Acquired Radioresistance Through Adaptive Evolution with Gamma Radiation as Selection Pressure: Increased Expression and Induction of Anti-Stress Genes
Abstract
1. Introduction
2. Results
2.1. Generation of Radioresistant E. coli via Adaptive Evolution
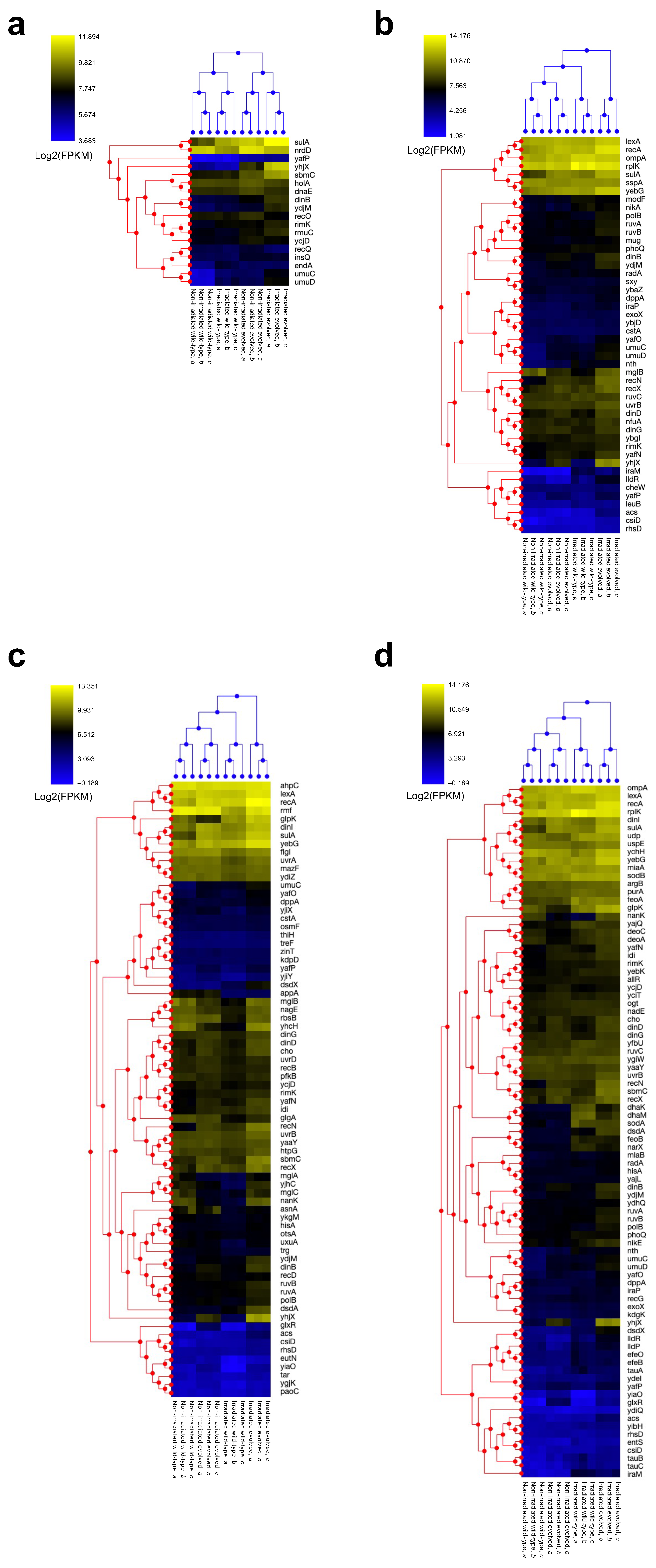
2.2. Alterations in Steady-State Gene Expression Through Evolution
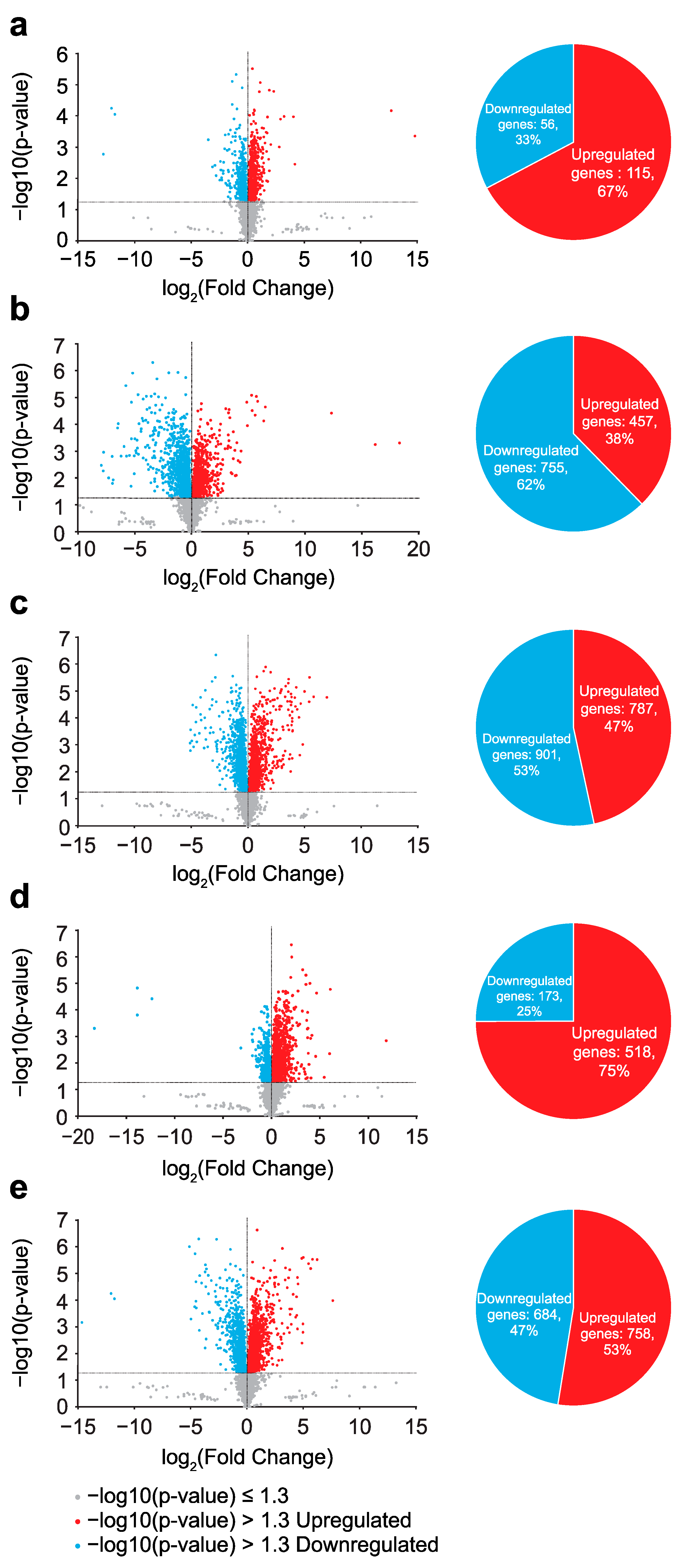
2.3. Gamma Irradiation-Induced Gene Expression Changes in Wild-Type E. coli
2.4. Gamma Irradiation-Induced Gene Expression Changes in Evolved E. coli
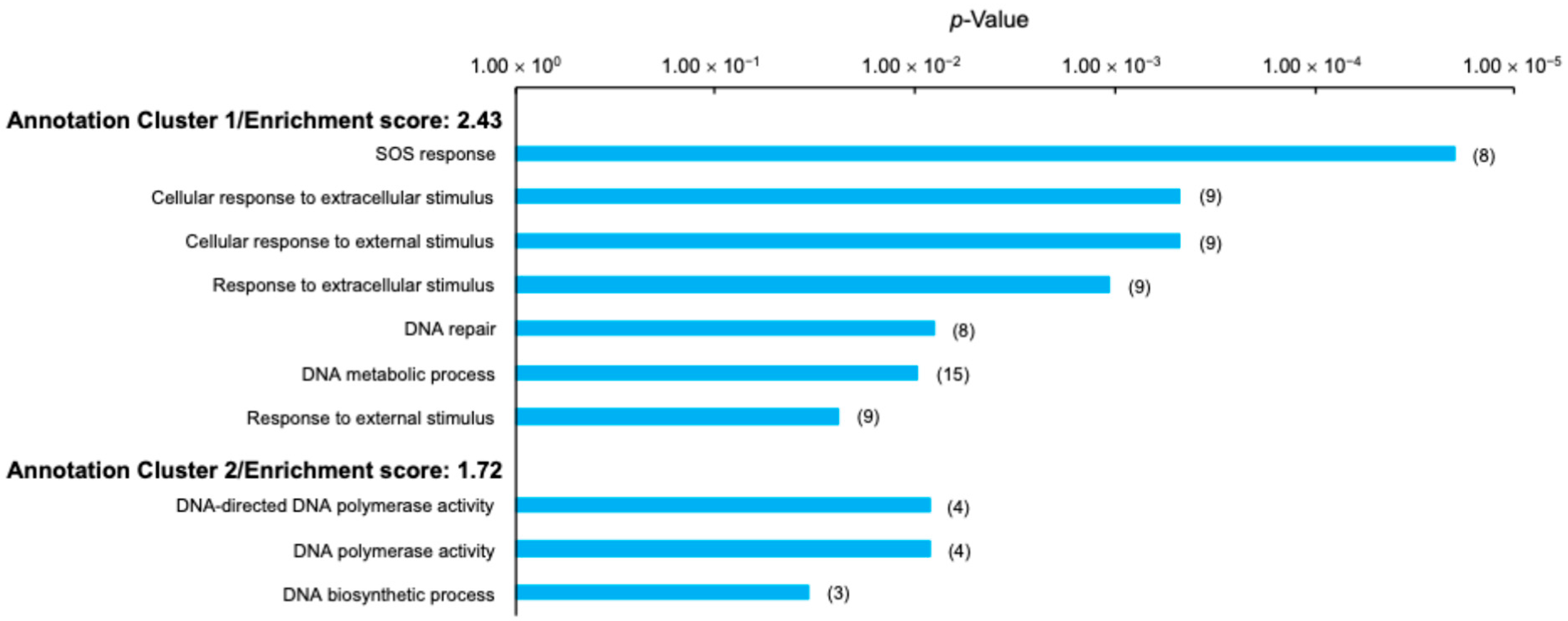
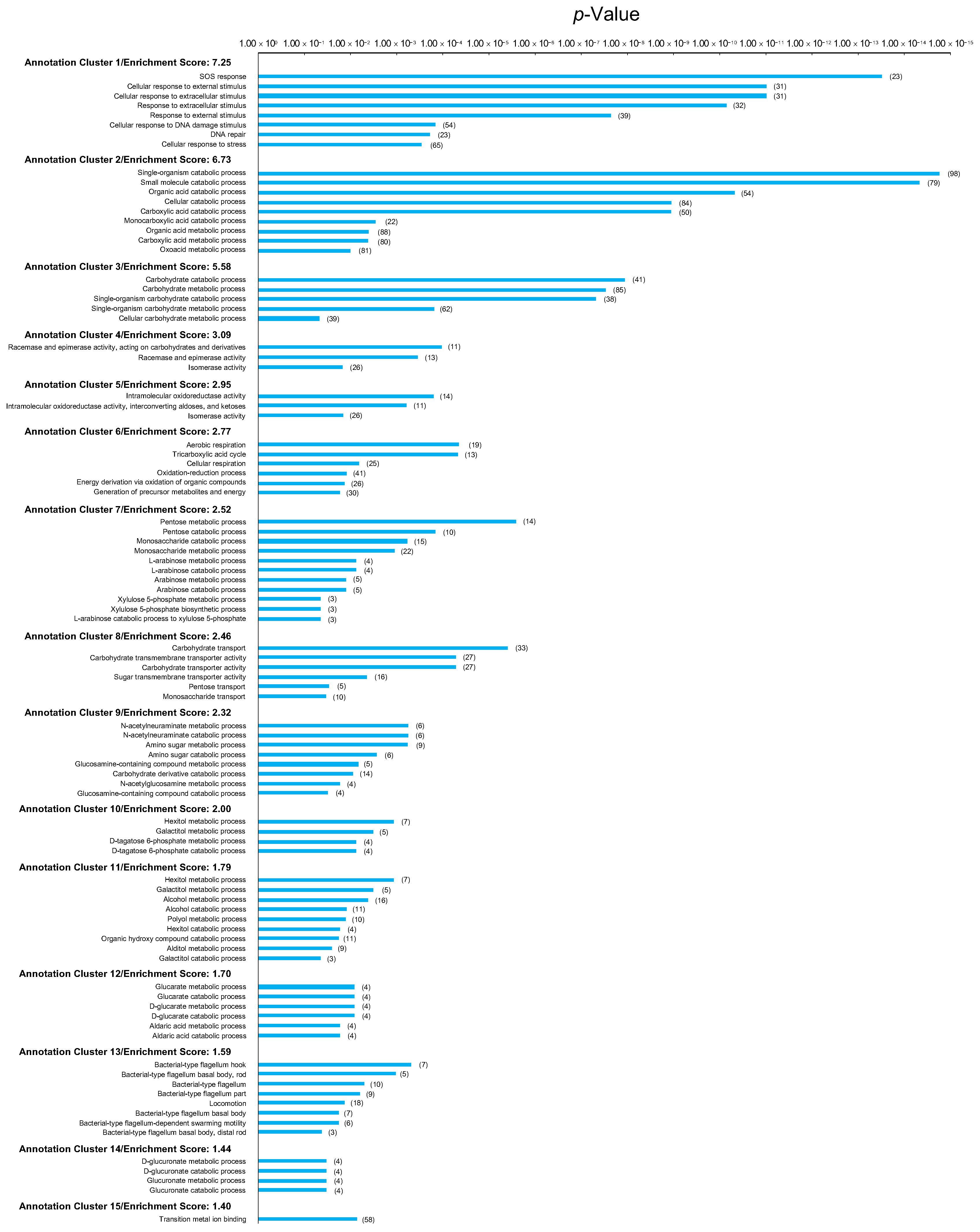
2.5. Changes in Effect of Gamma Irradiation on Gene Expression Alteration Through Evolution
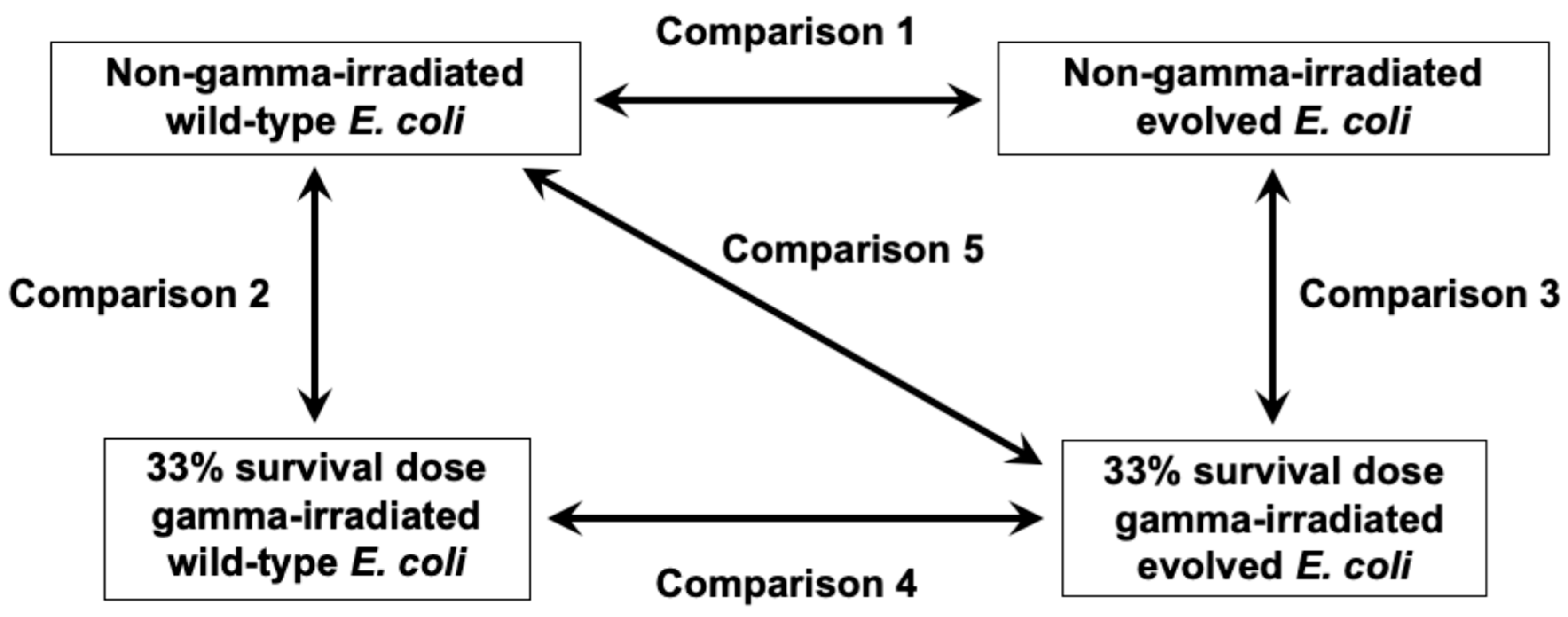
2.6. Effects of Evolution and Gamma Irradiation on Gene Expression Changes
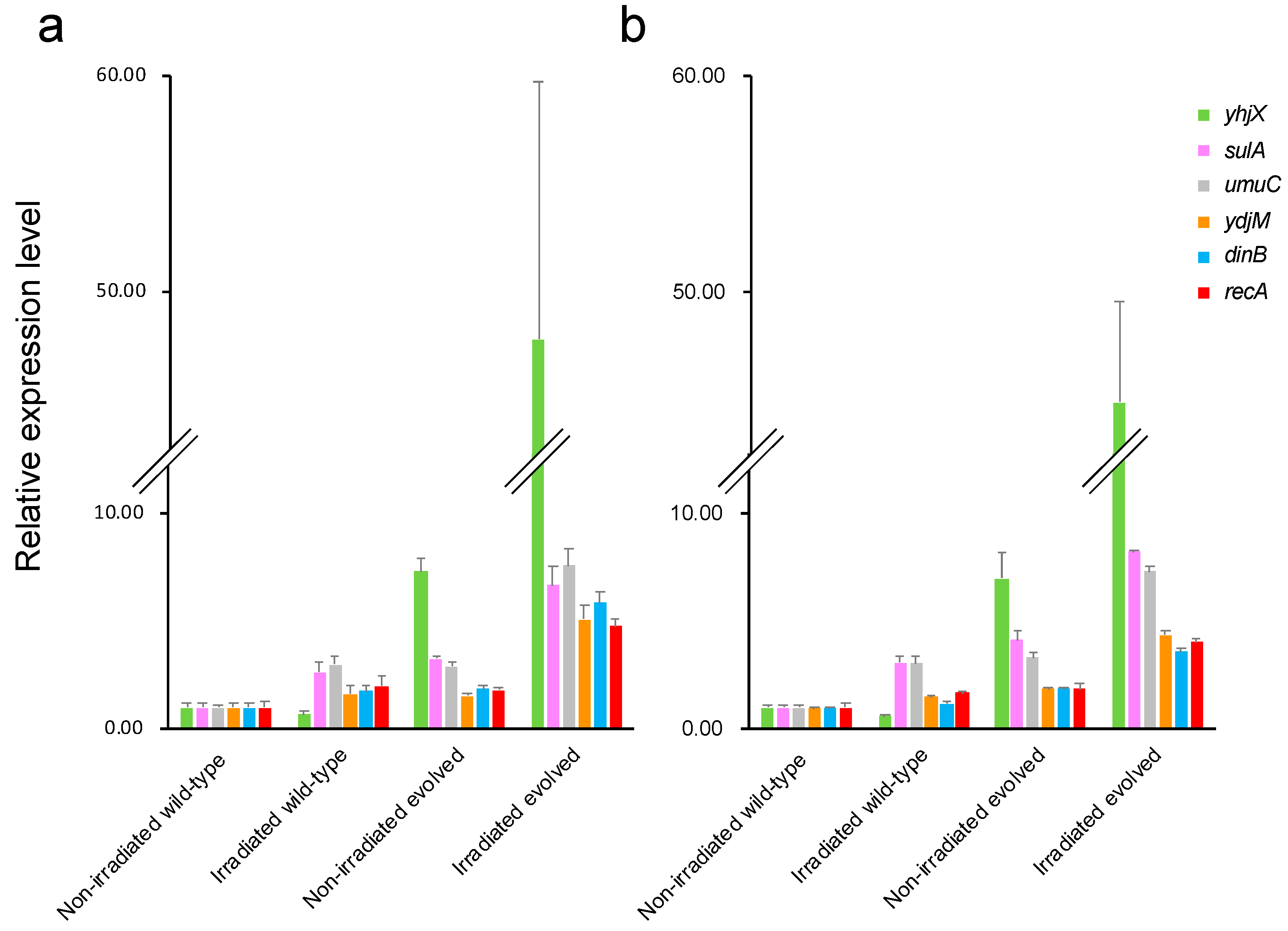
2.7. Changes in Expression Levels of Anti-Stress Genes Induced by Evolution and Gamma Irradiation
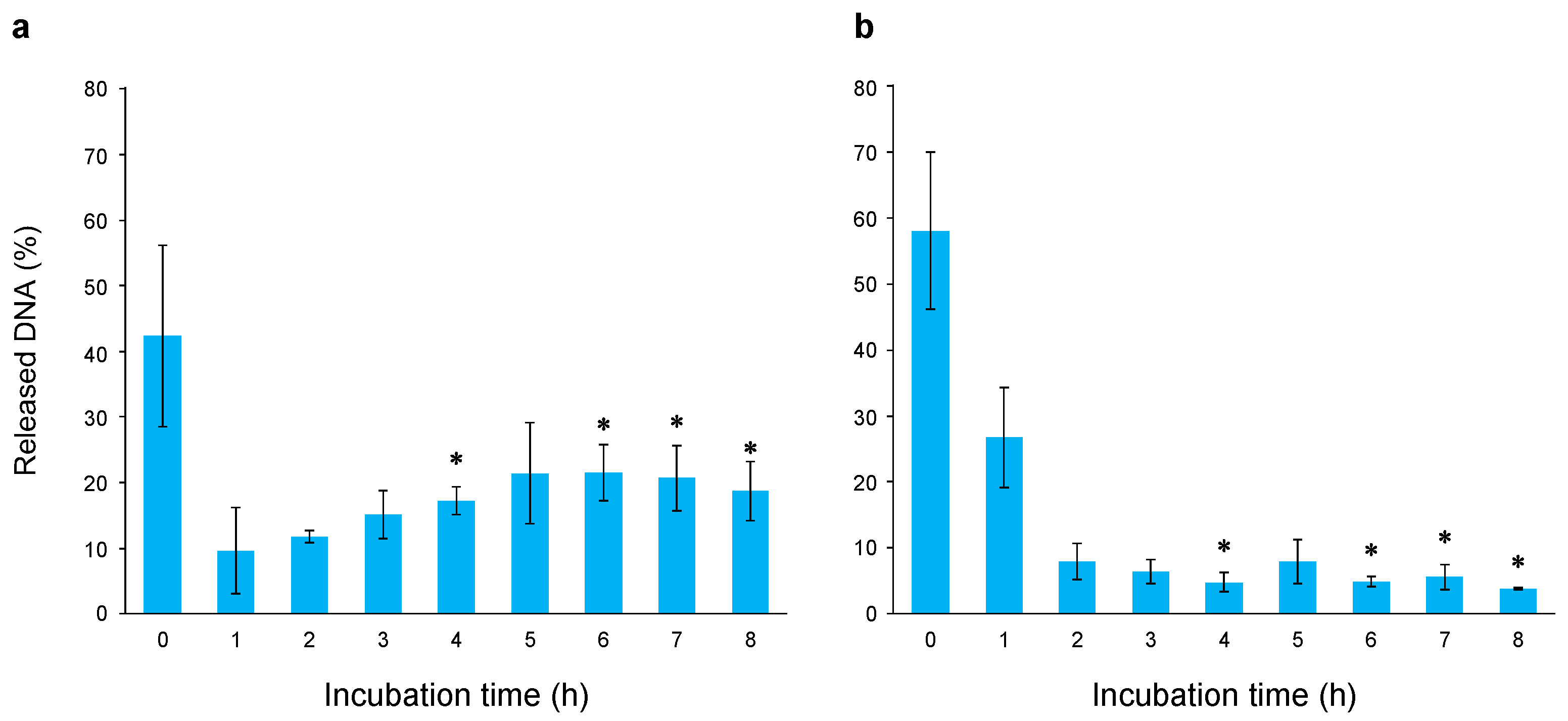
2.8. Comparison of Protective and Repair Activities Against Gamma Irradiation-Induced Genomic DNA Damage Between Wild-Type and Evolved E. coli
3. Discussion
4. Materials and Methods
4.1. Sensitivity of E. coli Cells to Gamma Irradiation
4.2. Adaptive Evolution Experiment with Gamma Radiation as Selection Pressure
4.3. Preparation of Cells for Gene Expression Analysis
4.4. Analysis of Gene Expression via RNA-Seq
4.5. GO, KEGG Pathway, and Hierarchical Clustering Analyses
4.6. qRT-PCR Analysis
4.7. Analysis of DNA Protective Activity
4.8. Analysis of Cell Proliferation After Gamma Irradiation
4.9. Analysis of DNA Repair Activity
4.10. Statistics and Reproducibility
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LB medium | Luria–Bertani medium |
