Modeling the Anti-Adhesive Role of Punicalagin Against Listeria Monocytogenes from the Analysis of the Interaction Between Internalin A and E-Cadherin
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Recognition Motifs at the InlA–Ecad Interface
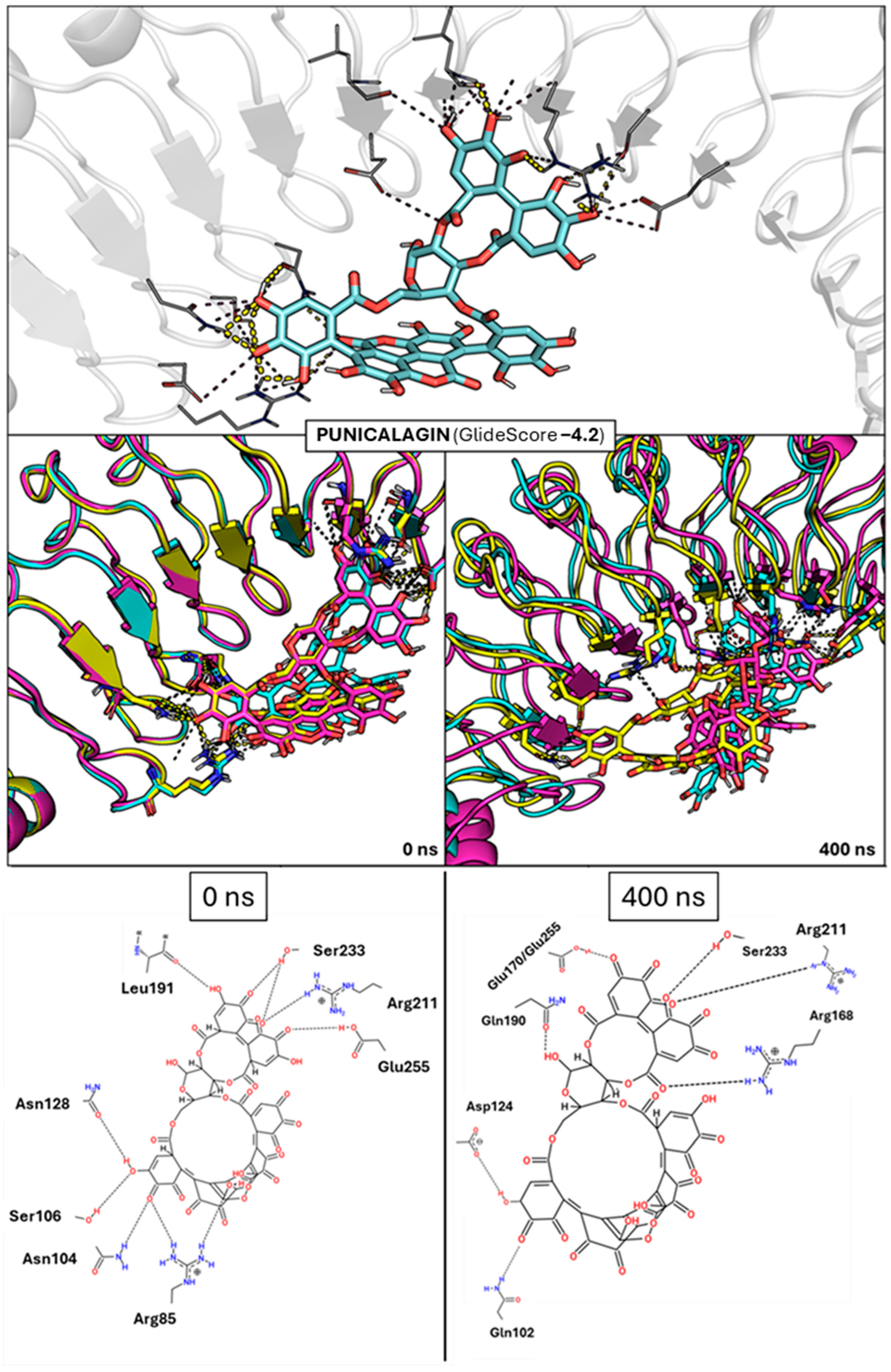
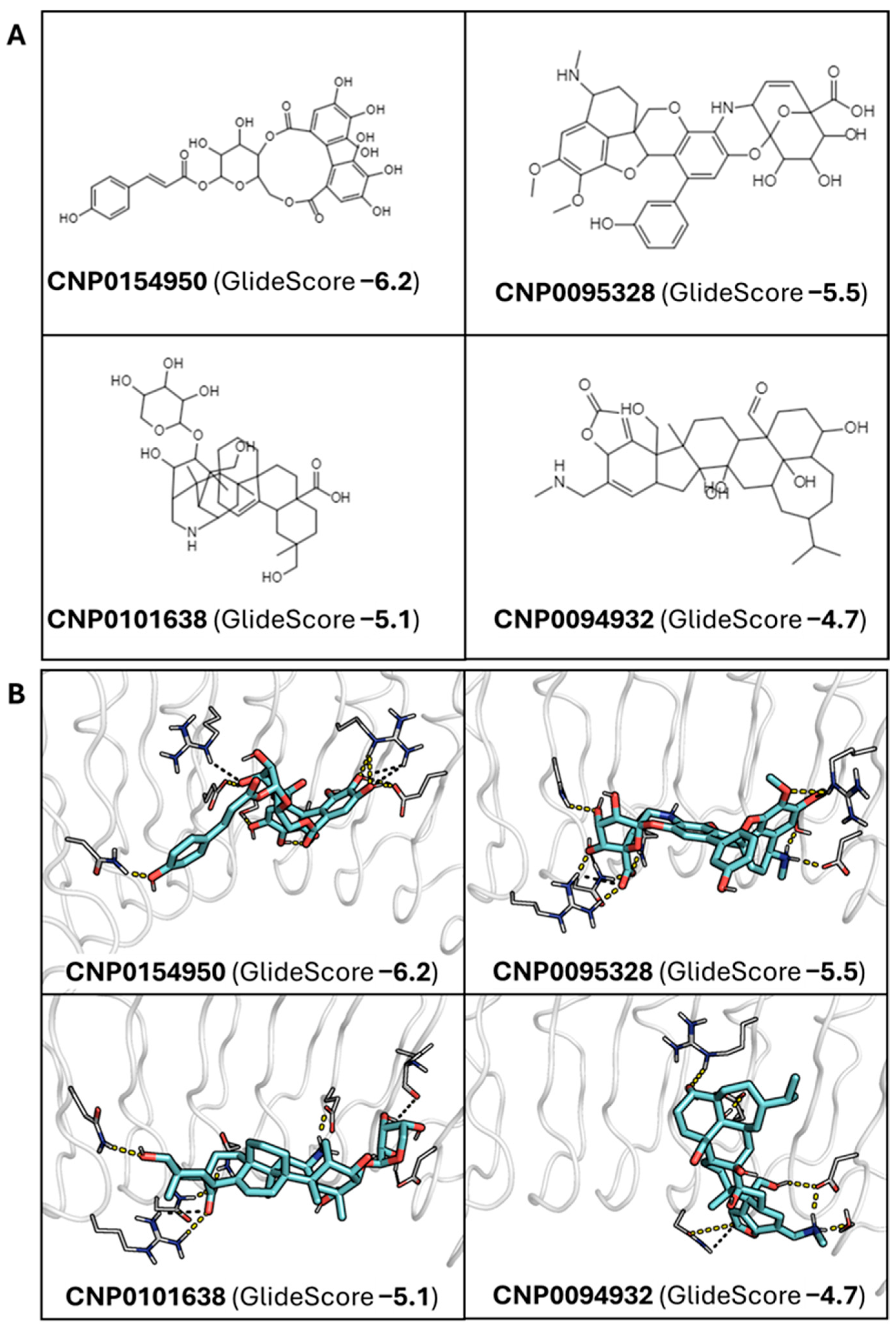
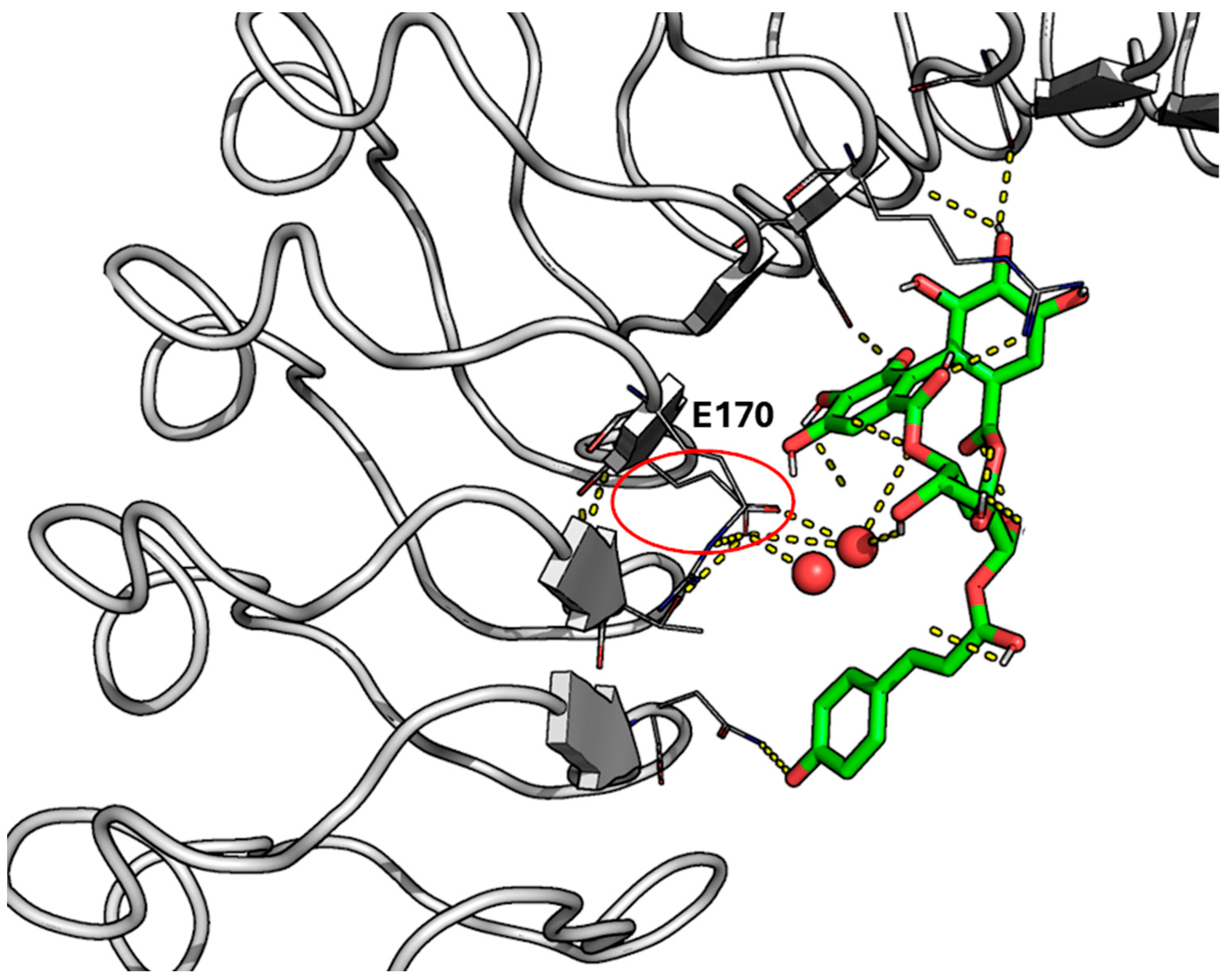
2.2. Docking-Based Screening and Analysis of InlA-Ligand Stability
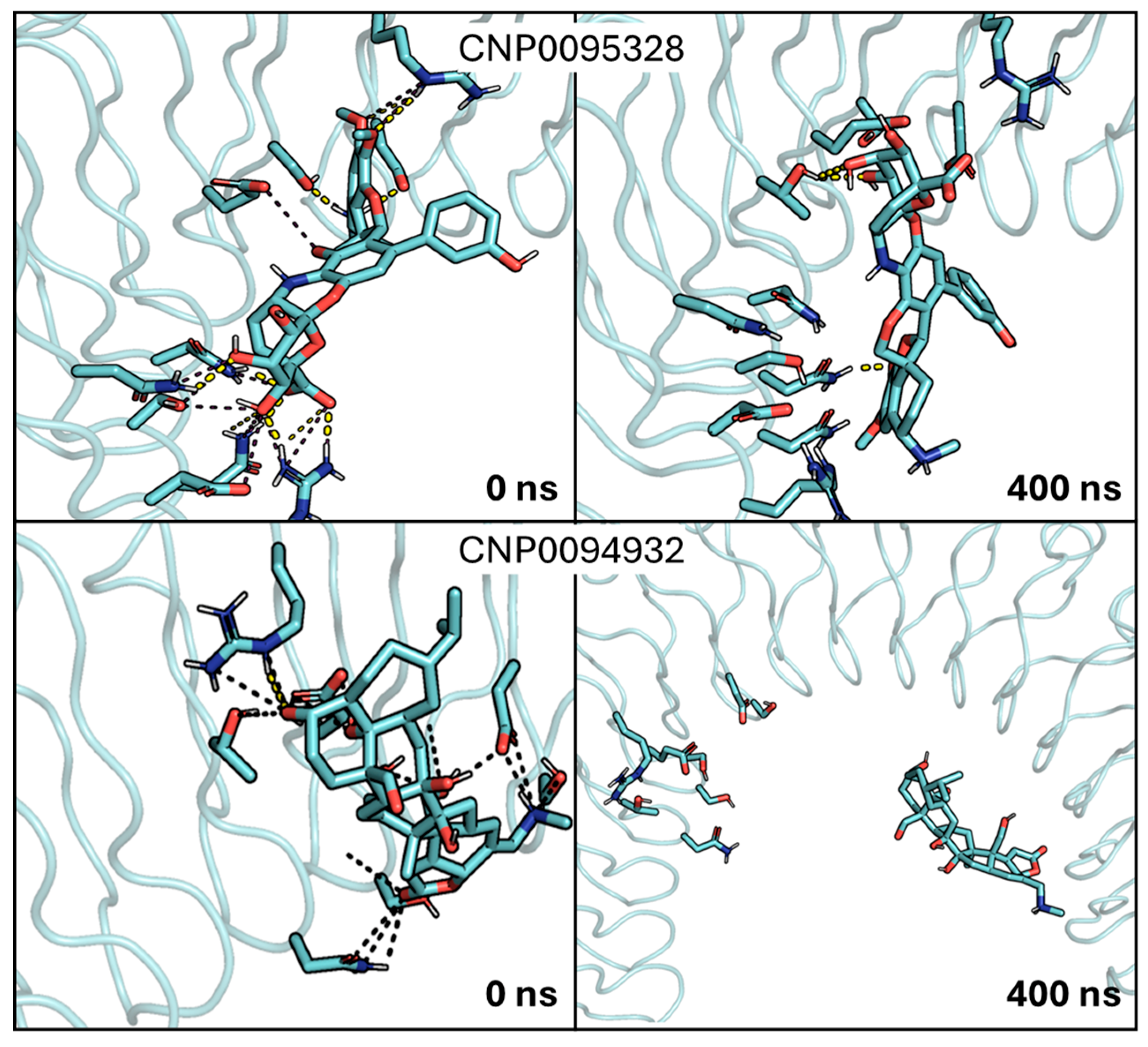
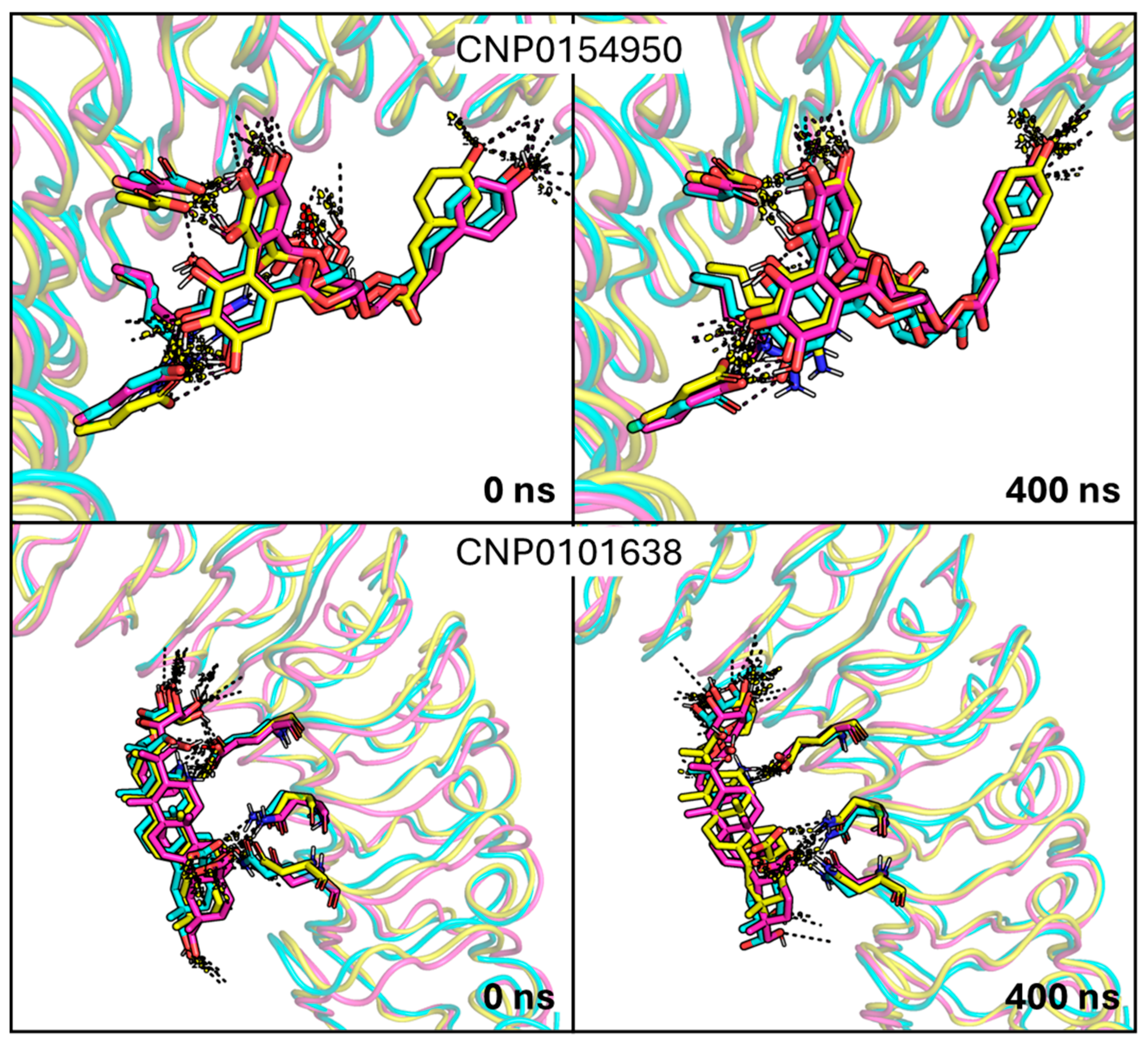
2.3. Analysis of MD Simulations of the InlA–Ligand Complexes
2.4. Possible Practical Application of the Outcomes in the Food Sector
3. Materials and Methods
3.1. Data Source
3.1.1. Protein Structure
3.1.2. Natural Compounds Library
3.2. Molecular Dynamics Simulations
3.3. Docking-Based Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSA | Contact Surface Area |
| Ecad | E-cadherin |
| InlA | Internalin A |
| LLR | Leucine-Rich Repeat |
| LM | Listeria monocytogenes |
| PDB | Protein Data Bank |
| MD | Molecular Dynamics |
| RMSD | Root Mean Squared Deviation |
References
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; et al. Persistence of microbiological hazards in food and feed production and processing environments. EFSA J. 2024, 22, e8521. [Google Scholar]
- Sar, T.; Kiraz, P.; Braho, V.; Harirchi, S.; Akbas, M.Y. Novel perspectives on food-based natural antimicrobials: A review of recent findings published since 2020. Microorganisms 2023, 11, 2234. [Google Scholar] [CrossRef]
- Karnwal, A.; Malik, T. Exploring the untapped potential of naturally occurring antimicrobial compounds: Novel advancements in food preservation for enhanced safety and sustainability. Front. Sustain. Food Syst. 2024, 8, 1307210. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Nakonechny, F.; Haddis, T.; Khalfin, B.; Dahan, A.; Ben-Shabat, S. Natural antimicrobial Ccompounds as Ppromising preservatives: A look at an old problem from new perspectives. Molecules 2024, 29, 5830. [Google Scholar] [CrossRef]
- Pan, Y.; Deng, Z.; Shahidi, F. Natural bioactive substances for the control of food-borne viruses and contaminants in food. Food Prod. Process. Nutr. 2020, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, L.; Ran, W.; Zhong, K.; Zhao, Y.; Gao, H. Antimicrobial activities of natural flavonoids against foodborne pathogens and their application in food industry. Food Chem. 2024, 460, 140476. [Google Scholar] [CrossRef] [PubMed]
