Molecular Insight into the Role of HLA Genotypes in Immunogenicity and Secondary Refractoriness to Anti-TNF Therapy in IBD Patients
Abstract
1. Introduction
2. HLA in Autoimmune Diseases
3. HLA Associations in Inflammatory Bowel Disease
3.1. HLA in Ulcerative Colitis
3.2. HLA in Crohn’s Disease
3.3. Role of HLA in Shaping IBD Precision Medicine
4. Genetic Modulation of Anti-TNF Pharmacokinetics in IBD
5. Contribution of HLA-DQ Alleles to Immunogenicity and Resistance to Anti-TNF Therapy
6. Ethnic and Geographic Limitations in HLA Studies and Molecular Pathways Linking HLA-DQ Genotypes to Immunogenicity and Therapeutic Resistance in IBD
7. HLA-Linked SNPs as Biomarkers of Anti-TNF Treatment Resistance
8. Gut Microbiota Signatures as Determinants of Refractoriness to Anti-TNF Therapy in IBD
9. Integrating HLA Genotyping into Pre-Therapeutic Assessment for Anti-TNF Agents
10. Future Perspectives
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| ADAs | Anti-drug antibodies |
| ADA (drug) | Adalimumab |
| APC | Antigen-presenting cell |
| ATG16L1 | Autophagy-related 16-like 1 |
| BSA | Body surface area |
| CAR-T | Chimeric antigen receptor T-cell therapy |
| CD | Crohn’s disease |
| C1orf106 | Chromosome 1 open reading frame 106 |
| DC | Dendritic cell |
| DQ | HLA-DQ locus |
| DR | HLA-DR locus |
| Fc | Fragment crystallizable region |
| FcγR | Fc gamma receptor |
| FCGRT | Neonatal Fc receptor transporter gene |
| FCal | Fecal calprotectin |
| GWASs | Genome-wide association studies |
| HLA | Human leukocyte antigen |
| IFX | Infliximab |
| IL | Interleukin |
| IL-6 | Interleukin 6 |
| IL-17 | Interleukin 17 |
| IL-23 | Interleukin 23 |
| JAK | Janus kinase |
| LOR | Loss of response |
| MHC | Major histocompatibility complex |
| MICA/MICB | MHC class I chain-related gene A/B |
| mAbs | Monoclonal antibodies |
| NOD2 | Nucleotide-binding oligomerization domain-containing protein 2 |
| PBMC | Peripheral blood mononuclear cell |
| pTDM | Proactive therapeutic drug monitoring |
| SERENE | Study of the Efficacy and Safety of Adalimumab |
| SNP | Single-nucleotide polymorphism |
| TCR | T-cell receptor |
| Th cell | T helper cell |
| TLR2 | Toll-like receptor 2 |
| TNF | Tumor necrosis factor |
| TNF-α | Tumor necrosis factor alpha |
| Treg | Regulatory T cell |
| UC | Ulcerative colitis |
| VNTR | Variable number tandem repeat |
References
- Annese, V. Genetics and epigenetics of IBD. Pharmacol. Res. 2020, 159, 104892. [Google Scholar] [CrossRef]
- Mirkov, M.U.; Verstockt, B.; Cleynen, I. Genetics of inflammatory bowel disease: Beyond NOD2. Lancet Gastroenterol. Hepatol. 2017, 2, 224–234. [Google Scholar] [CrossRef]
- Ye, L.; Lin, Y.; Fan, X.D.; Chen, Y.; Deng, Z.; Yang, Q.; Lei, X.; Mao, J.; Cui, C. Identify Inflammatory Bowel Disease-Related Genes Based on Machine Learning. Front. Cell Dev. Biol. 2021, 9, 722410. [Google Scholar] [CrossRef]
- Degenhardt, F.; Mayr, G.; Wendorff, M.; Boucher, G.; Ellinghaus, E.; Ellinghaus, D.; ElAbd, H.; Rosati, E.; Hübenthal, M.; Juzenas, S.; et al. Transethnic analysis of the human leukocyte antigen region for ulcerative colitis reveals not only shared but also ethnicity-specific disease associations. Hum. Mol. Genet. 2021, 30, 356–369. [Google Scholar] [CrossRef]
- Ahmad, T.; Armuzzi, A.; Neville, M.; Bunce, M.; Ling, K.L.; Welsh, K.I.; Marshall, S.E.; Jewell, D.P. The contribution of human leucocyte antigen complex genes to disease phenotype in ulcerative colitis. Tissue Antigens 2003, 62, 527–535. [Google Scholar] [CrossRef]
- Yamamoto-Furusho, J.K.; Uscanga, L.F.; Vargas-Alarcón, G.; Ruiz-Morales, J.A.; Higuera, L.; Cutiño, T.; Rodríguez-Pérez, J.M.; Villarreal-Garza, C.; Granados, J. Clinical and genetic heterogeneity in Mexican patients with ulcerative colitis. Hum. Immunol. 2003, 64, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Tsianos, E.V.; Katsanos, K.H.; Tsianos, V.E. Role of genetics in the diagnosis and prognosis of Crohn’s disease. World J. Gastroenterol. 2012, 18, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Marsal, J.; Barreiro-de Acosta, M.; Blumenstein, I.; Cappello, M.; Bazin, T.; Sebastian, S. Management of non-response and loss of response to anti-tumor necrosis factor therapy in inflammatory bowel disease. Front. Med. 2022, 9, 897936. [Google Scholar] [CrossRef] [PubMed]
- Doherty, J.; Ryan, A.W.; Quinn, E.; Conroy, J.; Dolan, J.; Corcoran, R.; Hara, F.O.; Cullen, G.; Sheridan, J.; Bailey, Y.; et al. HLA-DQA1*05 allele carriage and anti-TNF therapy persistence in inflammatory bowel disease. Inflamm. Bowel Dis. 2025, 31, 903–911. [Google Scholar] [CrossRef]
- Powell Doherty, R.D.; Liao, H.; Satsangi, J.J.; Ternette, N. Extended analysis identifies drug-specific association of 2 distinct HLA class II haplotypes for development of immunogenicity to adalimumab and infliximab. Gastroenterology 2020, 159, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Choo, S.Y. The HLA system: Genetics, immunology, clinical testing, and clinical implications. Yonsei Med. J. 2007, 48, 11–23. [Google Scholar] [CrossRef]
- Hoek, M.; Demmers, L.C.; Wu, W.; Heck, A.J.R. Allotype-specific glycosylation and cellular localization of human leukocyte antigen class I proteins. J. Proteome Res. 2021, 20, 4518–4528. [Google Scholar] [CrossRef] [PubMed]
- Matzaraki, V.; Kumar, V.; Wijmenga, C.; Zhernakova, A. The MHC locus and genetic susceptibility to autoimmune and infectious diseases. Genome Biol. 2017, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Medhasi, S.; Chantratita, N. Human leukocyte antigen (HLA) system: Genetics and association with bacterial and viral infections. J. Immunol. Res. 2022, 2022, 9710376. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Sato, A. The HLA system. First of two parts. N. Engl. J. Med. 2000, 343, 702–709. [Google Scholar] [CrossRef]
