Fighting HER2 in Gastric Cancer: Current Approaches and Future Landscapes
Abstract
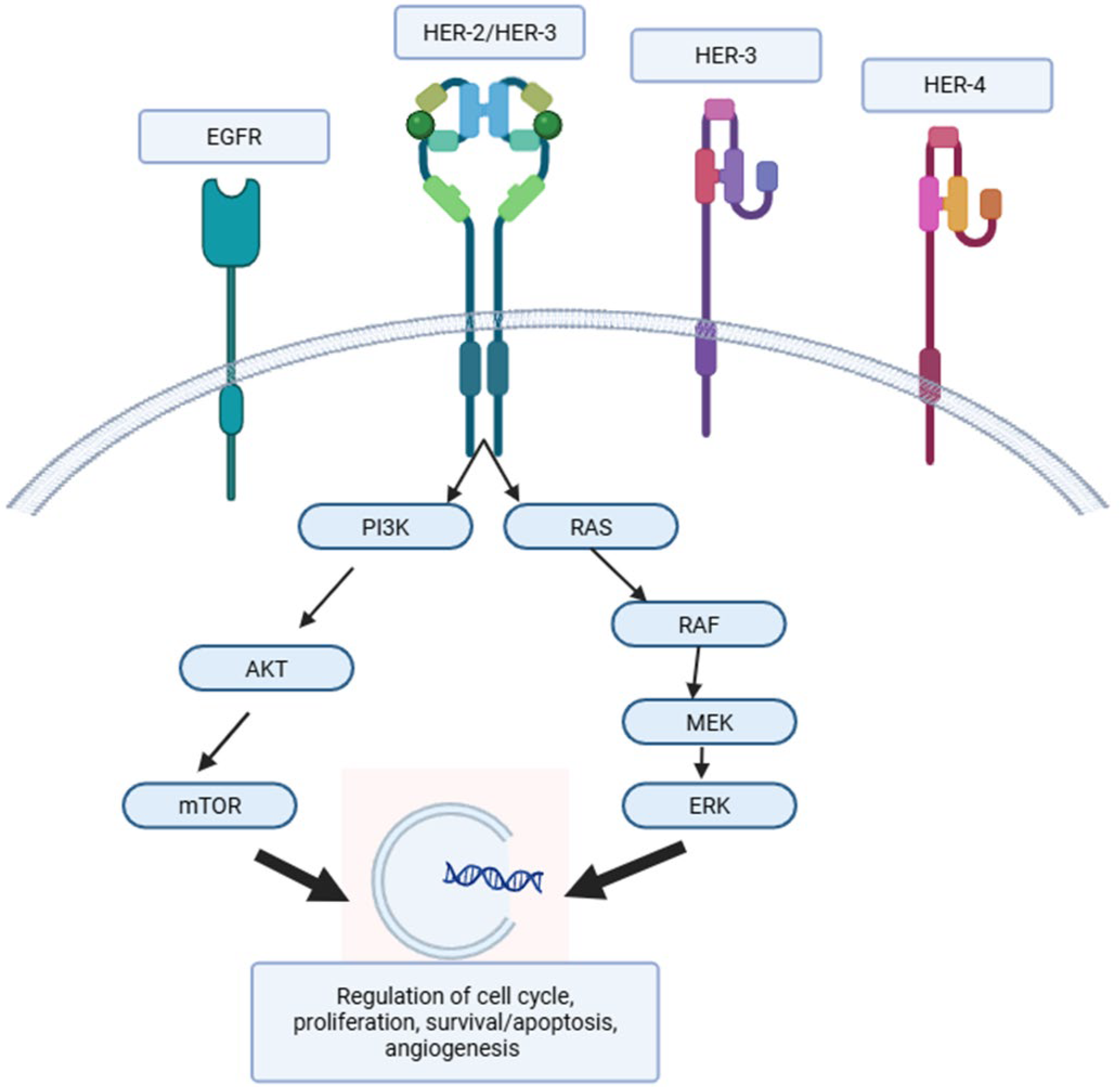
1. Introduction
2. Classification of Anti-HER 2 Agents
3. Targeting HER2 in Perioperative and Neoadjuvant Settings
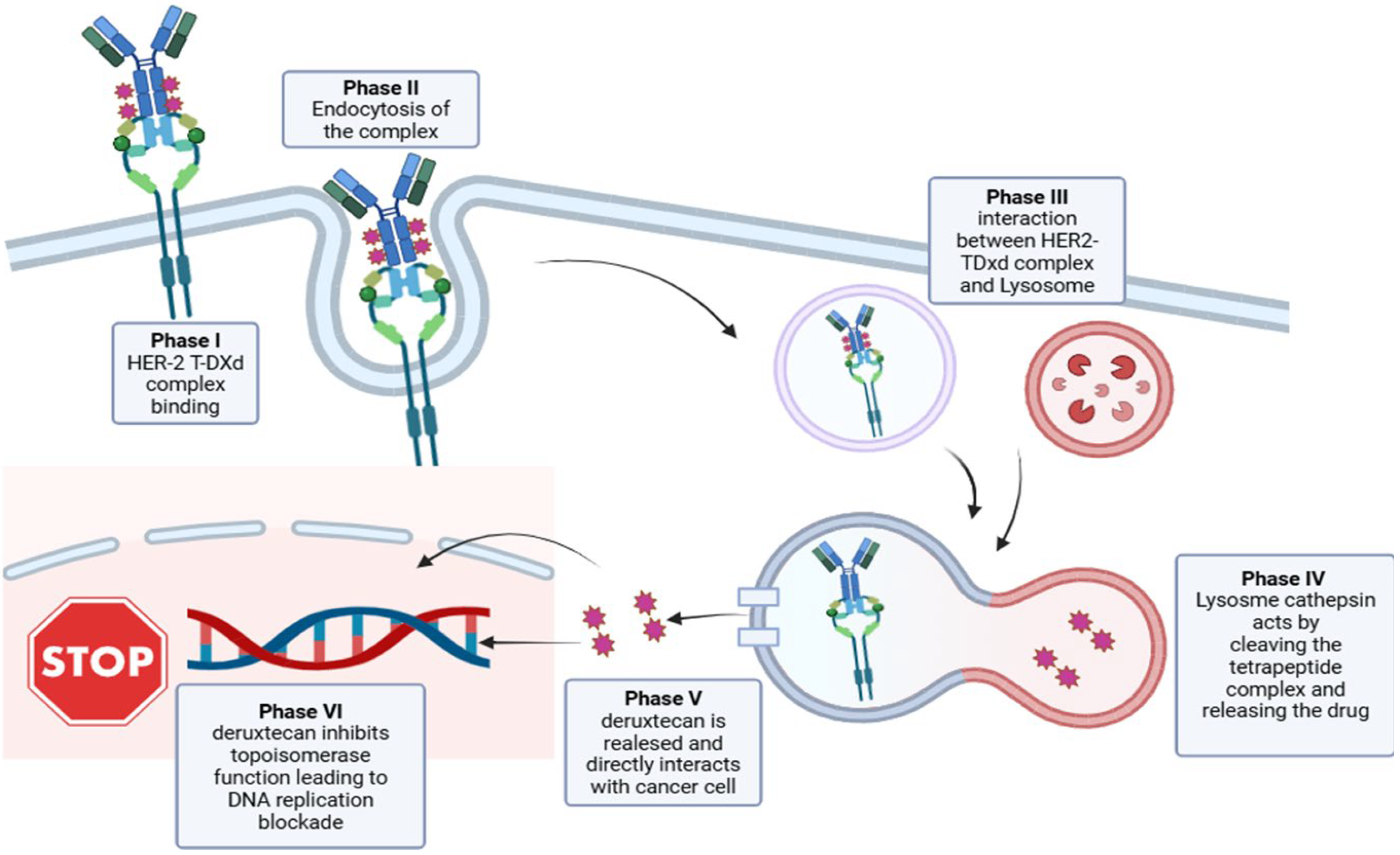
4. Targeting HER2 in Advanced Disease
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| ADCC | Antibody-dependent cell-mediated cytotoxicity |
| ADC | Antibody–drug conjugate |
| BsAb | Bispecific antibody |
| CAR-T | Chimeric antigen receptor T (cells) |
| CNS | Central nervous system |
| CROSS | Paclitaxel and carboplatin with radiotherapy |
| DFS | Disease-free survival |
| EGFR | Epidermal growth factor receptor |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| FLOT | 5-fluorouracil, leucovorin, oxaliplatin, docetaxel |
| FOLFOX | 5-fluorouracil, leucovorin, oxaliplatin |
| GC | Gastric cancer |
| GEJ | Gastroesophageal junction |
| HER2 | Human epidermal growth factor receptor 2 |
| ISAC | Immune-stimulating antibody conjugate |
| mAb | Monoclonal antibody |
| ORR | Objective response rate |
| OS | Overall survival |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death ligand 1 |
| PFS | Progression-free survival |
| PR | Partial response |
| SD | Stable disease |
| T-DXd | Trastuzumab deruxtecan |
| TKI | Tyrosine kinase inhibitor |
| VEGF | Vascular endothelial growth factor |
| XELOX | Capecitabine, oxaliplatin |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Huang, Q.; He, A.; Wu, X.; Lin, X.; Zhang, Y.; Sun, Y.; Zhang, H. The Global Cancer Burden of Gastric Cancer and Its Impact on Survival: An Epidemiological Study. Eur. J. Cancer 2022, 58, 98–107. [Google Scholar] [CrossRef]
- Ilson, D.H. Advances in the treatment of gastric cancer: 2020-2021. Curr. Opin. Gastroenterol. 2021, 37, 615–618. [Google Scholar] [CrossRef]
- De Vita, F.; Di Martino, N.; Fabozzi, A.; Laterza, M.M.; Ventriglia, J.; Savastano, B.; Petrillo, A.; Gambardella, V.; Sforza, V.; Marano, L.; et al. Clinical management of advanced gastric cancer: The role of new molecular drugs. World J. Gastroenterol. 2014, 20, 14537–14558. [Google Scholar] [CrossRef]
- Sexton, R.E.; Al Hallak, M.N.; Diab, M.; Azmi, A.S. Gastric cancer: A comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020, 39, 1179–1203. [Google Scholar] [CrossRef]
- Wei, P.; Zhang, X.; Du, X.; Yang, Q. Chemotherapy in Advanced Gastric Cancer: A Review. Crit. Rev. Oncol. Hematol. 2020, 148, 102896. [Google Scholar] [CrossRef]
- Huang, Q.; Li, Y. Surgical Approaches to Gastric Cancer. J. Gastrointest. Oncol. 2018, 9, 654–660. [Google Scholar] [CrossRef]
- Hsu, J.T.; Lin, Y.N.; Chen, Y.F.; Kou, H.W.; Wang, S.Y.; Chou, W.C.; Wu, T.R.; Yeh, T.S. A comprehensive overview of gastric cancer management from a surgical point of view. Biomed. J. 2024, 100817. [Google Scholar] [CrossRef]
- Chen, J.; Bu, Z.; Ji, J. Surgical treatment of gastric cancer: Current status and future directions. Chin. J. Cancer Res. 2021, 33, 159–167. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Li, M.; Zhou, Y. Chemotherapy for Gastric Cancer: Clinical Strategies and Efficacy. Ann. Transl. Med. 2014, 2, 114. [Google Scholar] [CrossRef]
- Huang, F.; Wang, M.; Wang, Z.; Li, C.; Zhang, Y. The Effectiveness of Radiotherapy in Gastric Cancer Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 682–690. [Google Scholar] [CrossRef][Green Version]
- Yang, H.; Ai, H.; Zhang, J.; Ma, J.; Liu, K.; Li, Z. UPS: Opportunities and challenges for gastric cancer treatment. Front. Oncol. 2023, 13, 1140452. [Google Scholar] [CrossRef]
- Meng, X.; Yang, J.; Zhou, Y.; Lu, Y.; Wang, C. New Developments in Targeted Therapy for Gastric Cancer. Int. J. Cancer 2020, 147, 28–38. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, Y.; Zhou, Y.; Shi, Y. Targeted Therapy in Gastric Cancer: A Review. Mol. Clin. Oncol. 2018, 9, 553–560. [Google Scholar] [CrossRef]
