Small Extracellular Vesicles in Neurodegenerative Disease: Emerging Roles in Pathogenesis, Biomarker Discovery, and Therapy
Abstract
1. Introduction
2. Emerging Roles of sEVs in Neurodegeneration
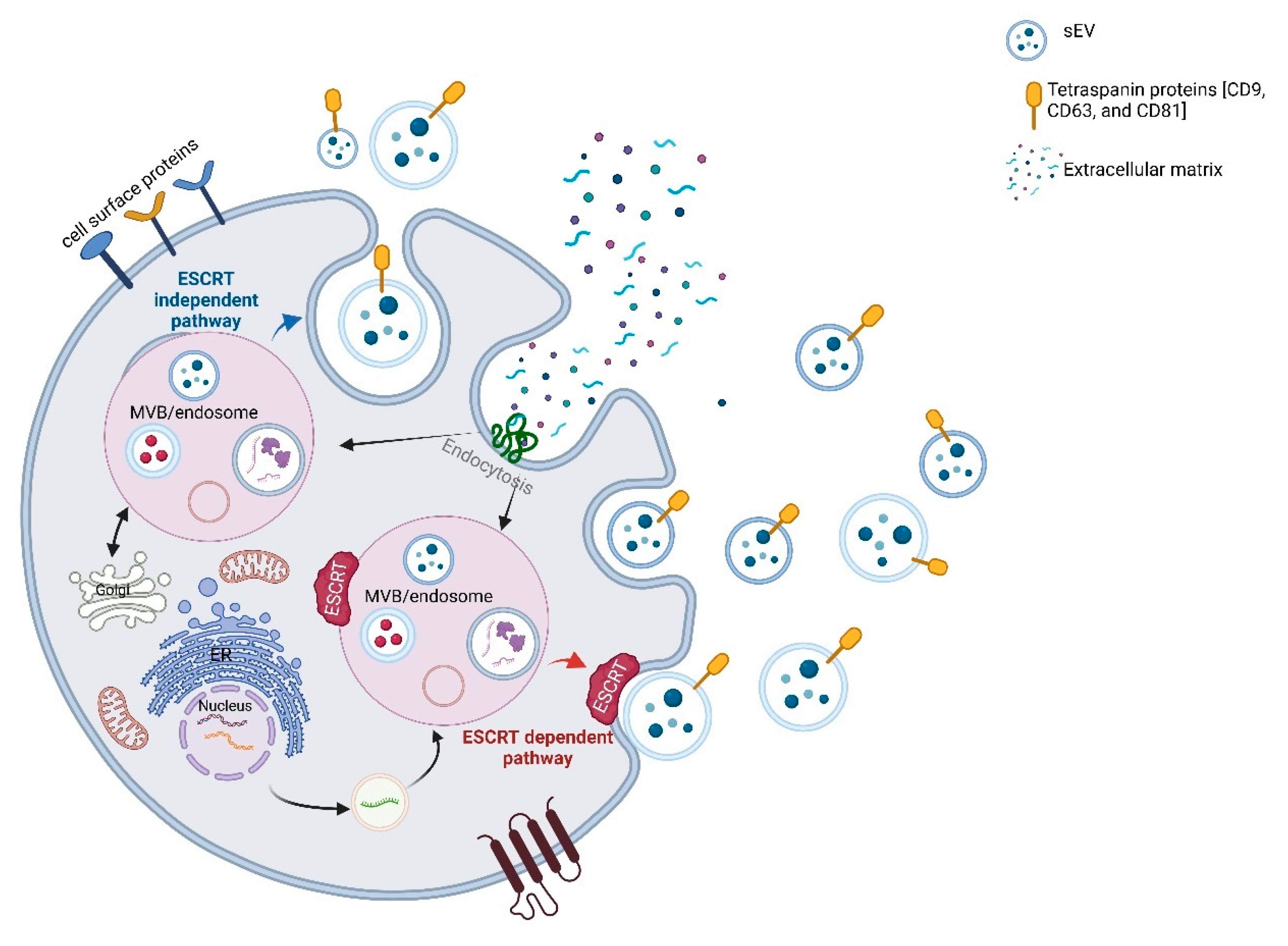
2.1. Biogenesis and Molecular Cargo
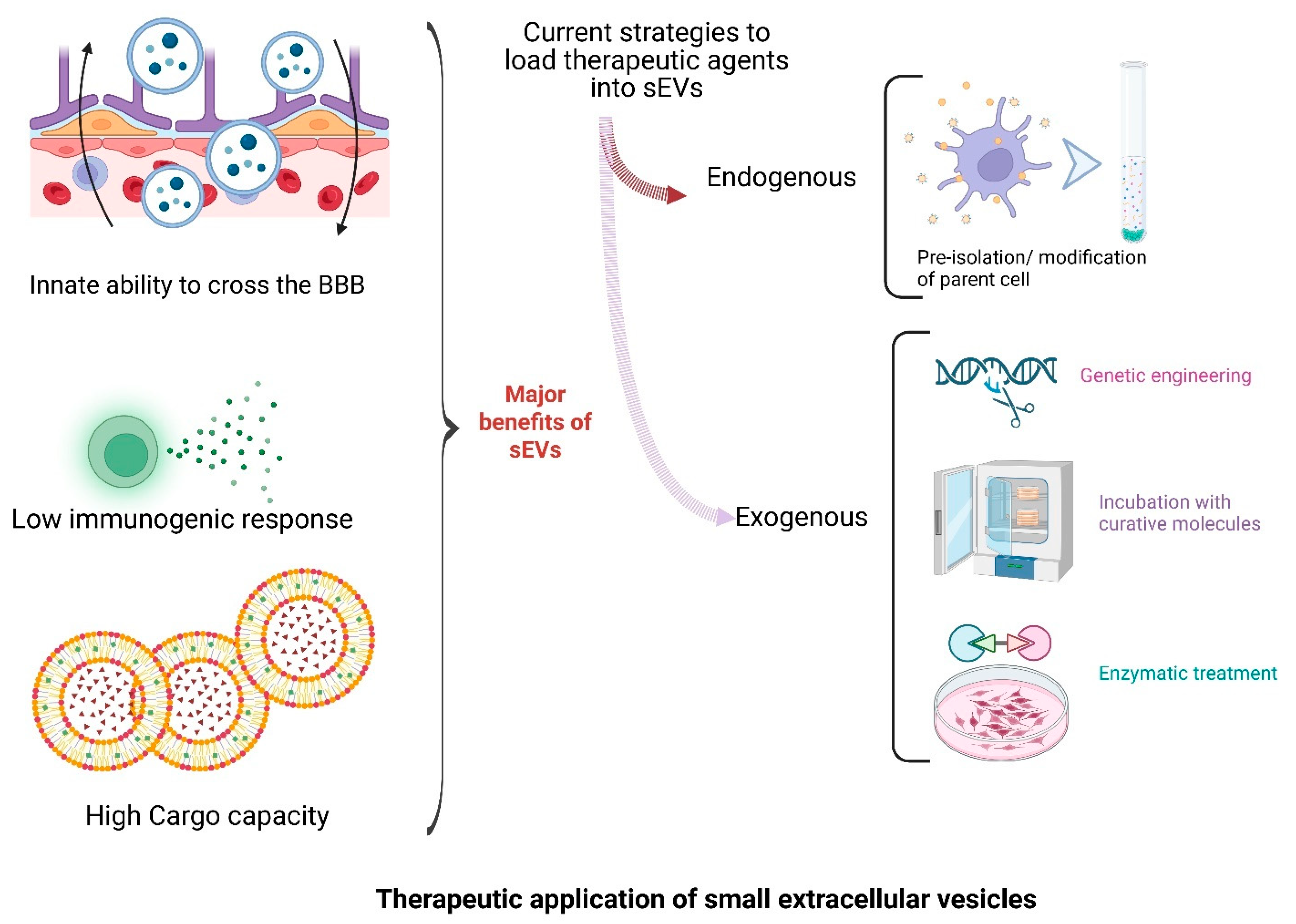
2.2. Crossing the Blood–Brain Barrier
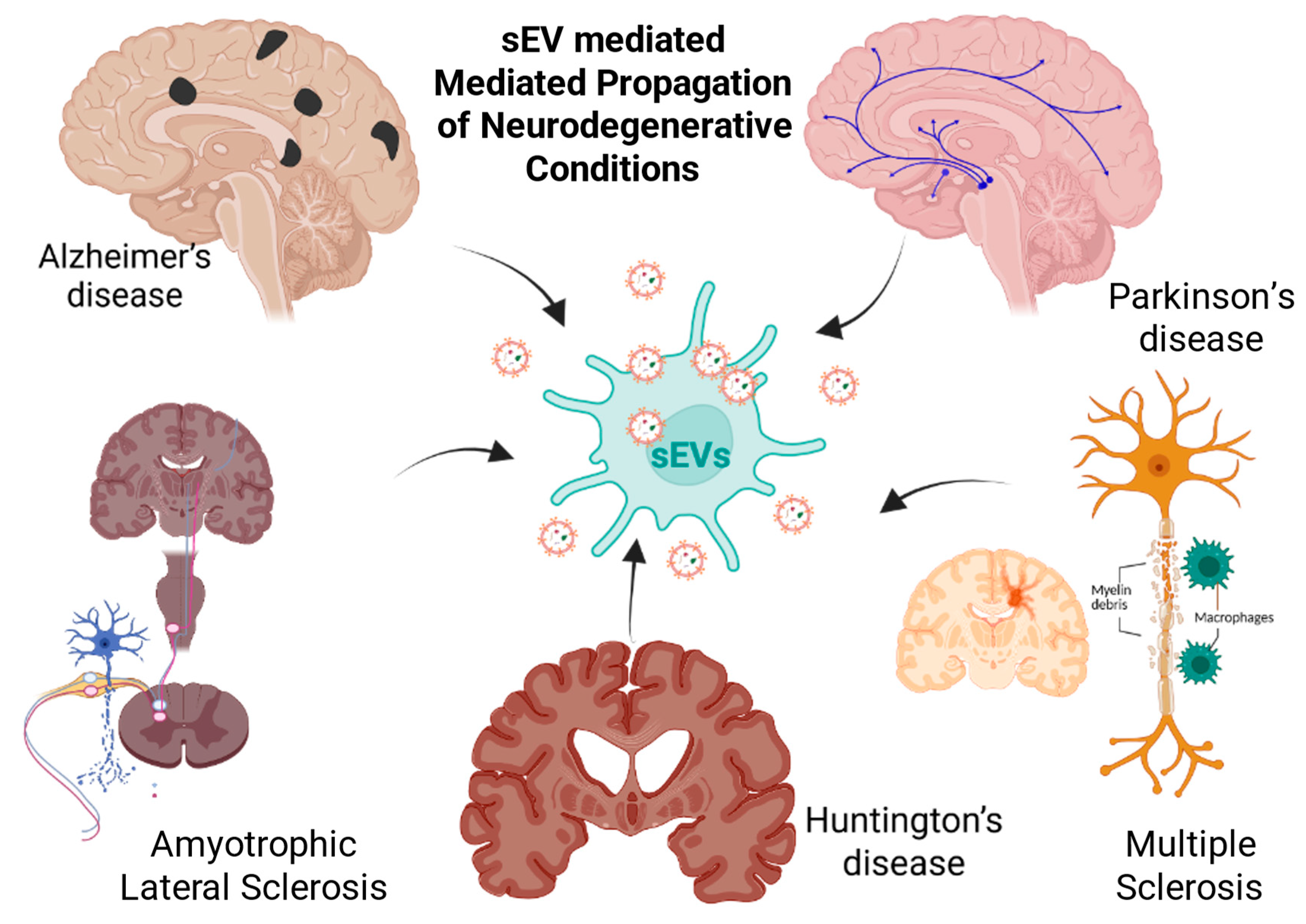
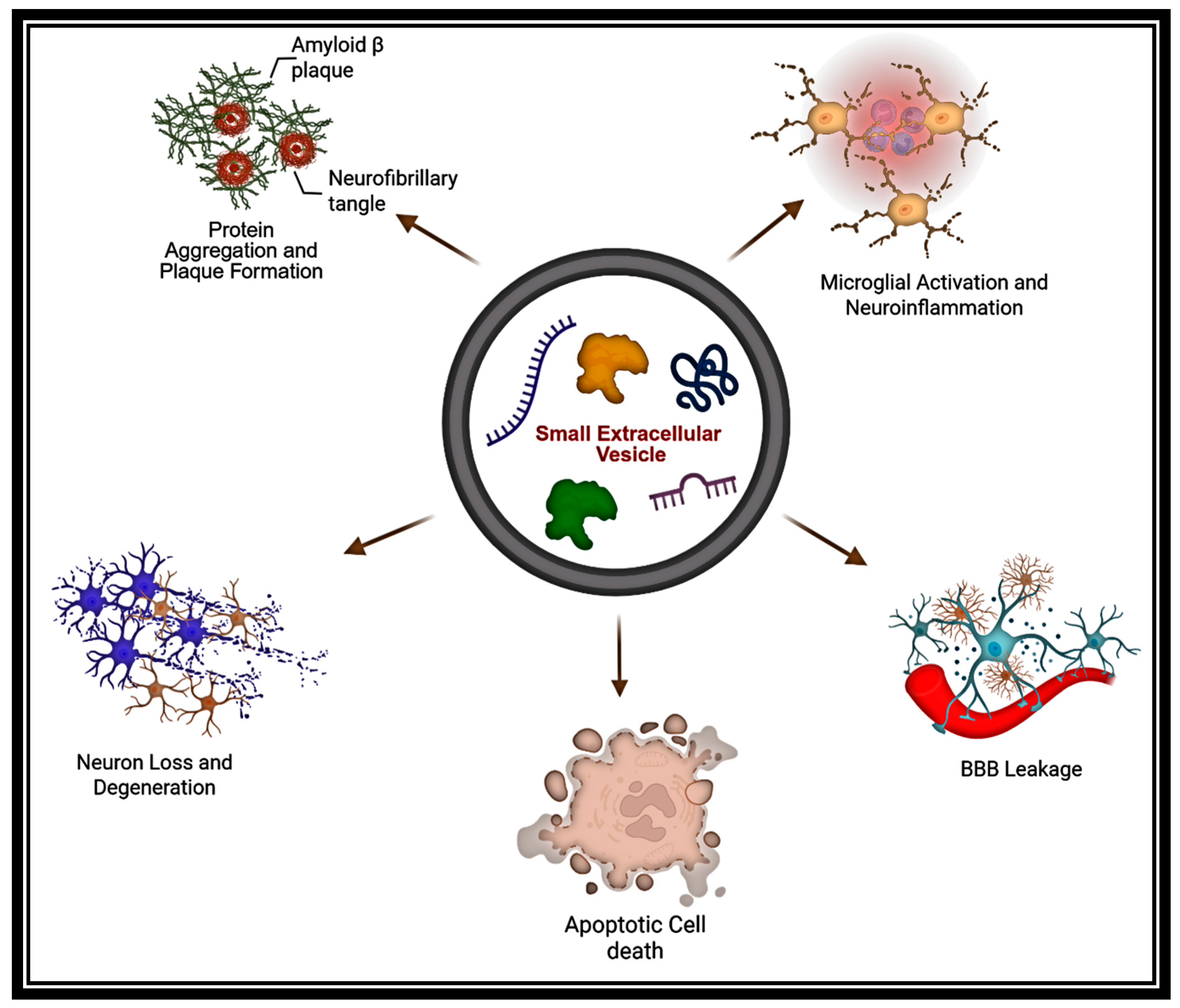
2.3. Vectors of Pathology
2.4. Liquid Biopsy Potential
2.5. Therapeutic Promise
2.6. Rationale and Scope of This Review
3. Determinants of sEV Composition in the CNS: Biogenesis, Cellular Origin, and Disease Modulation
3.1. Biogenesis and Molecular Determinants of Cargo Loading in sEVs
3.2. Cell-Type-Specific sEV Profiles in the CNS
3.3. Influence of Stress and Disease on the Composition of sEVs
4. Physiological Functions of Small EVs in the Healthy CNS
4.1. Neuronal sEVs: Tuning Synapse Formation, Maturation, and Plasticity
4.2. Microglial sEVs and Complement-Driven Synaptic Pruning
4.3. Oligodendrocyte-Derived sEVs: Metabolic Lifelines for Axons and Myelin Upkeep
4.4. Integrated Glia–Neuron Crosstalk and Myelin Maintenance
5. Small EVs in NDD Pathogenesis
5.1. Small EVs in Alzheimer’s Disease: Biomarker Signatures, Pathogenic Spread, and Therapeutic Modulation
5.2. Parkinson’s Disease (PD)
5.3. Amyotrophic Lateral Sclerosis (ALS)
5.4. Multiple Sclerosis (MS)
5.5. Huntington’s Disease (HD)
5.6. sEVs in Chronic CNS Injury (Chronic SCI and TBI)
| Disease/Condition | sEV Function/Role | Key sEV Cargo | References |
|---|---|---|---|
| Alzheimer’s Disease | Spread of Aβ and tau; modulate microglial inflammation and synaptic dysfunction | Aβ, phosphorylated tau, miR-132-5p, miR-193b, miR-125b, miR-485-5p, miR-23a, miR-125b | [46,111] |
| Parkinson’s Disease | Transmit misfolded α-synuclein; influence dopaminergic neuron survival | α-synuclein, DJ-1, LRRK2, Let-7f-5p and miR-125a-5p, miR-10b-5p and miR-151a-3p, miR-24, miR-195, POU3F3 | [112,113] |
| Amyotrophic Lateral Sclerosis | Transfer TDP-43, SOD1; promote motor neuron degeneration | TDP-43, SOD1, FUS, miR-124, miR-146a-5p; miR-199a-3p; miR-151a-3p; miR-151a-5p; miR-199a-5p, MiR-151a-5p | [114,115] |
| Huntington’s Disease | Disseminate mHTT aggregates; modulate synaptic toxicity and inflammation | mutant huntingtin (mHTT), miR-124 | [116] |
| Multiple Sclerosis | Amplify inflammatory responses; alter T cell and BBB function | miR-155, CD29, CD31, inflammatory cytokines | [95,117,118] |
| Chronic Spinal Cord Injury | Carry pro-inflammatory miRNAs and cytokines; inhibit regeneration, sustain gliosis | miR-21, cytokines (TNF-α, IL-1β), GFAP | [104] |
| Traumatic Brain Injury | Alter neurovascular signaling; deliver injury-responsive miRNAs and DAMPs | miR-873, HSP70, NLRP3, mitochondrial DNA | [119] |
6. Small EV Biomarkers: Current Landscape, Technical Hurdles, and Clinical Promise
7. Small EVs as Therapeutic Platforms for CNS Disorders: Engineering, Delivery, and Clinical Translation
8. Comparative Assessment of Diverse Carrier Platforms for CNS Drug Delivery
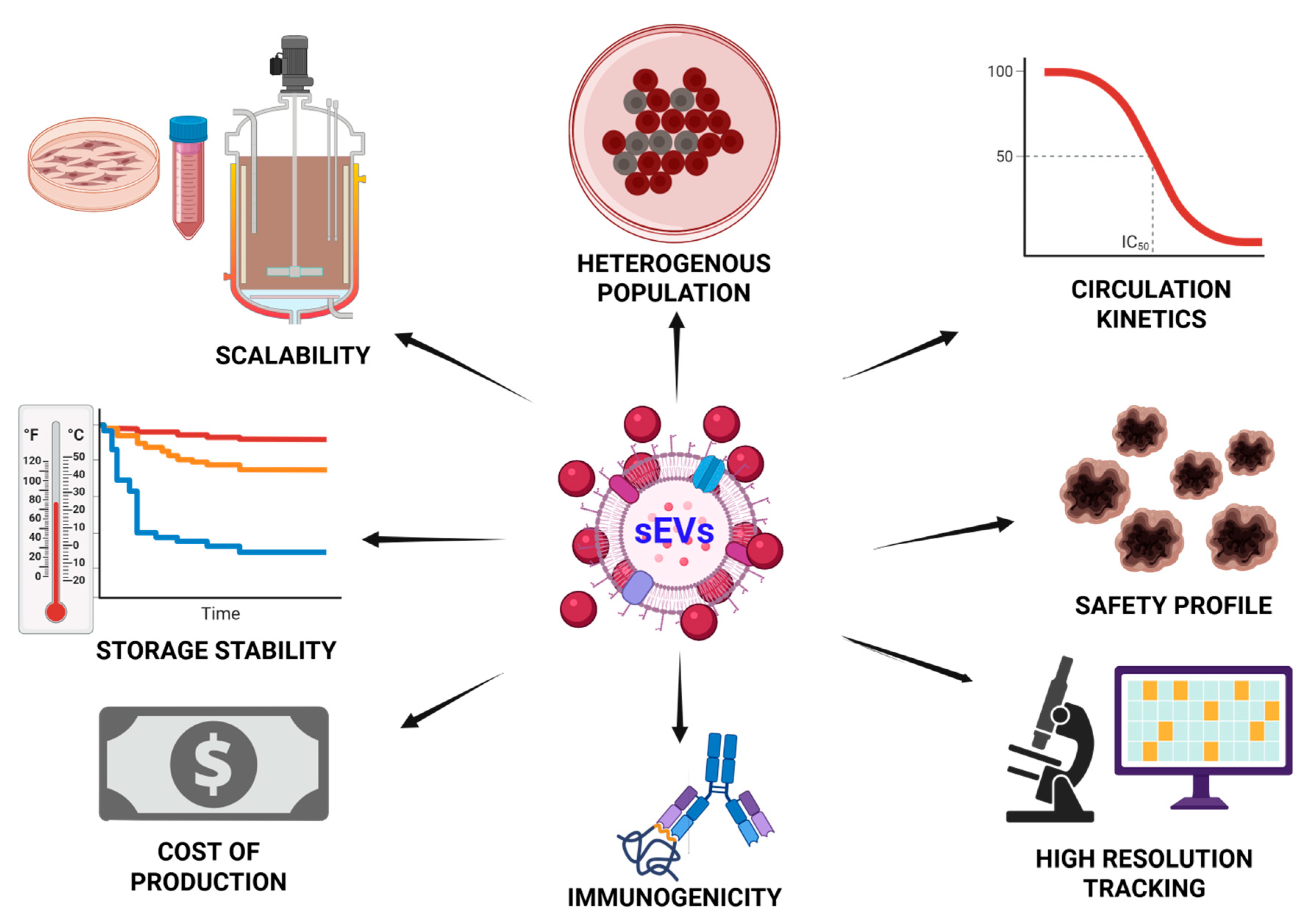
9. Challenges and Future Directions
9.1. Standardization of Isolation and Characterization
9.2. Scaling Manufacturing Without Compromising Identity
9.3. Immunogenicity and Biodistribution Concerns
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANT1 | Adenine nucleotide translocators |
| AD | Alzheimer’s disease |
| AMPK | AMP-activated protein kinase |
| ALS | Amyotrophic lateral sclerosis |
| APCs | Antigen-presenting cells |
| ApoE | Apolipoprotein E |
| AGO2 | Argonaute 2 |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| CaMKK | Calcium/calmodulin-dependent protein kinase kinase |
| CADM1 | Cell adhesion molecule 1 |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| C9orf72 | Chromosome 9 open reading frame 72 |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| CDK5 | Cyclin-dependent kinase 5 |
| DAMPs | Damage-associated molecular patterns |
| ESCRT | Endosomal sorting complexes required for transport |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EMA | European Medicines Agency |
| EAE | Experimental autoimmune encephalomyelitis |
| EV | Extracellular vesicle |
| FDA | Food and Drug Administration |
| FOXJ3 | Forkhead box O3 |
| GABRB3 | Gamma-aminobutyric acid; receptor subunit beta-3 |
| GFAP | Glial fibrillary acidic protein |
| GLAST | Glutamate/aspartate transporter |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GSK3β | Glycogen synthase kinase 3 beta |
| hnRNPA2B1 | Heterogeneous nuclear ribonucleoprotein A2/B1 |
| HDAC2 | Histone deacetylase 2 |
| HD | Huntington’s disease |
| IL-1β | Interleukin-1β |
| ISEV | International Society for Extracellular Vesicles |
| IND | Investigational New Drug |
| L1CAM | L1 cell adhesion molecule |
| L1CAM/CD171 | L1 cell adhesion molecule |
| LRRK2 | Leucine-rich repeat kinase 2 |
| LIMK1 | LIM domain kinase 1 |
| Lamp2b | Lysosomal-associated membrane protein 2 |
| LNP | Lipid nanoparticle |
| MHC | Major histocompatibility complex |
| MSC | Mesenchymal stem cell |
| MSC-EVs | Mesenchymal-stem-cell-derived EVs |
| MISEV-2023 | Minimal Information for Studies of Extracellular Vesicles guidelines 2023 |
| MCT1 | Monocarboxylate transporters |
| MS | Multiple sclerosis |
| MVBs | Multivesicular bodies |
| mHTT | Mutant huntingtin protein |
| MBP | Myelin basic protein |
| MOG | Myelin oligodendrocyte glycoprotein |
| NGF | Nerve growth factor |
| NDDs | Neurodegenerative diseases |
| NFL | Neurofilament light |
| nSMase2 | Neutral sphingomyelinase 2 |
| NMDA | N-methyl-D-aspartate |
| NP | Nanoparticle |
| PD | Parkinson’s disease |
| PTEN | Phosphatase and tensin |
| PEGylation | Polyethylene glycol |
| PSD-95 | Postsynaptic density protein 95 |
| PDCD4 | Programmed cell death protein 4 |
| PLP | Proteolipid protein |
| RVG | Rabies virus glycoprotein |
| RBPs | RNA-binding proteins |
| SCEVs | Schwann cell (SC)-derived exosomal vesicles |
| STAT3 | Signal transducer and activator of transcription 3 |
| sEVs | Small extracellular vesicles |
| SCI | Spinal cord injury |
| SOD1 | Superoxide dismutase 1 |
| TDP-43 | TAR DNA-binding protein 43 |
| TLR7/8 | Toll-like receptors 7 and 8 |
| TBI | Traumatic brain injury |
| TNF-α | Tumor necrosis factor alpha |
| YBX1 | Y-box binding protein 1 |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chetelat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Pramotton, F.M.; Spitz, S.; Kamm, R.D. Challenges and Future Perspectives in Modeling Neurodegenerative Diseases Using Organ-on-a-Chip Technology. Adv. Sci. 2024, 11, e2403892. [Google Scholar] [CrossRef]