- Wang, M.; Claesson, M.H. Classification of human leukocyte antigen (HLA) supertypes. Methods Mol. Biol. 2014, 1184, 309–317. [Google Scholar] [CrossRef]
- Robinson, J.; Halliwell, J.A.; Hayhurst, J.D.; Flicek, P.; Parham, P.; Marsh, S.G. The IPD and IMGT/HLA database: Allele variant databases. Nucleic Acids Res. 2015, 43, D423–D431. [Google Scholar] [CrossRef]
- Raghavan, M.; Geng, J. HLA-B polymorphisms and intracellular assembly modes. Mol. Immunol. 2015, 68, 89–93. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Szeto, C.; Gras, S. The pockets guide to HLA class I molecules. Biochem. Soc. Trans. 2021, 49, 2319–2331. [Google Scholar] [CrossRef]
- Noble, J.A.; Erlich, H.A. Genetics of type 1 diabetes. Cold Spring Harb. Perspect. Med. 2012, 2, a007732. [Google Scholar] [CrossRef]
- Akassou, A.; Bakri, Y. Does HLA-B27 status influence ankylosing spondylitis phenotype? Clin. Med. Insights Arthritis Musculoskelet. Disord. 2018, 11, 1179544117751627. [Google Scholar] [CrossRef]
- Ishina, I.A.; Zakharova, M.Y.; Kurbatskaia, I.N.; Mamedov, A.E.; Belogurov, A.A., Jr.; Gabibov, A.G. MHC class II presentation in autoimmunity. Cells 2023, 12, 314. [Google Scholar] [CrossRef]
- Fröhlich, F.; Micheroli, R.; Hebeisen, M.; Kissling, S.; Bürki, K.; Exer, P.; Bräm, R.; Niedermann, K.; Möller, B.; Nissen, M.J.; et al. HLA-B27 as a predictor of effectiveness of treatment with TNF inhibitors in axial spondyloarthritis: Data from the Swiss Clinical Quality Management Registry. Clin. Rheumatol. 2023, 42, 1267–1274. [Google Scholar] [CrossRef]
- Liu, W.; Wu, Y.H.; Zhang, L.; Liu, X.Y.; Xue, B.; Liu, B.; Wang, Y.; Ji, Y. Efficacy and safety of TNF-α inhibitors for active ankylosing spondylitis patients: Multiple treatment comparisons in a network meta-analysis. Sci. Rep. 2016, 6, 32768. [Google Scholar] [CrossRef]
- Frison, E.; Breban, M.; Costantino, F. How to translate genetic findings into clinical applications in spondyloarthritis? Front. Immunol. 2024, 15, 1301735. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Marshall, S.E.; Jewell, D. Genetics of inflammatory bowel disease: The role of the HLA complex. World J. Gastroenterol. 2006, 12, 3628–3635. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.V.; Nøhr, A.K.; Allin, K.H.; Krarup, H.; Larsen, L.; Sazonovs, A.; Jess, T. HLA-DRB1*01:03 and Severe Ulcerative Colitis. JAMA 2024, 332, 1941–1943. [Google Scholar] [CrossRef] [PubMed]
- Goyette, P.; Boucher, G.; Mallon, D.; Ellinghaus, E.; Jostins, L.; Huang, H.; Ripke, S.; Gusareva, E.S.; Annese, V.; Hauser, S.L.; et al. High-density mapping of the MHC identifies a shared role for HLA-DRB1*01:03 in inflammatory bowel diseases and heterozygous advantage in ulcerative colitis. Nat. Genet. 2015, 47, 172–179. [Google Scholar] [CrossRef]
- Annese, V.; Piepoli, A.; Latiano, A.; Lombardi, G.; Napolitano, G.; Caruso, N.; Cocchiara, E.; Accadia, L.; Perri, F.; Andriulli, A. HLA-DRB1 alleles may influence disease phenotype in patients with inflammatory bowel disease: A critical reappraisal with review of the literature. Dis. Colon Rectum 2005, 48, 57–64. [Google Scholar] [CrossRef]
- Matsumura, Y.; Kinouchi, Y.; Nomura, E.; Negoro, K.; Kakuta, Y.; Endo, K.; Aizawa, H.; Takagi, S.; Takahashi, S.; Shimosegawa, T. HLA-DRB1 alleles influence clinical phenotypes in Japanese patients with ulcerative colitis. Tissue Antigens 2008, 71, 447–452. [Google Scholar] [CrossRef]
- Venkateswaran, S.; Prince, J.; Cutler, D.J.; Marigorta, U.M.; Okou, D.T.; Prahalad, S.; Mack, D.; Boyle, B.; Walters, T.; Griffiths, A.; et al. Enhanced Contribution of HLA in Pediatric Onset Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 829–838. [Google Scholar] [CrossRef]
- Arimura, Y.; Isshiki, H.; Onodera, K.; Nagaishi, K.; Yamashita, K.; Sonoda, T.; Matsumoto, T.; Takahashi, A.; Takazoe, M.; Yamazaki, K.; et al. Characteristics of Japanese inflammatory bowel disease susceptibility loci. J. Gastroenterol. 2014, 49, 1217–1230. [Google Scholar] [CrossRef]
- Mahdi, B.M. Role of HLA typing on Crohn’s disease pathogenesis. Ann. Med. Surg. 2015, 4, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.R.; Thiyagaraja, S.; Welsh, K.I.; Wordsworth, B.P.; Hill Gaston, J.S.; Jewell, D.P. Clinical phenotype is related to HLA genotype in the peripheral arthropathies of inflammatory bowel disease. Gastroenterology 2000, 118, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.R.; Chua, C.N.; Ahmad, T.; Cheng, H.; Welsh, K.I.; Jewell, D.P. Uveitis and erythema nodosum in inflammatory bowel disease: Clinical features and the role of HLA genes. Gastroenterology 2002, 123, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Rios Rodriguez, V.; Duran, T.I.; Torgutalp, M.; López-Medina, C.; Dougados, M.; Kishimoto, M.; Ono, K.; Protopopov, M.; Haibel, H.; Rademacher, J.; et al. Comparing clinical profiles in spondyloarthritis with Crohn’s disease or ulcerative colitis: Insights from the ASAS-PerSpA study. Rheumatol. Adv. Pract. 2024, 8, rkae064. [Google Scholar] [CrossRef]
- Vanaki, N.; Aslani, S.; Jamshidi, A.; Mahmoudi, M. Role of innate immune system in the pathogenesis of ankylosing spondylitis. Biomed. Pharmacother. 2018, 105, 130–143. [Google Scholar] [CrossRef]
- Naser, S.A.; Arce, M.; Khaja, A.; Fernandez, M.; Naser, N.; Elwasila, S.; Thanigachalam, S. Role of ATG16L, NOD2 and IL23R in Crohn’s disease pathogenesis. World J. Gastroenterol. 2012, 18, 412–424. [Google Scholar] [CrossRef]
