Important Agronomic Traits. Proc. Natl. Acad. Sci. USA 2017, 114, 5277–5282. [Google Scholar] [CrossRef]
- Li, Y.; Cao, X.-L.; Zhu, Y.; Yang, X.-M.; Zhang, K.-N.; Xiao, Z.-Y.; Wang, H.; Zhao, J.-H.; Zhang, L.-L.; Li, G.-B.; et al. Osa-miR398b Boosts H2 O2 Production and Rice Blast Disease-Resistance via Multiple Superoxide Dismutases. New Phytol. 2019, 222, 1507–1522. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Zhang, J.; Wu, L.; Qi, Y.; Zhou, J.-M. Identification of microRNAs Involved in Pathogen-Associated Molecular Pattern-Triggered Plant Innate Immunity. Plant Physiol. 2010, 152, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Meng, Y.; Wise, R.P. Mla- and Rom1-Mediated Control of microRNA398 and Chloroplast Copper/Zinc Superoxide Dismutase Regulates Cell Death in Response to the Barley Powdery Mildew Fungus. New Phytol. 2014, 201, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, P.; Mei, H.; Wang, D.; Sun, J.; Yang, C.; Hao, L.; Cao, S.; Chu, C.; Hu, S.; et al. Fine-Tuning of MiR528 Accumulation Modulates Flowering Time in Rice. Mol. Plant 2019, 12, 1103–1113. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; He, R.-R.; Lian, J.-P.; Zhou, Y.-F.; Zhang, F.; Li, Q.-F.; Yu, Y.; Feng, Y.-Z.; Yang, Y.-W.; Lei, M.-Q.; et al. OsmiR528 Regulates Rice-Pollen Intine Formation by Targeting an Uclacyanin to Influence Flavonoid Metabolism. Proc. Natl. Acad. Sci. USA 2020, 117, 727–732. [Google Scholar] [CrossRef]
- Yuan, S.; Li, Z.; Li, D.; Yuan, N.; Hu, Q.; Luo, H. Constitutive Expression of Rice MicroRNA528 Alters Plant Development and Enhances Tolerance to Salinity Stress and Nitrogen Starvation in Creeping Bentgrass. Plant Physiol. 2015, 169, 576–593. [Google Scholar] [CrossRef]
- Jin, X.; Fu, Z.; Lv, P.; Peng, Q.; Ding, D.; Li, W.; Tang, J. Identification and Characterization of microRNAs during Maize Grain Filling. PLoS ONE 2015, 10, e0125800. [Google Scholar] [CrossRef]
- Wu, J.; Yang, R.; Yang, Z.; Yao, S.; Zhao, S.; Wang, Y.; Li, P.; Song, X.; Jin, L.; Zhou, T.; et al. ROS Accumulation and Antiviral Defence Control by microRNA528 in Rice. Nat. Plants 2017, 3, 16203. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, Y.; Tang, R.; Qu, H.; Duan, X.; Jiang, Y. Banana sRNAome and Degradome Identify microRNAs Functioning in Differential Responses to Temperature Stress. BMC Genom. 2019, 20, 33. [Google Scholar] [CrossRef]
- Kantar, M.; Lucas, S.J.; Budak, H. miRNA Expression Patterns of Triticum Dicoccoides in Response to Shock Drought Stress. Planta 2011, 233, 471–484. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, H.; Zhu, L.; Li, R.; Feng, Y.; Zhang, L.; Yang, Y.; Liu, X.; Zhang, H. Involvement of miR528 in the Regulation of Arsenite Tolerance in Rice (Oryza sativa L.). J. Agric. Food Chem. 2015, 63, 8849–8861. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Zhang, Y.-X.; Liu, J.-Y. Identification and Analysis of Seven H2O2-Responsive miRNAs and 32 New miRNAs in the Seedlings of Rice (Oryza sativa L. Ssp. Indica). Nucleic Acids Res. 2011, 39, 2821–2833. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, M.; Xu, X.; Li, X.; Li, C.; Ding, Z. System Analysis of microRNAs in the Development and Aluminium Stress Responses of the Maize Root System. Plant Biotechnol. J. 2014, 12, 1108–1121. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, G.; Hu, X.; Wang, H.; Du, L.; Zhu, Y. Role of microRNAs in Plant Responses to Nutrient Stress. Plant Soil 2014, 374, 1005–1021. [Google Scholar] [CrossRef]
- Liu, B.; Li, P.; Li, X.; Liu, C.; Cao, S.; Chu, C.; Cao, X. Loss of Function of OsDCL1 Affects microRNA Accumulation and Causes Developmental Defects in Rice. Plant Physiol. 2005, 139, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Kurtoglu, K.Y.; Kantar, M.; Budak, H. New Wheat microRNA Using Whole-Genome Sequence. Funct. Integr. Genom. 2014, 14, 363–379. [Google Scholar] [CrossRef]
- Zhang, L.; Chia, J.-M.; Kumari, S.; Stein, J.C.; Liu, Z.; Narechania, A.; Maher, C.A.; Guill, K.; McMullen, M.D.; Ware, D. A Genome-Wide Characterization of microRNA Genes in Maize. PLOS Genet. 2009, 5, e1000716. [Google Scholar] [CrossRef]
- Paterson, A.H.; Bowers, J.E.; Bruggmann, R.; Dubchak, I.; Grimwood, J.; Gundlach, H.; Haberer, G.; Hellsten, U.; Mitros, T.; Poliakov, A.; et al. The Sorghum Bicolor Genome and the Diversification of Grasses. Nature 2009, 457, 551–556. [Google Scholar] [CrossRef]
- Zanca, A.S.; Vicentini, R.; Ortiz-Morea, F.A.; Del Bem, L.E.V.; da Silva, M.J.; Vincentz, M.; Nogueira, F.T.S. Identification and Expression Analysis of microRNAs and Targets in the Biofuel Crop Sugarcane. BMC Plant Biol. 2010, 10, 260. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Kuang, Z.; Zhao, Y.; Deng, Y.; He, H.; Wan, M.; Tao, Y.; Wang, D.; Wei, J.; Li, L.; et al. PmiREN2.0: From Data Annotation to Functional Exploration of Plant microRNAs. Nucleic Acids Res. 2022, 50, D1475–D1482. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Van Bel, M.; Diels, T.; Vancaester, E.; Kreft, L.; Botzki, A.; Van de Peer, Y.; Coppens, F.; Vandepoele, K. PLAZA 4.0: An Integrative Resource for Functional, Evolutionary and Comparative Plant Genomics. Nucleic Acids Res. 2018, 46, D1190–D1196. [Google Scholar] [CrossRef]
- Soreng, R.J.; Peterson, P.M.; Romaschenko, K.; Davidse, G.; Zuloaga, F.O.; Judziewicz, E.J.; Filgueiras, T.S.; Davis, J.I.; Morrone, O. A Worldwide Phylogenetic Classification of the Poaceae (Gramineae). J. Syst. Evol. 2015, 53, 117–137. [Google Scholar] [CrossRef]
- Grass Phylogeny Working Group II New Grass Phylogeny Resolves Deep Evolutionary Relationships and Discovers C4 Origins. New Phytol. 2012, 193, 304–312. [CrossRef]
- Zhu, H.; Chen, C.; Zeng, J.; Yun, Z.; Liu, Y.; Qu, H.; Jiang, Y.; Duan, X.; Xia, R. MicroRNA528, a Hub Regulator Modulating ROS Homeostasis via Targeting of a Diverse Set of Genes Encoding Copper-Containing Proteins in Monocots. New Phytol. 2020, 225, 385–399. [Google Scholar] [CrossRef]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A Plant Small RNA Target Analysis Server (2017 Release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef]
