The Olfactory System of Dolichogenidea gelechiidivoris (Marsh) (Hymenoptera: Braconidae), a Natural Enemy of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae)
Abstract
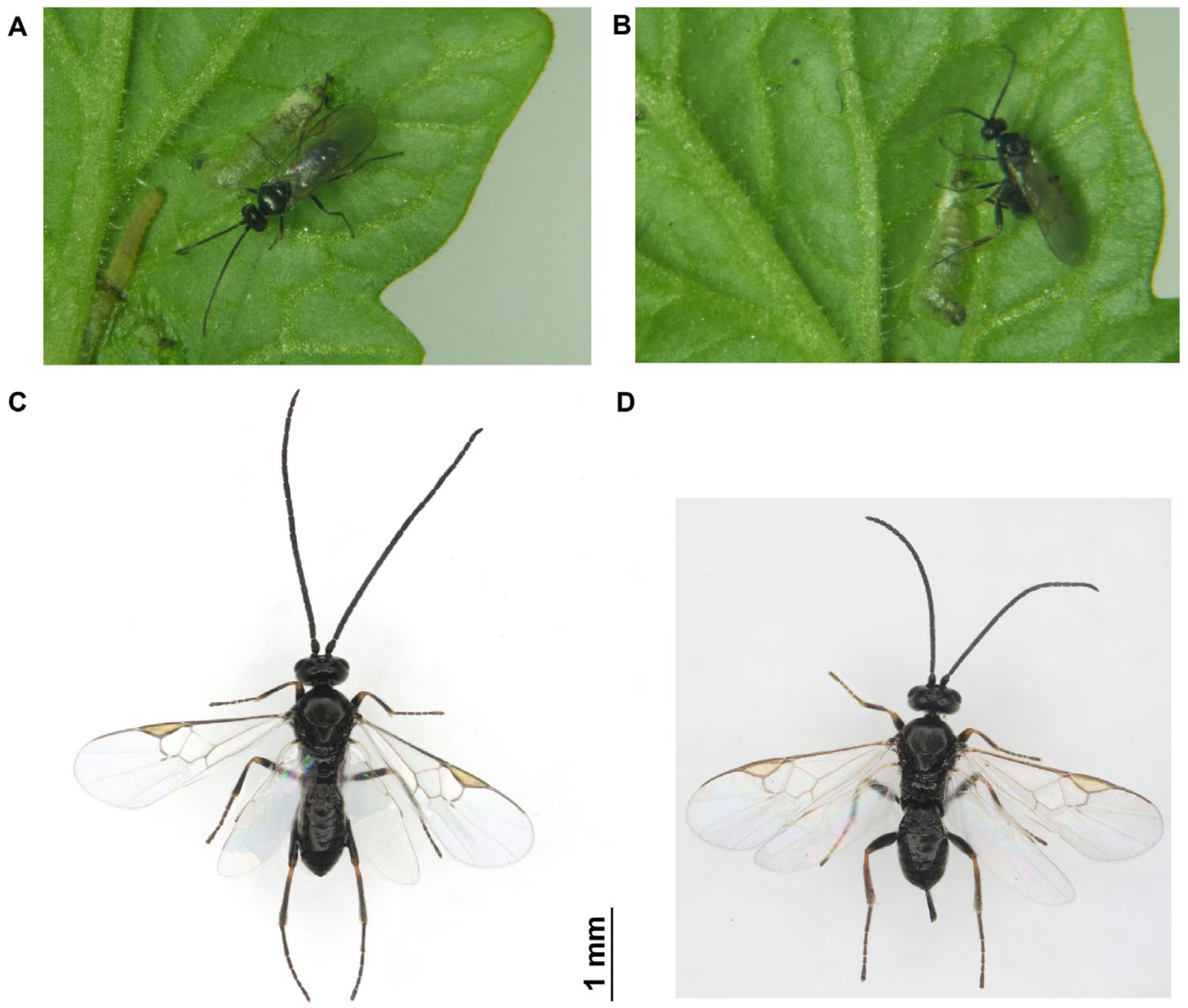
1. Introduction
2. Results
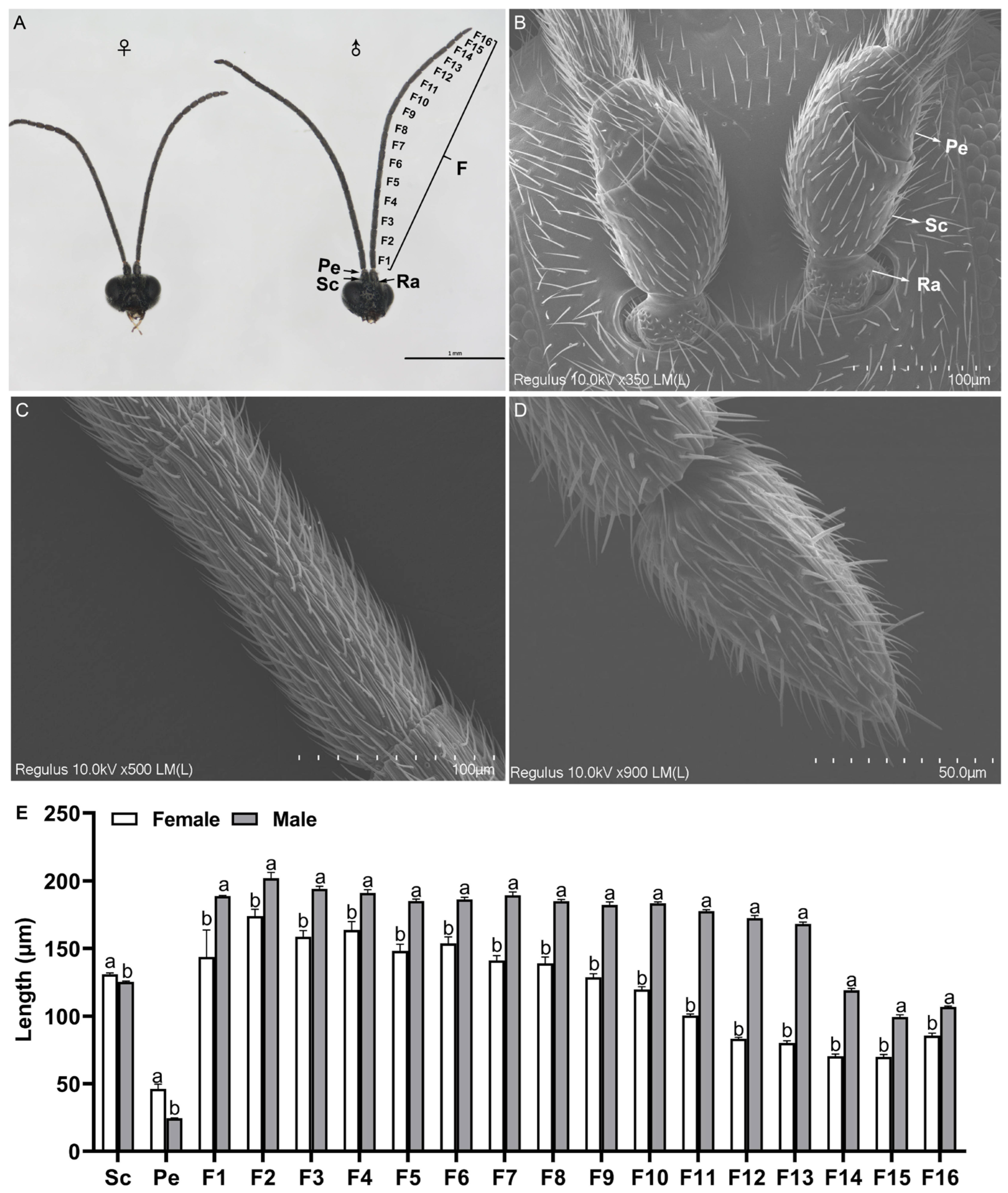
2.1. General Description of Antennae
2.2. Scanning Electron Microscopy of the Adult Antennae
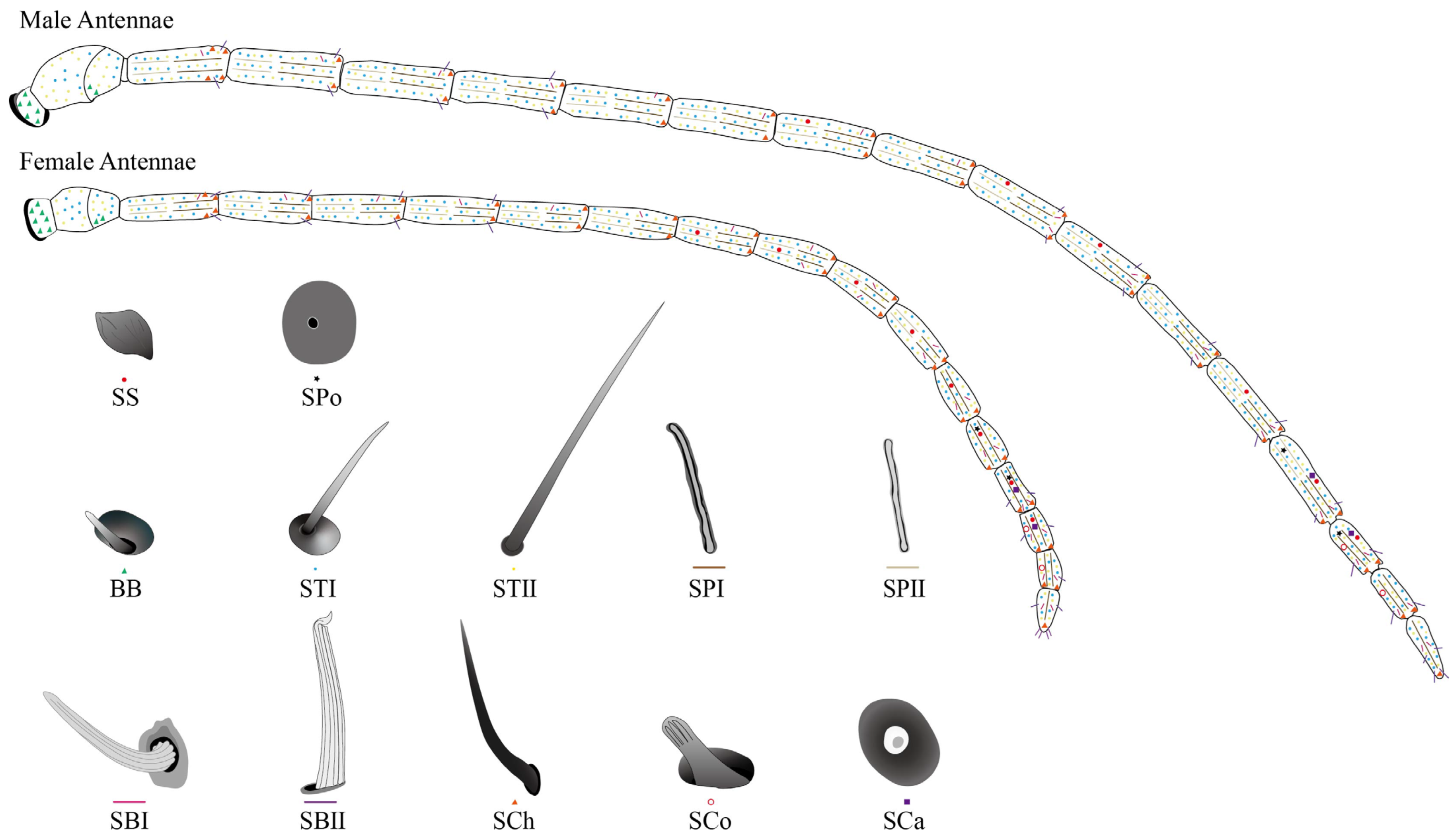
2.2.1. Different Types and Morphology of Sensilla with Distribution
2.2.2. Böhm Bristles (BBs)
2.2.3. Sensilla Trichodea (ST)
2.2.4. Sensilla Placodea (SP)
2.2.5. Sensilla Basiconica (SB)
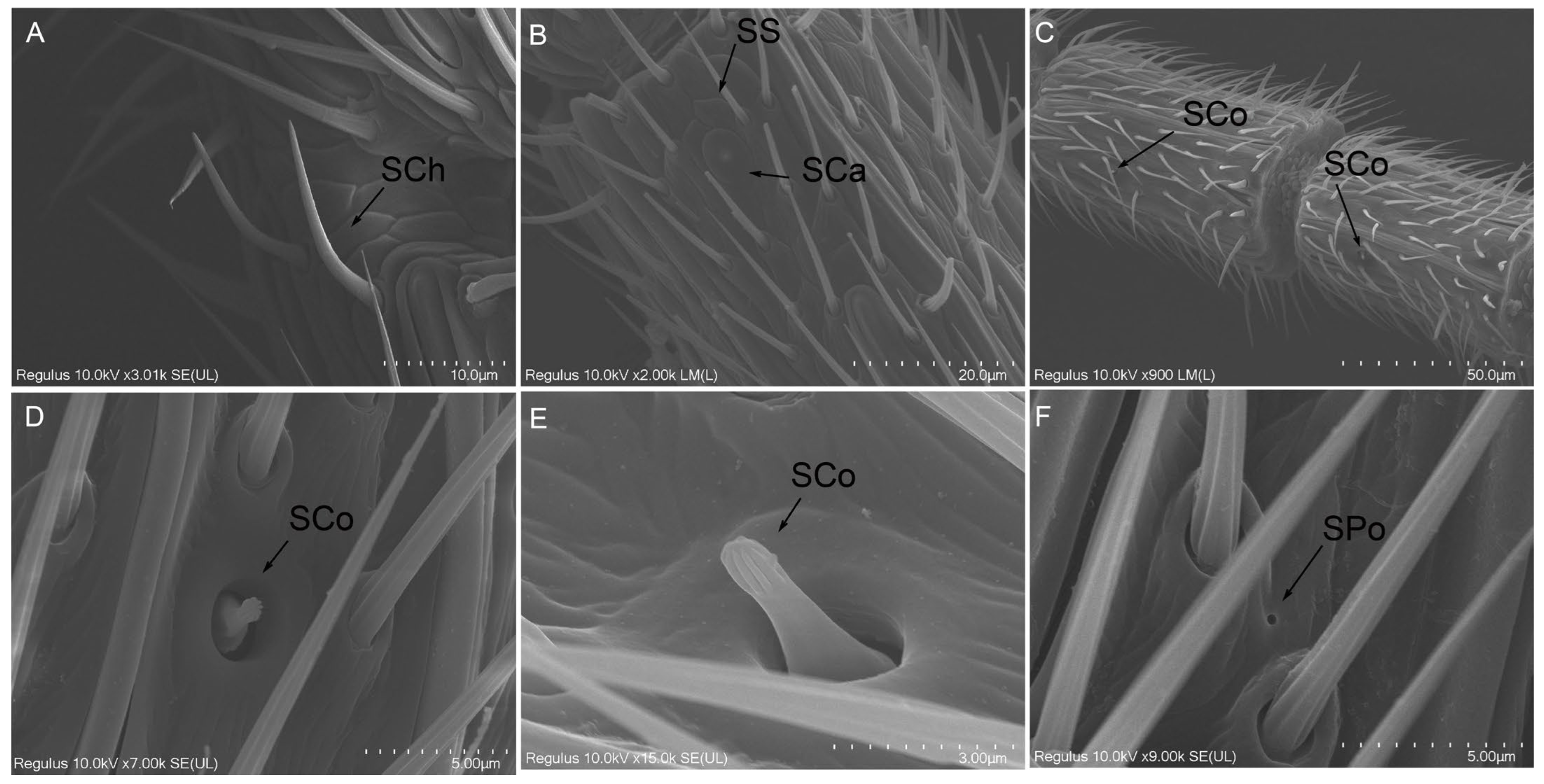
2.2.6. Sensilla Chaetica (SCh)
2.2.7. Sensilla Coeloconica (SCo)
2.2.8. Sensilla Campaniformia (SCa)
2.2.9. Sensilla Squamous (SS)
2.2.10. Smell Pores (SPo)
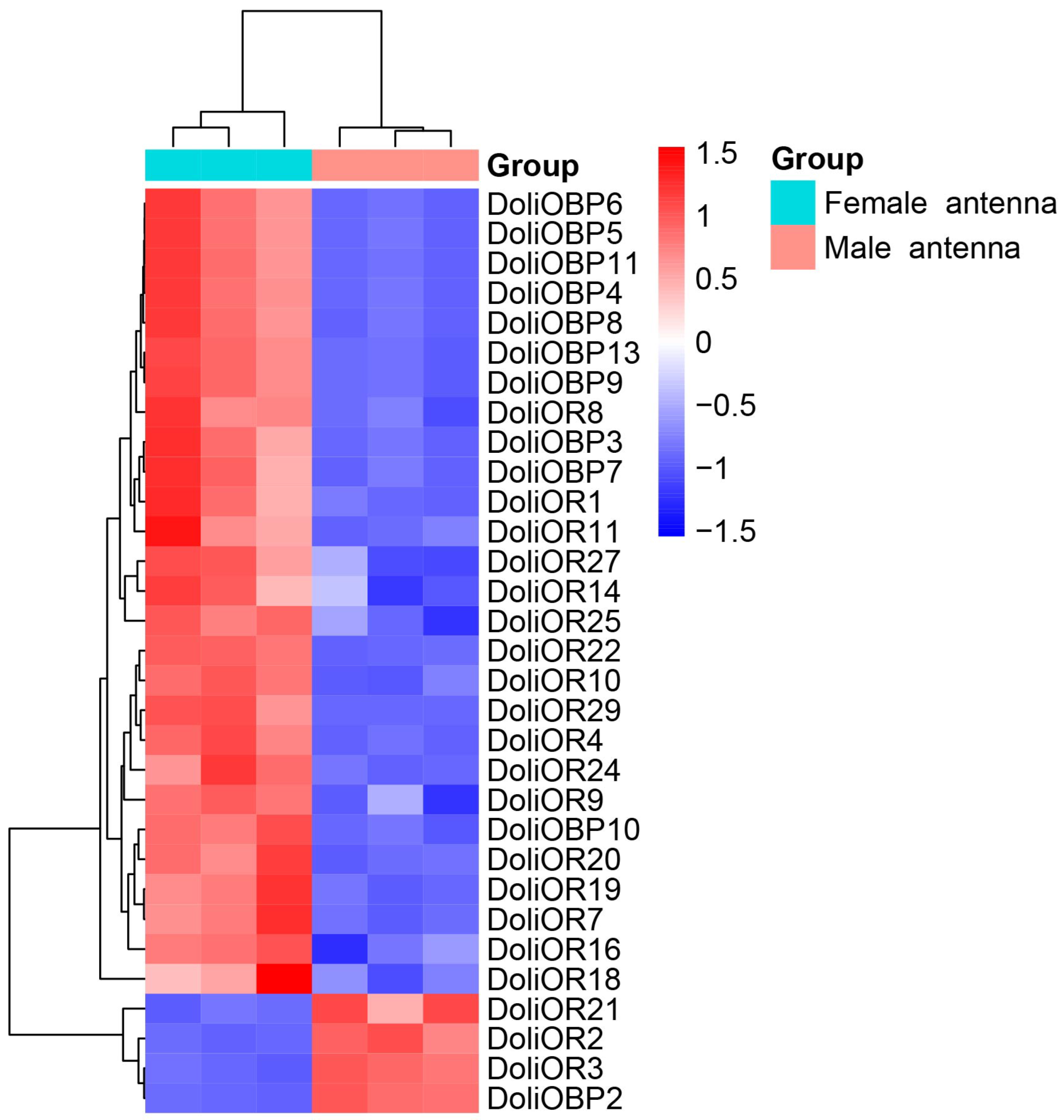
2.3. Transcriptome-Based Identification of Differentially Expressed DoliOBPs and DoliORs
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Scanning Electron Microscopy
4.3. RNA Isolation
4.4. RNA-Seq and Transcriptome Assembly
4.5. Differential Gene Expression Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, F.; Zhang, G.N.; Wang, J.J. Scanning Electron Microscopy Studies of Antennal Sensilla of Bruchid Beetles, Callosobruchus chinensis (L.) and Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). Micron 2009, 40, 320–326. [Google Scholar] [CrossRef]
- Liu, H.X.; Liu, Z.X.; Zheng, H.X.; Jin, Z.R.; Zhang, J.T.; Zhang, P.Q. Sensilla on the Antennae and Ovipositor of the Carpenterworm, Streltzoviella insularis (Staudinger, 1892) (Lepidoptera, Cossidae). Orient. Insects 2018, 52, 420–433. [Google Scholar] [CrossRef]
- Nowińska, A. Report on the Types and Distribution of Antennal Sensilla in Lygaeidae (Heteroptera: Lygaeoidea) and Their Putative Functions. Insects 2025, 16, 44. [Google Scholar] [CrossRef]
- Haddad, S.; Clarke, D.J.; Jeong, S.H.; Mitchell, R.F.; McKenna, D.D. Antennal Sensilla in Longhorn Beetles (Coleoptera: Cerambycidae). Ann. Entomol. Soc. Am. 2023, 116, 83–113. [Google Scholar] [CrossRef]
- Lemon, W.C.; Getz, W.M. Neural Coding of General Odors in Insects. Ann. Entomol. Soc. Am. 1999, 92, 861–872. [Google Scholar] [CrossRef]
- Wang, J.J.; Liu, P.C.; Wang, J.G.; Luan, Q.S.; Jiang, X.; Cao, C.W. Morphology and Distribution of Antennal Sensilla in an Egg Parasitoid Wasp, Anastatus disparis (Hymenoptera: Eupelmidae). J. Insect Sci. 2022, 22, 6. [Google Scholar] [CrossRef]