- Körfer, J.; Lordick, F.; Hacker, U.T. Molecular Targets for Gastric Cancer Treatment and Future Perspectives from a Clinical and Translational Point of View. Cancers 2021, 13, 5216. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in Combination with Chemotherapy for HER2-Positive Gastric Cancer: A Randomized Study. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamanaka, T.; Esaki, T.; Tsuji, A.; Doi, T.; Naito, Y.; Shimoyama, T. Advances in Anti-HER2 Therapeutics for Gastric Cancer. Oncol. Rep. 2021, 45, 1329–1339. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Wei, Z.; Song, J.; Zha, X.; Wang, D.; Xu, M. Advancements and challenges in immunotherapy for gastric cancer: Current approaches and future directions. Front. Immunol. 2025, 16, 1592733. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Z. Recent advances in the progress of immune checkpoint inhibitors in the treatment of advanced gastric cancer: A review. Front. Oncol. 2022, 12, 934249. [Google Scholar] [CrossRef]
- Baselga, J.; Albanell, J. Mechanism of Action of Anti-HER2 Monoclonal Antibodies. Ann. Oncol. 2001, 12 (Suppl. S1), S35–S41. [Google Scholar] [CrossRef]
- Junttila, T.T.; Akita, R.W.; Parsons, K.; Fields, C.; Lewis Phillips, G.D.; Friedman, L.S.; Sampath, D.; Sliwkowski, M.X. Ligand-Independent HER2/HER3/PI3K Complex Is Disrupted by Trastuzumab and Is Effectively Inhibited by the PI3K Inhibitor GDC-0941. Cancer Cell 2009, 15, 429–440. [Google Scholar] [CrossRef]
- Park, S.; Jiang, Z.; Mortenson, E.D.; Deng, L.; Radkevich-Brown, O.; Yang, X.; Sattar, H.; Wang, Y.; Brown, N.K.; Greene, M.; et al. The Therapeutic Effect of Anti-HER2/neu Antibody Depends on Both Innate and Adaptive Immunity. Cancer Cell 2010, 18, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Morii, N.; Yamashiro, H. Pertuzumab in the Treatment of HER2-Positive Breast Cancer: An Evidence-Based Review of Its Safety, Efficacy, and Place in Therapy. Core Evid. 2019, 14, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Lee-Hoeflich, S.T.; Crocker, L.; Yao, E.; Pham, T.; Munroe, X.; Hoeflich, K.P.; Sliwkowski, M.X.; Stern, H.M. A Central Role for HER3 in HER2-Amplified Breast Cancer: Implications for Targeted Therapy. Cancer Res. 2008, 68, 5878–5887. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, S.; Cinci, M.; Hasmann, M.; Fehring, V.; Kirschfink, M. Lipoplex Mediated Silencing of Membrane Regulators (CD46, CD55 and CD59) Enhances Complement-Dependent Anti-Tumor Activity of Trastuzumab and Pertuzumab. Mol. Oncol. 2013, 7, 580–594. [Google Scholar] [CrossRef]
- Schroeder, R.L.; Stevens, C.L.; Sridhar, J. Small Molecule Tyrosine Kinase Inhibitors of ErbB2/HER2/Neu in the Treatment of Aggressive Breast Cancer. Molecules 2014, 19, 15196–15212. [Google Scholar] [CrossRef]
- Schlam, I.; Nunes, R.; Lynce, F. Profile of Margetuximab: Evidence to Date in the Targeted Treatment of Metastatic HER2-Positive Breast Cancer. OncoTargets Ther. 2022, 15, 471–478. [Google Scholar] [CrossRef]
- Spector, N.; Xia, W.; El-Hariry, I.; Yarden, Y.; Bacus, S. HER2 Therapy: Small Molecule HER-2 Tyrosine Kinase Inhibitors. Breast Cancer Res. 2007, 9, 205. [Google Scholar] [CrossRef]
- Scaltriti, M.; Verma, C.; Guzman, M.; Jimenez, J.; Parra, J.L.; Pedersen, K.; Smith, D.J.; Landolfi, S.; Ramon y Cajal, S.; Arribas, J.; et al. Lapatinib, a HER2 Tyrosine Kinase Inhibitor, Induces Stabilization and Accumulation of HER2 and Potentiates Trastuzumab-Dependent Cell Cytotoxicity. Oncogene 2009, 28, 803–814. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, S.; Shi, Y. Tyrosine Kinase Inhibitors for Solid Tumors in the Past 20 Years (2001–2020). J. Hematol. Oncol. 2020, 13, 143. [Google Scholar] [CrossRef]