- Pilon, M. The Copper microRNAs. New Phytol. 2017, 213, 1030–1035. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Li, X.-K.; Qin, X.-M.; Cui, J.-L. Transcriptional Regulatory Mechanisms of Flavonoid Metabolism from Flower Bud to Flower in Hemerocallis Citrina. Sci. Hortic. 2024, 326, 112766. [Google Scholar] [CrossRef]
- Li, T.; Tang, S.; Li, W.; Zhang, S.; Wang, J.; Pan, D.; Lin, Z.; Ma, X.; Chang, Y.; Liu, B.; et al. Genome Evolution and Initial Breeding of the Triticeae Grass Leymus Chinensis Dominating the Eurasian Steppe. Proc. Natl. Acad. Sci. USA 2023, 120, e2308984120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, X.; Wang, M.; Xie, L.; Wu, Z.; Yu, J.; Wang, Y.; Zhang, Z.; Jia, Y.; Liu, Q. The miR528-D3 Module Regulates Plant Height in Rice by Modulating the Gibberellin and Abscisic Acid Metabolisms. Rice 2022, 15, 27. [Google Scholar] [CrossRef]
- Luján-Soto, E.; Aguirre de la Cruz, P.I.; Juárez-González, V.T.; Reyes, J.L.; Sanchez, M.d.l.P.; Dinkova, T.D. Transcriptional Regulation of Zma-MIR528a by Action of Nitrate and Auxin in Maize. Int. J. Mol. Sci. 2022, 23, 15718. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, Z.; Gao, L.; Wang, L.; Gao, M.; Jiao, Z.; Qiao, H.; Yang, J.; Chen, M.; Yao, L.; et al. Genome-Wide Identification and Characterization of microRNAs in Developing Grains of Zea mays L. PLoS ONE 2016, 11, e0153168. [Google Scholar] [CrossRef]
- Kang, M.; Zhao, Q.; Zhu, D.; Yu, J. Characterization of microRNAs Expression during Maize Seed Development. BMC Genom. 2012, 13, 360. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-H.; Spriggs, A.; Matthew, L.; Fan, L.; Kennedy, G.; Gubler, F.; Helliwell, C. A Diverse Set of microRNAs and microRNA-like Small RNAs in Developing Rice Grains. Genome Res. 2008, 18, 1456–1465. [Google Scholar] [CrossRef]
- Xue, L.-J.; Zhang, J.-J.; Xue, H.-W. Characterization and Expression Profiles of miRNAs in Rice Seeds. Nucleic Acids Res. 2009, 37, 916–930. [Google Scholar] [CrossRef]
- Luo, Y.-C.; Zhou, H.; Li, Y.; Chen, J.-Y.; Yang, J.-H.; Chen, Y.-Q.; Qu, L.-H. Rice Embryogenic Calli Express a Unique Set of microRNAs, Suggesting Regulatory Roles of microRNAs in Plant Post-Embryogenic Development. FEBS Lett. 2006, 580, 5111–5116. [Google Scholar] [CrossRef]
- Alejandri-Ramírez, N.D.; Chávez-Hernández, E.C.; Contreras-Guerra, J.L.; Reyes, J.L.; Dinkova, T.D. Small RNA Differential Expression and Regulation in Tuxpeño Maize Embryogenic Callus Induction and Establishment. Plant Physiol. Biochem. PPB 2018, 122, 78–89. [Google Scholar] [CrossRef]
- Chávez-Hernández, E.C.; Alejandri-Ramírez, N.D.; Juárez-González, V.T.; Dinkova, T.D. Maize miRNA and Target Regulation in Response to Hormone Depletion and Light Exposure during Somatic Embryogenesis. Front. Plant Sci. 2015, 6, 555. [Google Scholar] [CrossRef]