- Ayeni, E.A.; Aldossary, A.M.; Ayejoto, D.A.; Gbadegesin, L.A.; Alshehri, A.A.; Alfassam, H.A.; Afewerky, H.K.; Almughem, F.A.; Bello, S.M.; Tawfik, E.A. Neurodegenerative Diseases: Implications of Environironmental and Climatic Influences on Neurotransmitters and Neuronal Hormones Activities. Int. J. Environ. Res. Public Health 2022, 19, 12495. [Google Scholar] [CrossRef] [PubMed]
- Brettschneider, J.; Del Tredici, K.; Lee, V.M.; Trojanowski, J.Q. Spreading of pathology in neurodegenerative diseases: A focus on human studies. Nat. Rev. Neurosci. 2015, 16, 109–120. [Google Scholar] [CrossRef]
- Satao, K.S.; Doshi, G.M. Intercellular communication via exosomes: A new paradigm in the pathophysiology of neurodegenerative disorders. Life Sci. 2025, 365, 123468. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Tkach, M.; Thery, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Wang, M.; Qin, L.; Tang, B. MicroRNAs in Alzheimer’s Disease. Front. Genet. 2019, 10, 153. [Google Scholar] [CrossRef]
- Abuelezz, N.Z.; Nasr, F.E.; AbdulKader, M.A.; Bassiouny, A.R.; Zaky, A. MicroRNAs as Potential Orchestrators of Alzheimer’s Disease-Related Pathologies: Insights on Current Status and Future Possibilities. Front. Aging Neurosci. 2021, 13, 743573. [Google Scholar] [CrossRef]
- Meder, D.; Siebner, H.R. Spectral signatures of neurodegenerative diseases: How to decipher them? Brain 2018, 141, 2241–2244. [Google Scholar] [CrossRef]
- Fraser, K.B.; Rawlins, A.B.; Clark, R.G.; Alcalay, R.N.; Standaert, D.G.; Liu, N.; Parkinson’s Disease Biomarker Program, C.; West, A.B. Ser(P)-1292 LRRK2 in urinary exosomes is elevated in idiopathic Parkinson’s disease. Mov. Disord. 2016, 31, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Thery, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Liu, L.; Ma, F.; Wong, C.W.; Guo, X.E.; Chacko, J.V.; Farhoodi, H.P.; Zhang, S.X.; Zimak, J.; Segaliny, A.; et al. Elucidation of Exosome Migration across the Blood-Brain Barrier Model In Vitro. Cell Mol. Bioeng. 2016, 9, 509–529. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Stewart, T.; Banks, W.A.; Zhang, J. The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Curr. Pharm. Des. 2017, 23, 6206–6214. [Google Scholar] [CrossRef]
- Pulliam, L.; Sun, B.; Mustapic, M.; Chawla, S.; Kapogiannis, D. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J. Neurovirol. 2019, 25, 702–709. [Google Scholar] [CrossRef]
- Osaid, Z.; Haider, M.; Hamoudi, R.; Harati, R. Exosomes Interactions with the Blood-Brain Barrier: Implications for Cerebral Disorders and Therapeutics. Int. J. Mol. Sci. 2023, 24, 15635. [Google Scholar] [CrossRef]
- Rajendran, L.; Bali, J.; Barr, M.M.; Court, F.A.; Kramer-Albers, E.M.; Picou, F.; Raposo, G.; van der Vos, K.E.; van Niel, G.; Wang, J.; et al. Emerging roles of extracellular vesicles in the nervous system. J. Neurosci. 2014, 34, 15482–15489. [Google Scholar] [CrossRef]
- Guo, J.L.; Lee, V.M. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat. Med. 2014, 20, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Sardar Sinha, M.; Ansell-Schultz, A.; Civitelli, L.; Hildesjo, C.; Larsson, M.; Lannfelt, L.; Ingelsson, M.; Hallbeck, M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018, 136, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Ray, S.K.; Kanwar, J.R.; Mukherjee, S. Exosome-based therapeutics: Advancing drug delivery for neurodegenerative diseases. Mol. Cell. Neurosci. 2025, 133, 104004. [Google Scholar] [CrossRef]
- Kapogiannis, D.; Mustapic, M.; Shardell, M.D.; Berkowitz, S.T.; Diehl, T.C.; Spangler, R.D.; Tran, J.; Lazaropoulos, M.P.; Chawla, S.; Gulyani, S.; et al. Association of Extracellular Vesicle Biomarkers With Alzheimer Disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol. 2019, 76, 1340–1351. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, F.; Li, C.; Mao, J.; Wang, Y.; Zhou, X.; Xie, H.; Wen, C. Application of Single Extracellular Vesicle Analysis Techniques. Int. J. Nanomed. 2023, 18, 5365–5376. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, M.; Xiao, Q.; Wang, H.; Chi, C.; Lin, T.; Wang, Y.; Yi, X.; Zhu, L. Recent Advances in Microfluidic-Based Extracellular Vesicle Analysis. Micromachines 2024, 15, 630. [Google Scholar] [CrossRef] [PubMed]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L.; et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 2015, 11, 600–607. [Google Scholar] [CrossRef]
- Park, C.; Weerakkody, J.S.; Schneider, R.; Miao, S.; Pitt, D. CNS cell-derived exosome signatures as blood-based biomarkers of neurodegenerative diseases. Front. Neurosci. 2024, 18, 1426700. [Google Scholar] [CrossRef]
- Schwarz, G.; Ren, X.; Xie, W.; Guo, H.; Jiang, Y.; Zhang, J. Engineered exosomes: A promising drug delivery platform with therapeutic potential. Front. Mol. Biosci. 2025, 12, 1583992. [Google Scholar] [CrossRef]
- Lin, E.Y.; Hsu, S.X.; Wu, B.H.; Deng, Y.C.; Wuli, W.; Li, Y.S.; Lee, J.H.; Lin, S.Z.; Harn, H.J.; Chiou, T.W. Engineered Exosomes Containing microRNA-29b-2 and Targeting the Somatostatin Receptor Reduce Presenilin 1 Expression and Decrease the beta-Amyloid Accumulation in the Brains of Mice with Alzheimer’s Disease. Int. J. Nanomed. 2024, 19, 4977–4994. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Feng, T.; Liu, B.; Qiu, F.; Xu, Y.; Zhao, Y.; Zheng, Y. Engineered exosomes: Desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics 2021, 11, 8926–8944. [Google Scholar] [CrossRef]
- Yang, J.; Luo, S.; Zhang, J.; Yu, T.; Fu, Z.; Zheng, Y.; Xu, X.; Liu, C.; Fan, M.; Zhang, Z. Exosome-mediated delivery of antisense oligonucleotides targeting alpha-synuclein ameliorates the pathology in a mouse model of Parkinson’s disease. Neurobiol. Dis. 2021, 148, 105218. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef]
- Jung, Y.H.; Jo, H.Y.; Kim, D.H.; Oh, Y.J.; Kim, M.; Na, S.; Song, H.Y.; Lee, H.J. Exosome-Mediated Mitochondrial Regulation: A Promising Therapeutic Tool for Alzheimer’s Disease and Parkinson’s Disease. Int. J. Nanomed. 2025, 20, 4903–4917. [Google Scholar] [CrossRef]
- Lee, Y.J.; Shin, K.J.; Chae, Y.C. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer. Exp. Mol. Med. 2024, 56, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Skibinski, G.; Parkinson, N.J.; Brown, J.M.; Chakrabarti, L.; Lloyd, S.L.; Hummerich, H.; Nielsen, J.E.; Hodges, J.R.; Spillantini, M.G.; Thusgaard, T.; et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat. Genet. 2005, 37, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Perrotta, C.; Novellino, L.; Francolini, M.; Riganti, L.; Menna, E.; Saglietti, L.; Schuchman, E.H.; Furlan, R.; Clementi, E.; et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009, 28, 1043–1054. [Google Scholar] [CrossRef]
