Regulation of Subcellular Protein Synthesis for Restoring Neural Connectivity
Abstract
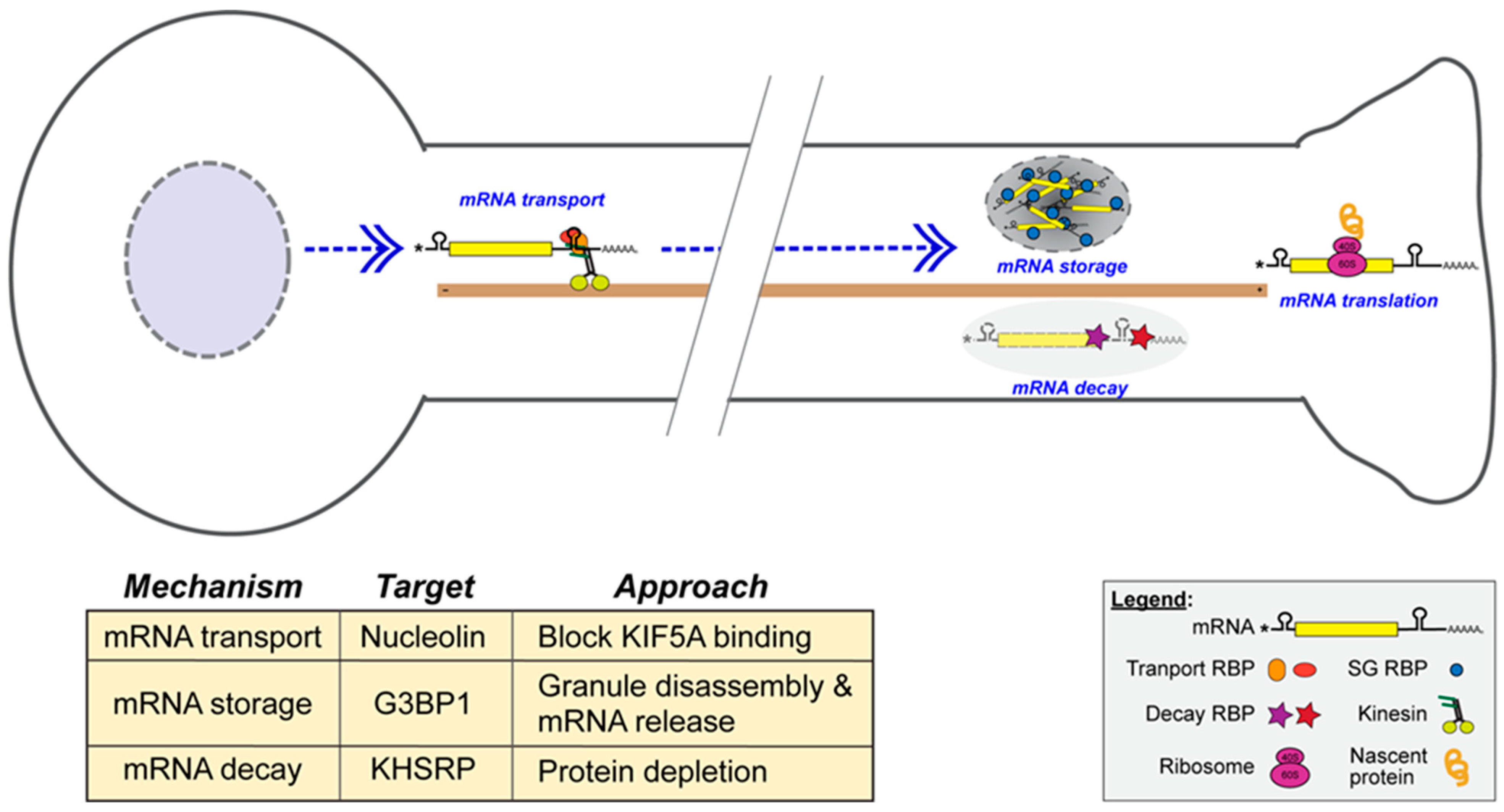
1. Introduction
2. Subcellular Synthesis of Neuronal Proteins
2.1. Functions of Locally Synthesized Proteins
| Function | Locally Synthesized Neuronal Proteins | Description |
|---|---|---|
| Synaptic plasticity in dendrites | ARC, CaMKIIα, PDS-95 | Allows for rapid modification of synaptic strength and plasticity, critical for memory formation and consolidation [7,8,9,10,11,35,36]. |
| Dendrite growth | Palmitoyl- and Prenyl-CDC42 isoforms | Promotes dendrite growth and spine maturation (Palm-CDC42) and branching (Prenyl-CDC42) [22,37] |
| Axonal growth and guidance | β-Actin, γ-Actin, Cofilin, RhoA, GAP43, Prenyl-CDC42 isoform, HMGB1, NRN1 | Drive growth cone dynamics, regulate axonal branching, and guide directional axon growth by enabling rapid, spatially restricted synthesis of cytoskeletal and signaling proteins [22,34,38,39] |
| Axonal injury response and retrograde signaling | Importin B1 (Kpnb1), RanBP1, Vimentin, mTOR, CALR, CREB3 | Rapidly synthesized after injury to initiate local regeneration processes, growth cone formation, retrograde signaling, and stress responses critical for neuronal survival and recovery [20,40,41,42,43]. |
| Axon survival | Bclw | Loss of Bclw mRNA transport into axons in neuropathy triggers axon degeneration [44,45] |
| Retrograde transport | LIS1 | Modulates activity of retrograde motor protein dynein [46] |
| mRNA stability | KHSRP | RNA-binding proteins can regulate mRNA transport, stability, storage, and translation, enabling compartment-specific control of proteins [47] |
| Nuclear transcriptional regulation | CREB, LUMAN/CREB3, STAT3, ATF4, HMGN5 | Allows for axonal synthesis of factors involved in nuclear transcriptional regulation with pro-growth or neurodegenerative outcomes [48,49,50,51,52]. |
| Vesicle, protein, and membrane trafficking | BIP (GRP78), CALR, SNAP25, TC10 | Facilitates local membrane repair, secretion pathways, and vesicle trafficking critical for axonal maintenance, growth, and injury responses [43,53,54,55,56,57] |
| Mitochondrial function and transport | Lamin B2, COXIV, COXVIIC, PINK1 | Ensures local mitochondrial biogenesis, energy metabolism, and transport within axons [58,59,60] |
2.2. RNA–Protein Interactions Are a Key Determinant for Where and When Individual mRNAs Are Translated in Axons and Dendrites
2.3. Axonal mRNA Transcriptomes
2.4. Ribonucleoprotein Complexes (RNPs) as Regulatory Platforms for RNA Transport, Stability, and Translational Regulation
2.5. Translational Regulation of Axonal mRNAs
| Stimulus | Effects on Translation | Target mRNA Examples | Mechanism and Outcome(s) [References] |
|---|---|---|---|
| Axotomy | Rapid Ca2+-dependent translation activation | Kpnb1, Calr, Luman/Creb3, mTor | Initiates retrograde signaling, supports growth cone formation, promotes further axonal mRNA translation [42,43,51,116] |
| mTOR activation | Stimulates CAP-dependent protein synthesis under normo-calcemic conditions | Csnk2a1, Nrn1 | Phosphorylates eIF4E, 4EBP1/2, and RP S6 [42,92,117] |
| Axon pathfinding/guidance cues | Receptor-based signaling | Rhoa, Actb, Cofilin | Signaling cascades converge on translation factors, with sequestration of mRNAs and translational machinery near receptors [118,119,120,121,122] |
| Neurotransmitter (Glutamate) | Unknown, presumably ionic alterations | unknown | [123] |
| Amyloid β peptide | Converges on intrinsic stress response (ISR) pathway for eIF2α phosphorylation | Atf4 | Induces neurodegeneration linked to Alzheimer’s disease [49] |
2.6. Approaches to Modifying the Axonal Translatome to Increase Axon Regeneration
3. Summary and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schuman, E.M. mRNA trafficking and local protein synthesis at the synapse. Neuron 1999, V23, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Steward, O. Translating axon guidance cues. Cell 2002, 110, 537–540. [Google Scholar] [CrossRef]
- Smith, T.P.; Sahoo, P.K.; Kar, A.N.; Twiss, J.L. Intra-axonal mechanisms driving axon regeneration. Brain Res. 2020, 1740, 146864. [Google Scholar] [CrossRef]
- Dalla Costa, I.; Buchanan, C.N.; Zdradzinski, M.D.; Sahoo, P.K.; Smith, T.P.; Thames, E.; Kar, A.N.; Twiss, J.L. The functional organization of axonal mRNA transport and translation. Nat. Rev. Neurosci. 2021, 22, 77–91. [Google Scholar] [CrossRef]