| PBS | phosphate-buffered saline |
| RNA-seq | RNA sequencing |
| DEGs | differentially expressed genes |
| GO | gene ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| qRT-PCR | quantitative real-time reverse transcription polymerase chain reaction |
| SFGE | static field gel electrophoresis |
| FPKMs | fragments per kilobase of transcript per million mapped fragments |
| DAVID | Database for Annotation, Visualization and Integrated Discovery |
| CFU | Colony-forming unit |
References
- Horikoshi, K. Alkaliphiles: Some Applications of Their Products for Biotechnology. Microbiol. Mol. Biol. Rev. 1999, 63, 735–750. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Dopson, M. Life in acid: pH homeostasis in acidophiles. Trends Microbiol. 2007, 15, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Falb, M.; Müller, K.; Königsmaier, L.; Oberwinkler, T.; Horn, P.; von Gronau, S.; Gonzalez, O.; Pfeiffer, F.; Bornberg-Bauer, E.; Oesterhelt, D. Metabolism of halophilic archaea. Extremophiles 2008, 12, 177–196. [Google Scholar] [CrossRef]
- Takai, K.; Nakamura, K. Archaeal diversity and community development in deep-sea hydrothermal vents. Curr. Opin. Microbiol. 2011, 14, 282–291. [Google Scholar] [CrossRef]
- Sarethy, I.P.; Saxena, Y.; Kapoor, A.; Sharma, M.; Sharma, S.K.; Gupta, V.; Gupta, S. Alkaliphilic bacteria: Applications in industrial biotechnology. J. Ind. Microbiol. Biotechnol. 2011, 38, 769–790. [Google Scholar] [CrossRef]
- Zhang, C.; Phillips, A.P.R.; Wipfler, R.L.; Olsen, G.J.; Whitaker, R.J. The essential genome of the crenarchaeal model Sulfolobus islandicus. Nat. Commun. 2018, 9, 4908. [Google Scholar] [CrossRef] [PubMed]
