The Human Mycobiome: Composition, Immune Interactions, and Impact on Disease
Abstract
1. Introduction
2. Components of a Healthy Mycobiome
2.1. Gastrointestinal Tract
2.2. Oral Cavity
2.3. Respiratory Tract
2.4. Skin
2.5. Genitourinary Tract
| Body Niche | Frequently Reported Fungal Taxa | Relative Abundance | Study |
|---|---|---|---|
| Gastrointestinal tract | Saccharomyces cerevisiae | 13.9% | [23] |
| Candida spp. | 0.5% | ||
| Penicillium | <1% | ||
| Aspergillus | 25.7% | ||
| Debaryomyces udenii | 19.9% | ||
| Cladosporium | 6.1% | [24] | |
| Pichia | 18.9% | ||
| Oral cavity | Malassezia spp. | 37.7% | [25] |
| Epicoccum spp. | 33.8% | ||
| Candida/Pichia spp. | 9.6% | ||
| Cladosporium/Davidiella spp. | 3.0% | ||
| Alternaria spp. | 1.9% | ||
| Respiratory tract | Saccharomyctes | 48.1% | [12] |
| Microsporidia | 23.6% | ||
| Dothideomycetes | 16.6% | ||
| Cryptomycota | 3.0% | ||
| Chytridiomyctes | 3.0% | ||
| Skin | Malassezia restricta | 89.5% | [26] |
| Malassezia globosa | 2.90% | ||
| Vaginal tract | Candida albicans | 63.50% | [27] |
| Leptosphaerulina chartarum | 9.90% | ||
| Clavispora lusitaniae | 9.10% |
3. Methods and Limitations in Mycobiome Research
4. Factors Influencing Mycobiome Composition
5. The Mycobiome’s Interactions
5.1. Interactions Between Fungi and Bacteria
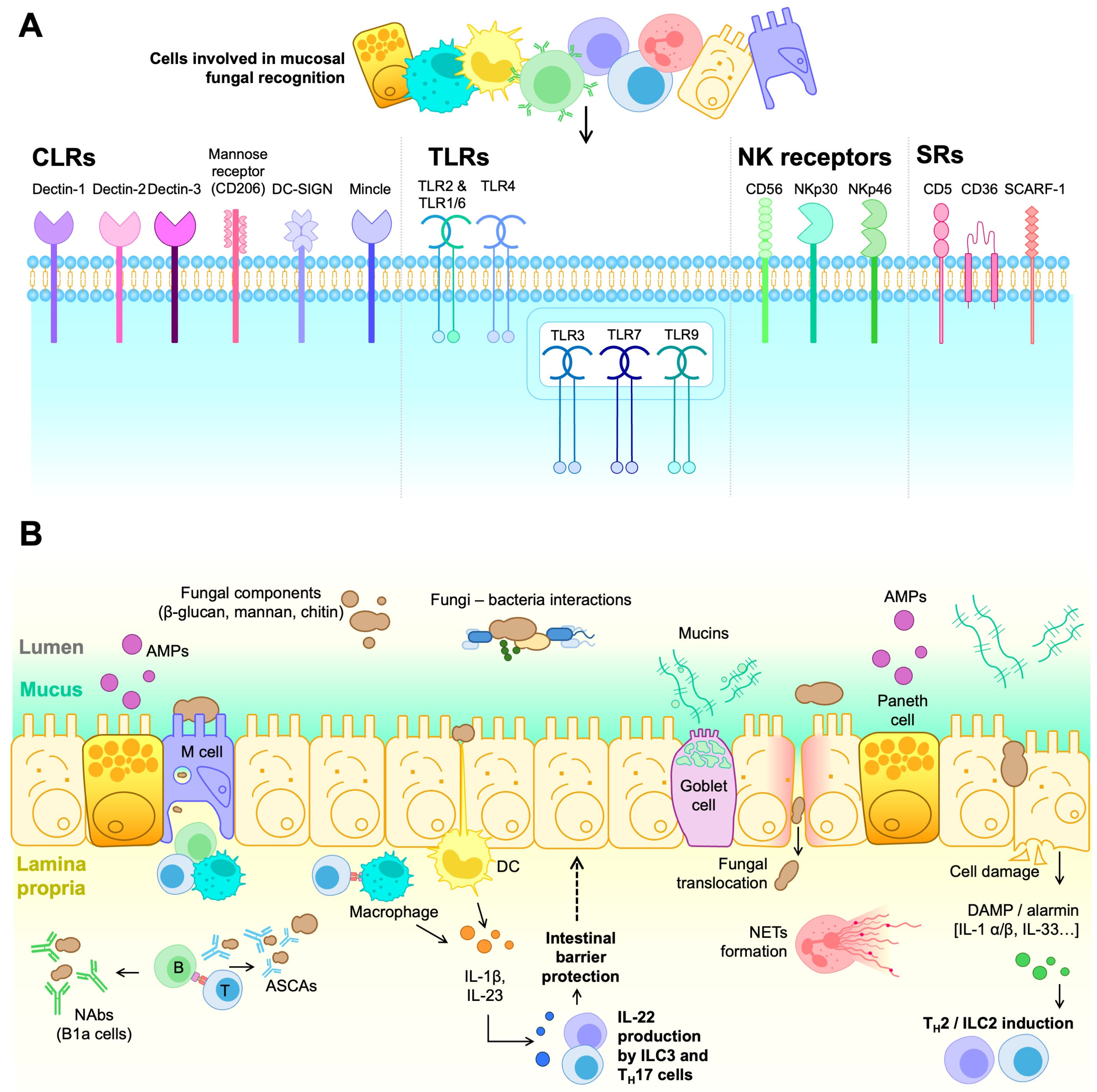
5.2. Mycobiome Interactions with the Immune System
6. The Gut Mycobiome in Health and Disease
6.1. GI Tract Diseases
6.2. Neurological Disorders
6.3. Lung Disorders
6.4. Systemic Infections
6.5. Liver Disorders
6.6. Pancreatic Disorders
6.7. Skin Disorders
6.8. Genitourinary Disorders
6.9. Cancer
6.10. Autoimmune Disorders
7. Clinical Applications of Mycobiome Modulation
8. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Dekaboruah, E.; Suryavanshi, M.V.; Chettri, D.; Verma, A.K. Human Microbiome: An Academic Update on Human Body Site Specific Surveillance and Its Possible Role. Arch. Microbiol. 2020, 202, 2147–2167. [Google Scholar] [CrossRef]
- Hallen-Adams, H.E.; Kachman, S.D.; Kim, J.; Legge, R.M.; Martínez, I. Fungi Inhabiting the Healthy Human Gastrointestinal Tract: A Diverse and Dynamic Community. Fungal Ecol. 2015, 15, 9–17. [Google Scholar] [CrossRef]
- Nash, A.K.; Auchtung, T.A.; Wong, M.C.; Smith, D.P.; Gesell, J.R.; Ross, M.C.; Stewart, C.J.; Metcalf, G.A.; Muzny, D.M.; Gibbs, R.A.; et al. The Gut Mycobiome of the Human Microbiome Project Healthy Cohort. Microbiome 2017, 5, 153. [Google Scholar] [CrossRef]
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018, 6, 116. [Google Scholar] [CrossRef]
- Proctor, L.M.; Creasy, H.H.; Fettweis, J.M.; Lloyd-Price, J.; Mahurkar, A.; Zhou, W.; Buck, G.A.; Snyder, M.P.; Strauss, J.F.; Weinstock, G.M.; et al. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Hallen-Adams, H.E.; Suhr, M.J. Fungi in the Healthy Human Gastrointestinal Tract. Virulence 2017, 8, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.J.; Kühbacher, T.; Musfeldt, M.; Rosenstiel, P.; Hellmig, S.; Rehman, A.; Drews, O.; Weichert, W.; Timmis, K.N.; Schreiber, S. Fungi and Inflammatory Bowel Diseases: Alterations of Composition and Diversity. Scand. J. Gastroenterol. 2008, 43, 831–841. [Google Scholar] [CrossRef]
- Ptasiewicz, M.; Grywalska, E.; Mertowska, P.; Korona-Głowniak, I.; Poniewierska-Baran, A.; Niedźwiedzka-Rystwej, P.; Chałas, R. Armed to the Teeth—The Oral Mucosa Immunity System and Microbiota. Int. J. Mol. Sci. 2022, 23, 882. [Google Scholar] [CrossRef]
- Belvoncikova, P.; Splichalova, P.; Videnska, P.; Gardlik, R. The Human Mycobiome: Colonization, Composition and the Role in Health and Disease. J. Fungi 2022, 8, 1046. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.H.N.; Panduwawala, C.P.; Samaranayake, L.P. Biodiversity of the Human Oral Mycobiome in Health and Disease. Oral Dis. 2019, 25, 363–371. [Google Scholar] [CrossRef]
- Nguyen, L.D.N.; Viscogliosi, E.; Delhaes, L. The Lung Mycobiome: An Emerging Field of the Human Respiratory Microbiome. Front. Microbiol. 2015, 6, 89. [Google Scholar] [CrossRef]
- Cuthbertson, L.; Felton, I.; James, P.; Cox, M.J.; Bilton, D.; Schelenz, S.; Loebinger, M.R.; Cookson, W.O.C.; Simmonds, N.J.; Moffatt, M.F. The Fungal Airway Microbiome in Cystic Fibrosis and Non-Cystic Fibrosis Bronchiectasis. J. Cyst. Fibros. 2021, 20, 295–302. [Google Scholar] [CrossRef]
- Weaver, D.; Gago, S.; Bromley, M.; Bowyer, P. The Human Lung Mycobiome in Chronic Respiratory Disease: Limitations of Methods and Our Current Understanding. Curr. Fungal Infect. Rep. 2019, 13, 109–119. [Google Scholar] [CrossRef]
- Ali, N.A.B.M.; Ivan, F.X.; Mac Aogáin, M.; Narayana, J.K.; Lee, S.Y.; Lim, C.L.; Chotirmall, S.H. The Healthy Airway Mycobiome in Individuals of Asian Descent. Chest 2021, 159, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Freeman, A.F.; Park, M.; Sokolic, R.; Candotti, F.; Holland, S.M.; Segre, J.A.; Kong, H.H. The Altered Landscape of the Human Skin Microbiome in Patients with Primary Immunodeficiencies. Genome Res. 2013, 23, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; Kong, H.H.; et al. Topographic Diversity of Fungal and Bacterial Communities in Human Skin. Nature 2013, 498, 367–370. [Google Scholar] [CrossRef]
- Li, H.; Goh, B.N.; Teh, W.K.; Jiang, Z.; Goh, J.P.Z.; Goh, A.; Wu, G.; Hoon, S.S.; Raida, M.; Camattari, A.; et al. Skin Commensal Malassezia Globosa Secreted Protease Attenuates Staphylococcus aureus Biofilm Formation. J. Investig. Dermatol. 2018, 138, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, A.L.; Underhill, D.M. The Mycobiome of the Human Urinary Tract: Potential Roles for Fungi in Urology. Ann. Transl. Med. 2017, 5, 31. [Google Scholar] [CrossRef]
- Willems, H.M.E.; Ahmed, S.S.; Liu, J.; Xu, Z.; Peters, B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi 2020, 6, 27. [Google Scholar] [CrossRef]
- Esposito, M.M.; Patsakos, S.; Borruso, L. The Role of the Mycobiome in Women’s Health. J. Fungi 2023, 9, 348. [Google Scholar] [CrossRef]
- Mishra, K.; Bukavina, L.; Ghannoum, M. Symbiosis and Dysbiosis of the Human Mycobiome. Front. Microbiol. 2021, 12, 636131. [Google Scholar] [CrossRef]
- Raimondi, S.; Amaretti, A.; Gozzoli, C.; Simone, M.; Righini, L.; Candeliere, F.; Brun, P.; Ardizzoni, A.; Colombari, B.; Paulone, S.; et al. Longitudinal Survey of Fungi in the Human Gut: ITS Profiling, Phenotyping, and Colonization. Front. Microbiol. 2019, 10, 1575. [Google Scholar] [CrossRef]
- Kabwe, M.H.; Vikram, S.; Mulaudzi, K.; Jansson, J.K.; Makhalanyane, T.P. The Gut Mycobiota of Rural and Urban Individuals Is Shaped by Geography. BMC Microbiol. 2020, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.K.; David, M.S.; Li, L.; Heider, T.N.; Peterson, J.D.; Montano, E.A.; Dongari-Bagtzoglou, A.; Diaz, P.I.; Strausbaugh, L.D. Redefining the Human Oral Mycobiome with Improved Practices in Amplicon-Based Taxonomy: Discovery of Malassezia as a Prominent Commensal. PLoS ONE 2014, 9, e90899. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.H.Y.; Chan, K.C.K.; Lee, P.K.H. Skin Fungal Community and Its Correlation with Bacterial Community of Urban Chinese Individuals. Microbiome 2016, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Lehtoranta, L.; Hibberd, A.A.; Yeung, N.; Laitila, A.; Maukonen, J.; Ouwehand, A.C. Characterization of Vaginal Fungal Communities in Healthy Women and Women with Bacterial Vaginosis (BV); a Pilot Study. Microb. Pathog. 2021, 161, 105055. [Google Scholar] [CrossRef]
