1. Introduction
Helicobacter pylori is a highly adapted Gram-negative bacterium that colonizes the human gastric mucosa. The infection is typically acquired in childhood and often persists for decades [
1]. Its presence is associated with a broad spectrum of gastroduodenal diseases, ranging from chronic gastritis and peptic ulcer disease to mucosa-associated lymphoid tissue (MALT) lymphoma and gastric adenocarcinoma [
2]. While classical studies have emphasized bacterial virulence factors such as cytotoxin-associated gene A (CagA) and vacuolating cytotoxin A (VacA), increasing attention has been directed toward the host’s endogenous modulators of immunity and epithelial integrity, particularly galectins. Galectins are a family of β-galactoside-binding lectins that exhibit diverse immunological and structural functions in both innate and adaptive immune responses [
3,
4]. Within the gastric mucosa, galectins participate in pathogen recognition, immune cell regulation, epithelial barrier stabilization, and modulation of inflammation and tissue remodeling [
4]. Their expression is dynamically regulated during
H. pylori infection and varies by age, tissue compartment, and inflammatory context. This review explores the multifaceted roles of key galectin family members—Galectin-1 (Gal-1), Galectin-2 (Gal-2), Galectin-3 (Gal-3), Galectin-8 (Gal-8), and Galectin-9 (Gal-9)—in shaping host–pathogen interactions, mediating mucosal defense, and contributing to either containment or persistence of infection as well as their implications in the early stages of gastric carcinogenesis.
In summary, this review provides a comprehensive and integrative overview of the multifaceted roles of galectins in H. pylori infection, emphasizing their dual capacity to enhance mucosal immunity while also contributing to bacterial persistence and immune evasion. By consolidating current evidence on galectin-mediated modulation of host–pathogen interactions, the review offers novel insights into potential diagnostic biomarkers and therapeutic targets that could improve clinical management of H. pylori-associated diseases.
2. Galectins: Conserved Structure, Diverse Functions, and Unique Classification
Galectins represent a distinct group of β-galactoside-binding lectins encoded by the
LGALS gene family [
1]. These proteins are evolutionarily conserved yet functionally diverse, defined by their specific affinity for glycoconjugates containing β-galactoside linkages, such as N-acetyllactosamine [
2]. Although 22 galectin genes have been identified across mammalian species, only 16 are known to be expressed in human tissues, with galectins-5, -6, -11, -15, -19, and -20 absent in humans [
2].
Despite sharing a conserved structural framework, particularly within their carbohydrate recognition domains (CRDs), galectins exhibit a broad range of biological roles that reflect both evolutionary conservation and specialization [
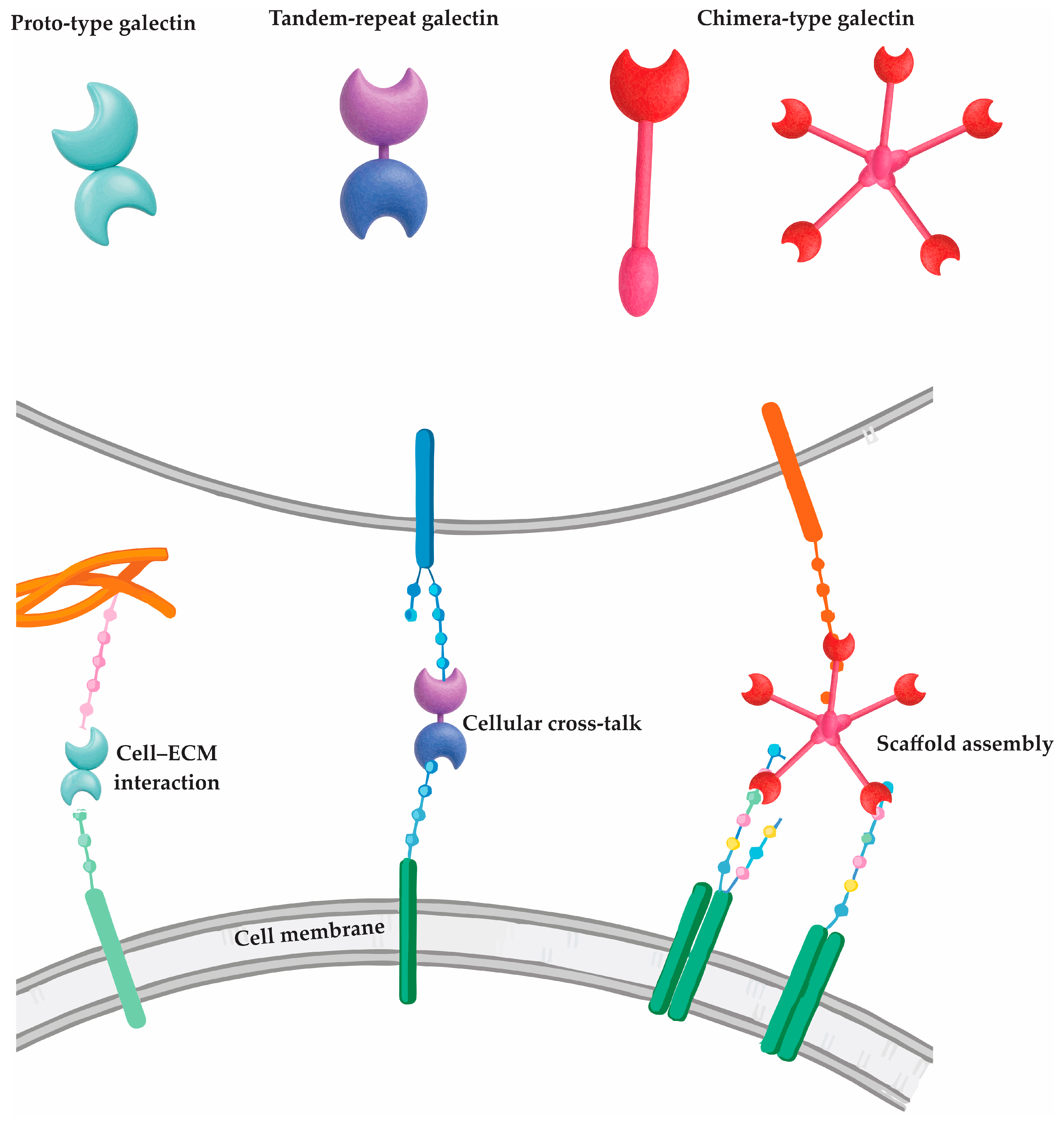
3]. Each galectin contains at least one CRD—a highly homologous motif that governs glycan binding—yet their structural architecture varies, enabling classification into three principal types [
4].
The proto-type galectins (e.g., Gal-1, -2, -5, -7, -10, -13) possess a single CRD and often form homodimers, primarily through non-covalent interactions [
5,
6]. These dimeric forms facilitate cross-linking of glycoprotein receptors and participate in diverse signaling cascades [
6]. In contrast, tandem-repeat galectins (e.g., Gal-4, -8, -9, -12) harbor two CRDs connected by a short linker peptide, allowing for simultaneous engagement with distinct glycan targets—an attribute that enhances their regulatory flexibility [
5]. Galectin-3, the sole chimera-type galectin, is unique in its combination of a single CRD and a non-lectin N-terminal domain that supports oligomerization into pentamers, enhancing its ability to form multivalent glycan lattices [
7]. The structural classification of galectins and their corresponding molecular interactions with cell surface and extracellular ligands are illustrated in
Figure 1.
Unlike most lectins that are restricted to membrane-associated roles, galectins exhibit a broader subcellular distribution [
5]. They localize to various cellular compartments, including the plasma membrane, cytosol, nucleus, endomembrane compartments, extracellular matrix, and even the bloodstream. [
5]. This dynamic localization underpins their capacity to regulate processes ranging from intracellular signaling to extracellular matrix remodeling and immune modulation [
4].
Furthermore, emerging evidence suggests that galectins can form heterodimers and higher-order hetero-oligomers when co-expressed within the same tissue environment, adding another layer of complexity to their regulatory potential [
6]. These interactions may confer context-dependent specificity and functional versatility, reinforcing their significance in both physiological and pathological settings.
2.1. Galectins as Multifaceted Regulators of Innate Immune Responses
Galectins, widely expressed in epithelial and immune cells, have emerged as pivotal regulators of innate immunity [
4]. Their ability to act both extracellularly through carbohydrate recognition and intracellularly through protein–protein interactions enables them to modulate key aspects of innate immune cell behavior [
7,
8,
9]. The complexity of their involvement stems not only from their structural diversity but also from their dual capacity to either amplify or restrain immune activity depending on the biological context [
8,
10].
In innate immune signaling, galectins are encountered by immune cells during inflammation, infection, or tissue injury [
4]. Gal-3, for instance, is released upon cellular damage and functions as a damage-associated molecular pattern (DAMP), activating innate immune cells [
11]. However, many intracellular roles of galectins—especially those independent of glycan binding—have gained increasing recognition [
8]. In dendritic cells, both galectin-1 (Gal-1) and Gal-3 regulate cytokine production and T cell-stimulating capacity [
12]. Galectin-1 supports immune tolerance, as evidenced in autoimmune disease models, while Gal-3 modulates cytokine output, influencing T helper cell polarization [
13,
14,
15,
16,
17]. Interestingly, Gal-3 may inhibit or facilitate dendritic cell-mediated T cell activation depending on the microenvironment, suggesting a finely tuned immunoregulatory role [
18].
Macrophages, central effectors of innate defense, are likewise under galectin regulation [
19]. Galectin-3 enhances NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome activity, leading to elevated secretion of interleukin-1 beta (IL-1β) and IL-18, as shown in colitis and hepatobiliary inflammation models [
20,
21]. Conversely, galectin-9 (Gal-9) attenuates inflammasome activation by promoting the autophagic degradation of NLRP3, highlighting opposing roles within the same pathway [
22]. Furthermore, galectins influence macrophage polarization: Gal-3 promotes M2-like, reparative macrophage phenotypes in response to IL-4 and IL-10, while galectin-12 (Gal-12) supports M1-type polarization through the activation of pro-inflammatory signaling cascades [
23,
24]. These findings underscore the role of galectins in directing macrophage functional specialization.
The phagocytic ability of innate cells is also shaped by intracellular galectins [
4]. Galectin-3, localized to the cytosolic side of phagosomes, facilitates actin rearrangement and enhances uptake of opsonized targets [
25,
26]. Its deficiency impairs both phagocytosis and cytoskeletal organization [
27]. Similarly, Gal-9 promotes phagocytic capacity in dendritic cells by supporting actin polymerization, indicating a conserved intracellular role in cytoskeletal regulation [
28].
In terms of immune cell trafficking, galectins exhibit context-dependent functions [
4]. In skin inflammation such as psoriasis, Gal-3 and galectin-7 (Gal-7) suppress neutrophil recruitment by downregulating pro-inflammatory chemokines and signaling pathways [
29,
30]. In contrast, Gal-3 promotes neutrophil infiltration in models of airway inflammation and parasitic infection, where it is actively secreted by myeloid cells [
31]. Galectin-3 is also involved in eosinophilic recruitment during allergic airway disease, whereas Gal-1 appears to suppress this response [
32]. These divergent actions suggest that the tissue environment and type of inflammatory trigger are critical determinants of galectin function.
Overall, galectins orchestrate a diverse set of responses within innate immunity, acting as intracellular regulators, extracellular ligands, and modulators of cytokine networks, phagocytosis, and immune cell migration. Their multifaceted roles offer both therapeutic opportunities and conceptual challenges, particularly as individual galectins can exhibit opposing functions in different contexts. Clarifying the spatiotemporal dynamics of galectin expression and interaction will be essential for harnessing their immunomodulatory potential in infection, autoimmunity, and chronic inflammation.
2.2. Galectins as Critical Modulators of T and B Lymphocyte Responses
Galectins are increasingly recognized as key modulators of adaptive immunity, capable of influencing T and B cell fate through both extracellular glycan interactions and intracellular signaling mechanisms [
3]. Their regulatory roles span the initiation, expansion, differentiation, and contraction of lymphocyte populations, revealing a dynamic contribution to immune balance and tolerance [
4].
Galectins can directly shape T cell activation by binding to glycosylated surface receptors, including cluster of differentiation 2 (CD2), CD7, CD8, CD43, CD45, and the T cell receptor (TCR) [
18,
33]. This binding alters membrane organization, affects receptor turnover, and modifies the spatial distribution of signaling platforms, ultimately modulating signal strength [
33]. Early studies using mannoside acetylglucosaminyltransferase 5 (Mgat5)-deficient mice, which lack the enzyme necessary for producing complex N-glycans, revealed that reduced galectin binding enhances T cell receptor (TCR) signaling and autoimmune susceptibility [
34]. These effects were reversible in wild-type cells by disrupting galectin binding with lactose, underscoring the importance of glycan–galectin interactions in controlling immune thresholds [
34].