- Farid, A.N.; Waheed, A.; Motwani, S. Synthetic and natural antimicrobials as a control against food borne pathogens: A review. Heliyon 2023, 9, e17021. [Google Scholar] [CrossRef] [PubMed]
- Kallipolitis, B.; Gahan, C.G.M.; Piveteau, P. Factors contributing to Listeria monocytogenes transmission and impact on food safety. Curr. Opin. Food Sci. 2020, 36, 9. [Google Scholar] [CrossRef]
- te Giffel, M.C.; Zwietering, M.H. Validation of predictive models describing the growth of Listeria monocytogenes. Int. J. Food Microbiol. 1999, 46, 135. [Google Scholar] [CrossRef]
- Li, X.; Zheng, J.Y.; Zhao, W.; Wu, Y.F. Prevalence of Listeria monocytogenes in milk and dairy product Supply chains: A global systematic review and meta-analysis. Foodborne Pathog. Dis. 2024, 21, 526. [Google Scholar] [CrossRef]
- Alegbeleye, O.; Sant’Ana, A.S. Survival and growth behaviour of Listeria monocytogenes in ready-to-eat vegetable salads. Food Control 2022, 138, 109023. [Google Scholar] [CrossRef]
- de Noordhout, C.M.; Devleesschauwer, B.; Angulo, F.J.; Verbeke, G.; Haagsma, J.; Kirk, M.; Havelaar, A.; Speybroeck, N. The global burden of listeriosis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 1073. [Google Scholar] [CrossRef]
- Khalil, A.; Samara, A.; O’Brien, P.; Ladhani, S. Listeria outbreaks cause maternal and perinatal mortality and morbidity: We must do better. Lancet Microbe 2023, 4, e206. [Google Scholar] [CrossRef] [PubMed]
- Ireton, K.; Mortuza, R.; Gyanwali, G.C.; Gianfelice, A.; Hussain, M. Role of internalin proteins in the pathogenesis of Listeria monocytogenes. Mol. Microbiol. 2021, 116, 1407. [Google Scholar] [CrossRef] [PubMed]
- Drolia, R.; Bhunia, A.K. Crossing the intestinal barrier via Listeria adhesion protein and Internalin A. Trends Microbiol. 2019, 27, 408. [Google Scholar] [CrossRef]
- Al-Shemy, M.T.; El-Demerdash, A.S.; Marzec, A.; Dawwam, G.E. Biocontrol of virulent Listeria monocytogenes using green carboxylated cellulose nanocrystals-silver nano-biohybrids. Int. J. Biol. Macromol. 2024, 290, 139012. [Google Scholar] [CrossRef]
- Lecuit, M. Listeria monocytogenes, a model in infection biology. Cell. Microbiol. 2020, 22, e13186. [Google Scholar] [CrossRef]
- Yamazaki, T.; Nagatoishi, S.; Yamawaki, T.; Nozawa, T.; Matsunaga, R.; Nakakido, M.; Caaveiro, J.M.M.; Nakagawa, I.; Tsumoto, K. Anti-InlA single-domain antibodies that inhibit the cell invasion of Listeria monocytogenes. J. Biol. Chem. 2023, 299, 105254. [Google Scholar] [CrossRef] [PubMed]