- Maida, R.; Mameli, M.; Müller, B.; Krieger, J.; Steinbrecht, R.A. The Expression Pattern of Four Odorant-Binding Proteins in Male and Female Silk Moths, Bombyx mori. J. Neurocytol. 2005, 34, 149–163. [Google Scholar] [CrossRef]
- Roh, H.S.; Park, K.C.; Oh, H.W.; Park, C.G. Morphology and Distribution of Antennal Sensilla of Two Tortricid Moths, Cydia pomonella and C. succedana (Lepidoptera). Microsc. Res. Tech. 2016, 79, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.B.; Dou, F.G.; Zhang, Y.J.; Huang, H.X.; Zheng, X.L.; Wang, X.Y.; Lu, W. Observations on the Ultrastructure of Antennal Sensilla of Adult Glenea cantor (Cerambycidae: Lamiinae). J. Insect Sci. 2020, 20, 7. [Google Scholar] [CrossRef]
- Iacovone, A.; Salerno, G.; French, A.S.; Conti, E.; Marion-Poll, F. Antennal Gustatory Perception and Behavioural Responses in Trissolcus brochymenae Females. J. Insect Physiol. 2015, 78, 15–25. [Google Scholar] [CrossRef]
- King, B.H.; Gunathunga, P.B. Gustation in Insects: Taste Qualities and Types of Evidence Used to Show Taste Function of Specific Body Parts. J. Insect Sci. 2023, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Alborn, H.T.; Lewis, W.J.; Tumlinson, J.H. Host-Specific Recognition Kairomone for the Parasitoid Microplitis croceipes (Cresson). J. Chem. Ecol. 1995, 21, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Stange, G.; Stowe, S. Carbon-Dioxide Sensing Structures in Terrestrial Arthropods. Microsc. Res. Tech. 1999, 47, 416–427. [Google Scholar] [CrossRef]
- Cingolani, M.F.; Barakat, M.C.; Cerretti, P.; Chirinos, D.T.; Ferrer, F.; Gaviria Vega, J.; Grenier, S.; Kondo, T.; Pape, T.; Plowes, R.; et al. Dipteran Parasitoids as Biocontrol Agents. BioControl 2025, 70, 285–300. [Google Scholar] [CrossRef]
- Turlings, T.C.; Loughrin, J.H.; McCall, P.J.; Röse, U.S.; Lewis, W.J.; Tumlinson, J.H. How Caterpillar-Damaged Plants Protect Themselves by Attracting Parasitic Wasps. Proc. Natl. Acad. Sci. USA 1995, 92, 4169–4174. [Google Scholar] [CrossRef]
- Fukushima, J.; Kainoh, Y.; Honda, H.; Takabayashi, J. Learning of Herbivore-Induced and Nonspecific Plant Volatiles by a Parasitoid, Cotesia kariyai. J. Chem. Ecol. 2002, 28, 579–586. [Google Scholar] [CrossRef]
- Afsheen, S.; Wang, X.; Li, R.; Zhu, C.S.; Lou, Y.G. Differential Attraction of Parasitoids in Relation to Specificity of Kairomones from Herbivores and Their By-products. Insect Sci. 2008, 5, 381–397. [Google Scholar] [CrossRef]