- Pavlopoulou, A.; Savva, G.D.; Louka, M.; Bagos, P.G.; Vorgias, C.E.; Michalopoulos, I.; Georgakilas, A.G. Unraveling the mechanisms of extreme radioresistance in prokaryotes: Lessons from nature. Mutat. Res. Rev. Mutat. Res. 2016, 767, 92–107. [Google Scholar] [CrossRef]
- Anderson, A.W.; Nordan, H.C.; Cain, R.F.; Parrish, G.; Duggan, D. Studies on a Radio-Resistant Micrococcus I. Isolation, Morphology, Cultural Characteristics and Resistance to Gamma Radiation. Food Technol. 1956, 10, 575–578. [Google Scholar]
- Moseley, B.E.; Copland, H.J. Isolation and properties of a recombination-deficient mutant of Micrococcus radiodurans. J. Bacteriol. 1975, 121, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Blasius, M.; Sommer, S.; Hübscher, U. Deinococcus radiodurans: What belongs to the survival kit? Crit. Rev. Biochem. Mol. Biol. 2008, 43, 221–238. [Google Scholar] [CrossRef]
- Ishino, Y.; Narumi, I. DNA repair in hyperthermophilic and hyperradioresistant microorganisms. Curr. Opin. Microbiol. 2015, 25, 103–112. [Google Scholar] [CrossRef]
- Lim, S.; Jung, J.H.; Blanchard, L.; de Groot, A. Conservation and diversity of radiation and oxidative stress resistance mechanisms in Deinococcus species. FEMS Microbiol. Rev. 2019, 43, 19–52. [Google Scholar] [CrossRef]
- Cox, M.M.; Battista, J.R. Deinococcus radiodurans—the consummate survivor. Nat. Rev. Microbiol. 2005, 3, 882–892. [Google Scholar] [CrossRef]
- Fong, S.S.; Joyce, A.R.; Palsson, B.Ø. Parallel adaptive evolution cultures of Escherichia coli lead to convergent growth phenotypes with different gene expression states. Genome Res. 2005, 15, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Herring, C.D.; Raghunathan, A.; Honisch, C.; Patel, T.; Applebee, M.K.; Joyce, A.R.; Albert, T.J.; Blattner, F.R.; van den Boom, D.; Cantor, C.R.; et al. Comparative genome sequencing of Escherichia coli allows observation of bacterial evolution on a laboratory timescale. Nat. Genet. 