- Wollert, T.; Heinz, D.W.; Schubert, W.D. Thermodynamically reengineering the listerial invasion complex InIA/E-cadherin. Proc. Nat. Acad. Sci. USA 2007, 104, 13960. [Google Scholar] [CrossRef] [PubMed]
- Schubert, W.D.; Urbanke, C.; Ziehm, T.; Beier, V.; Machner, M.P.; Domann, E.; Wehland, J.; Chakraborty, T.; Heinz, D.W. Structure of internalin, a major invasion protein of Listeria monocytogenes, in complex with its human receptor E-cadherin. Cell 2002, 111, 825. [Google Scholar] [CrossRef]
- Mengaud, J.; Ohayon, H.; Gounon, P.; Mege, R.M.; Cossart, P. E-cadherin is the receptor for internalin, a surface protein required for entry of L-monocytogenes into epithelial cells. Cell 1996, 84, 923. [Google Scholar] [CrossRef] [PubMed]
- Van Stelten, A.; Roberts, A.R.; Manuel, C.S.; Nightingale, K.K. Listeria monocytogenesIsolates carrying virulence-attenuating mutations in Internalin A are commonly isolated from ready-to-eat food processing plant and retail environments. J. Food Protect. 2016, 79, 1733. [Google Scholar] [CrossRef]
- Nightingale, K.K.; Ivy, R.A.; Ho, A.J.; Fortes, E.D.; Njaa, B.L.; Peters, R.M.; Wiedmann, M. InlA premature stop codons are common among Listeria monocytogenes isolates from foods and yield virulence-attenuated strains that confer protection against fully virulent strains. Appl. Environ. Microbiol. 2008, 74, 6570. [Google Scholar] [CrossRef] [PubMed]
- Dellafiora, L.; Filipello, V.; Dall’Asta, C.; Finazzi, G.; Galaverna, G.; Losio, M.N. A Structural study on the Listeria Monocytogenes Internalin A-human E-cadherin interaction: A molecular tool to investigate the effects of missense mutations. Toxins 2020, 12, 60. [Google Scholar] [CrossRef]
- Genheden, S.; Eriksson, L.A. Of mice and men: Dissecting the interaction between Listeria Monocytogenes internalin A and E-cadherin. Comput. Struct. Biotech. J. 2013, 6, e201303022. [Google Scholar] [CrossRef]
- Wollert, T.; Pasche, B.; Rochon, M.; Deppenmeier, S.; van den Heuvel, J.; Gruber, A.D.; Heinz, D.W.; Lengeling, A.; Schubert, W.D. Extending the host range of Listeria monocytogenes by rational protein design. Cell 2007, 129, 891. [Google Scholar] [CrossRef] [PubMed]
- Lecuit, M.; Dramsi, S.; Gottardi, C.; Fedor-Chaiken, M.; Gumbiner, B.; Cossart, P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. Embo J. 1999, 18, 3956. [Google Scholar] [CrossRef]