- Steward, O. Polyribosomes at the base of dendritic spines of central nervous system neurons--their possible role in synapse construction and modification. Cold Spring Harb. Symp. Quant. Biol. 1983, 48, 745–759. [Google Scholar] [CrossRef]
- Schuman, E.M.; Dynes, J.L.; Steward, O. Synaptic regulation of translation of dendritic mRNAs. J. Neurosci. 2006, 26, 7143–7146. [Google Scholar] [CrossRef]
- Steward, O.; Worley, P.F. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron 2001, 30, 227–240. [Google Scholar] [CrossRef]
- Farris, S.; Lewandowski, G.; Cox, C.D.; Steward, O. Selective localization of arc mRNA in dendrites involves activity- and translation-dependent mRNA degradation. J. Neurosci. 2014, 34, 4481–4493. [Google Scholar] [CrossRef]
- Huang, F.; Chotiner, J.K.; Steward, O. Actin polymerization and ERK phosphorylation are required for Arc/Arg3.1 mRNA targeting to activated synaptic sites on dendrites. J. Neurosci. 2007, 27, 9054–9067. [Google Scholar] [CrossRef]
- McCurry, C.L.; Shepherd, J.D.; Tropea, D.; Wang, K.H.; Bear, M.F.; Sur, M. Loss of Arc renders the visual cortex impervious to the effects of sensory experience or deprivation. Nat. Neurosci. 2010, 13, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chuang, Y.A.; Na, Y.; Ye, Z.; Yang, L.; Lin, R.; Zhou, J.; Wu, J.; Qiu, J.; Savonenko, A.; et al. Arc oligomerization is regulated by CaMKII phosphorylation of the GAG domain: An essential mechanism for plasticity and memory formation. Mol. Cell 2019, 75, 13–25.E5. [Google Scholar] [CrossRef] [PubMed]
- Koenig, E. Synthetic mechanisms in the axon—I: Local axonal synthesis of acetylcholinesterase. J. Neurochem. 1965, 12, 343–355. [Google Scholar] [CrossRef]
- Koenig, E. Synthetic mechanisms in the axon—II: RNA in myelin-free axons of the cat. J. Neurochem. 1965, 12, 357–361. [Google Scholar] [CrossRef]
- Koenig, E. Synthetic mechanisms in the axon—IV: In Vitro incorporation of [3H] precursors into axonal protein and RNA. J. Neurochem. 1967, 14, 437–446. [Google Scholar] [CrossRef]
- Koenig, E. Synthetic mechanisms in the axon—III: Stimulation of acetylcholinesterase synthesis by actinomycin-D in the hypoglossal nerve. J. Neurochem. 1967, 14, 429–435. [Google Scholar] [CrossRef]
- Tobias, G.; Koenig, E. Axonal protein synthesizing activity during the early outgrowth period following neurotomy. Exp. Neurol. 1975, 49, 221–234. [Google Scholar] [CrossRef]
- Zheng, J.Q.; Kelly, T.K.; Chang, B.; Ryazantsev, S.; Rajasekaran, A.K.; Martin, K.C.; Twiss, J.L. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J. Neurosci. 2001, 21, 9291–9303. [Google Scholar] [CrossRef]
- Bassell, G.J.; Zhang, H.; Byrd, A.L.; Femino, A.M.; Singer, R.H.; Taneja, K.L.; Lifshitz, L.M.; Herman, I.M.; Kosik, K.S. Sorting of beta-actin mRNA and protein to neurites and growth cones in culture. J. Neurosci. 1998, 18, 251–265. [Google Scholar] [CrossRef]
- Campbell, D.S.; Holt, C.E. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron 2001, 32, 1013–1026. [Google Scholar] [CrossRef]
- Hanz, S.; Perlson, E.; Willis, D.; Zheng, J.Q.; Massarwa, R.; Huerta, J.J.; Koltzenburg, M.; Kohler, M.; van-Minnen, J.; Twiss, J.L.; et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 2003, 40, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.E. Biological roles of local protein synthesis in axons: A journey of discovery. Annu. Rev. Genet. 2024, 58, 1–8. [Google Scholar] [CrossRef]
- Lee, S.J.; Zdradzinski, M.D.; Sahoo, P.K.; Kar, A.N.; Patel, P.; Kawaguchi, R.; Aguilar, B.J.; Lantz, K.D.; McCain, C.R.; Coppola, G.; et al. Selective axonal translation of the mRNA isoform encoding prenylated Cdc42 supports axon growth. J. Cell Sci. 2021, 134, jcs251967. [Google Scholar] [CrossRef]
- Poulopoulos, A.; Murphy, A.J.; Ozkan, A.; Davis, P.; Hatch, J.; Kirchner, R.; Macklis, J.D. Subcellular transcriptomes and proteomes of developing axon projections in the cerebral cortex. Nature 2019, 565, 356–360. [Google Scholar] [CrossRef]
- Cajigas, I.J.; Tushev, G.; Will, T.J.; tom Dieck, S.; Fuerst, N.; Schuman, E.M. The local transcriptome in the synaptic neuropil revealed by deep sequencing and high-resolution imaging. Neuron 2012, 74, 453–466. [Google Scholar] [CrossRef]