Further insights revealed that Gal-3, whose expression is regulated by IL-10 via the upregulation of Mgat5, can elevate the threshold for T cell activation by enhancing TCR glycosylation [
34,
35]. This impairs TCR-CD8 co-localization, thereby dampening T cell sensitivity to antigen stimulation [
35]. Moreover, Gal-3 functions not only at the membrane but also intracellularly. Upon T cell activation, it relocates to the cytosolic side of the immunological synapse, where it may downregulate the surface expression of key TCR components [
18,
36]. These intracellular effects may be mediated through interactions with adaptor proteins, such as ALG-2-interacting protein X (ALIX), independent of carbohydrate recognition [
37]. Notably, Gal-3 knockout mice exhibit stronger CD8
+ T cell responses to viral antigens, affirming its role in tempering cytotoxic T cell activation [
38].
Galectin-9, like Gal-3, is recruited intracellularly to the immune synapse [
39]. It has been linked to enhanced production of pro-inflammatory cytokines, such as IL-17, by activated CD4
+ T cells. Mice lacking Gal-9 demonstrate impaired T helper 17 (Th17) cell differentiation and reduced immunoglobulin A (IgA) production following oral antigen exposure, suggesting that galectin-9 promotes mucosal immunity by favoring Th17 lineage commitment [
40].
Beyond activation, galectins are central to T cell contraction following the resolution of immune responses [
4]. Galectin-1 was among the first to be identified as a mediator of T cell apoptosis, with similar apoptotic functions later attributed to galectins -2, -3, -8, and -9 [
41,
42,
43,
44]. This pro-apoptotic role is most pronounced in effector T cells, suggesting a selective mechanism to limit immune overactivation. In contrast, intracellular Gal-3 appears to prevent apoptosis by interacting with anti-apoptotic proteins such as B-cell lymphoma 2 (BCL-2), revealing a cell-intrinsic role in T cell survival [
45]. In vivo, the deletion of Gal-1, -8, or -9 exacerbates autoimmunity in the experimental autoimmune encephalomyelitis (EAE) model, reinforcing their importance in T cell homeostasis [
4]. The regulatory influence of Gal-9 extends to CD8
+ T cells, as seen in viral infection models where its deficiency results in enhanced cytotoxic responses [
46].
The effects of galectins on T cells inevitably impact B cell responses, particularly in T cell-dependent antibody production [
4]. Galectin-9 facilitates IgA class switching indirectly by supporting Th17 cell differentiation [
40]. In contrast, intracellular Gal-3 impairs B cell maturation into IgA-producing plasma cells [
47]. This inhibitory effect was evident in vitro, where B1 cells lacking Gal-3 showed enhanced plasma cell differentiation in response to IL-5 and transforming growth factor-beta 1 (TGF-β1).
Moreover, galectins can directly influence B cell function through surface glycan recognition [
48]. Endogenous Gal-9 expressed on naïve B cells regulates receptor clustering and signaling by modulating CD22 and CD45 localization, thereby dampening B cell activation [
49]. The disruption of this mechanism through Gal-9 deficiency reduces Src homology region 2 domain-containing phosphatase-1 (SHP1) recruitment, enhances B-cell receptor (BCR) signaling, and accelerates plasma cell differentiation [
50]. Similarly, Gal-1 and galectin-8 (Gal-8) affect B cell responses via glycan-mediated interactions, and their inhibition results in diminished plasma cell generation in vitro [
51].
2.3. Galectins in Host Responses to Bacterial Infections
Galectins play multifaceted roles in shaping the host response to bacterial pathogens, acting both as pattern recognition molecules and as modulators of immune signaling [
5]. Through their affinity for β-galactoside-containing glycans, galectins can directly bind to bacterial surface structures such as lipopolysaccharides (LPS) and glycoproteins, influencing bacterial adhesion, immune recognition, and the outcome of infection [
3,
52,
53].
Among Gram-negative bacteria, Gal-3 has been extensively studied for its interaction with LPS, where it can either promote or restrain inflammation depending on the context [
54,
55,
56]. In infections caused by
Salmonella or
Neisseria meningitidis, Gal-3 binds to outer membrane components, influencing cytokine production and dampening excessive inflammatory responses. However, it may also facilitate bacterial adhesion and immune evasion by modulating host–pathogen interfaces [
56,
57].
In contrast, Gal-9 can exert immunosuppressive effects by inducing apoptosis of pro-inflammatory T cell subsets, such as T helper 1 (Th1) and Th17 cells, thereby weakening antibacterial defenses in infections like those caused by
Klebsiella pneumonia [
58]. Similarly, Gal-1 may enhance bacterial invasion by bridging microbial glycoproteins with host integrins, as observed in
Chlamydia trachomatis and
Porphyromonas gingivalis infections [
59,
60].
Intracellularly, galectins also regulate antibacterial autophagy [
25]. Galectin-8 typically promotes autophagy by recognizing damaged vacuolar membranes, while Gal-3 can antagonize this process, favoring bacterial persistence [
25]. This antagonism is evident in infections with
Listeria monocytogenes and
Group A Streptococcus, where galectin-3 suppresses autophagy-associated ubiquitination, thereby limiting bacterial clearance [
25].
Interestingly, galectin expression can be modulated by bacterial virulence factors [
52]. For instance, during
Yersinia enterocolitica infection, bacterial effector proteins stimulate Gal-1 expression via mitogen-activated protein kinase (MAPK) signaling, thereby contributing to a tempered mucosal inflammatory response [
61].
Together, these findings underscore the context-dependent nature of galectin functions in bacterial infections. While some galectins contribute to host defense by promoting autophagy or enhancing pathogen recognition, others may facilitate immune evasion and microbial persistence. The interplay between galectin subtype, pathogen structure, and host immune status ultimately determines whether their influence is protective or pathogenic. A comparative overview of the immunoregulatory roles of different galectins across innate immunity, adaptive immunity, and bacterial infections is presented in
Table 1.
3. Helicobacter pylori Infection and the Gastric Mucosal Response
Helicobacter pylori (H. pylori) is a helical, flagellated Gram-negative bacterium that exhibits remarkable adaptation to the human gastric niche [
62]. Colonization typically begins in early childhood and, if untreated, may persist for decades [
63]. The bacterium primarily targets the surface of gastric epithelial cells, where it establishes a stable but chronic infection [
64]. One of its key survival strategies is the production of urease, an enzyme that catalyzes the hydrolysis of urea into ammonia, thereby buffering gastric acidity and creating a more hospitable microenvironment within the gastric mucus layer [
65].
Globally,
H. pylori infects approximately half of the population, yet clinical outcomes vary widely [
66]. While many carriers remain asymptomatic, persistent infection is associated with a continuum of gastroduodenal diseases. These include chronic gastritis, peptic ulcers, and MALT lymphoma as well as systemic manifestations such as iron deficiency anemia and immune thrombocytopenia [
66]. Of particular concern is the role of
H. pylori in gastric carcinogenesis [
67]. Long-standing inflammation and epithelial damage promote the progression of atrophic gastritis, intestinal metaplasia, and ultimately gastric adenocarcinoma, leading the International Agency for Research on Cancer to classify
H. pylori as a Group 1 carcinogen [
67,
68].
One of the bacterium’s most effective immune evasion mechanisms is molecular mimicry [
69]. The outer membrane of
H. pylori contains LPS with a fucosylated O-antigen that closely resembles Lewis antigens expressed on host epithelial surfaces [
69,
70]. This mimicry enables the bacterium to camouflage itself and modulate host immune recognition, contributing to its persistence within the gastric mucosa [
69].
Recent studies have drawn attention to the role of endogenous lectins—particularly galectins—in shaping the host response to
H. pylori. Several galectins, including galectin-3, -4, and -9, are expressed in the gastrointestinal tract and are capable of recognizing bacterial glycans such as those found on
H. pylori LPS [
71]. These lectins may contribute to pathogen recognition and immune signaling, yet their function in chronic infection is complex [
71]. While galectin binding may support bacterial clearance, it can also influence immune regulation and epithelial responses in ways that inadvertently facilitate persistent colonization [
71]. Thus, the interaction between
H. pylori and host galectins represents a subtle yet important aspect of the host–pathogen interplay within the chronically inflamed gastric mucosa.
3.1. Pathogenesis of Helicobacter pylori Infection: Molecular Strategies of Colonization and Immune Evasion
The ability of
Helicobacter pylori to establish persistent infection within the human gastric mucosa depends on its capacity to overcome mucosal defenses and firmly adhere to the epithelial surface [
72]. This initial adhesion is a decisive step in pathogenesis, enabling the bacterium to resist mechanical clearance and initiate downstream virulence mechanisms [
73].
The gastric mucosa is equipped with several protective barriers, including surfactant protein D (SP-D) and mucins, which serve as the first line of defense [
74]. SP-D, a collagen-containing C-type lectin originally identified in pulmonary surfactant, is also expressed in the gastric lumen, where its concentration increases during
H. pylori-associated inflammation [
74,
75]. It binds to bacterial LPS, inhibits motility, and induces aggregation of
H. pylori, potentially limiting its ability to colonize deeper epithelial layers [
74]. In parallel, mucins—highly glycosylated glycoproteins secreted by mucosal epithelial cells—contribute to a biochemical barrier [
76]. Particularly, gland mucins rich in O-glycans with terminal α1,4-linked N-acetylglucosamine exhibit direct antimicrobial effects against
H. pylori, which may explain the confinement of bacteria to the superficial mucous layer [
77].
Once in proximity to epithelial cells,
H. pylori utilizes a repertoire of surface molecules to establish firm adhesion [
78]. A key structural feature of its outer membrane is LPS, whose O-antigen chains mimic human blood group antigens, notably Lewis X (LeX), Lewis Y (LeY), Lewis A (LeA), and Lewis B (LeB) [
79]. This molecular mimicry serves a dual function—facilitating adhesion and subverting host immune recognition [
78]. The expression of these mimicry antigens is phase-variable, allowing
H. pylori to alternate between different O-antigen structures in successive bacterial generations, a strategy that enhances immune evasion and persistence [
80].
Importantly, these glycan structures are not merely passive mimics. They actively participate in adhesion, as demonstrated by the diminished binding capacity of
H. pylori mutants lacking O-antigen and the ability of LeX-coated particles to replicate bacterial binding patterns on human gastric tissue [
81]. The interaction between
H. pylori surface glycans and host receptors facilitates the delivery of bacterial effector proteins, such as CagA, via the type IV secretion system encoded by the
cag pathogenicity island [
82]. Once translocated into host cells, CagA disrupts epithelial signaling, promotes cellular transformation, and contributes to gastric carcinogenesis [
82].
Collectively, H. pylori pathogenesis relies on a sophisticated interplay between bacterial glycan mimicry, adhesion molecules, and host epithelial defenses.
3.2. Immune Response in Helicobacter pylori Infection: Balancing Inflammation and Persistence
The host immune response to
Helicobacter pylori is characterized by a complex interplay of pro-inflammatory and immunoregulatory mechanisms that both drive mucosal inflammation and paradoxically support long-term bacterial persistence [
83,
84]. Central to this response are Th1 and Th17 cells, which mediate protective immunity but are also involved in the development of gastric mucosal pathology [
85].
Infection with
H. pylori activates both Th1 and Th17 pathways, leading to the production of pro-inflammatory cytokines such as interferon-gamma (IFN-γ) and IL-17, which contribute to epithelial inflammation, atrophic changes, and the onset of intestinal metaplasia [
85,
86,
87]. These responses are essential for immune-mediated clearance of the bacterium; indeed, experimental models have demonstrated that the absence of Th1 or Th17 cells results in the failure to eradicate
H. pylori even after vaccination [
85,
88]. However, this protective inflammation comes at the cost of tissue damage and long-term gastric alterations.
Interestingly, the magnitude of the Th1 and Th17 responses differs between age groups [
89]. In children,
H. pylori-induced immune activation is generally less intense, resulting in milder histological inflammation compared to adults [
89]. This subdued immune profile may facilitate bacterial survival and colonization during early life, a critical period when most persistent infections are established [
73,
89]. The mechanisms underlying this age-dependent modulation remain incompletely understood but likely involve a combination of host and microbial factors.
H. pylori has also evolved virulence strategies that actively suppress protective immune responses [
90,
91]. Two key effectors—CagA and VacA—play central roles in immune modulation [
90,
91]. CagA, delivered into host cells via the type IV secretion system, alters intracellular signaling and suppresses co-stimulatory molecule expression, including B7 homolog 2 (B7-H2), thereby dampening Th17 cell development [
92]. VacA further skews the immune balance by promoting the differentiation of regulatory T cells (Tregs), which suppress Th1 and Th17 responses and create a tolerogenic environment favorable for persistent colonization [
93]. In experimental settings,
H. pylori strains lacking functional VacA induce more robust inflammatory responses and fail to establish chronic infection, underscoring the immunosuppressive role of this toxin [
63].
