Recent Advances in Experimental Functional Characterization of GWAS Candidate Genes in Osteoporosis
Abstract
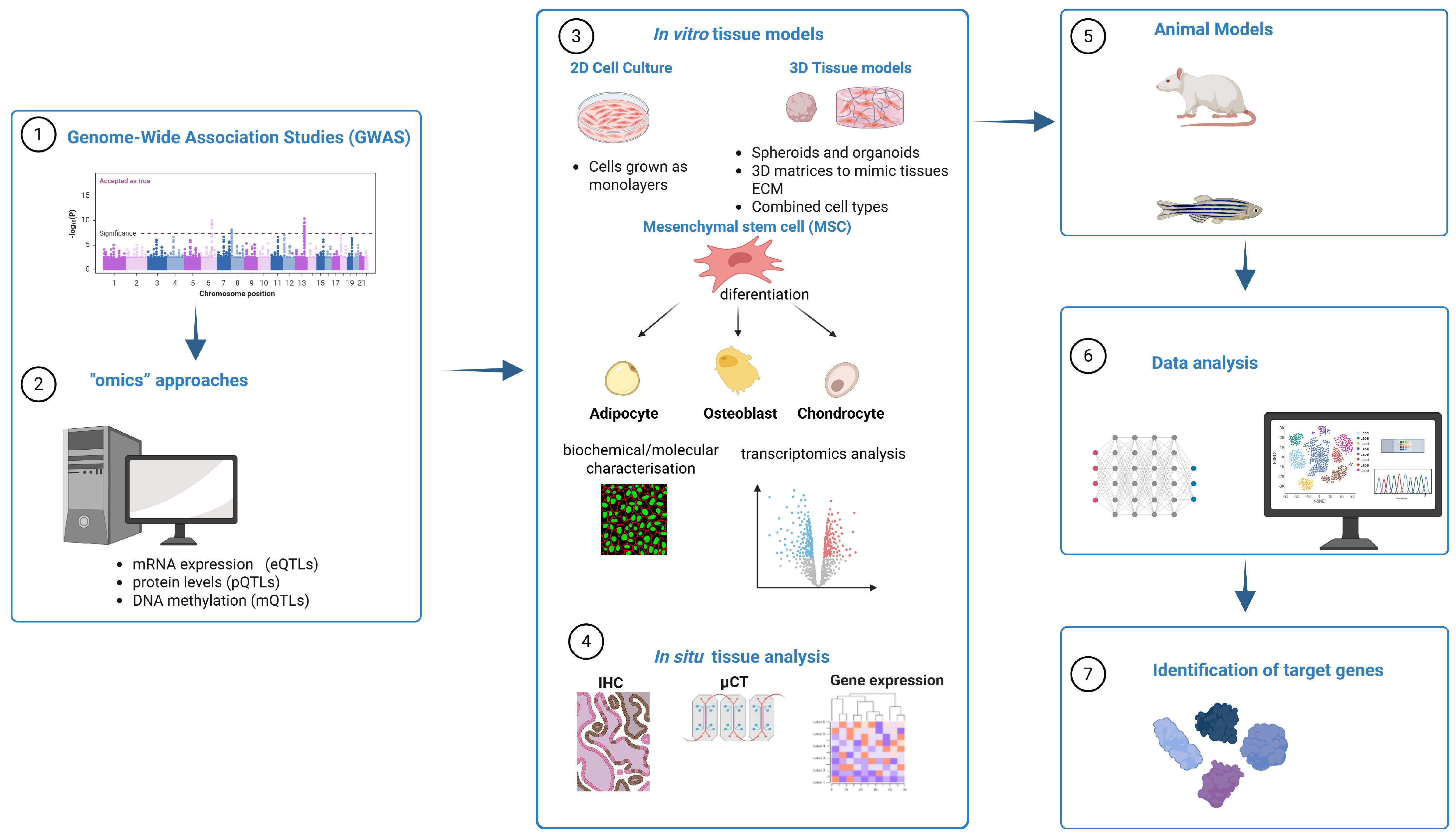
1. Introduction
2. Osteoporosis as a Multifactorial Disease
3. Osteoporosis Genome-Wide Association Studies
4. Post-GWAS In Silico Studies
5. Experimental Functional Characterization of GWAS Hits
5.1. Selection of In Vitro Cell Model
5.2. Gain- and Loss-of-Function Approaches in Cell Models
5.3. Methods and Approaches for Evaluation of Bone-Specific Outcomes
5.3.1. Cell Differentiation Evaluation
5.3.2. Functional Assessment—Matrix Mineralization and Resorption
5.4. Animal Models for Bone Research
5.5. In Situ Tissue Gene Expression
6. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sozen, T.; Ozisik, L.; Calik Basaran, N. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement 2000, 17, 1–45.
- Kiel, D.P.; Demissie, S.; Dupuis, J.; Lunetta, K.L.; Murabito, J.M.; Karasik, D. Genome-wide association with bone mass and geometry in the Framingham Heart Study. BMC Med. Genet. 2007, 8 (Suppl. S1), S14. [Google Scholar] [CrossRef]
- Zhu, X.; Bai, W.; Zheng, H. Twelve years of GWAS discoveries for osteoporosis and related traits: Advances, challenges and applications. Bone Res. 2021, 9, 23. [Google Scholar] [CrossRef]
- Kague, E. Finding the genes for fragile bones. eLife 2022, 11, e85161. [Google Scholar] [CrossRef]
- Tam, V.; Patel, N.; Turcotte, M.; Bossé, Y.; Paré, G.; Meyre, D. Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 2019, 20, 467–484. [Google Scholar] [CrossRef]
- Medina-Gomez, C.; Kemp, J.; Trajanoska, K.; Luan, J.; Chesi, A.; Ahluwalia, T.; Mook-Kanamori, D.O.; Ham, A.; Hartwig, F.P.; Evans, D.S.; et al. Life-Course Genome-wide Association Study Meta-analysis of Total Body BMD and Assessment of Age-Specific Effects. Am. J. Hum. Genet. 2018, 102, 88–102. [Google Scholar] [CrossRef]
- Rauner, M.; Foessl, I.; Formosa, M.; Kague, E.; Prijatelj, V.; Lopez, N.; Banerjee, B.; Bergen, D.; Busse, B.; Calado, Â.; et al. Perspective of the GEMSTONE Consortium on Current and Future Approaches to Functional Validation for Skeletal Genetic Disease Using Cellular, Molecular and Animal-Modeling Techniques. Front. Endocrinol. 2021, 12, 731217. [Google Scholar] [CrossRef]
- Estrada, K.; Styrkarsdottir, U.; Evangelou, E.; Hsu, Y.-H.; Duncan, E.L.; Ntzani, E.E.; Oei, L.; Albagha, O.M.E.; Amin, N.; Kemp, J.P.; et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat. Genet. 2012, 44, 491–501. [Google Scholar] [CrossRef]
- Yang, T.-L.; Shen, H.; Liu, A.; Dong, S.-S.; Zhang, L.; Deng, F.-Y.; Zhao, Q.; Deng, H.-W. A road map for understanding molecular and genetic determinants of osteoporosis. Nat. Rev. Endocrinol. 2020, 16, 91–103. [Google Scholar] [CrossRef]
- Fabo, T.; Khavari, P. Functional characterization of human genomic variation linked to polygenic diseases. Trends Genet. 2023, 39, 462–490. [Google Scholar] [CrossRef]
- Flynn, E.D.; Lappalainen, T. Functional Characterization of Genetic Variant Effects on Expression. Annu. Rev. Biomed. Data Sci. 2022, 5, 119–139. [Google Scholar] [CrossRef]
- Lappalainen, T.; MacArthur, D.G. From variant to function in human disease genetics. Science 2021, 373, 1464–1468. [Google Scholar] [CrossRef]
- Amroodi, M.N.; Maghsoudloo, M.; Amiri, S.; Mokhtari, K.; Mohseni, P.; Pourmarjani, A.; Jamali, B.; Khosroshahi, E.M.; Asadi, S.; Tabrizian, P.; et al. Unraveling the molecular and immunological landscape: Exploring signaling pathways in osteoporosis. Biomed. Pharmacother. 2024, 177, 116954. [Google Scholar] [CrossRef]
- Ng, M.Y.M.; Sham, P.C.; Paterson, A.D.; Chan, V.; Kung, A.W.C. Effect of environmental factors and gender on the heritability of bone mineral density and bone size. Ann. Hum. Genet. 2006, 70 Pt 4, 428–438. [Google Scholar] [CrossRef]
- Ralston, S.H. Genetic control of susceptibility to osteoporosis. J. Clin. Endocrinol. Metab. 2002, 87, 2460–2466. [Google Scholar] [CrossRef]
- Holroyd, C.; Harvey, N.; Dennison, E.; Cooper, C. Epigenetic influences in the developmental origins of osteoporosis. Osteoporos. Int. 2012, 23, 401–410. [Google Scholar] [CrossRef]
- Pouresmaeili, F.; Kamali Dehghan, B.; Kamarehei, M.; Yong Meng, G. A comprehensive overview on osteoporosis and its risk factors. Ther. Clin. Risk Manag. 2018, 14, 2029–2049. [Google Scholar] [CrossRef]
- Morris, J.A.; Kemp, J.P.; Youlten, S.E.; Laurent, L.; Logan, J.G.; Chai, R.C.; Vulpescu, N.A.; Forgetta, V.; Kleinman, A.; Mohanty, S.T.; et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat. Genet. 2019, 51, 258–266. [Google Scholar] [CrossRef]
- Kim, S.K. Identification of 613 new loci associated with heel bone mineral density and a polygenic risk score for bone mineral density, osteoporosis and fracture. PLoS ONE 2018, 13, e0213962. [Google Scholar] [CrossRef]
- Kichaev, G.; Bhatia, G.; Loh, P.-R.; Gazal, S.; Burch, K.; Freund, M.K.; Schoech, A.; Pasaniuc, B.; Price, A.L. Leveraging Polygenic Functional Enrichment to Improve GWAS Power. Am. J. Hum. Genet. 2019, 104, 65–75. [Google Scholar] [CrossRef]
