Enantioseparation of Proton Pump Inhibitors by HPLC on Polysaccharide-Type Stationary Phases: Enantiomer Elution Order Reversal, Thermodynamic Characterization, and Hysteretic Effect
Abstract
1. Introduction
2. Results and Discussions
2.1. Enantioseparation Screening in Pure Eluents
2.2. Enantiomer Elution Order Reversal
2.3. Thermodynamic Study of the Enantioseparations
2.4. Multivariate Statistical Analysis and Selection of Minimal Representative CSP Set
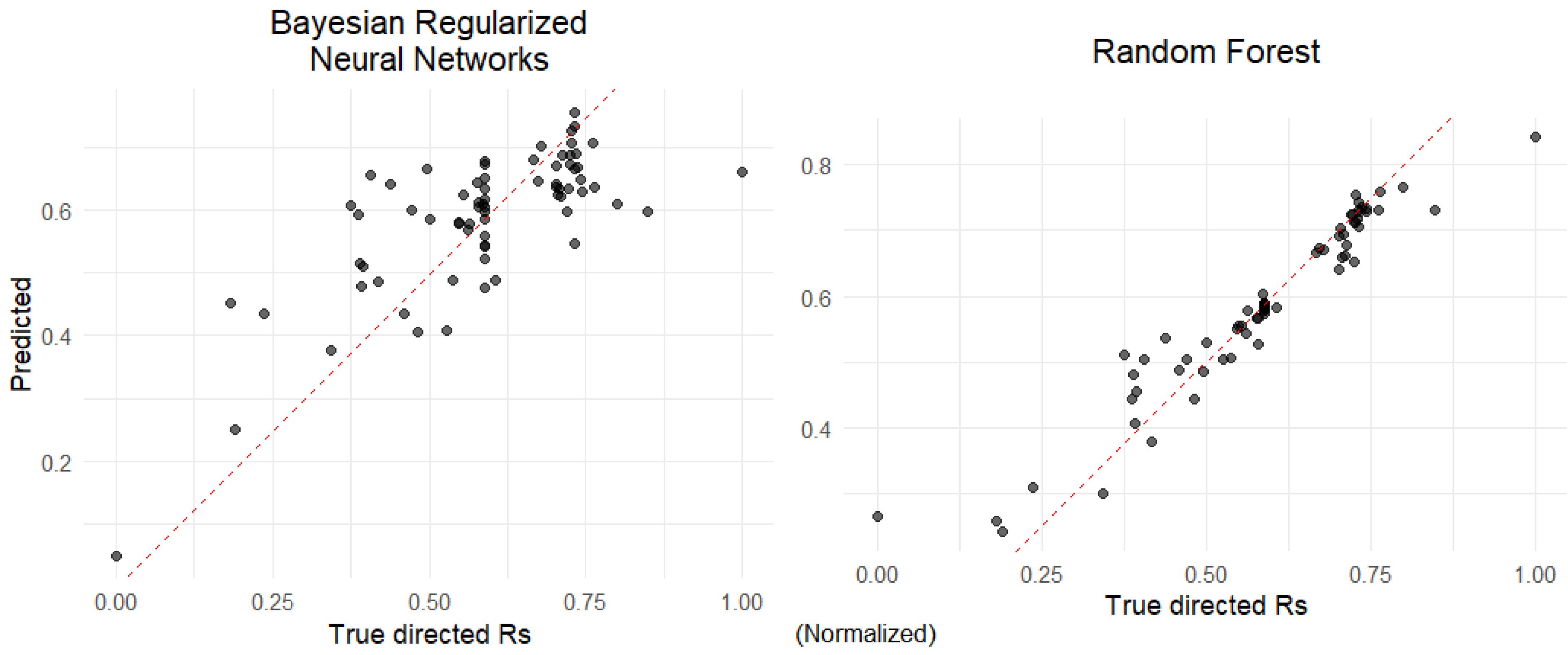
2.5. Machine Learning for Predicting Directed Resolution
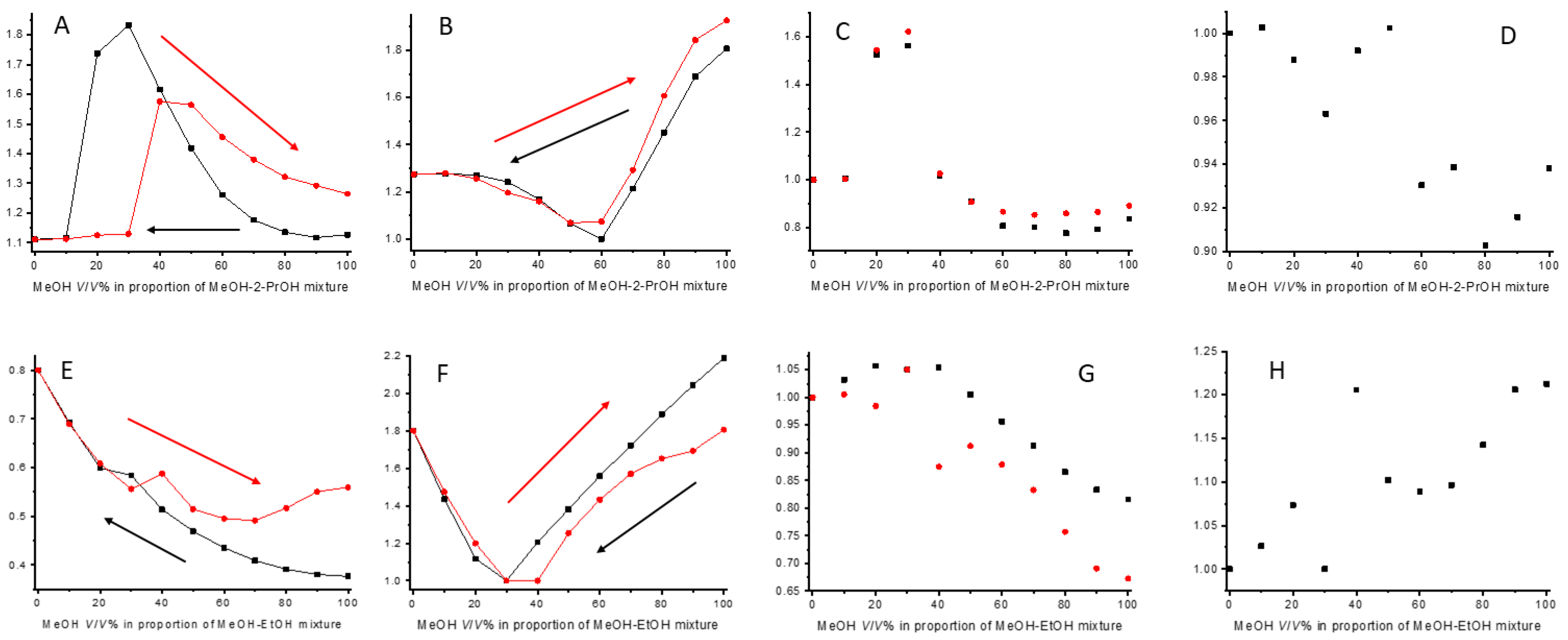
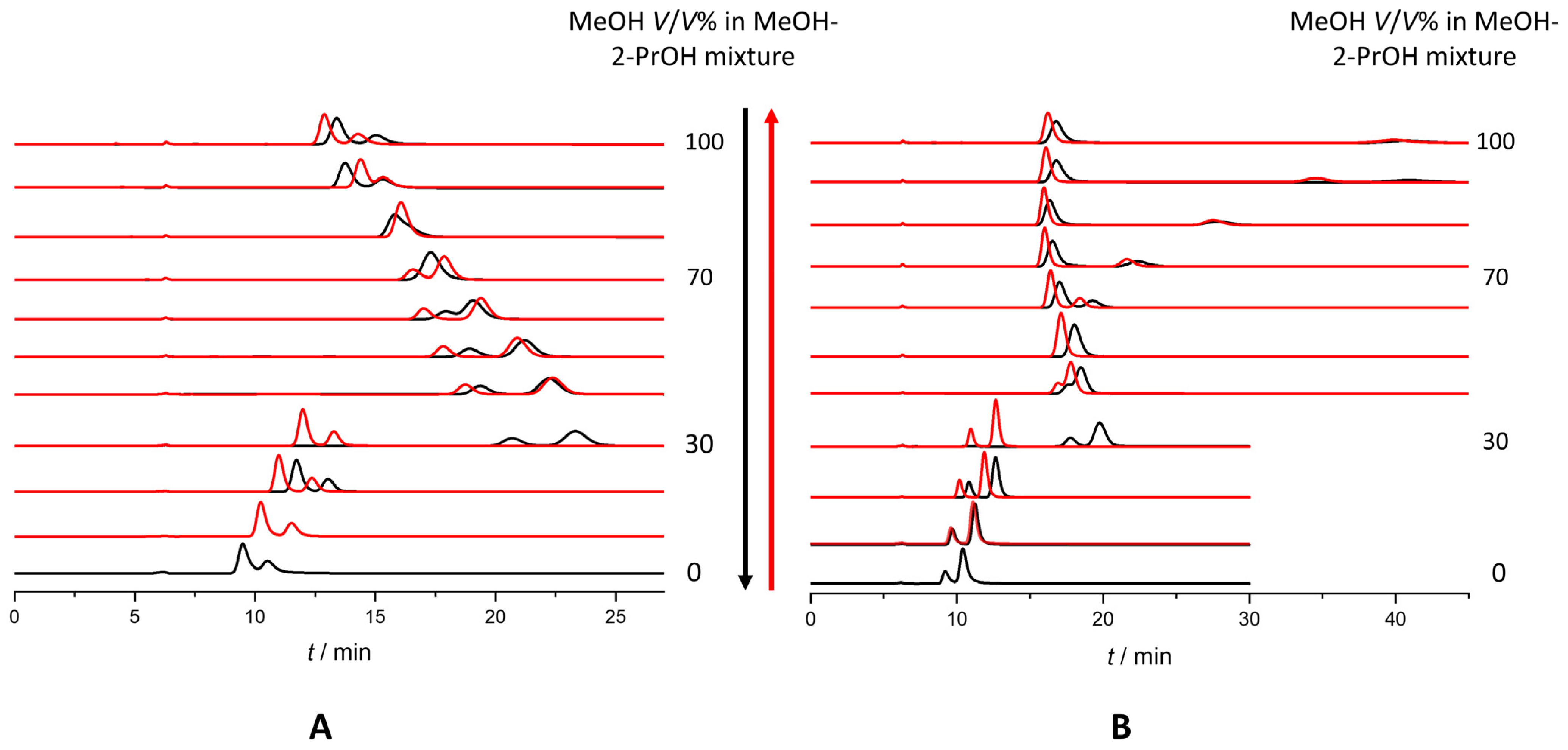
2.6. Use of Solvent Mixtures as Mobile Phases: Hysteresis of Retention and Selectivity
- (A) The two enantiomers separate (let kA < kB, accordingly EEO A > B);
- (B) Coelution occurs (kA = kB).
- υA = υB, meaning αforward = αreverse.