Beyond T cell regulation,
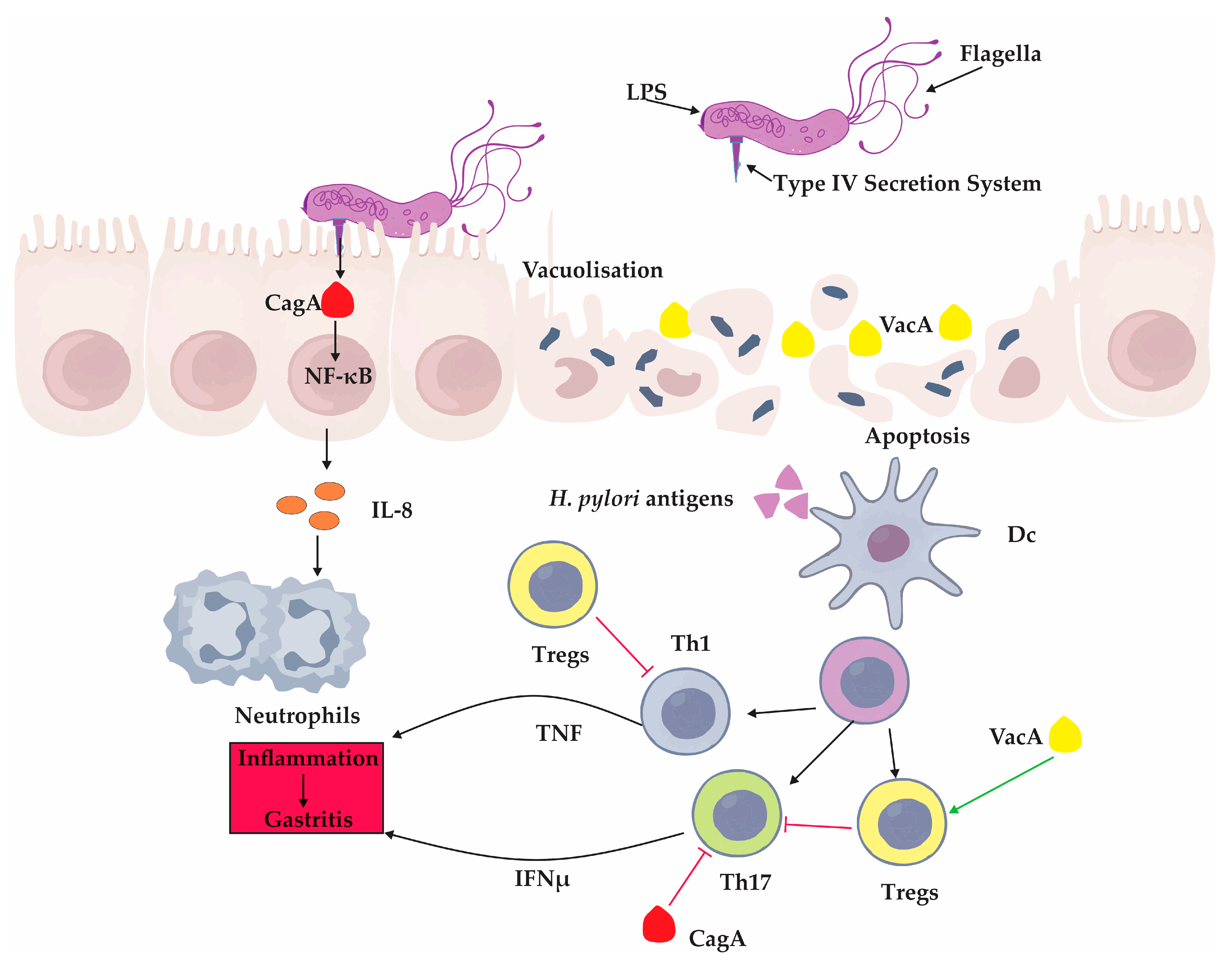
H. pylori infection triggers a cascade of innate immune events that amplify inflammation [
94]. Activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is a hallmark feature, leading to the production of chemokines such as IL-8, which recruits neutrophils to the site of infection [
94]. This influx of immune cells contributes to the oxidative stress and epithelial injury observed in chronic gastritis and promotes a cycle of persistent inflammation [
95].
Thus,
H. pylori orchestrates a finely tuned manipulation of the host immune response: provoking sufficient inflammation to secure a niche within the gastric mucosa while simultaneously dampening specific immune pathways that would otherwise lead to its eradication [
95]. Age-related alterations in mucosal immunity significantly influence the host response to
Helicobacter pylori infection [
96]. Immunosenescence, characterized by impaired immune surveillance and chronic low-grade inflammation, contributes to distinct patterns of gastric immunopathology in older adults. Aging is accompanied by gastric atrophy, reduced epithelial regeneration, and changes in cytokine production, collectively reshaping the gastric microenvironment [
96]. It is known that Gal-1 expression is increased in chronic gastritis, both in the epithelial and stromal compartments, contributing to the modulation of local immune responses [
97]. However, the impact of aging on the expression of galectins within the gastric mucosa, as well as their potential role in age-associated mucosal alterations during
H. pylori infection, remains unexplored.
The balance between immune activation and immune suppression is pivotal to the bacterium’s long-term survival and the pathogenesis of disease. These key interactions between
H. pylori virulence factors and the host immune system—including epithelial signaling disruption, cytokine release, and T cell modulation—are summarized in
Figure 2.
4. Galectin-1: Structure, Immune Regulation, and Its Role in Helicobacter pylori Infection
Galectin-1 (Gal-1), a prototypical β-galactoside-binding lectin encoded by the LGALS1 gene, is a multifunctional molecule that integrates structural, immunological, and context-dependent regulatory functions across physiological and pathological settings. Structurally characterized by its homodimeric β-sandwich configuration and conserved CRDs, Gal-1 engages in diverse cellular processes, including signal transduction, immune regulation, and extracellular matrix interactions. Its ability to modulate both innate and adaptive immune responses, promote immune tolerance, and resolve inflammation underlies its central role in maintaining tissue homeostasis. In the setting of Helicobacter pylori infection, Gal-1’s immunosuppressive actions—particularly its attenuation of Th1/Th17-mediated inflammation—have garnered interest for their contribution to bacterial persistence, especially in pediatric populations. The following sections explore the structural biology of Gal-1, its role in host–bacteria interactions, and its emerging significance in H. pylori-induced immune modulation and gastritis pathogenesis.
4.1. Galectin-1: Structural Features and Multifaceted Immunomodulatory Functions
Galectin-1 is a highly conserved β-galactoside-binding lectin with a molecular weight of approximately 14 kDa, encoded by the
LGALS1 gene [
8,
98]. Structurally, Gal-1 exists as a homodimer, with each subunit forming a characteristic 135-amino acid β-sandwich fold composed of anti-parallel β-strands [
5]. Each monomer harbors a CRD, essential for binding glycosylated ligands in the extracellular environment [
8].
Gal-1 is ubiquitously expressed across various tissues and cell types, functioning in both intracellular and extracellular compartments [
99]. Intracellularly, Gal-1 has been associated with nuclear processes such as mRNA splicing and signal transduction through weak protein–protein interactions [
99]. Extracellularly, Gal-1 can bind to glycosylated receptors on cell surfaces or within the extracellular matrix, where it exerts effects by clustering glycoproteins and modulating cell–cell and cell–matrix adhesion [
100,
101]. Interestingly, Gal-1 immobilized in the extracellular matrix demonstrates enhanced biological activity compared to its soluble counterpart—capable of inducing T cell apoptosis [
100].
Functionally, Gal-1 is involved in a broad spectrum of physiological and pathological processes. It plays key roles in embryonic development, tissue remodeling, muscle differentiation, neuroregeneration, and angiogenesis [
102]. In the context of the immune system, Gal-1 modulates inflammation, promotes immune tolerance, and contributes to the resolution of immune responses [
103]. These immunomodulatory functions are especially relevant in cancer, where Gal-1 promotes tumor immune escape, and in pregnancy, where it supports maternal–fetal tolerance [
103]. Additionally, Gal-1 is implicated in infectious diseases, transplant immunology, and autoimmune disorders, highlighting its context-dependent roles in regulating immune equilibrium [
99].
Due to its structural versatility and diverse biological activities, Gal-1 has emerged as a critical node in the network of glycoimmune interactions. Ongoing research continues to uncover its multifaceted contributions to host–pathogen interactions, including in chronic infections such as Helicobacter pylori, where its immune-suppressive properties may influence bacterial persistence and disease progression.
4.2. Galectin-1 as a Context-Dependent Regulator of Host–Bacterial Interactions
Galectin-1, a β-galactoside-binding lectin widely expressed in mammalian tissues, plays a multifaceted role in bacterial infections, exerting both pro-pathogenic and protective effects depending on the pathogen and host context [
99]. This lectin can modulate the course of infection by influencing immune cell activation, cytokine production, and the interactions between bacterial ligands and host glycan structures [
99].
In several bacterial models, Gal-1 appears to facilitate microbial persistence. For instance, during
Yersinia enterocolitica infection, Gal-1 attenuates the host inflammatory response by downregulating the production of interferon-γ, tumor necrosis factor-α, interleukin-17, and nitric oxide [
61]. This immunosuppressive effect is further supported by Gal-1’s ability to bind to bacterial virulence factors such as Yersinia outer proteins (Yops), protecting them from proteolytic degradation and thereby maintaining their capacity to subvert host immunity [
104].
Similarly, in infections caused by
Tropheryma whipplei and
Chlamydia trachomatis, Gal-1 enhances bacterial adherence and entry into host cells [
59,
105]. This is achieved through a bridging mechanism between bacterial surface glycoproteins and complex N-glycan structures on host membranes, particularly those containing β1,6-branches [
59]. Gal-1-mediated enhancement of pathogen–host interaction involves key receptors such as β1 integrins and platelet-derived growth factor receptor beta (PDGFRβ), facilitating internalization and intracellular survival [
59].
The role of Gal-1 is not limited to facilitating infection. In
Pseudomonas aeruginosa-induced corneal keratitis, Gal-1 exhibits tissue-protective properties by mitigating neutrophilic infiltration and suppressing pathogenic Th17 responses, thereby reducing inflammatory damage [
106]. This illustrates its dual capacity to limit destructive immune responses while potentially preserving tissue integrity.
Gal-1 also modulates neutrophil behavior, a critical component of early antibacterial defense. Depending on the inflammatory context, it can promote or suppress neutrophil migration and oxidative activity [
107,
108]. Gal-1 encourages the clearance of apoptotic neutrophils by macrophages, thereby aiding in the resolution of inflammation [
109]. However, during acute or sustained inflammation, Gal-1 can inhibit neutrophil adhesion and transmigration, tempering excessive immune infiltration [
107].
Experimental models underscore Gal-1’s significance in regulating infection outcomes. Gal-1-deficient mice show heightened inflammatory responses and increased bacterial clearance, particularly in the context of
Yersinia infection. In contrast, the administration of exogenous Gal-1 can impair microbial eradication, confirming its role in immune suppression under certain conditions [
61].
4.3. Galectin-1 as a Modulator of Immune Homeostasis in Helicobacter pylori Infection
In the context of
Helicobacter pylori infection, Gal-1 has emerged as a potent immunomodulatory molecule with distinct regulatory roles in shaping mucosal immunity, particularly in the pediatric population [
89]. Its known capacity to suppress pro-inflammatory Th1 and Th17 responses is of particular relevance to the localized immune landscape of
H. pylori-induced gastritis [
110]. Galectin-1 exerts these effects by inducing apoptosis in Th1 and Th17 cells, dampening the secretion of pro-inflammatory mediators such as IL-12, IFN-γ, and tumor necrosis factor-alpha (TNF-α) while simultaneously promoting the anti-inflammatory cytokine IL-10 [
110,
111]. This pattern of cytokine regulation supports a shift toward immune tolerance and attenuation of chronic inflammation.
Experimental findings in both human and animal models underscore Gal-1’s anti-inflammatory role beyond T cell regulation. Galectin-1 has been shown to inhibit neutrophil infiltration, suppress the release of arachidonic acid from LPS-activated macrophages, and reduce inducible nitric oxide synthase (iNOS) production [
112]. These effects collectively contribute to a muted inflammatory response, which is characteristic of pediatric
H. pylori gastritis and may facilitate persistent bacterial colonization during early life [
89].
In clinical observations of
H. pylori-induced gastritis, the distribution and regulation of Gal-1 within the gastric mucosa reveal a striking regional specificity [
113]. In pediatric patients, inflammation tends to be more pronounced in the antrum compared to the corpus [
113,
114]. Correspondingly, Gal-1 expression is found to be significantly higher in epithelial cells of the corpus, with
H. pylori infection selectively enhancing its expression in corpus stromal cells but not in the antrum [
114]. This differential expression suggests that Gal-1 may mitigate corpus inflammation, contributing to the antral-dominant pattern of gastritis commonly seen in children [
113]. In contrast, studies in adults show no infection-induced change in Gal-1 expression in either epithelial or stromal compartments of the antrum, implying an age-dependent regulation of this lectin in response to infection [
114]. A summary of the immunomodulatory functions and region-specific expression patterns of Galectin-1 in
Helicobacter pylori infection is provided in
Table 2.