- Saura, C.; Oliveira, M.; Feng, Y.H.; Dai, M.S.; Chen, S.W.; Hurvitz, S.A.; Kim, S.B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated with ≥2 HER2-Directed Regimens: Phase III NALA Trial. J. Clin. Oncol. 2020, 38, 3138–3149. [Google Scholar] [CrossRef]
- Lin, N.U.; Murthy, R.K.; Abramson, V.; Anders, C.; Bachelot, T.; Bedard, P.L.; Borges, V.; Cameron, D.; Carey, L.A.; Chien, A.J.; et al. Tucatinib vs. Placebo, Both in Combination with Trastuzumab and Capecitabine, for Previously Treated ERBB2 (HER2)-Positive Metastatic Breast Cancer in Patients with Brain Metastases: Updated Exploratory Analysis of the HER2CLIMB Randomized Clinical Trial. JAMA Oncol. 2023, 9, 197–205. [Google Scholar] [CrossRef]
- Rassy, E.; Rached, L.; Pistilli, B. Antibody drug conjugates targeting HER2: Clinical development in metastatic breast cancer. Breast 2022, 66, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Hamilton, E.P.; Beeram, M.; Hanna, D.L.; El-Khoueiry, A.B.; Kang, Y.-K.; Lee, K.W.; Lee, J.; Rha, S.Y.; Chaves, J.M. Zanidatamab (ZW25) in HER2-Expressing Gastroesophageal Adenocarcinoma (GEA): Results from a Phase I Study. J. Clin. Oncol. 2021, 39 (Suppl. S3), 164. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Kawazoe, A.; Yañez, P.; Li, N.; Lonardi, S.; Kolesnik, O.; Barajas, O.; Bai, Y.; Shen, L.; Tang, Y.; et al. The KEYNOTE-811 Trial of Dual PD-1 and HER2 Blockade in HER2-Positive Gastric Cancer. Nature 2021, 600, 727–730. [Google Scholar] [CrossRef]
- Tabernero, J.; Shen, L.; Elimova, E.; Ku, G.; Liu, T.; Shitara, K.; Lin, X.; Boyken, L.; Li, H.; Grim, J. HERIZON-GEA-01: Zanidatamab+Chemo±Tislelizumab for 1L Treatment of HER2-Positive Gastroesophageal Adenocarcinoma. Fut. Oncol. 2022, 18, 3255–3266. [Google Scholar] [CrossRef]
- Xu, J.; Ying, J.; Liu, R.; Wu, J.; Ye, F.; Xu, N.; Zhang, Y.; Zhao, R.; Xiang, X.; Wang, J.; et al. KN026 (Anti-HER2 Bispecific Antibody) in Patients with Previously Treated, Advanced HER2-Expressing Gastric or Gastroesophageal Junction Cancer. Eur. J. Cancer 2023, 178, 1–12. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Ychou, M.; Boige, V.; Pignon, J.-P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.-M.; Saint-Aubert, B.; et al. Perioperative Chemotherapy Compared with Surgery Alone for Resectable Gastroesophageal Adenocarcinoma: An FNCLCC and FFCD Multicenter Phase III Trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative Chemotherapy with Fluorouracil Plus Leucovorin, Oxaliplatin, and Docetaxel versus Fluorouracil or Capecitabine Plus Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Randomised, Phase 2/3 Trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef]
- ESMO Guidelines. Available online: https://www.esmo.org/guidelines (accessed on 15 May 2025).
- Marx, A.H.; Tharun, L.; Muth, J.; Dancau, A.M.; Simon, R.; Yekebas, E.; Kaifi, J.T.; Mirlacher, M.; Brümmendorf, T.H.; Bokemeyer, C.; et al. HER-2 amplification is highly homogenous in gastric cancer. Hum. Pathol. 2009, 40, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Gravalos, C.; Jimeno, A. HER2 in gastric cancer: A new prognostic factor and a novel therapeutic target. Ann. Oncol. 2008, 19, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.Y.; Huang, J.Y.; Zhao, Q.R.; Jiang, N.; Xu, H.M.; Wang, Z.N.; Li, H.Q.; Zhang, S.B.; Sun, Z. The clinicopathological parameters and prognostic significance of HER2 expression in gastric cancer patients: A meta-analysis of literature. World J. Surg. Oncol. 2017, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Allgayer, H.; Babic, R.; Gruetzner, K.U.; Tarabichi, A.; Schildberg, F.W.; Heiss, M.M. C-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J. Clin. Oncol. 2000, 18, 2201–2209. [Google Scholar] [CrossRef]
- Begnami, M.D.; Fukuda, E.; Fregnani, J.H.; Nonogaki, S.; Montagnini, A.L.; da Costa, W.L.; Soares, F.A. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J. Clin. Oncol. 2011, 29, 3030–3036. [Google Scholar] [CrossRef]