- Shivaee, A.; Bahonar, S.; Goudarzi, M.; Hematian, A.; Hajikhani, B.; Kalani, B.S. Investigating the effect of the inhibitory peptide on L. monocytogenes cell invasion: An in silico and in vitro study. Gut Pathog. 2023, 15, 51. [Google Scholar] [CrossRef]
- Sahu, S.C.; Gaines, D.W.; Williams, K.M.; Raybourne, R.B. A synthetic polypeptide based on human E-cadherin inhibits invasion of human intestinal and liver cell lines by Listeria monocytogenes. J. Med. Microbiol. 2007, 56, 1011. [Google Scholar] [CrossRef]
- Upadhyay, A.; Johny, A.K.; Amalaradjou, M.A.R.; Baskaran, S.A.; Kim, K.S.; Venkitanarayanan, K. Plant-derived antimicrobials reduce Listeria monocytogenes virulence factors in vitro, and down-regulate expression of virulence genes. Int. J. Food Microbiol. 2012, 157, 88. [Google Scholar] [CrossRef]
- Xu, Y.F.; Li, G.H.; Zhang, B.G.; Wu, Q.; Wang, X.; Xia, X.D. Tannin-rich pomegranate rind extracts reduce adhesion to and invasion of Caco-2 cells by Listeria monocytogenes and decrease its expression of virulence genes. J. Food Protect. 2015, 78, 128. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Pérez-Alvarez, J.A.; Viuda-Martos, M. Assessment of polyphenolic profile and antibacterial activity of pomegranate peel (Punica granatum) flour obtained from co-product of juice extraction. Food Control 2016, 59, 94. [Google Scholar] [CrossRef]
- Shahbazi, Y. Antibacterial and antioxidant properties of methanolic extracts of apple (Malus pumila), grape (Vitis vinifera), pomegranate (Punica granatum L.) and common fig (Ficus carica L.) fruits. Pharm. Sci. 2017, 23, 308. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res. Int. 2019, 115, 167. [Google Scholar] [CrossRef] [PubMed]
- Mendes, P.M.; Fontoura, G.M.G.; Rodrigues, L.D.; Souza, A.S.; Viana, J.P.M.; Pereira, A.L.F.; Dutra, R.P.; Ferreira, A.G.N.; Neto, M.S.; Reis, A.S.; et al. Therapeutic potential of Punica granatum and isolated compounds: Evidence-based advances to treat bacterial infections. Int. J. Microbiol. 2023, 2023, 4026440. [Google Scholar] [CrossRef]
- Persuric, Z.; Martinovic, L.S.; Malenica, M.; Gobin, I.; Pedisic, S.; Dragovic-Uzelac, V.; Pavelic, S.K. Assessment of the biological activity and phenolic composition of ethanol extracts of pomegranate (Punica granatum L.) peels. Molecules 2020, 25, 5916. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, C.; Hao, P.; Peng, L.; Zhang, S.Y.; Zhao, Q. Phenolic components and biological activity of pomegranate. Chem. Biodivers. 2025, 22, e202402301. [Google Scholar] [CrossRef] [PubMed]