- Kemp, J.P.; Morris, J.A.; Medina-Gomez, C.; Forgetta, V.; Warrington, N.M.; Youlten, S.E.; Zheng, J.; Gregson, C.L.; Grundberg, E.; Trajanoska, K.; et al. Identification of 153 new loci associated with heel bone mineral density and functional involvement of GPC6 in osteoporosis. Nat. Genet. 2017, 49, 1468–1475. [Google Scholar] [CrossRef]
- Bush, W.S.; Moore, J.H. Chapter 11: Genome-Wide Association Studies. PLoS Comput. Biol. 2012, 8, e1002822. [Google Scholar] [CrossRef]
- Spain, S.L.; Barrett, J.C. Strategies for fine-mapping complex traits. Hum. Mol. Genet. 2015, 24, R111–R119. [Google Scholar] [CrossRef]
- Gusev, A.; Ko, A.; Shi, H.; Bhatia, G.; Chung, W.; Penninx, B.W.J.H.; Jansen, R.; de Geus, E.J.C.; Boomsma, D.I.; Wright, F.A.; et al. Integrative approaches for large-scale transcriptome-wide association studies. Nat. Genet. 2016, 48, 245–252. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, F.; Hu, H.; Bakshi, A.; Robinson, M.R.; Powell, J.E.; Montgomery, G.W.; Goddard, M.E.; Wray, N.R.; Visscher, P.M.; et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 2016, 48, 481–487. [Google Scholar] [CrossRef]
- Rivadeneira, F.; Styrkársdottir, U.; Estrada, K.; Halldórsson, B.V.; Hsu, Y.-H.; Richards, J.B.; Zillikens, M.C.; Kavvoura, F.K.; Amin, N.; Aulchenko, Y.S.; et al. Twenty bone mineral density loci identified by large-scale meta-analysis of genome-wide association studies. Nat. Genet. 2009, 41, 1199–1206. [Google Scholar] [CrossRef]
- Pei, Y.-F.; Liu, L.; Liu, T.-L.; Yang, X.-L.; Zhang, H.; Wei, X.-T.; Feng, G.-J.; Hai, R.; Ran, S.; Zhang, L. Joint Association Analysis Identified 18 New Loci for Bone Mineral Density. J. Bone Miner. Res. 2019, 34, 1086–1094. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, M.; Xie, Z.-G.; Liu, J.; Peng, H.-P.; Pei, Y.-F.; Sun, H.-P.; Zhang, L. Twelve New Genomic Loci Associated with Bone Mineral Density. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, F.; Zeng, J.; Wu, Y.; Kemper, K.E.; Xue, A.; Zhang, M.; Powell, J.E.; Goddard, M.E.; Wray, N.R.; et al. Genotype-by-environment interactions inferred from genetic effects on phenotypic variability in the UK Biobank. Sci. Adv. 2019, 5, eaaw3538. [Google Scholar] [CrossRef]
- Manolio, T.A.; Collins, F.S.; Cox, N.J.; Goldstein, D.B.; Hindorff, L.A.; Hunter, D.J.; McCarthy, M.I.; Ramos, E.M.; Cardon, L.R.; Chakravarti, A.; et al. Finding the missing heritability of complex diseases. Nature 2009, 461, 747–753. [Google Scholar] [CrossRef]
- Pers, T.H.; Karjalainen, J.M.; Chan, Y.; Westra, H.-J.; Wood, A.R.; Yang, J.; Lui, J.C.; Vedantam, S.; Gustafsson, S.; Esko, T.; et al. Biological interpretation of genome-wide association studies using predicted gene functions. Nat. Commun. 2015, 6, 5890. [Google Scholar] [CrossRef]
- Benner, C.; Spencer, C.C.A.; Havulinna, A.S.; Salomaa, V.; Ripatti, S.; Pirinen, M. FINEMAP: Efficient variable selection using summary data from genome-wide association studies. Bioinformatics 2016, 32, 1493–1501. [Google Scholar] [CrossRef]
- Kichaev, G.; Yang, W.-Y.; Lindstrom, S.; Hormozdiari, F.; Eskin, E.; Price, A.L.; Kraft, P.; Pasaniuc, B. Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies. PLoS Genet. 2014, 10, e1004722. [Google Scholar] [CrossRef]
- The GTEx Consortium; Aguet, F.; Anand, S.; Ardlie, K.G.; Gabriel, S.; Getz, G.A.; Graubert, A.; Hadley, K.; Handsaker, R.E.; Huang, K.H.; et al. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef]
- Watanabe, K.; Taskesen, E.; Van Bochoven, A.; Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 2017, 8, 1826. [Google Scholar] [CrossRef]
- Hormozdiari, F.; van de Bunt, M.; Segrè, A.V.; Li, X.; Joo, J.W.J.; Bilow, M.; Sul, J.H.; Sankararaman, S.; Pasaniuc, B.; Eskin, E. Colocalization of GWAS and eQTL Signals Detects Target Genes. Am. J. Hum. Genet. 2016, 99, 1245–1260. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Ng, P.C. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003, 31, 3812–3814. [Google Scholar] [CrossRef]
- Li, B.; Krishnan, V.G.; Mort, M.E.; Xin, F.; Kamati, K.K.; Cooper, D.N.; Mooney, S.D.; Radivojac, P. Automated inference of molecular mechanisms of disease from amino acid substitutions. Bioinformatics 2009, 25, 2744–2750. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Ward, L.D.; Kellis, M. HaploReg: A resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012, 40, D930–D934. [Google Scholar] [CrossRef]
- Boyle, A.P.; Hong, E.L.; Hariharan, M.; Cheng, Y.; Schaub, M.A.; Kasowski, M.; Karczewski, K.J.; Park, J.; Hitz, B.C.; Weng, S.; et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012, 22, 1790–1797. [Google Scholar] [CrossRef]
- De Leeuw, C.A.; Mooij, J.M.; Heskes, T.; Posthuma, D. MAGMA: Generalized Gene-Set Analysis of GWAS Data. PLoS Comput. Biol. 2015, 11, e1004219. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38 (Suppl. S2), W214–W220. [Google Scholar] [CrossRef]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-omics approaches to disease. Genome Biol. 2017, 18, 83. [Google Scholar] [CrossRef]