- Gumy, L.F.; Yeo, G.S.; Tung, Y.C.; Zivraj, K.H.; Willis, D.; Coppola, G.; Lam, B.Y.; Twiss, J.L.; Holt, C.E.; Fawcett, J.W. Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA 2011, 17, 85–98. [Google Scholar] [CrossRef]
- Shigeoka, T.; Jung, H.; Jung, J.; Turner-Bridger, B.; Ohk, J.; Lin, J.Q.; Amieux, P.S.; Holt, C.E. Dynamic axonal translation in developing and mature visual circuits. Cell 2016, 166, 181–192. [Google Scholar] [CrossRef]
- Briese, M.; Saal, L.; Appenzeller, S.; Moradi, M.; Baluapuri, A.; Sendtner, M. Whole transcriptome profiling reveals the RNA content of motor axons. Nucleic Acids Res. 2016, 44, e33. [Google Scholar] [CrossRef]
- Bigler, R.L.; Kamande, J.W.; Dumitru, R.; Niedringhaus, M.; Taylor, A.M. Messenger RNAs localized to distal projections of human stem cell derived neurons. Sci. Rep. 2017, 7, 611. [Google Scholar] [CrossRef]
- Minis, A.; Dahary, D.; Manor, O.; Leshkowitz, D.; Pilpel, Y.; Yaron, A. Subcellular transcriptomics-dissection of the mRNA composition in the axonal compartment of sensory neurons. Dev. Neurobiol. 2014, 74, 365–381. [Google Scholar] [CrossRef]
- Merianda, T.T.; Lin, A.C.; Lam, J.S.; Vuppalanchi, D.; Willis, D.E.; Karin, N.; Holt, C.E.; Twiss, J.L. A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol. Cell. Neurosci. 2009, 40, 128–142. [Google Scholar] [CrossRef]
- Gonzalez, C.; Canovas, J.; Fresno, J.; Couve, E.; Court, F.A.; Couve, A. Axons provide the secretory machinery for trafficking of voltage-gated sodium channels in peripheral nerve. Proc. Natl. Acad. Sci. USA 2016, 113, 1823–1828. [Google Scholar] [CrossRef]
- Kar, A.; Lee, S.; Twiss, J. Expanding axonal transcriptome brings new functions for axonally synthesized proteins in health and disease. Neurosci. 2018, 24, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.J.; Park, M.; Spillane, M.; Yoo, S.; Pacheco, A.; Gomes, C.; Vuppalanchi, D.; McDonald, M.; Kim, H.H.; Merianda, T.T.; et al. Axonally synthesized beta-actin and GAP-43 proteins support distinct modes of axonal growth. J. Neurosci. 2013, 33, 3311–3322. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Sivadasan, R.; Saal, L.; Luningschror, P.; Dombert, B.; Rathod, R.J.; Dieterich, D.C.; Blum, R.; Sendtner, M. Differential roles of alpha-, beta-, and gamma-actin in axon growth and collateral branch formation in motoneurons. J. Cell Biol. 2017, 216, 793–814. [Google Scholar] [CrossRef]
- Muddashetty, R.S.; Kelic, S.; Gross, C.; Xu, M.; Bassell, G.J. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J. Neurosci. 2007, 27, 5338–5348. [Google Scholar] [CrossRef]
- Muddashetty, R.S.; Nalavadi, V.C.; Gross, C.; Yao, X.; Xing, L.; Laur, O.; Warren, S.T.; Bassell, G.J. Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling. Mol. Cell 2011, 42, 673–688. [Google Scholar] [CrossRef]
- Yap, K.; Xiao, Y.; Friedman, B.A.; Je, H.S.; Makeyev, E.V. Polarizing the neuron Through sustained co-expression of alternatively spliced isoforms. Cell Rep. 2016, 15, 1316–1328. [Google Scholar] [CrossRef]
- Merianda, T.T.; Coleman, J.; Kim, H.H.; Kumar Sahoo, P.; Gomes, C.; Brito-Vargas, P.; Rauvala, H.; Blesch, A.; Yoo, S.; Twiss, J.L. Axonal amphoterin mRNA is regulated by translational control and enhances axon outgrowth. J. Neurosci. 2015, 35, 5693–5706. [Google Scholar] [CrossRef] [PubMed]
- Merianda, T.T.; Gomes, C.; Yoo, S.; Vuppalanchi, D.; Twiss, J.L. Axonal localization of neuritin/CPG15 mRNA in neuronal populations through distinct 5′ and 3′ UTR elements. J. Neurosci. 2013, 33, 13735–13742. [Google Scholar] [CrossRef]
- Yudin, D.; Hanz, S.; Yoo, S.; Iavnilovitch, E.; Willis, D.; Gradus, T.; Vuppalanchi, D.; Segal-Ruder, Y.; Ben-Yaakov, K.; Hieda, M.; et al. Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve. Neuron 2008, 59, 241–252. [Google Scholar] [CrossRef]
- Perlson, E.; Michaelevski, I.; Kowalsman, N.; Ben-Yaakov, K.; Shaked, M.; Seger, R.; Eisenstein, M.; Fainzilber, M. Vimentin binding to phosphorylated Erk sterically hinders enzymatic dephosphorylation of the kinase. J. Mol. Biol. 2006, 364, 938–944. [Google Scholar] [CrossRef]
- Terenzio, M.; Koley, S.; Samra, N.; Rishal, I.; Zhao, Q.; Sahoo, P.K.; Urisman, A.; Marvaldi, L.; Oses-Prieto, J.A.; Forester, C.; et al. Locally translated mTOR controls axonal local translation in nerve injury. Science 2018, 359, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.; Merianda, T.T.; Twiss, J.L.; Gallo, G. Mechanism and role of the intra-axonal Calreticulin translation in response to axonal injury. Exp. Neurol. 2020, 323, 113072. [Google Scholar] [CrossRef] [PubMed]
