Green Synthesis of Copper Oxide Nanoparticles Using Camellia sinensis: Anticancer Potential and Apoptotic Mechanism in HT-29 and MCF-7 Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. High-Resolution Transmission Electron Microscopy
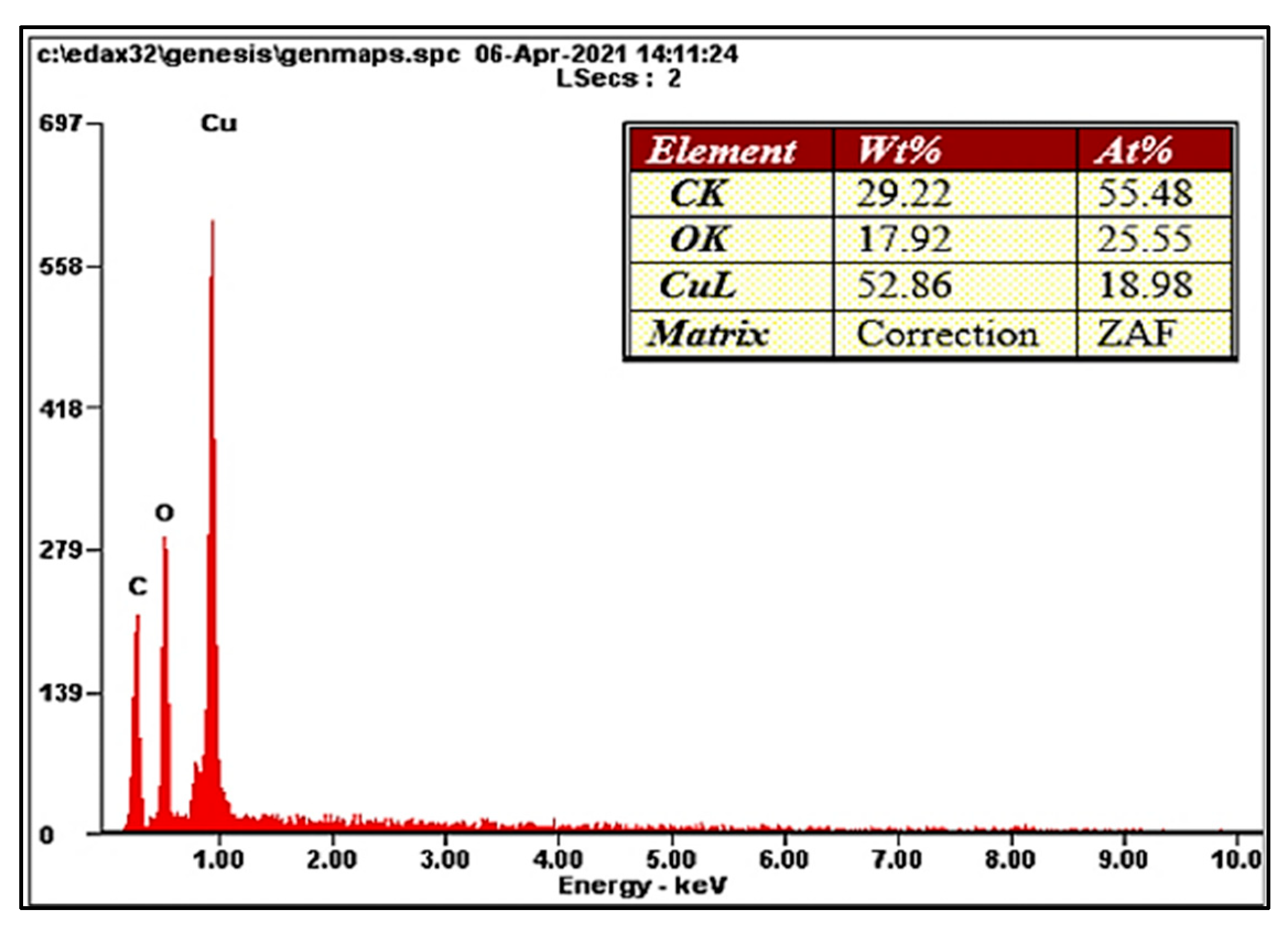
2.2. Elemental Analysis
2.3. Fourier-Transform Infrared Spectroscopy
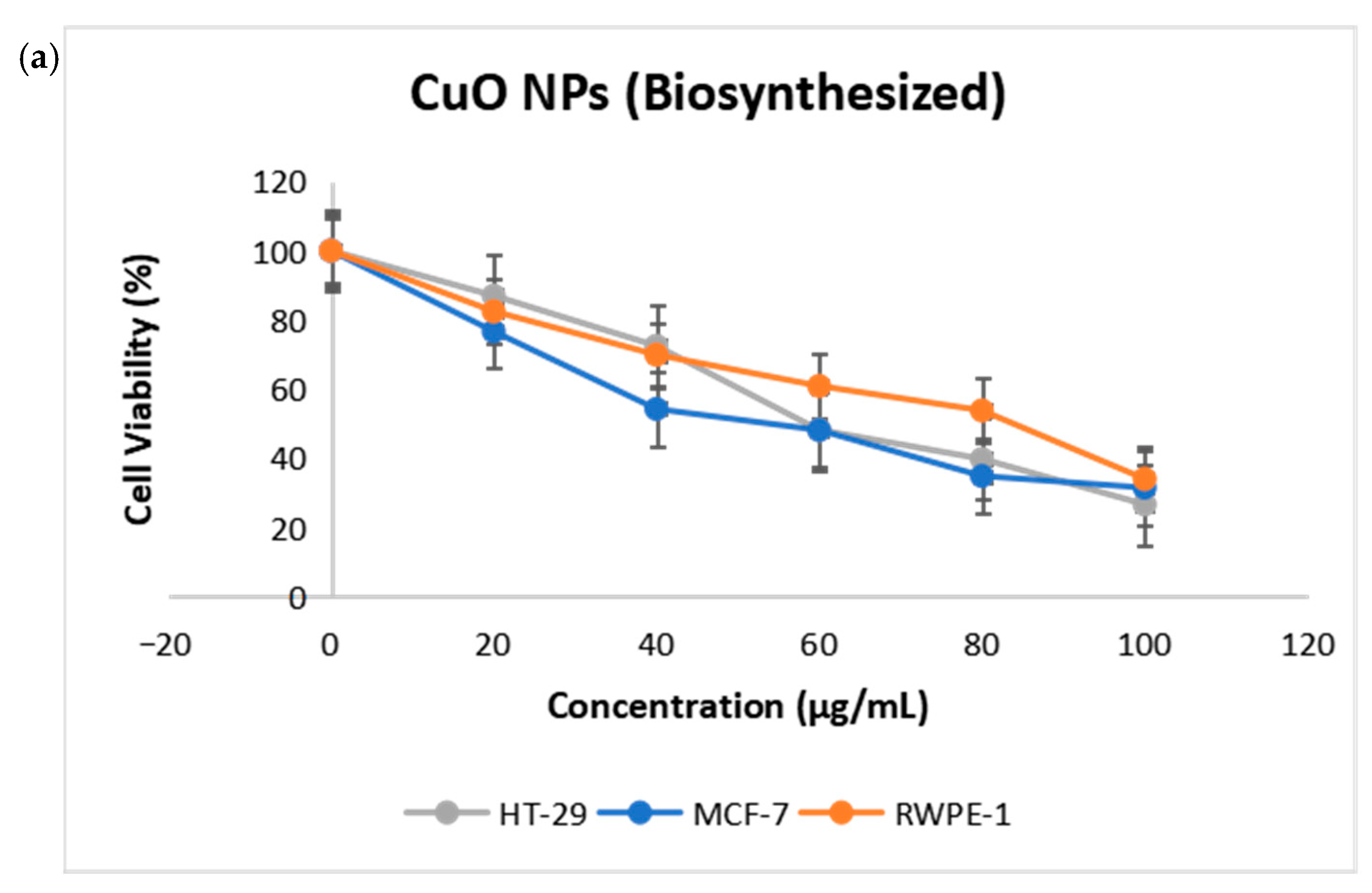
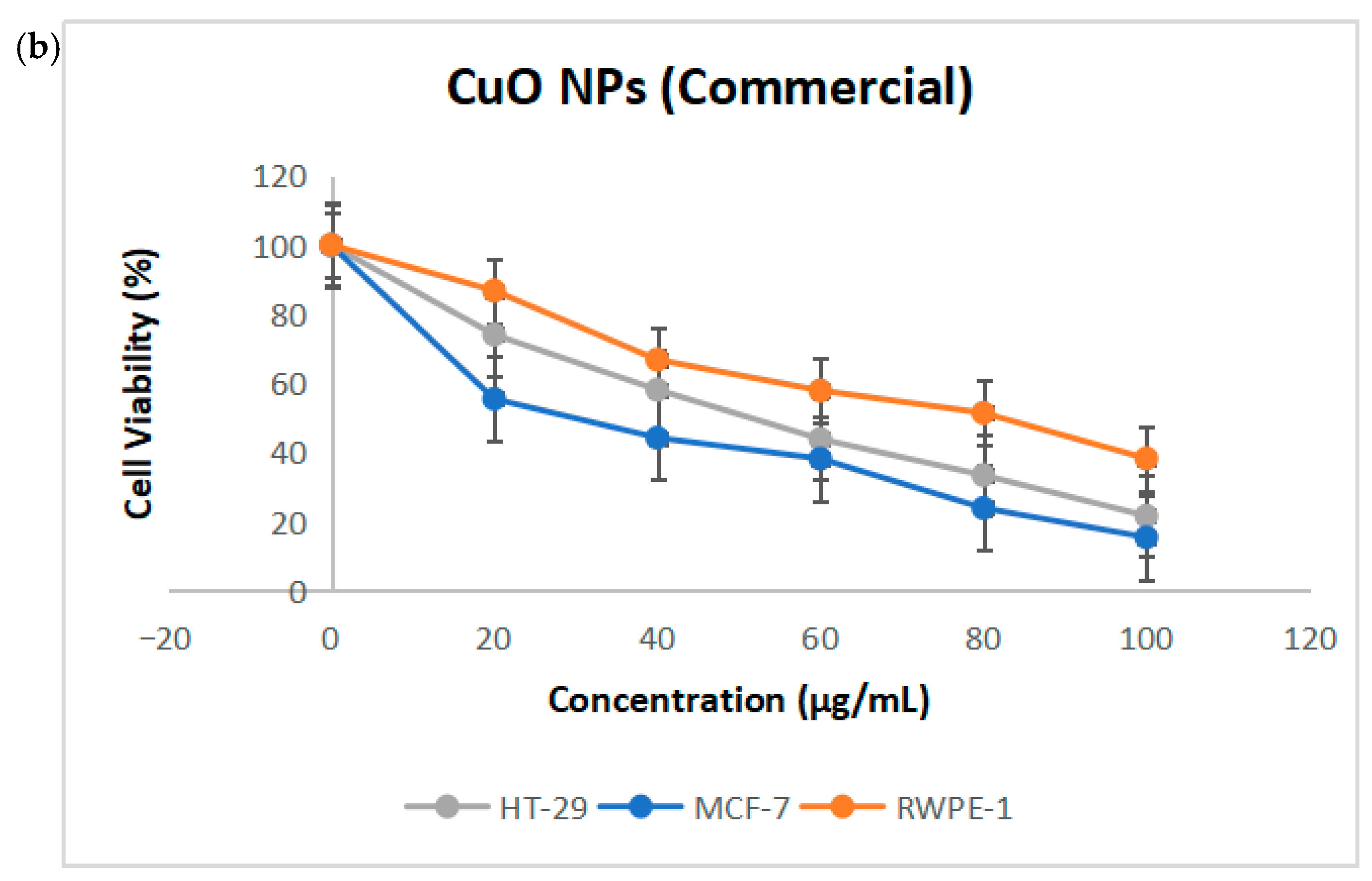
2.4. Cytotoxicity of CuO NPs
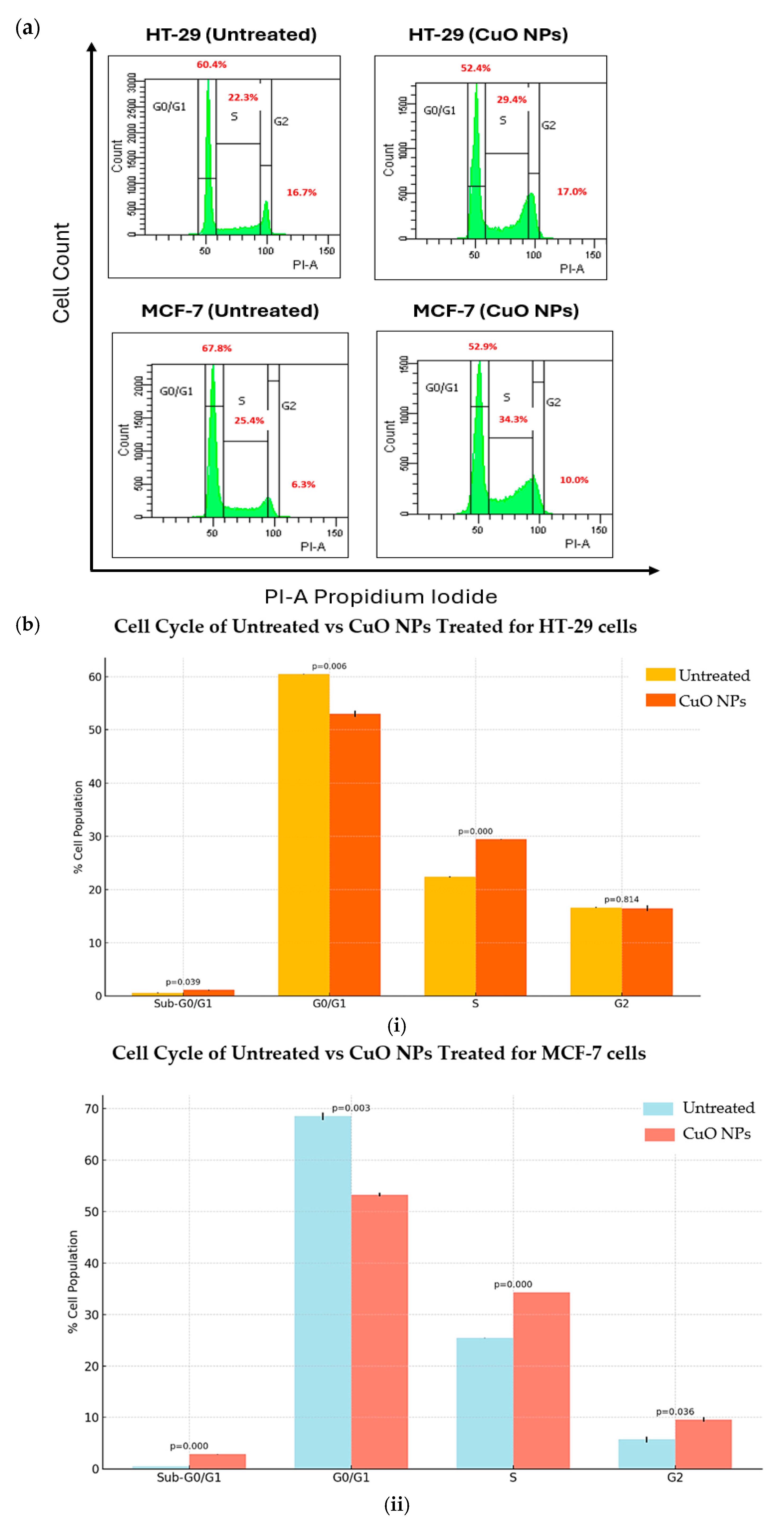
2.5. CuO NPs Alter the Progression of Cancer Cell Growth
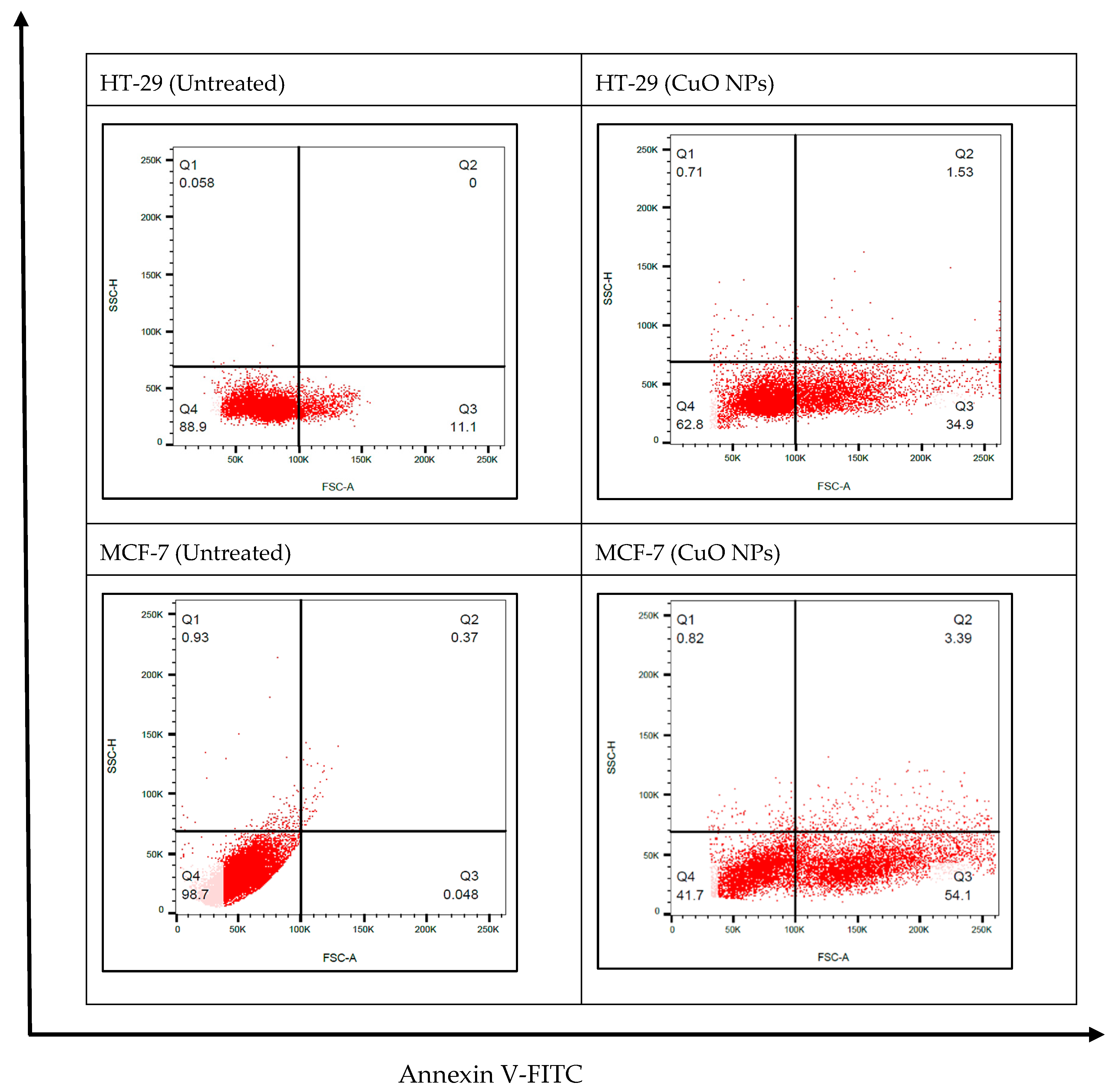
2.6. CuO NPs Induces Apoptosis in Cancer Cells: Annexin V-FITC Assay Test
2.7. CuO NPs Induces Apoptosis in Cancer Cells: Activation of Caspase Pathways
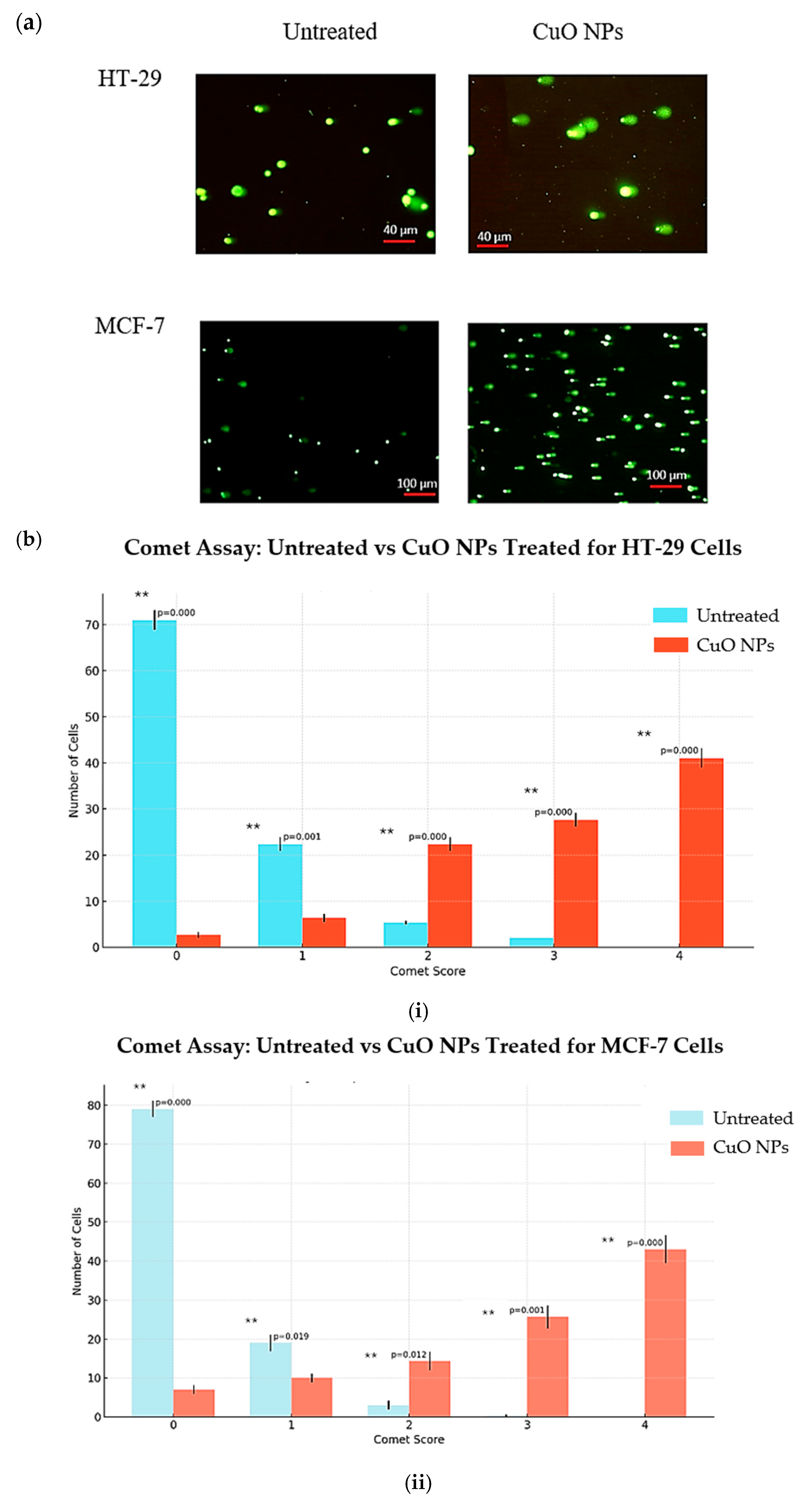
2.8. CuO NPs Damages the DNA of the Cancer Cells
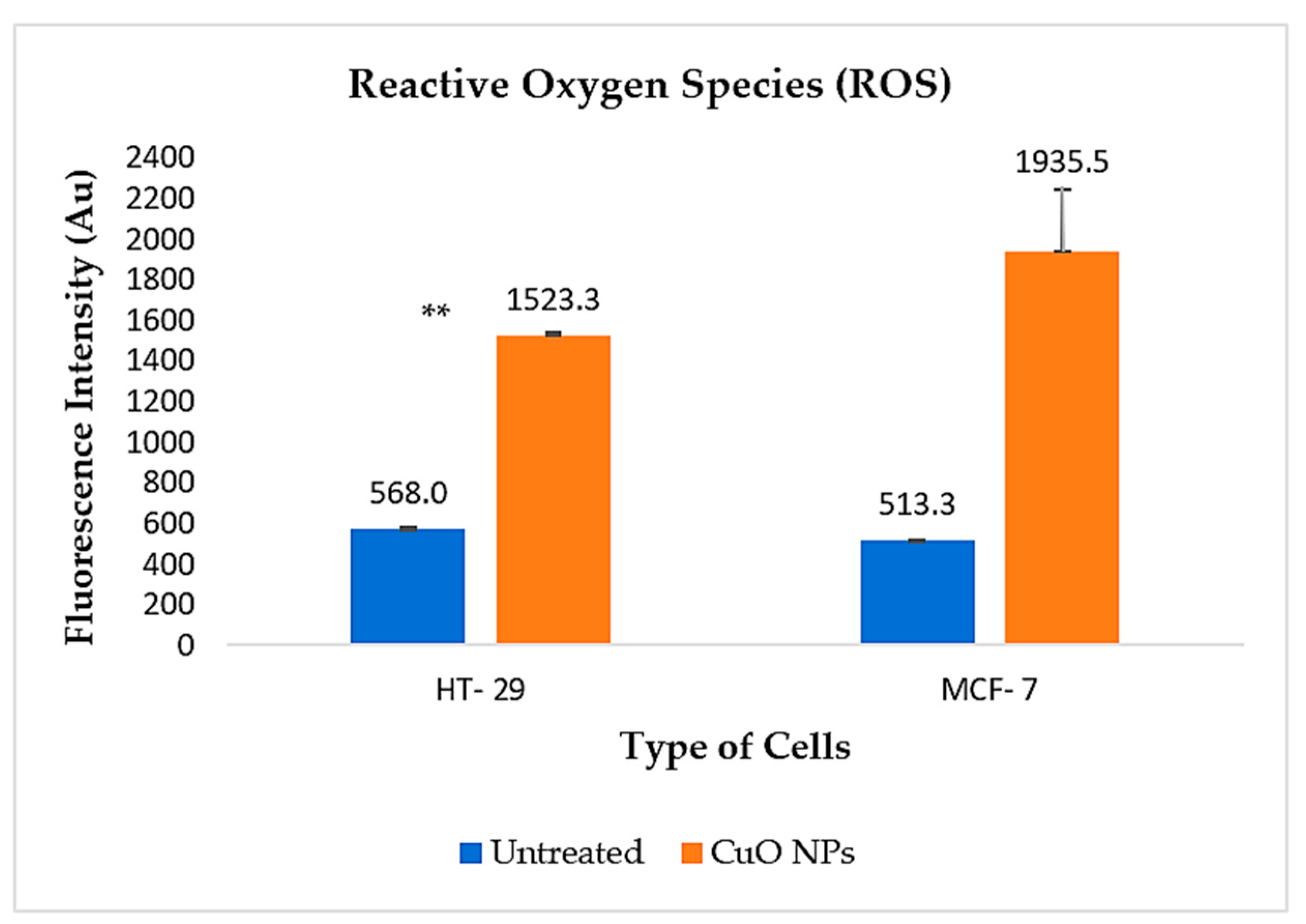
2.9. Mechanisms of Cancer Cell Death
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Green Synthesis of Copper Oxide Nanoparticles
3.3. Material Characterization
3.3.1. High-Resolution Transmission Electron Microscopy (HRTEM)
3.3.2. Energy-Dispersive X-Ray Analysis (EDX)
3.3.3. Fourier-Transform Infrared Spectroscopy
3.4. Cytotoxic Effects on Cancer
3.5. Apoptotic Effects on Cancer
3.6. Genotoxic Effects on Cancer Cells
3.7. Mechanisms of Cell Death