- Zhang, L.; Zhan, H.; Xu, W.; Yan, S.; Ng, S.C. The Role of Gut Mycobiome in Health and Diseases. Ther. Adv. Gastroenterol. 2021, 14, 17562848211047130. [Google Scholar] [CrossRef]
- Chin, V.K.; Yong, V.C.; Chong, P.P.; Amin Nordin, S.; Basir, R.; Abdullah, M. Mycobiome in the Gut: A Multiperspective Review. Mediat. Inflamm. 2020, 2020, 9560684. [Google Scholar] [CrossRef]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal Evolution: Diversity, Taxonomy and Phylogeny of the Fungi. Fungi. Biol. Rev. Camb. Philos. Soc. 2019, 94, 2101–2137. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zinger, L.; Nilsson, R.H.; Kennedy, P.G.; Yang, T.; Anslan, S.; Mikryukov, V. Best Practices in Metabarcoding of Fungi: From Experimental Design to Results. Mol. Ecol. 2022, 31, 2769–2795. [Google Scholar] [CrossRef]
- Steenwyk, J.L.; Knowles, S.; Bastos, R.W.; Balamurugan, C.; Rinker, D.; Mead, M.E.; Roberts, C.D.; Raja, H.A.; Li, Y.; Colabardini, A.C.; et al. Evolutionary Origin and Population Diversity of a Cryptic Hybrid Pathogen. Nat. Commun. 2024, 15, 8412. [Google Scholar] [CrossRef]
- Kauserud, H. ITS Alchemy: On the Use of ITS as a DNA Marker in Fungal Ecology. Fungal Ecol. 2023, 65, 101274. [Google Scholar] [CrossRef]
- Avershina, E.; Qureshi, A.I.; Winther-Larsen, H.C.; Rounge, T.B. Challenges in Capturing the Mycobiome from Shotgun Metagenome Data: Lack of Software and Databases. Microbiome 2025, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Khannous-lleiffe, O.; Gabaldón, T. B-GUT Reference Genome Database Improves Biomarker Discovery and Fungal Identification in Gut Metagenomes. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.P.; Munro, C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5, 267–292. [Google Scholar] [CrossRef]
- Rittenour, W.R.; Park, J.H.; Cox-Ganser, J.M.; Beezhold, D.H.; Green, B.J. Comparison of DNA Extraction Methodologies Used for Assessing Fungal Diversity via ITS Sequencing. J. Environ. Monit. 2012, 14, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Boix-Amorós, A.; Puente-Sánchez, F.; du Toit, E.; Linderborg, K.M.; Zhang, Y.; Yang, B.; Salminen, S.; Isolauri, E.; Tamames, J.; Mira, A.; et al. Mycobiome Profiles in Breast Milk from Healthy Women Depend on Mode of Delivery, Geographic Location, and Interaction with Bacteria. Appl. Environ. Microbiol. 2019, 85, e02994-18. [Google Scholar] [CrossRef]
- Ward, T.L.; Knights, D.; Gale, C.A. Infant Fungal Communities: Current Knowledge and Research Opportunities. BMC Med. 2017, 15, 30. [Google Scholar] [CrossRef]
- Zhu, T.; Duan, Y.-Y.; Kong, F.-Q.; Galzote, C.; Quan, Z.-X. Dynamics of Skin Mycobiome in Infants. Front. Microbiol. 2020, 11, 1790. [Google Scholar] [CrossRef]
- Schei, K.; Avershina, E.; Øien, T.; Rudi, K.; Follestad, T.; Salamati, S.; Ødegård, R.A. Early Gut Mycobiota and Mother-Offspring Transfer. Microbiome 2017, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.F.; Castro Mejia, J.L.; Krych, L.; Khakimov, B.; Kot, W.; Bechshøft, R.L.; Reitelseder, S.; Højfeldt, G.W.; Engelsen, S.B.; Holm, L.; et al. Gut Mycobiome Dysbiosis Is Linked to Hypertriglyceridemia Among Home Dwelling Elderly Danes. biorXiv 2020. [Google Scholar] [CrossRef]
- Wu, L.; Zeng, T.; Deligios, M.; Milanesi, L.; Langille, M.G.I.; Zinellu, A.; Rubino, S.; Carru, C.; Kelvin, D.J. Age-Related Variation of Bacterial and Fungal Communities in Different Body Habitats across the Young, Elderly, and Centenarians in Sardinia. mSphere 2020, 5, e00558-19. [Google Scholar] [CrossRef] [PubMed]
- Szóstak, N.; Handschuh, L.; Samelak-Czajka, A.; Tomela, K.; Schmidt, M.; Pruss, Ł.; Milanowska-Zabel, K.; Kozlowski, P.; Philips, A. Host Factors Associated with Gut Mycobiome Structure. mSystems 2023, 8, e0098622. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Dollive, S.; Grunberg, S.; Chen, J.; Li, H.; Wu, G.D.; Lewis, J.D.; Bushman, F.D. Archaea and Fungi of the Human Gut Microbiome: Correlations with Diet and Bacterial Residents. PLoS ONE 2013, 8, e66019. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Strati, F.; Di Paola, M.; Stefanini, I.; Albanese, D.; Rizzetto, L.; Lionetti, P.; Calabrò, A.; Jousson, O.; Donati, C.; Cavalieri, D.; et al. Age and Gender Affect the Composition of Fungal Population of the Human Gastrointestinal Tract. Front. Microbiol. 2016, 7, 1227. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, F.; Yang, X.; Wu, N.; Jiang, W.; Li, X.; Li, X.; Liu, Y. Changes in the Composition of Intestinal Fungi and Their Role in Mice with Dextran Sulfate Sodium-Induced Colitis. Sci. Rep. 2015, 5, 10416. [Google Scholar] [CrossRef]
- Jiang, T.T.; Shao, T.Y.; Ang, W.X.G.; Kinder, J.M.; Turner, L.H.; Pham, G.; Whitt, J.; Alenghat, T.; Way, S.S. Commensal Fungi Recapitulate the Protective Benefits of Intestinal Bacteria. Cell Host Microbe 2017, 22, 809–816.e4. [Google Scholar] [CrossRef]
- Castagliuolo, I.; Riegler, M.F.; Valenick, L.; LaMont, J.T.; Pothoulakis, C. Saccharomyces boulardii Protease Inhibits the Effects of Clostridium Difficile Toxins A and B in Human Colonic Mucosa. Infect. Immun. 1999, 67, 302–307. [Google Scholar] [CrossRef]
- Czerucka, D.; Dahan, S.; Mograbi, B.; Rossi, B.; Rampal, P. Saccharomyces boulardii Preserves the Barrier Function and Modulates the Signal Transduction Pathway Induced in Enteropathogenic Escherichia coli-Infected T84 Cells. Infect. Immun. 2000, 68, 5998–6004. [Google Scholar] [CrossRef]
- García, C.; Tebbji, F.; Daigneault, M.; Liu, N.-N.; Köhler, J.R.; Allen-Vercoe, E.; Sellam, A. The Human Gut Microbial Metabolome Modulates Fungal Growth via the TOR Signaling Pathway. mSphere 2017, 2, e00555-17. [Google Scholar] [CrossRef]
- Das, S.; Konwar, B.K. Inhibiting Pathogenicity of Vaginal Candida albicans by Lactic Acid Bacteria and MS Analysis of Their Extracellular Compounds. APMIS 2024, 132, 161–186. [Google Scholar] [CrossRef]
- Lan, J.; Chen, C. The Role of Lactic Acid Bacteria in Maintaining Vaginal Internal Environment Homeostasis in Patients with Infertility. Microb. Pathog. 2023, 176, 106004. [Google Scholar] [CrossRef]
- Osset, J.; García, E.; Bartolomé, R.M.; Andreu, A. Role of Lactobacillus as Protector against Vaginal Candidiasis. Med. Clin. 2001, 117, 285–288. [Google Scholar] [CrossRef]
- Hui, W.W.; Emerson, L.E.; Clapp, B.; Sheppe, A.E.; Sharma, J.; del Castillo, J.; Ou, M.; Maegawa, G.H.B.; Hoffman, C.; Larkin, J.; et al. The Cross-Kingdom Interaction between Helicobacter Pylori and Candida albicans. PLoS Pathog. 2021, 17, e1009515. [Google Scholar] [CrossRef]