5. Galectin-2: Structural Characteristics and Antimicrobial Defense in Helicobacter pylori Infection
Galectin-2 (Gal-2) is a 14 kDa β-galactoside-binding lectin, structurally related to Gal-1 and predominantly expressed in gastrointestinal epithelial cells. It contributes to mucosal integrity by stabilizing cell–cell junctions and crosslinking mucins within the gastric mucus layer. In Helicobacter pylori infection, Gal-2 plays a direct antimicrobial role by binding to bacterial surface glycoconjugates, promoting aggregation, and inducing bacterial death. These actions highlight Gal-2 as both a barrier-strengthening and bactericidal molecule essential for gastric mucosal defense.
5.1. Galectin-2: Structural Insights and Its Role in Gastric Mucosal Integrity
Galectin-2, a proto-type member of the galectin family, shares significant structural homology with Gal-1, with which it has approximately 43% amino acid sequence identity [
43,
115]. First identified in human hepatoma HepG2 cells, Gal-2 is a 14 kDa protein encoded by the
LGALS2 gene located on chromosome 22, in close proximity—though on the opposite strand—to the
LGALS1 gene encoding Gal-1 [
116]. This proximity may suggest a shared evolutionary origin or coordinated regulatory mechanisms.
Functionally, Gal-2 has garnered attention for its immunomodulatory and barrier-enhancing properties within the gastrointestinal tract [
117]. Unlike many other galectins, Gal-2 is predominantly expressed in the gastrointestinal epithelium, including the surface mucous and neck cells of the stomach, goblet cells of the small intestine, and brush border of colonic enterocytes [
118,
119,
120]. It is also present in extra-intestinal tissues such as the placenta and cardiovascular system [
121,
122]. Upon synthesis in the cytoplasm, Gal-2 can be transported to the cell membrane, transported into the nucleus, or secreted extracellularly via non-classical pathways likely involving endosomal vesicles [
4].
One of the most notable roles of Gal-2 is its ability to enhance mucosal barrier function. It achieves this by binding to mucin proteins, such as mucin 5AC (MUC5AC), and facilitating their crosslinking within the mucus layer [
117,
123]. This interaction strengthens the viscoelastic properties of gastric mucus, reinforcing its protective barrier against luminal insults, including acid, enzymes, and microbial pathogens like
Helicobacter pylori [
117,
123]. Gal-2 also binds various glycoconjugates, including β1 integrin, ganglioside GM1, mucin 1 (MUC1), β-catenin, and epithelial cadherin (E-cadherin) [
124]. Its interaction with β-catenin is particularly relevant in the context of epithelial cell adhesion, as it enhances the stability of β-catenin/E-cadherin complexes at adherens junctions, thus contributing to epithelial integrity [
124].
Beyond mechanical reinforcement of the mucosal barrier, Gal-2 has demonstrated immunoregulatory capabilities. It is implicated in the induction of T cell apoptosis and modulation of inflammation, with roles identified in conditions such as colitis and contact dermatitis [
43,
115,
125]. Gal-2 preferentially binds to complex oligosaccharides such as N-acetyllactosamine (LacNAc), with significantly higher affinity than to simple galactose-containing monosaccharides. Structural modifications such as 3-O-sulfation of galactose residues further enhance this binding affinity [
126].
5.2. Galectin-2 as a Bactericidal Mediator in Gastric Defense Against Helicobacter pylori
Galectin-2 plays a direct antimicrobial role in the gastric mucosa, acting as part of the innate defense system against
H. pylori [
127]. Its protective activity is mediated through specific interactions with β-galactoside-containing glycoconjugates present on the bacterial surface, particularly within LPS structures [
127]. Galectin-2 demonstrates a selective affinity for the H type I blood group antigen expressed in the O-antigen portion of
H. pylori LPS, enabling it to recognize and bind bacterial targets in a pH-dependent manner, especially at pH 6.0, which mimics the local gastric environment [
127].
Functionally, Gal-2 exhibits dual antibacterial mechanisms: it induces bacterial aggregation and exerts direct bactericidal effects [
68]. The protein forms homodimers via its CRDs, enabling it to crosslink surface-expressed β-galactoside residues on neighboring
H. pylori cells [
68]. This crosslinking promotes aggregation of bacterial cells into dense clumps, a process that is concentration-dependent and competitively inhibited by lactose, a β-galactoside-containing disaccharide, indicating the specificity of Gal-2–glycan interactions [
68]. This aggregation not only impairs motility but also correlates with bacterial cell death, particularly in the central regions of the clumps where metabolic disruption and membrane compromise are most pronounced [
68].
Microscopic and viability analyses reveal that
H. pylori cells located at the core of Gal-2-induced aggregates lose viability, whereas peripheral bacteria exhibit partial resistance, suggesting a dose-dependent bactericidal gradient [
68]. Although the precise molecular mechanism remains to be fully elucidated, it is hypothesized that Gal-2 may interfere with bacterial membrane integrity and essential metabolic pathways upon glycan binding, ultimately leading to bacterial death.
Interestingly,
H. pylori infection is associated with the downregulation of Gal-2 expression in gastric tissues, suggesting that the pathogen may evade host defenses by modulating galectin levels [
68]. Nevertheless, Gal-2’s ability to agglutinate and kill
H. pylori—even in the absence of opsonins or immune cell involvement—highlights its role as a non-immune effector molecule capable of reinforcing mucosal barrier function and contributing to the containment of infection near the epithelial surface. The key antimicrobial properties and mechanisms of action of Galectin-2 in
Helicobacter pylori infection are summarized in
Table 3.
6. Galectin-3: A Key Regulator of Gastric Mucosal Immunity and Carcinogenic Signaling During Helicobacter pylori Infection
Galectin-3 (Gal-3), a chimera-type β-galactoside-binding lectin, plays diverse roles in immunity, epithelial defense, and carcinogenesis. Structurally equipped to form multivalent lattices, Gal-3 is expressed in both immune and epithelial cells, particularly in the gastrointestinal tract. During Helicobacter pylori infection, Gal-3 participates in early immune responses, modulates bacterial adhesion, and promotes macrophage activation. Simultaneously, Gal-3 contributes to epithelial survival and proliferative signaling through pathways initiated by bacterial virulence factors such as CagA. This duality positions Gal-3 at the intersection of host defense and gastric oncogenic transformation, reflecting its context-dependent function in shaping infection outcomes.
6.1. Galectin-3: A Multifunctional Mediator at the Interface of Immunity and Gastric Mucosal Defense
Galectin-3 is a structurally distinct member of the galectin family, categorized as a chimera-type galectin due to its composite structure comprising a single CRD tethered to a non-lectin N-terminal domain enriched in proline and glycine residues [
128,
129]. This configuration enables Gal-3 to multimerize upon ligand binding, forming oligomeric lattices that potentiate its biological interactions with glycosylated receptors and extracellular matrix components [
129].
Galectin-3 is constitutively expressed in a variety of cell types, with particularly high levels observed in epithelial cells of the gastrointestinal tract and in immune cells, especially activated macrophages [
130,
131,
132]. Within immune compartments, Gal-3 contributes to a broad spectrum of functions, including the enhancement of phagocytosis, prolongation of neutrophil and macrophage survival, and facilitation of neutrophil extravasation during inflammation [
31,
133]. Its localization in phagocytic cups and bacterium-containing phagosomes underscores its role in intracellular host defense mechanisms, particularly against bacterial pathogens such as
Mycobacterium tuberculosis [
26,
134].
Notably, Gal-3 is not confined to intracellular compartments. It is also secreted via non-classical pathways and localizes to the cell membrane and extracellular space, where it engages in additional immunoregulatory activities [
5]. One prominent extracellular function of Gal-3 is its ability to bind LPS from various Gram-negative bacteria, thereby modulating the host’s inflammatory response [
135]. In this context, Gal-3 has been implicated in dampening excessive responses to LPS, potentially offering protection against endotoxin-induced shock [
56].
In the stomach, Gal-3 is selectively expressed by surface mucous epithelial cells and is abundantly secreted into the overlying mucus layer [
118,
136]. This strategic localization enables it to serve as a first-line defense molecule within the gastric mucosal barrier [
136]. Immunohistochemical and immunoblot analyses consistently demonstrate its enrichment at the luminal interface, suggesting a specialized function in mucosal immunity [
136]. By interacting with microbial glycoconjugates and stabilizing mucin networks, Gal-3 may contribute to maintaining epithelial integrity while also exerting direct antimicrobial actions.
6.2. Galectin-3 in Bacterial Pathogenesis: Dual Roles in Host Defense and Pathogen Survival
Galectin-3 plays a versatile role in bacterial infections by recognizing specific glycans on microbial surfaces [
52,
129]. In Gram-negative bacteria, it primarily binds to LPS structures—most notably the β-galactoside residues in the outer core and O-antigen [
135,
137]. Remarkably, even atypical LPS, such as from
Salmonella minnesota, can be recognized through lipid A, reflecting Gal-3’s structural adaptability [
138]. Gram-positive bacteria, although lacking LPS, are not exempt from Gal-3 binding; here, hydrophobic interactions mediate recognition [
139].
Functionally, Gal-3 helps regulate the host’s inflammatory response to bacterial LPS, preventing excessive cytokine release and reducing susceptibility to endotoxin-induced shock [
56]. However, this regulatory role may come at the cost of impaired bacterial clearance, as seen in Gal-3-deficient models, where stronger Th1 responses and reactive oxygen production promote better pathogen control [
11].
Galectin-3 also interferes with intracellular antimicrobial defenses [
57]. In
Listeria monocytogenes infections, it is recruited to disrupted phagosomal membranes, where it suppresses autophagic clearance [
25]. Gal-3-deficient macrophages show more efficient bacterial killing, further potentiated by inhibition of autophagy, suggesting Gal-3 subverts this process [
25]. Similarly, in
Group A Streptococcus infection, Gal-3 antagonizes Gal-8-mediated ubiquitin recruitment, impairing bacterial degradation and promoting intracellular persistence, particularly in endothelial cells [
140].
Beyond immunity, Gal-3 facilitates bacterial adhesion and invasion [
57]. In
Neisseria meningitidis, it binds both LPS and pilins, enhancing bacterial attachment to immune cells [
141]. In ocular and urinary tract infections (
P. aeruginosa,
P. mirabilis), Gal-3 acts as a molecular bridge, promoting pathogen–host interactions through glycan recognition [
142,
143].
6.3. Galectin-3 in Helicobacter pylori Infection: A Dual Modulator of Mucosal Defense and Carcinogenic Transformation
The interaction between
Helicobacter pylori and host gastric epithelium triggers a multifaceted response in which Gal-3 plays a pivotal role. One of the early defensive mechanisms involves Gal-3 secretion by gastric epithelial cells into the mucus layer, where it functions as a physical barrier, entrapping
H. pylori and limiting its direct contact with epithelial surfaces [
11,
136]. In murine models deficient in Gal-3, this defensive line is compromised—
H. pylori penetrates deeper into gastric tissues, and the bacterial load remains significantly elevated both at early and chronic stages of infection [
136]. These observations suggest that Gal-3 contributes to mucosal integrity by restricting initial colonization.
The release of Gal-3 appears to be induced by direct bacterial contact, potentially through interactions between bacterial O-antigen side chains and membrane-associated Gal-3 [
81]. Intriguingly, the application of recombinant extracellular Gal-3 has been shown to reduce bacterial adhesion, reinforcing its role as a soluble defensive mediator [
144]. Beyond preventing attachment, Gal-3 exerts bactericidal effects—its CRD-dependent activity promotes the elimination of phagocytosed
H. pylori by macrophages [
136]. Macrophages lacking Gal-3 exhibit impaired bacterial killing despite unaffected phagocytosis, highlighting lectin’s role in the intracellular handling of the pathogen [
136].
However, the role of Gal-3 is not confined to antimicrobial defense. Upon
H. pylori infection, gastric epithelial and cancer cells upregulate Gal-3 expression, not only increasing its extracellular presence but also accumulating it in the cytoplasm [
94]. In this intracellular context, Gal-3 contributes to enhanced proliferation and resistance to apoptosis—traits that align with early tumorigenic transformation [
145]. It has also been implicated in conferring resistance to IFN-γ-mediated growth suppression in hyperproliferative gastric cancer cell lines, linking bacterial infection, immune evasion, and cancer progression through Gal-3-mediated pathways [
145].
Given the established association between
H. pylori infection and the development of gastric adenocarcinoma and MALT lymphoma, Gal-3’s upregulation during infection may represent a key endogenous factor contributing to the altered cellular environment that precedes neoplastic transformation [
114]. In this context, Gal-3 emerges not only as a modulator of host defense but also as a potential participant in the early molecular events of gastric carcinogenesis.
6.4. Galectin-3 Expression and Interaction with H. pylori: A Molecular Axis in Gastric Colonization
Infection of the gastric mucosa by
Helicobacter pylori prompts a rapid and sustained upregulation of Gal-3, particularly within the gastric stroma and epithelial compartments [
114]. This early host response is more than a defensive maneuver—it appears to be intimately linked to the bacterium’s strategy for mucosal colonization [
81].