- Pease-Raissi, S.E.; Pazyra-Murphy, M.F.; Li, Y.; Wachter, F.; Fukuda, Y.; Fenstermacher, S.J.; Barclay, L.A.; Bird, G.H.; Walensky, L.D.; Segal, R.A. Paclitaxel reduces axonal Bclw to initiate IP3R1-dependent axon degeneration. Neuron 2017, 96, 373–386.E6. [Google Scholar] [CrossRef]
- Cosker, K.E.; Fenstermacher, S.J.; Pazyra-Murphy, M.F.; Elliott, H.L.; Segal, R.A. The RNA-binding protein SFPQ orchestrates an RNA regulon to promote axon viability. Nat. Neurosci. 2016, 19, 690–696. [Google Scholar] [CrossRef]
- Villarin, J.M.; McCurdy, E.P.; Martinez, J.C.; Hengst, U. Local synthesis of dynein cofactors matches retrograde transport to acutely changing demands. Nat. Commun. 2016, 7, 13865. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Buchanan, C.N.; Zdradzinski, M.D.; Sahoo, P.K.; Kar, A.N.; Lee, S.J.; Urisman, A.; Oses-Prieto, J.A.; Dell’Orco, M.; Cassiday, D.E.; et al. Intra-axonal translation of Khsrp mRNA slows axon regeneration by destabilizing localized mRNAs. Nucleic Acids Res. 2022, 50, 5772–5792. [Google Scholar] [CrossRef]
- Ben-Yaakov, K.; Dagan, S.Y.; Segal-Ruder, Y.; Shalem, O.; Vuppalanchi, D.; Willis, D.E.; Yudin, D.; Rishal, I.; Rother, F.; Bader, M.; et al. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J. 2012, 31, 1350–1363. [Google Scholar] [CrossRef]
- Baleriola, J.; Walker, C.A.; Jean, Y.Y.; Crary, J.F.; Troy, C.M.; Nagy, P.L.; Hengst, U. Axonally synthesized ATF4 transmits a neurodegenerative signal across brain regions. Cell 2014, 158, 1159–1172. [Google Scholar] [CrossRef]
- Ying, Z.; Misra, V.; Verge, V.M. Sensing nerve injury at the axonal ER: Activated Luman/CREB3 serves as a novel axonally synthesized retrograde regeneration signal. Proc. Natl. Acad. Sci. USA 2014, 111, 16142–16147. [Google Scholar] [CrossRef]
- Ying, Z.; Zhai, R.; McLean, N.A.; Johnston, J.M.; Misra, V.; Verge, V.M. The unfolded protein response and cholesterol biosynthesis link Luman/CREB3 to regenerative axon growth in sensory neurons. J. Neurosci. 2015, 35, 14557–14570. [Google Scholar] [CrossRef]
- Moretti, F.; Rolando, C.; Winker, M.; Ivanek, R.; Rodriguez, J.; Von Kriegsheim, A.; Taylor, V.; Bustin, M.; Pertz, O. Growth Cone Localization of the mRNA Encoding the Chromatin Regulator HMGN5 Modulates Neurite Outgrowth. Mol. Cell. Biol. 2015, 35, 2035–2050. [Google Scholar] [CrossRef]
- Vuppalanchi, D.; Merianda, T.T.; Donnelly, C.; Pacheco, A.; Williams, G.; Yoo, S.; Ratan, R.R.; Willis, D.E.; Twiss, J.L. Lysophosphatidic acid differentially regulates axonal mRNA translation through 5′UTR elements. Mol. Cell. Neurosci. 2012, 50, 136–146. [Google Scholar] [CrossRef]
- Gracias, N.G.; Shirkey-Son, N.J.; Hengst, U. Local translation of TC10 is required for membrane expansion during axon outgrowth. Nat. Commun. 2014, 5, 3506. [Google Scholar] [CrossRef] [PubMed]
- Aschrafi, A.; Natera-Naranjo, O.; Gioio, A.E.; Kaplan, B.B. Regulation of axonal trafficking of cytochrome c oxidase IV mRNA. Mol. Cell. Neurosci. 2010, 43, 422–430. [Google Scholar] [CrossRef]
- Aschrafi, A.; Schwechter, A.D.; Mameza, M.G.; Natera-Naranjo, O.; Gioio, A.E.; Kaplan, B.B. MicroRNA-338 regulates local cytochrome c oxidase IV mRNA levels and oxidative phosphorylation in the axons of sympathetic neurons. J. Neurosci. 2008, 28, 12581–12590. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.F.R.; Martinez, J.C.; Hengst, U. Intra-axonal synthesis of SNAP25 is required for the formation of presynaptic terminals. Cell Rep. 2017, 20, 3085–3098. [Google Scholar] [CrossRef]
- Harbauer, A.B.; Hees, J.T.; Wanderoy, S.; Segura, I.; Gibbs, W.; Cheng, Y.; Ordonez, M.; Cai, Z.; Cartoni, R.; Ashrafi, G.; et al. Neuronal mitochondria transport Pink1 mRNA via synaptojanin 2 to support local mitophagy. Neuron 2022, 110, 1516–1531.E9. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.; Altman, T.; Golani-Armon, A.; Savulescu, A.F.; Ibraheem, A.; Mhlanga, M.M.; Perlson, E.; Arava, Y.S. Co-transport of the nuclear-encoded Cox7c mRNA with mitochondria along axons occurs through a coding-region-dependent mechanism. J. Cell Sci. 2022, 135, jcs259436. [Google Scholar] [CrossRef]
