Characterization of Tellurite Toxicity to Escherichia coli Under Aerobic and Anaerobic Conditions
Abstract
1. Introduction
2. Results
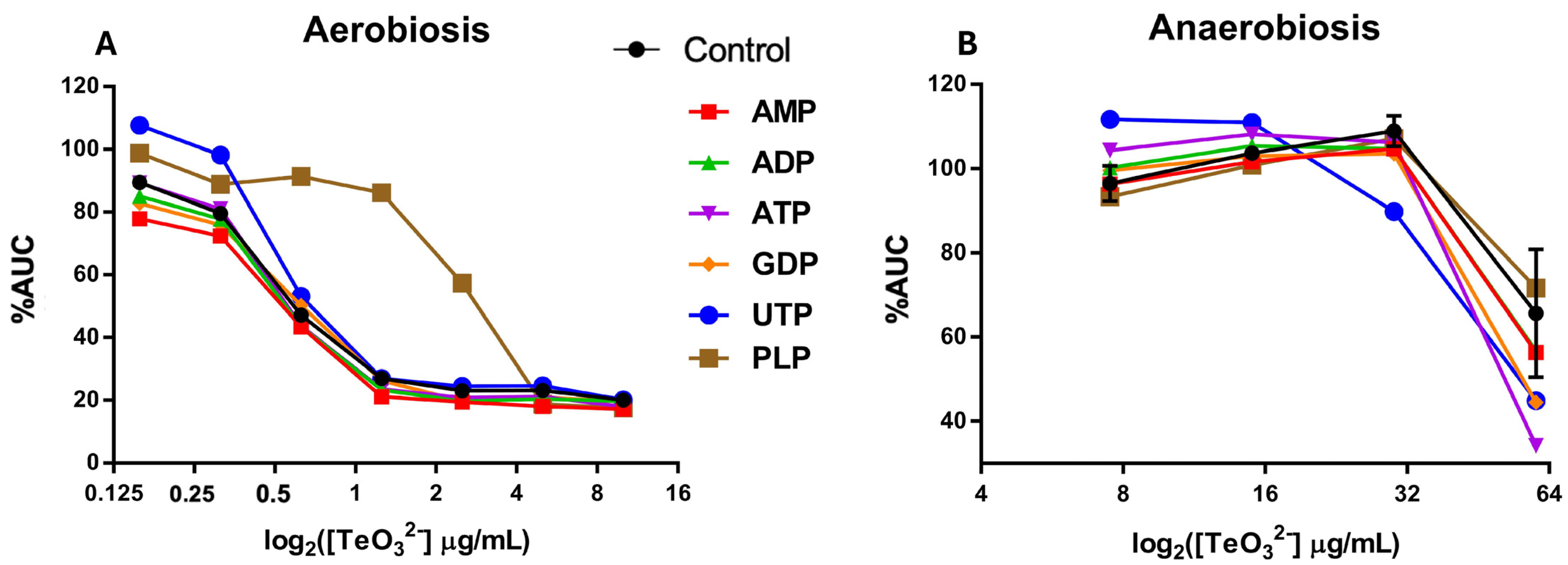
2.1. Tellurite Resistance of E. coli Under Aerobic vs. Anaerobic Conditions
2.2. Evidence for Tellurite Targeting [4Fe–4S] Cluster-Containing Enzymes
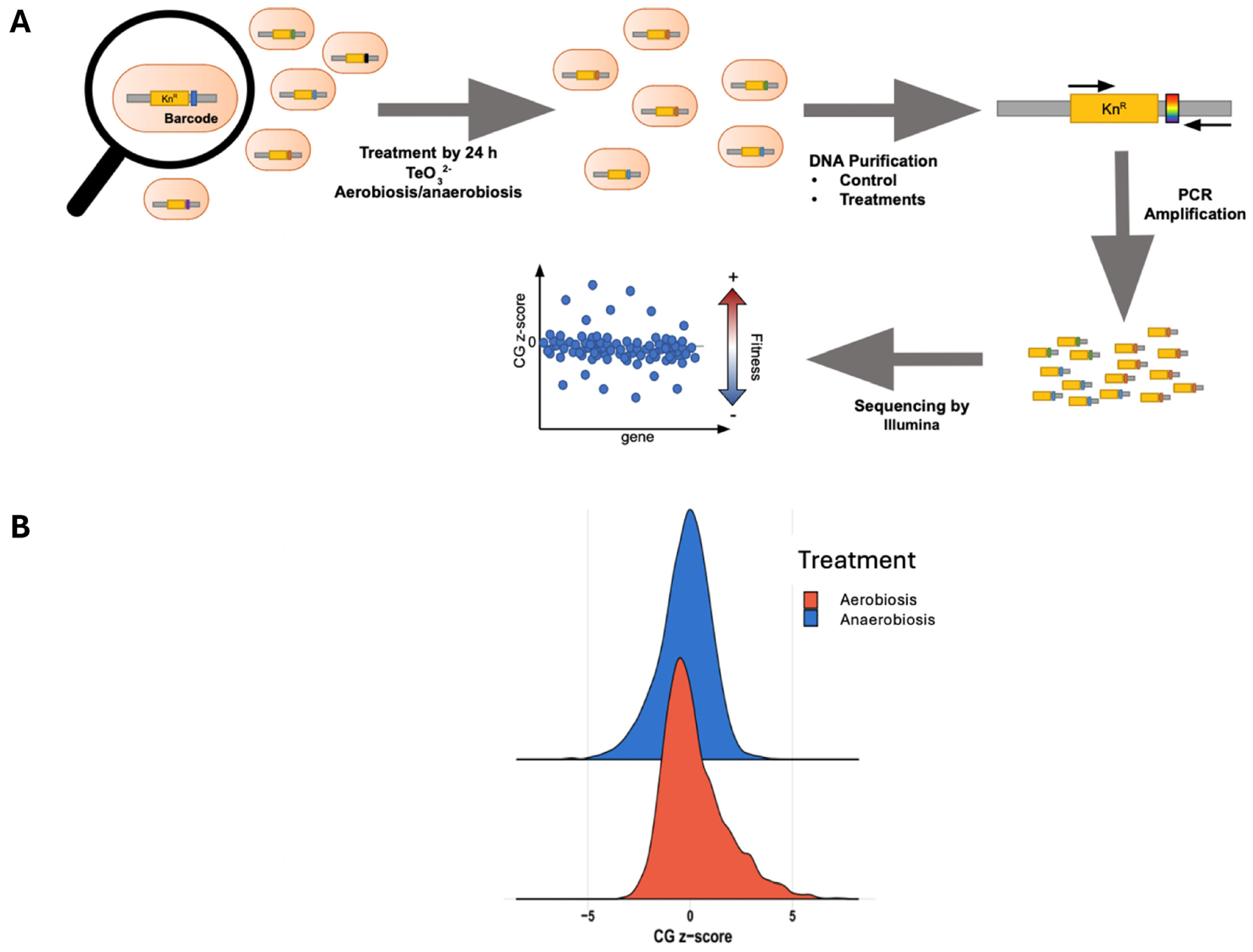
2.3. Chemical-Genomic Profile of Tellurite Toxicity: Common Genes Under Aerobiosis and Anaerobiosis
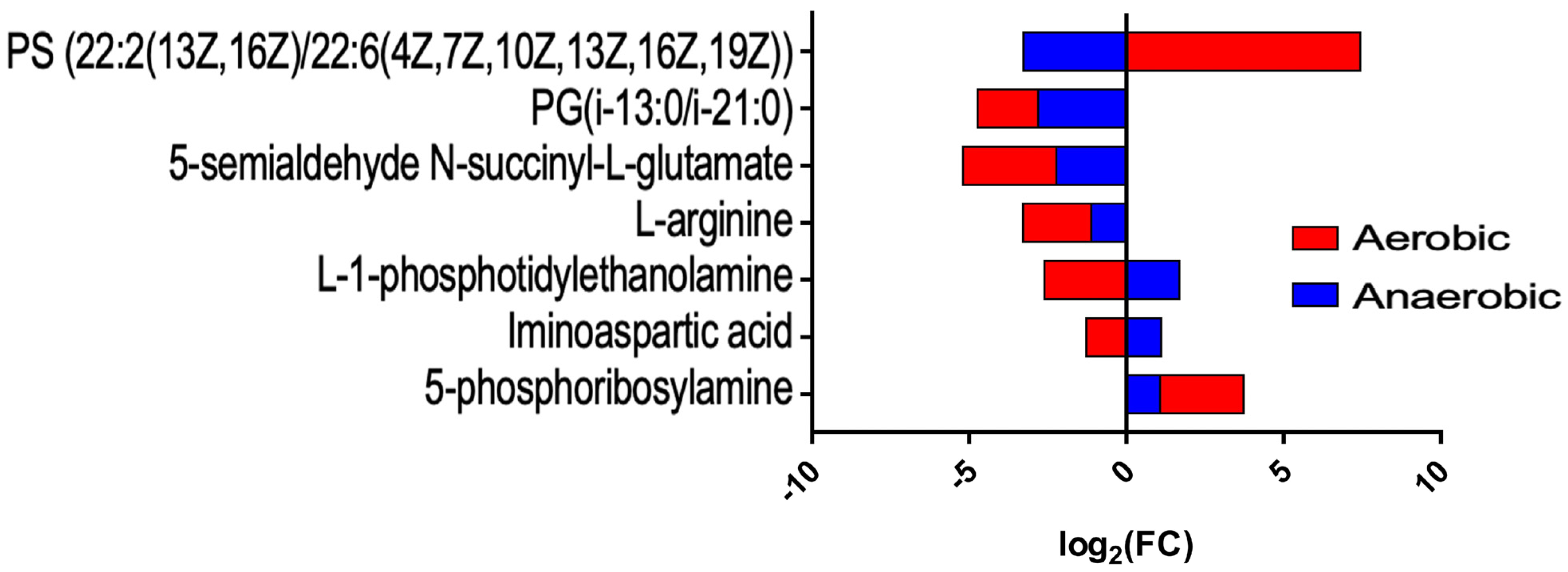
2.4. Metabolomic Changes Induced by Tellurite in Aerobic and Anaerobic Conditions
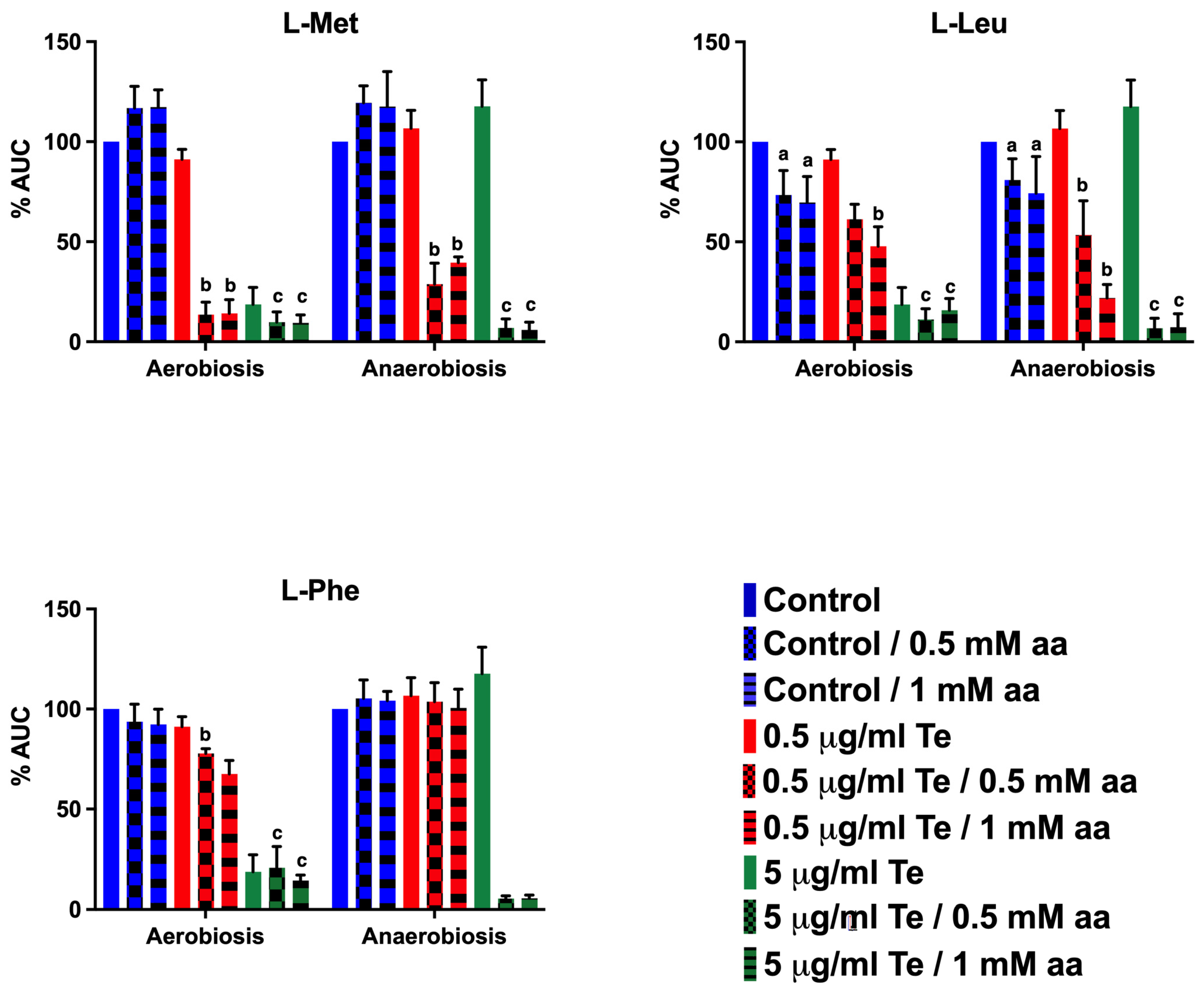
2.5. Effect of Supplementing Key Metabolites on Tellurite Toxicity
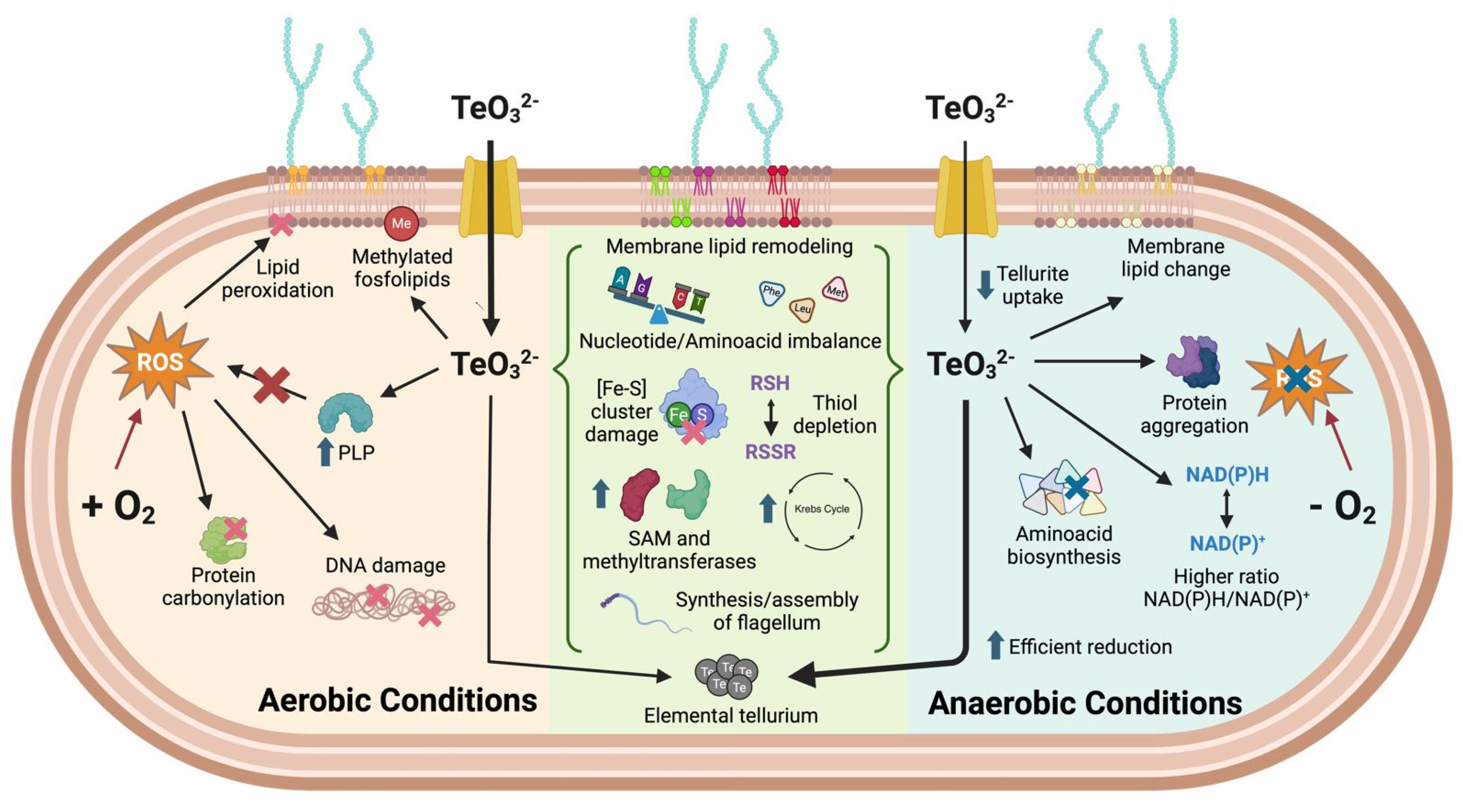
3. Discussion
3.1. Tellurite Resistance of E. coli Under Aerobic vs. Anaerobic Conditions
3.2. Evidence for Tellurite Targeting [4Fe–4S] Cluster-Containing Enzymes
3.3. Chemical-Genomic Profile of Tellurite Toxicity: Common Genes Under Aerobiosis and Anaerobiosis
3.4. Metabolomic Changes Induced by Tellurite Under Aerobic and Anaerobic Conditions
3.5. Effect of Supplementing Key Metabolites on Tellurite Toxicity
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Cell Viability
4.3. Quantification of Tellurite E. coli Cultures
4.4. Determination of Free Thiol Content
4.5. Determination of NADH and NAD+ Content
4.6. Growth Curve Analysis and Area Under Curve (AUC) Calculation
4.7. Chemical-Genomic Profiling (Barcode Sequencing)
4.8. Metabolomic Profiling of Tellurite-Treated Cells
4.9. Metabolite Supplementation Experiments
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chasteen, T.G.; Fuentes, D.E.; Tantaleán, J.C.; Vásquez, C.C. Tellurite: History, Oxidative Stress, and Molecular Mechanisms of Resistance. FEMS Microbiol. Rev. 2009, 33, 820–832. [Google Scholar] [CrossRef]
- Molina-Quiroz, R.C.; Loyola, D.E.; Díaz-Vásquez, W.A.; Arenas, F.A.; Urzúa, U.; Pérez-Donoso, J.M.; Vásquez, C.C. Global Transcriptomic Analysis Uncovers a Switch to Anaerobic Metabolism in Tellurite-Exposed Escherichia coli. Res. Microbiol. 2014, 165, 566–570. [Google Scholar] [CrossRef]
- Turner, R.J.; Weiner, J.H.; Taylor, D.E. Tellurite-Mediated Thiol Oxidation in Escherichia coli. Microbiology 1999, 145, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Nies, D.H. Microbial Heavy-Metal Resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. [Google Scholar] [CrossRef]
- Hill, S.M.; Jobling, M.G.; Lloyd, B.H.; Strike, P.; Ritchie, D.A. Functional Expression of the Tellurite Resistance Determinant from the IncHI-2 Plasmid pMER610. Mol. Gen. Genet. 1993, 241, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.F.; Colleran, E.; Taylor, D.E. Phage Inhibition, Colicin Resistance, and Tellurite Resistance Are Encoded by a Single Cluster of Genes on the IncHI2 Plasmid R478. J. Bacteriol. 1995, 177, 5016–5027. [Google Scholar] [CrossRef]
- Taylor, D.E. Bacterial Tellurite Resistance. Trends Microbiol. 1999, 7, 111–115. [Google Scholar] [CrossRef]