- Knolhoff, L.M.; Heckel, D.G. Behavioral Assays for Studies of Host Plant Choice and Adaptation in Herbivorous Insects. Annu. Rev. Entomol. 2014, 59, 263–278. [Google Scholar] [CrossRef]
- Suh, E.; Bohbot, J.D.; Zwiebel, L.J. Peripheral Olfactory Signaling in Insects. Curr. Opin. Insect Sci. 2014, 6, 86–92. [Google Scholar] [CrossRef]
- Kang, Z.W.; Liu, F.H.; Xu, Y.Y.; Cheng, J.H.; Lin, X.L.; Jing, X.F.; Tian, H.G.; Liu, T.X. Identification of Candidate Odorant-degrading Enzyme Genes in the Antennal Transcriptome of Aphidius gifuensis. Entomol. Res. 2021, 51, 36–54. [Google Scholar] [CrossRef]
- Leal, W.S. Odorant Reception in Insects: Roles of Receptors, Binding Proteins, and Degrading Enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Iovinella, I.; Zhu, J.; Wang, G.R.; Dani, F.R. Beyond Chemoreception: Diverse Tasks of Soluble Olfactory Proteins in Insects. Biol. Rev. 2018, 93, 184–200. [Google Scholar] [CrossRef]
- Cassau, S.; Krieger, J. The Role of SNMPs in Insect Olfaction. Cell Tissue Res. 2021, 383, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, J.; Pregitzer, P.; Breer, H.; Krieger, J. Access to the Odor World: Olfactory Receptors and Their Role for Signal Transduction in Insects. Cell. Mol. Life Sci. 2018, 75, 485–508. [Google Scholar] [CrossRef]
- Das, P.; Morawo, T.; Fadamiro, H. Plant-Associated Odor Perception and Processing in Two Parasitoid Species with Different Degrees of Host Specificity: Implications for Host Location Strategies. J. Insect Physiol. 2017, 101, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Biondi, A.; Guedes, R.N.C.; Wan, F.H.; Desneux, N. Ecology, Worldwide Spread, and Management of the Invasive South American Tomato Pinworm, Tuta absoluta: Past, Present, and Future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Han, P.; Mansour, R.; Arno, J.; Brevault, T.; Campos, M.R.; Chailleux, A.; Guedes, R.N.C.; Karimi, J.; Konan, K.A.J.; et al. Integrated Pest Management of Tuta absoluta: Practical Implementations across Different World Regions. J. Pest Sci. 2022, 95, 17–39. [Google Scholar] [CrossRef]
- Zhang, G.F.; Xian, X.Q.; Zhang, Y.B.; Liu, W.X.; Liu, H.; Feng, X.D.; Ma, D.Y.; Wang, Y.S.; Gao, Y.H.; Zhang, R.; et al. Outbreak of the South American Tomato Leafminer, Tuta absoluta, in the Chinese Mainland: Geographic and Potential Host Range Expansion. Pest Manag. Sci. 2021, 77, 5475–5488. [Google Scholar] [CrossRef]