- Gosset-Erard, C.; Zhao, M.J.; Lordel-Madeleine, S.; Ennahar, S. Identification of punicalagin as the bioactive compound behind the antimicrobial activity of pomegranate (Punica granatum L.) peels. Food Chem. 2021, 352, 129396. [Google Scholar] [CrossRef]
- Sorokina, M.; Merseburger, P.; Rajan, K.; Yirik, M.A.; Steinbeck, C. COCONUT online: Collection of open natural products database. J. Cheminform. 2021, 13, 2. [Google Scholar] [CrossRef]
- Rutz, A.; Sorokina, M.; Galgonek, J.; Mietchen, D.; Willighagen, E.; Gaudry, A.; Graham, J.G.; Stephan, R.; Page, R.; Vondrásek, J.; et al. The LOTUS initiative for open knowledge management in natural products research. Elife 2022, 11, 70780. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739. [Google Scholar] [CrossRef]
- Ginex, T.; Muñoz-Muriedas, J.; Herrero, E.; Gibert, E.; Cozzini, P.; Luque, F.J. Development and validation of hydrophobic molecular fields derived from the quantum mechanical IEF/PCM-MST solvation model in 3D-QSAR. J. Comput. Chem. 2016, 37, 1147. [Google Scholar] [CrossRef]
- Vazquez, J.; Deplano, A.; Herrero, A.; Ginex, T.; Gibert, E.; Rabal, O.; Oyarzabal, J.; Herrero, E.; Luque, F.J. Development and validation of molecular overlays derived from three-dimensional hydrophobic similarity with PharmScreen. J. Chem. Inf. Model. 2018, 58, 1596. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Kokubo, H. Exploring the stability of ligand binding modes to proteins by Molecular Dynamics simulations: A cross-docking study. J. Chem. Inf. Model. 2017, 57, 2514. [Google Scholar] [CrossRef]
- Liu, K.; Kokubo, H. Prediction of ligand binding mode among multiple cross-docking poses by molecular dynamics simulations. J. Comput-Aided Mol. Des. 2020, 34, 1195. [Google Scholar] [CrossRef] [PubMed]
- Pancu, D.F.; Scurtu, A.; Macasoi, I.G.; Marti, D.; Mioc, M.; Soica, C.; Coricovac, D.; Horhat, D.; Poenaru, M.; Dehelean, C. Antibiotics: Conventional therapy and natural compounds with antibacterial activity—A pharmaco-toxicological screening. Antibiotics 2021, 10, 401. [Google Scholar] [CrossRef]
- de Oliveira, I.; Santos-Buelga, C.; Aquino, Y.; Barros, L.; Heleno, S.A. New frontiers in the exploration of phenolic compounds and other bioactives as natural preservatives. Food Biosci. 2025, 68, 106571. [Google Scholar] [CrossRef]