- Navajas Hernández, P.; Mouhtar El Halabi, S.; González Parra, A.C.; Valdés Delgado, T.; Maldonado Pérez, B.; Castro Laria, L.; Charpentier, C.; Argüelles-Arias, F. Carriage of the HLA-DQA1*05 haplotype is associated with a higher risk of infratherapeutic drug concentration and higher immunogenicity in patients undergoing treatment with anti-TNF for inflammatory bowel disease. Therap. Adv. Gastroenterol. 2024, 17, 17562848241278145. [Google Scholar] [CrossRef]
- Mosch, R.; Guchelaar, H.J. Immunogenicity of Monoclonal Antibodies and the Potential Use of HLA Haplotypes to Predict Vulnerable Patients. Front. Immunol. 2022, 13, 885672. [Google Scholar] [CrossRef]
- Fan, W.L.; Shiao, M.S.; Hui, R.C.; Su, S.C.; Wang, C.W.; Chang, Y.C.; Chung, W.H. HLA Association with Drug-Induced Adverse Reactions. J. Immunol. Res. 2017, 2017, 3186328. [Google Scholar] [CrossRef]
- Ashton, J.J.; Mossotto, E.; Ennis, S.; Beattie, R.M. Personalising medicine in inflammatory bowel disease-current and future perspectives. Transl. Pediatr. 2019, 8, 56–69. [Google Scholar] [CrossRef]
- Vieujean, S.; Louis, E. Precision medicine and drug optimization in adult inflammatory bowel disease patients. Therap. Adv. Gastroenterol. 2023, 16, 17562848231173331. [Google Scholar] [CrossRef]
- Jan, Z.; El Assadi, F.; Velayutham, D.; Mifsud, B.; Jithesh, P.V. Pharmacogenomics of TNF inhibitors. Front. Immunol. 2025, 16, 1521794. [Google Scholar] [CrossRef] [PubMed]
- Ballesta-López, O.; Gil-Candel, M.; Centelles-Oria, M.; Megías-Vericat, J.E.; Solana-Altabella, A.; Ribes-Artero, H.; Nos-Mateu, P.; García-Pellicer, J.; Poveda-Andrés, J.L. Pharmacogenetics in Response to Biological Agents in Inflammatory Bowel Disease: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 1760. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.E.; Werida, R.H.; Radwan, M.A.; Askar, S.R.; Omran, G.A.; El-Mohamdy, M.A.; Hagag, R.S. Efficacy and safety of infliximab and adalimumab in inflammatory bowel disease patients. Inflammopharmacology 2024, 32, 3259–3269. [Google Scholar] [CrossRef] [PubMed]
- Atay, A.; Cagir, Y.; Ergul, M.; Ozturk, O.; Durak, M.B.; Yuksel, I. Comparative Outcomes of Adalimumab and Infliximab Dose Escalation in Inflammatory Bowel Disease Patients Failing First-Line Biologic Treatment. J. Clin. Med. 2025, 14, 1228. [Google Scholar] [CrossRef]
- Adegbola, S.O.; Sahnan, K.; Warusavitarne, J.; Hart, A.; Tozer, P. Anti-TNF Therapy in Crohn’s Disease. Int. J. Mol. Sci. 2018, 19, 2244. [Google Scholar] [CrossRef]
- Kopylov, U.; Seidman, E. Predicting durable response or resistance to antitumor necrosis factor therapy in inflammatory bowel disease. Therap. Adv. Gastroenterol. 2016, 9, 513–526. [Google Scholar] [CrossRef]
- Ordás, I.; Mould, D.R.; Feagan, B.G.; Sandborn, W.J. Anti-TNF monoclonal antibodies in inflammatory bowel disease: Pharmacokinetics-based dosing paradigms. Clin. Pharmacol. Ther. 2012, 91, 635–646. [Google Scholar] [CrossRef]
- Ternant, D.; Bejan-Angoulvant, T.; Passot, C.; Mulleman, D.; Paintaud, G. Clinical pharmacokinetics and pharmacodynamics of monoclonal antibodies approved to treat rheumatoid arthritis. Clin. Pharmacokinet. 2015, 54, 1107–1123. [Google Scholar] [CrossRef]
- Levy, R.A.; Guzman, R.; Castañeda-Hernández, G.; Martinez-Vazquez, M.; Damian, G.; Cara, C. Biology of anti-TNF agents in immune-mediated inflammatory diseases: Therapeutic implications. Immunotherapy 2016, 8, 1427–1436. [Google Scholar] [CrossRef]
- Hicks, J.K.; El Rouby, N.; Ong, H.H.; Schildcrout, J.S.; Ramsey, L.B.; Shi, Y.; Anne Tang, L.; Aquilante, C.L.; Beitelshees, A.L.; Blake, K.V.; et al. Opportunity for genotype-guided prescribing among adult patients in 11 US health systems. Clin. Pharmacol. Ther. 2021, 110, 179–188. [Google Scholar] [CrossRef]
- Dömling, A.; Li, X. TNF-α: The shape of small molecules to come? Drug Discov. Today 2022, 27, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Vande Casteele, N.; Khanna, R.; Levesque, B.G.; Stitt, L.; Zou, G.Y.; Singh, S.; Lockton, S.; Hauenstein, S.; Ohrmund, L.; Greenberg, G.R.; et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut 2015, 64, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Cao, L.; Li, Y.; Cai, X.; Ge, Y.; Zhu, W. Visceral fat is associated with mucosal healing of infliximab treatment in Crohn’s disease. Dis. Colon Rectum 2018, 61, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, C.B.; Lyu, K.S.; Jin, Z.M.; Guan, S.X.; You, N.; Huang, M.; Wang, X.D.; Gao, X. Association of polymorphisms in C1orf106, IL1RN, and IL10 with post-induction infliximab trough level in Crohn’s disease patients. Gastroenterol. Rep. 2019, 8, 367–373. [Google Scholar] [CrossRef]
- Salvador-Martín, S.; Pujol-Muncunill, G.; Bossacoma, F.; Navas, V.; Viada, F.J.; Muñoz, R.; Magallares, L.; Moreno, A.; Segarra, O.; Clemente, S.; et al. Pharmacogenetics of anti-TNF therapy in paediatric Crohn’s disease and ulcerative colitis: Comparison with adults. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 11–12. [Google Scholar] [CrossRef]
- Juanola, O.; Moratalla, A.; Gutiérrez, A.; Sempere, L.; Zapater, P.; Giménez, P.; Almenta, I.; Peiró, G.; González-Navajas, J.M.; Such, J.F.; et al. Anti-TNF-α loss of response is associated with a decreased percentage of FoxP3+ T cells and a variant NOD2 genotype in patients with Crohn’s disease. J. Gastroenterol. 2015, 50, 758–768. [Google Scholar] [CrossRef]
- Schäffler, H.; Geiss, D.; Gittel, N.; Rohde, S.; Huth, A.; Glass, Ä.; Brandhorst, G.; Jaster, R.; Lamprecht, G. Mutations in the NOD2 gene are associated with a specific phenotype and lower anti-tumor necrosis factor trough levels in Crohn’s disease. J. Dig. Dis. 2018, 19, 678–684. [Google Scholar] [CrossRef]