- Taylor, D.E.; Rooker, M.; Keelan, M.; Ng, L.-K.; Martin, I.; Perna, N.T.; Burland, N.T.V.; Blattner, F.R. Genomic Variability of O Islands Encoding Tellurite Resistance in Enterohemorrhagic Escherichia coli O157:H7 Isolates. J. Bacteriol. 2002, 184, 4690–4698. [Google Scholar] [CrossRef]
- Turner, R.J.; Weiner, J.H.; Taylor, D.E. The Tellurite-Resistance Determinants tehAtehB and klaAklaBtelB Have Different Biochemical Requirements. Microbiology 1995, 141, 3133–3140. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.E.; Hou, Y.; Turner, R.J.; Weiner, J.H. Location of a Potassium Tellurite Resistance Operon (tehA tehB) within the Terminus of Escherichia coli K-12. J. Bacteriol. 1994, 176, 2740–2742. [Google Scholar] [CrossRef]
- Turner, R.J.; Hou, Y.; Weiner, J.H.; Taylor, D.E. The Arsenical ATPase Efflux Pump Mediates Tellurite Resistance. J. Bacteriol. 1992, 174, 3092–3094. [Google Scholar] [CrossRef]
- Elías, A.O.; Abarca, M.J.; Montes, R.A.; Chasteen, T.G.; Pérez-Donoso, J.M.; Vásquez, C.C. Tellurite Enters Escherichia coli Mainly through the PitA Phosphate Transporter. MicrobiologyOpen 2012, 1, 259–267. [Google Scholar] [CrossRef]
- Elías, A.; Díaz-Vásquez, W.; Abarca-Lagunas, M.J.; Chasteen, T.G.; Arenas, F.; Vásquez, C.C. The ActP Acetate Transporter Acts Prior to the PitA Phosphate Carrier in Tellurite Uptake by Escherichia coli. Microbiol. Res. 2015, 177, 15–21. [Google Scholar] [CrossRef]
- Arenas, F.A.; Covarrubias, P.C.; Sandoval, J.M.; Pérez-Donoso, J.M.; Imlay, J.A.; Vásquez, C.C. The Escherichia coli BtuE Protein Functions as a Resistance Determinant against Reactive Oxygen Species. PLoS ONE 2011, 6, e15979. [Google Scholar] [CrossRef]
- Calderón, I.L.; Elías, A.O.; Fuentes, E.L.; Pradenas, G.A.; Castro, M.E.; Arenas, F.A.; Pérez, J.M.; Vásquez, C.C. Tellurite-Mediated Disabling of [4Fe–4S] Clusters of Escherichia coli Dehydratases. Microbiology 2009, 155, 1840–1846. [Google Scholar] [CrossRef]
- Morales, E.H.; Pinto, C.A.; Luraschi, R.; Muñoz-Villagrán, C.M.; Cornejo, F.A.; Simpkins, S.W.; Nelson, J.; Arenas, F.A.; Piotrowski, J.S.; Myers, C.L.; et al. Accumulation of Heme Biosynthetic Intermediates Contributes to the Antibacterial Action of the Metalloid Tellurite. Nat. Commun. 2017, 8, 15320. [Google Scholar] [CrossRef]
- Tantaleán, J.C.; Araya, M.A.; Saavedra, C.P.; Fuentes, D.E.; Pérez, J.M.; Calderón, I.L.; Youderian, P.; Vásquez, C.C. The Geobacillus stearothermophilus V iscS Gene, Encoding Cysteine Desulfurase, Confers Resistance to Potassium Tellurite in Escherichia coli K-12. J. Bacteriol. 2003, 185, 5831–5837. [Google Scholar] [CrossRef]
- Macomber, L.; Imlay, J.A. The Iron-Sulfur Clusters of Dehydratases Are Primary Intracellular Targets of Copper Toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef]
- Leonardo, M.R.; Dailly, Y.; Clark, D.P. Role of NAD in Regulating the adhE Gene of Escherichia coli. J. Bacteriol. 1996, 178, 6013–6018. [Google Scholar] [CrossRef]
- Fontecave, M.; Ollagnier-de-Choudens, S. Iron–Sulfur Cluster Biosynthesis in Bacteria: Mechanisms of Cluster Assembly and Transfer. Arch. Biochem. Biophys. 2008, 474, 226–237. [Google Scholar] [CrossRef]