- Yoon, B.C.; Jung, H.; Dwivedy, A.; O’Hare, C.M.; Zivraj, K.H.; Holt, C.E. Local translation of extranuclear lamin B promotes axon maintenance. Cell 2012, 148, 752–764. [Google Scholar] [CrossRef]
- Eom, T.; Antar, L.N.; Singer, R.H.; Bassell, G.J. Localization of a beta-actin messenger ribonucleoprotein complex with zipcode-binding protein modulates the density of dendritic filopodia and filopodial synapses. J. Neurosci. 2003, 23, 10433–10444. [Google Scholar] [CrossRef]
- Ross, A.F.; Oleynikov, Y.; Kislauskis, E.H.; Taneja, K.L.; Singer, R.H. Characterization of a beta-actin mRNA zipcode-binding protein. Mol. Cell. Biol. 1997, 17, 2158–2165. [Google Scholar] [CrossRef]
- Huttelmaier, S.; Zenklusen, D.; Lederer, M.; Dictenberg, J.; Lorenz, M.; Meng, X.; Bassell, G.J.; Condeelis, J.; Singer, R.H. Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature 2005, 438, 512–515. [Google Scholar] [CrossRef]
- Vuppalanchi, D.; Coleman, J.; Yoo, S.; Merianda, T.T.; Yadhati, A.G.; Hossain, J.; Blesch, A.; Willis, D.E.; Twiss, J.L. Conserved 3′-untranslated region sequences direct subcellular localization of chaperone protein mRNAs in neurons. J. Biol. Chem. 2010, 285, 18025–18038. [Google Scholar] [CrossRef]
- Pacheco, A.; Twiss, J.L. Localized IRES-dependent translation of ER chaperone protein mRNA in sensory axons. PLoS ONE 2012, 7, e40788. [Google Scholar] [CrossRef]
- Hachet, O.; Ephrussi, A. Splicing of oskar RNA in the nucleus is coupled to its cytoplasmic localization. Nature 2004, 428, 959–963. [Google Scholar] [CrossRef]
- Kilchert, C.; Spang, A. Cotranslational transport of ABP140 mRNA to the distal pole of S. cerevisiae. EMBO J. 2011, 30, 3567–3580. [Google Scholar] [CrossRef]
- Mendonsa, S.; von Kugelgen, N.; Dantsuji, S.; Ron, M.; Breimann, L.; Baranovskii, A.; Lodige, I.; Kirchner, M.; Fischer, M.; Zerna, N.; et al. Massively parallel identification of mRNA localization elements in primary cortical neurons. Nat. Neurosci. 2023, 26, 394–405. [Google Scholar] [CrossRef]
- Haseltine, W.A.; Hazel, K.; Patarca, R. RNA Structure: Past, Future, and Gene Therapy Applications. Int. J. Mol. Sci. 2024, 26, 110. [Google Scholar] [CrossRef]
- Bolognani, F.; Contente-Cuomo, T.; Perrone-Bizzozero, N.I. Novel recognition motifs and biological functions of the RNA-binding protein HuD revealed by genome-wide identification of its targets. Nucleic Acids Res. 2010, 38, 117–130. [Google Scholar] [CrossRef]
- Olguin, S.L.; Patel, P.; Dell’Orco, M.; Buchanan, C.N.; Gardiner, A.S.; Cole, R.; Sundarajan, A.; Mudge, J.; Allan, A.M.; Ortinski, P.; et al. The RNA binding protein KHSRP attenuates axonal and dendritic growth, synaptic transmission, and memory consolidation via dysregulation of neuronal gene expression. Commun. Biol. 2022, 5, 672. [Google Scholar] [CrossRef]
- Martinez, J.C.; Randolph, L.K.; Iascone, D.M.; Pernice, H.F.; Polleux, F.; Hengst, U. Pum2 Shapes the Transcriptome in Developing Axons through Retention of Target mRNAs in the Cell Body. Neuron 2019, 104, 931–946. [Google Scholar] [CrossRef]
- Zdradzinksi, M.; Vaughan, L.; Buchanan, C.; Trumbull, K.; Perrone-Bizzero, N.; Lu, Q.; Larsen, J.; Twiss, J. KHSRP-mediated Decay of Axonally Localized Prenyl-Cdc42 mRNA Slows Nerve Regeneration. bioRxiv 2025, preprint. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Twiss, J.L. Profiling Locally Translated mRNAs in Regenerating Axons. Methods Mol. Biol. 2023, 2636, 145–161. [Google Scholar] [CrossRef]
- Doron-Mandel, E.; Alber, S.; Oses, J.A.; Medzihradszky, K.F.; Burlingame, A.L.; Fainzilber, M.; Twiss, J.L.; Lee, S.J. Isolation and analyses of axonal ribonucleoprotein complexes. Methods Cell Biol. 2016, 131, 467–486. [Google Scholar] [CrossRef]
- Alber, S.; Di Matteo, P.; Zdradzinski, M.D.; Dalla Costa, I.; Medzihradszky, K.F.; Kawaguchi, R.; Di Pizio, A.; Freund, P.; Panayotis, N.; Marvaldi, L.; et al. PTBP1 regulates injury responses and sensory pathways in adult peripheral neurons. Sci. Adv. 2023, 9, eadi0286. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Oses-Prieto, J.A.; Kawaguchi, R.; Sahoo, P.K.; Kar, A.N.; Rozenbaum, M.; Oliver, D.; Chand, S.; Ji, H.; Shtutman, M.; et al. hnRNPs Interacting with mRNA Localization Motifs Define Axonal RNA Regulons. Mol. Cell. Proteom. MCP 2018, 17, 2091–2106. [Google Scholar] [CrossRef] [PubMed]
- Keene, J.D. RNA regulons: Coordination of post-transcriptional events. Nat. Rev. Genet. 2007, 8, 533–543. [Google Scholar] [CrossRef]