- Liu, X.; Meng, P.; Liu, Z.; Tian, X.; Xi, J.; Du, M.; Yang, H.; Long, Q. New insights on targeting extracellular vesicle release by GW4869 to modulate lipopolysaccharide-induced neuroinflammation in mice model. Nanomedicine 2024, 19, 2619–2632. [Google Scholar] [CrossRef]
- Duke, L.C.; Cone, A.S.; Sun, L.; Dittmer, D.P.; Meckes, D.G., Jr.; Tomko, R.J., Jr. Tetraspanin CD9 alters cellular trafficking and endocytosis of tetraspanin CD63, affecting CD63 packaging into small extracellular vesicles. J. Biol. Chem. 2025, 301, 108255. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Yin, Y.; Jia, X.; Mao, L. Mechanism of cargo sorting into small extracellular vesicles. Bioengineered 2021, 12, 8186–8201. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Yang, Z.; Wang, B.; Gong, H.; Zhang, K.; Lin, Y.; Sun, M. Extracellular vesicles: Biological mechanisms and emerging therapeutic opportunities in neurodegenerative diseases. Transl. Neurodegener. 2024, 13, 60. [Google Scholar] [CrossRef]
- Fabbiano, F.; Corsi, J.; Gurrieri, E.; Trevisan, C.; Notarangelo, M.; D’Agostino, V.G. RNA packaging into extracellular vesicles: An orchestra of RNA-binding proteins? J. Extracell. Vesicles 2020, 10, e12043. [Google Scholar] [CrossRef]
- Long, Y.; Liu, J.; Wang, Y.; Guo, H.; Cui, G. The complex effects of miR-146a in the pathogenesis of Alzheimer’s disease. Neural Regen. Res. 2025, 20, 1309–1323. [Google Scholar] [CrossRef]
- Alhenaky, A.; Alhazmi, S.; Alamri, S.H.; Alkhatabi, H.A.; Alharthi, A.; Alsaleem, M.A.; Abdelnour, S.A.; Hassan, S.M. Exosomal MicroRNAs in Alzheimer’s Disease: Unveiling Their Role and Pioneering Tools for Diagnosis and Treatment. J. Clin. Med. 2024, 13, 6960. [Google Scholar] [CrossRef]
- Faure, J.; Lachenal, G.; Court, M.; Hirrlinger, J.; Chatellard-Causse, C.; Blot, B.; Grange, J.; Schoehn, G.; Goldberg, Y.; Boyer, V.; et al. Exosomes are released by cultured cortical neurones. Mol. Cell Neurosci. 2006, 31, 642–648. [Google Scholar] [CrossRef]
- Lachenal, G.; Pernet-Gallay, K.; Chivet, M.; Hemming, F.J.; Belly, A.; Bodon, G.; Blot, B.; Haase, G.; Goldberg, Y.; Sadoul, R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell Neurosci. 2011, 46, 409–418. [Google Scholar] [CrossRef]
- Antoniou, A.; Auderset, L.; Kaurani, L.; Sebastian, E.; Zeng, Y.; Allahham, M.; Cases-Cunillera, S.; Schoch, S.; Grundemann, J.; Fischer, A.; et al. Neuronal extracellular vesicles and associated microRNAs induce circuit connectivity downstream BDNF. Cell Rep. 2023, 42, 112063. [Google Scholar] [CrossRef] [PubMed]
- Nogueras-Ortiz, C.J.; Eren, E.; Yao, P.; Calzada, E.; Dunn, C.; Volpert, O.; Delgado-Peraza, F.; Mustapic, M.; Lyashkov, A.; Rubio, F.J.; et al. Single-extracellular vesicle (EV) analyses validate the use of L1 Cell Adhesion Molecule (L1CAM) as a reliable biomarker of neuron-derived EVs. J. Extracell. Vesicles 2024, 13, e12459. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.; Pozzi, S.; Tortarolo, M.; Fiordaliso, F.; Bisighini, C.; Pasetto, L.; Spaltro, G.; Lidonnici, D.; Gensano, F.; Battaglia, E.; et al. Mutant copper-zinc superoxide dismutase (SOD1) induces protein secretion pathway alterations and exosome release in astrocytes: Implications for disease spreading and motor neuron pathology in amyotrophic lateral sclerosis. J. Biol. Chem. 2013, 288, 15699–15711. [Google Scholar] [CrossRef]
- Zhao, S.; Sheng, S.; Wang, Y.; Ding, L.; Xu, X.; Xia, X.; Zheng, J.C. Astrocyte-derived extracellular vesicles: A double-edged sword in central nervous system disorders. Neurosci. Biobehav. Rev. 2021, 125, 148–159. [Google Scholar] [CrossRef]
- Wang, G.; Dinkins, M.; He, Q.; Zhu, G.; Poirier, C.; Campbell, A.; Mayer-Proschel, M.; Bieberich, E. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): Potential mechanism of apoptosis induction in Alzheimer disease (AD). J. Biol. Chem. 2012, 287, 21384–21395. [Google Scholar] [CrossRef]
- Aires, I.D.; Santiago, A.R. Microglial exosomes in retinal neuroinflammation: Focus in glaucoma. Neural Regen. Res. 2021, 16, 1801–1802. [Google Scholar] [CrossRef]
- Ghosh, M.; Pearse, D.D. The Yin and Yang of Microglia-Derived Extracellular Vesicles in CNS Injury and Diseases. Cells 2024, 13, 1834. [Google Scholar] [CrossRef]
- Fruhbeis, C.; Kuo-Elsner, W.P.; Muller, C.; Barth, K.; Peris, L.; Tenzer, S.; Mobius, W.; Werner, H.B.; Nave, K.A.; Frohlich, D.; et al. Oligodendrocytes support axonal transport and maintenance via exosome secretion. PLoS Biol. 2020, 18, e3000621. [Google Scholar] [CrossRef]
- Bolivar, D.A.; Mosquera-Heredia, M.I.; Vidal, O.M.; Barcelo, E.; Allegri, R.; Morales, L.C.; Silvera-Redondo, C.; Arcos-Burgos, M.; Garavito-Galofre, P.; Velez, J.I. Exosomal mRNA Signatures as Predictive Biomarkers for Risk and Age of Onset in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 12293. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Geng, D. Key developments and hotspots of exosomes in Alzheimer’s disease: A bibliometric study spanning 2003 to 2023. Front. Aging Neurosci. 2024, 16, 1377672. [Google Scholar] [CrossRef] [PubMed]
- Almasi, F.; Abbasloo, F.; Soltani, N.; Dehbozorgi, M.; Moghadam Fard, A.; Kiani, A.; Ghasemzadeh, N.; Mesgari, H.; Zadeh Hosseingholi, E.; Payandeh, Z.; et al. Biology, Pathology, and Targeted Therapy of Exosomal Cargoes in Parkinson’s Disease: Advances and Challenges. Mol. Neurobiol. 2025, 62, 8381–8399. [Google Scholar] [CrossRef] [PubMed]
- Akbari-Gharalari, N.; Ghahremani-Nasab, M.; Naderi, R.; Chodari, L.; Nezhadshahmohammad, F. The potential of exosomal biomarkers: Revolutionizing Parkinson’s disease: How do they influence pathogenesis, diagnosis, and therapeutic strategies? AIMS Neurosci. 2024, 11, 374–397. [Google Scholar] [CrossRef]
- Bao, F.; Fleming, J.C.; Golshani, R.; Pearse, D.D.; Kasabov, L.; Brown, A.; Weaver, L.C. A selective phosphodiesterase-4 inhibitor reduces leukocyte infiltration, oxidative processes, and tissue damage after spinal cord injury. J. Neurotrauma 2011, 28, 1035–1049. [Google Scholar] [CrossRef]
- Solana-Balaguer, J.; Garcia-Segura, P.; Campoy-Campos, G.; Chicote-Gonzalez, A.; Fernandez-Irigoyen, J.; Santamaria, E.; Perez-Navarro, E.; Masana, M.; Alberch, J.; Malagelada, C. Motor skill learning modulates striatal extracellular vesicles’ content in a mouse model of Huntington’s disease. Cell Commun. Signal 2024, 22, 321. [Google Scholar] [CrossRef]