- Choi, S.W.; O’Reilly, P.F. PRSice-2: Polygenic Risk Score software for biobank-scale data. GigaScience 2019, 8, giz082. [Google Scholar] [CrossRef]
- Vilhjálmsson, B.J.; Yang, J.; Finucane, H.K.; Gusev, A.; Lindström, S.; Ripke, S.; Genovese, G.; Loh, P.-R.; Bhatia, G.; Do, R.; et al. Modeling Linkage Disequilibrium Increases Accuracy of Polygenic Risk Scores. Am. J. Hum. Genet. 2015, 97, 576–592. [Google Scholar] [CrossRef]
- Komori, T. Regulation of osteoblast differentiation by transcription factors. J. Cell. Biochem. 2006, 99, 1233–1239. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Ralphs, J.R.; Richards, R.G.; Hayes, J.S. A phenotypic comparison of osteoblast cell lines versus human primary osteoblasts for biomaterials testing. J. Biomed. Mater. Res. 2014, 102, 2636–2643. [Google Scholar] [CrossRef]
- Ansari, S.; Ito, K.; Hofmann, S. Cell Sources for Human In vitro Bone Models. Curr. Osteoporos. Rep. 2021, 19, 88–100. [Google Scholar] [CrossRef]
- Ye, W.; Wang, Y.; Hou, S.; Mei, B.; Liu, X.; Huang, H.; Zhou, Q.; Niu, Y.; Chen, Y.; Zhang, M.; et al. USF3 modulates osteoporosis risk by targeting WNT16, RANKL, RUNX2, and two GWAS lead SNPs rs2908007 and rs4531631. Hum. Mutat. 2021, 42, 37–49. [Google Scholar] [CrossRef]
- Malavašič, P.; Polajžer, S.; Lovšin, N. Anaphase-Promoting Complex Subunit 1 Associates with Bone Mineral Density in Human Osteoporotic Bone. Int. J. Mol. Sci. 2023, 24, 12895. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.-G.; Tan, F.; Liu, J.; Yi, R.; Zhao, X. Three functional polymorphisms in CCDC170 were associated with osteoporosis phenotype. Biol. Open 2021, 10, bio050930. [Google Scholar] [CrossRef]
- Dvorakova, J.; Wiesnerova, L.; Chocholata, P.; Kulda, V.; Landsmann, L.; Cedikova, M.; Kripnerova, M.; Eberlova, L.; Babuska, V. Human cells with osteogenic potential in bone tissue research. BioMed Eng. OnLine 2023, 22, 33. [Google Scholar] [CrossRef]
- Wischmann, J.; Lenze, F.; Thiel, A.; Bookbinder, S.; Querido, W.; Schmidt, O.; Burgkart, R.; Von Eisenhart-Rothe, R.; Richter, G.H.S.; Pleshko, N.; et al. Matrix mineralization controls gene expression in osteoblastic cells. Exp. Cell Res. 2018, 372, 25–34. [Google Scholar] [CrossRef]
- Chesi, A.; Wagley, Y.; Johnson, M.E.; Manduchi, E.; Su, C.; Lu, S.; Leonard, M.E.; Hodge, K.M.; Pippin, J.A.; Hankenson, K.D.; et al. Genome-scale Capture C promoter interactions implicate effector genes at GWAS loci for bone mineral density. Nat. Commun. 2019, 10, 1260. [Google Scholar] [CrossRef]
- Määttä, J.A.; Bendre, A.; Laanti, M.; Büki, K.G.; Rantakari, P.; Tervola, P.; Saarimäki, J.; Poutanen, M.; Härkönen, P.; Väänänen, K. Fam3c modulates osteogenic cell differentiation and affects bone volume and cortical bone mineral density. BoneKEy Rep. 2016, 5, 787. [Google Scholar] [CrossRef]
- Kaur, G.; Pippin, J.A.; Chang, S.; Redmond, J.; Chesi, A.; Wells, A.D.; Maerz, T.; Grant, S.F.A.; Coleman, R.M.; Hankenson, K.D.; et al. Osteoporosis GWAS-implicated DNM3 locus contextually regulates osteoblastic and chondrogenic fate of mesenchymal stem/progenitor cells through oscillating miR-199a-5p levels. JBMR Plus 2024, 8, ziae051. [Google Scholar] [CrossRef]
- Bieback, K.; Schallmoser, K.; Klüter, H.; Strunk, D. Clinical Protocols for the Isolation and Expansion of Mesenchymal Stromal Cells. Transfus. Med. Hemother. 2008, 35, 286–294. [Google Scholar] [CrossRef]
- Camernik, K.; Zupan, J. Complete Assessment of Multilineage Differentiation Potential of Human Skeletal Muscle-Derived Mesenchymal Stem/Stromal Cells. Methods Mol. Biol. 2019, 2045, 131–144. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Grotheer, V.; Skrynecki, N.; Oezel, L.; Windolf, J.; Grassmann, J. Osteogenic differentiation of human mesenchymal stromal cells and fibroblasts differs depending on tissue origin and replicative senescence. Sci. Rep. 2021, 11, 11968. [Google Scholar] [CrossRef]
- Kodama, J.; Kaito, T. Osteoclast Multinucleation: Review of Current Literature. Int. J. Mol. Sci. 2020, 21, 5685. [Google Scholar] [CrossRef]
- Gallois, A.; Lachuer, J.; Yvert, G.; Wierinckx, A.; Brunet, F.; Rabourdin-Combe, C.; Delprat, C.; Jurdic, P.; Mazzorana, M. Genome-wide expression analyses establish dendritic cells as a new osteoclast precursor able to generate bone-resorbing cells more efficiently than monocytes. J. Bone Miner. Res. 2010, 25, 661–672. [Google Scholar] [CrossRef]
- Kodrič, K.; Zupan, J.; Kranjc, T.; Komadina, R.; Mlakar, V.; Marc, J.; Lovšin, N. Sex-determining region Y (SRY) attributes to gender differences in RANKL expression and incidence of osteoporosis. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef]
- Roca-Ayats, N.; Martínez-Gil, N.; Cozar, M.; Gerousi, M.; Garcia-Giralt, N.; Ovejero, D.; Mellibovsky, L.; Nogués, X.; Díez-Pérez, A.; Grinberg, D.; et al. Functional characterization of the C7ORF76 genomic region, a prominent GWAS signal for osteoporosis in 7q21.3. Bone 2019, 123, 39–47. [Google Scholar] [CrossRef]