- Grabsch, H.; Sivakumar, S.; Gray, S.; Gabbert, H.E.; Müller, W. HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value—Conclusions from 924 cases of two independent series. Cell Oncol. 2010, 32, 57–65. [Google Scholar] [CrossRef]
- Okines, A.F.; Thompson, L.C.; Cunningham, D.; Wotherspoon, A.; Reis-Filho, J.S.; Langley, R.E.; Waddell, T.S.; Noor, D.; Eltahir, Z.; Wong, R.; et al. Effect of HER2 on prognosis and benefit from peri-operative chemotherapy in early oesophago-gastric adenocarcinoma in the MAGIC trial. Ann. Oncol. 2013, 24, 1253–1261. [Google Scholar] [CrossRef]
- Gianni, L.; Eiermann, W.; Semiglazov, V.; Manikhas, A.; Lluch, A.; Tjulandin, S.; Zambetti, M.; Vazquez, F.; Byakhow, M.; Lichinitser, M.; et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone in patients with HER2-positive locally advanced breast cancer (the NOAH trial): A randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 2010, 375, 377–384. [Google Scholar] [CrossRef]
- Rivera, F.; Izquierdo-Manuel, M.; García-Alfonso, P.; Martínez de Castro, E.; Gallego, J.; Limón, M.L.; Alsina, M.; López, L.; Galán, M.; Falcó, E.; et al. Perioperative trastuzumab, capecitabine and oxaliplatin in patients with HER2-positive resectable gastric or gastro-oesophageal junction adenocarcinoma: NEOHX phase II trial. Eur. J. Cancer 2021, 145, 158–167. [Google Scholar] [CrossRef]
- Hofheinz, R.-D.; Hegewisch-Becker, S.; Kunzmann, V.; Thuss-Patience, P.; Fuchs, M.; Homann, N.; Graeven, U.; Schulte, N.; Merx, K.; Pohl, M.; et al. Trastuzumab in combination with 5-fluorouracil, leucovorin, oxaliplatin and docetaxel as perioperative treatment for patients with human epidermal growth factor receptor 2-positive locally advanced esophagogastric adenocarcinoma: A phase II trial of the Arbeitsgemeinschaft Internistische Onkologie Gastric Cancer Study Group. Int. J. Cancer 2021, 149, 1322–1331. [Google Scholar] [CrossRef]
- Hofheinz, R.D.; Haag, G.M.; Ettrich, T.J.; Borchert, K.; Kretzschmar, A.; Teschendorf, C.; Siegler, G.M.; Ebert, M.P.; Goekkurt, E.; Welslau, M.; et al. Perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2-positive resectable esophagogastric adenocarcinoma: Final results of the PETRARCA multicenter randomized phase II trial of the AIO. J. Clin. Oncol. 2020, 38, S4502. [Google Scholar] [CrossRef]
- Stroes, C.I.; Schokker, S.; Creemers, A.; Molenaar, R.J.; Hulshof, M.C.C.M.; van der Woude, S.O.; Bennink, R.J.; Mathôt, R.A.A.; Krishnadath, K.K.; Punt, C.J.A.; et al. Phase II feasibility and biomarker study of neoadjuvant trastuzumab and pertuzumab with chemoradiotherapy for resectable human epidermal growth factor receptor 2-positive esophageal adenocarcinoma: TRAP study. J. Clin. Oncol. 2020, 38, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Safran, H.P.; Winter, K.; Ilson, D.H.; Wigle, D.; DiPetrillo, T.; Haddock, M.G.; Hong, T.S.; Leichman, L.P.; Rajdev, L.; Resnick, M.; et al. Trastuzumab with trimodality treatment for oesophageal adenocarcinoma with HER2 overexpression (NRG Oncology/RTOG 1010): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 259. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Grabsch, H.; Mauer, M.E.; Lorenzen, S.; Bouche, O.; Thuss-Patience, P.C.; Elme, A.; Moehler, M.H.; Romario, U.F.; Kang, Y.-K.; et al. EORTC-1203 GITC “INNOVATION”: Integration of trastuzumab (T), with or without pertuzumab (P), into perioperative chemotherapy of HER-2 positive stomach cancer: Overall survival results. J. Clin. Oncol. 2025, 43 (Suppl. S4), LBA331. [Google Scholar] [CrossRef]
- Smyth, E.C.; Griffiths, D.; Cozens, K.; Turkington, R.C.; Roy, R.; Ngan, S.; Owen, R.; Foley, K.; Waugh, R.; Steele, C.; et al. A single-arm phase II trial of trastuzumab deruxtecan in patients with gastrooesophageal adenocarcinoma cancer who are ctDNA and HER2 positive: DECIPHER. J. Clin. Oncol. 2025, 43, TPS512. [Google Scholar] [CrossRef]
