Physiological and Transcriptomic Analyses Reveal Regulatory Mechanisms of Adventitious Root Formation in In Vitro Culture of Cinnamomum camphora
Abstract
1. Introduction
2. Results
2.1. Morphological and Anatomical Changes During AR Formation
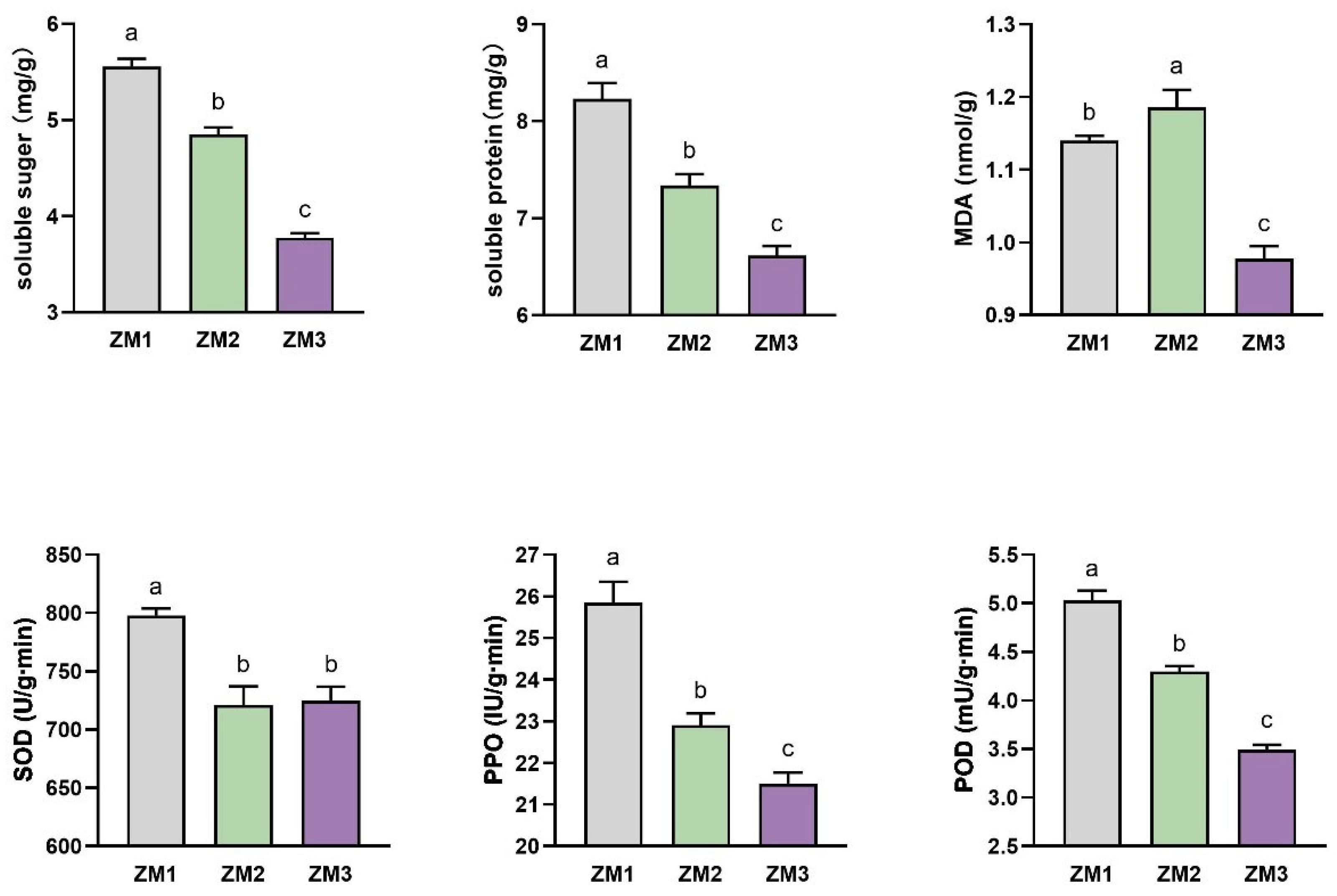
2.2. Changes in Nutrient Content and Enzyme Activities During AR Formation
2.3. Changes in Endogenous Hormone Levels During AR Formation
2.4. Transcriptomic Analysis of Critical Stages of AR Formation
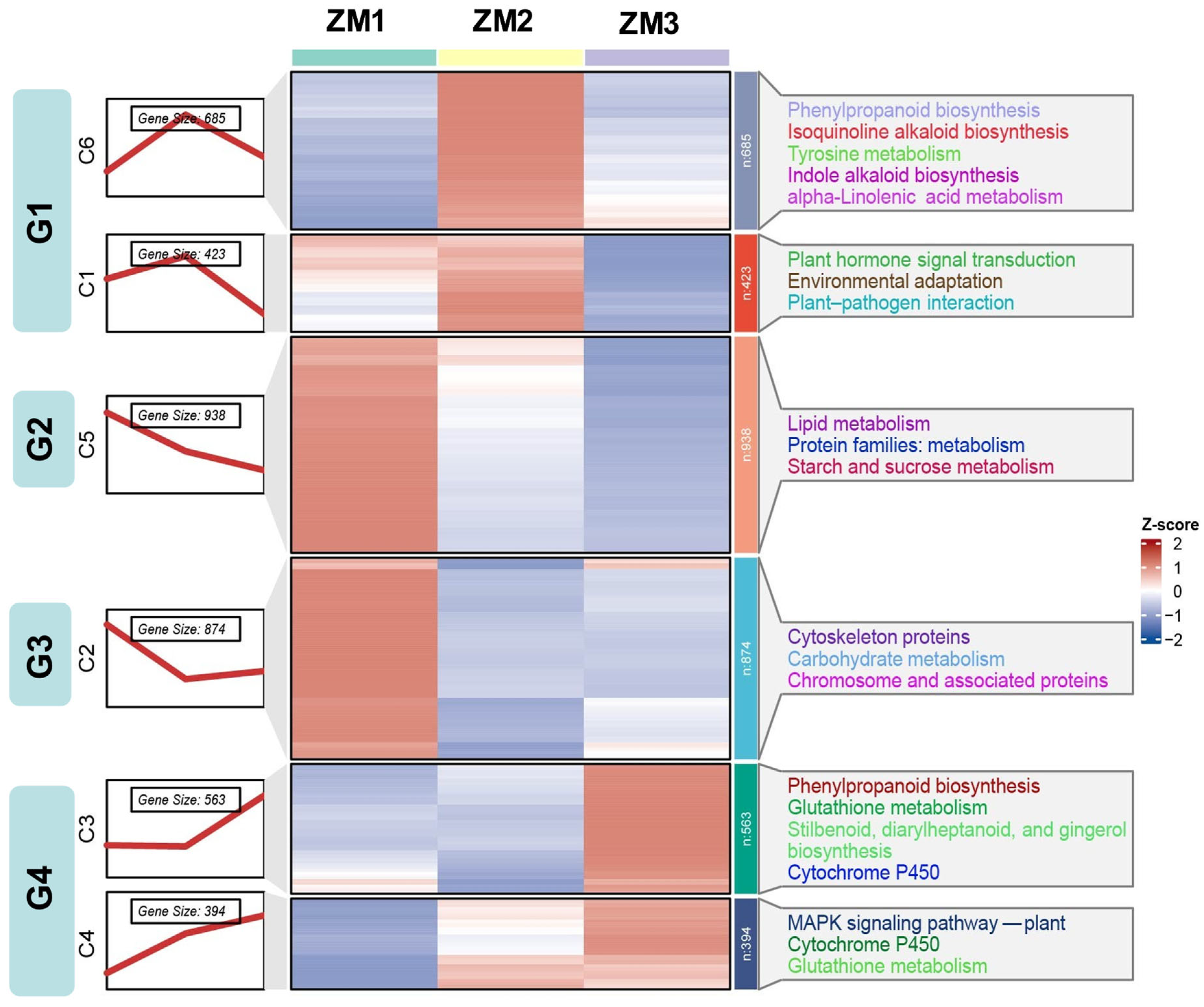
2.5. Temporal Dynamics of DEGs
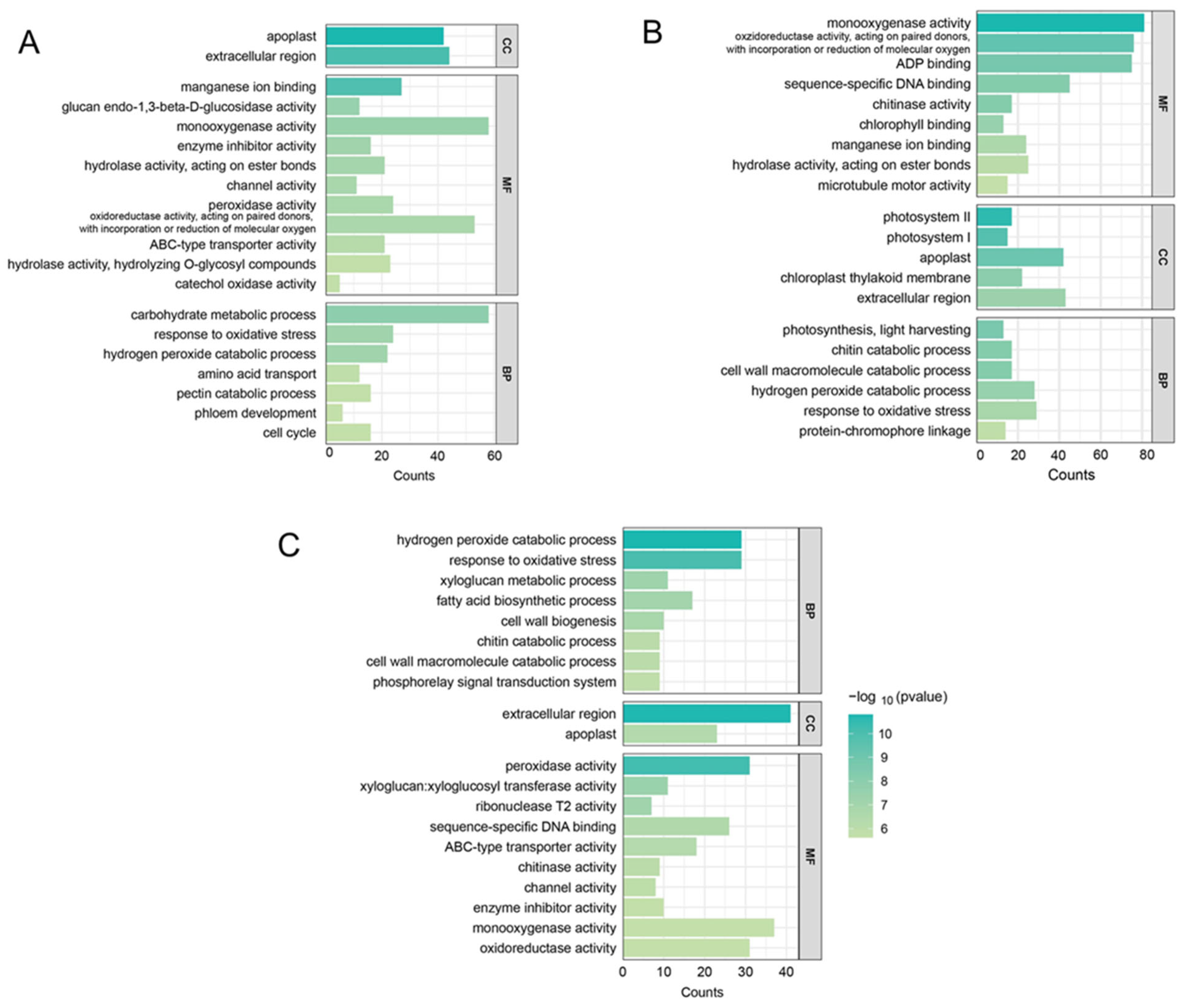
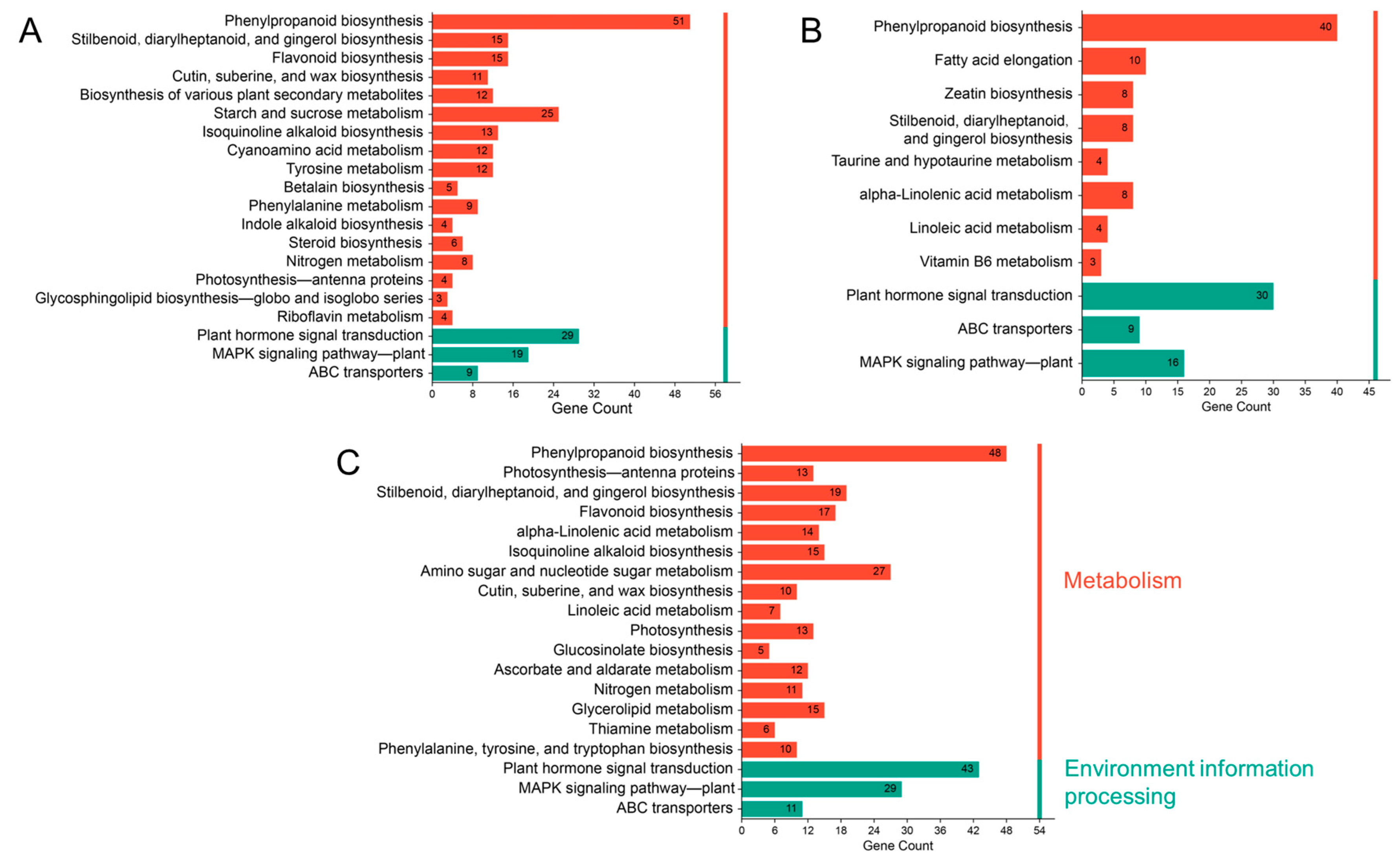
2.6. GO and KEGG Enrichment Analysis of DEGs
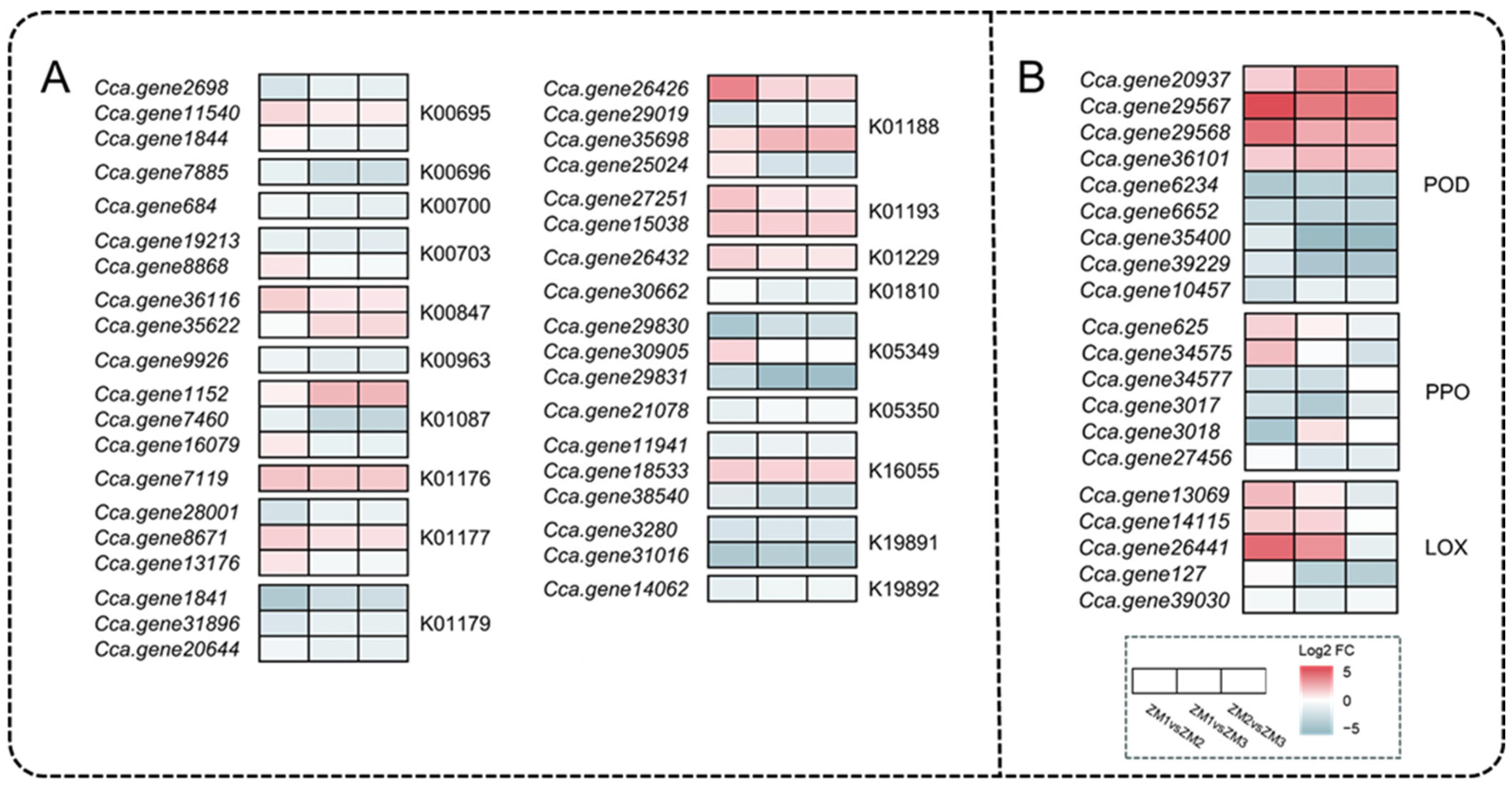
2.7. Analysis of DEGs Related to Nutrients and Oxidoreductases
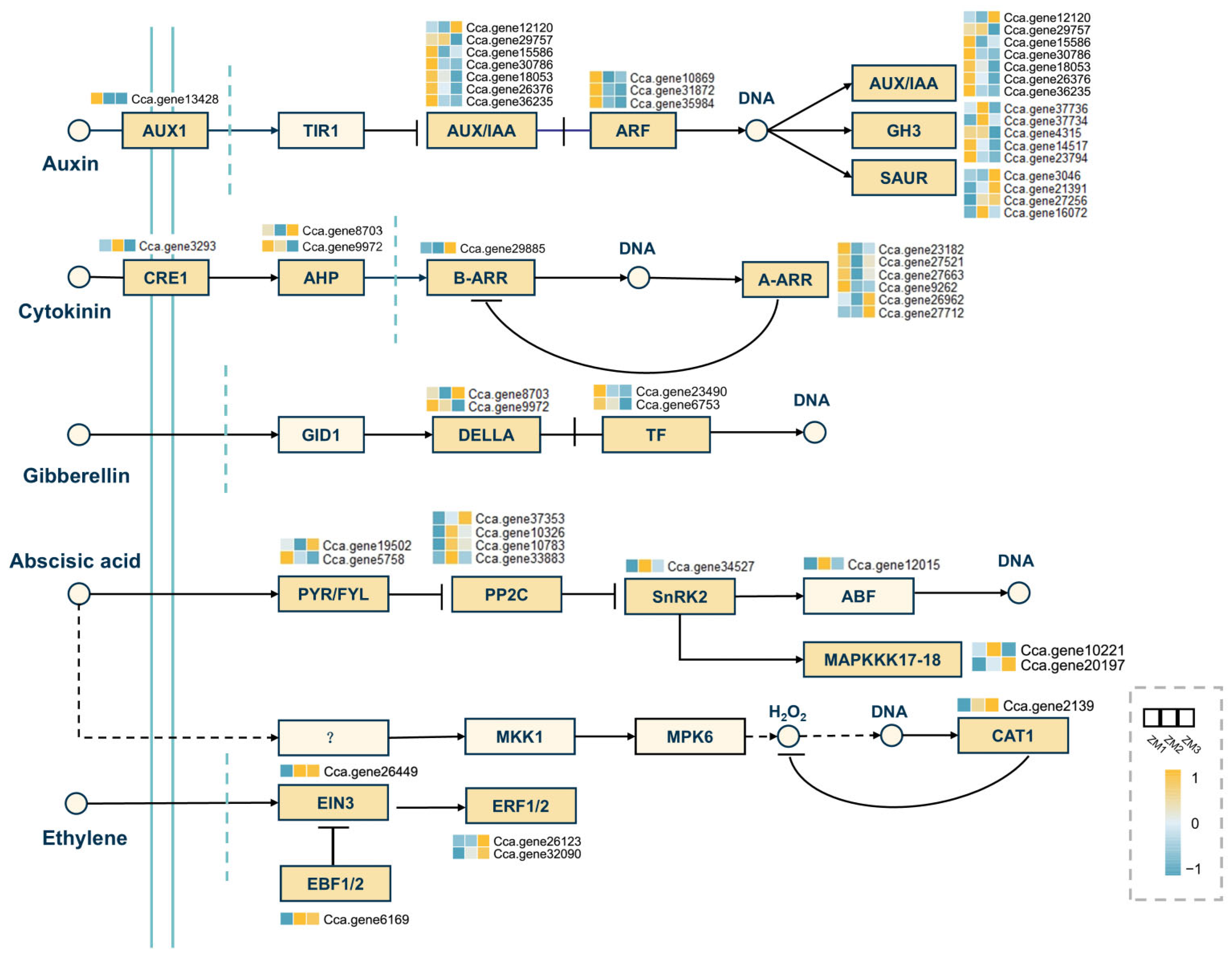
2.8. Analysis of DEGs in Key Signaling Pathways
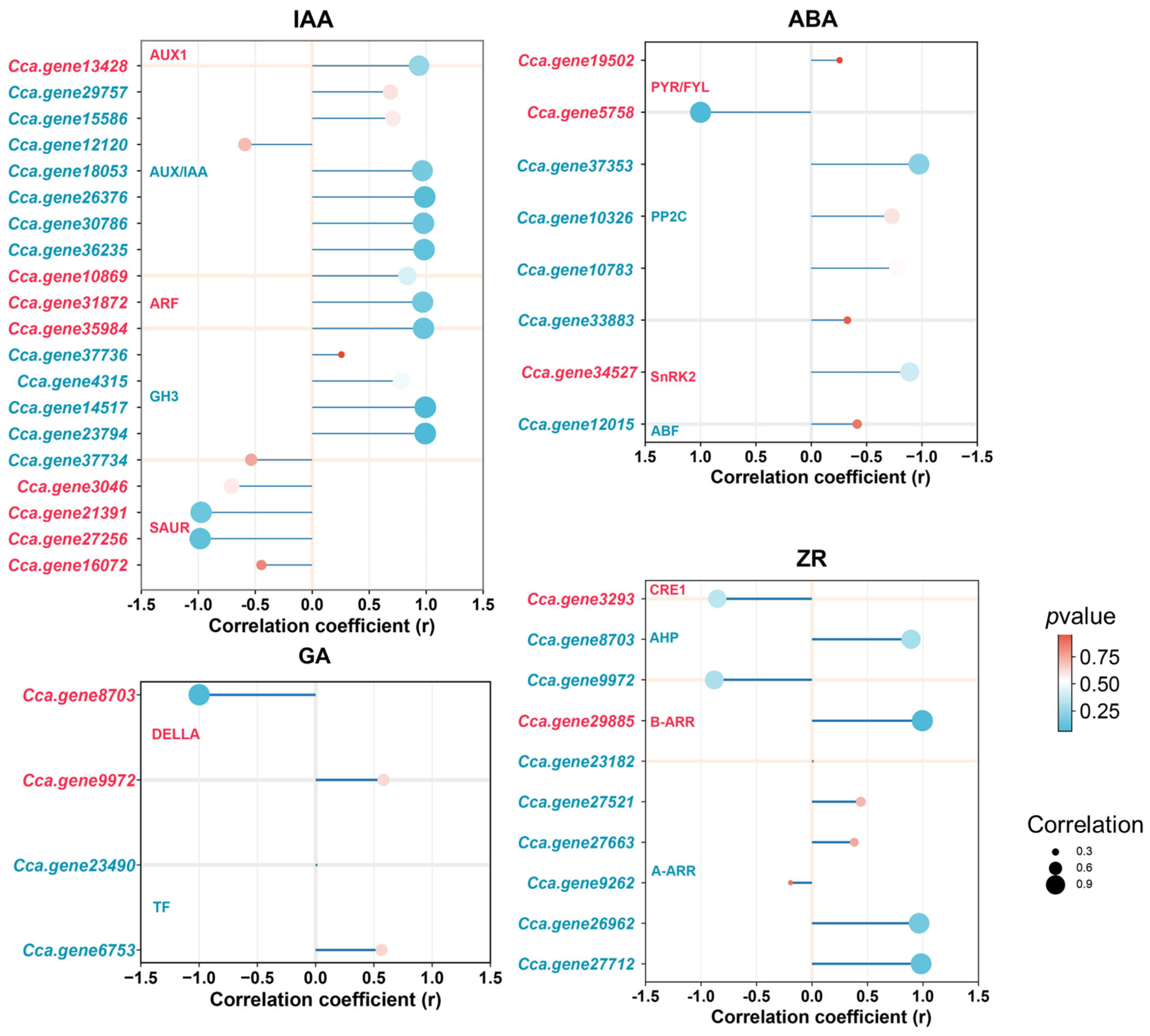
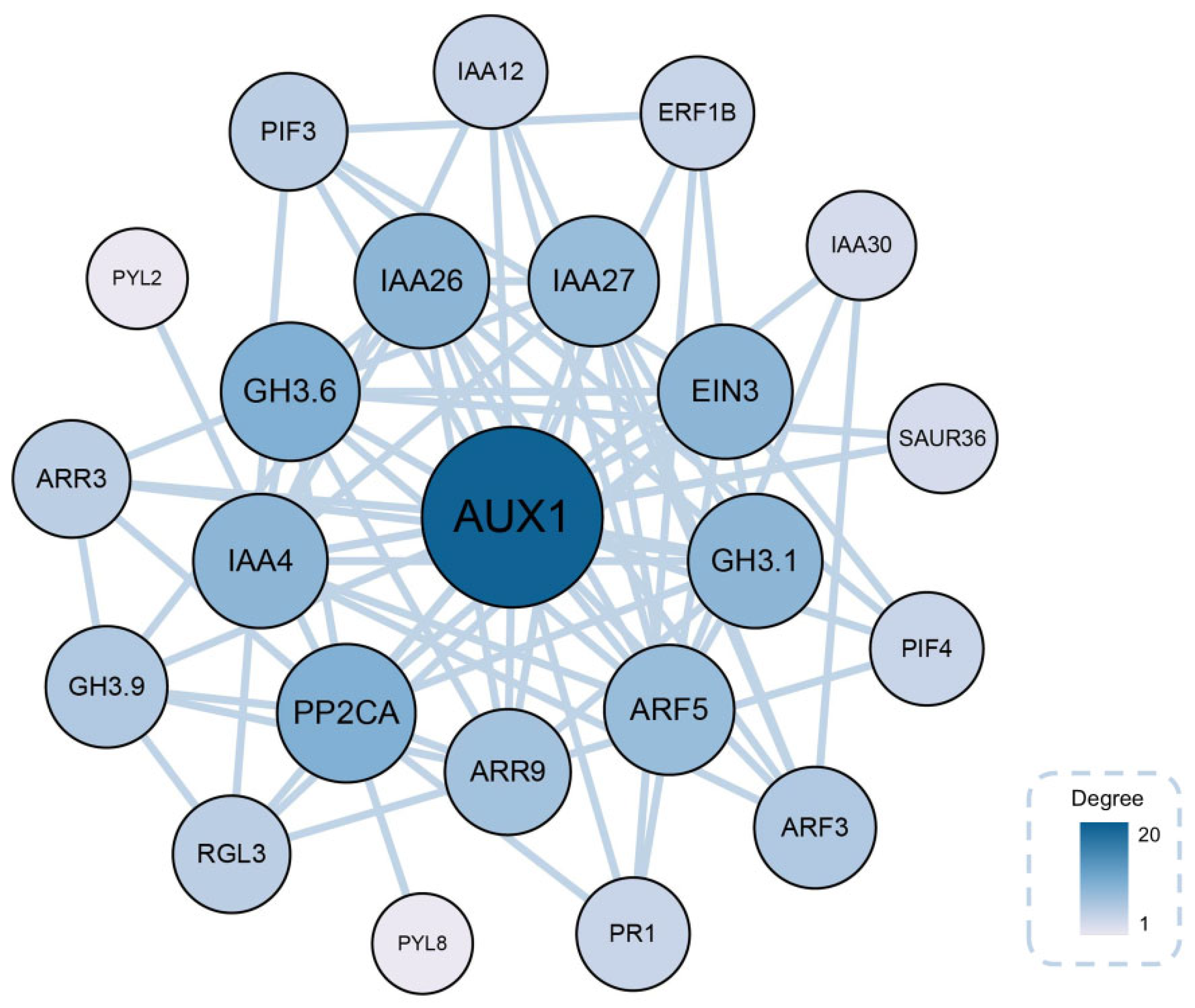
2.9. Identification of Key Genes Associated with AR Formation
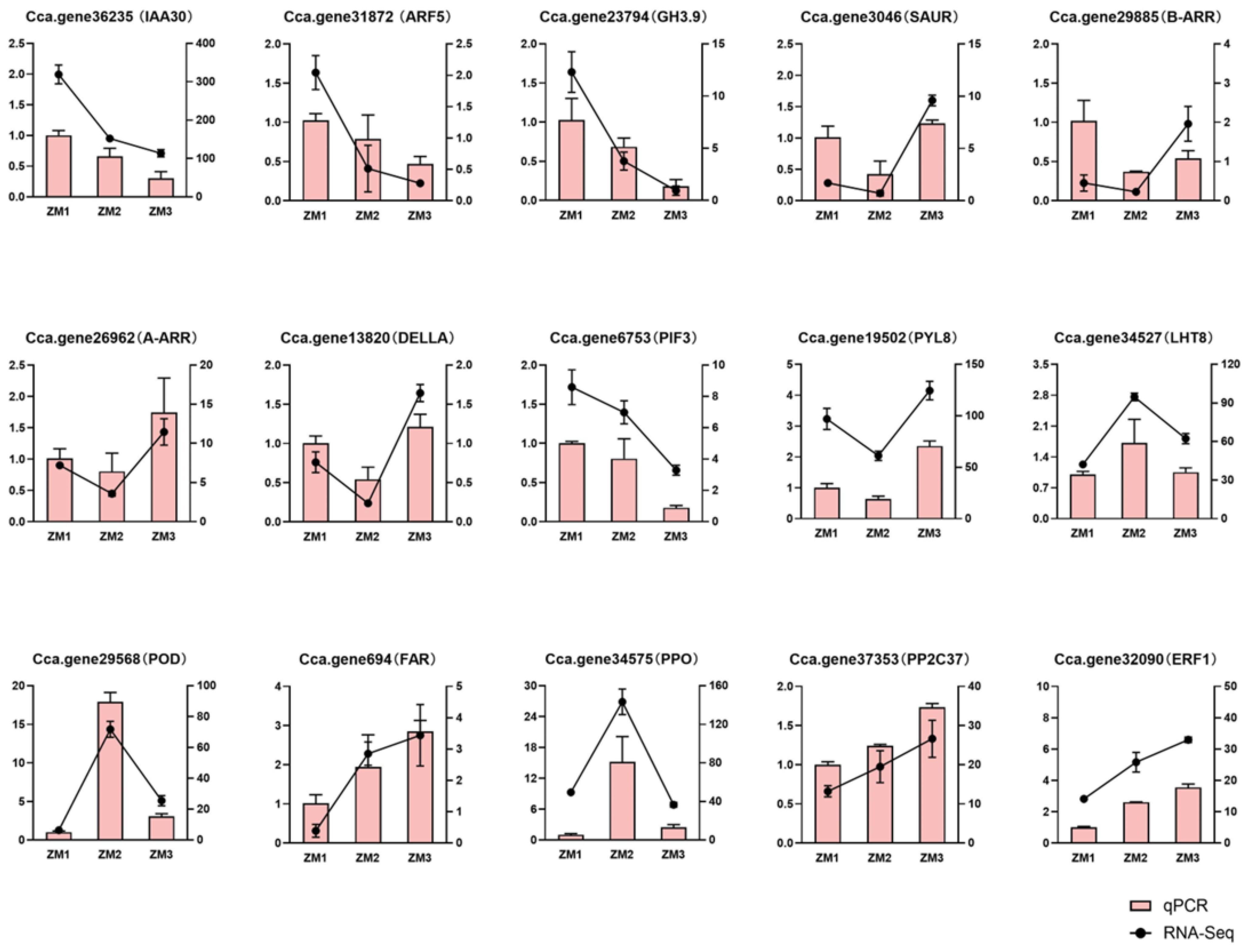
2.10. Validation of Gene Expression
3. Discussion
3.1. The Process of AR Formation in C. camphora
3.2. Regulation Mechanism of Nutrients and Oxidoreductases During AR Formation
3.3. Regulation Mechanism of Auxin
3.4. Regulation Mechanism of Endogenous ZR
3.5. Regulation Mechanism of Endogenous ABA and ET
4. Materials and Methods
4.1. Plant Materials
4.2. Morphological and Anatomical Observation
4.3. Physiological Index Measurement
4.4. RNA-Seq and Data Analysis
4.5. Identification and Analysis of DEGs
4.6. Quantitative Real-Time PCR (qRT-PCR) Validation
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Takaoka, D. Sesquiterpene alcohols in camphor oil. Phytochemistry 1976, 15, 425–426. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, Y.; Liu, S.; Zhong, Y.; Wu, Y.; Li, J.; Xu, L.A.; Xu, M. The complete chloroplast genome of Cinnamomum camphora and its comparison with related Lauraceae species. PeerJ 2017, 5, e3820. [Google Scholar] [CrossRef]
- Nirmal Babu, K.; Sajina, A.; Minoo, D.; John, C.Z.; Mini, P.M.; Tushar, K.V.; Rema, J.; Ravindran, P.N. Micropropagation of camphor tree (Cinnamomum camphora). Plant Cell Tissue Organ Cult. 2003, 74, 179–183. [Google Scholar] [CrossRef]
- Zuzarte, M.; Salgueiro, L.; Canhoto, J. Plant tissue culture: Industrial relevance and future directions. Adv. Biochem. Eng./Biotechnol. 2024, 188, 1–15. [Google Scholar] [CrossRef]