- Granillo, A.R.; Canales, M.G.M.; Espíndola, M.E.S.; Rivera, M.A.M.; De Lucio, V.M.B.; Tovar, A.V.R. Antibiosis Interaction of Staphylococccus Aureus on Aspergillus fumigatus Assessed In Vitro by Mixed Biofilm Formation. BMC Microbiol. 2015, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.M.; Jabra-Rizk, M.A.; Scheper, M.A.; Leid, J.G.; Costerton, J.W.; Shirtliff, M.E. Microbial Interactions and Differential Protein Expression in Staphylococcus aureus–Candida albicans Dual-Species Biofilms. FEMS Immunol. Med. Microbiol. 2010, 59, 493–503. [Google Scholar] [CrossRef]
- Koo, H.; Andes, D.R.; Krysan, D.J. Candida–Streptococcal Interactions in Biofilm-Associated Oral Diseases. PLoS Pathog. 2018, 14, e1007342. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.J.; Nobbs, A.H.; Vickerman, M.M.; Barbour, M.E.; Jenkinson, H.F. Interaction of Candida albicans Cell Wall Als3 Protein with Streptococcus Gordonii SspB Adhesin Promotes Development of Mixed-Species Communities. Infect. Immun. 2010, 78, 4644–4652. [Google Scholar] [CrossRef]
- Kalan, L.; Loesche, M.; Hodkinson, B.P.; Heilmann, K.; Ruthel, G.; Gardner, S.E.; Grice, E.A. Redefining the Chronic-Wound Microbiome: Fungal Communities Are Prevalent, Dynamic, and Associated with Delayed Healing. mBio 2016, 7, e01058-16. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, G.; Mukherjee, P.K.; Gower-Rousseau, C.; Hager, C.; Chandra, J.; Retuerto, M.A.; Neut, C.; Vermeire, S.; Clemente, J.; Colombel, J.F.; et al. Bacteriome and Mycobiome Interactions Underscore Microbial Dysbiosis in Familial Crohn’s Disease. mBio 2016, 7, e01250-16. [Google Scholar] [CrossRef] [PubMed]
- De Brucker, K.; Tan, Y.; Vints, K.; De Cremer, K.; Braem, A.; Verstraeten, N.; Michiels, J.; Vleugels, J.; Cammue, B.P.A.; Thevissen, K. Fungal β-1,3-Glucan Increases Ofloxacin Tolerance of Escherichia coli in a Polymicrobial E. coli/Candida albicans Biofilm. Antimicrob. Agents Chemother. 2015, 59, 3052–3058. [Google Scholar] [CrossRef]
- Kong, E.F.; Tsui, C.; Kucharíková, S.; Andes, D.; Van Dijck, P.; Jabra-Rizk, M.A. Commensal Protection of Staphylococcus aureus against Antimicrobials by Candida albicans Biofilm Matrix. mBio 2016, 7, e01365-16. [Google Scholar] [CrossRef]
- Hatinguais, R.; Willment, J.A.; Brown, G.D. C-type Lectin Receptors in Antifungal Immunity: Current Knowledge and Future Developments. Parasite Immunol. 2022, 45, e12951. [Google Scholar] [CrossRef]
- Huysamen, C.; Brown, G.D. The Fungal Pattern Recognition Receptor, Dectin-1, and the Associated Cluster of C-Type Lectin-like Receptors. FEMS Microbiol. Lett. 2008, 290, 121–128. [Google Scholar] [CrossRef]
- Li, M.; Vultorius, C.; Bethi, M.; Yu, Y. Spatial Organization of Dectin-1 and TLR2 during Synergistic Crosstalk Revealed by Super-Resolution Imaging. J. Phys. Chem. B 2022, 126, 5781–5792. [Google Scholar] [CrossRef]
- Li, W.; Yan, J.; Yu, Y. Geometrical Reorganization of Dectin-1 and TLR2 on Single Phagosomes Alters Their Synergistic Immune Signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 25106–25114. [Google Scholar] [CrossRef]
- Esteban, A.; Popp, M.W.; Vyas, V.K.; Strijbis, K.; Ploegh, H.L.; Fink, G.R. Fungal Recognition Is Mediated by the Association of Dectin-1 and Galectin-3 in Macrophages. Proc. Natl. Acad. Sci. USA 2011, 108, 14270–14275. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; da Fonseca, D.M.; Walker, L.; Griffiths, J.S.; Taylor, P.R.; Gow, N.A.R.; Orr, S.J. Dependence on Mincle and Dectin-2 Varies with Multiple Candida Species During Systemic Infection. Front. Microbiol. 2021, 12, 633229. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.L.; Zhao, X.Q.; Jiang, C.; You, Y.; Chen, X.P.; Jiang, Y.Y.; Jia, X.M.; Lin, X. C-Type Lectin Receptors Dectin-3 and Dectin-2 Form a Heterodimeric Pattern-Recognition Receptor for Host Defense against Fungal Infection. Immunity 2013, 39, 324–334. [Google Scholar] [CrossRef]
- Drummond, R.A.; Franco, L.M.; Lionakis, M.S. Human CARD9: A Critical Molecule of Fungal Immune Surveillance. Front. Immunol. 2018, 9, 1836. [Google Scholar] [CrossRef]
- Speakman, E.A.; Dambuza, I.M.; Salazar, F.; Brown, G.D. T Cell Antifungal Immunity and the Role of C-Type Lectin Receptors. Trends Immunol. 2020, 41, 61–76. [Google Scholar] [CrossRef]
- Hatinguais, R.; Willment, J.A.; Brown, G.D. PAMPs of the Fungal Cell Wall and Mammalian PRRs. Curr. Top. Microbiol. Immunol. 2020, 425, 187–223. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Salazar, F.; Brown, G.D. Antifungal Innate Immunity: A Perspective from the Last 10 Years. J. Innate Immun. 2018, 10, 373–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Y.; Shen, S.; Hou, Y.; Chen, Y.; Wang, T. The Mycobiota of the Human Body: A Spark Can Start a Prairie Fire. Gut Microbes 2020, 11, 655–679. [Google Scholar] [CrossRef]
- Heilig, L.; Natasha, F.; Trinks, N.; Aimanianda, V.; Wong, S.S.W.; Fontaine, T.; Terpitz, U.; Strobel, L.; Mauff, F.L.; Sheppard, D.C.; et al. CD56-Mediated Activation of Human Natural Killer Cells Is Triggered by Aspergillus fumigatus Galactosaminogalactan. PLoS Pathog. 2024, 20, e1012315. [Google Scholar] [CrossRef]
- Li, S.S.; Ogbomo, H.; Mansour, M.K.; Xiang, R.F.; Szabo, L.; Munro, F.; Mukherjee, P.; Mariuzza, R.A.; Amrein, M.; Vyas, J.M.; et al. Identification of the Fungal Ligand Triggering Cytotoxic PRR-Mediated NK Cell Killing of Cryptococcus and Candida. Nat. Commun. 2018, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Vitenshtein, A.; Charpak-Amikam, Y.; Yamin, R.; Bauman, Y.; Isaacson, B.; Stein, N.; Berhani, O.; Dassa, L.; Gamliel, M.; Gur, C.; et al. NK Cell Recognition of Candida Glabrata through Binding of NKp46 and NCR1 to Fungal Ligands Epa1, Epa6, and Epa7. Cell Host Microbe 2016, 20, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Vera, J.; Fenutria, R.; Cañadas, O.; Figueras, M.; Mota, R.; Sarrias, M.R.; Williams, D.L.; Casals, C.; Yelamos, J.; Lozano, F. The CD5 Ectodomain Interacts with Conserved Fungal Cell Wall Components and Protects from Zymosan-Induced Septic Shock-like Syndrome. Proc. Natl. Acad. Sci. USA 2009, 106, 1506–1511. [Google Scholar] [CrossRef]
- Velasco-de-Andrés, M.; Català, C.; Casadó-Llombart, S.; Simões, I.; Zaragoza, O.; Carreras, E.; Lozano, F. The Lymphocyte Scavenger Receptor CD5 Plays a Nonredundant Role in Fungal Infection. Cell Mol. Immunol. 2021, 18, 498–500. [Google Scholar] [CrossRef]
- Means, T.K.; Mylonakis, E.; Tampakakis, E.; Colvin, R.A.; Seung, E.; Puckett, L.; Tai, M.F.; Stewart, C.R.; Pukkila-Worley, R.; Hickman, S.E.; et al. Evolutionarily Conserved Recognition and Innate Immunity to Fungal Pathogens by the Scavenger Receptors SCARF1 and CD36. J. Exp. Med. 2009, 206, 637–653. [Google Scholar] [CrossRef]
- Means, T.K. Fungal Pathogen Recognition by Scavenger Receptors in Nematodes and Mammals. Virulence 2010, 1, 37–41. [Google Scholar] [CrossRef]
- Ruchti, F.; LeibundGut-Landmann, S. New Insights into Immunity to Skin Fungi Shape Our Understanding of Health and Disease. Parasite Immunol. 2022, 45, e12948. [Google Scholar] [CrossRef]
- Swidergall, M.; Filler, S.G. Oropharyngeal Candidiasis: Fungal Invasion and Epithelial Cell Responses. PLoS Pathog. 2017, 13, e1006056. [Google Scholar] [CrossRef] [PubMed]