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gebreslassie, Y.T.; Gebremeskel, F.G.J.B.R. Green and cost-effective biofabrication of copper oxide nanoparticles: Exploring antimicrobial and anticancer applications. Biotechnol. Rep. 2024, 41, e00828. [Google Scholar] [CrossRef] [PubMed]
- Velumani, P.; Palani, N.; Casmie, A.A.; Senthilvel, R.; Parthasarthy, V. Cellular and chromosomal interaction of bio-synthesized copper oxide nanoparticles-Induced nano-cytotoxicity and genotoxicity. Toxicol. Vitr. 2025, 104, 106000. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Charinpanitkul, T.; Kobatake, E.; Sriyudthsak, M. Nanowires nickel oxide and nanospherical manganese oxide synthesized via low temperature hydrothermal technique for hydrogen peroxide sensor. J. Chem. 2016, 2016, 9138961. [Google Scholar] [CrossRef]
- Kelsall, R.W.; Hamley, I.W.; Geoghegan, M. Nanoscale Science and Technology; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Liu, H.; Zheng, S.; Xiong, H.; Alwahibi, M.S.; Niu, X. Biosynthesis of copperoxide nanoparticles using Abies spectabilis plant extract and analyzing its antinociceptive and anti-inflammatory potency in various mice models. Arab. J. Chem. 2020, 13, 6995–7006. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Rostami-Vartooni, A.; Hussin, S.M. Green synthesis of CuO nanoparticles using aqueous extract of Thymus vulgaris L. leaves and their catalytic performance for N-arylation of indoles and amines. J. Colloid Interface Sci. 2016, 466, 113–119. [Google Scholar] [CrossRef]