- Aigbedion-Atalor, P.O.; Mohamed, S.A.; Hill, M.P.; Zalucki, M.P.; Azrag, A.G.A.; Srinivasan, R.; Ekesi, S. Host Stage Preference and Performance of Dolichogenidea gelechiidivoris (Hymenoptera: Braconidae), a Candidate for Classical Biological Control of Tuta absoluta in Africa. Biol. Control 2020, 144, 104215. [Google Scholar] [CrossRef]
- Salas Gervassio, N.G.; Aquino, D.; Vallina, C.; Biondi, A.; Luna, M.G. A Re-Examination of Tuta absoluta Parasitoids in South America for Optimized Biological Control. J. Pest Sci. 2019, 92, 1343–1357. [Google Scholar] [CrossRef]
- Aigbedion-Atalor, P.O.; Hill, M.P.; Ayelo, P.M.; Ndlela, S.; Zalucki, M.P.; Mohamed, S.A. Can the Combined Use of the Mirid Predator Nesidiocoris tenuis and a Braconid Larval Endoparasitoid Dolichogenidea gelechiidivoris Improve the Biological Control of Tuta absoluta? Insects 2021, 12, 1004. [Google Scholar] [CrossRef]
- Denis, C.; Riudavets, J.; Alomar, O.; Agusti, N.; Gonzalez-Valero, H.; Cubi, M.; Matas, M.; Rodriguez, D.; van Achterberg, K.; Arno, J. Naturalized Dolichogenidea gelechiidivoris Complement the Resident Parasitoid Complex of Tuta absoluta in North-Eastern Spain. J. Appl. Entomol. 2022, 146, 461–464. [Google Scholar] [CrossRef]
- Syropoulou, A.; Gonzalez-Cabrera, J.; Arno, J.; Urbaneja-Bernat, P. Role of Tomato Plant-Derived Food Sources on Dolichogenidea gelechiidivoris, of Tuta absoluta. Biol. Control 2025, 202, 105719. [Google Scholar] [CrossRef]
- Urbaneja-Bernat, P.; Riudavets, J.; Denis, C.; Ojeda, J.; Alomar, O.; Arno, J. Lobularia maritima as a Nutrient-Rich Floral Food Source for Two Parasitoid Wasps of Tuta absoluta. Entomol. Gen. 2024, 44, 339–346. [Google Scholar] [CrossRef]
- Gao, Y.; Luo, L.Z.; Hammond, A. Antennal Morphology, Structure and Sensilla Distribution in Microplitis pallidipes (Hymenoptera: Braconidae). Micron 2007, 38, 684–693. [Google Scholar] [CrossRef]
- Chen, H.H.; Zhao, Y.X.; Kang, L. Antennal Sensilla of Grasshoppers (Orthoptera: Acrididae) in Relation to Food Preferences and Habits. J. Biosci. 2003, 28, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Zhang, T.T.; Wang, Z.Y.; He, K.L.; Bai, S.X. Morphology and Ultrastructure of Antennal Sensilla of Morphology and Ultrastructure of Antennal Macrocentrus cingulum Brischke (Hymenoptera: Braconidae) and Their Probable Functions. Micron 2013, 50, 35–43. [Google Scholar] [CrossRef]