- Igyártó, B.Z.; Haley, K.; Ortner, D.; Bobr, A.; Gerami-Nejad, M.; Edelson, B.T.; Zurawski, S.M.; Malissen, B.; Zurawski, G.; Berman, J.; et al. Skin-Resident Murine Dendritic Cell Subsets Promote Distinct and Opposing Antigen-Specific T Helper Responses. Immunity 2011, 35, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Ruchti, F.; Tuor, M.; Mathew, L.; McCarthy, N.E.; LeibundGut-Landmann, S. Γδ T Cells Respond Directly and Selectively to the Skin Commensal Yeast Malassezia for IL-17-Dependent Fungal Control. PLoS Pathog. 2024, 20, e1011668. [Google Scholar] [CrossRef]
- Jensen, O.; Trujillo, E.; Hanson, L.; Ost, K.S. Controlling Candida: Immune Regulation of Commensal Fungi in the Gut. Infect. Immun. 2024, 92, e00516–e00523. [Google Scholar] [CrossRef] [PubMed]
- Albac, S.; Schmitz, A.; Lopez-Alayon, C.; d’Enfert, C.; Sautour, M.; Ducreux, A.; Labruère-Chazal, C.; Laue, M.; Holland, G.; Bonnin, A.; et al. Candida albicans Is Able to Use M Cells as a Portal of Entry across the Intestinal Barrier In Vitro. Cell Microbiol. 2016, 18, 195–210. [Google Scholar] [CrossRef]
- Ayabe, T.; Satchell, D.P.; Wilson, C.L.; Parks, W.C.; Selsted, M.E.; Ouellette, A.J. Secretion of Microbicidal α-Defensins by Intestinal Paneth Cells in Response to Bacteria. Nat. Immunol. 2000, 1, 113–118. [Google Scholar] [CrossRef]
- Pierre, J.F.; Peters, B.M.; Torre, D.L.; Sidebottom, A.M.; Tao, Y.; Zhu, X.; Cham, C.M.; Wang, L.; Kambal, A.; Harris, K.G.; et al. Peptide YY: A Paneth Cell Antimicrobial Peptide That Maintains Candida Gut Commensalism. Science 2023, 381, 502–508. [Google Scholar] [CrossRef]
- Elphick, D.A.; Mahida, Y.R. Paneth Cells: Their Role in Innate Immunity and Inflammatory Disease. Gut 2005, 54, 1802–1809. [Google Scholar] [CrossRef]
- Alonso-Monge, R.; Gresnigt, M.S.; Román, E.; Hube, B.; Pla, J.; Jarosz, D. Candida albicans colonization of the gastrointestinal tract: A double-edged sword. PLoS Pathog. 2021, 17, e1009710. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, T.; Lu, X.; Xu, Z.; Qu, J.; Zhang, Z.; Shi, G.; Shen, S.; Hou, Y.; Chen, Y.; et al. Fungal-induced Glycolysis in Macrophages Promotes Colon Cancer by Enhancing Innate Lymphoid Cell Secretion of IL-22. EMBO J. 2021, 40, e105320. [Google Scholar] [CrossRef]
- Leonardi, I.; Gao, I.H.; Lin, W.Y.; Allen, M.; Li, X.V.; Fiers, W.D.; De Celie, M.B.; Putzel, G.G.; Yantiss, R.K.; Johncilla, M.; et al. Mucosal Fungi Promote Gut Barrier Function and Social Behavior via Type 17 Immunity. Cell 2022, 185, 831–846.e14. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, Y.; Rui, B.; Lei, Z.; Ning, X.; Liu, Y.; Li, M. Crosstalk between the Gut Microbiota and Innate Lymphoid Cells in Intestinal Mucosal Immunity. Front. Immunol. 2023, 14, 1171680. [Google Scholar] [CrossRef] [PubMed]
- Castleman, M.J.; Dillon, S.M.; Purba, C.M.; Cogswell, A.C.; Kibbie, J.J.; McCarter, M.D.; Santiago, M.L.; Barker, E.; Wilson, C.C. Commensal and Pathogenic Bacteria Indirectly Induce IL-22 but Not IFNγ Production from Human Colonic ILC3s via Multiple Mechanisms. Front. Immunol. 2019, 10, 649. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.S.; Camilli, G.; Kotowicz, N.K.; Ho, J.; Richardson, J.P.; Naglik, J.R. Role for IL-1 Family Cytokines in Fungal Infections. Front. Microbiol. 2021, 12, 633047. [Google Scholar] [CrossRef]
- Vonk, A.G.; Netea, M.G.; Van Krieken, J.H.; Iwakura, Y.; Van Der Meer, J.W.M.; Kullberg, B.J. Endogenous Interleukin (IL)-1α and IL-1β Are Crucial for Host Defense against Disseminated Candidiasis. J. Infect. Dis. 2006, 193, 1419–1426. [Google Scholar] [CrossRef]
- Zheng, Y.; Dang, E.V. Novel Mechanistic Insights Underlying Fungal Allergic Inflammation. PLoS Pathog. 2023, 19, e1011623. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Gomes, M.; Wich, M.; Böde, S.; Hube, B.; Jacobsen, I.D.; Jungnickel, B. B Cell Recognition of Candida albicans Hyphae via TLR 2 Promotes IgG1 and IL-6 Secretion for TH17 Differentiation. Front. Immunol. 2021, 12, 698849. [Google Scholar] [CrossRef]
- Wu, X.; Xia, Y.; He, F.; Zhu, C.; Ren, W. Intestinal Mycobiota in Health and Diseases: From a Disrupted Equilibrium to Clinical Opportunities. Microbiome 2021, 9, 60. [Google Scholar] [CrossRef]
- Li, R.; Rezk, A.; Li, H.; Gommerman, J.L.; Prat, A.; Bar-Or, A.; Canadian B Cells in MS Team. Antibody-Independent Function of Human B Cells Contributes to Antifungal T Cell Responses. J. Immunol. 2017, 198, 3245–3254. [Google Scholar] [CrossRef]
- Tian, R.; Fu, M.; Zhang, Z.; Ren, J.; An, J.; Liu, Y.; Li, W. In Situ IgM Production and Clonal Expansion of B-1 Cells in Peritoneal Cavity Promote Elimination of C. Albicans Infection in IgH Transgenic Mice with VH Derived from a Natural Antibody. PLoS ONE 2013, 8, e60779. [Google Scholar] [CrossRef]
- Chen, L.; Ruan, G.; Cheng, Y.; Yi, A.; Chen, D.; Wei, Y. The Role of Th17 Cells in Inflammatory Bowel Disease and the Research Progress. Front. Immunol. 2023, 13, 1055914. [Google Scholar] [CrossRef] [PubMed]
- Chehoud, C.; Albenberg, L.G.; Judge, C.; Hoffmann, C.; Grunberg, S.; Bittinger, K.; Baldassano, R.N.; Lewis, J.D.; Bushman, F.D.; Wu, G.D. Fungal Signature in the Gut Microbiota of Pediatric Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1948–1956. [Google Scholar] [CrossRef]
- Gutierrez, M.W.; van Tilburg Bernardes, E.; Changirwa, D.; McDonald, B.; Arrieta, M.C. “Molding” Immunity—Modulation of Mucosal and Systemic Immunity by the Intestinal Mycobiome in Health and Disease. Mucosal Immunol. 2022, 15, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Iliev, I.D.; Funari, V.A.; Taylor, K.D.; Nguyen, Q.; Reyes, C.N.; Strom, S.P.; Brown, J.; Becker, C.A.; Fleshner, P.R.; Dubinsky, M.; et al. Interactions between Commensal Fungi and the C-Type Lectin Receptor Dectin-1 Influence Colitis. Science 2012, 336, 1314–1317. [Google Scholar] [CrossRef]
- Leonardi, I.; Li, X.; Semon, A.; Li, D.; Doron, I.; Putzel, G.; Bar, A.; Prieto, D.; Rescigno, M.; McGovern, D.P.B.; et al. CX3CR1+, Mononuclear Phagocytes Control Immunity to Intestinal Fungi. Science 2018, 359, 232–236. [Google Scholar] [CrossRef]
- Kumamoto, C.A. The Fungal Mycobiota: Small Numbers, Large Impacts. In Cell Host and Microbe; Cell Press: Cambridge, MA, USA, 2016; pp. 750–751. [Google Scholar] [CrossRef]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal Microbiota Dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef]
- Luo, P.; Yang, Z.; Chen, B.; Zhong, X. The Multifaceted Role of CARD9 in Inflammatory Bowel Disease. J. Cell. Mol. Med. 2019, 24, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, A.M.; Faccenda, D.; Garcia-Cela, E. Unravelling the Impact of Mycotoxins on Gut Health: Implications for Inflammatory Bowel Disease. Curr. Opin. Food Sci. 2025, 64, 101316. [Google Scholar] [CrossRef]
- Richard, M.L.; Sokol, H. The Gut Mycobiota: Insights into Analysis, Environmental Interactions and Role in Gastrointestinal Diseases. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Sivignon, A.; De Vallée, A.; Barnich, N.; Denizot, J.; Darcha, C.; Pignède, G.; Vandekerckove, P.; Darfeuille-Michaud, A. Saccharomyces cerevisiae CNCM I-3856 Prevents Colitis Induced by AIEC Bacteria in the Transgenic Mouse Model Mimicking Crohn’s Disease. Inflamm. Bowel Dis. 2015, 21, 276–286. [Google Scholar] [CrossRef]