2006, 38, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Barrick, J.E.; Yu, D.S.; Yoon, S.H.; Jeong, H.; Oh, T.K.; Schneider, D.; Lenski, R.E.; Kim, J.F. Genome evolution and adaptation in a long-term experiment with Escherichia coli. Nature 2009, 461, 1243–1247. [Google Scholar] [CrossRef]
- Dhar, R.; Sägesser, R.; Weikert, C.; Yuan, J.; Wagner, A. Adaptation of Saccharomyces cerevisiae to saline stress through laboratory evolution. J. Evol. Biol. 2011, 24, 1135–1153. [Google Scholar] [CrossRef]
- Ying, B.-W.; Matsumoto, Y.; Kitahara, K.; Suzuki, S.; Ono, N.; Furusawa, C.; Kishimoto, T.; Yomo, T. Bacterial transcriptome reorganization in thermal adaptive evolution. BMC Genom. 2015, 16, 802. [Google Scholar] [CrossRef] [PubMed]
- Erdman, I.E.; Thatcher, F.S.; MacQueen, K.F. Studies on the Irradiation of Microorganisms in Relation to Food Preservation: II. Irradiation Resistant Mutants. Can. J. Microbiol. 1961, 7, 207–215. [Google Scholar] [CrossRef]
- Ewing, D. The Directed Evolution of Radiation Resistance in E. coli. Biochem. Biophys. Res. Commun. 1995, 216, 549–553. [Google Scholar] [CrossRef]
- Harris, D.R.; Pollock, S.V.; Wood, E.A.; Goiffon, R.J.; Klingele, A.J.; Cabot, E.L.; Schackwitz, W.; Martin, J.; Eggington, J.; Durfee, T.J.; et al. Directed Evolution of Ionizing Radiation Resistance in Escherichia coli. J. Bacteriol. 2009, 191, 5240–5252. [Google Scholar] [CrossRef]