- Legué, V.; Rigal, A.; Bhalerao, R.P. Adventitious root formation in tree species: Involvement of transcription factors. Physiol. Plant. 2014, 151, 192–198. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, L.; Xu, Y.; Li, J.; Yang, Z.; Huang, H.; Xu, L. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell 2014, 26, 1081–1093. [Google Scholar] [CrossRef]
- Yu, J.; Liu, W.; Liu, J.; Qin, P.; Xu, L. Auxin control of root organogenesis from callus in tissue culture. Front. Plant Sci. 2017, 8, 1385. [Google Scholar] [CrossRef]
- Khanam, M.; Anis, M.; Javed, S.; Mottaghipisheh, J.; Csupor, D. Adventitious root culture—An alternative strategy for secondary metabolite production: A review. Agronomy 2022, 12, 1178. [Google Scholar] [CrossRef]
- Gonin, M.; Bergougnoux, V.; Nguyen, T.D.; Gantet, P.; Champion, A. What makes adventitious roots? Plants 2019, 8, 240. [Google Scholar] [CrossRef]
- Zahra, A.F.; Ilham, E.Q.; Nagla, A.; Mohammed, I.; Rabea, Z.; Najiba, B. Effect of medium culture, pH, activated carbon, and light on germination, rooting and growth of tomato (Lycopersicum esculentum Mill.). Univers. J. Agric. Res. 2023, 11, 389–402. [Google Scholar] [CrossRef]
- Jagannathan, L.; Cuddapah, S.; Costa, M. Oxidative stress under ambient and physiological oxygen tension in tissue culture. Curr. Pharmacol. Rep. 2016, 2, 64–72. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant defense system in plants: Reactive oxygen species production, signaling, and scavenging during abiotic stress-induced oxidative damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Jiang, C.; Lu, M.Z.; Zhang, J. Exogenous hormones supplementation improve adventitious root formation in woody plants. Front. Bioeng. Biotechnol. 2022, 10, 1009531. [Google Scholar] [CrossRef]
- Paciorek, T.; Zažímalová, E.; Ruthardt, N.; Petrášek, J.; Stierhof, Y.-D.; Kleine-Vehn, J.; Morris, D.A.; Emans, N.; Jürgens, G.; Geldner, N.; et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 2005, 435, 1251–1256. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Zhang, L.; Chen, B. Study on the mechanism of the regulation of NAA promoted the adventitious root formation of the Dalbergia odorifera. T. Chen Hardwood Cuttings. In Proceedings of the International Conference on Mechatronics, Materials, Chemistry and Computer Engineering, Chongqing, China, 24–25 July 2017; Atlantis Press: Dordrecht, The Netherlands, 2017. [Google Scholar]