- Chen, C.-J.; Liu, Q.; Zhang, Y.-C.; Qu, L.-H.; Chen, Y.-Q.; Gautheret, D. Genome-Wide Discovery and Analysis of microRNAs and Other Small RNAs from Rice Embryogenic Callus. RNA Biol. 2011, 8, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Luján-Soto, E.; Juárez-González, V.T.; Reyes, J.L.; Dinkova, T.D. MicroRNA Zma-miR528 Versatile Regulation on Target mRNAs during Maize Somatic Embryogenesis. Int. J. Mol. Sci. 2021, 22, 5310. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, Z.; Lu, S.; Lin, H.; Gao, S.; Peng, H.; Yuan, G.; Liu, L.; Zhang, Z.; Zhao, M.; et al. Combined Small RNA and Degradome Sequencing Reveals microRNA Regulation during Immature Maize Embryo Dedifferentiation. Biochem. Biophys. Res. Commun. 2013, 441, 425–430. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Z.; Wang, Y.; Zheng, L.; Ye, R.; Ji, Y.; Zhao, S.; Ji, S.; Liu, R.; Xu, L.; et al. Viral-Inducible Argonaute18 Confers Broad-Spectrum Virus Resistance in Rice by Sequestering a Host microRNA. Elife 2015, 4, e05733. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Yang, Z.; Yang, R.; Huang, Y.; Guo, G.; Kong, X.; Lan, Y.; Zhou, T.; Wang, H.; Wang, W.; et al. Transcriptional Regulation of miR528 by OsSPL9 Orchestrates Antiviral Response in Rice. Mol. Plant 2019, 12, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Kang, J.; Guo, G.; Yang, Z.; Huang, Y.; Lan, Y.; Zhou, T.; Wang, L.; Wei, C.; Xu, Z.; et al. The Key Micronutrient Copper Orchestrates Broad-Spectrum Virus Resistance in Rice. Sci. Adv. 2022, 8, eabm0660. [Google Scholar] [CrossRef]
- Liu, K.; Ma, X.; Zhao, L.; Lai, X.; Chen, J.; Lang, X.; Han, Q.; Wan, X.; Li, C. Comprehensive Transcriptomic Analysis of Three Varieties with Different Brown Planthopper-Resistance Identifies Leaf Sheath lncRNAs in Rice. BMC Plant Biol. 2023, 23, 367. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Liu, N.; Su, Q.; Liu, X.; Jia, H.; Liu, Y.; Sun, M.; Cao, Z.; Dong, J. MicroRNAs Involved in the Trans-Kingdom Gene Regulation in the Interaction of Maize Kernels and Fusarium Verticillioides. Int. J. Biol. Macromol. 2023, 242, 125046. [Google Scholar] [CrossRef]
- Ding, D.; Zhang, L.; Wang, H.; Liu, Z.; Zhang, Z.; Zheng, Y. Differential Expression of miRNAs in Response to Salt Stress in Maize Roots. Ann. Bot. 2009, 103, 29–38. [Google Scholar] [CrossRef]
- Wang, M.; Guo, W.; Li, J.; Pan, X.; Pan, L.; Zhao, J.; Zhang, Y.; Cai, S.; Huang, X.; Wang, A.; et al. The miR528-AO Module Confers Enhanced Salt Tolerance in Rice by Modulating the Ascorbic Acid and Abscisic Acid Metabolism and ROS Scavenging. J. Agric. Food Chem. 2021, 69, 8634–8648. [Google Scholar] [CrossRef]
- Hivrale, V.; Zheng, Y.; Puli, C.O.R.; Jagadeeswaran, G.; Gowdu, K.; Kakani, V.G.; Barakat, A.; Sunkar, R. Characterization of Drought- and Heat-Responsive microRNAs in Switchgrass. Plant Sci. Int. J. Exp. Plant Biol. 2016, 242, 214–223. [Google Scholar] [CrossRef]