- Byrne, R.T.; Klingele, A.J.; Cabot, E.L.; Schackwitz, W.S.; Martin, J.A.; Martin, J.; Wang, Z.; Wood, E.A.; Pennacchio, C.; Pennacchio, L.A.; et al. Evolution of extreme resistance to ionizing radiation via genetic adaptation of DNA repair. eLife 2014, 3, e01322. [Google Scholar] [CrossRef] [PubMed]
- Bruckbauer, S.T.; Trimarco, J.D.; Henry, C.; Wood, E.A.; Battista, J.R.; Cox, M.M. A variant of the Escherichia coli anaerobic transcription factor FNR exhibiting diminished promoter activation function enhances ionizing radiation resistance. PLoS ONE 2019, 14, e0199482. [Google Scholar] [CrossRef] [PubMed]
- Bruckbauer, S.T.; Trimarco, J.D.; Martin, J.; Bushnell, B.; Senn, K.A.; Schackwitz, W.; Lipzen, A.; Blow, M.; Wood, E.A.; Culberson, W.S.; et al. Experimental Evolution of Extreme Resistance to Ionizing Radiation in Escherichia coli after 50 Cycles of Selection. J. Bacteriol. 2019, 201, e00784-18. [Google Scholar] [CrossRef] [PubMed]
- Bruckbauer, S.T.; Martin, J.; Minkoff, B.B.; Veling, M.T.; Lancaster, I.; Liu, J.; Trimarco, J.D.; Bushnell, B.; Lipzen, A.; Wood, E.A.; et al. Physiology of Highly Radioresistant Escherichia coli After Experimental Evolution for 100 Cycles of Selection. Front. Microbiol. 2020, 11, 582590. [Google Scholar] [CrossRef]
- Hall, J.; Giaccia, A. Molecular Mechanisms of DNA and Chromosome Damage and Repair in Radiobiology for the Radiologist, 7th ed.; Lippincott Williams & Wilkin: Philadelphia, PA, USA, 2012; pp. 12–34. [Google Scholar]
- Hall, B.; Hallgrímsson, B. Before Darwin in Strickberger’s Evolution, 4th ed.; Jones & Bartlett Publishers: Boston, MA, USA, 2008; pp. 2–19. [Google Scholar]
- Kawai, K.; Kanesaki, Y.; Yoshikawa, H.; Hirasawa, T. Identification of metabolic engineering targets for improving glycerol assimilation ability of Saccharomyces cerevisiae based on adaptive laboratory evolution and transcriptome analysis. J. Biosci. Bioeng. 2019, 128, 162–169. [Google Scholar] [CrossRef]