- Ye, R.; Tong, Z.; Zhang, J.; Zhu, Y. Tissue culture and rapid propagation for stems of Cinnamomum camphora. J. Zhejiang AF Univ. 2016, 33, 177–182. [Google Scholar] [CrossRef]
- Xiao, Z.; Zheng, L.; Zhang, H.; Wang, Y.; Li, F.; Jiang, R.; Peng, Y.; Jin, Z. Study on tissue culture technology of stem segment of Cinnamomum camphora ‘Ganfang 1’. J. Nanchang Inst. Technol. 2022, 41, 71–77. [Google Scholar]
- Wang, Y.; Pang, D.; Ruan, L.; Liang, J.; Zhang, Q.; Qian, Y.; Zhang, Y.; Bai, P.; Wu, L.; Cheng, H.; et al. Integrated transcriptome and hormonal analysis of naphthalene acetic acid-induced adventitious root formation of tea cuttings (Camellia sinensis). BMC Plant Biol. 2022, 22, 319. [Google Scholar] [CrossRef]
- Chen, X.; Gao, H.; Wang, S.S. Studies on endogenous hormone levels in cuttings of three poplar species during rooting process. Sci. Silvae Sin. 1994, 30, 1–7. [Google Scholar]
- Delbarre, A.; Muller, P.; Imhoff, V.; Morgat, J.-L.; Barbier-Brygoo, H. Uptake, Accumulation and metabolism of auxins in tobacco leaf protoplasts. Planta 1994, 195, 159–167. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Z.; Luo, L.; Wang, P.; Zhu, X.; Liu, J.; Wang, C. Exogenous auxin regulates the growth and development of peach fruit at the expansion stage by mediating multiple-hormone signaling. BMC Plant Biol. 2023, 23, 499. [Google Scholar] [CrossRef]
- Swarup, R.; Péret, B. AUX/LAX family of auxin influx carriers—An overview. Front. Plant Sci. 2012, 3, 225. [Google Scholar] [CrossRef]
- Hayashi, K. The interaction and integration of auxin signaling components. Plant Cell Physiol. 2012, 53, 965–975. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef]
- Lee, H.W.; Cho, C.; Pandey, S.K.; Park, Y.; Kim, M.-J.; Kim, J. LBD16 and LBD18 acting downstream of ARF7 and ARF19 are involved in adventitious root formation in Arabidopsis. BMC Plant Biol. 2019, 19, 46. [Google Scholar] [CrossRef]
- Kong, X.; Lu, S.; Tian, H.; Ding, Z. WOX5 is shining in the root stem cell niche. Trends Plant Sci. 2015, 20, 601–603. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Jia, H.; Liu, B.; Sun, P.; Hu, J.; Wang, L.; Lu, M. The WUSCHEL-Related Homeobox 5a (PtoWOX5a) is involved in adventitious root development in Poplar. Tree Physiol. 2018, 38, 139–153. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Luijten, M.; Miyashima, S.; Lenhard, M.; Hashimoto, T.; Nakajima, K.; Scheres, B.; Heidstra, R.; Laux, T. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 2007, 446, 811–814. [Google Scholar] [CrossRef]