- Wang, Z.; Pu, H.; Shan, S.; Zhang, P.; Li, J.; Song, H.; Xu, X. Melatonin Enhanced Chilling Tolerance and Alleviated Peel Browning of Banana Fruit under Low Temperature Storage. Postharvest Biol. Technol. 2021, 179, 111571. [Google Scholar] [CrossRef]
- Hong, Z.; Xu, H.; Shen, Y.; Liu, C.; Guo, F.; Muhammad, S.; Zhang, Y.; Niu, H.; Li, S.; Zhou, W.; et al. Bioengineering for Robust Tolerance against Cold and Drought Stresses via Co-Overexpressing Three Cu-miRNAs in Major Food Crops. Cell Rep. 2024, 43, 114828. [Google Scholar] [CrossRef]
- Wang, H.; Jia, Y.; Bai, X.; Wang, J.; Liu, G.; Wang, H.; Wu, Y.; Xin, J.; Ma, H.; Liu, Z.; et al. Whole-Transcriptome Profiling and Identification of Cold Tolerance-Related ceRNA Networks in Japonica Rice Varieties. Front. Plant Sci. 2024, 15, 1260591. [Google Scholar] [CrossRef]
- Ferreira, T.H.; Gentile, A.; Vilela, R.D.; Costa, G.G.L.; Dias, L.I.; Endres, L.; Menossi, M. microRNAs Associated with Drought Response in the Bioenergy Crop Sugarcane (Saccharum spp.). PLoS ONE 2012, 7, e46703. [Google Scholar] [CrossRef]
- Liu, Z.; Kumari, S.; Zhang, L.; Zheng, Y.; Ware, D. Characterization of miRNAs in Response to Short-Term Waterlogging in Three Inbred Lines of Zea mays. PLoS ONE 2012, 7, e39786. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, Y.; Qi, X. LncRNA TCONS_00021861 Is Functionally Associated with Drought Tolerance in Rice (Oryza sativa L.) via Competing Endogenous RNA Regulation. BMC Plant Biol. 2021, 21, 410. [Google Scholar] [CrossRef]
- Fan, B.; Sun, F.; Yu, Z.; Zhang, X.; Yu, X.; Wu, J.; Yan, X.; Zhao, Y.; Nie, L.; Fang, Y.; et al. Integrated Analysis of Small RNAs, Transcriptome and Degradome Sequencing Reveal the Drought Stress Network in Agropyron Mongolicum Keng. Front. Plant Sci. 2022, 13, 976684. [Google Scholar] [CrossRef]
- Kumar, D.; Ramkumar, M.K.; Dutta, B.; Kumar, A.; Pandey, R.; Jain, P.K.; Gaikwad, K.; Mishra, D.C.; Chaturvedi, K.K.; Rai, A.; et al. Integration of miRNA Dynamics and Drought Tolerant QTLs in Rice Reveals the Role of miR2919 in Drought Stress Response. BMC Genom. 2023, 24, 526. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, Z.; Hamid, R.; Jacob, F.; Mirzaei, M.; Zeinalabedini, M.; Abdirad, S.; Atwell, B.J.; Haynes, P.A.; Ghaffari, M.R.; Salekdeh, G.H. MicroRNA Profiling of Root Meristematic Zone in Contrasting Genotypes Reveals Novel Insight into in Rice Response to Water Deficiency. J. Plant Growth Regul. 2023, 42, 3814–3834. [Google Scholar] [CrossRef]
- Ali, W.; Isayenkov, S.V.; Zhao, F.-J.; Maathuis, F.J.M. Arsenite Transport in Plants. Cell. Mol. Life Sci. CMLS 2009, 66, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A. Arsenic in Rice—Understanding a New Disaster for South-East Asia. Trends Plant Sci. 2004, 9, 415–417. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H. Molecular Identification and Analysis of Arsenite Stress-Responsive miRNAs in Rice. J. Agric. Food Chem. 2012, 60, 6524–6536. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Tiwari, M.; Lakhwani, D.; Tripathi, R.D.; Trivedi, P.K. Differential Expression of microRNAs by Arsenate and Arsenite Stress in Natural Accessions of Rice. Met. Integr. Biometal Sci. 2015, 7, 174–187. [Google Scholar] [CrossRef]