- Vera, A.; Arís, A.; Carrió, M.; González-Montalbán, N.; Villaverde, A. Lon and ClpP Proteases Participate in the Physiological Disintegration of Bacterial Inclusion Bodies. J. Biotechnol. 2005, 119, 163–171. [Google Scholar] [CrossRef]
- Lemire, J.; Alhasawi, A.; Appanna, V.P.; Tharmalingam, S.; Appanna, V.D. Metabolic Defence against Oxidative Stress: The Road Less Travelled so Far. J. Appl. Microbiol. 2017, 123, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Kleetz, J.; Welter, L.; Mizza, A.-S.; Aktas, M.; Narberhaus, F. Phospholipid N-Methyltransferases Produce Various Methylated Phosphatidylethanolamine Derivatives in Thermophilic Bacteria. Appl. Environ. Microbiol. 2021, 87, e01105-21. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Bilski, P.; Li, M.Y.; Ehrenshaft, M.; Daub, M.E.; Chignell, C.F. Symposium-in-Print Vitamin B6 (Pyridoxine) and Its Derivatives Are Efficient Singlet Oxygen Quenchers and Potential Fungal Antioxidants. Photochem. Photobiol. 2000, 71, 129–134. [Google Scholar] [CrossRef]
- Levine, R.L.; Mosoni, L.; Berlett, B.S.; Stadtman, E.R. Methionine Residues as Endogenous Antioxidants in Proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 15036–15040. [Google Scholar] [CrossRef]
- Umbarger, H.E. Amino Acid Biosynthesis and Its Regulation. Annu. Rev. Biochem. 1978, 47, 533–606. [Google Scholar] [CrossRef]
- Díaz-Vásquez, W.A.; Abarca-Lagunas, M.J.; Arenas, F.A.; Pinto, C.A.; Cornejo, F.A.; Wansapura, P.T.; Appuhamillage, G.A.; Chasteen, T.G.; Vásquez, C.C. Tellurite Reduction by Escherichia coli NDH-II Dehydrogenase Results in Superoxide Production in Membranes of Toxicant-Exposed Cells. Biometals 2014, 27, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J.; Aharonowitz, Y.; Weiner, J.H.; Taylor, D.E. Glutathione Is a Target in Tellurite Toxicity and Is Protected by Tellurite Resistance Determinants in Escherichia coli. Can. J. Microbiol. 2001, 47, 33–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Arenas-Salinas, M.; Vargas-Pérez, J.I.; Morales, W.; Pinto, C.; Muñoz-Díaz, P.; Cornejo, F.A.; Pugin, B.; Sandoval, J.M.; Díaz-Vásquez, W.A.; Muñoz-Villagrán, C.; et al. Flavoprotein-Mediated Tellurite Reduction: Structural Basis and Applications to the Synthesis of Tellurium-Containing Nanostructures. Front. Microbiol. 2016, 7, 1160. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Vásquez, W.A.; Abarca-Lagunas, M.J.; Cornejo, F.A.; Pinto, C.A.; Arenas, F.A.; Vásquez, C.C. Tellurite-Mediated Damage to the Escherichia coli NDH-Dehydrogenases and Terminal Oxidases in Aerobic Conditions. Arch. Biochem. Biophys. 2015, 566, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Anaganti, N.; Basu, B.; Gupta, A.; Joseph, D.; Apte, S.K. Depletion of Reduction Potential and Key Energy Generation Metabolic Enzymes Underlies Tellurite Toxicity in Deinococcus radiodurans. Proteomics 2015, 15, 89–97. [Google Scholar] [CrossRef]
- Guzmán, G.I.; Utrilla, J.; Nurk, S.; Brunk, E.; Monk, J.M.; Ebrahim, A.; Palsson, B.O.; Feist, A.M. Model-Driven Discovery of Underground Metabolic Functions in Escherichia coli. Proc. Natl. Acad. Sci. USA 2015, 112, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.F.; Imlay, J.A. Silver(I), Mercury(II), Cadmium(II), and Zinc(II) Target Exposed Enzymic Iron-Sulfur Clusters When They Toxify Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 3614–3621. [Google Scholar] [CrossRef]