- Mandal, D.; Bolander, M.E.; Mukhopadhyay, D.; Sarkar, G.; Mukherjee, P. The use of microorganisms for the formation of metal nanoparticles and their application. Appl. Microbiol. Biotechnol. 2006, 69, 485–492. [Google Scholar] [CrossRef]
- Kowshik, M.; Ashtaputre, S.; Kharrazi, S.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 2002, 14, 95. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.; Alam, M.; Kumar, R. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: A novel biological approach to nanoparticle synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Kumar, P.V.; Pammi, S.; Kollu, P.; Satyanarayana, K.; Shameem, U. Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their anti bacterial activity. Ind. Crops Prod. 2014, 52, 562–566. [Google Scholar] [CrossRef]
- Ibrahim, S.; Jakaria@Zakaria, N.Z.; Rozali, S.; Ghazali, N.N.N.; Karim, M.S.A.; Sabri, M.F.M. Biosynthesis of Copper Oxide Nanoparticles Using Camellia Sinensis Plant Powder. In Advances in Material Sciences and Engineering; Springer: Singapore, 2019; pp. 233–238. [Google Scholar]
- Kumar, V.V.; Nithya, S.; Shyam, A.; Subramanian, N.S.; Anthuvan, J.; Anthony, S.P. Natural amino acid based phenolic derivatives for synthesizing silver nanoparticles with tunable morphology and antibacterial studies. Bull. Korean Chem. Soc. 2013, 34, 2702–2706. [Google Scholar] [CrossRef]