- Zhao, C.; Meng, X.; Shan, Z.; Jiang, J.; Liu, X.; Li, H.; Sun, J.; Ding, C.; Xia, J.; Liu, Z.; et al. Efficacy and safety of perioperative chemotherapy combined with tislelizumab and trastuzumab for HER2-positive resectable gastric/gastroesophageal junction cancer (GC/EGJC): Preliminary results of a phase 2, single-arm trial. J. Clin. Oncol. 2023, 41, e16084. [Google Scholar] [CrossRef]
- Tajiri, R.; Ooi, A.; Fujimura, T.; Dobashi, Y.; Oyama, T.; Nakamura, R.; Ikeda, H. Intratumoral heterogeneous amplification of ERBB2 and subclonal genetic diversity in gastric cancers revealed by multiple ligation-dependent probe amplification and fluorescence in situ hybridization. Hum. Pathol. 2014, 45, 725–734. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Caporale, M.; Morano, F.; Scartozzi, M.; Gloghini, A.; De Vita, F.; Giommoni, E.; Fornaro, L.; Aprile, G.; Melisi, D.; et al. HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: Implication for further clinical research. Int. J. Cancer 2016, 139, 2859–2864. [Google Scholar] [CrossRef]
- Stein, A.; Paschold, L.; Tintelnot, J.; Goekkurt, E.; Henkes, S.-S.; Simnica, D.; Schultheiss, C.; Willscher, E.; Bauer, M.; Wickenhauser, C.; et al. Efficacy of ipilimumab vs FOLFOX in combination with nivolumab and trastuzumab in patients with previously untreated ERBB2-positive esophagogastric adenocarcinoma: The AIO INTEGA randomized clinical trial. JAMA Oncol. 2022, 8, 1150–1158. [Google Scholar] [CrossRef]
- Elimova, E.; Ajani, J.; Burris, H.; Denlinger, C.; Iqbal, S.; Kang, Y.-K.; Kim, J.; Lee, K.-W.; Lin, B.; Mehta, R.; et al. Zanidatamab + chemotherapy for first-line treatment of HER2+ advanced or metastatic gastro-oesophageal adenocarcinoma (mGEA): New and updated data from a phase II trial. Ann. Oncol. 2024, 35, S887–S888. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; van Laarhoven, H.W.M.; Rha, S.Y.; Kozlov, V.; Oh, D.-Y.; Gravina, A.; Rapatoni, L.; Shoji, H.; Hofheinz, R.D.; Chen, L.-T.; et al. Trastuzumab deruxtecan (T-DXd) monotherapy and combinations in patients with advanced/metastatic HER2-positive esophageal, gastric, or gastroesophageal junction adenocarcinoma: Initial results from a phase 1b/2 study (DESTINY-Gastric03). Ann. Oncol. 2024, 35, S878. [Google Scholar] [CrossRef]
- Thuss-Patience, P.C.; Shah, M.A.; Ohtsu, A.; Cutsem, E.V.; Ajani, J.A.; Castro, H.; Mansoor, W.; Chung, H.C.; Bodoky, G.; Shitara, K.; et al. Trastuzumab emtansine versus taxane use for HER2-positive advanced gastric cancer: GATSBY, a randomised, open-label phase II/III study. Lancet Oncol. 2017, 18, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Ryu, M.H.; Park, Y.S.; Ahn, J.Y.; Park, Y.; Park, S.R.; Ryoo, B.-Y.; Lee, G.H.; Jung, H.-Y.; Kang, Y.-K. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: Results of the GASTHER3 study. Gastric Cancer 2019, 22, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, X.; Liu, Y.; Li, S.; Zhang, D.; Meng, X.; Lu, L.; Li, Y. MMP7 induces T-DM1 resistance and leads to poor prognosis of gastric adenocarcinoma via a DKK1-dependent manner. Anticancer Agents Med. Chem. 2018, 18, 2010–2016. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Deng, J.; Wu, H.; Huang, Z. HER2-positive gastric cancer: From targeted therapy to CAR-T cell therapy. Front. Immunol. 2025, 16, 1560280. [Google Scholar] [CrossRef]
- Song, Y.; Tong, C.; Wang, Y.; Gao, Y.; Dai, H.; Guo, Y.; Zhao, X.; Wang, Y.; Wang, Z.; Han, W.; et al. Effective and persistent antitumor activity of HER2-directed CAR-T cells against gastric cancer cells in vitro and xentransplanted tumors in vivo. Protein Cell 2017, 8, 984–994. [Google Scholar] [CrossRef]