- Ananbeh, H.; Vodicka, P.; Kupcova Skalnikova, H. Emerging Roles of Exosomes in Huntington’s Disease. Int. J. Mol. Sci. 2021, 22, 4085. [Google Scholar] [CrossRef]
- Huo, L.; Du, X.; Li, X.; Liu, S.; Xu, Y. The Emerging Role of Neural Cell-Derived Exosomes in Intercellular Communication in Health and Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 738442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, T.V.; Yuan, Q.; Sadoul, R.; Lam, T.T.; Bordey, A. Small Extracellular Vesicles Control Dendritic Spine Development through Regulation of HDAC2 Signaling. J. Neurosci. 2021, 41, 3799–3807. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Teng, T. Exosomes: New targets for understanding axon guidance in the developing central nervous system. Front. Cell Dev. Biol. 2024, 12, 1510862. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Chen, J.; Huang, D.; Feng, S.; Yang, T.; Li, Z.; Wang, X.; Zhao, M.; Wu, J.; Zhong, T. Current perspectives on clinical use of exosomes as novel biomarkers for cancer diagnosis. Front. Oncol. 2022, 12, 966981. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, Y.F.; Lei, L.; Zhang, Y. MicroRNA-specific targets for neuronal plasticity, neurotransmitters, neurotrophic factors, and gut microbes in the pathogenesis and therapeutics of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 136, 111186. [Google Scholar] [CrossRef]
- Scott-Hewitt, N.; Mahoney, M.; Huang, Y.; Korte, N.; Yvanka de Soysa, T.; Wilton, D.K.; Knorr, E.; Mastro, K.; Chang, A.; Zhang, A.; et al. Microglial-derived C1q integrates into neuronal ribonucleoprotein complexes and impacts protein homeostasis in the aging brain. Cell 2024, 187, 4193–4212 e4124. [Google Scholar] [CrossRef]
- Delaveris, C.S.; Wang, C.L.; Riley, N.M.; Li, S.; Kulkarni, R.U.; Bertozzi, C.R. Microglia mediate contact-independent neuronal pruning via secreted Neuraminidase-3 associated with extracellular vesicles. bioRxiv 2023. [Google Scholar] [CrossRef]
- Wang, G.; Liu, H.Y.; Meng, X.W.; Chen, Y.; Zhao, W.M.; Li, W.T.; Xu, H.B.; Peng, K.; Ji, F.H. Complement C1q-mediated microglial synaptic elimination by enhancing desialylation underlies sevoflurane-induced developmental neurotoxicity. Cell Biosci. 2024, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, X.H.; Xu, S.X.; Wang, C.; Sun, S.; Song, X.; Li, R.; Li, N.; Feng, Y.; Duan, H.; et al. Oligodendrocyte-derived exosomes-containing SIRT2 ameliorates depressive-like behaviors and restores hippocampal neurogenesis and synaptic plasticity via the AKT/GSK-3beta pathway in depressed mice. CNS Neurosci. Ther. 2024, 30, e14661. [Google Scholar] [CrossRef]
- Chamberlain, K.A.; Huang, N.; Xie, Y.; LiCausi, F.; Li, S.; Li, Y.; Sheng, Z.H. Oligodendrocytes enhance axonal energy metabolism by deacetylation of mitochondrial proteins through transcellular delivery of SIRT2. Neuron 2021, 109, 3456–3472 e3458. [Google Scholar] [CrossRef]
- Fruhbeis, C.; Frohlich, D.; Kuo, W.P.; Kramer-Albers, E.M. Extracellular vesicles as mediators of neuron-glia communication. Front. Cell. Neurosci. 2013, 7, 182. [Google Scholar] [CrossRef]
- Li, S.; Sheng, Z.H. Oligodendrocyte-derived transcellular signaling regulates axonal energy metabolism. Curr. Opin. Neurobiol. 2023, 80, 102722. [Google Scholar] [CrossRef]
- Cyr, B.; Cabrera Ranaldi, E.; Hadad, R.; Dietrich, W.D.; Keane, R.W.; de Rivero Vaccari, J.P. Extracellular vesicles mediate inflammasome signaling in the brain and heart of Alzheimer’s disease mice. Front. Mol. Neurosci. 2024, 17, 1369781. [Google Scholar] [CrossRef]
- Ikezu, T.; Zhang, Z.; Yu, K.; Bao, H.; Ikezu, T.; Ravula, A.R.; Melvin, B.; Scholes, C.; You, Y.; Ellison, J.; et al. Unique lipid cargoes in APOE4 human brain-derived extracellular vesicles recruit cell adhesion molecules and promote tauopathy in Alzheimer’s disease. Res. Sq. 2025, rs.3, rs-6508552. [Google Scholar]
- Song, L.; Oseid, D.E.; Wells, E.A.; Robinson, A.S. The Interplay between GSK3beta and Tau Ser262 Phosphorylation during the Progression of Tau Pathology. Int. J. Mol. Sci. 2022, 23, 11610. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Deng, C.; Meng, Z.; Zhu, M.; Yang, R.; Yuan, J.; Meng, S. Combined Catalpol and Tetramethylpyrazine Promote Axonal Plasticity in Alzheimer’s Disease by Inducing Astrocytes to Secrete Exosomes Carrying CDK5 mRNA and Regulating STAT3 Phosphorylation. Mol. Neurobiol. 2024, 61, 10770–10791. [Google Scholar] [CrossRef]
- Dinkins, M.B.; Enasko, J.; Hernandez, C.; Wang, G.; Kong, J.; Helwa, I.; Liu, Y.; Terry, A.V., Jr.; Bieberich, E. Neutral Sphingomyelinase-2 Deficiency Ameliorates Alzheimer’s Disease Pathology and Improves Cognition in the 5XFAD Mouse. J. Neurosci. 2016, 36, 8653–8667. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, K.W.; Lee, E.J. Gut-brain axis and Environironmental factors in Parkinson’s disease: Bidirectional link between disease onset and progression. Neural Regen. Res. 2025, 20, 3416–3429. [Google Scholar] [CrossRef] [PubMed]
- Pan-Montojo, F.; Schwarz, M.; Winkler, C.; Arnhold, M.; O’Sullivan, G.A.; Pal, A.; Said, J.; Marsico, G.; Verbavatz, J.M.; Rodrigo-Angulo, M.; et al. Environironmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci. Rep. 2012, 2, 898. [Google Scholar] [CrossRef]
- Cortes, A.; Phung, T.K.; de Mena, L.; Garrido, A.; Infante, J.; Ruiz-Martinez, J.; Galmes-Ordinas, M.A.; Glendinning, S.; Perez, J.; Roig, A.; et al. In-depth mass-spectrometry reveals phospho-RAB12 as a blood biomarker of G2019S LRRK2-driven Parkinson’s disease. Brain 2025, 148, 2075–2092. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Hu, X.; Liu, H.; Yu, Q.; Kuang, G.; Liu, L.; Yu, D.; Lin, Z.; Xiong, N. Brain-derived extracellular vesicles: A promising avenue for Parkinson’s disease pathogenesis, diagnosis, and treatment. Neural Regen. Res. 2025, 21, 1447–1467. [Google Scholar] [CrossRef]
- Kong, W.; Li, X.; Guo, X.; Sun, Y.; Chai, W.; Chang, Y.; Huang, Q.; Wang, P.; Wang, X. Ultrasound-Assisted CRISPRi-Exosome for Epigenetic Modification of alpha-Synuclein Gene in a Mouse Model of Parkinson’s Disease. ACS Nano 2024, 18, 7837–7851. [Google Scholar] [CrossRef]
- Bahadorani, M.; Nasiri, M.; Dellinger, K.; Aravamudhan, S.; Zadegan, R. Engineering Exosomes for Therapeutic Applications: Decoding Biogenesis, Content Modification, and Cargo Loading Strategies. Int. J. Nanomed. 2024, 19, 7137–7164. [Google Scholar] [CrossRef]