Upon contact with
H. pylori, gastric epithelial cells, including AGS cell lines, swiftly elevate Gal-3 synthesis and promote its secretion into the extracellular space [
81]. This response has been shown to depend on the presence of a functional type IV secretion system, as infections with
cagA or
cagE mutants fail to elicit Gal-3 expression [
81]. Inhibiting MAPK signaling also suppresses Gal-3 induction, suggesting that translocation of CagA and downstream kinase activation is central to Gal-3 regulation during infection [
81].
One of the most intriguing aspects of Gal-3 in
H. pylori infection is its role as a microbial receptor. The bacterium expresses Lewis-type antigens, particularly polymeric Lewis X motifs, along the O-antigen side chains of its LPS [
146,
147]. Gal-3 binds with high specificity to these carbohydrate structures, recognizing repeating N-acetyllactosamine motifs, a well-established ligand for its CRD [
81]. This binding is inhibited by lactose and specific monoclonal antibodies, confirming the involvement of both the CRD and N-terminal domain in the interaction [
81].
Beyond its role in recognizing Lewis antigen-like structures on
H. pylori LPS, the potential influence of bacterial strain heterogeneity on Gal-3 interactions remains underexplored. Polymorphisms in key virulence factors, particularly CagA and VacA, determine the pathogenic potential of
H. pylori and shape the host inflammatory response [
148]. CagA-positive strains with specific EPIYA motif patterns are linked to stronger oncogenic signaling and more severe gastric pathology [
148]. These genetic differences among strains are also associated with variations in the expression of outer membrane structures, including Lewis antigens, which could modulate Gal-3 binding affinity and downstream immune responses. Although direct evidence connecting
H. pylori strain diversity to functional differences in Gal-3 interactions is still lacking, this represents a significant gap in understanding the pathogen’s immune evasion strategies and warrants future investigation.
The Gal-3–O-antigen binding not only anchors
H. pylori to the epithelial surface but may also facilitate localized bacterial clustering and enhance mucosal retention. While this binding strengthens adhesion to AGS epithelial cells, it is not the sole mechanism employed by
H. pylori, indicating a multifactorial adhesion process [
81].
6.5. Galectin-3 and the Innate Immune Response to H. pylori: Beyond Adhesion and Aggregation
Galectin-3 is increasingly recognized as an active participant in the early immune response to
Helicobacter pylori, functioning at the crossroads of epithelial signaling, immune cell recruitment, and inflammatory amplification [
136]. Its release from infected gastric epithelial cells occurs rapidly and independently of classical secretory pathways, indicating an unconventional, possibly ectocytic, mechanism that allows Gal-3 to act as a first-line alarmin [
81,
136].
Once in the extracellular milieu, Gal-3 assumes an immunomodulatory role. It promotes chemotaxis of monocytes and neutrophils, helping to mobilize phagocytic cells toward the infected gastric epithelium [
11,
144]. Furthermore, recombinant Gal-3 has been shown to increase the phagocytic potential of human neutrophils and stimulate the respiratory burst through the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, thereby enhancing the antimicrobial capacity of the recruited immune cells [
149]. These features position Gal-3 not merely as a physical barrier but as an amplifier of innate defense mechanisms.
Importantly, the upregulation of Gal-3 in response to
H. pylori is tightly linked to bacterial virulence mechanisms. The delivery of the CagA effector protein into host epithelial cells activates the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) signaling pathway, a cascade known to regulate Gal-3 expression in other cell types undergoing stress or differentiation [
81]. Thus, the bacterium exploits its own pathogenic machinery to trigger host cell responses that may initially serve defensive functions but could later contribute to pathological remodeling of the gastric mucosa.
Intracellularly, Gal-3’s functions diverge further. Under certain conditions, post-translational modifications such as phosphorylation can alter its lectin properties and transform it into an anti-apoptotic molecule [
150]. This effect, possibly relevant in the setting of persistent
H. pylori infection, could prolong the survival of epithelial cells harboring oncogenic CagA signaling—creating a microenvironment permissive to early neoplastic transformation [
151]. Notably, Gal-3 contains a conserved anti-death motif resembling domains found in the Bcl-2 family, suggesting a convergence of lectin activity and cell survival pathways [
152].
6.6. Galectin-3 in the Interplay Between H. pylori Infection and Gastric Carcinogenesis
Galectin-3 has emerged as a critical intracellular mediator linking
Helicobacter pylori infection with oncogenic transformation in gastric epithelial cells [
152]. Upon bacterial challenge, Gal-3 undergoes dynamic intracellular redistribution—from a predominantly nuclear localization to cytoplasmic accumulation—facilitated via chromosomal region maintenance 1 (CRM1)-mediated export [
94]. This shift is not merely spatial but functional: cytoplasmic Gal-3 contributes to cell survival by modulating NF-κB signaling and enhancing IL-8 secretion, thereby supporting a proinflammatory and pro-proliferative environment [
94].
Galectin-3 expression is upregulated in response to CagA translocation via ERK/MAPK activation, linking bacterial virulence to host immune modulation [
81]. Elevated Gal-3 levels, in turn, inhibit infection-induced apoptosis and prolong the viability of infected epithelial cells [
94]. This anti-apoptotic effect is tightly linked to Gal-3’s capacity to delay cell cycle arrest, a mechanism that may permit the persistence of CagA-injected cells prone to malignant transformation.
Extracellular Gal-3 also plays a complementary role in shaping the tumor-promoting microenvironment. Its presence reduces bacterial adhesion, indirectly suppressing apoptosis, and promotes the recruitment of monocytes to the site of infection—events that contribute to sustained, low-grade inflammation [
144]. Such a milieu, marked by chronic immune activation and epithelial hyperproliferation, is a well-established precursor to gastric cancer.
Galectin-3 undergoes notable context-dependent reprogramming that links chronic inflammation to cancer development. In the early stages of
H. pylori infection, Gal-3 acts as an alarmin and antimicrobial effector by recognizing bacterial LPS, aggregating the pathogen, recruiting neutrophils and monocytes, and amplifying their respiratory burst to limit bacterial load and mucosal invasion [
11,
81,
136,
144,
149]. However, chronic infection driven by bacterial virulence factors like CagA sustains ERK/MAPK signaling in epithelial cells and upregulates LGALS3 expression [
81]. Concurrently, post-translational modifications, such as phosphorylation, transform cytoplasmic Gal-3 into an anti-apoptotic and pro-survival molecule that stabilizes NF-κB, promotes IL-8 production, and fosters epithelial proliferation [
94]. This shift also remodels key pathways, including beta-catenin/T cell factor 4 (β-catenin/TCF-4) and protein kinase B/glycogen synthase kinase 3 beta (AKT/GSK-3β); induces expression of invasion-promoting molecules like Fascin-1, protease-activated receptor 1 (PAR-1), and matrix metalloproteinase-1 (MMP-1); and disrupts E-cadherin-mediated adhesion, collectively driving epithelial–mesenchymal transition and invasive behavior [
153,
154]. Although direct evidence in
H. pylori gastritis is scarce, Gal-3 likely interacts with other lectin receptors such as sialic acid-binding Siglecs and C-type lectins like dectin-1, which modulate immune responses, including neutrophil apoptosis, dendritic cell cytokine secretion, and NLRP3 inflammasome activation [
129,
155,
156]. Considering the high sialylation of gastric mucins and Gal-3’s capacity to engage non-canonical inflammasome pathways, similar cross-talk in chronic
H. pylori infection may amplify pro-tumorigenic inflammation and represents an important area for further investigation [
4].
A comprehensive overview of Gal-3’s multifaceted roles during
H. pylori infection and associated carcinogenic transformation is summarized in
Table 4.
7. Galectin-8: A Dual-Function Sentinel in Intracellular Defense and Inflammatory Signaling During Helicobacter pylori Infection
Galectin-8 (Gal-8), a tandem-repeat lectin with distinct glycan-binding domains, plays a pivotal role in cellular responses to membrane damage and bacterial invasion. It acts both as a sensor of lysosomal injury and an inducer of antibacterial autophagy, particularly during infections with Helicobacter pylori. Through recognition of exposed intracellular glycans and recruitment of autophagy adaptors, Gal-8 facilitates pathogen clearance. Simultaneously, its extracellular functions contribute to immune activation and cytokine release, positioning Gal-8 at the interface between intracellular immunity and mucosal inflammation.
7.1. Structural Characteristics and Biological Functions of Galectin-8
Galectin-8 is a tandem-repeat type β-galactoside-binding lectin encoded by the
LGALS8 gene [
157]. It exists in multiple isoforms generated through alternative splicing, with the canonical form comprising two distinct CRDs—N-terminal and C-terminal—linked by a flexible peptide [
158]. The two CRDs display different glycan-binding specificities: the N-terminal domain prefers α2,3-sialylated or sulfated β-galactosides, while the C-terminal domain favors branched N-glycans [
159]. Full biological activity of Gal-8 depends on the integrity of both CRDs, as inhibition or deletion of either domain abrogates its function [
10]. Galectin-8 can multimerize and cross-link glycosylated receptors, thereby influencing a broad range of cellular processes [
8].
Biologically, Gal-8 regulates cell adhesion, migration, proliferation, and apoptosis [
158]. It mediates integrin clustering and turnover, modulates signaling pathways such as mechanistic target of rapamycin (mTOR) and MAPK, and can act extracellularly by binding to glycoproteins in the extracellular matrix or on the surface of immune and epithelial cells [
4,
160,
161]. Gal-8 also participates in endocytic trafficking and autophagic responses by recognizing intracellular glycans exposed on damaged endosomes and lysosomes [
158].
In the immune system, Gal-8 is expressed in lymphoid organs and is rapidly upregulated in response to inflammatory stimuli [
162]. In adaptive immunity, it has context-dependent effects on T cells, promoting activation and proliferation at low concentrations and inducing apoptosis or proliferation arrest at higher levels. Gal-8 also supports regulatory T cell differentiation and can suppress effector responses during prolonged immune activation [
162]. In B cells, it enhances antigen retention and presentation in germinal centers and promotes IL-10 production [
162]. Within the innate immune compartment, Gal-8 activates dendritic cells by upregulating co-stimulatory molecules and cytokine release, promotes neutrophil adhesion and reactive oxygen species production through integrin binding, and modulates natural killer (NK) cell activity by interacting with surface receptors such as bone marrow stromal antigen 2 (BST2) [
162]. Collectively, these functions place Gal-8 as an important regulator of immune homeostasis and inflammation.
7.2. Galectin-8 in Bacterial Infections: A Key Sentinel in Intracellular Immunity
Galectin-8 has emerged as a key intracellular sensor of membrane damage during bacterial infection [
57]. Unlike Gal-3, which may suppress autophagy under certain conditions, Gal-8 actively promotes antibacterial autophagy by recognizing glycans exposed on damaged vacuolar membranes [
25,
163]. This function is particularly critical in infections caused by cytosol-invading pathogens such as
Listeria monocytogenes and
Group A Streptococcus (GAS) [
25,
140,
164].
In epithelial cells infected with GAS, Gal-8 is recruited to damaged vacuoles, where it facilitates the targeting of bacteria for degradation [
140]. This recruitment correlates with the enhanced binding of parkin, an E3 ubiquitin ligase that orchestrates ubiquitin-dependent autophagy [
140]. Experimental models have shown that the absence of Gal-8 impairs this process, leading to reduced bacterial clearance [
140]. Interestingly, in cells lacking both Gal-8 and Gal-3, bacterial replication is even more limited than in Gal-8-deficient cells alone—suggesting a counter-regulatory interaction, where Gal-3 inhibits while Gal-8 promotes autophagic degradation [
140].
Further, Gal-8 appears to play a dominant role in epithelial cells, where its expression is higher than in endothelial cells [
140]. This cell-type specificity influences the efficiency of autophagic killing of intracellular bacteria and shapes the trajectory of infection. In the absence of Gal-8, bacteria more readily escape immune clearance, emphasizing its function as a molecular bridge between pathogen recognition and intracellular defense pathways [
140].
7.3. Galectin-8 as a Molecular Sensor of Lysosomal Damage and Modulator of Autophagy in H. pylori Infection
Galectin-8, a tandem-repeat lectin with distinct affinity for O-glycosylated host glycans, emerges as a critical mediator in the cellular response to
Helicobacter pylori infection [
165]. Evidence from in vivo studies in rhesus macaques as well as in vitro human gastric epithelial models indicates that
H. pylori infection robustly upregulates Gal-8 mRNA expression in gastric tissue [
166]. This transcriptional activation correlates with a marked intracellular accumulation of Gal-8 aggregates in infected cells—an effect that reflects underlying lysosomal membrane damage induced by the bacterium [
165].