- Kikui, S.; Sasaki, T.; Maekawa, M.; Miyao, A.; Hirochika, H.; Matsumoto, H.; Yamamoto, Y. Physiological and Genetic Analyses of Aluminium Tolerance in Rice, Focusing on Root Growth during Germination. J. Inorg. Biochem. 2005, 99, 1837–1844. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, X.; Li, C.; Zhang, B.; Zhang, C.; Zhang, X.-S.; Ding, Z. Auxin Efflux Carrier ZmPGP1 Mediates Root Growth Inhibition under Aluminum Stress. Plant Physiol. 2018, 177, 819–832. [Google Scholar] [CrossRef]
- Lima, J.C.; Arenhart, R.A.; Margis-Pinheiro, M.; Margis, R. Aluminum Triggers Broad Changes in microRNA Expression in Rice Roots. Genet. Mol. Res. GMR 2011, 10, 2817–2832. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Z.; Zhu, C. Microarray-Based Analysis of Cadmium-Responsive microRNAs in Rice (Oryza sativa). J. Exp. Bot. 2011, 62, 3563–3573. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, L.; Liu, C.; Hong, Z.; Wu, X.; Zhang, Y.; Fahad, M.; Shen, Y.; Bian, J.; He, H.; et al. UCL23 Hierarchically Regulated by WRKY51-miR528 Mediates Cadmium Uptake, Tolerance, and Accumulation in Rice. Cell Rep. 2025, 44, 115336. [Google Scholar] [CrossRef]
- Adhikari, A.; Roy, D.; Adhikari, S.; Saha, S.; Ghosh, P.K.; Shaw, A.K.; Hossain, Z. microRNAomic Profiling of Maize Root Reveals Multifaceted Mechanisms to Cope with Cr (VI) Stress. Plant Physiol. Biochem. PPB 2023, 198, 107693. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Anwar, S.; Kuai, J.; Noman, A.; Shahid, M.; Din, M.; Ali, A.; Zhou, G. Alteration in Yield and Oil Quality Traits of Winter Rapeseed by Lodging at Different Planting Density and Nitrogen Rates. Sci. Rep. 2018, 8, 634. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, X.; Yang, J.; Liu, W.; Du, Q.; Wang, H.; Fu, C.; Li, W.-X. MicroRNA528 Affects Lodging Resistance of Maize by Regulating Lignin Biosynthesis under Nitrogen-Luxury Conditions. Mol. Plant 2018, 11, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Y.; Chen, H.; Du, Q.; Wang, Z.; Gong, X.; Sun, Q.; Li, W.-X. Nitrogen Supply Affects Ion Homeostasis by Modifying Root Casparian Strip Formation through the miR528-LAC3 Module in Maize. Plant Commun. 2023, 4, 100553. [Google Scholar] [CrossRef] [PubMed]
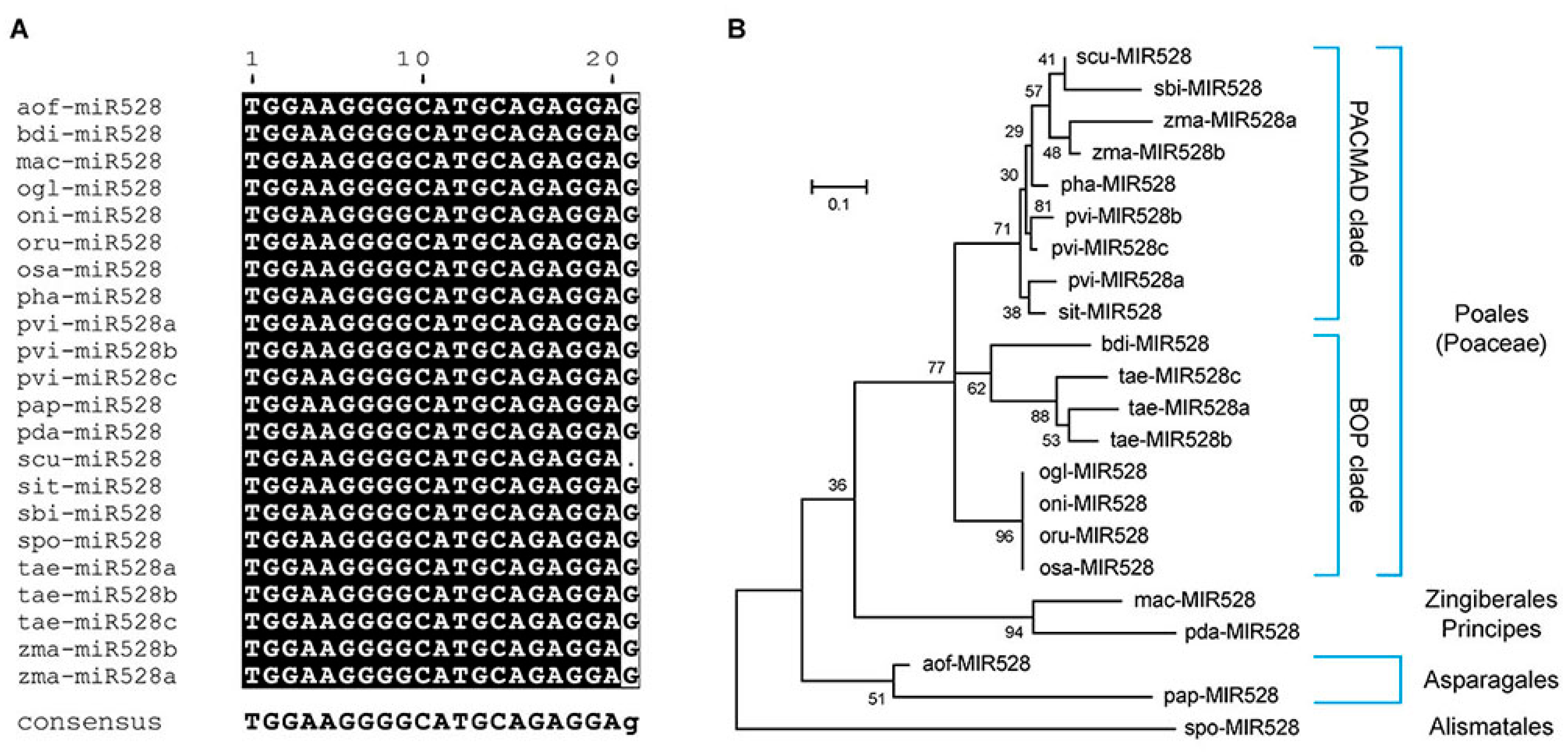
| Locus ID | Organism | Genome Location a | Overlapping Gene b |
|---|---|---|---|
| aof-MIR528 | Asparagus officinalis L. | NC_033797.1:10579184~10579302,+ | × |
| bdi-MIR528 | Brachypodium distachyon (L.) P.Beauv. | Bd1:73295140~73295266,− | BRADI_1g76465v3 |
| mac-MIR528 | Musa acuminata Colla | Chr8:10341398~10341507,− | GSMUA_Achr8G13592_001 |
| ogl-MIR528 | Oryza glaberrima Steud. | GL455988.1:1549995~1550120,+ | ORGLA03G0021200 |
| oni-MIR528 | Oryza nivara S.D.Sharma and Shastry | Chr3:1341691~1341816,+ | ONIVA03G01960 |
| oru-MIR528 | Oryza rufipogon Griff. | HG417167.1:1412714~1412839,+ | ORUFI03G02090 |