- υA > υB, i.e., υA/υB > 1, which can only occur if kAreverse < kBreverse and either kAforward > kAreverse or kBforward < kBreverse. This means that either enantiomer A elutes earlier or enantiomer B elutes later, leading to an increase in selectivity.
- υA < υB, i.e., υA/υB < 1, which can only occur if kAreverse ≥ kBreverse. If equality holds, the two enantiomers coelute, whereas if kAreverse > kBreverse, an EEO reversal occurs.
- υA = υB, meaning αforward = αreverse = 0.
- υA > υB, i.e., υA/υB > 1, which can only happen if kAreverse < kBreverse, meaning the elution order is A > B.
- υA < υB, i.e., υA/υB < 1, which can only occur if kAreverse > kBreverse, meaning the elution order is B > A.
2.7. Semi-Preparative Separations
3. Materials and Methods
3.1. Materials
3.2. HPLC Instrumentation and Conditions
3.3. Multivariate Analysis
3.3.1. Data Preparation
3.3.2. Molecular Descriptor Extraction
3.3.3. Visualization and Correlation Analysis
3.3.4. Clustering and Principal Component Analysis (PCA)
3.3.5. Machine Learning Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chankvetadze, B. Recent trends in preparation, investigation and application of polysaccharide-based chiral stationary phases for separation of enantiomers in high-performance liquid chromatography. TrAC Trends Anal. Chem. 2020, 122, 115709. [Google Scholar] [CrossRef]
- Chankvetadze, B.; Kartozia, I.; Yamamoto, C.; Okamoto, Y. Comparative enantioseparation of selected chiral drugs on four different polysaccharide-type chiral stationary phases using polar organic mobile phases. J. Pharm. Biomed. Anal. 2002, 27, 467–478. [Google Scholar] [CrossRef]
- Dessi, A.; Sechi, B.; Dallocchio, R.; Chankvetadze, B.; Perez-Baeza, M.; Cossu, S.; Mamane, V.; Pale, P.; Peluso, P. Comparative enantioseparation of planar chiral ferrocenes on polysaccharide-based chiral stationary phases. Chirality 2022, 34, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Dobo, M.; Foroughbakhshfasaei, M.; Horvath, P.; Szabo, Z.I.; Toth, G. Chiral separation of oxazolidinone analogues by liquid chromatography on polysaccharide stationary phases using polar organic mode. J. Chromatogr. A 2022, 1662, 462741. [Google Scholar] [CrossRef]
- Foroughbakhshfasaei, M.; Dobo, M.; Boda, F.; Szabo, Z.I.; Toth, G. Comparative Chiral Separation of Thalidomide Class of Drugs Using Polysaccharide-Type Stationary Phases with Emphasis on Elution Order and Hysteresis in Polar Organic Mode. Molecules 2021, 27, 111. [Google Scholar] [CrossRef]
- Peluso, P.; Mamane, V.; Dallocchio, R.; Dessi, A.; Cossu, S. Noncovalent interactions in high-performance liquid chromatography enantioseparations on polysaccharide-based chiral selectors. J. Chromatogr. A 2020, 1623, 461202. [Google Scholar] [CrossRef] [PubMed]
- Sechi, B.; Mamane, V.; Dallocchio, R.; Dessì, A.; Cossu, S.; Jibuti, G.; Peluso, P. Enantioseparation of new axially chiral carboxylic acids on polysaccharide-based chiral stationary phases under normal phase elution conditions. J. Pharm. Biomed. Anal. Open 2023, 1, 100011. [Google Scholar] [CrossRef]
- Tanacs, D.; Orosz, T.; Szakonyi, Z.; Le, T.M.; Fulop, F.; Lindner, W.; Ilisz, I.; Peter, A. High-performance liquid chromatographic enantioseparation of isopulegol-based ss-amino lactone and ss-amino amide analogs on polysaccharide-based chiral stationary phases focusing on the change of the enantiomer elution order. J. Chromatogr. A 2020, 1621, 461054. [Google Scholar] [CrossRef]
- Nehra, A.K.; Alexander, J.A.; Loftus, C.G.; Nehra, V. Proton Pump Inhibitors: Review of Emerging Concerns. Mayo Clin. Proc. 2018, 93, 240–246. [Google Scholar] [CrossRef]
- Klinkenberg-Knol, E.C.; Jansen, J.M.; Festen, H.P.; Meuwissen, S.G.; Lamers, C.B. Double-blind multicentre comparison of omeprazole and ranitidine in the treatment of reflux oesophagitis. Lancet 1987, 1, 349–351. [Google Scholar] [CrossRef]
- Calcaterra, A.; D’Acquarica, I. The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 2018, 147, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Hancu, G.; Modroiu, A. Chiral Switch: Between Therapeutical Benefit and Marketing Strategy. Pharmaceuticals 2022, 15, 240. [Google Scholar] [CrossRef]
- Bui, C.V.; Rosenau, T.; Hettegger, H. Polysaccharide- and beta-Cyclodextrin-Based Chiral Selectors for Enantiomer Resolution: Recent Developments and Applications. Molecules 2021, 26, 4322. [Google Scholar] [CrossRef]
- Gumustas, M.; Ozkan, S.A.; Chankvetadze, B. Analytical and Preparative Scale Separation of Enantiomers of Chiral Drugs by Chromatography and Related Methods. Curr. Med. Chem. 2018, 25, 4152–4188. [Google Scholar] [CrossRef]