One pivotal mechanism driving Gal-8 aggregation is its interaction with exposed host O-glycans on the cytoplasmic face of disrupted lysosomes [
165]. The integrity of lysosomal membranes is compromised during infection, exposing glycan structures typically sequestered within the lysosomal lumen [
165]. Gal-8 recognizes these glycans, accumulating around sites of damage and initiating downstream autophagic responses. This glycan-dependent process is supported by data showing that the inhibition of O-glycosylation significantly attenuates Gal-8 aggregation, whereas N-glycosylation blockade has a lesser effect [
165].
Interestingly, Gal-8 not only marks damaged organelles but also actively participates in the recruitment of autophagic machinery. Colocalization studies reveal that Gal-8 aggregates are frequently found alongside autophagosomes and the autophagy adaptor protein NDP52, implicating Gal-8 in selective autophagy [
165]. The functional depletion of Gal-8 results in diminished autophagic flux during
H. pylori infection, highlighting a bidirectional regulatory loop whereby Gal-8 facilitates and is sustained by autophagy.
The vacuolating cytotoxin A (VacA) produced by
H. pylori further compounds this process. As a pore-forming toxin, VacA directly compromises intracellular vesicles, including lysosomes and mitochondria, exacerbating cellular stress [
167]. Three mechanisms have been proposed to explain VacA’s role in enhancing Gal-8 accumulation: first, by directly destabilizing lysosomal membranes; second, by promoting mitophagy-related oxidative damage that disrupts lysosomal stability via hydroxyl radical formation; and third, by manipulating autophagic pathways to favor bacterial persistence while still inducing sufficient organelle stress to promote Gal-8 recruitment [
165].
Beyond its intracellular functions, Gal-8 may also act as a secreted immunomodulator during infection [
4]. Infected epithelial cells demonstrate increased Gal-8 release, which in turn can activate dendritic cells and enhance the production of proinflammatory cytokines [
168]. These findings suggest Gal-8 not only orchestrates intracellular defenses but also contributes to the broader inflammatory landscape of
H. pylori-associated gastritis. The principal mechanisms and functions of Galectin-8 in
Helicobacter pylori infection are summarized in
Table 5.
8. Galectin-9: Immune Checkpoint Modulator in Bacterial Infections and Helicobacter pylori-Driven Mucosal Tolerance
Galectin-9 (Gal-9) is a tandem-repeat lectin with dual carbohydrate recognition domains that mediate diverse immunoregulatory functions. Its ability to suppress proinflammatory Th1 and Th17 cells while enhancing regulatory T cell differentiation underscores its role in maintaining immune homeostasis during bacterial infections. In the context of Helicobacter pylori, Gal-9 expression—particularly in pediatric gastric mucosa—fosters an immunosuppressive environment that facilitates bacterial persistence and reduces tissue damage, thereby contributing to chronic colonization.
8.1. Structural Features and Functional Versatility of Galectin-9
Galectin-9 is a member of the tandem-repeat subclass of galectins, distinguished by the presence of two CRDs located at opposite ends of the protein—namely the N-terminal CRD (N-CRD) and the C-terminal CRD (C-CRD) [
169]. These domains, each comprising approximately 148–149 amino acids, are connected by a flexible linker whose length varies among isoforms [
170]. Despite their structural resemblance, subtle variations in amino acid composition between the two domains endow them with distinct binding specificities, influencing Gal-9’s interactions with cellular receptors and glycan ligands [
171].
Comprehensive structural analyses have revealed that the biological functions of Gal-9 are intricately tied to the specialization of its CRDs [
171]. The N-CRD is particularly effective in stimulating dendritic cells, while the C-CRD plays a dominant role in modulating T cell fate, including the induction of apoptosis through receptor-mediated signaling [
170]. This dual functionality allows Gal-9 to modulate immune responses through both innate and adaptive pathways.
Functionally, Gal-9 exhibits remarkable plasticity across immune contexts [
171]. Under conditions of immune hyperactivation, Gal-9 typically exerts a suppressive effect, curbing excessive inflammation and promoting regulatory T cell activity [
172]. Conversely, in settings of immune insufficiency, Gal-9 can amplify immune responses, enhancing the function of antigen-presenting cells and facilitating immune activation [
172]. This bidirectional immunoregulatory potential underscores Gal-9’s role as a homeostatic checkpoint within the immune system [
172].
Given its broad spectrum of activity—from modulating dendritic cell phenotype to regulating T cell viability—Galectin-9 has emerged as a promising therapeutic target in both inflammatory and neoplastic diseases.
8.2. Galectin-9 in the Regulation of Host Immunity During Bacterial Infections
Although Gal-9 is primarily known for its role in immune regulation within viral and tumor contexts, emerging evidence has highlighted its functional relevance in bacterial infections, where it modulates both pathogen clearance and host immune tolerance [
4]. Its immunomodulatory properties stem largely from its ability to engage the T cell immunoglobulin and mucin domain-containing protein 3 (Tim-3), which is expressed on various immune subsets, particularly Th1 and Th17 lymphocytes [
39].
In the setting of
Klebsiella pneumoniae infection, Gal-9 demonstrates a prominent immunosuppressive function [
58]. The experimental administration of exogenous Gal-9 in infected murine models leads to apoptosis of Th1 and Th17 cells, reducing IL-17A production—a cytokine critical for neutrophil recruitment and antimicrobial defense [
58]. This suppression also correlates with decreased levels of granulocyte colony-stimulating factor (G-CSF) and macrophage inflammatory protein 2 (MIP-2), two key mediators of granulopoiesis and chemotaxis [
58]. As a consequence, bacterial clearance is impaired, and overall survival is diminished in Gal-9-treated animals [
58]. These findings suggest that Gal-9 dampens protective immunity during acute bacterial infections by curtailing Th17-driven responses, which may otherwise contribute to efficient pathogen elimination.
Beyond systemic infections, Gal-9 expression has also been implicated at mucosal surfaces [
173]. In corneal models of
Pseudomonas aeruginosa infection, transcriptional profiling of infected tissues revealed an upregulation of Gal-9 expression alongside other galectin family members [
174]. Although its precise role in ocular immunity remains under investigation, the coordinated induction of Gal-9 suggests it may participate in localized immune regulation and tissue repair mechanisms during bacterial challenge.
Moreover, Gal-9’s modulatory role is not restricted to direct immune suppression. Through its interactions with Tim-3, Gal-9 may influence the dynamics of antigen-presenting cells and dendritic cell maturation, thereby shaping the early innate immune response and subsequent adaptive immunity [
175]. This is particularly relevant in infections characterized by excessive inflammation, where Gal-9-mediated signaling may serve to contain immunopathology, albeit at the cost of compromised bacterial clearance.
8.3. Galectin-9 and Immune Modulation in Helicobacter pylori Infection
Galectin-9, a potent immunoregulatory protein, plays a pivotal role in shaping the host immune landscape during
Helicobacter pylori infection, particularly in the pediatric gastric mucosa [
89]. Recent findings indicate that Gal-9 expression is markedly elevated in children with
H. pylori-positive gastritis, where it appears to orchestrate a regulatory immune profile conducive to bacterial persistence [
89].
One of the principal mechanisms through which Gal-9 exerts its effects is via engagement with the checkpoint receptor Tim-3, prominently expressed on Th1 and Th17 cells [
42]. This interaction promotes intracellular calcium flux and leads to apoptotic elimination of these proinflammatory T cell subsets [
42]. In parallel, Gal-9 enhances the differentiation of inducible regulatory T (Treg) cells by facilitating the co-localization of transforming growth factor-β (TGF-β) receptor I and CD44, a known Treg marker [
176]. This dual effect—a contraction of Th1/Th17 responses and expansion of the Treg population—contributes to an immune environment characterized by low-grade inflammation and high tolerance to bacterial antigens.
Importantly, this Gal-9-driven immunological shift is more pronounced in children than in adults [
89]. Pediatric gastric mucosa demonstrates increased orkhead box P3-positive (FoxP3
+) Treg cell infiltration during infection, which correlates with attenuated inflammatory cell recruitment and reduced mucosal damage [
177]. This age-dependent regulatory dominance may explain why spontaneous clearance of
H. pylori is less frequent in children and why colonization often leads to chronic infection rather than acute inflammation.
The spatial distribution of Gal-9 further supports its role in modulating superficial mucosal immunity. Immunohistochemical analyses reveal that Gal-9 is predominantly expressed in the gastric epithelium rather than in the deeper stromal compartments [
89]. This localization suggests that Gal-9 primarily influences immune responses in the epithelial barrier zone—precisely where
H. pylori establishes colonization.
Beyond its immunosuppressive effects, Gal-9 may also contribute directly to bacterial containment. It has been shown to bind LPS structures on Gram-negative bacteria, potentially acting as an opsonin that enhances macrophage recognition and antimicrobial activity [
178]. This dual role—immune suppression and bacterial control—may create a finely balanced host–microbial relationship that permits
H. pylori survival without provoking destructive inflammation.
Interestingly, while Gal-9 expression rises significantly in pediatric infection, similar induction is not observed in adult patients, highlighting a developmental divergence in mucosal immune regulation [
114]. This discrepancy reinforces the notion that Gal-9 contributes to the age-specific immune tolerance that underlies persistent
H. pylori colonization in children [
89]. Moreover, inverse correlations between bacterial load and Th17 responses and the observed positive association between Tregs and
H. pylori density further support Gal-9’s role as a permissive factor for colonization [
89]. The immunomodulatory functions and mucosal localization of Galectin-9 in
H. pylori infection are summarized in
Table 6.
To provide an integrative overview of the diverse yet complementary functions of individual galectins discussed in this review, we summarized their structural features, immunomodulatory roles, and specific contributions to
Helicobacter pylori infection and gastric pathology in
Table 7. This comprehensive synthesis underscores the complexity of galectin-mediated host–pathogen interactions and their dual potential to either contain infection or promote bacterial persistence and disease progression.
9. Galectins as Emerging Biomarkers and Therapeutic Targets in Helicobacter pylori-Associated Pathologies
Galectins hold significant potential as biomarkers for the diagnosis, prognosis, and therapeutic stratification of H. pylori-associated diseases, including chronic gastritis, peptic ulcer disease, and gastric cancer. Their differential expression in the gastric mucosa in response to H. pylori infection, alongside their regulatory roles in inflammation, immune tolerance, and tissue remodeling, positions them as promising candidates for clinical application.
Galectin-1, with its immunosuppressive profile and region-specific expression in the pediatric gastric mucosa, could serve as a biomarker for early-life infection persistence and for stratifying patients at risk for chronic infection with minimal inflammatory damage [
89,
110,
111]. Similarly, galectin-3 levels, which increase in both epithelial and stromal compartments during infection, correlate with enhanced proliferative signaling and resistance to apoptosis, suggesting its potential as a biomarker for identifying individuals at higher risk of gastric carcinogenesis [
110,
111,
114,
145].
Galectin-8 and galectin-9, as modulators of autophagy and immune checkpoint pathways, respectively, present dual opportunities: first, as indicators of the host’s autophagic and immunoregulatory responses to infection and, second, as therapeutic targets to enhance bacterial clearance or modulate pathological inflammation [
89,
165].
Future clinical studies are warranted to validate galectin-based biomarkers in diverse patient populations and to explore galectin-targeted interventions as adjunctive therapies alongside standard H. pylori eradication regimens.
10. Clinical Perspectives and Future Directions
Although considerable preclinical evidence underscores the multifaceted roles of galectins in Helicobacter pylori infection, their clinical relevance remains insufficiently addressed. Experimental studies have illuminated the dualistic nature of galectin functions in both host defense and bacterial persistence as well as their contribution to mucosal immune modulation and epithelial transformation. However, clinical trials evaluating galectin-targeted therapies in H. pylori-associated diseases are currently lacking.
Insights derived from oncology and immunotherapy suggest that pharmacological modulation of galectins, particularly Gal-1 and Gal-3, may have therapeutic potential in conditions where chronic infection contributes to carcinogenesis. In this context, the gastric mucosa represents a compelling model for exploring galectin inhibition strategies aimed at restoring immune competence and mitigating the long-term consequences of persistent infection.
Future research should prioritize translational studies that integrate molecular profiling of galectin expression with clinical parameters in H. pylori-infected individuals. Such efforts could clarify the prognostic value of galectin signatures and inform personalized approaches to infection management. Furthermore, exploring galectin inhibition in preclinical models of H. pylori-induced gastric pathology may yield novel adjunctive therapies capable of enhancing bacterial clearance while limiting tissue damage and carcinogenic progression.
A comprehensive understanding of the spatial and temporal dynamics of galectin expression during infection as well as their interaction with bacterial virulence factors is critical for advancing this field. These investigations hold promise for the development of innovative therapeutic strategies that target the immunoregulatory axis of galectins, ultimately contributing to more effective control of H. pylori infection and its associated diseases.
11. Concluding Remarks
This review has synthesized current insights into the roles of galectins in Helicobacter pylori infection, highlighting their context-dependent functions in immunity, bacterial persistence, and gastric pathologies. Galectin-1 and Galectin-9 predominantly contribute to immune tolerance by inducing regulatory T cells and suppressing proinflammatory Th1 and Th17 responses, which facilitates chronic colonization, particularly in pediatric mucosa. In contrast, Galectin-2, Galectin-3, and Galectin-8 participate in direct antibacterial defenses through mechanisms such as bacterial aggregation, modulation of phagocytosis, and autophagy induction.