- Wall, M.E.; Wani, M.C. Camptothecin and taxol: Discovery to clinic—Thirteenth Bruce F. Cain Memorial Award. Lecture.Cancer Res. 1995, 55, 753–760. [Google Scholar]
- Kano, Y.; Ohnuma, T.; Okano, T.; Holland, J.F. Effects of vincristine in combination with methotrexate and other antitumor agents in human acute lymphoblastic leukemia cells in culture. Cancer Res. 1988, 48, 351–356. [Google Scholar]
- Sharma, S.; Sultana, S. Effect of Hibiscus rosa sinensis extract on hyperproliferation and oxidative damage caused by benzoyl peroxide and ultraviolet radiations in mouse skin. Basic Clin. Pharmacol. Toxicol. 2004, 95, 220–225. [Google Scholar] [CrossRef]
- Sathaye, S.; Bagul, Y.; Gupta, S.; Kaur, H.; Redkar, R. Hepatoprotective effects of aqueous leaf extract and crude isolates of Murraya koenigii against in vitro ethanol-induced hepatotoxicity model. Exp. Toxicol. Pathol. 2011, 63, 587–591. [Google Scholar] [CrossRef]
- Gupta, R.; Kannan, G.M.; Sharma, M.; Flora, S.J.S. Therapeutic effects of Moringa oleifera on arsenic-induced toxicity in rats. Environ. Toxicol. Pharmacol. 2005, 20, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Sreelatha, S.; Jeyachitra, A.; Padma, P. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem. Toxicol. 2011, 49, 1270–1275. [Google Scholar] [CrossRef]