- Khadka, K.K.; Shek, J.; Hoffman, J.; Vulin, R.; Foellmer, M. Longer Antennae for Romeo: Assessing Effect of Antennae Length on Courtship and Mating Success in Male Crickets, Acheta domesticus (Orthoptera, Gryllidae). J. Insect Behav. 2012, 25, 96–103. [Google Scholar] [CrossRef]
- Piersanti, S.; Saitta, V.; Rebora, M.; Salerno, G. Olfaction in Phytophagous Ladybird Beetles: Antennal Sensilla and Sensitivity to Volatiles from Host Plants in Chnootriba elaterii. Arthropod-Plant Interact. 2022, 16, 617–630. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, X.J.; Ullah, H.S.; Tang, Y.; Zhu, D.Y.; Xu, H.L.; Wen, Q.; Tian, X.X.; Tan, J.L. Comparative SEM Study of Sensilla and Tyloid Structures in the Antennae of Vespinae (Hymenoptera: Vespidae). Insects 2024, 15, 448. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Liu, Y.; Li, X.J.; Cheng, W.N.; Zhu-Salzman, K. Morphology, Ultrastructure and Possible Functions of Antennal Sensilla of Sitodiplosis mosellana Géhin (Diptera: Cecidomyiidae). J. Insect Sci. 2016, 16, 93. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.H. Morphology and Location of Sensilla in the Antennae and Ovipositor of Sirex noctilio (Hymenoptera: Siricidae). Arthropod Struct. Dev. 2023, 73, 101252. [Google Scholar] [CrossRef]
- Ong, C.Y.; Loo, X.J.; Tee, C.S.; Wong, W.L. Distribution and Morphometric Studies on Antennal Sensilla of Female and Male Pediobius imbreus (Hymenoptera: Eulophidae). Zoomorphology 2024, 143, 653–665. [Google Scholar] [CrossRef]
- Chakilam, S.; Brożek, J.; Chajec, Ł.; Poprawa, I.; Gaidys, R. Ultra-Morphology and Mechanical Function of the Trichoideum Sensillum in Nabis rugosus (Linnaeus, 1758) (Insecta: Heteroptera: Cimicomorpha). Insects 2022, 13, 799. [Google Scholar] [CrossRef]
- Chakilam, S.; Gaidys, R.; Brożek, J. Ultrastructure of a Mechanoreceptor of the Trichoid Sensilla of the Insect Nabis rugosus: Stimulus-Transmitting and Bio-Sensory Architecture. Bioengineering 2023, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.C.; Berg, B.G.; Wang, G.R. Editorial: Recent Advances in Insect Olfaction: Characterization of Neural Circuits from Sensory Input to Motor Output. Front. Cell. Neurosci. 2023, 17, 1282499. [Google Scholar] [CrossRef]
- Altner, H.; Prillinger, L. Ultrastructure of Invertebrate Chemo-, Thermo-, and Hygroreceptors and Its Functional Significance. In International Review of Cytology; Academic Press: Cambridge, MA, USA, 1980; Volume 67, pp. 69–139. [Google Scholar]