- Darabi, S.; Ariaei, A.; Rustamzadeh, A.; Afshari, D.; Charkhat Gorgich, E.A.; Darabi, L. Cerebrospinal fluid and blood exosomes as biomarkers for amyotrophic lateral sclerosis; a systematic review. Diagn. Pathol. 2024, 19, 47. [Google Scholar] [CrossRef]
- Carata, E.; Muci, M.; Di Giulio, S.; Di Giulio, T.; Mariano, S.; Panzarini, E. The Neuromuscular Disorder Mediated by Extracellular Vesicles in Amyotrophic Lateral Sclerosis. Curr. Issues Mol. Biol. 2024, 46, 5999–6017. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ozdemir, S.; Fritz, C.; Mobius, W.; Kleineidam, L.; Mandelkow, E.; Biernat, J.; Dogdu, C.; Peters, O.; Cosma, N.C.; et al. Plasma extracellular vesicle tau and TDP-43 as diagnostic biomarkers in FTD and ALS. Nat. Med. 2024, 30, 1771–1783. [Google Scholar] [CrossRef] [PubMed]
- Menke, R.A.; Gray, E.; Lu, C.H.; Kuhle, J.; Talbot, K.; Malaspina, A.; Turner, M.R. CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Ann. Clin. Transl. Neurol. 2015, 2, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wang, H.; Li, Q.; Chen, Z. Extracellular Vesicles: A Review of Their Therapeutic Potentials, Sources, Biodistribution, and Administration Routes. Int. J. Nanobiotechnol. 2025, 20, 3175–3199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, F.; Jia, B.; Wu, Z.; Huang, Z.; He, M.; Weng, H.; So, K.F.; Qu, W.; Fu, Q.L.; et al. Intranasal delivery of small extracellular vesicles reduces the progress of amyotrophic lateral sclerosis and the overactivation of complement-coagulation cascade and NF-kB signaling in SOD1(G93A) mice. J. Nanobiotechnol. 2024, 22, 503. [Google Scholar] [CrossRef]
- Manna, I.; De Benedittis, S.; Porro, D. Extracellular Vesicles in Multiple Sclerosis: Their Significance in the Development and Possible Applications as Therapeutic Agents and Biomarkers. Genes 2024, 15, 772. [Google Scholar] [CrossRef]
- Casella, G.; Rasouli, J.; Boehm, A.; Zhang, W.; Xiao, D.; Ishikawa, L.L.W.; Thome, R.; Li, X.; Hwang, D.; Porazzi, P.; et al. Oligodendrocyte-derived extracellular vesicles as antigen-specific therapy for autoimmune neuroinflammation in mice. Sci. Transl. Med. 2020, 12, eaba0599. [Google Scholar] [CrossRef] [PubMed]
- Lim Falk, V.; Mueller-Wirth, N.; Karathanasis, D.; Evangelopoulos, M.E.; Maleska Maceski, A.; Zadic, A.; Kuhle, J.; Schlup, C.; Marti, S.; Guse, K.; et al. Extracellular Vesicle Marker Changes Associated With Disease Activity in Relapsing-Remitting Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2025, 12, e200404. [Google Scholar] [CrossRef]
- Krakenes, T.; Sandvik, C.E.; Ytterdal, M.; Gavasso, S.; Evjenth, E.C.; Bo, L.; Kvistad, C.E. The Therapeutic Potential of Exosomes from Mesenchymal Stem Cells in Multiple Sclerosis. Int. J. Mol. Sci. 2024, 25, 10292. [Google Scholar] [CrossRef]
- Tang, B.L. Unconventional Secretion and Intercellular Transfer of Mutant Huntingtin. Cells 2018, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Ananbeh, H.; Novak, J.; Juhas, S.; Juhasova, J.; Klempir, J.; Doleckova, K.; Rysankova, I.; Turnovcova, K.; Hanus, J.; Hansikova, H.; et al. Huntingtin Co-Isolates with Small Extracellular Vesicles from Blood Plasma of TgHD and KI-HD Pig Models of Huntington’s Disease and Human Blood Plasma. Int. J. Mol. Sci. 2022, 23, 5598. [Google Scholar] [CrossRef]
- Miguez, A.; Gomis, C.; Vila, C.; Monguio-Tortajada, M.; Fernandez-Garcia, S.; Bombau, G.; Galofre, M.; Garcia-Bravo, M.; Sanders, P.; Fernandez-Medina, H.; et al. Soluble mutant huntingtin drives early human pathogenesis in Huntington’s disease. Cell Mol. Life Sci. 2023, 80, 238. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhong, S.; Zhang, R.; Kang, K.; Zhang, X.; Xu, Y.; Zhao, C.; Zhao, M. Functional analysis of brain derived neurotrophic factor (BDNF) in Huntington’s disease. Aging 2021, 13, 6103–6114. [Google Scholar] [CrossRef]
- Wilton, D.K.; Mastro, K.; Heller, M.D.; Gergits, F.W.; Willing, C.R.; Fahey, J.B.; Frouin, A.; Daggett, A.; Gu, X.; Kim, Y.A.; et al. Microglia and complement mediate early corticostriatal synapse loss and cognitive dysfunction in Huntington’s disease. Nat. Med. 2023, 29, 2866–2884. [Google Scholar] [CrossRef]
- Guo, M.; Wang, L.; Yin, Z.; Chen, F.; Lei, P. Small extracellular vesicles as potential theranostic tools in central nervous system disorders. Biomed. Pharmacother. 2023, 167, 115407. [Google Scholar] [CrossRef]
- Li, Y.; Khan, N.; Ritzel, R.M.; Lei, Z.; Allen, S.; Faden, A.I.; Wu, J. Sexually dimorphic extracellular vesicle responses after chronic spinal cord injury are associated with neuroinflammation and neurodegeneration in the aged brain. J. Neuroinflamm. 2023, 20, 197. [Google Scholar] [CrossRef]
- Hasan, A.; Ardizzone, A.; Giosa, D.; Scuderi, S.A.; Calcaterra, E.; Esposito, E.; Capra, A.P. The Therapeutic Potential of MicroRNA-21 in the Treatment of Spinal Cord Injury. Curr. Issues Mol. Biol. 2025, 47, 70. [Google Scholar] [CrossRef]
- Lin, M.; Alimerzaloo, F.; Wang, X.; Alhalabi, O.; Krieg, S.M.; Skutella, T.; Younsi, A. Harnessing stem cell-derived exosomes: A promising cell-free approach for spinal cord injury. Stem Cell Res. Ther. 2025, 16, 182. [Google Scholar] [CrossRef] [PubMed]
- Nakazaki, M.; Yokoyama, T.; Lankford, K.L.; Hirota, R.; Kocsis, J.D.; Honmou, O. Mesenchymal Stem Cells and Their Extracellular Vesicles: Therapeutic Mechanisms for Blood-Spinal Cord Barrier Repair Following Spinal Cord Injury. Int. J. Mol. Sci. 2024, 25, 13460. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Asim, M.; El-Menyar, A.; Biswas, K.H.; Rizoli, S.; Al-Thani, H. The evolving role of extracellular vesicles (exosomes) as biomarkers in traumatic brain injury: Clinical perspectives and therapeutic implications. Front. Aging Neurosci. 2022, 14, 933434. [Google Scholar] [CrossRef]
- de Rivero Vaccari, J.P.; Brand, F., 3rd; Adamczak, S.; Lee, S.W.; Perez-Barcena, J.; Wang, M.Y.; Bullock, M.R.; Dietrich, W.D.; Keane, R.W. Exosome-mediated inflammasome signaling after central nervous system injury. J. Neurochem. 2016, 136 (Suppl. S1), 39–48. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Li, P.; Bai, F.; Liu, C.; Huang, X. Serum exosomes miR-206 and miR-549a-3p as potential biomarkers of traumatic brain injury. Sci. Rep. 2024, 14, 10082. [Google Scholar]