- Paula, F.S.; Kabeya, L.M.; Kanashiro, A.; de Figueiredo, A.S.; Azzolini, A.E.C.; Uyemura, S.A.; Lucisano-Valim, Y.M. Modulation of human neutrophil oxidative metabolism and degranulation by extract of Tamarindus indica L. fruit pulp. Food Chem. Toxicol. 2009, 47, 163–170. [Google Scholar] [CrossRef]
- Aardra, B.; Sundar, S.; Shanmugam, R.; Ramadoss, R.; Panneerselvam, S.; Ramani, P. Camellia sinensis assisted synthesis of copper oxide nanoparticles (CuONPs) and assessment of its antioxidant activity and zebrafish embryonic toxicology evaluation. Cureus 2023, 15, e50220. [Google Scholar] [CrossRef]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Evaluation of antioxidant and anticancer activity of copper oxide nanoparticles synthesized using medicinally important plant extracts. Biomed. Pharmacother. 2017, 89, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Manna, S.; Chattopadhyay, S.; Mondal, D.; Chattopadhyay, D.; Raj, A.; Das, S.; Bag, B.G.; Roy, S. Azadirachta indica leaves mediated green synthesized copper oxide nanoparticles induce apoptosis through activation of TNF-α and caspases signaling pathway against cancer cells. J. Saudi Chem. Soc. 2019, 23, 222–238. [Google Scholar] [CrossRef]
- Zou, X.; Cheng, S.; You, B.; Yang, C. Technology. Bio-mediated synthesis of copper oxide nanoparticles using Pogestemon benghalensis extract for treatment of the esophageal cancer in nursing care. J. Drug Deliv. Sci. Technol. 2020, 58, 101759. [Google Scholar] [CrossRef]