- Cossarizza, A.; Chang, H.; Radbruch, A.; Abrignani, S.; Addo, R.; Akdis, M.; Andrä, I.; Andreata, F.; Annunziato, F.; Arranz, E.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (third edition). Eur. J. Immunol. 2021, 51, 2708–3145. [Google Scholar] [CrossRef]
- Meital, L.T.; Coward, A.S.; Windsor, M.T.; Bailey, T.G.; Kuballa, A.; Russell, F.D. A simple and effective method for the isolation and culture of human monocytes from small volumes of peripheral blood. J. Immunol. Methods 2019, 472, 75–78. [Google Scholar] [CrossRef]
- Cui, C.; Schoenfelt, K.Q.; Becker, K.M.; Becker, L. Isolation of polymorphonuclear neutrophils and monocytes from a single sample of human peripheral blood. STAR Protoc. 2021, 2, 100845. [Google Scholar] [CrossRef]
- Miltenyi, S.; Müller, W.; Weichel, W.; Radbruch, A. High gradient magnetic cell separation with MACS. Cytometry 1990, 11, 231–238. [Google Scholar] [CrossRef]
- Nielsen, M.C.; Andersen, M.N.; Møller, H.J. Monocyte isolation techniques significantly impact the phenotype of both isolated monocytes and derived macrophages in vitro. Immunology 2020, 159, 63–74. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone Resorption by Osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Alonso, N.; Estrada, K.; Albagha, O.M.E.; Herrera, L.; Reppe, S.; Olstad, O.K.; Gautvik, K.M.; Ryan, N.M.; Evans, K.L.; Nielson, C.M.; et al. Identification of a novel locus on chromosome 2q13, which predisposes to clinical vertebral fractures independently of bone density. Ann. Rheum. Dis. 2018, 77, 378–385. [Google Scholar] [CrossRef]
- Kramer, I.; Baertschi, S.; Halleux, C.; Keller, H.; Kneissel, M. Mef2c deletion in osteocytes results in increased bone mass. J. Bone Miner. Res. 2012, 27, 360–373. [Google Scholar] [CrossRef]
- Sebastian, A.; Hum, N.R.; Morfin, C.; Murugesh, D.K.; Loots, G.G. Global gene expression analysis identifies Mef2c as a potential player in Wnt16-mediated transcriptional regulation. Gene 2018, 675, 312–321. [Google Scholar] [CrossRef]
- Regenerative Medicine Institute, National Centre for Biomedical Engineering Science, National University of Ireland, Galway; Czekanska, E.; Stoddart, M.; Richards, R.; Hayes, J. In search of an osteoblast cell model for in vitro research. eCM 2012, 24, 1–17. [Google Scholar] [CrossRef]
- Bicer, M.; Cottrell, G.S.; Widera, D. Impact of 3D cell culture on bone regeneration potential of mesenchymal stromal cells. Stem Cell Res. Ther. 2021, 12, 31. [Google Scholar] [CrossRef]
- Cesarz, Z.; Tamama, K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 9176357. [Google Scholar] [CrossRef]
- Koblenzer, M.; Weiler, M.; Fragoulis, A.; Rütten, S.; Pufe, T.; Jahr, H. Physiological Mineralization during In Vitro Osteogenesis in a Biomimetic Spheroid Culture Model. Cells 2022, 11, 2702. [Google Scholar] [CrossRef]
- Favi, P.M.; Benson, R.S.; Neilsen, N.R.; Hammonds, R.L.; Bates, C.C.; Stephens, C.P.; Dhar, M.S. Cell proliferation, viability, and in vitro differentiation of equine mesenchymal stem cells seeded on bacterial cellulose hydrogel scaffolds. Mater. Sci. Eng. C 2013, 33, 1935–1944. [Google Scholar] [CrossRef]
- Ho, S.S.; Murphy, K.C.; Binder, B.Y.K.; Vissers, C.B.; Leach, J.K. Increased Survival and Function of Mesenchymal Stem Cell Spheroids Entrapped in Instructive Alginate Hydrogels. Stem Cells Transl. Med. 2016, 5, 773–781. [Google Scholar] [CrossRef]
- Frith, J.E.; Thomson, B.; Genever, P.G. Dynamic Three-Dimensional Culture Methods Enhance Mesenchymal Stem Cell Properties and Increase Therapeutic Potential. Tissue Eng. Part C Methods 2010, 16, 735–749. [Google Scholar] [CrossRef]
- Lo, Y.-P.; Liu, Y.-S.; Rimando, M.G.; Ho, J.H.-C.; Lin, K.; Lee, O.K. Three-dimensional spherical spatial boundary conditions differentially regulate osteogenic differentiation of mesenchymal stromal cells. Sci. Rep. 2016, 6, 21253. [Google Scholar] [CrossRef]
- Borciani, G.; Montalbano, G.; Baldini, N.; Cerqueni, G.; Vitale-Brovarone, C.; Ciapetti, G. Co–culture systems of osteoblasts and osteoclasts: Simulating in vitro bone remodeling in regenerative approaches. Acta Biomater. 2020, 108, 22–45. [Google Scholar] [CrossRef]
- Guillaumin, S.; Rossoni, A.; Zeugolis, D. State-of the-art and future perspective in co-culture systems for tendon engineering. Biomater. Biosyst. 2025, 17, 100110. [Google Scholar] [CrossRef]
- Cao, S.; Li, H.; Li, K.; Lu, J.; Zhang, L. In vitro mineralization of MC3T3-E1 osteoblast-like cells on collagen/nano-hydroxyapatite scaffolds coated carbon/carbon composites. J. Biomed. Mater. Res. 2016, 104, 533–543. [Google Scholar] [CrossRef]
- Marino, S.; Bishop, R.T.; De Ridder, D.; Delgado-Calle, J.; Reagan, M.R. 2D and 3D In Vitro Co-Culture for Cancer and Bone Cell Interaction Studies. In Bone Research Protocols; Idris, A.I., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1914, pp. 71–98. ISBN 978-1-4939-8996-6. Available online: http://link.springer.com/10.1007/978-1-4939-8997-3_5 (accessed on 14 May 2025).