- Otsuka, Y.; Muto, A.; Takeuchi, R.; Okada, C.; Ishikawa, M.; Nakamura, K.; Yamamoto, N.; Dose, H.; Nakahigashi, K.; Tanishima, S.; et al. GenoBase: Comprehensive Resource Database of Escherichia coli K-12. Nucleic Acids Res. 2015, 43, D606–D617. [Google Scholar] [CrossRef]
- Choudhury, H.G.; Cameron, A.D.; Iwata, S.; Beis, K. Structure and Mechanism of the Chalcogen-Detoxifying Protein TehB from Escherichia coli. Biochem. J. 2011, 435, 85–91. [Google Scholar] [CrossRef]
- Prigent-Combaret, C.; Sanguin, H.; Champier, L.; Bertrand, C.; Monnez, C.; Colinon, C.; Blaha, D.; Ghigo, J.; Cournoyer, B. The Bacterial Thiopurine Methyltransferase Tellurite Resistance Process Is Highly Dependent upon Aggregation Properties and Oxidative Stress Response. Environ. Microbiol. 2012, 14, 2645–2660. [Google Scholar] [CrossRef]
- Chasteen, T.G.; Bentley, R. Biomethylation of Selenium and Tellurium: Microorganisms and Plants. Chem. Rev. 2003, 103, 1–26. [Google Scholar] [CrossRef]
- Hondorp, E.R.; Matthews, R.G. Oxidative Stress Inactivates Cobalamin-Independent Methionine Synthase (MetE) in Escherichia coli. PLoS Biol. 2004, 2, e336. [Google Scholar] [CrossRef]
- Spencer, J.B.; Stolowich, N.J.; Roessner, C.A.; Scott, A.I. The Escherichia coli cysG Gene Encodes the Multifunctional Protein, Siroheme Synthase. FEBS Lett. 1993, 335, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.J.; Siegel, L.M.; Tove, S.R.; Kamin, H. Siroheme: A New Prosthetic Group Participating in Six-Electron Reduction Reactions Catalyzed by Both Sulfite and Nitrite Reductases. Proc. Natl. Acad. Sci. USA 1974, 71, 612–616. [Google Scholar] [CrossRef]
- Kredich, N.M. Biosynthesis of Cysteine. EcoSal Plus 2008, 3, 10–1128. [Google Scholar] [CrossRef]
- Fuentes, D.E.; Fuentes, E.L.; Castro, M.E.; Pérez, J.M.; Araya, M.A.; Chasteen, T.G.; Pichuantes, S.E.; Vásquez, C.C. Cysteine Metabolism-Related Genes and Bacterial Resistance to Potassium Tellurite. J. Bacteriol. 2007, 189, 8953–8960. [Google Scholar] [CrossRef]
- Golovina, A.Y.; Sergiev, P.V.; Golovin, A.V.; Serebryakova, M.V.; Demina, I.; Govorun, V.M.; Dontsova, O.A. The yfiC Gene of E. coli Encodes an Adenine-N6 Methyltransferase That Specifically Modifies A37 of tRNA1Val (Cmo5 UAC). RNA 2009, 15, 1134–1141. [Google Scholar] [CrossRef]
- Basturea, G.N.; Dague, D.R.; Deutscher, M.P.; Rudd, K.E. YhiQ Is RsmJ, the Methyltransferase Responsible for Methylation of G1516 in 16S rRNA of E. coli. J. Mol. Biol. 2012, 415, 16–21. [Google Scholar] [CrossRef]
- Arora, S.; Bhamidimarri, S.P.; Bhattacharyya, M.; Govindan, A.; Weber, M.H.W.; Vishveshwara, S.; Varshney, U. Distinctive Contributions of the Ribosomal P-Site Elements m2G966, m5C967 and the C-Terminal Tail of the S9 Protein in the Fidelity of Initiation of Translation in Escherichia coli. Nucleic Acids Res. 2013, 41, 4963–4975. [Google Scholar] [CrossRef]