| osa-MIR528 | Oryza sativa L. | Chr3:1667310~1667435,+ | LOC_Os03g03724 |
| pha-MIR528 | Panicum hallii Vasey | Chr09:69425755~69425882,− | × |
| pvi-MIR528a | Panicum virgatum L. | Chr01N:69192314~69192445,+ | Pavir.Aa02527.1 |
| pvi-MIR528b | Panicum virgatum L. | Chr09K:87418444~87418571,− | × |
| pvi-MIR528c | Panicum virgatum L. | Chr09N:120070864~120070991,− | × |
| pap-MIR528 | Phalaenopsis aphrodite Rchb.f. | NEWO01000071.1:538724~538838,+ | × |
| pda-MIR528 | Phoenix dactylifera L. | NW_008247750.1:18899~19050,− | × |
| scu-MIR528 | Saccharum cultivar | JXQF01030556.1:160~293,+ | × |
| sit-MIR528 | Setaria italica (L.) P. Beauvois | Scaffold_9:57157041~57157170,− | SETIT_040610mg |
| sbi-MIR528 | Sorghum bicolor (L.) Moench | Chr1:79165414~79165537,− | sbi-MIR528 |
| spo-MIR528 | Spirodela polyrhiza (L.) Schleid. | Pseudo17:196564~196705,− | × |
| tae-MIR528a | Triticum aestivum L. | Chr4B:162371969~162372094,+ | × |
| tae-MIR528b | Triticum aestivum L. | Chr4D:32161888~32162013,+ | × |
| tae-MIR528c | Triticum aestivum L. | Chr5A:210090100~210090222,+ | × |
| zma-MIR528a | Zea mays L. | Chr1:6409229~6409390,+ | zma-MIR528a |
| zma-MIR528b | Zea mays L. | Chr9:153752320~153752436,− | zma-MIR528b |
| Regulated Trait | Crop Species | Experimental Approach | Regulatory Mechanism and Trait Outcomes | Potential Application | Reference |
|---|---|---|---|---|---|
| Flowering time | Rice | Overexpression | Represses OsRFI2 via OsSPL7 activation, promoting early flowering under long-day conditions | Photoperiod adaptation and regional cultivation optimization | [14] |
| Plant architecture | Creeping bentgrass | Transgenic overexpression of pri-osa-miR528 | Reduces internode length, increases tillering and vascular bundles, and enhances lodging resistance | Breeding for lodging-resistant cultivars | [16] |
| Seed development | Rice | Expression profiling | Targets copper-binding proteins and ascorbate oxidases during grain filling | Enhancing grain development and seed vigor | [45,46] |
| Embryogenic development | Maize | Functional validation | Regulates SOD, PLC, and transcription factors to promote somatic embryogenesis | Optimization of somatic embryogenesis and plant regeneration protocols | [48,49] |
| Biotic stress (viral) | Rice | Mutant analysis | AGO18 sequestration de-represses AO, increasing ROS-mediated antiviral defense | Breeding virus-resistant varieties | [18,54] |
| Biotic stress (insect) | Rice | Expression analysis | lncRNA-mediated AO de-repression activates ascorbate defense against brown planthopper | Development of brown planthopper-resistant cultivars | [56] |
| Biotic stress (fungal) | Maize | Cross-kingdom study | Targets fungal FvTTP to reduce mycotoxins and enhance SA signaling | Breeding Fusarium-resistant maize with reduced mycotoxin accumulation | [57] |
| Salt stress | Creeping bentgrass | Transgenic overexpression of pri-osa-miR528 | Suppresses AAO and CBP1, improving ion homeostasis | Breeding salt-tolerant cultivars | [16] |