- Renga, G.; Bellet, M.M.; Stincardini, C.; Pariano, M.; Oikonomou, V.; Villella, V.R.; Brancorsini, S.; Clerici, C.; Romani, L.; Costantini, C. To Be or Not to Be a Pathogen: Candida albicans and Celiac Disease. Front. Immunol. 2019, 10, 2844. [Google Scholar] [CrossRef]
- Renga, G.; Pariano, M.; D’onofrio, F.; Pieraccini, G.; Di Serio, C.; Villella, V.R.; Abbate, C.; Puccetti, M.; Giovagnoli, S.; Stincardini, C.; et al. The Immune and Microbial Homeostasis Determines the Candida–Mast Cells Cross-Talk in Celiac Disease. Life Sci. Alliance 2024, 7, e202302441. [Google Scholar] [CrossRef]
- Gwak, M.G.; Chang, S.Y. Gut-Brain Connection: Microbiome, Gut Barrier, and Environmental Sensors. Immune Netw. 2021, 21, e20. [Google Scholar] [CrossRef]
- Markey, L.; Hooper, A.; Melon, L.C.; Baglot, S.; Hill, M.N.; Maguire, J.; Kumamoto, C.A. Colonization with the Commensal Fungus Candida albicans Perturbs the Gut-Brain Axis through Dysregulation of Endocannabinoid Signaling. Psychoneuroendocrinology 2020, 121, 104808. [Google Scholar] [CrossRef]
- Takata, K.; Tomita, T.; Okuno, T.; Kinoshita, M.; Koda, T.; Honorat, J.A.; Takei, M.; Hagihara, K.; Sugimoto, T.; Mochizuki, H.; et al. Dietary Yeasts Reduce Inflammation in Central Nerve System via Microflora. Ann. Clin. Transl. Neurol. 2014, 2, 56–66. [Google Scholar] [CrossRef]
- Yuan, X.; Li, X.; Hei, G.; Zhang, X.; Song, X. Intestinal Mycobiota Dysbiosis Associated Inflammation Activation in Chronic Schizophrenia. Behav. Brain Res. 2024, 472, 115149. [Google Scholar] [CrossRef]
- Zhou, K.; Baranova, A.; Cao, H.; Sun, J.; Zhang, F. Gut Microbiome and Schizophrenia: Insights from Two-Sample Mendelian Randomization. Schizophrenia 2024, 10, 75. [Google Scholar] [CrossRef]
- Tiew, P.Y.; Mac Aogain, M.; Ali, N.A.B.M.; Thng, K.X.; Goh, K.; Lau, K.J.X.; Chotirmall, S.H. The Mycobiome in Health and Disease: Emerging Concepts, Methodologies and Challenges. Mycopathologia 2020, 185, 207–231. [Google Scholar] [CrossRef]
- Kanj, A.N.; Skalski, J.H. Gut Mycobiome and Asthma. J. Fungi 2024, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Sey, E.A.; Warris, A. The Gut-Lung Axis: The Impact of the Gut Mycobiome on Pulmonary Diseases and Infections. Oxf. Open Immunol. 2024, 5, iqae008. [Google Scholar] [CrossRef] [PubMed]
- Soret, P.; Vandenborght, L.E.; Francis, F.; Coron, N.; Enaud, R.; Avalos, M.; Schaeverbeke, T.; Berger, P.; Fayon, M.; Thiebaut, R.; et al. Respiratory Mycobiome and Suggestion of Inter-Kingdom Network during Acute Pulmonary Exacerbation in Cystic Fibrosis. Sci. Rep. 2020, 10, 3589. [Google Scholar] [CrossRef]
- Liang, M.; Xu, J.; Luo, Y.; Qu, J. Epidemiology, Pathogenesis, Clinical Characteristics, and Treatment of Mucormycosis: A Review. Ann. Med. 2024, 56, 2396570. [Google Scholar] [CrossRef]
- Hoenigl, M.; Seidel, D.; Carvalho, A.; Rudramurthy, S.M.; Arastehfar, A.; Gangneux, J.P.; Nasir, N.; Bonifaz, A.; Araiza, J.; Klimko, N.; et al. The Emergence of COVID-19 Associated Mucormycosis: A Review of Cases from 18 Countries. Lancet Microbe 2022, 3, e543. [Google Scholar] [CrossRef]
- Alqarihi, A.; Kontoyiannis, D.P.; Ibrahim, A.S. Mucormycosis in 2023: An Update on Pathogenesis and Management. Front. Cell. Infect. Microbiol. 2023, 13, 1254919. [Google Scholar] [CrossRef] [PubMed]
- Axell-House, D.B.; Wurster, S.; Jiang, Y.; Kyvernitakis, A.; Lewis, R.E.; Tarrand, J.J.; Raad, I.I.; Kontoyiannis, D.P. Breakthrough Mucormycosis Developing on Mucorales-Active Antifungals Portrays a Poor Prognosis in Patients with Hematologic Cancer. J. Fungi 2021, 7, 217. [Google Scholar] [CrossRef]
- Gullì, S.P.; Hallur, V.; Kale, P.; Menezes, G.A.; Russo, A.; Singla, N. From Spores to Solutions: A Comprehensive Narrative Review on Mucormycosis. Diagnostics 2024, 14, 314. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Munley, J.A.; Kelly, L.S.; Kannan, K.B.; Mankowski, R.T.; Sharma, A.; Upchurch, G.; Casadesus, G.; Chakrabarty, P.; Wallet, S.M.; et al. Gut Mycobiome Dysbiosis after Sepsis and Trauma. Crit. Care 2024, 28, 18. [Google Scholar] [CrossRef] [PubMed]
- Krivonos, D.V.; Fedorov, D.E.; Klimina, K.M.; Veselovsky, V.A.; Kovalchuk, S.N.; Pavlenko, A.V.; Yanushevich, O.O.; Andreev, D.N.; Sokolov, F.S.; Fomenko, A.K.; et al. Gut Mycobiome Changes During COVID-19 Disease. J. Fungi 2025, 11, 194. [Google Scholar] [CrossRef]
- Yin, Y.; Tuohutaerbieke, M.; Feng, C.; Li, X.; Zhang, Y.; Xu, Q.; Tu, J.; Yang, E.; Zou, Q.; Shen, T. Characterization of the Intestinal Fungal Microbiome in HIV and HCV Mono-Infected or Co-Infected Patients. Viruses 2022, 14, 1811. [Google Scholar] [CrossRef]
- Feys, S.; Carvalho, A.; Clancy, C.J.; Gangneux, J.P.; Hoenigl, M.; Lagrou, K.; Rijnders, B.J.A.; Seldeslachts, L.; Vanderbeke, L.; van de Veerdonk, F.L.; et al. Influenza-Associated and COVID-19-Associated Pulmonary Aspergillosis in Critically Ill Patients. Lancet Respir. Med. 2024, 12, 728–742. [Google Scholar] [CrossRef]
- Lamoth, F.; Lewis, R.E.; Walsh, T.J.; Kontoyiannis, D.P. Navigating the Uncertainties of COVID-19-Associated Aspergillosis: A Comparison with Influenza-Associated Aspergillosis. J. Infect. Dis. 2021, 224, 1631–1640. [Google Scholar] [CrossRef]
- Dewi, I.M.; Janssen, N.A.; Rosati, D.; Bruno, M.; Netea, M.G.; Brüggemann, R.J.; Verweij, P.E.; van de Veerdonk, F.L. Invasive Pulmonary Aspergillosis Associated with Viral Pneumonitis. Curr. Opin. Microbiol. 2021, 62, 21–27. [Google Scholar] [CrossRef]
- Lv, L.; Gu, S.; Jiang, H.; Yan, R.; Chen, Y.; Chen, Y.; Luo, R.; Huang, C.; Lu, H.; Zheng, B.; et al. Gut Mycobiota Alterations in Patients with COVID-19 and H1N1 Infections and Their Associations with Clinical Features. Commun. Biol. 2021, 4, 480. [Google Scholar] [CrossRef]
- Maurer, H.C.; Schult, D.; Koyumdzhieva, P.; Reitmeier, S.; Middelhoff, M.; Rasch, S.; List, M.; Janssen, K.P.; Steiger, K.; Protzer, U.; et al. Gut Microbial Disruption in Critically Ill Patients with COVID-19-Associated Pulmonary Aspergillosis. J. Fungi 2022, 8, 1265. [Google Scholar] [CrossRef]
- Dakalbab, S.; Hamdy, R.; Holigová, P.; Abuzaid, E.J.; Abu-Qiyas, A.; Lashine, Y.; Mohammad, M.G.; Soliman, S.S.M. Uniqueness of Candida Auris Cell Wall in Morphogenesis, Virulence, Resistance, and Immune Evasion. Microbiol. Res. 2024, 286, 127797. [Google Scholar] [CrossRef] [PubMed]
- Holt, A.M.; Nett, J.E. Innate Immune Response to Candida auris. Curr. Opin. Microbiol. 2024, 80, 102510. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; McClain, C.J.; Feng, W. Microbiome dysbiosis and alcoholic liver disease. Liver Res. 2019, 3, 218–226. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, L.; Stärkel, P.; Fan, J.G.; Fouts, D.E.; Bacher, P.; Schnabl, B. The Gut Mycobiome: A Novel Player in Chronic Liver Diseases. J. Gastroenterol. 2020, 56, 1–11. [Google Scholar] [CrossRef]
- Borgo, F.; Verduci, E.; Riva, A.; Lassandro, C.; Riva, E.; Morace, G.; Borghi, E. Relative Abundance in Bacterial and Fungal Gut Microbes in Obese Children: A Case Control Study. Child. Obes. 2017, 13, 78–84. [Google Scholar] [CrossRef]