- Feng, K.; Liu, Y.; Guo, Y.; Qiu, J.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Han, W. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell 2017, 8, 1034–1042. [Google Scholar] [CrossRef]
- Reiss, K.A.; Yuan, Y.; Ueno, N.T.; Johnson, M.L.; Gill, S.; Dees, E.C.; Chao, J.; Angelos, M.; Shestova, O.; Serody, J.S.; et al. Phase 1, first-in-human (FIH) study of the anti-HER2 CAR macrophage CT-0508 in subjects with HER2 overexpressing solid tumours. J. Clin. Oncol. 2022, 40 (Suppl. S16), 2533. [Google Scholar] [CrossRef]
- Schlechter, B.L.; Olson, D.; George, M.A.; Saibil, S.; Giordano, A.; Bouvier, R.; Gavriliuc, M.; Pieke, B.; Gruber, K.; Lichtenstein, E.; et al. A phase I/II trial investigating safety and efficacy of autologous TAC01-HER2 in relapsed or refractory solid tumours. J. Clin. Oncol. 2023, 41 (Suppl. S16), 2519. [Google Scholar] [CrossRef]
- Kono, K.; Takahashi, A.; Sugai, H.; Fujii, H.; Choudhury, A.R.; Kiessling, R.; Matsumoto, Y. Dendritic Cells Pulsed with HER-2/neu-derived Peptides Can Induce Specific T-Cell Responses in Patients with Gastric Cancer. Clin. Cancer Res. 2002, 8, 921–930. [Google Scholar]
- Maeng, H.M.; Moore, B.N.; Bagheri, H.; Steinberg, S.M.; Inglefield, J.; Dunham, K.; Wei, W.-Z.; Morris, J.C.; Terabe, M.; England, L.C.; et al. Phase I Clinical Trial of an Autologous Dendritic Cell Vaccine Against HER2 Shows Safety and Preliminary Clinical Efficacy. Front. Oncol. 2021, 11, 789078. [Google Scholar] [CrossRef]
- Jung, M.; Lee, J.B.; Kim, H.S.; Kwon, W.S.; Kim, H.O.; Kim, S.; Park, M.; Kim, W.; Choi, K.-Y.; Oh, T.; et al. First-in-Human Phase 1 Study of a B Cell- and Monocyte-Based Immunotherapeutic Vaccine against HER2-Positive Advanced Gastric Cancer. Cancer Res. Treat. 2022, 54, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.; Maglakelidze, M.; Andrić, Z.; Ryspayeva, D.; Bulat, I.; Nikolić, I.; Petrović, Z.; Chawla, T.; Nagarkar, R.; Garner-Spitzer, E.; et al. Phase II Trial of HER-Vaxx, a B-cell Peptide-Based Vaccine, in HER2-Overexpressing Advanced Gastric Cancer Patients Under Platinum-Based Chemotherapy (HERIZON). Clin. Cancer Res. 2024, 30, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Pegram, M.D.; Lee, K.-W.; Sharma, M.; Lee, J.; Spira, A.I.; Hanna, G.J.; Kang, Y.-K.; Rasco, D.W.; Moore, K.N.; et al. A phase 1/2 study of a first-in-human immune-stimulating antibody conjugate (ISAC) BDC-1001 in patients with advanced HER2-expressing solid tumours. J. Clin. Oncol. 2023, 41 (Suppl. S16), 2538. [Google Scholar] [CrossRef]
- Shimozaki, K.; Fukuoka, S.; Ooki, A.; Yamaguchi, K. HER2-low gastric cancer: Is the subgroup targetable? ESMO Open 2024, 9, 103679. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Raoufmoghaddam, S.; Sztachelska, M.; Winter, M.; Das, S. Phase 1b/2, open-label dose-escalation and -expansion study evaluating trastuzumab deruxtecan (T-DXd) monotherapy and combinations in patients with HER2+ and HER2-low gastric cancer: DESTINY-Gastric03 (DG-03). J. Clin. Oncol. 2024, 42 (Suppl. S3), TPS424. [Google Scholar] [CrossRef]
- Li, J.; Luo, S.; Liu, T.; Dong, L.; Yuan, X.; Feng, J.; Wang, Y.; Deng, Y.; Chen, J.; Zhang, M.; et al. 684P phase I trial of SHR-A1811 in HER2-expressing advanced gastric cancer (GC) or gas-troesophageal junction adenocarcinoma (GEJ) and colorectal cancer (CRC). Ann. Oncol. 2023, 34, S478. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, J.; Wang, A.; Wei, J.; Peng, Z.; Wang, X.; Zhou, J.; Qi, C.; Liu, D.; Li, J.; et al. Disitamab vedotin (RC48) plus toripalimab for HER2-expressing advanced gastric or gastroesophageal junction and other solid tumours: A multicentre, open label, dose escalation and expansion phase 1 trial. eClinicalMedicine 2024, 68, 102415. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Q.; Zhang, F.; Meng, F.; Liu, S.; Zhou, R.; Wu, Q.; Li, X.; Shen, L.; Huang, J.; et al. HER2 recruits AKT1 to disrupt STING signalling and suppress antiviral defence and antitumour immunity. Nat. Cell Biol. 2019, 21, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Duvall, J.R.; Bukhalid, R.A.; Cetinbas, N.M.; Catcott, K.C.; Lancaster, K.; Bentley, K.W.; Clark, S.; Clardy, S.; Collins, S.D.; Dirksen, A.; et al. Abstract 3503: XMT-2056, a HER2-targeted Immunosynthen STING-agonist antibody-drug conjugate, binds a novel epitope of HER2 and shows increased anti-tumor activity in combination with trastuzumab and pertuzumab. Cancer Res. 2022, 82, 3503. [Google Scholar] [CrossRef]
- Segal, N.H.; Logan, T.F.; Hodi, F.S.; McDermott, D.; Melero, I.; Hamid, O.; Schmidt, H.; Robert, C.; Chiarion-Sileni, V.; Ascierto, P.A.; et al. Results from an Integrated Safety Analysis of Urelumab, an Agonist Anti-CD137 Monoclonal Antibody. Clin. Cancer Res. 2017, 23, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Ku, G.; Lee, J.; Aviano, K.; Demuth, T.; Hasenkamp, L.C.; Olwill, S.A. Abstract CT154: Combination of cinrebafusp alfa with ramucirumab and paclitaxel is well tolerated and elicits encouraging clinical activity in patients with HER2-positive gastric/gastroesophageal junction (GEJ) adenocarcinoma. Cancer Res. 2023, 83 (Suppl. S8), CT154. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, X.; Jia, Y.; Dai, Y.; Xu, F.; Huang, Y.; Wang, X.; Wu, H.; Shao, Y.; Long, J.; et al. The design, preclinical study and phase I dose escalation plan of a HER2 targeted immunoliposome (HF-K1) for HER2 low solid tumor treatment. J. Clin. Oncol. 2024, 42 (Suppl. S16), 3035. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Johnson, A.M.; Dumbrava, E.E.I.; Raghav, K.; Balaji, K.; Bhatt, M.; Murthy, R.K.; Rodon, J.; Piha-Paul, S.A. Advances in HER2-Targeted Therapy: Novel Agents and Opportunities Beyond Breast and Gastric Cancer. Clin. Cancer Res. 2019, 25, 2033–2041. [Google Scholar] [CrossRef]
- Matsuoka, T.; Yashiro, M. Recent advances in the HER2 targeted therapy of gastric cancer. World J. Clin. Cases 2015, 3, 42–51. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Van Laarhoven, H.W.M.; Rha, S.Y.; Kozlov, V.; Oh, D.Y.; Gravina, A.; Rapatoni, L.; Shoji, H.; Hofheinz, R.D.; Chen, L.T. Updated results from the trastuzumab deruxtecan (T-DXd) 5.4 mg/kg triplet combination of DESTINY-Gastric03 (DG-03): First-line (1L) T-DXd with fluoropyrimidine (FP) and pembrolizumab in advanced/metastatic HER2-positive (HER2+) esophageal adenocarcinoma, gastric cancer (GC), or gastroesophageal junction adenocarcinoma (GEJA). J. Clin. Oncol. 2025, 43 (Suppl. S1), 448. [Google Scholar] [CrossRef]
- Mishima, S.; Shitara, K. Trastuzumab deruxtecan for the treatment of HER2-positive gastric cancer. Expert Opin. Biol. Ther. 2021, 21, 825–830. [Google Scholar] [CrossRef]
- Ocaña, A.; Amir, E.; Pandiella, A. HER2 heterogeneity and resistance to anti-HER2 antibody-drug conjugates. Breast Cancer Res. 2020, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Nasca, V.; Bergamo, F.; Foltran, L.; Antonuzzo, L.; Bencardino, K.; Dell’Aquila, E.; Corallo, S.; Spallanzani, A.; Brunetti, O.; Spada, D.; et al. Adjuvant TRastuzumab deruxtecan plus fluoropyrimidine versus standard chemotherapy in HER2-positive gastric or gastroesophageal cancer patients with persistence of minimal residual disease in liquid biopsy after pre-operative chemotherapy and radical surgery: The multicentre, phase II randomized TRINITY trial. BMC Cancer 2025, 25, 633. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Wei, X.; Tang, C.; Zhang, W. Disitamab vedotin, a novel HER2-directed antibody-drug conjugate in gastric cancer and other solid tumors. Drugs Today 2022, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, W.; Narjis, K.; Musani, S.; Nancy, F.; Qureshi, L.; Mudasir, M.; Naseem, R.; Tooba, F.; Yousuf, J.; Farhan, K.; et al. Exploring Zanidatamab’s efficacy across HER2-positive Malignancies: A narrative review. BMC Cancer 2025, 25, 382. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Kawazoe, A.; Bai, Y.; Xu, J.; Lonardi, S.; Metges, J.P.; Yanez, P.; Wyrwicz, L.S.; Shen, L.; Ostapenko, Y.; et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric cancer: KEYNOTE-811. Lancet 2023, 402, 1121–1130. [Google Scholar] [CrossRef]