- Brommage, R. Genetic Approaches to Identifying Novel Osteoporosis Drug Targets. J. Cell. Biochem. 2015, 116, 2139–2145. [Google Scholar] [CrossRef]
- Wu, N.; Liu, B.; Du, H.; Zhao, S.; Li, Y.; Cheng, X.; Wang, S.; Lin, J.; Zhou, J.; Qiu, G.; et al. The Progress of CRISPR/Cas9-Mediated Gene Editing in Generating Mouse/Zebrafish Models of Human Skeletal Diseases. Comput. Struct. Biotechnol. J. 2019, 17, 954–962. [Google Scholar] [CrossRef]
- Back, S.; Manfredi, J.J. Knockdown of Target Genes by siRNA In Vitro. In Cell Cycle Checkpoints; Manfredi, J.J., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2267, pp. 159–163. ISBN 978-1-07-161216-3. [Google Scholar]
- Taxman, D.J.; Moore, C.B.; Guthrie, E.H.; Huang, M.T.-H. Short Hairpin RNA (shRNA): Design, Delivery, and Assessment of Gene Knockdown. In RNA Therapeutics; Sioud, M., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 629, pp. 139–156. ISBN 978-1-60761-656-6. Available online: https://link.springer.com/10.1007/978-1-60761-657-3_10 (accessed on 14 May 2025).
- Nascimento, S.D.; Mansur, F.; Rocha, F.; Marti, L. siRNA transfection and shRNA transduction optimization for CXCL12 mRNA silencing in human bone marrow mesenchymal stem cells. Authorea 2024. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Fitzgerald, J. Applications of CRISPR for musculoskeletal research. Bone Jt. Res. 2020, 9, 351–359. [Google Scholar] [CrossRef]
- Chen, L.; Shi, K.; Qiu, W.; Aagaard, L.; Kassem, M. Generation of Inducible CRISPRi and CRISPRa Human Stromal/Stem Cell Lines for Controlled Target Gene Transcription during Lineage Differentiation. Stem Cells Int. 2020, 2020, 8857344. [Google Scholar] [CrossRef]
- Chong, Z.X.; Yeap, S.K.; Ho, W.Y. Transfection types, methods and strategies: A technical review. PeerJ 2021, 9, e11165. [Google Scholar] [CrossRef]
- Fu, Y.; Han, Z.; Cheng, W.; Niu, S.; Wang, T.; Wang, X. Improvement strategies for transient gene expression in mammalian cells. Appl. Microbiol. Biotechnol. 2024, 108, 480. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, L.; Li, Z.; Qiu, Q.; Wang, H.; Huang, X.; Ma, D. Impact of Different Cell Types on the Osteogenic Differentiation Process of Mesenchymal Stem Cells. Stem Cells Int. 2025, 2025, 5551222. [Google Scholar] [CrossRef]
- Mollentze, J.; Durandt, C.; Pepper, M.S. An In Vitro and In Vivo Comparison of Osteogenic Differentiation of Human Mesenchymal Stromal/Stem Cells. Stem Cells Int. 2021, 2021, 9919361. [Google Scholar] [CrossRef]
- Huang, W. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci. 2007, 12, 3068–3092. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Osteoblast Differentiation by Runx2. In Osteoimmunology: Advances in Experimental Medicine and Biology; Choi, Y., Ed.; Springer: Boston, MA, USA, 2009; Volume 658, pp. 43–49. Available online: http://link.springer.com/10.1007/978-1-4419-1050-9_5 (accessed on 12 May 2025).