A key theme emerging from these findings is the functional duality of certain galectins, especially Gal-3 and Gal-9, which can protect the host in acute infection yet contribute to immune evasion and pre-neoplastic changes under chronic conditions. This dualism underscores the delicate balance between effective host defense and the risk of pathology driven by prolonged infection and inflammation.
Nonetheless, significant limitations persist in the field. Much of the current evidence is derived from experimental models with variable translational relevance to human disease, particularly in distinguishing pediatric from adult immune responses. Additionally, the spatial and temporal dynamics of galectin expression in vivo remain incompletely characterized. The contribution of galectins to the immunopathology of H. pylori also appears to be modulated by bacterial strain heterogeneity and host glycosylation patterns—areas that require further elucidation.
A comprehensive understanding of these aspects is critical to refining our knowledge of galectin-mediated immunity and their implications in gastric diseases. Bridging these gaps will enable the development of targeted therapies that can selectively modulate galectin functions to restore effective immunity without exacerbating tissue damage or carcinogenic risk.
12. Materials and Methods
A systematic literature search was conducted using PubMed, Scopus, and Web of Science databases to identify peer-reviewed publications addressing the role of galectins in Helicobacter pylori (H. pylori) infection. The search encompassed articles published from database inception to 1 June 2024. Medical subject headings (MeSH) and keyword combinations were used, including “galectin”, “Helicobacter pylori”, “innate immunity”, “adaptive immunity”, “autophagy”, “gastric mucosa”, “gastric cancer”, “lectin–glycan interaction”, and “bacterial persistence”. Boolean operators (AND/OR) were employed to optimize sensitivity and specificity of the retrieved records.
Eligible publications included original experimental research conducted in vitro, research conducted in vivo (animal models), and clinical studies in humans as well as systematic reviews and meta-analyses focused on the immunomodulatory role of galectins in H. pylori pathogenesis. Inclusion criteria required that studies directly explore the functional or mechanistic involvement of galectin family members (especially Gal-1, Gal-2, Gal-3, Gal-8, and Gal-9) in host–pathogen interactions during H. pylori infection. Articles addressing downstream effects, such as gastric inflammation, immune evasion, epithelial integrity, autophagic responses, or carcinogenesis, were prioritized. Only English-language publications were considered.
Exclusion criteria encompassed studies that did not include galectin-related mechanisms, lacked relevance to H. pylori infection, focused solely on unrelated bacterial pathogens, or presented findings without empirical data (e.g., opinion pieces, commentaries, or conference abstracts). In cases of duplicate data, the most comprehensive or recent version was retained. Special attention was given to studies elucidating the dualistic roles of galectins in enhancing mucosal defense versus facilitating bacterial persistence and their contributions to gastric carcinogenic transformation.
The selected studies were critically appraised and synthesized thematically to reflect the multifaceted roles of galectins in the context of
H. pylori colonization, immune modulation, and disease progression. The search initially identified 970 records. After removing 120 duplicates, 850 records remained for title and abstract screening. Of these, 640 were excluded due to irrelevance to the research topic. A total of 210 full-text articles were assessed for eligibility. Twenty-seven reports were excluded: 17 did not address galectins or
H. pylori, and 10 lacked original or eligible data. Ultimately, 175 studies met all inclusion criteria and were incorporated into this review. The study selection process is depicted in
Figure 3.
13. Conclusions
Galectins have emerged as central regulators in the complex immunobiological landscape of Helicobacter pylori infection. Through their context-dependent actions, these lectins contribute to both the containment of bacterial colonization and the maintenance of immune homeostasis while paradoxically enabling immune evasion and chronic persistence. Their involvement spans a wide array of processes—from innate and adaptive immune modulation, epithelial barrier reinforcement, and autophagic regulation to participation in the early events of gastric carcinogenesis. Notably, differences in galectin expression and function across age groups and gastric compartments point to a nuanced and finely tuned regulatory network that evolves with host development and disease progression. Continued investigation into galectin-mediated pathways holds promise for identifying biomarkers of disease risk and progression as well as uncovering novel targets for immunomodulatory or antimicrobial therapies. As our understanding deepens, galectins may not only illuminate the pathogenesis of H. pylori-related diseases but also offer broader insights into host–microbe interactions at mucosal surfaces.
Author Contributions
Conceptualization, B.S., I.J. and A.C.; data curation, B.S., B.S.S., M.D.S., I.J., I.M.B. and A.C.; investigation, J.N., M.P. (Milan Paunovic), M.P. (Mladen Pavlovic) and J.Z.; resources, M.P. (Marko Petrovic), D.T.-U., G.C. and N.M.; writing—original draft preparation, B.S., B.S.S., M.D.S., B.M. and J.Z.; writing—review and editing, N.Z. (Natasa Zdravkovic), M.P. (Marko Petrovic), M.P. (Mladen Pavlovic), N.Z. (Nenad Zornic) and J.N.; visualization, B.S., M.P. (Milan Paunovic) and N.M.; supervision, N.Z. (Natasa Zdravkovic), I.M.B., D.T.-U. and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Data Availability Statement
As a review article, this manuscript does not contain original research data but offers a comprehensive synthesis and critical evaluation of previously published studies. All referenced data and materials are properly cited and accessible through the cited sources. Therefore, a data availability statement is not applicable.
Acknowledgments
The authors sincerely thank the Faculty of Medical Sciences, University of Kragujevac, for their continuous support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ALIX | ALG-2-interacting protein X |
| B7-H2 | B7 homolog 2 |
| BCL-2 | B-cell lymphoma 2 |
| BCR | B-cell receptor |
| BST2 | bone marrow stromal antigen 2 |
| C-CRD | C-terminal CRD |
| CagA | cytotoxin-associated gene A |
| CD | cluster of differentiation |
| CRM1 | chromosomal region maintenance 1 |
| CRDs | carbohydrate recognition domains |
| DAMP | damage-associated molecular pattern |
| E-cadherin | epithelial cadherin |
| EAE | experimental autoimmune encephalomyelitis |
| ECM | extracellular matrix |
| ERK/MAPK | extracellular signal-regulated kinase/mitogen-activated protein kinase |
| FoxP3+ | forkhead box P3-positive |
| Gal | Galectin |
| Gal-1 | Galectin-1 |
| Gal-3 | Galectin-3 |
| Gal-7 | Galectin-7 |
| Gal-8 | Galectin-8 |
| Gal-9 | Galectin-9 |
| Gal-12 | Galectin-12 |
| GAS | Group A Streptococcus |
| G-CSF | granulocyte colony-stimulating factor |
| GM1 | ganglioside GM1 |
| IFN-γ | interferon-gamma |
| IgA | immunoglobulin A |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| LacNAc | N-acetyllactosamine |
| LeA | Lewis A |
| LeB | Lewis B |
| LeX | Lewis X |
| LeY | Lewis Y |
| LPS | lipopolysaccharides |
| MAPK | mitogen-activated protein kinase |
| MALT | mucosa-associated lymphoid tissue |
| Mgat5 | mannoside acetylglucosaminyltransferase 5 |
| MUC1 | mucin 1 |
| MUC5AC | mucin 5AC |
| mTOR | mechanistic target of rapamycin |
| N-CRD | N-terminal CRD |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK | natural killer |
| NLRP3 | NOD-like receptor family pyrin domain containing 3 |
| PDGFRβ | platelet-derived growth factor receptor beta |
| SHP1 | Src homology region 2 domain-containing phosphatase-1 |
| SP-D | surfactant protein D |
| TCR | T cell receptor |
| TGF-β | transforming growth factor-β |
| TGF-β1 | transforming growth factor-beta 1 |
| Th1 | T helper 1 |
| Th17 | T helper 17 |
| Tim-3 | T cell immunoglobulin and mucin domain-containing protein 3 |
| TNF-α | tumor necrosis factor-alpha |
| Tregs | T cells |
| VacA | vacuolating cytotoxin A |
| Yops | Yersinia outer proteins |
References
- Ali, A.; AlHussaini, K.I. Helicobacter pylori: A Contemporary Perspective on Pathogenesis, Diagnosis and Treatment Strategies. Microorganisms 2024, 12, 222. [Google Scholar] [CrossRef]
- Pereira, M.I.; Medeiros, J.A. Role of Helicobacter pylori in gastric mucosa-associated lymphoid tissue lymphomas. World J. Gastroenterol. 2014, 20, 684–698. [Google Scholar] [CrossRef]
- Vasta, G.R.; Ahmed, H.; Nita-Lazar, M.; Banerjee, A.; Pasek, M.; Shridhar, S.; Guha, P.; Fernández-Robledo, J.A. Galectins as self/non-self recognition receptors in innate and adaptive immunity: An unresolved paradox. Front. Immunol. 2012, 3, 199. [Google Scholar] [CrossRef]
- Liu, F.T.; Stowell, S.R. The role of galectins in immunity and infection. Nat. Rev. Immunol. 2023, 23, 479–494. [Google Scholar] [CrossRef]
- Sato, S.; Iwaki, J.; Hirabayashi, J. Decoding the multifaceted roles of galectins in self-defense. Semin. Immunol. 2025, 77, 101926. [Google Scholar] [CrossRef]
- Mayo, K.H. Heterologous Interactions with Galectins and Chemokines and Their Functional Consequences. Int. J. Mol. Sci. 2023, 24, 14083. [Google Scholar] [CrossRef]
- Verkerke, H.; Dias-Baruffi, M.; Cummings, R.D.; Arthur, C.M.; Stowell, S.R. Galectins: An Ancient Family of Carbohydrate Binding Proteins with Modern Functions. Methods Mol. Biol. 2022, 2442, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, Q.; Wang, D.; Wang, X.; Pan, Z.; Han, B.; He, G. Multifaceted roles of Galectins: From carbohydrate binding to targeted cancer therapy. Biomark. Res. 2025, 13, 49. [Google Scholar] [CrossRef]
- Stojanovic, M.; Stojanovic, B.; Arsenijevic, N.; Stojanovic, B. The Role of TLR-4 and Galectin-3 Interaction in Acute Pancreatitis. SJECR, 2020; in press. [Google Scholar] [CrossRef]
- Troncoso, M.F.; Elola, M.T.; Blidner, A.G.; Sarrias, L.; Espelt, M.V.; Rabinovich, G.A. The universe of galectin-binding partners and their functions in health and disease. J. Biol. Chem. 2023, 299, 105400. [Google Scholar] [CrossRef]
- Díaz-Alvarez, L.; Ortega, E. The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediat. Inflamm 2017, 2017, 9247574. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Qian, Y.; Karmakar, S.; Koyama, N.S.; Dias-Baruffi, M.; Leffler, H.; McEver, R.P.; Cummings, R.D. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J. Immunol. 2008, 180, 3091–3102. [Google Scholar] [CrossRef]
- Xu, G.; Tu, W.; Xu, C. Immunological tolerance induced by galectin-1 in rat allogeneic renal transplantation. Int. Immunopharmacol. 2010, 10, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Ilarregui, J.M.; Croci, D.O.; Bianco, G.A.; Toscano, M.A.; Salatino, M.; Vermeulen, M.E.; Geffner, J.R.; Rabinovich, G.A. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat. Immunol. 2009, 10, 981–991. [Google Scholar] [CrossRef]