- Chen, M.; Trotter, V.V.; Walian, P.J.; Chen, Y.; Lopez, R.; Lui, L.M.; Nielsen, T.N.; Malana, R.G.; Thorgersen, M.P.; Hendrickson, A.J.; et al. Molecular Mechanisms and Environmental Adaptations of Flagellar Loss and Biofilm Growth of Rhodanobacter under Environmental Stress. ISME J. 2024, 18, wrae151. [Google Scholar] [CrossRef]
- Henrich, B.; Becker, S.; Schroeder, U.; Plapp, R. Dcp Gene of Escherichia coli: Cloning, Sequencing, Transcript Mapping, and Characterization of the Gene Product. J. Bacteriol. 1993, 175, 7290–7300. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.J.; Lee, A.A.; Knox, T.M.; Miller, C.G. Regulation of the Salmonella Typhimurium pepT Gene by Cyclic AMP Receptor Protein (CRP) and FNR Acting at a Hybrid CRP-FNR Site. J. Bacteriol. 1997, 179, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Marceau, M.; McFall, E.; Lewis, S.D.; Shafer, J.A. D-Serine Dehydratase from Escherichia coli. DNA Sequence and Identification of Catalytically Inactive Glycine to Aspartic Acid Variants. J. Biol. Chem. 1988, 263, 16926–16933. [Google Scholar] [CrossRef]
- Soutourina, J.; Blanquet, S.; Plateau, P. Role of D-Cysteine Desulfhydrase in the Adaptation of Escherichia coli to d-Cysteine. J. Biol. Chem. 2001, 276, 40864–40872. [Google Scholar] [CrossRef]
- Cornejo, F.A.; Muñoz-Villagrán, C.; Luraschi, R.A.; Sandoval-Díaz, M.P.; Cancino, C.A.; Pugin, B.; Morales, E.H.; Piotrowski, J.S.; Sandoval, J.M.; Vásquez, C.C.; et al. Soft-Metal(Loid)s Induce Protein Aggregation in Escherichia coli. Front. Microbiol. 2023, 14, 1281058. [Google Scholar] [CrossRef]
- Sahu, S.K.; Parida, D.; Dalua, R.B.; Sahoo, S.; Ghosh, S.; Padi, S.; Gupta, S.; Behuria, H. Cobalt-Induced Cytotoxicity in E. coli (DH5α) Is Mediated by Modulation of Cellular Phospholipid Composition. J. Adv. Mircobiol. 2016, 2, 215–223. [Google Scholar] [CrossRef]
- Kumar Sahu, S.; Behuria, H.G. Biochemical Characterization of H2O2-Induced Oxidative Stress in E. coli. J. Appl. Microbiol. Biochem. 2018, 2, 10. [Google Scholar] [CrossRef][Green Version]
- Behuria, H.G.; Gupta, S.; Sahu, S.K. Copper and Iron Overload Protect Escherichia coli from Exogenous H2O2 by Modulating Membrane Phospholipid Composition. Environ. Sustain. 2019, 2, 23–32. [Google Scholar] [CrossRef]
- Borghese, R.; Borsetti, F.; Foladori, P.; Ziglio, G.; Zannoni, D. Effects of the Metalloid Oxyanion Tellurite (TeO32−) on Growth Characteristics of the Phototrophic Bacterium Rhodobacter capsulatus. Appl. Environ. Microbiol. 2004, 70, 6595–6602. [Google Scholar] [CrossRef]
- Lohmeier-Vogel, E.M.; Ung, S.; Turner, R.J. In Vivo31 P Nuclear Magnetic Resonance Investigation of Tellurite Toxicity in Escherichia coli. Appl. Environ. Microbiol. 2004, 70, 7342–7347. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.M.; Arenas, F.A.; Pradenas, G.A.; Sandoval, J.M.; Vásquez, C.C. Escherichia coli YqhD Exhibits Aldehyde Reductase Activity and Protects from the Harmful Effect of Lipid Peroxidation-Derived Aldehydes. J. Biol. Chem. 2008, 283, 7346–7353. [Google Scholar] [CrossRef]