- Maslowska, K.H.; Makiela-Dzbenska, K.; Fijalkowska, I.J. The SOS system: A complex and tightly regulated response to DNA damage. Environ. Mol. Mutagen. 2019, 60, 368–384. [Google Scholar] [CrossRef]
- Daly, M.J.; Gaidamakova, E.K.; Matrosova, V.Y.; Vasilenko, A.; Zhai, M.; Venkateswaran, A.; Hess, M.; Omelchenko, M.V.; Kostandarithes, H.M.; Makarova, K.S.; et al. Accumulation of Mn(II) in Deinococcus radiodurans Facilitates Gamma-Radiation Resistance. Science 2004, 306, 1025–1028. [Google Scholar] [CrossRef]
- Daly, M.J.; Gaidamakova, E.K.; Matrosova, V.Y.; Vasilenko, A.; Zhai, M.; Leapman, R.D.; Lai, B.; Ravel, B.; Li, S.-M.W.; Kemner, K.M.; et al. Protein Oxidation Implicated as the Primary Determinant of Bacterial Radioresistance. PLOS Biol. 2007, 5, e92. [Google Scholar] [CrossRef]
- Daly, M.J. Death by protein damage in irradiated cells. DNA Repair 2012, 11, 12–21. [Google Scholar] [CrossRef]
- de Toledo Arruda-Neto, J.D.; Righi, H.; Cabrera Gomez, J.G.; Ferreira da Silva, L.; Drigo, E.; da Costa Lemos, A.C. Radioresistance and radiosensitivity: A biophysical approach on bacterial cells robustness. Theory Biosci. 2023, 142, 13–28. [Google Scholar] [CrossRef]
- Saito, T.; Terato, H.; Yamamoto, O. Pigments of Rubrobacter radiotolerans. Arch. Microbiol. 1994, 162, 414–421. [Google Scholar] [CrossRef]
- Saito, T.; Miyabe, Y.; Ide, H.; Yamamoto, O. Hydroxyl radical scavenging ability of bacterioruberin. Radiat. Phys. Chem. 1997, 50, 267–269. [Google Scholar] [CrossRef]
- Saito, T.; Ohyama, Y.; Ide, H.; Ohta, S.; Yamamoto, O. A carotenoid pigment of the radioresistant bacterium Deinococcus radiodurans. Microbios 1998, 95, 79–90. [Google Scholar]