- Ibrahim, A.E.; El Gohary, N.A.; Aboushady, D.; Samir, L.; Karim, S.E.A.; Herz, M.; Salman, B.I.; Al-Harrasi, A.; Hanafi, R.; El Deeb, S. Recent advances in chiral selectors immobilization and chiral mobile phase additives in liquid chromatographic enantio-separations: A review. J. Chromatogr. A 2023, 1706, 464214. [Google Scholar] [CrossRef]
- Ferencz, E.; Kovacs, B.; Boda, F.; Foroughbakhshfasaei, M.; Kelemen, E.K.; Toth, G.; Szabo, Z.I. Simultaneous determination of chiral and achiral impurities of ivabradine on a cellulose tris(3-chloro-4-methylphenylcarbamate) chiral column using polar organic mode. J. Pharm. Biomed. Anal. 2020, 177, 112851. [Google Scholar] [CrossRef]
- Vankatova, P.; Kalikova, K.; Kubickova, A. Advantages of polar organic solvent chromatography for enantioseparation of chiral liquid crystals. J. Chromatogr. A 2023, 1709, 464383. [Google Scholar] [CrossRef]
- Arafah, R.S.; Ribeiro, A.E.; Rodrigues, A.E.; Pais, L.S. Separation of Nadolol Stereoisomers Using Chiralpak IA Chiral Stationary Phase. Chirality 2016, 28, 399–408. [Google Scholar] [CrossRef]
- Cheng, L.; Cai, J.; Fu, Q.; Ke, Y. Efficient preparative separation of 6-(4-aminophenyl)-5-methyl-4, 5-dihydro-3(2H)-pyridazinone enantiomers on polysaccharide-based stationary phases in polar organic solvent chromatography and supercritical fluid chromatography. J. Sep. Sci. 2019, 42, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.A.; Hancu, G.; Gyéresi, A.; Kelemen, H.; Szabó, Z.I.; Noszál, B.; Dubsky, P.; Tóth, G. Chiral separation of lansoprazole and rabeprazole by capillary electrophoresis using dual cyclodextrin systems. Electrophoresis 2019, 40, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- del Nozal, M.J.; Toribio, L.; Bernal, J.L.; Alonso, C.; Jimenez, J.J. Chiral separation of omeprazole and several related benzimidazoles using supercritical fluid chromatography. J. Sep. Sci. 2004, 27, 1023–1029. [Google Scholar] [CrossRef]
- Cirilli, R.; Ferretti, R.; Gallinella, B.; De Santis, E.; Zanitti, L.; La Torre, F. High-performance liquid chromatography enantioseparation of proton pump inhibitors using the immobilized amylose-based Chiralpak IA chiral stationary phase in normal-phase, polar organic and reversed-phase conditions. J. Chromatogr. A 2008, 1177, 105–113. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Z.; Guo, X.; Di, X.; Yu, J. Enantioseparation and modelling study of six proton pump inhibitors on a novel 3, 5-dichloro-phenylcarbamated β-cyclodextrin chemically bonded chiral stationary phase by high performance liquid chromatography. Microchem. J. 2021, 166, 106211. [Google Scholar] [CrossRef]
- Papp, L.A.; Hancu, G.; Kelemen, H.; Toth, G. Chiral separation in the class of proton pump inhibitors by chromatographic and electromigration techniques: An overview. Electrophoresis 2021, 42, 1761–1789. [Google Scholar] [CrossRef]
- Horvath, S.; Nemeth, G. Hysteresis of retention and enantioselectivity on amylose tris(3,5-dimethylphenylcarbamate) chiral stationary phases in mixtures of 2-propanol and methanol. J. Chromatogr. A 2018, 1568, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Nemeti, G.; Berkecz, R.; Le, T.M.; Szakonyi, Z.; Peter, A.; Ilisz, I. High-performance liquid chromatographic enantioseparation of azole analogs of monoterpene lactones and amides focusing on the separation characteristics of polysaccharide-based chiral stationary phases. J. Chromatogr. A 2024, 1717, 464660. [Google Scholar] [CrossRef] [PubMed]
- Toribio, L.; Bernal, J.L.; del Nozal, M.J.; Jimenez, J.J.; Nieto, E.M. Applications of the Chiralpak AD and Chiralcel OD chiral columns in the enantiomeric separation of several dioxolane compounds by supercritical fluid chromatography. J. Chromatogr. A 2001, 921, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Grecso, N.; Palko, M.; Fulop, F.; Lindner, W.; Peter, A. Structural and temperature effects on enantiomer separations of bicyclo[2.2.2]octane-based 3-amino-2-carboxylic acids on cinchona alkaloid-based zwitterionic chiral stationary phases. J. Pharm. Biomed. Anal. 2014, 98, 130–139. [Google Scholar] [CrossRef]
- Dombi, G.; Tyukodi, L.; Dobo, M.; Molnar, G.; Rozmer, Z.; Szabo, Z.I.; Fiser, B.; Toth, G. Enantioselective Binding of Proton Pump Inhibitors to Alpha1-Acid Glycoprotein and Human Serum Albumin-A Chromatographic, Spectroscopic, and In Silico Study. Int. J. Mol. Sci. 2024, 25, 10575. [Google Scholar] [CrossRef]
- Wickham, H. Reshaping Data with the reshape Package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation, R package version 1.1.4. 2025. Available online: https://dplyr.tidyverse.org (accessed on 22 July 2025).
- Slenter, D.N.B.C.; van Iersel, M.P.; Eijssen, L.M.T.; Evelo, C.T. PubChemR: Interface to the PubChem Database. 2022, Volume 16, pp. 150–186. Available online: https://cran.r-project.org/web/packages/PubChemR/PubChemR.pdf (accessed on 23 July 2025).