- Kwon, S.; Chin, K.; Nederlof, M.; Gray, J.W. Quantitative, in situ analysis of mRNAs and proteins with subcellular resolution. Sci. Rep. 2017, 7, 16459. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Mittal, S.; Kadamberi, I.P.; Pradeep, S.; Chaluvally-Raghavan, P. Optimized proximity ligation assay (PLA) for detection of RNA-protein complex interactions in cell lines. STAR Protoc. 2022, 3, 101340. [Google Scholar] [CrossRef]
- Guo, P.; Deng, Y. Spatial Omics: Navigating neuroscience research into the new era. Adv. Neurobiol. 2024, 41, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, E.; Chartrand, P.; Schaefer, M.; Shenoy, S.M.; Singer, R.H.; Long, R.M. Localization of ASH1 RNA particles in living yeast. Mol. Cell 1998, 2, 437–445. [Google Scholar] [CrossRef]
- Chao, J.A.; Patskovsky, Y.; Almo, S.C.; Singer, R.H. Structural basis for the coevolution of a viral RNA-protein complex. Nat. Struct. Mol. Biol. 2008, 15, 103–105. [Google Scholar] [CrossRef]
- Yan, X.; Hoek, T.A.; Vale, R.D.; Tanenbaum, M.E. Dynamics of translation of single mRNA molecules In Vivo. Cell 2016, 165, 976–989. [Google Scholar] [CrossRef]
- Moon, H.C.; Park, H.Y. Imaging Single mRNA dynamics in live neurons and brains. Methods Enzymol. 2016, 572, 51–64. [Google Scholar] [CrossRef]
- Ogawa, Y.; Lim, B.C.; George, S.; Oses-Prieto, J.A.; Rasband, J.M.; Eshed-Eisenbach, Y.; Hamdan, H.; Nair, S.; Boato, F.; Peles, E.; et al. Antibody-directed extracellular proximity biotinylation reveals that Contactin-1 regulates axo-axonic innervation of axon initial segments. Nat. Commun. 2023, 14, 6797. [Google Scholar] [CrossRef] [PubMed]
- Strack, R.L.; Jaffrey, S.R. Live-cell imaging of mammalian RNAs with Spinach2. Methods Enzymol. 2015, 550, 129–146. [Google Scholar] [CrossRef]
- Trachman, R.J.; Ferre-D’Amare, A.R. Tracking RNA with light: Selection, structure, and design of fluorescence turn-on RNA aptamers. Q. Rev. Biophys. 2019, 52, e8. [Google Scholar] [CrossRef]
- Bauer, K.E.; de Queiroz, B.R.; Kiebler, M.A.; Besse, F. RNA granules in neuronal plasticity and disease. Trends Neurosci. 2023, 46, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.C.; Fernandopulle, M.S.; Wang, G.; Choi, H.; Hao, L.; Drerup, C.M.; Patel, R.; Qamar, S.; Nixon-Abell, J.; Shen, Y.; et al. RNA granules hitchhike on lysosomes for long-distance transport, using Annexin A11 as a molecular tether. Cell 2019, 179, 147–164. [Google Scholar] [CrossRef]
- Sahoo, P.; Hanovice, N.; Ward, P.; Agrawal, M.; Smith, T.P.; Dulin, J.; Tuszynski, M.; Vaughn, L.S.; Welshhans, K.; Benowitz, L.; et al. Disruption of G3BP1 granules promotes CNS axon regeneration. Proc. Natl. Acad. Sci. USA 2025, 122, e2411811122. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.; Kar, A.; Samra, N.; Terenzio, M.; Patell, P.; Lee, S.; Miller, S.; Thames, E.; Jones, B.; Kawaguchi, R.; et al. A translational switch drives axonal stress granule disassembly through Casein Kinase 2α. Curr. Biol. CB 2020, 30, E4886. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.K.; Lee, S.J.; Jaiswal, P.B.; Alber, S.; Kar, A.N.; Miller-Randolph, S.; Thames, E.; Taylor, E.E.; Smith, T.; Singh, B.; et al. Axonal G3BP1 stress granule protein limits axonal mRNA translation and nerve regeneration. Nat. Commun. 2018, 9, 3358. [Google Scholar] [CrossRef]
- Taha, M.S.; Ahmadian, M.R. Fragile X Messenger Ribonucleoprotein Protein and Its Multifunctionality: From Cytosol to Nucleolus and Back. Biomolecules 2024, 14, 399. [Google Scholar] [CrossRef] [PubMed]
- Briata, P.; Bordo, D.; Puppo, M.; Gorlero, F.; Rossi, M.; Perrone-Bizzozero, N.; Gherzi, R. Diverse roles of the nucleic acid-binding protein KHSRP in cell differentiation and disease. Wiley Interdiscip. Rev. RNA 2016, 7, 227–240. [Google Scholar] [CrossRef]
- Kiebler, M.A.; Bauer, K.E. RNA granules in flux: Dynamics to balance physiology and pathology. Nat. Rev. Neurosci. 2024, 25, 711–725. [Google Scholar] [CrossRef]
- Hentze, M.W.; Castello, A.; Schwarzl, T.; Preiss, T. A brave new world of RNA-binding proteins. Nat. Rev. Mol. Cell Biol. 2018, 19, 327–341. [Google Scholar] [CrossRef]
- Varadi, M.; Zsolyomi, F.; Guharoy, M.; Tompa, P. Functional advantages of conserved intrinsic disorder in RNA-binding proteins. PLoS ONE 2015, 10, e0139731. [Google Scholar] [CrossRef]