- Liu, M.W.; Li, H.; Xiong, G.F.; Zhang, B.R.; Zhang, Q.J.; Gao, S.J.; Zhu, Y.L.; Zhang, L.M. Mesenchymal stem cell exosomes therapy for the treatment of traumatic brain injury: Mechanism, progress, challenges and prospects. J. Transl. Med. 2025, 23, 427. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gu, Y.; Zhang, Q.; Liu, H.; Liu, Y. The Potential Roles of Exosomes Carrying APP and Tau Cleavage Products in Alzheimer’s Disease. J. Clin. Med. 2023, 12, 1883. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Chen, Z.T.; Zhou, R.L.; Zhang, X.; Ye, Q.Y.; Wang, Y.Z. Increased DJ-1 and alpha-Synuclein in Plasma Neural-Derived Exosomes as Potential Markers for Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 438. [Google Scholar]
- Zhang, P.; Rasheed, M.; Liang, J.; Wang, C.; Feng, L.; Chen, Z. Emerging Potential of Exosomal Non-coding RNA in Parkinson’s Disease: A Review. Front. Aging Neurosci. 2022, 14, 819836. [Google Scholar] [CrossRef]
- Gagliardi, D.; Bresolin, N.; Comi, G.P.; Corti, S. Extracellular vesicles and amyotrophic lateral sclerosis: From misfolded protein vehicles to promising clinical biomarkers. Cell Mol. Life Sci. 2021, 78, 561–572. [Google Scholar] [CrossRef]
- Banack, S.A.; Dunlop, R.A.; Cox, P.A. An miRNA fingerprint using neural-enriched extracellular vesicles from blood plasma: Towards a biomarker for amyotrophic lateral sclerosis/motor neuron disease. Open Biol. 2020, 10, 200116. [Google Scholar] [CrossRef]
- Lee, S.T.; Im, W.; Ban, J.J.; Lee, M.; Jung, K.H.; Lee, S.K.; Chu, K.; Kim, M. Exosome-Based Delivery of miR-124 in a Huntington’s Disease Model. J. Mov. Disord. 2017, 10, 45–52. [Google Scholar] [CrossRef]
- Mazzucco, M.; Mannheim, W.; Shetty, S.V.; Linden, J.R. CNS endothelial derived extracellular vesicles are biomarkers of active disease in multiple sclerosis. Fluids Barriers CNS 2022, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Maciak, K.; Dziedzic, A.; Miller, E.; Saluk-Bijak, J. miR-155 as an Important Regulator of Multiple Sclerosis Pathogenesis. A Review. Int. J. Mol. Sci. 2021, 22, 4332. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ye, L.; Sun, S.; Wang, Y.; Chen, Y.; Chang, J.; Yin, R.; Liu, X.; Zuo, W.; Xu, H.; et al. Molecular intersections of traumatic brain injury and Alzheimer’s disease: The role of ADMSC-derived exosomes and hub genes in microglial polarization. Metab. Brain Dis. 2024, 40, 77. [Google Scholar] [CrossRef]
- Rong, Y.; Liu, W.; Wang, J.; Fan, J.; Luo, Y.; Li, L.; Kong, F.; Chen, J.; Tang, P.; Cai, W. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, Z.; Bao, Q.; Chen, S.; Xu, W.; Jiang, J. Extracellular Vesicles as Emerging Therapeutic Strategies in Spinal Cord Injury: Ready to Go. Biomedicines 2025, 13, 1262. [Google Scholar] [CrossRef] [PubMed]
- Kodali, M.; Madhu, L.N.; Reger, R.L.; Milutinovic, B.; Upadhya, R.; Gonzalez, J.J.; Attaluri, S.; Shuai, B.; Gitai, D.L.G.; Rao, S.; et al. Intranasally administered human MSC-derived extracellular vesicles inhibit NLRP3-p38/MAPK signaling after TBI and prevent chronic brain dysfunction. Brain Behav. Immun. 2023, 108, 118–134. [Google Scholar] [CrossRef]
- Blaya, M.O.; Pressman, Y.; Andreu, M.; Moreno, W.J.; Sanchez-Molano, J.; Kerr, N.A.; Umland, O.; Khan, A.; Bramlett, H.M.; Dietrich, W.D. Human Schwann cell exosome treatment attenuates secondary injury mechanisms, histopathological consequences, and behavioral deficits after traumatic brain injury. Neurotherapeutics 2025, 22, e00555. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Sanchez-Molano, J.; Kerr, N.; Pressman, Y.; Silvera, R.; Khan, A.; Gajavelli, S.; Bramlett, H.M.; Dietrich, W.D. Beneficial Effects of Human Schwann Cell-Derived Exosomes in Mitigating Secondary Damage After Penetrating Ballistic-Like Brain Injury. J. Neurotrauma 2024, 41, 2395–2412. [Google Scholar] [CrossRef]
- Ghosh, M.; Pearse, D.D. Schwann Cell-Derived Exosomal Vesicles: A Promising Therapy for the Injured Spinal Cord. Int. J. Mol. Sci. 2023, 24, 17317. [Google Scholar] [CrossRef]
- Ramos-Zaldivar, H.M.; Polakovicova, I.; Salas-Huenuleo, E.; Corvalan, A.H.; Kogan, M.J.; Yefi, C.P.; Andia, M.E. Extracellular vesicles through the blood-brain barrier: A review. Fluids Barriers CNS 2022, 19, 60. [Google Scholar] [CrossRef]
- Bravo-Miana, R.D.C.; Arizaga-Echebarria, J.K.; Otaegui, D. Central nervous system-derived extracellular vesicles: The next generation of neural circulating biomarkers? Transl. Neurodegener. 2024, 13, 32. [Google Scholar] [CrossRef]
- Utz, J.; Berner, J.; Munoz, L.E.; Oberstein, T.J.; Kornhuber, J.; Herrmann, M.; Maler, J.M.; Spitzer, P. Cerebrospinal Fluid of Patients With Alzheimer’s Disease Contains Increased Percentages of Synaptophysin-Bearing Microvesicles. Front. Aging Neurosci. 2021, 13, 682115. [Google Scholar] [CrossRef]
- Kluge, A.; Schaeffer, E.; Bunk, J.; Sommerauer, M.; Rottgen, S.; Schulte, C.; Roeben, B.; von Thaler, A.K.; Welzel, J.; Lucius, R.; et al. Detecting Misfolded alpha-Synuclein in Blood Years before the Diagnosis of Parkinson’s Disease. Mov. Disord. 2024, 39, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Scaroni, F.; Visconte, C.; Serpente, M.; Golia, M.T.; Gabrielli, M.; Huiskamp, M.; Hulst, H.E.; Carandini, T.; De Riz, M.; Pietroboni, A.; et al. miR-150-5p and let-7b-5p in Blood Myeloid Extracellular Vesicles Track Cognitive Symptoms in Patients with Multiple Sclerosis. Cells 2022, 11, 1551. [Google Scholar] [CrossRef]
- Babaee, A.; Wichmann, T.O.; Rasmussen, M.M.; Brink, O.; Olsen, D.A.; Borris, L.C.; Lesbo, M.; Rasmussen, R.W.; Salomon, C.; Handberg, A.; et al. Extracellular Vesicle Glial Fibrillary Acidic Protein as a Circulating Biomarker of Traumatic Brain Injury Severity. J. Mol. Neurosci. 2025, 75, 69. [Google Scholar] [CrossRef]
- Ljungstrom, M.; Oltra, E. Methods for Extracellular Vesicle Isolation: Relevance for Encapsulated miRNAs in Disease Diagnosis and Treatment. Genes 2025, 16, 330. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrugger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Ramirez, M.I.; Amorim, M.G.; Gadelha, C.; Milic, I.; Welsh, J.A.; Freitas, V.M.; Nawaz, M.; Akbar, N.; Couch, Y.; Makin, L.; et al. Technical challenges of working with extracellular vesicles. Nanoscale 2018, 10, 881–906. [Google Scholar] [CrossRef]
- Saftics, A.; Purnell, B.; Beres, B.; Thompson, S.; Jiang, N.; Ghaeli, I.; Lima, C.; Armstrong, B.; Van Keuren-Jensen, K.; Jovanovic-Talisman, T. Single Extracellular VEsicle Nanoscopy-Universal Protocol (SEVEN-UP): Accessible Imaging Platform for Quantitative Characterization of Single Extracellular Vesicles. Anal. Chem. 2025, 97, 1654–1664. [Google Scholar] [CrossRef]
- Cohn, W.; Melnik, M.; Huang, C.; Teter, B.; Chandra, S.; Zhu, C.; McIntire, L.B.; John, V.; Gylys, K.H.; Bilousova, T. Multi-Omics Analysis of Microglial Extracellular Vesicles From Human Alzheimer’s Disease Brain Tissue Reveals Disease-Associated Signatures. Front. Pharmacol. 2021, 12, 766082. [Google Scholar] [CrossRef]