- Stan, D.; Enciu, A.M.; Mateescu, A.L.; Ion, A.C.; Brezeanu, A.C.; Stan, D.; Tanase, C. Natural compounds with antimicrobial and antiviral effect and nanocarriers used for their transportation. Front. Pharmacol. 2021, 12, 723233. [Google Scholar] [CrossRef]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Natural plant-derived chemical compounds as Listeria monocytogenes Inhibitors in vitro and in food model systems. Pathogens 2021, 10, 12. [Google Scholar] [CrossRef]
- Kong, Y.; Yan, H.; Hu, J.; Dang, Y.; Han, Z.; Tian, B.; Wang, P. Antibacterial activity and mechanism of action of osthole against Listeria monocytogenes. J. Agric. Food Chem. 2024, 72, 10853. [Google Scholar] [CrossRef]
- Chen, J.C.; Ho, T.Y.; Chang, Y.S.; Wu, S.L.; Li, C.C.; Hsiang, C.Y. Identification of Escherichia coli enterotoxin inhibitors from traditional medicinal herbs by in silico, in vitro, and in vivo analyses. J. Ethnopharmacol. 2009, 121, 372. [Google Scholar] [CrossRef]
- Wang, J.; Ren, H.; Xu, Q.L.; Zhou, Z.Y.; Wu, P.; Wei, X.Y.; Cao, Y.; Chen, X.X.; Tan, J.W. Antibacterial oleanane-type triterpenoids from pericarps of Akebia trifoliata. Food Chem. 2015, 168, 623. [Google Scholar] [CrossRef]
- Engels, C.; Schieber, A.; Gänzle, M.G. Inhibitory spectra and modes of antimicrobial action of gallotannins from mango kernels (Mangifera indica L.). Appl. Environ. Microbiol. 2011, 77, 2215. [Google Scholar] [CrossRef]
- Wu, J.; Jahncke, M.L.; Eifert, J.D.; O’Keefe, S.F.; Welbaum, G.E. Pomegranate peel (Punica granatum L) extract and Chinese gall (Galla chinensis) extract inhibit Vibrio parahaemolyticus and Listeria monocytogenes on cooked shrimp and raw tuna. Food Control 2016, 59, 695. [Google Scholar] [CrossRef]
- Penduka, D.; Mosa, R.; Simelane, M.; Basson, A.; Okoh, A.; Opoku, A. Evaluation of the anti-Listeria potentials of some plant-derived triterpenes. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Kurek, A.; Markowska, K.; Grudniak, A.M.; Janiszowska, W.; Wolska, K.I. The effect of oleanolic and ursolic acids on the hemolytic properties and biofilm formation of Listeria monocytogenes. Pol. J. Microbiol. 2014, 63, 21. [Google Scholar] [CrossRef]
- Suntar, I.; Labanca, F.; Milella, L. Gallotannins in food. In Handbook of Dietary Phytochemicals; Springer: Singapore, 2021; pp. 1173–1200. [Google Scholar]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10. [Google Scholar] [CrossRef]