- Wei, K.; Ruan, L.; Wang, L.; Cheng, H. Auxin-induced adventitious root formation in nodal cuttings of Camellia sinensis. Int. J. Mol. Sci. 2019, 20, 4817. [Google Scholar] [CrossRef]
- Kuroha, T.; Satoh, S. Involvement of cytokinins in adventitious and lateral root formation. Plant Root 2007, 1, 27–33. [Google Scholar] [CrossRef]
- Ramírez-Carvajal, G.A.; Morse, A.M.; Dervinis, C.; Davis, J.M. The cytokinin type-b response regulator PtRR13 is a negative regulator of adventitious root development in Populus. Plant Physiol. 2009, 150, 759–771. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, R.; Zi, H.; Li, Y.; Cao, X.; Li, D.; Guo, L.; Tong, J.; Pan, Y.; Jiao, Y.; et al. AUXIN RESPONSE FACTOR3 regulates floral meristem determinacy by repressing cytokinin biosynthesis and signaling. Plant Cell 2018, 30, 324–346. [Google Scholar] [CrossRef]
- Luo, X.; Chen, Z.; Gao, J.; Gong, Z. Abscisic acid inhibits root growth in Arabidopsis through ethylene biosynthesis. Plant J. 2014, 79, 44–55. [Google Scholar] [CrossRef]
- Joshi-Saha, A.; Valon, C.; Leung, J. Abscisic acid signal off the STARting block. Mol. Plant 2011, 4, 562–580. [Google Scholar] [CrossRef]
- Miyazono, K.; Miyakawa, T.; Sawano, Y.; Kubota, K.; Kang, H.-J.; Asano, A.; Miyauchi, Y.; Takahashi, M.; Zhi, Y.; Fujita, Y.; et al. Structural basis of abscisic acid signalling. Nature 2009, 462, 609–614. [Google Scholar] [CrossRef]
- Kumar, L.; Futschik, E.M. Mfuzz: A software package for soft clustering of microarray data. Bioinformation 2007, 2, 5–7. [Google Scholar] [CrossRef]
- Lakehal, A.; Bellini, C. Control of adventitious root formation: Insights into synergistic and antagonistic hormonal interactions. Physiol. Plant. 2019, 165, 90–100. [Google Scholar] [CrossRef]
- Yu, W.; Li, Y.; Zhang, Y.; Luo, C.; Zheng, Y.; Zhang, T.; Fu, C.; Liu, X. Integrated physiological and transcriptomic analyses reveal mechanisms regulating endogenous phytohormones in adventitious root formation during Cinnamomum bodinieri cutting propagation. Forests 2025, 16, 509. [Google Scholar] [CrossRef]
- Luo, C.; Liu, X.; Zheng, Y.; Dai, X.; Tang, X.; Zhang, T.; Zhang, X. Physiological and transcriptomic analysis reveal the regulation of adventitious root formation in Cinnamomum parthenoxylon cuttings. BMC Plant Biol. 2024, 24, 1217. [Google Scholar] [CrossRef]
- Welander, T. Influence of nitrogen and sucrose in the medium and of irradiance of the stock plants on root formation in Pelargonium petioles grown in vitro. Physiol. Plant. 1978, 43, 136–141. [Google Scholar] [CrossRef]