- Khan, S.H.; Choi, Y.; Veena, M.; Lee, J.K.; Shin, D.S. Advances in CAR T cell therapy: Antigen selection, modifications, and current trials for solid tumors. Front. Immunol. 2025, 15, 1489827. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, J.; Jiang, H.; Zhou, M. Strategies to Overcome Antigen Heterogeneity in CAR-T Cell Therapy. Cells 2025, 14, 320. [Google Scholar] [CrossRef]
- Kim, I.H. Emerging Targets for Systemic Treatment of Gastric Cancer: HER2 and Beyond. J. Gastric Cancer 2024, 24, 29–56. [Google Scholar] [CrossRef]
- Sato, Y.; Okamoto, K.; Kawano, Y.; Kasai, A.; Kawaguchi, T.; Sagawa, T.; Sogabe, M.; Miyamoto, H.; Takayama, T. Novel Biomarkers of Gastric Cancer: Current Research and Future Perspectives. J. Clin. Med. 2023, 12, 4646. [Google Scholar] [CrossRef]
| Study Name | Study DESIGN | Treatment Arms | Sample Size | Primary Site | pCR | R0 Resection | DFS | OS | G3–G4 AEs |
|---|---|---|---|---|---|---|---|---|---|
| NEOHX [52] | Phase II, single-arm | XELOX + T | 36 pts | GA, GEJA | 9.6% | 90% | 18-month DFS: 71% | mOS:79.9 5-yr OS: 58% | Diarrhea (33%), nausea, and vomiting (8%) |
| HER-FLOT [53] | Phase II, single-arm | FLOT + T | 56 pts | GA, GEJA | 21.4% | 92.9% | mDFS: 42.5 months | 3-yr OS: 82.1 | Neutropenia (46.6%), diarrhea (17.0%) |
| PETRARCA [54] | Phase II/III, randomized | FLOT + T + P | 80 pts | GA, GEJA | 35% | 93% | 2-yr DFS: 70% | 2-yr OS: 84% | Diarrhea (41%), leukopenia (23%) |
| TRAP [55] | Phase II, single-arm | CROSS + T + P | 40 pts | EA, GEJA | 34% | 100% | 3-yr PFS: 57% | 3-yr OS: 71% | Diarrhea (20%), dysphagia (18%) |
| RTOG-1010 [56] | Phase III, randomized | CROSS + T + P | 203 pts | EA, GEJA | 27% | 98% | 4-yr DFS: 33.2% | 4-yr OS: 47.6% | Hematological (56%), gastrointestinal disorders |
| INNOVATION [57] | Phase II, randomized | A: Chemo ° B: Chemo + T C: Chemo + T + P | 161 pts A: 33 pts B: 64 pts C: 64 pts | GA, GEJA | A: 33.3% °°″ B: 53.3% C: 37.9% | A: 83.9% B: 90.3% C: 85.9% | 3-yr PFS °° A: 68.4% B: 60.5% C: 50.4% | 3-yr OS °° A: 73.3% B: 72.2% C: 62.2% | Diarrhea (A: 5.9%, B: 3.0%, C: 26.1%), Neutropenia (A: 32.4%, B: 21.2%, C: 21.7%). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratti, M.; Citterio, C.; Orlandi, E.; Vecchia, S.; Anselmi, E.; Toscani, I.; Rotolo, M.; Salati, M.; Ghidini, M. Fighting HER2 in Gastric Cancer: Current Approaches and Future Landscapes. Int. J. Mol. Sci. 2025, 26, 7285. https://doi.org/10.3390/ijms26157285
Ratti M, Citterio C, Orlandi E, Vecchia S, Anselmi E, Toscani I, Rotolo M, Salati M, Ghidini M. Fighting HER2 in Gastric Cancer: Current Approaches and Future Landscapes. International Journal of Molecular Sciences. 2025; 26(15):7285. https://doi.org/10.3390/ijms26157285
Chicago/Turabian StyleRatti, Margherita, Chiara Citterio, Elena Orlandi, Stefano Vecchia, Elisa Anselmi, Ilaria Toscani, Martina Rotolo, Massimiliano Salati, and Michele Ghidini. 2025. "Fighting HER2 in Gastric Cancer: Current Approaches and Future Landscapes" International Journal of Molecular Sciences 26, no. 15: 7285. https://doi.org/10.3390/ijms26157285
APA StyleRatti, M., Citterio, C., Orlandi, E., Vecchia, S., Anselmi, E., Toscani, I., Rotolo, M., Salati, M., & Ghidini, M. (2025). Fighting HER2 in Gastric Cancer: Current Approaches and Future Landscapes. International Journal of Molecular Sciences, 26(15), 7285. https://doi.org/10.3390/ijms26157285