- Pradenas, G.A.; Paillavil, B.A.; Reyes-Cerpa, S.; Pérez-Donoso, J.M.; Vásquez, C.C. Reduction of the Monounsaturated Fatty Acid Content of Escherichia coli Results in Increased Resistance to Oxidative Damage. Microbiology 2012, 158, 1279–1283. [Google Scholar] [CrossRef]
- Pradenas, G.A.; Díaz-Vásquez, W.A.; Pérez-Donoso, J.M.; Vásquez, C.C. Monounsaturated Fatty Acids Are Substrates for Aldehyde Generation in Tellurite-Exposed Escherichia coli. BioMed Res. Int. 2013, 2013, 563756. [Google Scholar] [CrossRef]
- Chang, Y.; Cronan, J.E. Membrane Cyclopropane Fatty Acid Content Is a Major Factor in Acid Resistance of Escherichia coli. Mol. Microbiol. 1999, 33, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.-G.; Marshall, D.L. Adaptation of Escherichia coli O157:H7 to pH Alters Membrane Lipid Composition, Verotoxin Secretion, and Resistance to Simulated Gastric Fluid Acid. Appl. Environ. Microbiol. 2004, 70, 3500–3505. [Google Scholar] [CrossRef]
- Russell, N.J. Cold Adaptation of Microorganisms. Philos. Trans. R. Soc. Lond. B 1990, 326, 595–611. [Google Scholar] [CrossRef]
- Shigapova, N.; Török, Z.; Balogh, G.; Goloubinoff, P.; Vígh, L.; Horváth, I. Membrane Fluidization Triggers Membrane Remodeling Which Affects the Thermotolerance in Escherichia coli. Biochem. Biophys. Res. Commun. 2005, 328, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L.; Clarke, N.D. Biochemistry, 5th ed.; Freeman: New York, NY, USA, 2003; ISBN 978-0-7167-3051-4. [Google Scholar]
- Scala, J.; Williams, H.H. A Comparison of Selenite and Tellurite Toxicity in Escherichia coli. Arch. Biochem. Biophys. 1963, 101, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Scala, J.; Williams, H.H. The Enhancement of Selenite Toxicity by Methionine in Escherichia coli. Arch. Biochem. Biophys. 1962, 99, 363–368. [Google Scholar] [CrossRef]
- Uchida, K. Histidine and Lysine as Targets of Oxidative Modification. Amino Acids 2003, 25, 249–257. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 2012; ISBN 978-1-936113-42-2. [Google Scholar]
- Muñoz-Diaz, P.; Jiménez, K.; Luraschi, R.; Cornejo, F.; Figueroa, M.; Vera, C.; Rivas-Pardo, A.; Sandoval, J.M.; Vásquez, C.; Arenas, F. Anaerobic RSH-Dependent Tellurite Reduction Contributes to Escherichia coli Tolerance against Tellurite. Biol. Res. 2022, 55, 13. [Google Scholar] [CrossRef]
- Sprouffske, K.; Wagner, A. Growthcurver: An R package for obtaining interpretable metrics from microbial growth curves. BMC Bioinform. 2016, 17, 172. [Google Scholar] [CrossRef]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 In-frame, Single-gene Knockout Mutants: The Keio Collection. Mol. Syst. Biol. 2006, 2, 2006.0008. [Google Scholar] [CrossRef]
- Typas, A.; Nichols, R.J.; Siegele, D.A.; Shales, M.; Collins, S.R.; Lim, B.; Braberg, H.; Yamamoto, N.; Takeuchi, R.; Wanner, B.L.; et al. High-Throughput, Quantitative Analyses of Genetic Interactions in E. coli. Nat. Methods 2008, 5, 781–787. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and Integrative Analysis of Large Gene Lists Using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. CP Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