- Bonilla-Luque, O.M.; Silva, B.N.; Ezzaky, Y.; Possas, A.; Achemchem, F.; Cadavez, V.; Gonzales-Barron, U.; Valero, A. Meta-analysis of antimicrobial activity of Allium, Ocimum, and Thymus spp. confirms their promising application for increasing food safety. Food Res. Int. 2024, 188, 114408. [Google Scholar] [CrossRef] [PubMed]
- Kurćubić, V.S.; Đurović, V.; Stajić, S.B.; Dmitrić, M.; Živković, S.; Kurćubić, L.V.; Mašković, P.Z.; Mašković, J.; Mitić, M.; Živković, V.; et al. Multitarget phytocomplex: Focus on antibacterial profiles of Grape Pomace and Sambucus ebulus L. lyophilisates against extensively drug-resistant (XDR) bacteria and in vitro antioxidative power. Antibiotics 2024, 13, 980. [Google Scholar] [CrossRef] [PubMed]
- Arruda, T.R.; Bernardes, P.C.; Moraes, A.; Soares, N.D.F. Natural bioactives in perspective: The future of active packaging based on essential oils and plant extracts themselves and those complexed by cyclodextrins. Food Res. Int. 2022, 156, 111160. [Google Scholar] [CrossRef]
- Tang, G.-Y. Why polyphenols have promiscuous actions? An investigation by chemical bioinformatics. Nat. Prod. Commun. 2016, 11, 655–656. [Google Scholar] [CrossRef]
- Lesnik, S.; Jukic, M.; Bren, U. Unveiling polyphenol-protein interactions: A comprehensive computational analysis. J. Cheminform. 2025, 17, 50. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235. [Google Scholar] [CrossRef]
- Ligprep, S. Schrödinger Release 2021-1: LigPrep; Schrödinger, LLC: New York, NY, USA, 2021. [Google Scholar]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Cerutti, D.S.; Cisneros, G.A.; Cruzeiro, V.W.D.; Forouzesh, N.; Giese, T.J.; Götz, A.W.; Gohlke, H.; et al. The AmberTools. J. Chem. Inform. Model. 2023, 63, 6183. [Google Scholar] [CrossRef]
- Tian, C.; Kasavajhala, K.; Belfon, K.A.A.; Raguette, L.; Huang, H.; Migues, A.N.; Bickel, J.; Wang, Y.Z.; Pincay, J.; Wu, Q.; et al. ff19SB: Amino-acid-specific protein backbone parameters trained against quantum mechanics energy surfaces in solution. J. Chem. Theory Comput. 2020, 16, 528. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cieplak, P.; Kollman, P.A. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J. Comput. Chem. 2000, 21, 1049. [Google Scholar] [CrossRef]
- Gaussian 16 Rev. C.01; Wallingford, CT, USA, 2016. Available online: https://gaussian.com/citation/ (accessed on 20 March 2024).
- Xiong, Y.Y.; Shabane, P.S.; Onufriev, A.V. Melting points of OPC and OPC3 water models. ACS Omega 2020, 5, 25087. [Google Scholar] [CrossRef]