- Zughaibi, T.A.; Mirza, A.A.; Suhail, M.; Jabir, N.R.; Zaidi, S.K.; Wasi, S.; Zawawi, A.; Tabrez, S. Evaluation of anticancer potential of biogenic copper oxide nanoparticles (CuO NPs) against breast cancer. J. Nanomater. 2022, 2022, 1–7. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Durán-Lara, E.F.; Vasantharaj, S.; Saravanan, M.; Sabour, A.; Alshiekheid, M.; Chi, N.T.L.; Brindhadevi, K.; Pugazhendhi, A. Green synthesis of copper oxide nanoparticles using Abutilon indicum leaves extract and their evaluation of antibacterial, anticancer in human A549 lung and MDA-MB-231 breast cancer cells. Food Chem. Toxicol. 2022, 168, 113330. [Google Scholar] [CrossRef]
- Pillai, R.R.; Sreelekshmi, P.; Meera, A.P. Enhanced biological performance of green sythesized copper oxide nanoparticles using Pimenta dioica leaf extract. Mater. Today Proc. 2022, 50, 163–172. [Google Scholar] [CrossRef]
- Shinde, S.; Parjane, S.; Turakane, H.; Basnet, P.; Oza, R.; Abhale, Y.; Pansambal, S.; Khoo, K.S.; Rahdar, A.; Ghotekar, S. Bio-inspired synthesis and characterizations of groundnut shells-mediated Cu/CuO/Cu2O nanoparticles for anticancer, antioxidant, and DNA damage activities. J. Sol-Gel Sci. Technol. 2023, 106, 737–747. [Google Scholar] [CrossRef]
- Robertson, G.S.; LaCasse, E.C.; Holcik, M. Programmed Cell Death. In Pharmacology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 455–473. [Google Scholar]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.-Y.; Lin, L.-T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 2015, 35, S78–S103. [Google Scholar] [CrossRef]