- Otaru, N.; Ye, K.; Mujezinovic, D.; Berchtold, L.; Constancias, F.; Cornejo, F.A.; Krzystek, A.; de Wouters, T.; Braegger, C.; Lacroix, C.; et al. GABA Production by Human Intestinal Bacteroides spp.: Prevalence, Regulation, and Role in Acid Stress Tolerance. Front Microbiol. 2021, 12, 656895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Compounds | KEGG IDs | Aerobic | Anaerobic | Metabolic Pathway | ||
|---|---|---|---|---|---|---|
| log2(FC) | log10(p) | log2(FC) | log10(p) | |||
| 5-phosphoribosylamine | C03090 | 2.659 | 1.854 | 1.071 | 1.865 | Biosynthesis of 5-aminoimidazole ribonucleotides I and II |
| Iminoaspartic acid | C05840 | −1.273 | 1.438 | 1.107 | 1.564 | NAD+ de novo biosynthesis I (from aspartate) |
| L-1-phosphatidylethanolamine | C00350 | −2.609 | 1.329 | 1.679 | 1.253 | Biosynthesis of cardiolipin III, PS and PE |
| L-arginine | C00062 | −2.164 | 1.507 | −1.120 | 1.116 | Degradation of L-arginine III, biosynthesis of putrescine |
| 5-semialdehyde N-succinyl-L-glutamate | C05932 | −2.965 | 1.204 | −1.226 | 1.440 | Degradation of L-arginine II |
| PG(i-13:0/i-21:0) | n/a | −1.918 | 2.045 | −2.811 | 1.392 | Lipid metabolism |
| PS(22:2(13Z,16Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | n/a | 7.438 | 2.266 | −3.277 | 1.147 | Lipid metabolism |
| Compound | KEGG IDs | log2(FC) | log10(p) | Metabolic Pathway | ||
|---|---|---|---|---|---|---|
| Aerobic | Negative Enrichment | PG(16:0/20:3(8Z,11Z,14Z)) | n/a | −2.149 | 1.693 | Lipid metabolism |
| PG(i-13:0/i-19:0) | n/a | −6.983 | 1.612 | Lipid metabolism | ||
| PGP(22:6(4Z,7Z,10Z,13Z,16, 19Z)/20:2(11Z,14Z)) | n/a | −1.668 | 1.526 | Lipid metabolism | ||
| L-tryptophan | C00078 | −1.164 | 1.124 | Biosynthesis and degradation II (pyruvate pathway) of L-tryptophan | ||
| S-lactoylglutathione | C03451 | −1.295 | 3.112 | Degradation I of methylglyoxal | ||
| Adenosine phosphosulfate | C00224 | −1.573 | 2.850 | Sulfate activation for sulfonation | ||
| Acetyl-CoA | C00024 | −4.385 | 2.208 | Krebs cycle I, biosynthesis of fatty acids, L-cysteine and L-leucine | ||
| Positive Enrichment | PE-NMe(16:1(9Z)/18:0) | n/a | 2.771 | 2.289 | Lipid metabolism | |
| PE-NMe2(14:0/16:1(9Z)) | n/a | 7.343 | 1.711 | Lipid metabolism | ||
| PE-NMe2(16:1(9Z)/16:1(9Z)) | n/a | 6.034 | 1.214 | Lipid metabolism | ||
| PS(18:0/20:3(8Z,11Z,14Z)) | n/a | 2.557 | 1.870 | Lipid metabolism | ||
| PS(22:5(7Z,10Z,13Z,16Z,19Z)/20:2(11Z,14Z)) | n/a | 1.251 | 1.146 | Lipid metabolism | ||
| Anaerobic | Negative Enrichment | PA(20:5(5Z,8Z,11Z,14Z,17Z)/24:1(15Z)) | n/a | −3.192 | 2.158 | Lipid metabolism |
| PA(24:1(15Z)/18:0) | n/a | −1.694 | 2.195 | Lipid metabolism | ||
| PG(a-13:0/i-24:0) | n/a | −2.014 | 1.543 | Lipid metabolism | ||
| PS(22:4(7Z,10Z,13Z,16Z)/22:5(7Z,10Z,13Z,16Z,19Z)) | n/a | −3.655 | 1.743 | Lipid metabolism | ||
| 2′-deoxycytidine 5′-monophosphate (dCMP) | C00239 | −1.860 | 1.100 | Phosphorylation of pyrimidine deoxyribonucleotides | ||
| Positive Enrichment | glycerol 2-phosphate | C02979 | 1.000 | 1.162 | Glycerol and phosphate precursor | |
| PA(20:4(5Z,8Z,11Z,14Z)/24:1(15Z)) | n/a | 1.679 | 1.173 | Lipid metabolism | ||