- Salvador-Martín, S.; López-Cauce, B.; Nuñez, O.; Laserna-Mendieta, E.J.; García, M.I.; Lobato, E.; Abarca-Zabalía, J.; Sanjurjo-Saez, M.; Lucendo, A.J.; Marín-Jiménez, I.; et al. Genetic predictors of long-term response and trough levels of infliximab in Crohn’s disease. Pharmacol. Res. 2019, 149, 104478. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Martín, S.; Pujol-Muncunill, G.; Bossacoma, F.; Navas-López, V.M.; Gallego-Fernández, C.; Segarra, O.; Clemente, S.; Muñoz-Codoceo, R.; Viada, J.; Magallares, L.; et al. Pharmacogenetics of trough serum anti-TNF levels in paediatric inflammatory bowel disease. Br. J. Clin. Pharmacol. 2021, 87, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Curci, D.; Lucafò, M.; Cifù, A.; Fabris, M.; Bramuzzo, M.; Martelossi, S.; Franca, R.; Decorti, G.; Stocco, G. Pharmacogenetic variants of infliximab response in young patients with inflammatory bowel disease. Clin. Transl. Sci. 2021, 14, 2184–2192. [Google Scholar] [CrossRef] [PubMed]
- Frymoyer, A.; Piester, T.L.; Park, K.T. Infliximab dosing strategies and predicted trough exposure in children with Crohn disease. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 723–727. [Google Scholar] [CrossRef]
- Kelsen, J.R.; Grossman, A.B.; Pauly-Hubbard, H.; Gupta, K.; Baldassano, R.N.; Mamula, P. Infliximab therapy in pediatric patients 7 years of age and younger. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 758–762. [Google Scholar] [CrossRef]
- Jongsma, M.M.E.; Winter, D.A.; Huynh, H.Q.; Norsa, L.; Hussey, S.; Kolho, K.L.; Bronsky, J.; Assa, A.; Cohen, S.; Lev-Tzion, R.; et al. Infliximab in young paediatric IBD patients: It is all about the dosing. Eur. J. Pediatr. 2020, 179, 1935–1944. [Google Scholar] [CrossRef]
- Fasanmade, A.A.; Adedokun, O.J.; Blank, M.; Zhou, H.; Davis, H.M. Pharmacokinetic properties of infliximab in children and adults with Crohn’s disease: A retrospective analysis of data from 2 phase III clinical trials. Clin. Ther. 2011, 33, 946–964. [Google Scholar] [CrossRef]
- Billiet, T.; Dreesen, E.; Cleynen, I.; Wollants, W.J.; Ferrante, M.; Van Assche, G.; Gils, A.; Vermeire, S. A genetic variation in the neonatal Fc-receptor affects anti-TNF drug concentrations in inflammatory bowel disease. Am. J. Gastroenterol. 2016, 111, 1438–1445. [Google Scholar] [CrossRef]
- Pauline, O.; Robert, M.; Bernardeau, C.; Hlavaty, A.; Fusaroli, M.; Roustit, M.; Cracowski, J.L.; Khouri, C. Assessment of reported adverse events after interchanging between TNF-α inhibitor biosimilars in the WHO pharmacovigilance database. BioDrugs 2023, 37, 699–707. [Google Scholar] [CrossRef]
- Mahmoud, I.; Moalla, M.; Ben Tekaya, A.; Charfi, R.; Rouached, L.; Bouden, S.; Tekaya, R.; Saidane, O.; Abdelmoula, L.; Sfar, I. Assessment of the influence of Fc-γ receptor polymorphisms on biologics’ pharmacokinetics in Tunisian rheumatoid arthritis patients. Br. J. Clin. Pharmacol. 2023, 89, 1834–1843. [Google Scholar] [CrossRef]
- Chan, J.C.N.; Chan, A.T.C. Biologics and biosimilars: What, why and how? ESMO Open 2017, 2, e000180. [Google Scholar] [CrossRef]
- Aggarwal, R.S. What’s fueling the biotech engine—2012 to 2013. Nat. Biotechnol. 2014, 32, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Grilo, A.L.; Mantalaris, A. The increasingly human and profitable monoclonal antibody market. Trends Biotechnol. 2019, 37, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, N.A.; Heap, G.A.; Green, H.D.; Hamilton, B.; Bewshea, C.; Walker, G.J.; Thomas, A.; Nice, R.; Perry, M.H.; Bouri, S.; et al. Predictors of anti-TNF treatment failure in anti-TNF-naïve patients with active luminal Crohn’s disease: A prospective, multicentre cohort study. Lancet Gastroenterol. Hepatol. 2019, 4, 341–353. [Google Scholar] [CrossRef]
- Dendrou, C.A.; Petersen, J.; Rossjohn, J.; Fugger, L. HLA variation and disease. Nat. Rev. Immunol. 2018, 18, 325–339. [Google Scholar] [CrossRef]
- Megiorni, F.; Pizzuti, A. HLA-DQA1 and HLA-DQB1 in celiac disease predisposition: Practical implications of the HLA molecular typing. J. Biomed. Sci. 2012, 19, 88. [Google Scholar] [CrossRef]
- Tafti, M.; Lammers, G.J.; Dauvilliers, Y.; Overeem, S.; Mayer, G.; Nowak, J.; Pfister, C.; Dubois, V.; Eliaou, J.F.; Eberhard, H.P.; et al. Narcolepsy-associated HLA class I alleles implicate cell-mediated cytotoxicity. Sleep 2016, 39, 581–587. [Google Scholar] [CrossRef]
- Reddy, M.V.; Wang, H.; Liu, S.; Bode, B.; Reed, J.C.; Steed, R.D.; Anderson, S.W.; Steed, L.; Hopkins, D.; She, J.X. Association between type 1 diabetes and GWAS SNPs in the southeast US Caucasian population. Genes Immun. 2011, 12, 208–212. [Google Scholar] [CrossRef]
- Sazonovs, A.; Kennedy, N.A.; Moutsianas, L.; Heap, G.A.; Rice, D.L.; Reppell, M.; Bewshea, C.M.; Chanchlani, N.; Walker, G.J.; Perry, M.H.; et al. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn’s disease. Gastroenterology 2020, 158, 189–199. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Q.; Zhao, J.; Liu, T.; Yao, J.; Peng, X.; Zhi, M.; Zhang, M. HLA-DQA1*05 correlates with increased risk of anti-drug antibody development and reduced response to infliximab in Chinese patients with Crohn’s disease. Gastroenterol. Rep. 2024, 12, goae074. [Google Scholar] [CrossRef]
- Ternette, N.; Liao, H.; Satsangi, J. Association of the HLA-DQA1*05 allelic gene variants with immunogenicity to anti-TNF therapeutics—Important differences between infliximab and adalimumab. J. Crohn’s Colitis 2025, 19, jjae172. [Google Scholar] [CrossRef]
- Reppell, M.; Zheng, X.; Dreher, I.; Blaes, J.; Regan, E.; Haslberger, T.; Guay, H.; Pivorunas, V.; Smaoui, N. HLA-DQA1*05 associates with anti-TNF immunogenicity and low adalimumab trough concentrations in inflammatory bowel disease patients from the SERENE UC and CD studies. J. Crohn’s Colitis 2024, 19, jjae129. [Google Scholar] [CrossRef]