- Heidarzadeh, M.; Gursoy-Ozdemir, Y.; Kaya, M.; Eslami Abriz, A.; Zarebkohan, A.; Rahbarghazi, R.; Sokullu, E. Exosomal delivery of therapeutic modulators through the blood-brain barrier; promise and pitfalls. Cell Biosci. 2021, 11, 142. [Google Scholar] [CrossRef]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Balaraman, A.K.; Babu, M.A.; Moglad, E.; Mandaliya, V.; Rekha, M.M.; Gupta, S.; Prasad, G.V.S.; Kumari, M.; Chauhan, A.S.; Ali, H.; et al. Exosome-mediated delivery of CRISPR-Cas9: A revolutionary approach to cancer gene editing. Pathol. Res. Pract. 2025, 266, 155785. [Google Scholar] [CrossRef]
- Song, L.L.; Tang, Y.P.; Qu, Y.Q.; Yun, Y.X.; Zhang, R.L.; Wang, C.R.; Wong, V.K.W.; Wang, H.M.; Liu, M.H.; Qu, L.Q.; et al. Exosomal delivery of rapamycin modulates blood-brain barrier penetration and VEGF axis in glioblastoma. J. Control. Release 2025, 381, 113605. [Google Scholar] [CrossRef]
- Bashirrohelleh, M.A.; Bavarsad, K.; Khodadadi, A.; Shohan, M.; Asadirad, A. Curcumin-enhanced stem cell exosomes: A novel approach to modulating neuroinflammation and improving cognitive function in a rat model of Alzheimer’s disease. Eur. J. Pharmacol. 2025, 999, 177695. [Google Scholar] [CrossRef]
- Han, Y.; Jones, T.W.; Dutta, S.; Zhu, Y.; Wang, X.; Narayanan, S.P.; Fagan, S.C.; Zhang, D. Overview and Update on Methods for Cargo Loading into Extracellular Vesicles. Process 2021, 9, 356. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Wu, L.; Zhu, L.; Lu, M.; Zhang, Z.; Lv, Y.; Zhu, X.; Yao, H. Rapamycin-induced small extracellular vesicles under GelMA scaffolds facilitate diabetic wound repair through accelerating angiogenesis and alleviating macrophage-mediated inflammation via PI3K/Akt signaling pathway. Mater. Today Bio 2025, 32, 101846. [Google Scholar] [CrossRef]
- Yang, D.; Deng, Z.; Zhou, H.; Zhang, Q.; Zhang, X.; Gong, J. Exosome-mediated dual drug delivery of curcumin and methylene blue for enhanced cognitive function and mechanistic elucidation in Alzheimer’s disease therapy. Front. Cell Dev. Biol. 2025, 13, 1562565. [Google Scholar] [CrossRef]
- Fajrial, A.K.; He, Q.Q.; Wirusanti, N.I.; Slansky, J.E.; Ding, X. A review of emerging physical transfection methods for CRISPR/Cas9-mediated gene editing. Theranostics 2020, 10, 5532–5549. [Google Scholar] [CrossRef]
- Hur, J.; Chung, A.J. Microfluidic and Nanofluidic Intracellular Delivery. Adv. Sci. 2021, 8, e2004595. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, S.; Qin, F.; Fu, A.; Fu, C. Application progress of RVG peptides to facilitate the delivery of therapeutic agents into the central nervous system. RSC Adv. 2021, 11, 8505–8515. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Q.; Chai, Y.; Rong, R.; He, L.; Zhang, Y.; Cui, H.; Xu, H.; Zhang, X.; Wang, Z.; et al. An anti-CD19-exosome delivery system navigates the blood-brain barrier for targeting of central nervous system lymphoma. J. Nanobiotechnol. 2025, 23, 173. [Google Scholar] [CrossRef]
- N’Diaye, E.R.; Orefice, N.S.; Ghezzi, C.; Boumendjel, A. Chemically Modified Extracellular Vesicles and Applications in Radiolabeling and Drug Delivery. Pharmaceutics 2022, 14, 653. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, P.; Chen, T.; Lin, L.; Ji, X.; Liu, J.; Huang, H.; Saravanan, T.; Fuehrer, H.; Ross, C.A.; et al. In vivo self-assembled siRNAs within small extracellular vesicles attenuate LRRK2-induced neurodegeneration in Parkinson’s disease models. J. Control. Release 2025, 378, 1139–1153. [Google Scholar] [CrossRef]
- Yang, J.; Wu, S.; Hou, L.; Zhu, D.; Yin, S.; Yang, G.; Wang, Y. Therapeutic Effects of Simultaneous Delivery of Nerve Growth Factor mRNA and Protein via Exosomes on Cerebral Ischemia. Mol. Ther. Nucleic Acids 2020, 21, 512–522. [Google Scholar] [CrossRef]
- Evora, A.; Garcia, G.; Rubi, A.; De Vitis, E.; Matos, A.T.; Vaz, A.R.; Gervaso, F.; Gigli, G.; Polini, A.; Brites, D. Exosomes enriched with miR-124-3p show therapeutic potential in a new microfluidic triculture model that recapitulates neuron-glia crosstalk in Alzheimer’s disease. Front. Pharmacol. 2025, 16, 1474012. [Google Scholar] [CrossRef]
- Han, D.; Dong, X.; Zheng, D.; Nao, J. MiR-124 and the Underlying Therapeutic Promise of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1555. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, Y.; Wang, L.; Liu, Y.; Wang, X.; Li, Y.; Chi, G. miR-124: A Promising Therapeutic Target for Central Nervous System Injuries and Diseases. Cell Mol. Neurobiol. 2022, 42, 2031–2053. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, W.; Ke, H.; Lan, C. Optical imaging detection of extracellular vesicles of miR-146 modified bone marrow mesenchymal stem cells promoting spinal cord injury repair. SLAS Technol. 2024, 29, 100172. [Google Scholar] [CrossRef]
- Crose, J.J.; Crose, A.; Ransom, J.T.; Lightner, A.L. Bone marrow mesenchymal stem cell-derived extracellular vesicle infusion for amyotrophic lateral sclerosis. Neurodegener. Dis. Manag. 2024, 14, 111–117. [Google Scholar] [CrossRef]
- Goldschmidt-Clermont, P.J.; Khan, A.; Jimsheleishvili, G.; Graham, P.; Brooks, A.; Silvera, R.; Goldschmidt, A.J.P.; Pearse, D.D.; Dietrich, W.D.; Levi, A.D.; et al. Treating amyotrophic lateral sclerosis with allogeneic Schwann cell-derived exosomal vesicles: A case report. Neural Regen. Res. 2025, 20, 1207–1216. [Google Scholar] [CrossRef]
- Grangier, A.; Branchu, J.; Volatron, J.; Piffoux, M.; Gazeau, F.; Wilhelm, C.; Silva, A.K.A. Technological advances towards extracellular vesicles mass production. Adv. Drug Deliv. Rev. 2021, 176, 113843. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Ichihara, M.; Wang, X.; Yamamoto, K.; Kimura, J.; Majima, E.; Kiwada, H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J. Control. Release 2006, 112, 15–25. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinis, M.A.; Rodriguez-Gascon, A.; Almeida, A.J.; Preat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Preat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Perez-Aranda, M.; Martinez, G.; Merinero, M.; Arguelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomater 2020, 10, 1403. [Google Scholar] [CrossRef]
- Mingozzi, F.; High, K.A. Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood 2013, 122, 23–36. [Google Scholar] [CrossRef]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L., 3rd; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef]