| Salt stress | Rice | Overexpression | AO suppression elevates AsA/ABA, enhancing osmotic adjustment and ROS scavenging | Improving salt tolerance via ABA–AsA–ROS modulation | [59] |
| Temperature stress (cold) | Banana | Melatonin treatment | MaPPOs repression reduces enzymatic browning, enhances antioxidant activity | Postharvest preservation via PPO suppression | [61] |
| Temperature stress (cold) | Rice | Co-overexpression | Synergistic action with miR397/miR408 reduces oxidative damage | Developing cold-tolerant rice through miRNA synergy | [62] |
| Water stress (drought) | Wheat | Expression profiling | Downregulation coordinates drought-responsive gene networks | Breeding drought-resilient wheat cultivars | [20] |
| Water stress (drought) | Rice | Overexpression | Increases IAA accumulation and reduces ROS, promoting root elongation | Enhancing drought tolerance via IAA and ROS regulation | [66] |
| Arsenic (As III) stress | Rice | Overexpression | Suppresses CBP and IAR1, disrupting As uptake and antioxidant defense | Enhancing arsenic tolerance and reducing arsenic accumulation | [21] |
| Heavy-metal stress (Al) | Maize | Tissue-specific profiling | Mediates root-specific responses to aluminum toxicity | Enhancing aluminum tolerance in maize via root-specific regulation | [23] |
| Heavy-metal stress (Cd) | Rice | Regulatory module analysis | WRKY51-miR528-UCL23 axis coordinates ROS homeostasis under cadmium stress | Mitigating cadmium toxicity by modulating ROS homeostasis | [78] |
| Nitrogen homeostasis | Maize | Functional characterization | Targets ZmLAC3 to modify lignin biosynthesis and Casparian strip formation | Improving nitrogen use efficiency | [81,82] |
| Nitrogen homeostasis | Creeping bentgrass | Transgenic overexpression of pri-osa-miR528 | Enhances N assimilation via increased NiR activity and chlorophyll synthesis | Enhancing nitrogen assimilation and growth under deficiency | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, H.; Zhang, L.; Hu, Y.; Liu, Z.; Wang, Z.; Shen, F.; Wang, W. MicroRNA528 and Its Regulatory Roles in Monocotyledonous Plants. Int. J. Mol. Sci. 2025, 26, 7334. https://doi.org/10.3390/ijms26157334
Fu H, Zhang L, Hu Y, Liu Z, Wang Z, Shen F, Wang W. MicroRNA528 and Its Regulatory Roles in Monocotyledonous Plants. International Journal of Molecular Sciences. 2025; 26(15):7334. https://doi.org/10.3390/ijms26157334
Chicago/Turabian StyleFu, Hailin, Liwei Zhang, Yulin Hu, Ziyi Liu, Zhenyu Wang, Fafu Shen, and Wei Wang. 2025. "MicroRNA528 and Its Regulatory Roles in Monocotyledonous Plants" International Journal of Molecular Sciences 26, no. 15: 7334. https://doi.org/10.3390/ijms26157334
APA StyleFu, H., Zhang, L., Hu, Y., Liu, Z., Wang, Z., Shen, F., & Wang, W. (2025). MicroRNA528 and Its Regulatory Roles in Monocotyledonous Plants. International Journal of Molecular Sciences, 26(15), 7334. https://doi.org/10.3390/ijms26157334