- Spencer, E.A.; Stachelski, J.; Dervieux, T.; Dubinsky, M.C. Failure to achieve target drug concentrations during induction and not HLA-DQA1*05 carriage is associated with antidrug antibody formation in patients with inflammatory bowel disease. Gastroenterology 2022, 162, 1746–1748.e3. [Google Scholar] [CrossRef]
- Fuentes-Valenzuela, E.; García-Alonso, F.J.; Maroto-Martín, C.; Juan Casamayor, L.; Garrote, J.A.; Almendros Muñoz, R.; De Prado, Á.; Vara Castrodeza, A.; Marinero, M.Á.; Calleja Carbajosa, R.; et al. Influence of HLA-DQA1*05 genotype in adults with inflammatory bowel disease and anti-TNF treatment with proactive therapeutic drug monitoring: A retrospective cohort study. Inflamm. Bowel Dis. 2023, 29, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Navajas Hernández, P.; Del Pino Bellido, P.; Lorenzo González, L.; González Rodríguez, C.; Pérez Pérez, A.; Argüelles Arias, F. The HLA-DQA1*05 genotype does not influence the clinical response to ustekinumab and vedolizumab. Rev. Esp. Enferm. Dig. 2023, 115, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; van Sommeren, S.; Huang, H.; Ng, S.C.; Alberts, R.; Takahashi, A.; Ripke, S.; Lee, J.C.; Jostins, L.; Shah, T.; et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 2015, 47, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Alcolado, L.; Grueso-Navarro, E.; Arias, Á.; Lucendo, A.J.; Laserna-Mendieta, E.J. Impact of HLA-DQA1*05 Genotype in Immunogenicity and Failure to Treatment with Tumour Necrosis Factor-alpha Antagonists in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J. Crohn’s Colitis 2024, 18, 1034–1052. [Google Scholar] [CrossRef]
- Pau, A.; Galliano, I.; Barnini, E.; Dini, M.; Pizzol, A.; Ponte, A.; Gambarino, S.; Calvo, P.L.; Bergallo, M. Involvement of HLADQA1*05 in Patients with Inflammatory Bowel Disease Treated with Anti-TNF Drugs. Medicina 2025, 61, 102. [Google Scholar] [CrossRef]
- Hu, J.; Wang, W.; Wang, M.; Wu, C.; Jiao, Y.; Li, Y.; Zhang, W.; Liang, C.; Lin, Z.; Yu, Y.; et al. Immunological pathogenesis of inflammatory bowel disease: Focus on tissue resident memory T cells. Front. Immunol. 2025, 16, 1591584. [Google Scholar] [CrossRef]
- Sollid, L.M.; Jabri, B. Triggers and drivers of autoimmunity: Lessons from coeliac disease. Nat. Rev. Immunol. 2013, 13, 294–302. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Lee, C.; Yu, Z.; Chen, C.; Liang, C. Ulcerative colitis: Molecular insights and intervention therapy. Mol Biomed. 2024, 5, 42. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T. Pathophysiology of inflammatory bowel diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Fasanmade, A.A.; Adedokun, O.J.; Ford, J.; Hernandez, D.; Johanns, J.; Hu, C.; Davis, H.M.; Zhou, H. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur. J. Clin. Pharmacol. 2009, 65, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Bendtzen, K. Immunogenicity of anti-TNF-α biotherapies: II. Clinical relevance of methods used for anti-drug antibody detection. Front. Immunol. 2015, 6, 109. [Google Scholar] [CrossRef]
- Pascual-Oliver, A.; Casas-Deza, D.; Cuarán, C.; García-López, S.; Corsino-Roche, P.; Sierra-Moros, E.; Olier-Martínez, P.; González-Tarancón, R.; Vicente-Lidón, R. HLA-DQA1*05 Was Not Associated With Primary Nonresponse or Loss of Response to First Anti-TNF in Real-World Inflammatory Bowel Disease Patients. Inflamm. Bowel Dis. 2024, 30, 922–929. [Google Scholar] [CrossRef]
- Danese, S.; Vuitton, L.; Peyrin-Biroulet, L. Biologic agents for IBD: Practical insights. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 537–545. [Google Scholar] [CrossRef]
- Dreesen, E.; Bossuyt, P.; Mulleman, D.; Gils, A.; Pascual-Salcedo, D. Practical recommendations for the use of therapeutic drug monitoring of biopharmaceuticals in inflammatory diseases. Clin. Pharmacol. 2017, 9, 101–111. [Google Scholar] [CrossRef]
- Papamichael, K.; Dubinsky, M.C.; Cheifetz, A.S. Proactive Therapeutic Drug Monitoring of Adalimumab in Patients with Crohn’s Disease. Gastroenterology 2023, 164, 164–165. [Google Scholar] [CrossRef]
- Wilson, A.; Peel, C.; Wang, Q.; Pananos, A.D.; Kim, R.B. HLADQA1*05 genotype predicts anti-drug antibody formation and loss of response during infliximab therapy for inflammatory bowel disease. Aliment. Pharmacol. Ther. 2020, 51, 356–363. [Google Scholar] [CrossRef]
- Dubinsky, M.C.; Mei, L.; Friedman, M.; Dhere, T.; Haritunians, T.; Hakonarson, H.; Kim, C.; Glessner, J.; Targan, S.R.; McGovern, D.P.; et al. Genome-wide association (GWA) predictors of anti-TNFα therapeutic responsiveness in pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2010, 16, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Martín, S.; Zapata-Cobo, P.; Velasco, M.; Palomino, L.M.; Clemente, S.; Segarra, O.; Sánchez, C.; Tolín, M.; Moreno-Álvarez, A.; Fernández-Lorenzo, A.; et al. Association between HLA DNA variants and long-term response to anti-TNF drugs in a Spanish pediatric inflammatory bowel disease cohort. Int. J. Mol. Sci. 2023, 24, 1797. [Google Scholar] [CrossRef] [PubMed]
- Billiet, T.; Van Casteele, N.; Van Stappen, T.; Princen, F.; Singh, S.; Gils, A.; Ferrante, M.; Van Assche, G.; Cleynen, I.; Vermeire, S. Immunogenicity to infliximab is associated with HLA-DRB1. Gut 2015, 64, 1344–1345. [Google Scholar] [CrossRef] [PubMed]
- Caenepeel, C.; Sadat Seyed Tabib, N.; Vieira-Silva, S.; Vermeire, S. How the intestinal microbiota may reflect disease activity and influence therapeutic outcome in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2020, 52, 1453–1468. [Google Scholar] [CrossRef]
- Kolho, K.L.; Korpela, K.; Jaakkola, T.; Pichai, M.V.; Zoetendal, E.G.; Salonen, A.; de Vos, W.M. Fecal microbiota in pediatric inflammatory bowel disease and its relation to inflammation. Am. J. Gastroenterol. 2015, 110, 921–930. [Google Scholar] [CrossRef]