- Mar Rodríguez, M.; Pérez, D.; Javier Chaves, F.; Esteve, E.; Marin-Garcia, P.; Xifra, G.; Vendrell, J.; Jové, M.; Pamplona, R.; Ricart, W.; et al. Obesity Changes the Human Gut Mycobiome. Sci. Rep. 2015, 5, 14600. [Google Scholar] [CrossRef]
- Shah, A.; MacDonald, G.A.; Morrison, M.; Holtmann, G. Targeting the Gut Microbiome as a Treatment for Primary Sclerosing Cholangitis: A Conceptional Framework. Am. J. Gastroenterol. 2020, 115, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Muratori, P.; Muratori, L.; Guidi, M.; Maccariello, S.; Pappas, G.; Ferrari, R.; Gionchetti, P.; Campieri, M.; Bianchi, F.B. Anti-Saccharomyces cerevisiae Antibodies (ASCA) and Autoimmune Liver Diseases. Clin. Exp. Immunol. 2003, 132, 473–476. [Google Scholar] [CrossRef]
- Gosiewski, T.; Salamon, D.; Szopa, M.; Sroka, A.; Malecki, M.T.; Bulanda, M. Quantitative Evaluation of Fungi of the Genus Candida in the Feces of Adult Patients with Type 1 and 2 Diabetes—A Pilot Study. Gut Pathog. 2014, 6, 43. [Google Scholar] [CrossRef]
- Soyucen, E.; Gulcan, A.; Aktuglu-Zeybek, A.C.; Onal, H.; Kiykim, E.; Aydin, A. Differences in the Gut Microbiota of Healthy Children and Those with Type 1 Diabetes. Pediatr. Int. 2014, 56, 336–343. [Google Scholar] [CrossRef]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.; Preiss, P.; Verma, N.; et al. The Fungal Mycobiome Promotes Pancreatic Oncogenesis via MBL Activation. Nature 2019, 574, 264–267. [Google Scholar] [CrossRef]
- Nguyen, U.T.; Kalan, L.R. Forgotten Fungi: The Importance of the Skin Mycobiome. Curr. Opin. Microbiol. 2022, 70, 102235. [Google Scholar] [CrossRef]
- Żychowska, M.; Bakuła, Z.; Decewicz, P.; Hryncewicz-Gwóźdź, A.; Dyląg, M.; Jankowska-Konsur, A.; Gawor, J.; Gromadka, R.; Żaczek, A.; Jagielski, T. The Skin Mycobiome of Patients With Atopic Dermatitis and Healthy Volunteers: A Case-Control Study. Exp. Dermatol. 2025, 34, e70085. [Google Scholar] [CrossRef] [PubMed]
- Koike, Y.; Kuwatsuka, S.; Motooka, D.; Murota, H. Dysbiosis of the Human Skin Mycobiome in Patients Receiving Systemic IL-23 Inhibitors. Allergol. Int. 2025, 74, 72–77. [Google Scholar] [CrossRef]
- Carr, M.M.; Pryce, D.M.; Ive, F.A. Treatment of Seborrhoeic Dermatitis with Ketoconazole: I. Response of Seborrhoeic Dermatitis of the Scalp to Topical Ketoconazole. Br. J. Dermatol. 1987, 116, 213–216. [Google Scholar] [CrossRef]
- Lober, C.W. Patch Tests with Killed Sonicated Microflora in Patients with Psoriasis. Arch. Dermatol. 1982, 118, 322–325. [Google Scholar] [CrossRef]
- Jury, C.S.; McHugh, L.; Shankland, G.S.; Burden, A.D. A Randomized, Placebo-Controlled Trial of Oral Itraconazole in Scalp Psoriasis. J. Dermatol. Treat. 2000, 11, 85–89. [Google Scholar] [CrossRef]
- Liu, X.; Cai, Q.; Yang, H.; Gao, Z.; Yang, L. Distribution of Malassezia Species on the Skin of Patients with Psoriasis. J. Mycol. Med. 2021, 31, 101111. [Google Scholar] [CrossRef]
- Łabędź, N.; Navarrete-Dechent, C.; Kubisiak-Rzepczyk, H.; Bowszyc-Dmochowska, M.; Pogorzelska-Antkowiak, A.; Pietkiewicz, P. Pityriasis Versicolor—A Narrative Review on the Diagnosis and Management. Life 2023, 13, 2097. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Dóczi, I.; Ábrók, M.; Lázár, A.; Burián, K. Epidemiology of Candiduria and Candida Urinary Tract Infections in Inpatients and Outpatients: Results from a 10-Year Retrospective Survey. Cent. Eur. J. Urol. 2019, 72, 209–214. [Google Scholar] [CrossRef]
- Wehedy, E.; Murugesan, S.; George, C.R.; Shatat, I.F.; Al Khodor, S. Characterization of the Urinary Metagenome and Virome in Healthy Children. Biomedicines 2022, 10, 2412. [Google Scholar] [CrossRef]
- Behzadi, P.; Behzadi, E.; Ranjbar, R. Urinary Tract Infections and Candida albicans. Cent. Eur. J. Urol. 2015, 68, 96–101. [Google Scholar] [CrossRef]
- Fidel, P.L.; Cutright, J.; Steele, C. Effects of Reproductive Hormones on Experimental Vaginal Candidiasis. Infect. Immun. 2000, 68, 651–657. [Google Scholar] [CrossRef]
- Kumwenda, P.; Cottier, F.; Hendry, A.C.; Kneafsey, D.; Keevan, B.; Gallagher, H.; Tsai, H.J.; Hall, R.A. Estrogen Promotes Innate Immune Evasion of Candida albicans through Inactivation of the Alternative Complement System. Cell Rep. 2022, 38, 110183. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Levanduski, E.; Denz, P.; Villavicencio, H.S.; Bhatta, M.; Alhorebi, L.; Zhang, Y.; Gomez, E.C.; Morreale, B.; Senchanthisai, S.; et al. Fungal Mycobiome Drives IL-33 Secretion and Type 2 Immunity in Pancreatic Cancer. Cancer Cell 2022, 40, 153–167.e11. [Google Scholar] [CrossRef]
- Wei, A.; Zhao, H.; Cong, X.; Wang, L.; Chen, Y.; Gou, J.; Hu, Z.; Hu, X.; Tian, Y.; Li, K.; et al. Oral Mycobiota and Pancreatic Ductal Adenocarcinoma. BMC Cancer 2022, 22, 1251. [Google Scholar] [CrossRef]
- Qin, X.; Gu, Y.; Liu, T.; Wang, C.; Zhong, W.; Wang, B.; Cao, H. Gut Mycobiome: A Promising Target for Colorectal Cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188489. [Google Scholar] [CrossRef]
- Coker, O.O.; Nakatsu, G.; Dai, R.Z.; Wu, W.K.K.; Wong, S.H.; Ng, S.C.; Chan, F.K.L.; Sung, J.J.Y.; Yu, J. Enteric Fungal Microbiota Dysbiosis and Ecological Alterations in Colorectal Cancer. Gut 2019, 68, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Sharma, D.; Malireddi, R.K.S.; Guy, C.S.; Chang, T.C.; Olsen, S.R.; Neale, G.; Vogel, P.; Kanneganti, T.D. SYK-CARD9 Signaling Axis Promotes Gut Fungi-Mediated Inflammasome Activation to Restrict Colitis and Colon Cancer. Immunity 2018, 49, 515–530.e5. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, X.; Xu, R.; Adeel, K.; Lu, X.; Chen, M.; Shen, H.; Li, Z.; Xu, Z. Fungal Microbiota Dysbiosis and Ecological Alterations in Gastric Cancer. Front. Microbiol. 2022, 13, 889694. [Google Scholar] [CrossRef] [PubMed]
- Johannesburg, W.; Kew, M.C. AAatoxins as a Cause of Hepatocellular Carcinoma. J. Gastrointest. Liver Dis. 2013, 22, 305–310. [Google Scholar] [CrossRef]
- Mohamed, N.; Litlekalsøy, J.; Ahmed, I.A.; Martinsen, E.M.H.; Furriol, J.; Javier-Lopez, R.; Elsheikh, M.; Gaafar, N.M.; Morgado, L.; Mundra, S.; et al. Analysis of Salivary Mycobiome in a Cohort of Oral Squamous Cell Carcinoma Patients from Sudan Identifies Higher Salivary Carriage of Malassezia as an Independent and Favorable Predictor of Overall Survival. Front. Cell. Infect. Microbiol. 2021, 11, 673465. [Google Scholar] [CrossRef]
- Mäkinen, A.; Nawaz, A.; Mäkitie, A.; Meurman, J.H. Role of Non-Albicans Candida and Candida albicans in Oral Squamous Cell Cancer Patients. J. Oral. Maxillofac. Surg. 2018, 76, 2564–2571. [Google Scholar] [CrossRef]
- Dohlman, A.B.; Klug, J.; Mesko, M.; Gao, I.H.; Lipkin, S.M.; Shen, X.; Iliev, I.D. A Pan-Cancer Mycobiome Analysis Reveals Fungal Involvement in Gastrointestinal and Lung Tumors. Cell 2022, 185, 3807–3822.e12. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, W.; Pei, Y.; Tao, H.; Ma, J.; Li, R.; Zhang, F.; Wang, L.; Shen, L.; Liu, Y.; et al. Gut Mycobiome as a Potential Non-Invasive Tool in Early Detection of Lung Adenocarcinoma: A Cross-Sectional Study. BMC Med. 2023, 21, 409. [Google Scholar] [CrossRef]