- Cao, Y.; Charisi, A.; Cheng, L.-C.; Jiang, T.; Girke, T. ChemmineR: A compound mining framework for R. Bioinformatics 2008, 24, 1733–1734. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.-S.; Xiao, N.; Xu, Q.-S.; Chen, A.F. Rcpi: R/Bioconductor package to generate various descriptors of proteins, compounds and their interactions. Bioinformatics 2015, 31, 279–281. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Auguie, B. gridExtra: Functions in Grid Graphics. 2017. Available online: https://CRAN.R-project.org/package=gridExtra (accessed on 23 July 2025).
- Wilke, C.O. cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’. 2025. Available online: https://wilkelab.r-universe.dev/cowplot#:~:text=The%20cowplot%20package%20provides%20various,or%20mix%20plots%20with%20images (accessed on 23 July 2025).
- Galili, T. dendextend: An R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Fabian, M. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. 2020. Available online: https://kassambara.r-universe.dev/factoextra (accessed on 23 July 2025).
- Ramey, J. brnn: Bayesian Regularized Neural Networks. 2021. Available online: https://cran.r-project.org/web/packages/brnn/brnn.pdf (accessed on 23 July 2025).
- Liaw, A.; Wiener, M. Classification and Regression by RandomForest. Forest 2001, 2, 18–22. [Google Scholar]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Microsoft Corporation; Weston, S. doParallel: Foreach Parallel Adaptor for the ‘parallel’ Package. 2022. Available online: https://revolutionanalytics.r-universe.dev/doParallel (accessed on 23 July 2025).
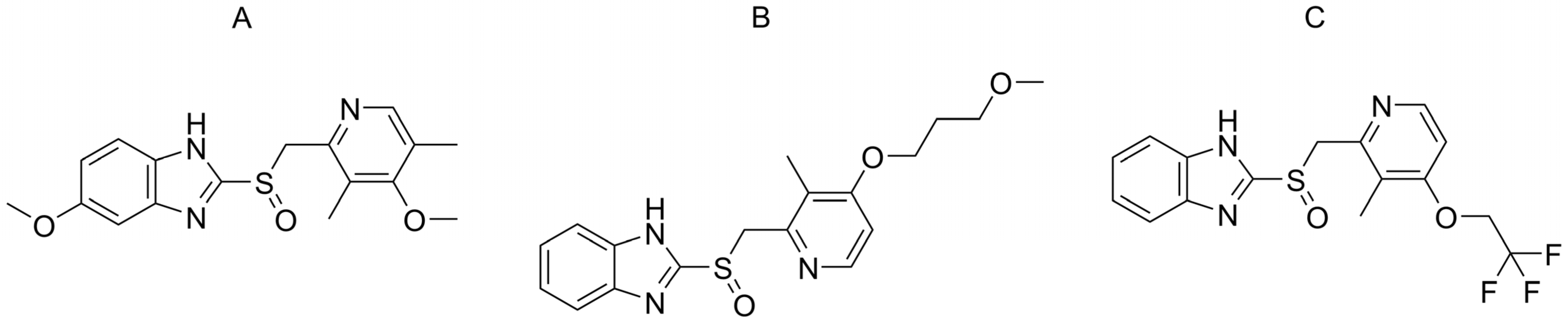