- Greenblatt, M.B.; Tsai, J.N.; Wein, M.N. Bone Turnover Markers in the Diagnosis and Monitoring of Metabolic Bone Disease. Clin. Chem. 2017, 63, 464–474. [Google Scholar] [CrossRef]
- Sun, H.; Ye, F.; Wang, J.; Shi, Y.; Tu, Z.; Bao, J.; Qin, M.; Bu, H.; Li, Y. The Upregulation of Osteoblast Marker Genes in Mesenchymal Stem Cells Prove the Osteoinductivity of Hydroxyapatite/Tricalcium Phosphate Biomaterial. Transplant. Proc. 2008, 40, 2645–2648. [Google Scholar] [CrossRef]
- Trivedi, S.; Srivastava, K.; Gupta, A.; Saluja, T.S.; Kumar, S.; Mehrotra, D.; Singh, S.K. A quantitative method to determine osteogenic differentiation aptness of scaffold. J. Oral Biol. Craniofacial Res. 2020, 10, 158–160. [Google Scholar] [CrossRef]
- Xiang, Q.; Li, L.; Ji, W.; Gawlitta, D.; Walboomers, X.F.; Van Den Beucken, J.J.J.P. Beyond resorption: Osteoclasts as drivers of bone formation. Cell Regen 2024, 13, 22. [Google Scholar] [CrossRef]
- Zainal Ariffin, S.H.; Megat Abdul Wahab, R.; Abdul Razak, M.; Yazid, M.D.; Shahidan, M.A.; Miskon, A.; Zainol Abidin, I.Z. Evaluation of in vitro osteoblast and osteoclast differentiation from stem cell: A systematic review of morphological assays and staining techniques. PeerJ 2024, 12, e17790. [Google Scholar] [CrossRef]
- Boscaro, D.; Sikorski, P. Spheroids as a 3D in vitro model to study bone and bone mineralization. Biomater. Adv. 2024, 157, 213727. [Google Scholar] [CrossRef]
- Bernar, A.; Gebetsberger, J.V.; Bauer, M.; Streif, W.; Schirmer, M. Optimization of the Alizarin Red S Assay by Enhancing Mineralization of Osteoblasts. Int. J. Mol. Sci. 2022, 24, 723. [Google Scholar] [CrossRef]
- Borciani, G.; Montalbano, G.; Baldini, N.; Vitale-Brovarone, C.; Ciapetti, G. Protocol of Co-Culture of Human Osteoblasts and Osteoclasts to Test Biomaterials for Bone Tissue Engineering. Methods Protoc. 2022, 5, 8. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, H.; He, H.; Ma, C. Assays for alkaline phosphatase activity: Progress and prospects. TrAC Trends Anal. Chem. 2019, 113, 32–43. [Google Scholar] [CrossRef]
- Morin, L.G. Direct Colorimetric Determination of Serum Calcium with o-Cresolphthalein Complexon. Am. J. Clin. Pathol. 1974, 61, 114–117. [Google Scholar] [CrossRef]
- Chaichanasakul, T.; Kang, B.; Bezouglaia, O.; Aghaloo, T.L.; Tetradis, S. Diverse Osteoclastogenesis of Bone Marrow from Mandible Versus Long Bone. J. Periodontol. 2014, 85, 829–836. [Google Scholar] [CrossRef]
- Bernhardt, A.; Koperski, K.; Schumacher, M.; Gelinsky, M. Relevance of osteoclast-specific enzyme activities in cell-based in vitro resorption assays. eCM 2017, 33, 28–42. [Google Scholar] [CrossRef]
- Stein, M.; Elefteriou, F.; Busse, B.; Fiedler, I.A.; Kwon, R.Y.; Farrell, E.; Ahmad, M.; Ignatius, A.; Grover, L.; Geris, L.; et al. Why Animal Experiments Are Still Indispensable in Bone Research: A Statement by the European Calcified Tissue Society. J. Bone Miner. Res. 2023, 38, 1045–1061. [Google Scholar] [CrossRef]
- Wiese, K.E.; Nusse, R.; Van Amerongen, R. Wnt signalling: Conquering complexity. Development 2018, 145, dev165902. [Google Scholar] [CrossRef]
- Koh, N.Y.Y.; Miszkiewicz, J.J.; Fac, M.L.; Wee, N.K.Y.; Sims, N.A. Preclinical Rodent Models for Human Bone Disease, Including a Focus on Cortical Bone. Endocr. Rev. 2024, 45, 493–520. [Google Scholar] [CrossRef]
- Jilka, R.L. The Relevance of Mouse Models for Investigating Age-Related Bone Loss in Humans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 1209–1217. [Google Scholar] [CrossRef]
- Bergen, D.J.M.; Kague, E.; Hammond, C.L. Zebrafish as an Emerging Model for Osteoporosis: A Primary Testing Platform for Screening New Osteo-Active Compounds. Front. Endocrinol. 2019, 10, 6. [Google Scholar] [CrossRef]
- Kague, E.; Karasik, D. Functional Validation of Osteoporosis Genetic Findings Using Small Fish Models. Genes 2022, 13, 279. [Google Scholar] [CrossRef]
- Sousa, S.; Valerio, F.; Jacinto, A. A new zebrafish bone crush injury model. Biol. Open 2012, 1, 915–921. [Google Scholar] [CrossRef]
- Ohlsson, C.; Henning, P.; Nilsson, K.H.; Wu, J.; Gustafsson, K.L.; Sjögren, K.; Törnqvist, A.; Koskela, A.; Zhang, F.-P.; Lagerquist, M.K.; et al. Inducible Wnt16 inactivation: WNT16 regulates cortical bone thickness in adult mice. J. Endocrinol. 2018, 237, 113–122. [Google Scholar] [CrossRef]
- McGowan, L.M.; Kague, E.; Vorster, A.; Newham, E.; Cross, S.; Hammond, C.L. Wnt16 Elicits a Protective Effect Against Fractures and Supports Bone Repair in Zebrafish. JBMR Plus 2021, 5, e10461. [Google Scholar] [CrossRef]
- Qu, X.; Liao, M.; Liu, W.; Cai, Y.; Yi, Q.; Long, J.; Tan, L.; Deng, Y.; Deng, H.; Chen, X. Loss of Wnt16 Leads to Skeletal Deformities and Downregulation of Bone Developmental Pathway in Zebrafish. Int. J. Mol. Sci. 2021, 22, 6673. [Google Scholar] [CrossRef]
- Watson, C.J.; Tang, W.J.; Rojas, M.F.; Fiedler, I.A.K.; Morfin Montes De Oca, E.; Cronrath, A.R.; Callies, L.K.; Swearer, A.A.; Ahmed, A.R.; Sethuraman, V.; et al. wnt16 regulates spine and muscle morphogenesis through parallel signals from notochord and dermomyotome. PLoS Genet. 2022, 18, e1010496. [Google Scholar] [CrossRef]
- Karasik, D.; Hsu, Y.-H.; Zhou, Y.; Cupples, L.A.; Kiel, D.P.; Demissie, S. Genome-wide pleiotropy of osteoporosis-related phenotypes: The framingham study. J. Bone Miner. Res. 2010, 25, 1555–1563. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, J.; Jia, Z.; Ha, P.; Soo, C.; Ting, K.; James, A.W.; Shi, B.; Zhang, X. NELL-1 in Genome-Wide Association Studies across Human Diseases. Am. J. Pathol. 2022, 192, 395–405. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Sig. Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- James, A.W.; Shen, J.; Zhang, X.; Asatrian, G.; Goyal, R.; Kwak, J.H.; Jiang, L.; Bengs, B.; Culiat, C.T.; Turner, A.S.; et al. NELL-1 in the treatment of osteoporotic bone loss. Nat. Commun. 2015, 6, 7362. [Google Scholar] [CrossRef]
- Bogoevski, K.; Woloszyk, A.; Blackwood, K.; Woodruff, M.A.; Glatt, V. Tissue Morphology and Antigenicity in Mouse and Rat Tibia: Comparing 12 Different Decalcification Conditions. J. Histochem. Cytochem. 2019, 67, 545–561. [Google Scholar] [CrossRef]
- Simpson, H.; Augat, P. Experimental Research Methods in Orthopedics and Trauma; Georg Thieme: Stuttgart, Germany, 2015; p. b-003-121999. ISBN 978-3-13-173111-1. Available online: http://www.thieme-connect.de/DOI/DOI?10.1055/b-003-121999 (accessed on 14 May 2025).