- Liu, N.N.; Yi, C.X.; Wei, L.Q.; Zhou, J.A.; Jiang, T.; Hu, C.C.; Wang, L.; Wang, Y.Y.; Zou, Y.; Zhao, Y.K.; et al. The Intratumor Mycobiome Promotes Lung Cancer Progression via Myeloid-Derived Suppressor Cells. Cancer Cell 2023, 41, 1927–1944.e9. [Google Scholar] [CrossRef]
- Alonso, R.; Fernández-Fernández, A.M.; Pisa, D.; Carrasco, L. Multiple sclerosis and mixed microbial infections. Direct identification of fungi and bacteria in nervous tissue. Neurobiol. Dis. 2018, 117, 42–61. [Google Scholar] [CrossRef]
- Li, B.Z.; Wang, H.; Li, X.B.; Zhang, Q.R.; Huang, R.G.; Wu, H.; Wang, Y.Y.; Li, K.D.; Chu, X.J.; Cao, N.W.; et al. Altered Gut Fungi in Systemic Lupus Erythematosus—A Pilot Study. Front. Microbiol. 2022, 13, 1031079. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Chang, F.C.; Hung, Y.M.; Chou, M.C.; Yip, H.T.; Chang, R.; Wei, J.C.C. Candida Infection as an Early Sign of Subsequent Sjögren’s Syndrome: A Population-Based Matched Cohort Study. Front. Med. 2022, 8, 796324. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.P.; Pathak, K.; Saikia, R.; Gogoi, U.; Ahmad, M.Z.; Patowary, P.; Das, A. Immunomodulatory Effect of Mushrooms and Their Bioactive Compounds in Cancer: A Comprehensive Review. Biomed. Pharmacother. 2022, 149, 112901. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Luo, F.; Ding, C.; Albeituni, S.; Hu, X.; Ma, Y.; Cai, Y.; McNally, L.; Sanders, M.A.; Jain, D.; et al. Dectin-1 Activation by a Natural Product β-Glucan Converts Immunosuppressive Macrophages into an M1-like Phenotype. J. Immunol. 2015, 195, 5055–5065. [Google Scholar] [CrossRef]
- Varnosfaderani, S.M.N.; Ebrahimzadeh, F.; Oryani, M.A.; Khalili, S.; Almasi, F.; Heris, R.M.; Payandeh, Z.; Li, C.; Afjadi, M.N.; Bahrami, A.A. Potential Promising Anticancer Applications of β-Glucans: A Review. Biosci. Rep. 2024, 44, BSR20231686. [Google Scholar] [CrossRef]
- Steimbach, L.; Borgmann, A.V.; Gomar, G.G.; Hoffmann, L.V.; Rutckeviski, R.; de Andrade, D.P.; Smiderle, F.R. Fungal Beta-Glucans as Adjuvants for Treating Cancer Patients—A Systematic Review of Clinical Trials. Clin. Nutr. 2021, 40, 3104–3113. [Google Scholar] [CrossRef] [PubMed]
- Kushner, B.H.; Cheung, I.Y.; Modak, S.; Kramer, K.; Ragupathi, G.; Cheung, N.K.V. Phase i Trial of a Bivalent Gangliosides Vaccine in Combination with β-Glucan for High-Risk Neuroblastoma in Second or Later Remission. Clin. Cancer Res. 2014, 20, 1375–1382. [Google Scholar] [CrossRef]
- Engel-Riedel, W.; Lowe, J.; Mattson, P.; Richard Trout, J.; Huhn, R.D.; Gargano, M.; Patchen, M.L.; Walsh, R.; Trinh, M.M.; Dupuis, M.; et al. A Randomized, Controlled Trial Evaluating the Efficacy and Safety of BTH1677 in Combination with Bevacizumab, Carboplatin, and Paclitaxel in First-Line Treatment of Advanced Non-Small Cell Lung Cancer. J. Immunother. Cancer 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Lu, Y.; Li, D.; Qin, L.; Qi, C.; Pu, X.; Zhuang, Y.; Zhu, Y.; Zha, Q.; Wang, G.; et al. β-Glucan Combined with Envafolimab and Endostar as Immune Rechallenge for Metastatic Non-Small Cell Lung Cancer. BMC Immunol. 2024, 25, 60. [Google Scholar] [CrossRef] [PubMed]
- Modak, S.; Koehne, G.; Vickers, A.; O’Reilly, R.J.; Cheung, N.K.V. Rituximab Therapy of Lymphoma Is Enhanced by Orally Administered (1 → 3),(1 → 4)-D-β-Glucan. Leuk. Res. 2005, 29, 679–683. [Google Scholar] [CrossRef]
- Zent, C.S.; Call, T.G.; Bowen, D.A.; Conte, M.J.; LaPlant, B.R.; Witzig, T.E.; Ansell, S.M.; Weiner, G.J. Early Treatment of High Risk Chronic Lymphocytic Leukemia with Alemtuzumab, Rituximab and PGG Beta Glucan Is Well Tolerated and Achieves High Complete Remission Rates. Leuk. Lymphoma 2015, 56, 2373–2378. [Google Scholar] [CrossRef]
- Segal, N.H.; Gada, P.; Senzer, N.; Gargano, M.A.; Patchen, M.L.; Saltz, L.B. A Phase 2 Efficacy and Safety, Open-Label, Multicenter Study of Imprime PGG Injection in Combination with Cetuximab in Patients with Stage IV KRAS-Mutant Colorectal Cancer. Clin. Color. Cancer 2016, 15, 222–227. [Google Scholar] [CrossRef]
- Dalmasso, G.; Cottrez, F.; Imbert, V.; Lagadec, P.; Peyron, J.F.; Rampal, P.; Czerucka, D.; Groux, H. Saccharomyces boulardii Inhibits Inflammatory Bowel Disease by Trapping T Cells in Mesenteric Lymph Nodes. Gastroenterology 2006, 131, 1812–1825. [Google Scholar] [CrossRef]
- Mourey, F.; Sureja, V.; Kheni, D.; Shah, P.; Parikh, D.; Upadhyay, U.; Satia, M.; Shah, D.; Troise, C.; Decherf, A. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of Saccharomyces boulardii in Infants and Children With Acute Diarrhea. Pediatr. Infect. Dis. J. 2020, 39, e347. [Google Scholar] [CrossRef]
- Pal, B.B.; Bandagi, R.V.; Pebbili, K.K.; Rathod, R.; Kotak, B.; Dhanaki, G.; Shah, S. Effectiveness of Saccharomyces boulardii CNCM I-745 in Adult Indian Patients with Diarrhoea: A Real-World, Multicentre, Retrospective, Comparative Study. Drugs Real World Outcomes 2024, 11, 309–316. [Google Scholar] [CrossRef]
- Guslandi, M.; Giollo, P.; Testoni, P.A. A Pilot Trial of Saccharomyces boulardii in Ulcerative Colitis. Eur. J. Gastroenterol. Hepatol. 2003, 15, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Heavey, M.K.; Hazelton, A.; Wang, Y.; Garner, M.; Anselmo, A.C.; Arthur, J.C.; Nguyen, J. Targeted Delivery of the Probiotic Saccharomyces boulardii to the Extracellular Matrix Enhances Gut Residence Time and Recovery in Murine Colitis. Nat. Commun. 2024, 15, 3784. [Google Scholar] [CrossRef] [PubMed]
- Pothoulakis, C. Review Article: Anti-Inflammatory Mechanisms of Action of Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2008, 30, 826–833. [Google Scholar] [CrossRef]
- Pineton de Chambrun, G.; Neut, C.; Chau, A.; Cazaubiel, M.; Pelerin, F.; Justen, P.; Desreumaux, P. A Randomized Clinical Trial of Saccharomyces cerevisiae versus Placebo in the Irritable Bowel Syndrome. Dig. Liver Dis. 2015, 47, 119–124. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, E. The Probiotic Effects of the Saccharomyces cerevisiae 28-7 Strain Isolated from Nuruk in a DSS-Induced Colitis Mouse Model. J. Microbiol. Biotechnol. 2022, 32, 877–884. [Google Scholar] [CrossRef]
- Etienne-Mesmin, L.; Livrelli, V.; Privat, M.; Denis, S.; Cardot, J.M.; Alric, M.; Blanquet-Diot, S. Effect of a New Probiotic Saccharomyces cerevisiae Strain on Survival of Escherichia Coli O157:H7 in a Dynamic Gastrointestinal Model. Appl. Environ. Microbiol. 2011, 77, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Duysburgh, C.; Miclotte, L.; Green, J.B.; Watts, K.T.; Sardi, M.I.; Chakrabarti, A.; Khafipour, E.; Marzorati, M. Saccharomyces cerevisiae Derived Postbiotic Alters Gut Microbiome Metabolism in the Human Distal Colon Resulting in Immunomodulatory Potential in Vitro. Front. Microbiol. 2024, 15, 1358456. [Google Scholar] [CrossRef]
- Poloni, V.L.; Pérez, M.E.; Escobar, F.; Luna, J.; Pereyra, Y.; Cristofolini, A.; Magnoli, A.; Cavaglieri, L. Postbiotics from Saccharomyces cerevisiae RC016 Cell Wall (Formerly Classified as a Prebiotic): Exploring In Vitro and In Vivo Benefits. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef]