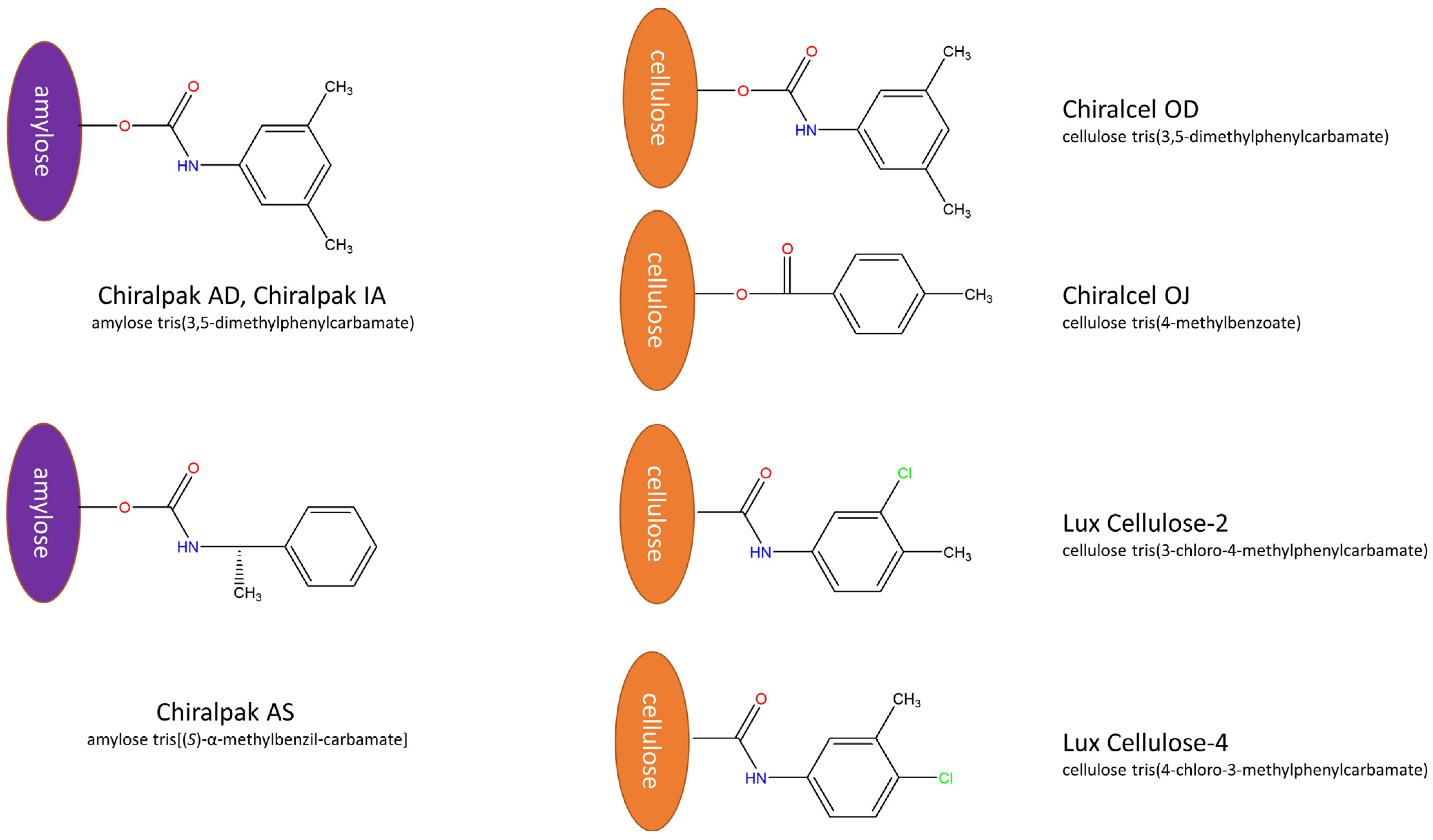
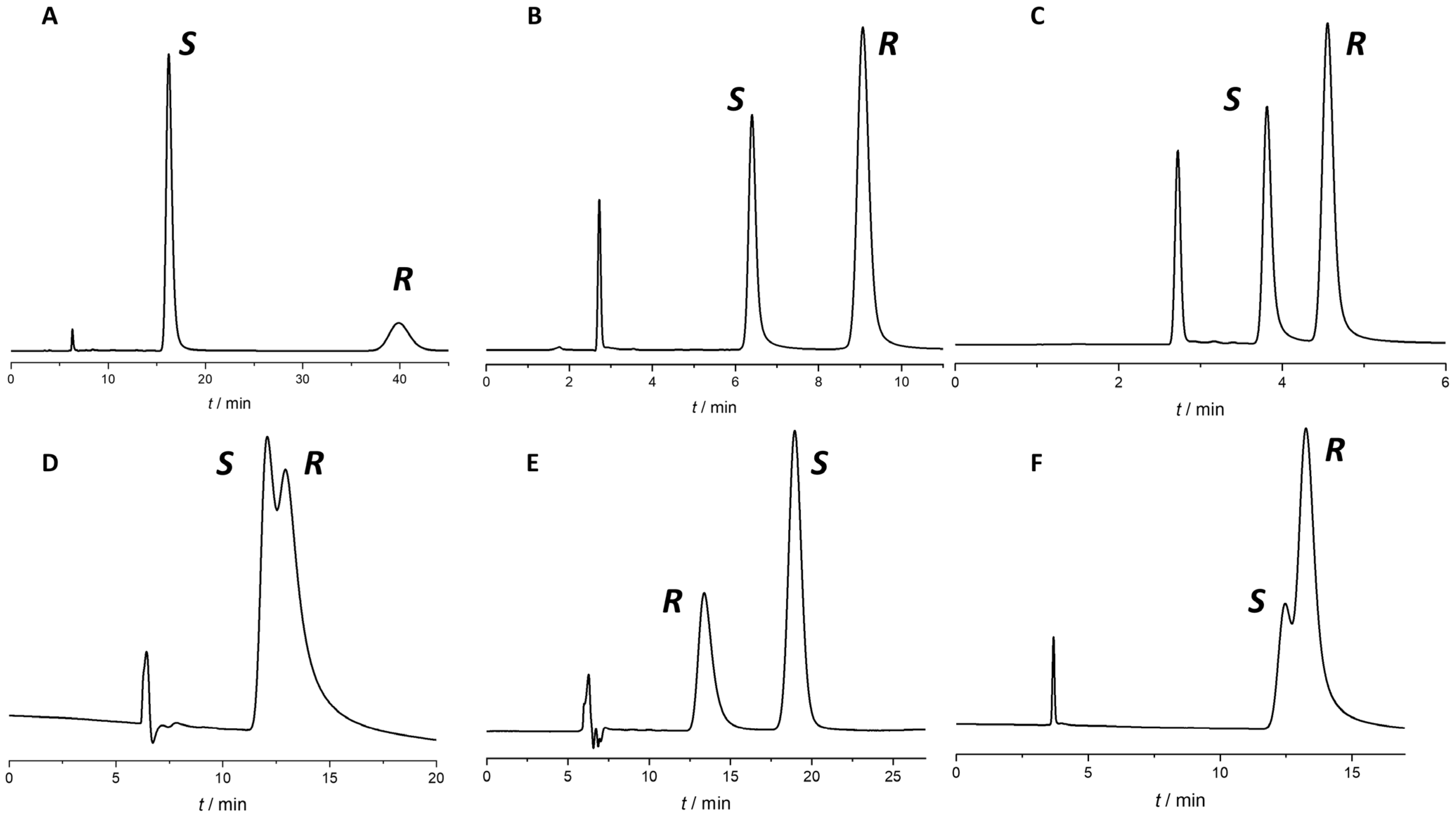

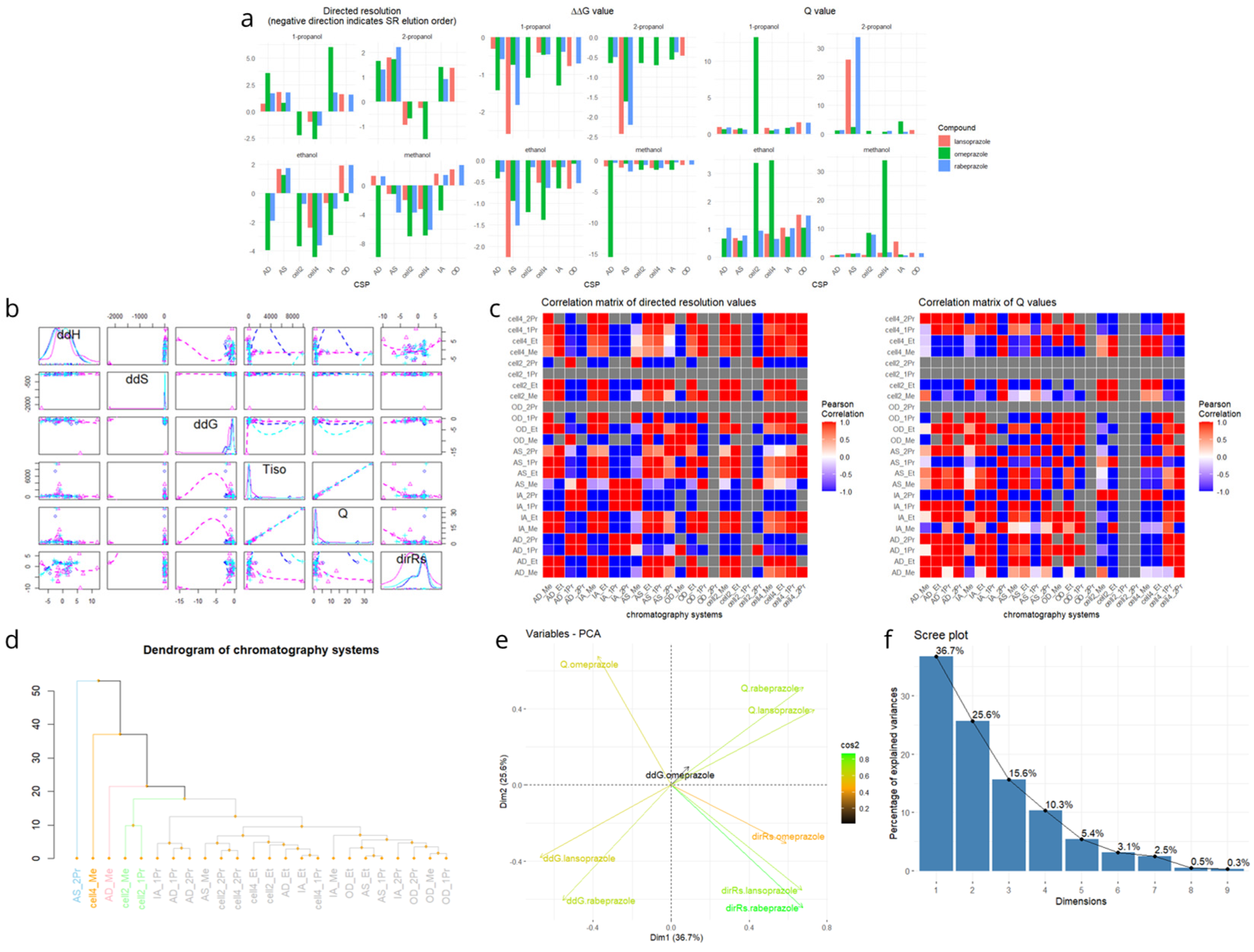
| Omeprazol | Rabeprazol | Lansoprazol | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Column | Eluent | k1 | k2 | Rs | EEO | k1 | k2 | Rs | EEO | k1 | k2 | Rs | EEO |
| OD | MeOH | 0.20 | 0.20 | - | - | 0.23 | 0.43 | 2.83 | R-S | 0.04 | 0.16 | 2.25 | R-S |
| EtOH | 0.42 | 0.47 | 0.57 | S-R | 0.51 | 0.69 | 1.95 | R-S | 0.33 | 0.48 | 1.92 | R-S | |
| 1-PrOH | 0.43 | 0.43 | - | - | 0.48 | 0.69 | 1.55 | R-S | 0.39 | 0.58 | 1.60 | R-S | |
| 2-PrOH | 0.88 | 0.88 | - | - | 1.17 | 1.17 | - | - | 0.87 | 1.24 | 1.38 | R-S | |
| Cell2 | MeOH | 1.02 | 1.90 | 7.02 | S-R | 1.54 | 2.11 | 3.76 | S-R | 0.53 | 0.68 | 2.00 | S-R |
| EtOH | 1.32 | 2.18 | 3.69 | S-R | 2.39 | 2.60 | 0.74 | S-R | 0.93 | 0.93 | - | - | |
| 1-PrOH | 1.54 | 2.41 | 2.24 | S-R | 2.90 | 2.90 | - | - | 1.32 | 1.32 | - | - | |
| 2-PrOH | 3.77 | 4.89 | 0.68 | S-R | 6.69 | 6.69 | - | - | 2.81 | 3.65 | 0.94 | S-R | |
| OJ | MeOH | 0.23 | 0.23 | - | - | 0.18 | 0.18 | - | - | 0.07 | 0.07 | - | - |
| EtOH | 0.22 | 0.22 | - | - | 0.15 | 0.15 | - | - | 0.05 | 0.05 | - | - | |
| 1-PrOH | 0.27 | 0.27 | - | - | 0.19 | 0.19 | - | - | 0.13 | 0.13 | - | - | |
| 2-PrOH | 0.41 | 0.41 | - | - | 0.31 | 0.31 | - | - | 0.23 | 0.23 | - | - | |
| Cell4 | MeOH | 0.85 | 1.79 | 6.89 | S-R | 1.35 | 2.33 | 6.16 | S-R | 0.40 | 0.67 | 3.26 | S-R |
| EtOH | 1.27 | 3.03 | 4.44 | S-R | 2.56 | 4.11 | 3.62 | S-R | 0.82 | 1.33 | 2.41 | S-R | |
| 1-PrOH | 1.45 | 2.45 | 2.60 | S-R | 3.21 | 3.97 | 1.36 | S-R | 1.08 | 1.37 | 0.97 | S-R | |
| 2-PrOH | 3.87 | 5.69 | 1.52 | S-R | 6.54 | 6.54 | - | - | 3.30 | 3.73 | 0.26 | S-R | |