- Calamar, A.; de Klerk, G.J. Effect of sucrose on adventitious root regeneration in apple. Plant Cell Tissue Organ Cult. 2002, 70, 207–212. [Google Scholar] [CrossRef]
- Takahashi, F.; Sato-Nara, K.; Kobayashi, K.; Suzuki, M.; Suzuki, H. Sugar-induced adventitious roots in Arabidopsis seedlings. J. Plant Res. 2003, 116, 83–91. [Google Scholar] [CrossRef]
- Williams, L.E.; Lemoine, R.; Sauer, N. Sugar transporters in higher plants—A diversity of roles and complex regulation. Trends Plant Sci. 2000, 5, 283–290. [Google Scholar] [CrossRef]
- Ahkami, A.; Scholz, U.; Steuernagel, B.; Strickert, M.; Haensch, K.-T.; Druege, U.; Reinhardt, D.; Nouri, E.; von Wirén, N.; Franken, P.; et al. Comprehensive transcriptome analysis unravels the existence of crucial genes regulating primary metabolism during adventitious root formation in Petunia hybrida. PLoS ONE 2014, 9, e100997. [Google Scholar] [CrossRef]
- Roussos, P.A. Adventitious root formation in plants: The implication of hydrogen peroxide and nitric oxide. Antioxidants 2023, 12, 862. [Google Scholar] [CrossRef]
- Sofo, A.; Dichio, B.; Xiloyannis, C.; Masia, A. Lipoxygenase activity and proline accumulation in leaves and roots of Olive trees in response to drought stress. Physiol. Plant. 2004, 121, 58–65. [Google Scholar] [CrossRef]
- Liu, N.; Lin, Z. Reactive oxygen species and relative enzyme activities in the development of aerial roots of Chinese banyan (Ficus microcarpa). J. Plant Growth Regul. 2014, 33, 160–168. [Google Scholar] [CrossRef]
- Yang, Y.; Xiang, Q.; Yang, Y.; Shu, D.; Yan, J.; Huang, L.; Yang, X.; Peng, J.; Chen, X.; Yang, G. Chemical composition and antimicrobial activity of essential oil of Camphora glanduliferum ‘Honganzhang’. Horticulturae 2025, 11, 67. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X. Recent advances in polyphenol oxidase-mediated plant stress responses. Phytochemistry 2021, 181, 112588. [Google Scholar] [CrossRef]
- Su, Y.; Wei, M.; Guo, Q.; Huang, J.; Zhao, K.; Huang, J. Investigating the relationships between callus browning in Isatis indigotica fortune, total phenol content, and PPO and POD activities. Plant Cell Tissue Organ Cult. 2023, 155, 175–182. [Google Scholar] [CrossRef]