- Palottini, F.; Fernández, C.; Balbuena, M.S. Antennal Sensilla Pattern Distribution and Odor Detection in Bombus pauloensis Foragers (Hymenoptera: Apidae). Apidologie 2024, 55, 83. [Google Scholar] [CrossRef]
- Ren, L.L.; Shi, J.; Zhang, Y.N.; Luo, Y.Q. Antennal Morphology and Sensillar Ultrastructure of Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae). Micron 2012, 43, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Must, A.; Merivee, E.; Mänd, M.; Luik, A.; Heidemaa, M. Electrophysiological Responses of the Antennal Campaniform Sensilla to Rapid Changes of Temperature in the Ground Beetles Pterostichus oblongopunctatus and Poecilus cupreus (Tribe Pterostichini) with Different Ecological Preferences. Physiol. Entomol. 2006, 31, 278–285. [Google Scholar] [CrossRef]
- Dietz, A.; Humphreys, W.J. Scanning Electron Microscopic Studies of Antennal Receptors of the Worker Honey Bee, Including Sensilla Campaniformia. Ann. Entomol. Soc. Am. 1971, 64, 919–925. [Google Scholar] [CrossRef]
- Abd El-Ghany, N.M.; Faucheux, M.J. The Mouthparts and Sensilla of the Adult Tomato Leafminer Moth, Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae). Arthropod Struct. Dev. 2022, 67, 101144. [Google Scholar] [CrossRef] [PubMed]
- Talukder, M.B.; Müller, C.H.G.; Zhang, D.D.; Schulz, S.; Löfstedt, C.; Wang, H.L.; Uhl, G.B. Olfaction with Legs—Spiders Use Wall-Pore Sensilla for Pheromone Detection. Proc. Natl. Acad. Sci. USA 2025, 122, e2415468121. [Google Scholar] [CrossRef]
- Jiang, J.; Xue, J.Y.; Yu, M.M.; Jiang, X.; Cheng, Y.M.; Wang, H.J.; Liu, Y.X.; Dou, W.; Fan, J.; Chen, J.L. Molecular Characterization of Chemosensory Protein (CSP) Genes and the Involvement of AgifCSP5 in the Perception of Host Location in the Aphid Parasitoid Aphidius gifuensis. Int. J. Mol. Sci. 2024, 25, 6392. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Yu, Q.L.; Gan, X.Y.; Song, L.W.; Zhang, K.P.; Zuo, T.T.; Zhang, J.J.; Hu, Y.; Chen, Q.; Ren, B.Z. Transcriptome Analysis and Identification of Chemosensory Genes in Baryscapus dioryctriae (Hymenoptera: Eulophidae). Insects 2022, 13, 1098. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.C.; Chen, H.C.; Hong, T.L.; Yan, M.W.; Wang, J.; Shao, Z.M.; Wu, F.A.; Sheng, S.; Wang, J. Identification of Chemosensory Genes by Antennal Transcriptome Analysis and Expression Profiles of Odorant-Binding Proteins in Parasitoid Wasp Aulacocentrum confusum. Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100881. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.N.; Xie, S.; Chen, J.N.; Wang, Z.H.; Yang, P.; Zhou, S.C.; Pang, L.; Li, F.; Shi, M.; Huang, J.H.; et al. Expression and Functional Characterization of Odorant-Binding Protein Genes in the Endoparasitic Wasp Cotesia vestalis. Insect Sci. 2021, 28, 1354–1368. [Google Scholar] [CrossRef]
- Ma, L.; Gu, S.H.; Liu, Z.W.; Wang, S.N.; Guo, Y.Y.; Zhou, J.J.; Zhang, Y.J. Molecular Characterization and Expression Profiles of Olfactory Receptor Genes in the Parasitic Wasp, Microplitis mediator (Hymenoptera: Braconidae). J. Insect Physiol. 2014, 60, 118–126. [Google Scholar] [CrossRef]
- Li, R.J.; Shan, S.; Song, X.; Khashaveh, A.; Wang, S.N.; Yin, Z.X.; Lu, Z.Y.; Dhiloo, K.H.; Zhang, Y.J. Plant Volatile Ligands for Male-Biased MmedOBP14 Stimulate Orientation Behavior of the Parasitoid Wasp Microplitis mediator. Int. J. Biol. Macromol. 2022, 223, 1521–1529. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Zhang, L.F.; Ze, S.Z.; Wang, D.W.; Yang, B. Identification and Tissue Distribution of Odorant Binding Protein Genes in the Beet Armyworm, Spodoptera exigua. J. Insect Physiol. 2013, 59, 722–728. [Google Scholar] [CrossRef]
- Yang, B.; Ozaki, K.; Ishikawa, Y.; Matsuo, T. Sexually Biased Expression of Odorant-Binding Proteins and Chemosensory Proteins in Asian Corn Borer Ostrinia furnacalis (Lepidoptera: Crambidae). Appl. Entomol. Zool. 2016, 51, 373–383. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, J.J.; Liu, J.T.; Huang, G.Z.; Xu, W.Y.; Zhang, Q.; Chen, J.L.; Zhang, Y.J.; Li, X.C.; Gu, S.H. Integrative Transcriptomic and Genomic Analysis of Odorant Binding Proteins and Chemosensory Proteins in Aphids. Insect Mol. Biol. 2019, 28, 1–22. [Google Scholar] [CrossRef]
- Li, H.C.; Li, W.Z.; Miao, C.J.; Wang, G.P.; Zhao, M.; Yuan, G.H.; Guo, X.R. Identification of the Differences in Olfactory System between Male and Female Oriental Tobacco Budworm Helicoverpa assulta. Arch. Insect Biochem. Physiol. 2021, 107, e21829. [Google Scholar] [CrossRef]
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Zacharuk, R.Y. Innervation of Sheath Cells of an Insect Sensillum by a Bipolar Type II Neuron. Can. J. Zool. 1980, 58, 1264–1276. [Google Scholar] [CrossRef]
- Ochieng, S.A.; Park, K.C.; Zhu, J.W.; Baker, T.C. Functional Morphology of Antennal Chemoreceptors of the Parasitoid Microplitis croceipes (Hymenoptera: Braconidae). Arthropod Struct. Dev. 2000, 29, 231–240. [Google Scholar] [CrossRef]