| AD | MeOH | 1.77 | 5.88 | 9.91 | S-R | 1.19 | 1.41 | 1.27 | R-S | 0.53 | 0.79 | 1.33 | R-S |
| EtOH | 1.82 | 2.65 | 3.95 | S-R | 1.80 | 2.14 | 1.93 | S-R | 1.02 | 1.02 | - | - | |
| 1-PrOH | 1.13 | 2.02 | 3.59 | R-S | 0.913 | 1.16 | 1.67 | R-S | 0.59 | 0.67 | 0.72 | R-S | |
| 2-PrOH | 0.53 | 0.75 | 1.65 | R-S | 0.573 | 0.76 | 1.30 | R-S | 0.35 | 0.35 | - | - | |
| AS | MeOH | 0.41 | 0.54 | 1.18 | S-R | 0.38 | 0.85 | 3.72 | S-R | 0.14 | 0.24 | 1.19 | S-R |
| EtOH | 0.52 | 0.73 | 1.27 | R-S | 0.41 | 0.69 | 1.75 | R-S | 0.15 | 0.36 | 1.68 | R-S | |
| 1-PrOH | 0.52 | 0.72 | 0.80 | R-S | 0.33 | 0.71 | 1.76 | R-S | 0.19 | 0.53 | 1.82 | R-S | |
| 2-PrOH | 1.15 | 2.18 | 1.73 | R-S | 0.76 | 1.77 | 2.22 | R-S | 0.43 | 1.08 | 1.80 | R-S | |
| IA | MeOH | 1.32 | 2.26 | 3.45 | S-R | 0.78 | 0.90 | 1.44 | R-S | 0.35 | 0.46 | 1.65 | R-S |
| EtOH | 1.39 | 1.84 | 2.92 | S-R | 1.29 | 1.42 | 1.08 | S-R | 0.64 | 0.69 | 0.68 | S-R | |
| 1-PrOH | 0.78 | 1.55 | 6.02 | R-S | 0.87 | 1.07 | 1.75 | R-S | 0.56 | 0.56 | - | - | |
| 2-PrOH | 0.87 | 1.09 | 1.41 | R-S | 0.99 | 1.15 | 0.91 | R-S | 0.64 | 0.64 | - | - | |
| Column | Alcohol | Rationale |
|---|---|---|
| Chiralpak AS | 2-PrOH | Most distant branch in dendrogram; unique behavior |
| Cellulose-4 | MeOH | Orthogonal to amylose; strong enantioselectivity |
| Chiralcel OD | 1-PrOH | Representative of moderate selectivity group |
| Chiralpak IA | EtOH | Bridges IA/AD cluster; distinct profile |
| Lux Cellulose-2 | EtOH | Covers Lux Cellulose-2 cluster with non-redundant behavior |
| Collected Enantiomers | Extraction Rate | Purity (%) |
|---|---|---|
| R-omeprazole | 88% | 99.93% |