- Shaw, K.A.; Bertha, M.; Hofmekler, T.; Chopra, P.; Vatanen, T.; Srivatsa, A.; Prince, J.; Kumar, A.; Sauer, C.; Zwick, M.E.; et al. Dysbiosis, inflammation, and response to treatment: A longitudinal study of pediatric subjects with newly diagnosed inflammatory bowel disease. Genome Med. 2016, 8, 75. [Google Scholar] [CrossRef]
- Magnusson, M.K.; Strid, H.; Sapnara, M.; Lasson, A.; Bajor, A.; Ung, K.A.; Öhman, L. Anti-TNF therapy response in ulcerative colitis is associated with colonic antimicrobial peptide expression and microbiota composition. J. Crohn’s Colitis 2016, 10, 943–952. [Google Scholar] [CrossRef]
- Rajca, S.; Grondin, V.; Louis, E.; Vernier-Massouille, G.; Grimaud, J.C.; Bouhnik, Y.; Laharie, D.; Dupas, J.L.; Pillant, H.; Picon, L.; et al. Alterations in the intestinal microbiome (dysbiosis) as a predictor of relapse after infliximab withdrawal in Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 978–986. [Google Scholar] [CrossRef]
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Grad, S.; Farcas, R.A.; Dumitrascu, D.L.; Surdea-Blaga, T.; Ismaiel, A.; Popa, S. Predictors of Immunogenicity and Loss of Response to ANTI-TNFα Therapy in Crohn Disease-A Systematic Review. Am. J. Ther. 2025, 32, e262–e268. [Google Scholar] [CrossRef] [PubMed]
- Melmed, G.Y.; Irving, P.M.; Jones, J.; Kaplan, G.G.; Kozuch, P.L.; Velayos, F.S.; Baidoo, L.; Sparrow, M.P.; Bressler, B.; Cheifetz, A.S.; et al. Appropriateness of testing for anti–tumor necrosis factor agent and antibody concentrations, and interpretation of results. Clin. Gastroenterol. Hepatol. 2016, 14, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Murugesan, S.; Ibrahim, N.; Elawad, M.; Al Khodor, S. Predictive biomarkers for anti-TNFα therapy in IBD patients. J. Transl. Med. 2024, 22, 284. [Google Scholar] [CrossRef] [PubMed]
- Tait, B.D.; Süsal, C.; Gebel, H.M.; Nickerson, P.W.; Zachary, A.A.; Claas, F.H.; Reed, E.F.; Bray, R.A.; Campbell, P.; Chapman, J.R.; et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation 2013, 95, 19–47. [Google Scholar] [CrossRef]
- DelBaugh, R.M.; Cook, L.J.; Siegel, C.A.; Tsongalis, G.J.; Khan, W.A. Validation of a rapid HLA-DQA1*05 pharmacogenomics assay to identify at-risk resistance to anti–tumor necrosis factor therapy among patients with inflammatory bowel disease. Am. J. Clin. Pathol. 2023, 160, 194–199. [Google Scholar] [CrossRef]
- Bergstein, S.; Spencer, E. HLA-DQA1*05 associates with immunogenicity and loss of response to anti-TNF therapy in the IBD population: A meta-analysis. Inflamm. Bowel Dis. 2023, 29, S58. [Google Scholar] [CrossRef]
- Kim, M.; Won, J.Y.; Choi, S.Y.; Ju, J.H.; Park, Y.H. Anti-TNFα treatment for HLA-B27-positive ankylosing spondylitis–related uveitis. Am. J. Ophthalmol. 2016, 170, 32–40. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Vande Casteele, N.; Boland, B.S.; Rivera-Nieves, J.; Ernst, P.B.; Eckmann, L.; Barrett, K.E.; Chang, J.T.; Sandborn, W.J. Should we divide Crohn’s disease into ileum-dominant and isolated colonic diseases? Clin. Gastroenterol. Hepatol. 2019, 17, 2634–2643. [Google Scholar] [CrossRef]
- Solitano, V.; Facciorusso, A.; McGovern, D.P.B.; Nguyen, T.; Colman, R.J.; Zou, L.; Boland, B.S.; Syversen, S.W.; Jørgensen, K.K.; Ma, C.; et al. HLA-DQA1*05 genotype and immunogenicity to tumor necrosis factor-α antagonists: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 3019–3029.e5. [Google Scholar] [CrossRef]
- Sucker, A.; Zhao, F.; Pieper, N.; Heeke, C.; Maltaner, R.; Stadtler, N.; Real, B.; Bielefeld, N.; Howe, S.; Weide, B.; et al. Acquired IFNγ resistance impairs anti-tumor immunity and gives rise to T-cell-resistant melanoma lesions. Nat. Commun. 2017, 8, 15440. [Google Scholar] [CrossRef]
- Adler, J.; Galanko, J.A.; Ammoury, R.; Benkov, K.J.; Bousvaros, A.; Boyle, B.; Cabrera, J.M.; Chun, K.Y.; Dorsey, J.; Ebach, D.R.; et al. HLA-DQA1*05 and risk of antitumor necrosis factor treatment failure and anti-drug antibody development in children with Crohn’s disease. Am. J. Gastroenterol. 2025, 120, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Kim, R.B. Letter: Genetic variation in the HLA-DQA1*05 allele predicts tumour necrosis factor-α antagonist immunogenicity—Does location matter? Aliment. Pharmacol. Ther. 2021, 53, 1055–1056. [Google Scholar] [CrossRef]
- Wientjes, M.H.M.; Den Broeder, A.A.; Welsing, P.M.J.; Verhoef, L.M.; Van Den Bemt, B.J.F. Prediction of response to anti-TNF treatment using laboratory biomarkers in patients with rheumatoid arthritis: A systematic review. RMD Open 2022, 8, e002570. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, S.; Beecham, A.; Gomez, L.; Dauer, R.; Khakoo, N.; Pascual, L.; Quintero, M.; Lopez, J.; Leavitt, J.S.; Solis, N.; et al. Ancestral Diversity in Pharmacogenomics Affects Treatment for Hispanic/Latine Populations with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2025, 23, 1008–1018.e7. [Google Scholar] [CrossRef]
- Fan, J.C.; Lu, Y.; Gan, J.H.; Lu, H. Identification of potential novel targets for treating inflammatory bowel disease using Mendelian randomization analysis. Int. J. Color. Dis. 2024, 39, 165. [Google Scholar] [CrossRef] [PubMed]
- Teva and Sanofi Announce Duvakitug (Anti-TL1A) Positive Phase 2b Results Demonstrating Best-in-Class Potential in Ulcerative Colitis and Crohn’s Disease. Available online: https://ir.tevapharm.com/news-and-events/press-releases/press-release-details/2024/Teva-and-Sanofi-Announce-Duvakitug-Anti-TL1A-Positive-Phase-2b-Results-Demonstrating-Best-in-Class-Potential-in-Ulcerative-Colitis-and-Crohns-Disease/default.aspx (accessed on 8 June 2025).