- Poutoglidou, F.; Saitis, A.; Pourzitaki, C.; Kouvelas, D. An Improved Method for Isolating High-Quality RNA From Rat Bone at Room Temperature Without the Need for Specialized Equipment. Cureus 2021, 13, e13806. [Google Scholar] [CrossRef]
- Pagani, S.; Maglio, M.; Sicuro, L.; Fini, M.; Giavaresi, G.; Brogini, S. RNA Extraction from Cartilage: Issues, Methods, Tips. Int. J. Mol. Sci. 2023, 24, 2120. [Google Scholar] [CrossRef]
- Xu, F.; Li, W.; Yang, X.; Na, L.; Chen, L.; Liu, G. The Roles of Epigenetics Regulation in Bone Metabolism and Osteoporosis. Front. Cell Dev. Biol. 2021, 8, 619301. [Google Scholar] [CrossRef]
- Ito, M. Assessment of bone quality using micro-computed tomography (micro-CT) and synchrotron micro-CT. J. Bone Miner. Metab. 2005, 23, 115–121. [Google Scholar] [CrossRef]
- Müller, R.; Rüegsegger, P. Micro-Tomographic Imaging for the Nondestructive evaluation of Trabecular Bone Architecture. In Studies in Health Technology and Informatics; IOS Press: Amsterdam, The Netherlands, 1997; Available online: https://www.medra.org/servlet/aliasResolver?alias=iospressISSNISBN&issn=0926-9630&volume=40&spage=61 (accessed on 14 May 2025).
- Mo, C.; Romero-Suarez, S.; Bonewald, L.; Johnson, M.; Brotto, M. Prostaglandin E2: From Clinical Applications to Its Potential Role in Bone- Muscle Crosstalk and Myogenic Differentiation. Recent Patents Biotechnol. 2012, 6, 223–229. [Google Scholar] [CrossRef]
- Kawai, M.; De Paula, F.J.A.; Rosen, C.J. New insights into osteoporosis: The bone–fat connection. J. Intern. Med. 2012, 272, 317–329. [Google Scholar] [CrossRef]
- Morris, J.A.; Sun, J.S.; Sanjana, N.E. Next-generation forward genetic screens: Uniting high-throughput perturbations with single-cell analysis. Trends Genet. 2024, 40, 118–133. [Google Scholar] [CrossRef]
- Park, B.-S.; Lee, M.; Kim, J.; Kim, T. Perturbomics: CRISPR–Cas screening-based functional genomics approach for drug target discovery. Exp. Mol. Med. 2025. [Google Scholar] [CrossRef]
- Long, E.; Wan, P.; Chen, Q.; Lu, Z.; Choi, J. From function to translation: Decoding genetic susceptibility to human diseases via artificial intelligence. Cell Genom. 2023, 3, 100320. [Google Scholar] [CrossRef]
- Bek, J.W.; Shochat, C.; De Clercq, A.; De Saffel, H.; Boel, A.; Metz, J.; Rodenburg, F.; Karasik, D.; Willaert, A.; Coucke, P.J. Lrp5 Mutant and Crispant Zebrafish Faithfully Model Human Osteoporosis, Establishing the Zebrafish as a Platform for CRISPR-Based Functional Screening of Osteoporosis Candidate Genes. J. Bone Miner. Res. 2020, 36, 1749–1764. [Google Scholar] [CrossRef]
- Pippin, J.A.; Chesi, A.; Wagley, Y.; Su, C.; Pahl, M.C.; Hodge, K.M.; Johnson, M.E.; Wells, A.D.; Hankenson, K.D.; Grant, S.F.A. CRISPR-CAS9–Mediated Genome Editing Confirms EPDR1 as an Effector Gene at the BMD GWAS Implicated ‘STARD3NL’ Locus. JBMR Plus 2021, 5, e10531. [Google Scholar] [CrossRef]
- Al-Barghouthi, B.M.; Rosenow, W.T.; Du, K.-P.; Heo, J.; Maynard, R.; Mesner, L.; Calabrese, G.; Nakasone, A.; Senwar, B.; Gerstenfeld, L.; et al. Transcriptome-wide association study and eQTL colocalization identify potentially causal genes responsible for human bone mineral density GWAS associations. eLife 2022, 11, e77285. [Google Scholar] [CrossRef]
- Jung, J.; Wu, Q. Revealing the Organ-Specific Expression of SPTBN1 using Single-Cell RNA Sequencing Analysis. bioRxiv 2023. [Google Scholar] [CrossRef]
- Tanaka, K.; Xue, Y.; Nguyen-Yamamoto, L.; Morris, J.A.; Kanazawa, I.; Sugimoto, T.; Wing, S.S.; Richards, J.B.; Goltzman, D. FAM210A is a novel determinant of bone and muscle structure and strength. Proc. Natl. Acad. Sci. USA 2018, 115, E3759–E3768. [Google Scholar] [CrossRef]

| Cell Line | Species | Cell Type | Differentiation Potential | Mineralization | 3D Culture Feasibility |
|---|---|---|---|---|---|
| HOS | Human | Osteoblast-like | Limited | Yes (under specific conditions) | Moderate |
| Saos-2 | Human | Mature osteoblast-like | Limited | Yes | High |
| MG-63 | Human | Pre-osteoblast | Limited | Low | Moderate |
| U2OS | Human | Osteosarcoma | Very limited | No | Low |
| MC3T3-E1 | Mouse | Pre-osteoblast | High (osteoblast lineage) | Yes | High |
| RAW264.7 | Mouse | Monocyte/macrophage | Differentiates into osteoclasts | Resorption | Moderate |
| THP-1 | Human | monocyte | Differentiates into osteoclasts | Resorption | Moderate |
| MSCs (primary) | Human | Mesenchymal stem cell | Osteogenic, chondrogenic, adipogenic | Yes (after induction) | High |
| hFOB 1.19 | Human | Immortalized osteoblast | High at permissive temperature | Yes | Moderate |
| CAL-72 | Human | Osteosarcoma | Limited | No | Low |
| TE-85 | Human | Osteosarcoma | Limited | No | Low |
| Model | Advantages | Limitations | Recommended Applications |
|---|---|---|---|
| Primary MSCs (bone-derived) | High physiological relevance; multilineage differentiation potential | Limited proliferation; donor variability; difficult to genetically manipulate | Functional validation of osteogenic genes gene expression profiling; differentiation studies |
| Primary monocytes (blood- or bone-derived) | Easily accessible; physiologically relevant; can generate mature osteoclasts | Fragile; difficult to genetically manipulate; batch variability | Osteoclastogenesis assays; gene expression; TRAP activity studies |
| Immortalized osteoblast-like and monocyte cell lines | Easy handling; unlimited proliferation; transfection and gene editing | Altered phenotype; reduced mineralization potential lower physiological relevance | Initial mechanistic screening; siRNA/shRNA or CRISPR studies; gene overexpression/knockdown |
| 3D spheroid | Mimics tissue-like environment; enhances osteogenesis; allows co-culture setups | Technically demanding; lower throughput; limited standardization | Bone remodeling studies; osteoblast–osteoclast interaction; scaffold testing |
| Mouse models (knock-out, knock-in, transgenic) | Whole-organism context; skeletal phenotype assessment; strong genetic tools | High cost; time-consuming | In vivo validation of gene function; developmental and systemic effect studies |
| Zebrafish (knock-out, knock-in, transgenic) | Transparent embryos; rapid bone development; easy genetic manipulation; regeneration studies | Lack of long bones and bone marrow; limited translational equivalence | Fast in vivo gene function screening; developmental studies; skeletal regeneration assays |
| Method | Advantages | Limitations | Recommended Applications |