- Miller, B.R.; McGee, T.D.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An efficient program for end-state free energy calculations. J. Chem. Theory Comput. 2012, 8, 3314. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177. [Google Scholar] [CrossRef]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Lombardo, G.E.; Cirmi, S.; Süntar, I.; Barreca, D.; Laganà, G.; Navarra, M. Pharmacology and toxicology of tannins. Arch. Toxicol. 2022, 96, 1257–1277. [Google Scholar] [CrossRef] [PubMed]


| E-Cadherin | Residue | Position | Average (kcal/mol) |
|---|---|---|---|
| Human | PRO | 16 | −7.0 |
| LYS | 28 | 0.6 | |
| GLY | 32 | 0.1 | |
| THR | 45 | 0.3 | |
| GLU | 64 | −3.9 | |
| ARG | 70 | 1.3 | |
| THR | 73 | 0.2 | |
| THR | 75 | 0.2 | |
| PHE | 77 | 0.1 | |
| ASN | 86 | 0.0 | |
| LEU | 95 | 0.2 | |
| Total contribution | −7.9 | ||
| Murine | GLU | 16 | −5.2 |
| ARG | 28 | 0.3 | |
| THR | 32 | 0.1 | |
| LYS | 45 | 0.3 | |
| GLN | 64 | −1.9 | |
| ALA | 70 | 0.1 | |
| LYS | 73 | 0.5 | |
| ILE | 75 | 0.1 | |
| TYR | 77 | 0.1 | |
| GLU | 86 | 0.1 | |
| VAL | 95 | 0.3 | |
| Total contribution | −5.1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedroni, L.; Ghidini, S.; Vázquez, J.; Luque, F.J.; Dellafiora, L. Modeling the Anti-Adhesive Role of Punicalagin Against Listeria Monocytogenes from the Analysis of the Interaction Between Internalin A and E-Cadherin. Int. J. Mol. Sci. 2025, 26, 7327. https://doi.org/10.3390/ijms26157327
Pedroni L, Ghidini S, Vázquez J, Luque FJ, Dellafiora L. Modeling the Anti-Adhesive Role of Punicalagin Against Listeria Monocytogenes from the Analysis of the Interaction Between Internalin A and E-Cadherin. International Journal of Molecular Sciences. 2025; 26(15):7327. https://doi.org/10.3390/ijms26157327
Chicago/Turabian StylePedroni, Lorenzo, Sergio Ghidini, Javier Vázquez, Francisco Javier Luque, and Luca Dellafiora. 2025. "Modeling the Anti-Adhesive Role of Punicalagin Against Listeria Monocytogenes from the Analysis of the Interaction Between Internalin A and E-Cadherin" International Journal of Molecular Sciences 26, no. 15: 7327. https://doi.org/10.3390/ijms26157327
APA StylePedroni, L., Ghidini, S., Vázquez, J., Luque, F. J., & Dellafiora, L. (2025). Modeling the Anti-Adhesive Role of Punicalagin Against Listeria Monocytogenes from the Analysis of the Interaction Between Internalin A and E-Cadherin. International Journal of Molecular Sciences, 26(15), 7327. https://doi.org/10.3390/ijms26157327