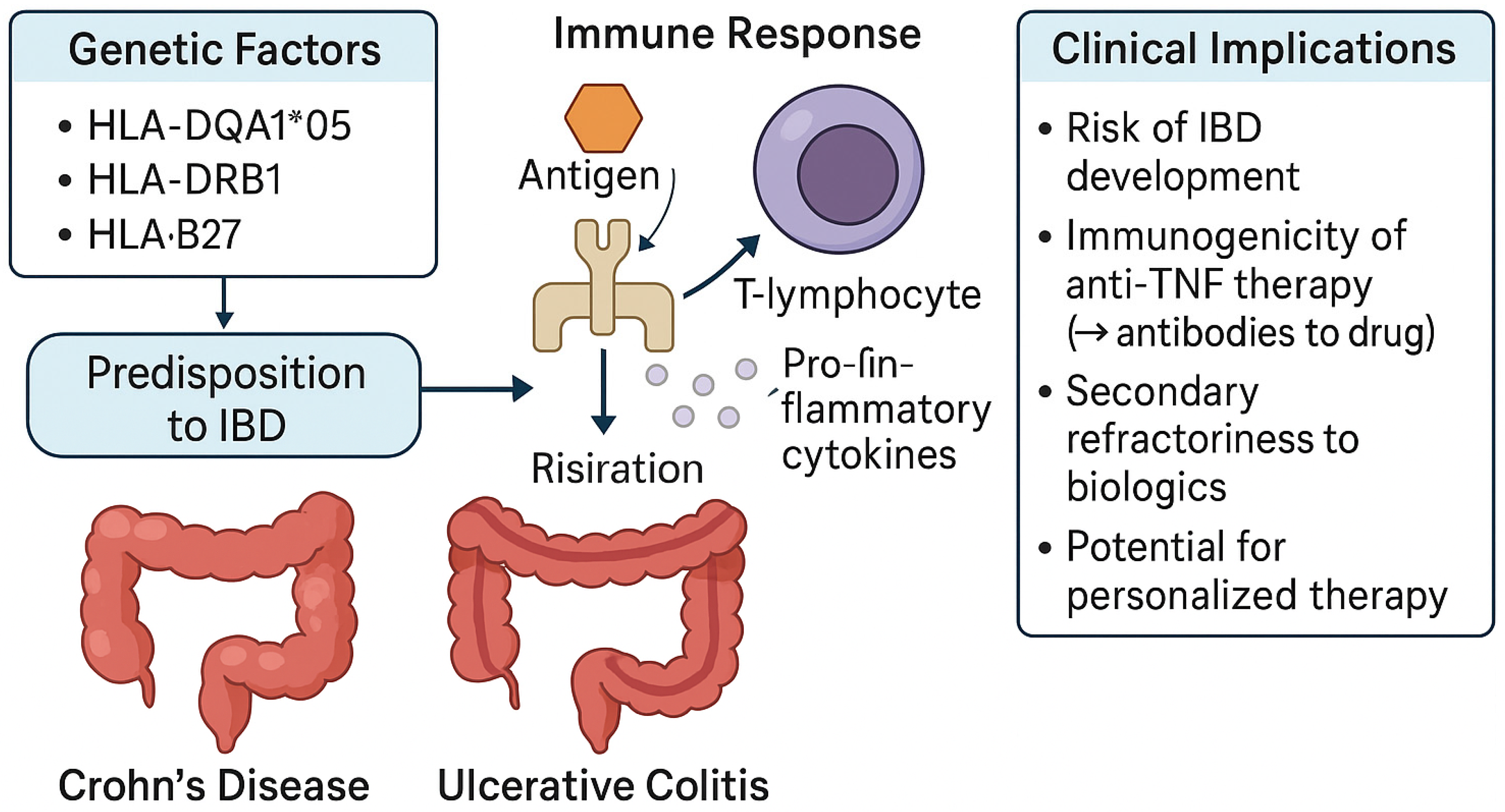
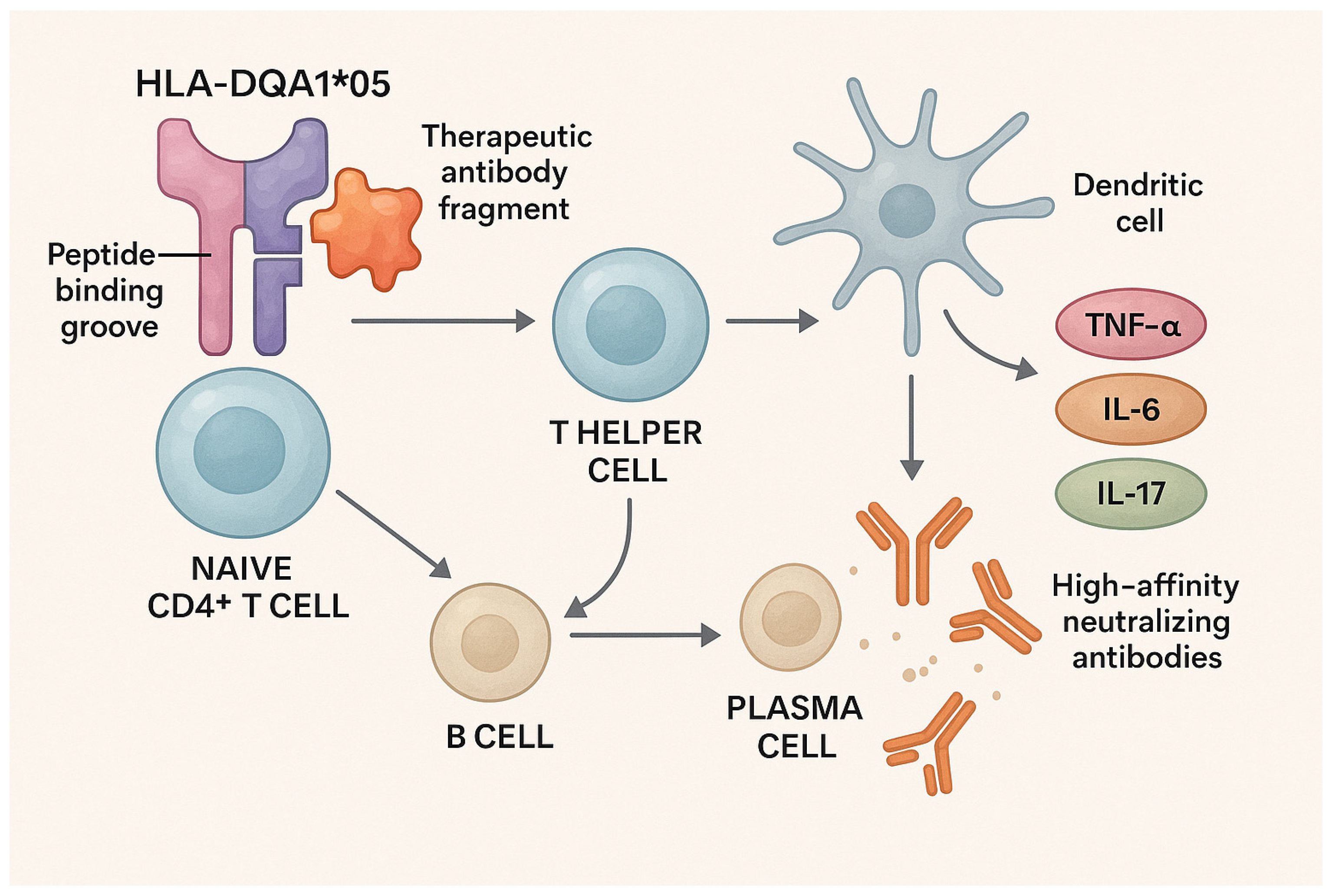
| HLA Allele/Haplotype | Associated Condition | Reported Effect |
|---|---|---|
| HLA-DRB1*01:03 | UC | Increased severity, risk of hospitalization, and colectomy |
| HLA-DRB1*13 (1309, 1320, 1325, 1329) | UC | Associated with pancolitis, surgery, and extraintestinal manifestations |
| HLA-DRB1*08 | UC | Associated with extensive disease |
| HLA-DRB1*09 | UC | Later age of diagnosis |
| HLA-DRB1*1502 | UC | Consistently associated with disease across populations |
| HLA-DRB1*13:01 | UC | Associated with extensive colitis in pediatric-onset UC |
| rs17188113 | UC | Genetic risk marker; female predominance; pediatric onset |
| HLA-B44 | UC | Linked to type II arthritis and erythema nodosum |
| TNF-1031C | UC | Associated with type II arthritis |
| HLA-DRB1*0450 | CD | Positive association with disease |
| HLA-DRB1*1502 | CD | Protective effect |
| HLA-DRB1*0405-DQB1*0401 | CD | Associated with fistulizing phenotype; ileocecal involvement |
| HLA-G, MICA/MICB | CD | Associated with disease phenotype |
| HLA-DRB1*0103 | CD | Linked to arthritis and uveitis |
| HLA-B*44 | CD | Associated with extraintestinal manifestations |
| HLA-B27 | CD | Linked to spondyloarthropathy |
| Gene/SNP | Effect on Pharmacokinetics |
|---|---|
| ATG16L1 (rs7587051, rs143063741) | Lower infliximab trough levels |
| C1orf106 (rs442905, rs59457695) | Variable levels (lower and higher) |
| IL6 (rs10499563) | Higher infliximab trough levels |
| TLR2 (rs1816702) | Higher adalimumab levels |
| NOD2 (rs2066844/45/47) | Dose intensification needed |
| Mechanism | Description | Consequence |
|---|---|---|
| Preferential peptide presentation | HLA-DQA1*05 has a unique binding groove that efficiently presents infliximab/adalimumab fragments to CD4+ T cells. | T-cell activation and B-cell help |
| B-cell stimulation | Activated Th cells promote differentiation of B cells into plasma cells producing ADAs. | High-titer ADA production |
| Pro-inflammatory dendritic cell polarization | HLA-DQA1*05 carriers show DC skewing toward Th1/Th17 profiles instead of Treg. | Chronic inflammation and therapy refractoriness |
| Reduced Treg-mediated suppression | Imbalance between effector and regulatory T cells. | Enhanced ADA generation and sustained inflammation |
| Upregulation of pro-inflammatory cytokines | Increased TNF-α, IL-6, and IL-17 expression. | Reduced drug levels and effectiveness |