- Zhao, J.-F.; Sun, A.-H.; Ruan, P.; Zhao, X.-H.; Lu, M.-Q.; Yan, J. Vibrio vulnificus cytolysin induces apoptosis in HUVEC, SGC-7901 and SMMC-7721 cells via caspase-9/3-dependent pathway. Microb. Pathog. 2009, 46, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Chasara, R.S.; Ajayi, T.O.; Leshilo, D.M.; Poka, M.S.; Witika, B.A. Exploring novel strategies to improve anti-tumour efficiency: The potential for targeting reactive oxygen species. Heliyon 2023, 9, e19896. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.; Vellayappan, B.A.; Jeyasekharan, A.D.J.R.b. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Kumari, S.; Badana, A.K.; Malla, R.R. Reactive oxygen species: A key constituent in cancer survival. Biomark. Insights 2018, 13, 1177271918755391. [Google Scholar] [CrossRef]
- Mayeen, A.; Shaji, L.K.; Nair, A.K.; Kalarikkal, N. Morphological characterization of nanomaterials. Charact. Nanomater. 2018, 335–364. [Google Scholar]
- Rajesh, K.; Ajitha, B.; Reddy, Y.A.K.; Suneetha, Y.; Reddy, P.S. Synthesis of copper nanoparticles and role of pH on particle size control. Mater. Today Proc. 2016, 3, 1985–1991. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Blanco Andujar, C. Sodium Carbonate Mediated Synthesis of Iron Oxide Nanoparticles to Improve Magnetic Hyperthermia Efficiency and Induce Apoptosis. Ph.D. Thesis, University College London, London, UK, 2014. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Letchumanan, D.; Ibrahim, S.; Nagoor, N.H.; Mohd Arshad, N. Green Synthesis of Copper Oxide Nanoparticles Using Camellia sinensis: Anticancer Potential and Apoptotic Mechanism in HT-29 and MCF-7 Cells. Int. J. Mol. Sci. 2025, 26, 7267. https://doi.org/10.3390/ijms26157267
Letchumanan D, Ibrahim S, Nagoor NH, Mohd Arshad N. Green Synthesis of Copper Oxide Nanoparticles Using Camellia sinensis: Anticancer Potential and Apoptotic Mechanism in HT-29 and MCF-7 Cells. International Journal of Molecular Sciences. 2025; 26(15):7267. https://doi.org/10.3390/ijms26157267
Chicago/Turabian StyleLetchumanan, Devanthiran, Suriani Ibrahim, Noor Hasima Nagoor, and Norhafiza Mohd Arshad. 2025. "Green Synthesis of Copper Oxide Nanoparticles Using Camellia sinensis: Anticancer Potential and Apoptotic Mechanism in HT-29 and MCF-7 Cells" International Journal of Molecular Sciences 26, no. 15: 7267. https://doi.org/10.3390/ijms26157267
APA StyleLetchumanan, D., Ibrahim, S., Nagoor, N. H., & Mohd Arshad, N. (2025). Green Synthesis of Copper Oxide Nanoparticles Using Camellia sinensis: Anticancer Potential and Apoptotic Mechanism in HT-29 and MCF-7 Cells. International Journal of Molecular Sciences, 26(15), 7267. https://doi.org/10.3390/ijms26157267