- Terato, H.; Suzuki, K.; Nishioka, N.; Okamoto, A.; Shimazaki-Tokuyama, Y.; Inoue, Y.; Saito, T. Characterization and Radio-resistant Function of Manganese Superoxide Dismutase of Rubrobacter radiotolerans. J. Radiat. Res. 2011, 52, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Fujii, N. Effects of carotenoids on damage of biological lipids induced by gamma irradiation. Radiat. Phys. Chem. 2014, 98, 57–63. [Google Scholar] [CrossRef]
- López-Maury, L.; Marguerat, S.; Bähler, J. Tuning gene expression to changing environments: From rapid responses to evolutionary adaptation. Nat. Rev. Genet. 2008, 9, 583–593. [Google Scholar] [CrossRef]
- Ishikawa, A.; Kusakabe, M.; Yoshida, K.; Ravinet, M.; Makino, T.; Toyoda, A.; Fujiyama, A.; Kitano, J. Different contributions of local- and distant-regulatory changes to transcriptome divergence between stickleback ecotypes. Evolution 2017, 71, 565–581. [Google Scholar] [CrossRef]
- Salem, A.M.H.; Nakano, T.; Takuwa, M.; Matoba, N.; Tsuboi, T.; Terato, H.; Yamamoto, K.; Yamada, M.; Nohmi, T.; Ide, H. Genetic Analysis of Repair and Damage Tolerance Mechanisms for DNA-Protein Cross-Links in Escherichia coli. J. Bacteriol. 2009, 191, 5657–5668. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Yendrek, C.R.; Ainsworth, E.A.; Thimmapuram, J. The bench scientist’s guide to statistical analysis of RNA-Seq data. BMC Res. Notes 2012, 5, 506. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Wang, Y.E.; Kutnetsov, L.; Partensky, A.; Farid, J.; Quackenbush, J. WebMeV: A Cloud Platform for Analyzing and Visualizing Cancer Genomic Data. Cancer Res. 2017, 77, e11–e14. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shahar, Y.; Robichon, A.; Sokolowski, M.B.; Robinson, G.E. Influence of Gene Action Across Different Time Scales on Behavior. Science 2002, 296, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Said-Salman, I.H.; Jebaii, F.A.; Yusef, H.H.; Moustafa, M.E. Global gene expression analysis of Escherichia coli K-12 DH5α after exposure to 2.4 GHz wireless fidelity radiation. Sci. Rep. 2019, 9, 14425. [Google Scholar] [CrossRef] [PubMed]
- Kudo, K.; Ito, H.; Ihara, S.; Terato, H. Oxidative DNA damage caused by pulsed discharge with cavitation on the bactericidal function. J. Phys. D Appl. Phys. 2015, 48, 365401. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, T.; Terato, H. Acquired Radioresistance Through Adaptive Evolution with Gamma Radiation as Selection Pressure: Increased Expression and Induction of Anti-Stress Genes. Int. J. Mol. Sci. 2025, 26, 7275. https://doi.org/10.3390/ijms26157275
Saito T, Terato H. Acquired Radioresistance Through Adaptive Evolution with Gamma Radiation as Selection Pressure: Increased Expression and Induction of Anti-Stress Genes. International Journal of Molecular Sciences. 2025; 26(15):7275. https://doi.org/10.3390/ijms26157275
Chicago/Turabian StyleSaito, Takeshi, and Hiroaki Terato. 2025. "Acquired Radioresistance Through Adaptive Evolution with Gamma Radiation as Selection Pressure: Increased Expression and Induction of Anti-Stress Genes" International Journal of Molecular Sciences 26, no. 15: 7275. https://doi.org/10.3390/ijms26157275
APA StyleSaito, T., & Terato, H. (2025). Acquired Radioresistance Through Adaptive Evolution with Gamma Radiation as Selection Pressure: Increased Expression and Induction of Anti-Stress Genes. International Journal of Molecular Sciences, 26(15), 7275. https://doi.org/10.3390/ijms26157275