| CRISPR/Cas9 Knock-out | Permanent gene disruption; high specificity; enables loss-of-function studies | Off-target effects; requires clonal selection; may induce compensatory pathways | Functional validation of essential genes; early developmental pathway analysis |
| CRISPR interference (CRISPRi) | Reversible gene silencing; targets non-coding regions; no DNA cleavage | Requires stable dCas9 expression; incomplete silencing possible | Regulation of enhancers/promoters; dose-dependent gene suppression |
| CRISPR activation (CRISPRa) | Gene upregulation from endogenous locus; no need for cDNA overexpression | Efficiency depends on chromatin context; requires guide RNA design and dCas9 fusion systems | Functional gain-of-function studies; promoter/enhancer mapping |
| RNA interference (siRNA) | Fast, transient gene knockdown; easy to apply in most cell lines | Off-target effects; transient; may not fully deplete target mRNA | Initial screening; pathway studies; short-term gene function testing |
| shRNA (short hairpin RNA) | Stable knockdown via integration; allows long-term silencing | Time-consuming cloning; potential for off-target effects; variable expression | Long-term gene silencing in immortalized or primary cells |
| Plasmid overexpression | Easy to design; widely used; applicable to many cell lines | Non-physiological expression levels; transient in most systems | Gain-of-function studies; rescue experiments |
| Marker | Stage of Expression | Function |
|---|---|---|
| SOX9 | Early | Transcription factor marking mesenchymal precursors |
| RUNX2 | Early to intermediate | Master regulator of osteoblast differentiation |
| ALP | Intermediate | Enzyme involved in the onset of matrix mineralization |
| COL1A1 | Early to late | Major structural protein of bone extracellular matrix |
| OSX (SP7) | Intermediate to late | Essential transcription factor for osteoblast maturation |
| OCN (BGLAP) | Late | Marker of mature osteoblasts; involved in bone mineralization |
| OPN (SPP1) | Late | Mediates cell adhesion and matrix remodeling |
| BSP | Late | Binds calcium; important for initial stages of mineral deposition |
| DLX5 | Early to intermediate | Promotes osteogenesis via signaling pathways such as Notch |
| ATF4 | Intermediate to late | Regulates osteoblast function and inhibits osteoclast differentiation |
| Gene | Study Approach | Function | Reference |
|---|---|---|---|
| ANAPC1 | -The expression of the ANAPC1 gene was examined in the human bone and muscle tissue samples from osteoporotic, osteoarthritic, and healthy individuals by quantitative PCR (q-PCR) -Osteogenic and adipogenic differentiation of MSCs -Silencing of ANAPC1 in HOS cells | ANAPC1 plays a role in bone physiology and osteoporosis development, with decreased expression in osteoporotic patients and altered expression during osteogenic differentiation of human mesenchymal stem cells. | [55] |
| CCDC170 | -Cloning of the different SNP alleles into a luciferase reporter vector, transfecting cells with the vectors along with miRNA mimics/inhibitors, and performing luciferase reporter assays -RNA isolation, cDNA synthesis, and qRT-PCR to measure gene expression levels -ELISA assays to measure protein levels of osteogenesis and osteoclastogenesis markers -In vivo mouse experiments with CCDC170 knockdown | The CCDC170 gene, through its interaction with microRNAs and specific genetic polymorphisms, plays a significant role in bone health and the risk of osteoporosis. | [56] |
| LRP5 | CRISPR/Cas9 gene editing, using a gRNA with high predicted out-of-frame efficiency | LRP5 acts as a co-receptor in the Wnt signaling pathway, binding Wnt ligands and interacting with Frizzled. Loss of LRP5 function leads to impaired Wnt signaling and reduced osteoblast differentiation. | [145] |
| USF3 | Overexpression and knockdown in U-2OS cells, luciferase reporter assay, biotin pull-down | Transcriptional regulator of osteogenesis and osteoclastogenesis. | [54] |
| EPDR1 | -CRISPR-Cas9 genome editing in osteoblast cells to delete the region containing the BMD-associated variants -Measurement of EPDR1 gene and protein expression in the edited cells using RT-qPCR and Western blotting -Assessment of alkaline phosphatase activity, a marker of osteoblast differentiation, in the edited cells | EPDR1 plays a key role in osteoblast differentiation and bone mineral density determination. | [146] |
| miR-199a-5p | Overexpression of miR-199a-5p in human mesenchymal stem/progenitor cells | miR-199a-5p regulates the terminal fate specification of MSCs into osteoblasts or chondrocytes, with overexpression favoring chondrogenic differentiation. | [61] |
| PPP6R3 | Deletion of Ppp6r3 gene in mice, TWAS/colocalization approach using GTEx | PPP6R3 is a regulatory subunit of protein phosphatase 6. Ppp6r deletion in mice decreased BMD. | [147] |
| SPTBN1 | Single-cell RNA sequencing | SPTBN1 is a cytoskeleton protein that contributes to organ development by establishing and maintaining cell structure and regulating various cellular functions. It is also involved in bone structure development and fracture healing. | [148] |
| FAM210A | Fam210a knock-out mice, to study the effects of Fam210a on bone and muscle biology -X-Gal staining to detect Fam210a expression in mouse tissues -Phenotypic analyses in the mouse models, including measurements of bone mineral density, bone biomechanical properties, muscle function, and gene expression | The function of the FAM210A protein is to regulate both bone and muscle structure and function. | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malavašič, P.; Lojk, J.; Lovšin, M.N.; Marc, J. Recent Advances in Experimental Functional Characterization of GWAS Candidate Genes in Osteoporosis. Int. J. Mol. Sci. 2025, 26, 7237. https://doi.org/10.3390/ijms26157237
Malavašič P, Lojk J, Lovšin MN, Marc J. Recent Advances in Experimental Functional Characterization of GWAS Candidate Genes in Osteoporosis. International Journal of Molecular Sciences. 2025; 26(15):7237. https://doi.org/10.3390/ijms26157237
Chicago/Turabian StyleMalavašič, Petra, Jasna Lojk, Marija Nika Lovšin, and Janja Marc. 2025. "Recent Advances in Experimental Functional Characterization of GWAS Candidate Genes in Osteoporosis" International Journal of Molecular Sciences 26, no. 15: 7237. https://doi.org/10.3390/ijms26157237
APA StyleMalavašič, P., Lojk, J., Lovšin, M. N., & Marc, J. (2025). Recent Advances in Experimental Functional Characterization of GWAS Candidate Genes in Osteoporosis. International Journal of Molecular Sciences, 26(15), 7237. https://doi.org/10.3390/ijms26157237