| Formation of ADA–drug immune complexes | Immune complexes neutralize drug activity and accelerate clearance. | Loss of therapeutic response (secondary LOR) |
| HLA Allele | Associated Disease | Impact |
|---|---|---|
| HLA-DRB1*01:03 | UC | Increased severity, risk of surgery |
| HLA-DRB1*1502 | UC | Consistent association across populations |
| HLA-DQA1*05 | CD and UC | Increased ADA formation, therapy failure |
| HLA-DRB1*03 | IBD | Immunogenicity to infliximab |
| HLA-DRB9 (rs239585) | IBD (pediatric) | Loss of response to infliximab |
| Microbial Feature | Association with Response |
|---|---|
| Faecalibacterium prausnitzii ↓ | Non-response, recurrence |
| Bifidobacterium ↑ | Response (pediatric) |
| Fusobacterium ↑ | Non-response |
| Akkermansia ↑ | Negative predictor |
| Streptococcus mitis ↑ | Non-response (↑ at baseline) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksic, M.; Corovic, I.; Maksic, T.; Zivic, J.; Zivic, M.; Zdravkovic, N.; Begovic, A.; Medovic, M.; Kralj, D.; Todorovic, Z.; et al. Molecular Insight into the Role of HLA Genotypes in Immunogenicity and Secondary Refractoriness to Anti-TNF Therapy in IBD Patients. Int. J. Mol. Sci. 2025, 26, 7274. https://doi.org/10.3390/ijms26157274
Maksic M, Corovic I, Maksic T, Zivic J, Zivic M, Zdravkovic N, Begovic A, Medovic M, Kralj D, Todorovic Z, et al. Molecular Insight into the Role of HLA Genotypes in Immunogenicity and Secondary Refractoriness to Anti-TNF Therapy in IBD Patients. International Journal of Molecular Sciences. 2025; 26(15):7274. https://doi.org/10.3390/ijms26157274
Chicago/Turabian StyleMaksic, Mladen, Irfan Corovic, Tijana Maksic, Jelena Zivic, Milos Zivic, Natasa Zdravkovic, Aleksa Begovic, Marija Medovic, Djordje Kralj, Zeljko Todorovic, and et al. 2025. "Molecular Insight into the Role of HLA Genotypes in Immunogenicity and Secondary Refractoriness to Anti-TNF Therapy in IBD Patients" International Journal of Molecular Sciences 26, no. 15: 7274. https://doi.org/10.3390/ijms26157274
APA StyleMaksic, M., Corovic, I., Maksic, T., Zivic, J., Zivic, M., Zdravkovic, N., Begovic, A., Medovic, M., Kralj, D., Todorovic, Z., Cekerevac, M., Medovic, R., & Nikolic, M. (2025). Molecular Insight into the Role of HLA Genotypes in Immunogenicity and Secondary Refractoriness to Anti-TNF Therapy in IBD Patients. International Journal of Molecular Sciences, 26(15), 7274. https://doi.org/10.3390/ijms26157274


_Kim.png)