- Yang, P.; Mathieu, C.; Kolaitis, R.M.; Zhang, P.; Messing, J.; Yurtsever, U.; Yang, Z.; Wu, J.; Li, Y.; Pan, Q.; et al. G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell 2020, 181, 325–345. [Google Scholar] [CrossRef]
- Gal, J.; Chen, J.; Na, D.Y.; Tichacek, L.; Barnett, K.R.; Zhu, H. The Acetylation of Lysine-376 of G3BP1 Regulates RNA Binding and Stress Granule Dynamics. Mol. Cell. Biol. 2019, 39, e00052-19. [Google Scholar] [CrossRef]
- Altman, T.; Ionescu, A.; Ibraheem, A.; Priesmann, D.; Gradus-Pery, T.; Farberov, L.; Alexandra, G.; Shelestovich, N.; Dafinca, R.; Shomron, N.; et al. Axonal TDP-43 condensates drive neuromuscular junction disruption through inhibition of local synthesis of nuclear encoded mitochondrial proteins. Nat. Commun. 2021, 12, 6914. [Google Scholar] [CrossRef]
- Verde, E.M.; Secco, V.; Ghezzi, A.; Mandrioli, J.; Carra, S. Molecular mechanisms of protein aggregation in ALS-FTD: Focus on TDP-43 and cellular protective responses. Cells 2025, 14, 680. [Google Scholar] [CrossRef]
- Kshirsagar, A.; Doroshev, S.M.; Gorelik, A.; Olender, T.; Sapir, T.; Tsuboi, D.; Rosenhek-Goldian, I.; Malitsky, S.; Itkin, M.; Argoetti, A.; et al. LIS1 RNA-binding orchestrates the mechanosensitive properties of embryonic stem cells in AGO2-dependent and independent ways. Nat. Commun. 2023, 14, 3293. [Google Scholar] [CrossRef]
- Wegmann, S. Liquid-liquid phase separation of tau protein in neurobiology and pathology. Adv. Exp. Med. Biol. 2019, 1184, 341–357. [Google Scholar] [CrossRef]
- Schaeffer, J.; Vilallongue, N.; Decourt, C.; Blot, B.; El Bakdouri, N.; Plissonnier, E.; Excoffier, B.; Paccard, A.; Diaz, J.J.; Humbert, S.; et al. Customization of the translational complex regulates mRNA-specific translation to control CNS regeneration. Neuron 2023, 111, 2881–2898. [Google Scholar] [CrossRef]
- Yadav, M.; Harding, R.J.; Li, T.; Xu, X.; Gall-Duncan, T.; Khan, M.; Bardile, C.F.; Sequiera, G.L.; Duan, S.; Chandrasekaran, R.; et al. Huntingtin is an RNA binding protein and participates in NEAT1-mediated paraspeckles. Sci. Adv. 2024, 10, eado5264. [Google Scholar] [CrossRef]
- Rishal, I.; Michaelevski, I.; Rozenbaum, M.; Shinder, V.; Medzihradszky, K.F.; Burlingame, A.L.; Fainzilber, M. Axoplasm isolation from peripheral nerve. Dev. Neurobiol. 2010, 70, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.J.; Willis, D.E.; Xu, M.; Tep, C.; Jiang, C.; Yoo, S.; Schanen, N.C.; Kirn-Safran, C.B.; van Minnen, J.; English, A.; et al. Limited availability of ZBP1 restricts axonal mRNA localization and nerve regeneration capacity. EMBO J. 2011, 30, 4665–4677. [Google Scholar] [CrossRef] [PubMed]
- Willis, D.E.; van Niekerk, E.A.; Sasaki, Y.; Mesngon, M.; Merianda, T.T.; Williams, G.G.; Kendall, M.; Smith, D.S.; Bassell, G.J.; Twiss, J.L. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J. Cell Biol. 2007, 178, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.B.; Rishal, I.; Doron-Mandel, E.; Kalinski, A.L.; Medzihradszky, K.F.; Terenzio, M.; Alber, S.; Koley, S.; Lin, A.; Rozenbaum, M.; et al. Nucleolin-Mediated RNA Localization Regulates Neuron Growth and Cycling Cell Size. Cell Rep. 2016, 16, 1664–1676. [Google Scholar] [CrossRef]
- Spaulding, E.L.; Burgess, R.W. Accumulating Evidence for Axonal Translation in Neuronal Homeostasis. Front. Neurosci. 2017, 11, 312. [Google Scholar] [CrossRef]
- Onate, M.; Catenaccio, A.; Martinez, G.; Armentano, D.; Parsons, G.; Kerr, B.; Hetz, C.; Court, F.A. Activation of the unfolded protein response promotes axonal regeneration after peripheral nerve injury. Sci. Rep. 2016, 6, 21709. [Google Scholar] [CrossRef]
- Harding, H.P.; Calfon, M.; Urano, F.; Novoa, I.; Ron, D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu. Rev. Cell Dev. Biol. 2002, 18, 575–599. [Google Scholar] [CrossRef]
- Cagnetta, R.; Wong, H.H.; Frese, C.K.; Mallucci, G.R.; Krijgsveld, J.; Holt, C.E. Noncanonical Modulation of the eIF2 Pathway Controls an Increase in Local Translation during Neural Wiring. Mol. Cell 2019, 73, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Cagnetta, R.; Flanagan, J.G.; Sonenberg, N. Control of Selective mRNA Translation in Neuronal Subcellular Compartments in Health and Disease. J. Neurosci. 2023, 43, 7247–7263. [Google Scholar] [CrossRef]