- Bleeker, M.A.K.; Smid, H.M.; Aelst, A.C.V.; Van Loon, J.J.; Vet, L.E.M. Antennal Sensilla of Two Parasitoid Wasps: A Comparative Scanning Electron Microscopy Study. Microsc. Res. Tech. 2004, 63, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Merivee, E.; Ploomi, A.; Rahi, M.; Bresciani, J.; Ravn, H.P.; Luik, A.; Sammelselg, V. Antennal Sensilla of the Ground Beetle Bembidion properans Steph. (Coleoptera, Carabidae). Micron 2002, 33, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Liu, T.X.; Zhang, S.Z. Observation of the Sensilla on the Adult Antennae of Cotesia ruficrus (Hymenoptera: Braconidae), a Parasitoid of Spodoptera frugiperda (Lepidoptera: Noctuidae), under Scanning Electron Microscope. Acta Entomol. Sin. 2023, 66, 1105–1116. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.-Y.; Yang, H.-S.; Huang, C.; Zhang, G.-F.; Arnó, J.; Collatz, J.; Li, C.-R.; Wan, F.-H.; Liu, W.-X.; Zhang, Y.-B. The Olfactory System of Dolichogenidea gelechiidivoris (Marsh) (Hymenoptera: Braconidae), a Natural Enemy of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Int. J. Mol. Sci. 2025, 26, 7312. https://doi.org/10.3390/ijms26157312
Yan S-Y, Yang H-S, Huang C, Zhang G-F, Arnó J, Collatz J, Li C-R, Wan F-H, Liu W-X, Zhang Y-B. The Olfactory System of Dolichogenidea gelechiidivoris (Marsh) (Hymenoptera: Braconidae), a Natural Enemy of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). International Journal of Molecular Sciences. 2025; 26(15):7312. https://doi.org/10.3390/ijms26157312
Chicago/Turabian StyleYan, Shu-Yan, He-Sen Yang, Cong Huang, Gui-Fen Zhang, Judit Arnó, Jana Collatz, Chuan-Ren Li, Fang-Hao Wan, Wan-Xue Liu, and Yi-Bo Zhang. 2025. "The Olfactory System of Dolichogenidea gelechiidivoris (Marsh) (Hymenoptera: Braconidae), a Natural Enemy of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae)" International Journal of Molecular Sciences 26, no. 15: 7312. https://doi.org/10.3390/ijms26157312
APA StyleYan, S.-Y., Yang, H.-S., Huang, C., Zhang, G.-F., Arnó, J., Collatz, J., Li, C.-R., Wan, F.-H., Liu, W.-X., & Zhang, Y.-B. (2025). The Olfactory System of Dolichogenidea gelechiidivoris (Marsh) (Hymenoptera: Braconidae), a Natural Enemy of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). International Journal of Molecular Sciences, 26(15), 7312. https://doi.org/10.3390/ijms26157312