- Munir, M.Z.; Ud Din, S.; Imran, M.; Zhang, Z.; Pervaiz, T.; Han, C.; Un Nisa, Z.; Bakhsh, A.; Atif Muneer, M.; Sun, Y.; et al. Transcriptomic and anatomic profiling reveal etiolation promotes adventitious rooting by exogenous application of 1-naphthalene acetic acid in Robinia pseudoacacia L. Forests 2021, 12, 789. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamazaki, C.; Mitsui, M.; Kakei, Y.; Mitani, Y.; Nakamura, A.; Ishii, T.; Soeno, K.; Shimada, Y. Transcriptional feedback regulation of YUCCA genes in response to auxin levels in Arabidopsis. Plant Cell Rep. 2015, 34, 1343–1352. [Google Scholar] [CrossRef]
- Druege, U.; Franken, P.; Hajirezaei, M.R. Plant hormone homeostasis, signaling, and function during adventitious root formation in cuttings. Front. Plant Sci. 2016, 7, 381. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Cao, P.; Xiao, Z.; Zhan, C.; Liu, M.; Nvsvrot, T.; Wang, N.; Vissenberg, K. The bZIP53-IAA4 module inhibits adventitious root development in Populus. J. Exp. Bot. 2020, 71, 3485–3498. [Google Scholar] [CrossRef]
- Bargmann, B.O.R.; Estelle, M. Auxin perception: In the IAA of the beholder. Physiol. Plant. 2014, 151, 52–61. [Google Scholar] [CrossRef]
- Wang, D.; Pei, K.; Fu, Y.; Sun, Z.; Li, S.; Liu, H.; Tang, K.; Han, B.; Tao, Y. Genome-wide analysis of the Auxin Response Factors (ARF) gene family in Rice (Oryza sativa). Gene 2007, 394, 13–24. [Google Scholar] [CrossRef]
- Simonini, S.; Bencivenga, S.; Trick, M.; Østergaard, L. Auxin-induced modulation of ETTIN activity orchestrates gene expression in Arabidopsis. Plant Cell 2017, 29, 1864–1882. [Google Scholar] [CrossRef]
- Luo, P.; Li, T.; Shi, W.; Ma, Q.; Di, D. The roles of GRETCHEN HAGEN3 (GH3)-dependent auxin conjugation in the regulation of plant development and stress adaptation. Plants 2023, 12, 4111. [Google Scholar] [CrossRef]
- Jia, C.; Shi, Y.; Wang, H.; Zhang, Y.; Luo, F.; Li, Z.; Tian, Y.; Lu, X.; Pei, Z. Genome-wide identification and ex-pression analysis of SMALL AUXIN UP RNA (SAUR) Genes in Rice (Oryza sativa). Plant Signal. Behav. 2024, 19, 2391658. [Google Scholar] [CrossRef]
- Khan, S.; Stone, J.M. Arabidopsis thaliana GH3.9 influences primary root growth. Planta 2007, 226, 21–34. [Google Scholar] [CrossRef]
- Hou, K.; Wu, W.; Gan, S.S. SAUR36, a small auxin up RNA gene, is involved in the promotion of leaf senescence in Arabidopsis. Plant Physiol. 2013, 161, 1002–1009. [Google Scholar] [CrossRef]
- Da Costa, C.T.; De Almeida, M.R.; Ruedell, C.M.; Schwambach, J.; Maraschin, F.D.S.; Fett-Neto, A.G. When stress and development go hand in hand: Main hormonal controls of adventitious rooting in cuttings. Front. Plant Sci. 2013, 4, 133. [Google Scholar] [CrossRef]