- Perry, R.B.; Doron-Mandel, E.; Iavnilovitch, E.; Rishal, I.; Dagan, S.Y.; Tsoory, M.; Coppola, G.; McDonald, M.K.; Gomes, C.; Geschwind, D.H.; et al. Subcellular knockout of importin beta1 perturbs axonal retrograde signaling. Neuron 2012, 75, 294–305. [Google Scholar] [CrossRef]
- Kye, M.J.; Niederst, E.D.; Wertz, M.H.; Goncalves Ido, C.; Akten, B.; Dover, K.Z.; Peters, M.; Riessland, M.; Neveu, P.; Wirth, B.; et al. SMN regulates axonal local translation via miR-183/mTOR pathway. Hum. Mol. Genet. 2014, 23, 6318–6331. [Google Scholar] [CrossRef] [PubMed]
- Welshhans, K.; Bassell, G.J. Netrin-1-induced local beta-actin synthesis and growth cone guidance requires zipcode binding protein 1. J. Neurosci. 2011, 31, 9800–9813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Eom, T.; Oleynikov, Y.; Shenoy, S.M.; Liebelt, D.A.; Dictenberg, J.B.; Singer, R.H.; Bassell, G.J. Neurotrophin-induced transport of a beta-actin mRNP complex increases beta-actin levels and stimulates growth cone motility. Neuron 2001, 31, 261–275. [Google Scholar] [CrossRef]
- Walker, B.A.; Ji, S.J.; Jaffrey, S.R. Intra-axonal translation of RhoA promotes axon growth inhibition by CSPG. J. Neurosci. 2012, 32, 14442–14447. [Google Scholar] [CrossRef]
- Wu, K.Y.; Hengst, U.; Cox, L.J.; Macosko, E.Z.; Jeromin, A.; Urquhart, E.R.; Jaffrey, S.R. Local translation of RhoA regulates growth cone collapse. Nature 2005, 436, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Piper, M.; Anderson, R.; Dwivedy, A.; Weinl, C.; van Horck, F.; Leung, K.M.; Cogill, E.; Holt, C. Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron 2006, 49, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.; HW, C.; Wu, C.; Wu, H.; Lee, Y.; Chen, E.; Fang, W.; Chang, Y. Glutamate Stimulates Local Protein Synthesis in the Axons of Rat Cortical Neurons by Activating α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptors and Metabotropic Glutamate Receptors. J. Biol. Chem. 2015, 290, 20748–20760. [Google Scholar] [CrossRef]
- Doron-Mandel, E.; Koppel, I.; Abraham, O.; Rishal, I.; Smith, T.P.; Buchanan, C.N.; Sahoo, P.K.; Kadlec, J.; Oses-Prieto, J.A.; Kawaguchi, R.; et al. The glycine arginine-rich domain of the RNA-binding protein nucleolin regulates its subcellular localization. EMBO J. 2021, 40, e107158. [Google Scholar] [CrossRef]
- Rishal, I.; Kam, N.; Perry, R.B.; Shinder, V.; Fisher, E.M.; Schiavo, G.; Fainzilber, M. A motor-driven mechanism for cell-length sensing. Cell Rep. 2012, 1, 608–616. [Google Scholar] [CrossRef]
- Albus, C.A.; Rishal, I.; Fainzilber, M. Cell length sensing for neuronal growth control. Trends Cell Biol. 2013, 23, 305–310. [Google Scholar] [CrossRef]
- Rotem, N.; Magen, I.; Ionescu, A.; Gershoni-Emek, N.; Altman, T.; Costa, C.J.; Gradus, T.; Pasmanik-Chor, M.; Willis, D.E.; Ben-Dov, I.Z.; et al. ALS Along the Axons—Expression of Coding and Noncoding RNA Differs in Axons of ALS models. Sci. Rep. 2017, 7, 44500. [Google Scholar] [CrossRef] [PubMed]
- Briese, M.; Saal-Bauernschubert, L.; Luningschror, P.; Moradi, M.; Dombert, B.; Surrey, V.; Appenzeller, S.; Deng, C.; Jablonka, S.; Sendtner, M. Loss of Tdp-43 disrupts the axonal transcriptome of motoneurons accompanied by impaired axonal translation and mitochondria function. Acta Neuropathol. Commun. 2020, 8, 116. [Google Scholar] [CrossRef]
- Price, T.J.; Inyang, K.E. Commonalities between pain and memory mechanisms and their meaning for understanding chronic pain. Prog. Mol. Biol. Transl. Sci. 2015, 131, 409–434. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Twiss, J.L.; Buchanan, C.N. Regulation of Subcellular Protein Synthesis for Restoring Neural Connectivity. Int. J. Mol. Sci. 2025, 26, 7283. https://doi.org/10.3390/ijms26157283
Twiss JL, Buchanan CN. Regulation of Subcellular Protein Synthesis for Restoring Neural Connectivity. International Journal of Molecular Sciences. 2025; 26(15):7283. https://doi.org/10.3390/ijms26157283
Chicago/Turabian StyleTwiss, Jeffery L., and Courtney N. Buchanan. 2025. "Regulation of Subcellular Protein Synthesis for Restoring Neural Connectivity" International Journal of Molecular Sciences 26, no. 15: 7283. https://doi.org/10.3390/ijms26157283
APA StyleTwiss, J. L., & Buchanan, C. N. (2025). Regulation of Subcellular Protein Synthesis for Restoring Neural Connectivity. International Journal of Molecular Sciences, 26(15), 7283. https://doi.org/10.3390/ijms26157283