- Tullio, V. Probiotic Yeasts: A Developing Reality? J. Fungi 2024, 10, 489. [Google Scholar] [CrossRef]
- Angulo, M.; Ramos, A.; Reyes-Becerril, M.; Guerra, K.; Monreal-Escalante, E.; Angulo, C. Probiotic Debaryomyces hansenii CBS 8339 yeast enhanced immune responses in mice. 3 Biotech 2022, 13, 28. [Google Scholar] [CrossRef]
- Ochangco, H.S.; Gamero, A.; Smith, I.M.; Christensen, J.E.; Jespersen, L.; Arneborg, N. In Vitro Investigation of Debaryomyces Hansenii Strains for Potential Probiotic Properties. World J. Microbiol. Biotechnol. 2016, 32, 141. [Google Scholar] [CrossRef]
- Ullah, M.; Rizwan, M.; Raza, A.; Xia, Y.; Han, J.; Ma, Y.; Chen, H. Snapshot of the Probiotic Potential of Kluveromyces marxianus DMKU-1042 Using a Comparative Probiogenomics Approach. Foods 2023, 12, 4329. [Google Scholar] [CrossRef]
- Keskin, P.; Kılıç Kanak, E.; Öztürk Yılmaz, S. Assessment of the Probiotic Properties of Yarrowia Lipolytica Isolated from Cold-Pressed Olive Oil. Microorganisms 2024, 12, 1905. [Google Scholar] [CrossRef]
- Ganapathiwar, S.; Bhukya, B. In Vitro Assessment for the Probiotic Potential of Pichia Kudriavzevii. Bioinformation 2023, 19, 441–444. [Google Scholar] [CrossRef]
- Helmy, E.A.; Abdel-Fadeel, R.H.; Yosri, M.; Hassan, E. Does Torulaspora Delbrueckii Has Some Probiotic Capabilities? In Vitro and in Vivo Assessment. Nutrire 2024, 49, 15. [Google Scholar] [CrossRef]
- Zuo, T.; Wong, S.H.; Cheung, C.P.; Lam, K.; Lui, R.; Cheung, K.; Zhang, F.; Tang, W.; Ching, J.Y.L.; Wu, J.C.Y.; et al. Gut Fungal Dysbiosis Correlates with Reduced Efficacy of Fecal Microbiota Transplantation in Clostridium Difficile Infection. Nat. Commun. 2018, 9, 3663. [Google Scholar] [CrossRef]
- Leonardi, I.; Paramsothy, S.; Doron, I.; Semon, A.; Kaakoush, N.O.; Clemente, J.C.; Faith, J.J.; Borody, T.J.; Mitchell, H.M.; Colombel, J.F.; et al. Fungal Trans-Kingdom Dynamics Linked to Responsiveness to Fecal Microbiota Transplantation (FMT) Therapy in Ulcerative Colitis. Cell Host Microbe 2020, 27, 823–829.e3. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wu, X.; Li, X.; Chen, M. Cancer-Associated Fungi: An Emerging Powerful Player in Cancer Immunotherapy. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2025, 1880, 189287. [Google Scholar] [CrossRef] [PubMed]
- El Jaddaoui, I.; Sehli, S.; Al Idrissi, N.; Bakri, Y.; Belyamani, L.; Ghazal, H. The Gut Mycobiome for Precision Medicine. J. Fungi 2025, 11, 279. [Google Scholar] [CrossRef]
- Quinton, J.F.; Sendid, B.; Reumaux, D.; Duthilleul, P.; Cortot, A.; Grandbastien, B.; Charrier, G.; Targan, S.R.; Colombel, J.F.; Poulain, D. Anti-Saccharomyces cerevisiae Mannan Antibodies Combined with Antineutrophil Cytoplasmic Autoantibodies in Inflammatory Bowel Disease: Prevalence and Diagnostic Role. Gut 1998, 42, 788–791. [Google Scholar] [CrossRef]
- Yao, G.; Zhang, X.; Zhang, T.; Jin, J.; Qin, Z.; Ren, X.; Wang, X.; Zhang, S.; Yin, X.; Tian, Z.; et al. The Role of Dysbiotic Gut Mycobiota in Modulating Risk for Abdominal Aortic Aneurysm. Microbiol. Spectr. 2024, 12, e0177624. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, R.R.; Miao, N.J.; Tang, J.J.; Zhang, Y.W.; Lu, X.R.; Yan, P.Y.; Wang, J.; Jia, X.M. PD-L1 Negatively Regulates Antifungal Immunity by Inhibiting Neutrophil Release from Bone Marrow. Nat. Commun. 2022, 13, 6857. [Google Scholar] [CrossRef]
- Turnbull, C.; Bones, J.; Stanley, M.; Medhavy, A.; Wang, H.; Lorenzo, A.M.D.; Cappello, J.; Shanmuganandam, S.; Pandey, A.; Seneviratne, S.; et al. DECTIN-1: A Modifier Protein in CTLA-4 Haploinsufficiency. Sci. Adv. 2023, 9, eadi9566. [Google Scholar] [CrossRef]
- Szostak, N.; Handschuh, L.; Samelak-Czajka, A.; Tomela, K.; Pietrzak, B.; Schmidt, M.; Galus, Ł.; Mackiewicz, J.; Mackiewicz, A.; Kozlowski, P.; et al. Gut Mycobiota Dysbiosis Is Associated with Melanoma and Response to Anti–PD-1 Therapy. Cancer Immunol. Res. 2024, 12, 427–439. [Google Scholar] [CrossRef]
- Huang, X.; Hu, M.; Sun, T.; Li, J.; Zhou, Y.; Yan, Y.; Xuan, B.; Wang, J.; Xiong, H.; Ji, L.; et al. Multi-Kingdom Gut Microbiota Analyses Define Bacterial-Fungal Interplay and Microbial Markers of Pan-Cancer Immunotherapy across Cohorts. Cell Host Microbe 2023, 31, 1930–1943.e4. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhu, X.; Huang, X.; Hua, L.; Lin, X.; Zhang, H.; Hu, Y.; Tong, T.; Li, L.; Xuan, B.; et al. Optimizing Anti-PD-1/PD-L1 Therapy Efficacy and Fecal Microbiota Transplantation Donor Selection through Gut Mycobiome-Based Enterotype. Cell Rep. 2025, 44, 115589. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, C.; Wang, S.; Xun, Z.; Zhang, D.; Lan, Z.; Zhang, L.; Chao, J.; Liang, Y.; Pu, Z.; et al. Characterizations of Multi-Kingdom Gut Microbiota in Immune Checkpoint Inhibitor-Treated Hepatocellular Carcinoma. J. Immunother. Cancer 2024, 12, e008686. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Huang, L.; Jiang, A.; Zhu, L.; Mou, W.; Li, Y.; Zhang, C.; Liu, Z.; Zhang, J.; Cheng, Q.; et al. Microbiota Boost Immunotherapy? A Meta-Analysis Dives into Fecal Microbiota Transplantation and Immune Checkpoint Inhibitors. BMC Med. 2025, 23, 341. [Google Scholar] [CrossRef] [PubMed]
| Age Group | Predominant Fungal Species | Study |
|---|---|---|
| 10 days to 3 months after birth | Rhodotorula mucilaginosa and Debaryomyces hansenii (from breast milk or formula) | [41] |
| >1–2 years | Ascomycota, Zygomycota, and Basidiomycota, with Candida species and Saccharomyces cerevisiae of Ascomycota being the most abundant due to the introduction of solid foods. | [41] |
| Adults | Candida spp., Saccharomyces, and Cladosporium | [3] |
| Elders (>65 years) | Penicillium, Candida, Aspergillus, and Saccharomyces | [42,43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo-Serradell, L.; Liu-Tindall, J.; Planells-Romeo, V.; Aragón-Serrano, L.; Isamat, M.; Gabaldón, T.; Lozano, F.; Velasco-de Andrés, M. The Human Mycobiome: Composition, Immune Interactions, and Impact on Disease. Int. J. Mol. Sci. 2025, 26, 7281. https://doi.org/10.3390/ijms26157281
Carrillo-Serradell L, Liu-Tindall J, Planells-Romeo V, Aragón-Serrano L, Isamat M, Gabaldón T, Lozano F, Velasco-de Andrés M. The Human Mycobiome: Composition, Immune Interactions, and Impact on Disease. International Journal of Molecular Sciences. 2025; 26(15):7281. https://doi.org/10.3390/ijms26157281
Chicago/Turabian StyleCarrillo-Serradell, Laura, Jade Liu-Tindall, Violeta Planells-Romeo, Lucía Aragón-Serrano, Marcos Isamat, Toni Gabaldón, Francisco Lozano, and María Velasco-de Andrés. 2025. "The Human Mycobiome: Composition, Immune Interactions, and Impact on Disease" International Journal of Molecular Sciences 26, no. 15: 7281. https://doi.org/10.3390/ijms26157281
APA StyleCarrillo-Serradell, L., Liu-Tindall, J., Planells-Romeo, V., Aragón-Serrano, L., Isamat, M., Gabaldón, T., Lozano, F., & Velasco-de Andrés, M. (2025). The Human Mycobiome: Composition, Immune Interactions, and Impact on Disease. International Journal of Molecular Sciences, 26(15), 7281. https://doi.org/10.3390/ijms26157281