| PA(22:4(7Z,10Z,13Z,16Z)/20:0) | n/a | 1.786 | 1.754 | Lipid metabolism | ||
| PE(18:4(6Z,9Z,12Z,15Z)/P-18:1(9Z)) | n/a | 2.158 | 1.147 | Lipid metabolism | ||
| PG(a-13:0/i-22:0) | n/a | 1011 | 1155 | Lipid metabolism | ||
| L-dihydroorotic acid | C00337 | 1.497 | 1.746 | Biosynthesis of UMP I |
| Amino Acid | Aerobiosis | Anaerobiosis | ||||
|---|---|---|---|---|---|---|
| µM/mg Protein | Control | Tellurite | p | Control | Tellurite | p |
| Alanine | 457.8 ± 48.1 | 687.6 ± 49.2 | ns | 1229.9 ± 147.6 | 1223.8 ± 211.2 | ns |
| Asparagine | 171.8 ± 6.3 | 16.2 ± 3.1 | ns | 90.4 ± 36.7 | 63.1 ± 12.3 | ns |
| Aspartic Acid | 4503.7 ± 188.6 | 440.2 ± 54.7 | *** | 1793.2 ± 764.4 | 2111.3 ± 526.7 | ns |
| Glutamic Acid | 1201.5 ± 44.5 | 1278.5 ± 151.1 | ns | 2607.7 ± 292.7 | 2257.7 ± 63.2 | ns |
| Glutamine | 47.7 ± 10.0 | 15.5 ± 3.0 | ns | 180.8 ± 10.2 | 105.1 ± 23.8 | ** |
| Glycine | 156.2 ± 4.1 | 63.0 ± 5.1 | **** | 128.5 ± 8.4 | 127.6 ± 3.9 | ns |
| Histidine | 22.2 ± 0.7 | 10.4 ± 2.1 | ns | 22.8 ± 7.3 | 14.5 ± 4.4 | ns |
| Isoleucine | 5.7 ± 2.0 | 2.3 ± 0.8 | * | 7.0 ± 1.7 | 7.5 ± 0.5 | ns |
| Lysine | 188.3 ± 5.1 | 364.9 ± 104.2 | * | 175.8 ± 16.1 | 146.5 ± 40.3 | ns |
| Methionine | 9.5 ± 4.4 | 16.0 ± 0.5 | * | 11.9 ± 1.7 | 12.6 ± 0.4 | ns |
| Phenylalanine/Leucine | 18.4 ± 3.0 | 28.3 ± 0.6 | * | 19.0 ± 5.7 | 16.8 ± 3.1 | ns |
| Proline | 4.7 ± 2.0 | 9.2 ± 1.1 | ns | 49.8 ± 2.6 | 67.4 ± 11.0 | * |
| Serine | 21.3 ± 1.4 | 10.8 ± 1.7 | *** | 9.0 ± 0.8 | 8.6 ± 0.9 | ns |
| Threonine | 500.4 ± 66.7 | 36.0 ± 4.8 | **** | 114.3 ± 14.0 | 96.1 ± 16.2 | ns |
| Tyrosine | 28.7 ± 9.4 | 99.2 ± 2.8 | ** | 58.0 ± 8.0 | 61.0 ± 61.0 | ns |
| Valine | 15.1 ± 0.1 | 21.4 ± 2.1 | * | 15.0 ± 2.5 | 18.5 ± 1.8 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luraschi, R.; Muñoz-Villagrán, C.; Cornejo, F.A.; Pugin, B.; Tobar, F.C.; Sandoval, J.M.; Rivas-Pardo, J.A.; Vera, C.; Arenas, F. Characterization of Tellurite Toxicity to Escherichia coli Under Aerobic and Anaerobic Conditions. Int. J. Mol. Sci. 2025, 26, 7287. https://doi.org/10.3390/ijms26157287
Luraschi R, Muñoz-Villagrán C, Cornejo FA, Pugin B, Tobar FC, Sandoval JM, Rivas-Pardo JA, Vera C, Arenas F. Characterization of Tellurite Toxicity to Escherichia coli Under Aerobic and Anaerobic Conditions. International Journal of Molecular Sciences. 2025; 26(15):7287. https://doi.org/10.3390/ijms26157287
Chicago/Turabian StyleLuraschi, Roberto, Claudia Muñoz-Villagrán, Fabián A. Cornejo, Benoit Pugin, Fernanda Contreras Tobar, Juan Marcelo Sandoval, Jaime Andrés Rivas-Pardo, Carlos Vera, and Felipe Arenas. 2025. "Characterization of Tellurite Toxicity to Escherichia coli Under Aerobic and Anaerobic Conditions" International Journal of Molecular Sciences 26, no. 15: 7287. https://doi.org/10.3390/ijms26157287
APA StyleLuraschi, R., Muñoz-Villagrán, C., Cornejo, F. A., Pugin, B., Tobar, F. C., Sandoval, J. M., Rivas-Pardo, J. A., Vera, C., & Arenas, F. (2025). Characterization of Tellurite Toxicity to Escherichia coli Under Aerobic and Anaerobic Conditions. International Journal of Molecular Sciences, 26(15), 7287. https://doi.org/10.3390/ijms26157287