- Su, Y.; Liu, Y.; Zhang, X. Auxin–cytokinin interaction regulates meristem development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef]
- Xie, M.; Chen, H.; Huang, L.; O’Neil, R.C.; Shokhirev, M.N.; Ecker, J.R. A B-ARR-mediated cytokinin transcriptional network directs hormone cross-regulation and shoot development. Nat. Commun. 2018, 9, 1604. [Google Scholar] [CrossRef]
- Sakai, H.; Honma, T.; Aoyama, T.; Sato, S.; Kato, T.; Tabata, S.; Oka, A. ARR1, a transcription factor for genes immediately responsive to cytokinins. Science 2001, 294, 1519–1521. [Google Scholar] [CrossRef]
- Liu, N. Effects of IAA and ABA on the immature peach fruit development process. Hortic. Plant J. 2019, 5, 145–154. [Google Scholar] [CrossRef]
- Fidler, J.; Graska, J.; Gietler, M.; Nykiel, M.; Prabucka, B.; Rybarczyk-Płońska, A.; Muszyńska, E.; Morkunas, I.; Labudda, M. PYR/PYL/RCAR receptors play a vital role in the abscisic-acid-dependent responses of plants to external or internal stimuli. Cells 2022, 11, 1352. [Google Scholar] [CrossRef]
- Fujii, H.; Zhu, J. Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc. Natl. Acad. Sci. USA 2009, 106, 8380–8385. [Google Scholar] [CrossRef]
- Zhai, Z.; Ao, Q.; Yang, L.; Lu, F.; Cheng, H.; Fang, Q.; Li, C.; Chen, Q.; Yan, J.; Wei, Y.; et al. Rapeseed PP2C37 interacts with PYR/PYL Abscisic Acid Receptors and negatively regulates drought tolerance. J. Agric. Food Chem. 2024, 72, 12445–12458. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, X.; Jing, R.; Chang, X.; Xie, H. Characterization of a common wheat (Triticum aestivum L.) TaSnRK2.7 Gene involved in abiotic stress responses. J. Exp. Bot. 2011, 62, 975–988. [Google Scholar] [CrossRef]
- Ichimura, K.; Shinozaki, K.; Tena, G.; Sheen, J.; Henry, Y.; Champion, A.; Kreis, M.; Zhang, S.; Hirt, H.; Wilson, C.; et al. MAPK group mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 2002, 7, 301–308. [Google Scholar] [CrossRef]
- Matsuoka, D.; Yasufuku, T.; Furuya, T.; Nanmori, T. An abscisic acid inducible Arabidopsis MAPKKK, MAPKKK18 regulates leaf senescence via its kinase activity. Plant Mol. Biol. 2015, 87, 565–575. [Google Scholar] [CrossRef]
- Zhao, G.; Cheng, Q.; Zhao, Y.; Wu, F.; Mu, B.; Gao, J.; Yang, L.; Yan, J.; Zhang, H.; Cui, X.; et al. The abscisic acid–responsive element binding factors MAPKKK18 module regulates abscisic acid–induced leaf senescence in Arabidopsis. J. Biol. Chem. 2023, 299, 103060. [Google Scholar] [CrossRef]