| S-rabeprazole | 74% | 99.89% |
| S-lansoprazole | 43% | 99.85% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobó, M.; Molnár, G.; Mhammad, A.; Dombi, G.; Mirzahosseini, A.; Szabó, Z.-I.; Tóth, G. Enantioseparation of Proton Pump Inhibitors by HPLC on Polysaccharide-Type Stationary Phases: Enantiomer Elution Order Reversal, Thermodynamic Characterization, and Hysteretic Effect. Int. J. Mol. Sci. 2025, 26, 7217. https://doi.org/10.3390/ijms26157217
Dobó M, Molnár G, Mhammad A, Dombi G, Mirzahosseini A, Szabó Z-I, Tóth G. Enantioseparation of Proton Pump Inhibitors by HPLC on Polysaccharide-Type Stationary Phases: Enantiomer Elution Order Reversal, Thermodynamic Characterization, and Hysteretic Effect. International Journal of Molecular Sciences. 2025; 26(15):7217. https://doi.org/10.3390/ijms26157217
Chicago/Turabian StyleDobó, Máté, Gergely Molnár, Ali Mhammad, Gergely Dombi, Arash Mirzahosseini, Zoltán-István Szabó, and Gergő Tóth. 2025. "Enantioseparation of Proton Pump Inhibitors by HPLC on Polysaccharide-Type Stationary Phases: Enantiomer Elution Order Reversal, Thermodynamic Characterization, and Hysteretic Effect" International Journal of Molecular Sciences 26, no. 15: 7217. https://doi.org/10.3390/ijms26157217
APA StyleDobó, M., Molnár, G., Mhammad, A., Dombi, G., Mirzahosseini, A., Szabó, Z.-I., & Tóth, G. (2025). Enantioseparation of Proton Pump Inhibitors by HPLC on Polysaccharide-Type Stationary Phases: Enantiomer Elution Order Reversal, Thermodynamic Characterization, and Hysteretic Effect. International Journal of Molecular Sciences, 26(15), 7217. https://doi.org/10.3390/ijms26157217