- Torres Crigna, A.; Fricke, F.; Nitschke, K.; Worst, T.; Erb, U.; Karremann, M.; Buschmann, D.; Elvers-Hornung, S.; Tucher, C.; Schiller, M.; et al. Inter-Laboratory Comparison of Extracellular Vesicle Isolation Based on Ultracentrifugation. Transfus. Med. Hemother 2021, 48, 48–59. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, L.; Ma, T.; Xu, W.; Qian, H.; Sun, Y.; Shi, H. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics 2022, 12, 6548–6575. [Google Scholar] [CrossRef]
- Garcia, S.G.; Sanroque-Munoz, M.; Clos-Sansalvador, M.; Font-Moron, M.; Monguio-Tortajada, M.; Borras, F.E.; Franquesa, M. Hollow fiber bioreactor allows sustained production of immortalized mesenchymal stromal cell-derived extracellular vesicles. Extracell. Vesicles Circ. Nucl. Acids 2024, 5, 201–220. [Google Scholar] [CrossRef]
- Yan, L.; Wu, X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol. Toxicol. 2020, 36, 165–178. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol. Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kim, M.S. Development and regulation of exosome-based therapy products. Wiley Interdiscip. Rev. Nanomed. Nanobiotechn. 2016, 8, 744–757. [Google Scholar] [CrossRef]
- Marzan, A.L.; Chitti, S.V. Unravelling the Role of Cancer Cell-Derived Extracellular Vesicles in Muscle Atrophy, Lipolysis, and Cancer-Associated Cachexia. Cells 2023, 12, 2598. [Google Scholar] [CrossRef]
- Wang, B.; Le, D.S.; Liu, L.; Zhang, X.X.; Yang, F.; Lai, G.R.; Zhang, C.; Zhao, M.L.; Shen, Y.P.; Liao, P.S.; et al. Targeting exosomal double-stranded RNA-TLR3 signaling pathway attenuates morphine tolerance and hyperalgesia. Cell Rep. Med. 2024, 5, 101782. [Google Scholar] [CrossRef]
- Muskan, M.; Abeysinghe, P.; Cecchin, R.; Branscome, H.; Morris, K.V.; Kashanchi, F. Therapeutic potential of RNA-enriched extracellular vesicles: The next generation in RNA delivery via biogenic nanoparticles. Mol. Ther. 2024, 32, 2939–2949. [Google Scholar] [CrossRef]
- Zhang, E.; Phan, P.; Zhao, Z. Cellular nanovesicles for therapeutic immunomodulation: A perspective on engineering strategies and new advances. Acta Pharm. Sin. B 2023, 13, 1789–1827. [Google Scholar] [CrossRef]
- Wan, Z.; Liu, T.; Xu, N.; Zhu, W.; Zhang, X.; Liu, Q.; Wang, H.; Wang, H. PKH Dyes Should Be Avoided in the EVs Biodistribution Study of the Brain: A Call for Caution. Int. J. Nanomed. 2024, 19, 10885–10898. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Wiklander, O.P.B.; Wood, M.J.A.; El-Andaloussi, S. Biodistribution of therapeutic extracellular vesicles. Extracell. Vesicles Circ. Nucl. Acids 2023, 4, 170–190. [Google Scholar] [CrossRef] [PubMed]
- Kunitake, K.; Mizuno, T.; Hattori, K.; Oneyama, C.; Kamiya, M.; Ota, S.; Urano, Y.; Kojima, R. Barcoding of small extracellular vesicles with CRISPR-gRNA enables comprehensive, subpopulation-specific analysis of their biogenesis and release regulators. Nat. Commun. 2024, 15, 9777. [Google Scholar] [CrossRef] [PubMed]
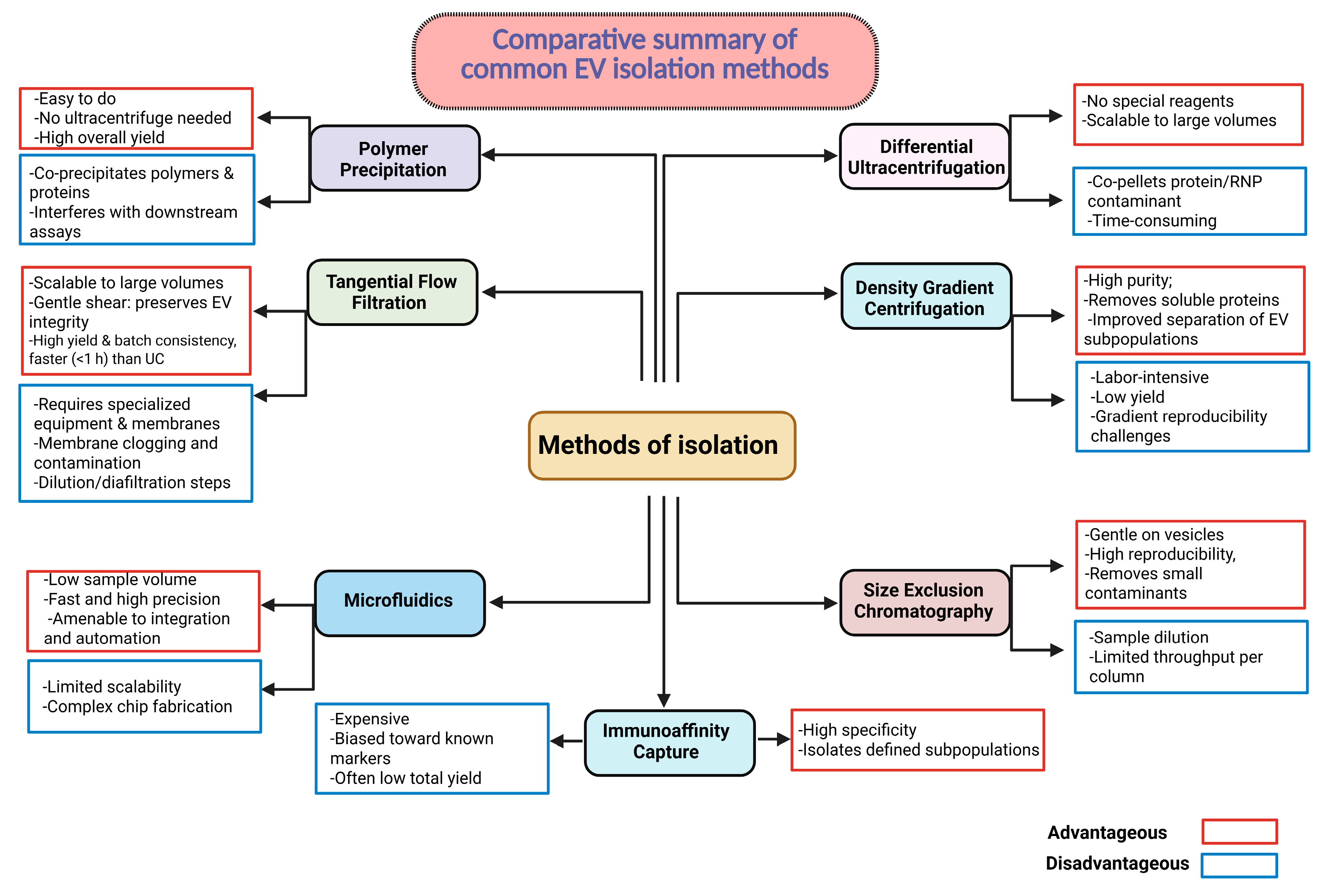
| Feature | sEVs | Liposomes/LNPs | Polymeric NPs | Viral Vectors |
| Typical Size | 30–150 nm | 20–200 nm (up to 1 µm) | 50–300 nm (tunable 20 nm–1 µm) | AAV: ~25 nm; lentivirus: ~100 nm |
| Biocompatibility | Very high (endogenous origin) | High (PEGylation improves circulation) | Moderate–high | Moderate–high (protein capsid; genomic risk) |
| Immunogenicity | Low | Moderate (anti-PEG antibodies) | Moderate–high (surface-charge-dependent) | High (neutralizing antibodies, innate/adaptive responses) |
| Cargo Capacity | Moderate, multiple protein molecules (~103); fewer nucleic-acid copies per vesicle | Very high (both hydrophilic and lipophilic drugs) | Moderate (<20% w/w, burst-release common) | Limited (AAV: ~4.7 kb; lenti: ~8–10 kb) |
| BBB Penetration | Modest; via transcytosis, hitchhiking | Poor intrinsic BBB crossing; requires ligands, FUS, or chemical disruption | Poor (<5%) unless actively targeted | Excellent when using neurotropic pseudotypes (e.g., AAV9, PHP.eB) |
| Targeting Strategies | Intrinsic tropism: surface engineering (RVG-Lamp2b), click-chemistry | Ligand Conjugation | PEGylation, surface modifications | Native tropism; pseudotyping envelope engineering, tissue-specific promoters |
| Scalability/Regulatory Status | Moderate; pilot GMP, heterogeneity challenges, several IND MSC-EV trials, one single patient SC-EV clinical investigation, no FDA approvals | High and well established, multiple FDA-approved | High; scalable, FDA-approved PLGA depots; no CNS-targeted PNPs yet | CMC more stringent, strict QA/QC, several FDA-approved gene therapies |
| Key Advantages | Biocompatible with native tropism and low immunogenic, can cross BBB, flexible loading (RNA, proteins, CRISPR), repeat dosing, surface easily engineered | Very high drug-loading flexibility, well-established GMP and analytics, robust regulatory track record | Tunable composition and degradation, controlled drug release profiles, broad polymer versatility | High transduction efficiency, long-term expression from single dose, extensive serotype availability |
| Limitations | Heterogeneity; lack of robust potency assays; isolation variability | Rapid clearance, stability issues; manufacturing complexity | Polymer degradation, burst release, scale-up variability | Immunogenicity, insertional risk, size limits, manufacturing cost |
| References | [10,138,161] | [162,163,164] | [165,166,167] | [168,169,170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, M.; Bayat, A.-H.; Pearse, D.D. Small Extracellular Vesicles in Neurodegenerative Disease: Emerging Roles in Pathogenesis, Biomarker Discovery, and Therapy. Int. J. Mol. Sci. 2025, 26, 7246. https://doi.org/10.3390/ijms26157246
Ghosh M, Bayat A-H, Pearse DD. Small Extracellular Vesicles in Neurodegenerative Disease: Emerging Roles in Pathogenesis, Biomarker Discovery, and Therapy. International Journal of Molecular Sciences. 2025; 26(15):7246. https://doi.org/10.3390/ijms26157246
Chicago/Turabian StyleGhosh, Mousumi, Amir-Hossein Bayat, and Damien D. Pearse. 2025. "Small Extracellular Vesicles in Neurodegenerative Disease: Emerging Roles in Pathogenesis, Biomarker Discovery, and Therapy" International Journal of Molecular Sciences 26, no. 15: 7246. https://doi.org/10.3390/ijms26157246
APA StyleGhosh, M., Bayat, A.-H., & Pearse, D. D. (2025). Small Extracellular Vesicles in Neurodegenerative Disease: Emerging Roles in Pathogenesis, Biomarker Discovery, and Therapy. International Journal of Molecular Sciences, 26(15), 7246. https://doi.org/10.3390/ijms26157246