- Martins, J.P.R.; Wawrzyniak, M.K.; Kalemba, E.M.; Ley-López, J.M.; Lira, J.M.S.; Chmielarz, P. In vitro rooting of Quercus robur, activated charcoal vs. exogenous auxin: A morphophysiological approach. Plant Cell Tissue Organ Cult. 2023, 156, 24. [Google Scholar] [CrossRef]
- Zhu, B.; Zhu, Y.; Zhang, Y.; Zhong, Z.; Pan, K.; Jiang, Y.; Wen, C.; Yang, Z.; Yao, X. Ethylene activates the EIN2-EIN3/EIL1 signaling pathway in tapetum and disturbs anther development in Arabidopsis. Cells 2022, 11, 3177. [Google Scholar] [CrossRef]
- Binder, B.M.; Walker, J.M.; Gagne, J.M.; Emborg, T.J.; Hemmann, G.; Bleecker, A.B.; Vierstra, R.D. The Arabidopsis EIN3 Binding F-Box Proteins EBF1 and EBF2 have distinct but overlapping roles in ethylene signaling. Plant Cell 2007, 19, 509–523. [Google Scholar] [CrossRef]
- Thirugnanasambantham, K.; Durairaj, S.; Saravanan, S.; Karikalan, K.; Muralidaran, S.; Islam, V.I.H. Role of Ethylene Response Transcription Factor (ERF) and its regulation in response to stress encountered by plants. Plant Mol. Biol. Rep. 2015, 33, 347–357. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one fastq preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, T.; Zheng, Y.; Wang, J.; Luo, C.; Li, Y.; Liu, X. Physiological and Transcriptomic Analyses Reveal Regulatory Mechanisms of Adventitious Root Formation in In Vitro Culture of Cinnamomum camphora. Int. J. Mol. Sci. 2025, 26, 7264. https://doi.org/10.3390/ijms26157264
Zhang Y, Zhang T, Zheng Y, Wang J, Luo C, Li Y, Liu X. Physiological and Transcriptomic Analyses Reveal Regulatory Mechanisms of Adventitious Root Formation in In Vitro Culture of Cinnamomum camphora. International Journal of Molecular Sciences. 2025; 26(15):7264. https://doi.org/10.3390/ijms26157264
Chicago/Turabian StyleZhang, Yuntong, Ting Zhang, Yongjie Zheng, Jun Wang, Chenglin Luo, Yuhua Li, and Xinliang Liu. 2025. "Physiological and Transcriptomic Analyses Reveal Regulatory Mechanisms of Adventitious Root Formation in In Vitro Culture of Cinnamomum camphora" International Journal of Molecular Sciences 26, no. 15: 7264. https://doi.org/10.3390/ijms26157264
APA StyleZhang, Y., Zhang, T., Zheng, Y., Wang, J., Luo, C., Li, Y., & Liu, X. (2025). Physiological and Transcriptomic Analyses Reveal Regulatory Mechanisms of Adventitious Root Formation in In Vitro Culture of Cinnamomum camphora. International Journal of Molecular Sciences, 26(15), 7264. https://doi.org/10.3390/ijms26157264