- Fermin Lee, A.; Chen, H.Y.; Wan, L.; Wu, S.Y.; Yu, J.S.; Huang, A.C.; Miaw, S.C.; Hsu, D.K.; Wu-Hsieh, B.A.; Liu, F.T. Galectin-3 modulates Th17 responses by regulating dendritic cell cytokines. Am. J. Pathol. 2013, 183, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.R.; Al Rasebi, Z.; Mensah-Brown, E.; Shahin, A.; Xu, D.; Goodyear, C.S.; Fukada, S.Y.; Liu, F.T.; Liew, F.Y.; Lukic, M.L. Galectin-3 deficiency reduces the severity of experimental autoimmune encephalomyelitis. J. Immunol. 2009, 182, 1167–1173. [Google Scholar] [CrossRef]
- Fermino, M.L.; Dylon, L.S.; Cecílio, N.T.; Santos, S.N.; Toscano, M.A.; Dias-Baruffi, M.; Roque-Barreira, M.C.; Rabinovich, G.A.; Bernardes, E.S. Lack of galectin-3 increases Jagged1/Notch activation in bone marrow-derived dendritic cells and promotes dysregulation of T helper cell polarization. Mol. Immunol. 2016, 76, 22–34. [Google Scholar] [CrossRef]
- Gilson, R.C.; Gunasinghe, S.D.; Johannes, L.; Gaus, K. Galectin-3 modulation of T-cell activation: Mechanisms of membrane remodelling. Prog. Lipid Res. 2019, 76, 101010. [Google Scholar] [CrossRef]
- MacKinnon, A.C.; Farnworth, S.L.; Hodkinson, P.S.; Henderson, N.C.; Atkinson, K.M.; Leffler, H.; Nilsson, U.J.; Haslett, C.; Forbes, S.J.; Sethi, T. Regulation of alternative macrophage activation by galectin-3. J. Immunol. 2008, 180, 2650–2658. [Google Scholar] [CrossRef]
- Markovic, B.S.; Nikolic, A.; Gazdic, M.; Bojic, S.; Vucicevic, L.; Kosic, M.; Mitrovic, S.; Milosavljevic, M.; Besra, G.; Trajkovic, V.; et al. Galectin-3 Plays an Important Pro-inflammatory Role in the Induction Phase of Acute Colitis by Promoting Activation of NLRP3 Inflammasome and Production of IL-1β in Macrophages. J. Crohns. Colitis. 2016, 10, 593–606. [Google Scholar] [CrossRef]
- Arsenijevic, A.; Stojanovic, B.; Milovanovic, J.; Arsenijevic, D.; Arsenijevic, N.; Milovanovic, M. Galectin-3 in Inflammasome Activation and Primary Biliary Cholangitis Development. Int. J. Mol. Sci. 2020, 21, 5097. [Google Scholar] [CrossRef]
- Wang, W.; Qin, Y.; Song, H.; Wang, L.; Jia, M.; Zhao, C.; Gong, M.; Zhao, W. Galectin-9 Targets NLRP3 for Autophagic Degradation to Limit Inflammation. J. Immunol. 2021, 206, 2692–2699. [Google Scholar] [CrossRef]
- Quenum Zangbede, F.O.; Chauhan, A.; Sharma, J.; Mishra, B.B. Galectin-3 in M2 Macrophages Plays a Protective Role in Resolution of Neuropathology in Brain Parasitic Infection by Regulating Neutrophil Turnover. J. Neurosci. 2018, 38, 6737–6750. [Google Scholar] [CrossRef]
- Wan, L.; Lin, H.J.; Huang, C.C.; Chen, Y.C.; Hsu, Y.A.; Lin, C.H.; Lin, H.C.; Chang, C.Y.; Huang, S.H.; Lin, J.M.; et al. Galectin-12 enhances inflammation by promoting M1 polarization of macrophages and reduces insulin sensitivity in adipocytes. Glycobiology 2016, 26, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Weng, I.C.; Chen, H.L.; Lo, T.H.; Lin, W.H.; Chen, H.Y.; Hsu, D.K.; Liu, F.T. Cytosolic galectin-3 and -8 regulate antibacterial autophagy through differential recognition of host glycans on damaged phagosomes. Glycobiology 2018, 28, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Hsu, D.K.; Apgar, J.R.; Yu, L.; Sharma, B.B.; Kuwabara, I.; Izui, S.; Liu, F.T. Critical role of galectin-3 in phagocytosis by macrophages. J. Clin. Investig. 2003, 112, 389–397. [Google Scholar] [CrossRef]
- Reichert, F.; Rotshenker, S. Galectin-3 (MAC-2) Controls Microglia Phenotype Whether Amoeboid and Phagocytic or Branched and Non-phagocytic by Regulating the Cytoskeleton. Front. Cell. Neurosci. 2019, 13, 90. [Google Scholar] [CrossRef]
- Querol Cano, L.; Tagit, O.; Dolen, Y.; van Duffelen, A.; Dieltjes, S.; Buschow, S.I.; Niki, T.; Hirashima, M.; Joosten, B.; van den Dries, K.; et al. Intracellular Galectin-9 Controls Dendritic Cell Function by Maintaining Plasma Membrane Rigidity. iScience 2019, 22, 240–255. [Google Scholar] [CrossRef]
- Chen, H.L.; Lo, C.H.; Huang, C.C.; Lu, M.P.; Hu, P.Y.; Chen, C.S.; Chueh, D.Y.; Chen, P.; Lin, T.N.; Lo, Y.H.; et al. Galectin-7 downregulation in lesional keratinocytes contributes to enhanced IL-17A signaling and skin pathology in psoriasis. J. Clin. Investig. 2021, 131, e130740. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.R.; Tan, G.Z.; Cao, C.X.; Han, Y.F.; Meng, Z.; Man, X.Y.; Jiang, Z.X.; Zhang, Y.P.; Dang, N.N.; Wei, K.H.; et al. Decrease of galectin-3 in keratinocytes: A potential diagnostic marker and a critical contributor to the pathogenesis of psoriasis. J. Autoimmun. 2018, 89, 30–40. [Google Scholar] [CrossRef]
- Robinson, B.S.; Arthur, C.M.; Evavold, B.; Roback, E.; Kamili, N.A.; Stowell, C.S.; Vallecillo-Zúniga, M.L.; Van Ry, P.M.; Dias-Baruffi, M.; Cummings, R.D.; et al. The Sweet-Side of Leukocytes: Galectins as Master Regulators of Neutrophil Function. Front. Immunol. 2019, 10, 1762. [Google Scholar] [CrossRef]
- Rao, S.P.; Ge, X.N.; Sriramarao, P. Regulation of Eosinophil Recruitment and Activation by Galectins in Allergic Asthma. Front. Med. 2017, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.S.; Alves, I.; Vicente, M.; Campar, A.; Silva, M.C.; Padrão, N.A.; Pinto, V.; Fernandes, Â.; Dias, A.M.; Pinho, S.S. Glycans as Key Checkpoints of T Cell Activity and Function. Front. Immunol. 2018, 9, 2754. [Google Scholar] [CrossRef]
- Demetriou, M.; Granovsky, M.; Quaggin, S.; Dennis, J.W. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 2001, 409, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.K.; Boukhaled, G.M.; Condotta, S.A.; Mazouz, S.; Guthmiller, J.J.; Vijay, R.; Butler, N.S.; Bruneau, J.; Shoukry, N.H.; Krawczyk, C.M.; et al. Interleukin-10 Directly Inhibits CD8(+) T Cell Function by Enhancing N-Glycan Branching to Decrease Antigen Sensitivity. Immunity 2018, 48, 299–312.e5. [Google Scholar] [CrossRef]
- Chen, H.Y.; Fermin, A.; Vardhana, S.; Weng, I.C.; Lo, K.F.; Chang, E.Y.; Maverakis, E.; Yang, R.Y.; Hsu, D.K.; Dustin, M.L.; et al. Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc. Natl. Acad. Sci. USA 2009, 106, 14496–14501. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Tsao, C.H.; Lin, Y.T.; Hsu, D.K.; Chiang, M.L.; Lo, C.H.; Chien, F.C.; Chen, P.; Arthur Chen, Y.M.; Chen, H.Y.; et al. Galectin-3 promotes HIV-1 budding via association with Alix and Gag p6. Glycobiology 2014, 24, 1022–1035. [Google Scholar] [CrossRef]
- Kaur, M.; Kumar, D.; Butty, V.; Singh, S.; Esteban, A.; Fink, G.R.; Ploegh, H.L.; Sehrawat, S. Galectin-3 Regulates γ-Herpesvirus Specific CD8 T Cell Immunity. iScience 2018, 9, 101–119. [Google Scholar] [CrossRef]
- Gossink, E.M.; Coffer, P.J.; Cutilli, A.; Lindemans, C.A. Immunomodulation by galectin-9: Distinct role in T cell populations, current therapeutic avenues and future potential. Cell. Immunol. 2025, 407, 104890. [Google Scholar] [CrossRef]
- Liang, C.C.; Li, C.S.; Weng, I.C.; Chen, H.Y.; Lu, H.H.; Huang, C.C.; Liu, F.T. Galectin-9 Is Critical for Mucosal Adaptive Immunity through the T Helper 17-IgA Axis. Am. J. Pathol. 2018, 188, 1225–1235. [Google Scholar] [CrossRef]
- Perillo, N.L.; Pace, K.E.; Seilhamer, J.J.; Baum, L.G. Apoptosis of T cells mediated by galectin-1. Nature 1995, 378, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef]
- Sturm, A.; Lensch, M.; André, S.; Kaltner, H.; Wiedenmann, B.; Rosewicz, S.; Dignass, A.U.; Gabius, H.J. Human galectin-2: Novel inducer of T cell apoptosis with distinct profile of caspase activation. J. Immunol. 2004, 173, 3825–3837. [Google Scholar] [CrossRef] [PubMed]
- Pardo, E.; Cárcamo, C.; Uribe-San Martín, R.; Ciampi, E.; Segovia-Miranda, F.; Curkovic-Peña, C.; Montecino, F.; Holmes, C.; Tichauer, J.E.; Acuña, E.; et al. Galectin-8 as an immunosuppressor in experimental autoimmune encephalomyelitis and a target of human early prognostic antibodies in multiple sclerosis. PLoS ONE 2017, 12, e0177472. [Google Scholar] [CrossRef]
- Hoyer, K.K.; Pang, M.; Gui, D.; Shintaku, I.P.; Kuwabara, I.; Liu, F.T.; Said, J.W.; Baum, L.G.; Teitell, M.A. An anti-apoptotic role for galectin-3 in diffuse large B-cell lymphomas. Am. J. Pathol. 2004, 164, 893–902. [Google Scholar] [CrossRef]
- Reddy, P.B.; Sehrawat, S.; Suryawanshi, A.; Rajasagi, N.K.; Mulik, S.; Hirashima, M.; Rouse, B.T. Influence of galectin-9/Tim-3 interaction on herpes simplex virus-1 latency. J. Immunol. 2011, 187, 5745–5755. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.L.; Bernardes, E.S.; Brand, C.; dos Santos, S.N.; Cabanel, M.P.; Arcanjo, K.D.; Brito, J.M.; Borojevic, R.; Chammas, R.; El-Cheikh, M.C. Lack of galectin-3 up-regulates IgA expression by peritoneal B1 lymphocytes during B cell differentiation. Cell Tissue Res. 2016, 363, 411–426. [Google Scholar] [CrossRef]
- Giovannone, N.; Smith, L.K.; Treanor, B.; Dimitroff, C.J. Galectin-Glycan Interactions as Regulators of B Cell Immunity. Front. Immunol. 2018, 9, 2839. [Google Scholar] [CrossRef] [PubMed]
- Giovannone, N.; Liang, J.; Antonopoulos, A.; Geddes Sweeney, J.; King, S.L.; Pochebit, S.M.; Bhattacharyya, N.; Lee, G.S.; Dell, A.; Widlund, H.R.; et al. Galectin-9 suppresses B cell receptor signaling and is regulated by I-branching of N-glycans. Nat. Commun. 2018, 9, 3287. [Google Scholar] [CrossRef]
- Smith, L.K.; Fawaz, K.; Treanor, B. Galectin-9 regulates the threshold of B cell activation and autoimmunity. Elife 2021, 10, e64557. [Google Scholar] [CrossRef]
- Tsai, C.M.; Guan, C.H.; Hsieh, H.W.; Hsu, T.L.; Tu, Z.; Wu, K.J.; Lin, C.H.; Lin, K.I. Galectin-1 and galectin-8 have redundant roles in promoting plasma cell formation. J. Immunol. 2011, 187, 1643–1652. [Google Scholar] [CrossRef]
- Vasta, G.R. Roles of galectins in infection. Nat. Rev. Microbiol. 2009, 7, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Campanero-Rhodes, M.A.; Kalograiaki, I.; Euba, B.; Llobet, E.; Ardá, A.; Jiménez-Barbero, J.; Garmendia, J.; Solís, D. Exploration of Galectin Ligands Displayed on Gram-Negative Respiratory Bacterial Pathogens with Different Cell Surface Architectures. Biomolecules 2021, 11, 595. [Google Scholar] [CrossRef]
- Pirone, L.; Lenza, M.P.; Di Gaetano, S.; Capasso, D.; Filocaso, M.; Russo, R.; Di Carluccio, C.; Saviano, M.; Silipo, A.; Pedone, E. Biophysical and Structural Characterization of the Interaction between Human Galectin-3 and the Lipopolysaccharide from Pseudomonas aeruginosa. Int. J. Mol. Sci. 2024, 25, 2895. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Liu, X.; Liu, B.; Wu, X.; Wu, J.; Yan, D.; Han, L.; Tang, Z.; Yuan, X.; et al. Intracellular galectin-3 is a lipopolysaccharide sensor that promotes glycolysis through mTORC1 activation. Nat. Commun. 2022, 13, 7578. [Google Scholar] [CrossRef]
- Li, Y.; Komai-Koma, M.; Gilchrist, D.S.; Hsu, D.K.; Liu, F.T.; Springall, T.; Xu, D. Galectin-3 is a negative regulator of lipopolysaccharide-mediated inflammation. J. Immunol. 2008, 181, 2781–2789. [Google Scholar] [CrossRef]
- Ayona, D.; Fournier, P.E.; Henrissat, B.; Desnues, B. Utilization of Galectins by Pathogens for Infection. Front. Immunol. 2020, 11, 1877. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Liao, Y.; Wang, Y.; Liu, C.; Zhu, X.; Chen, Z.K.; Sun, Z. Tim-3 ligand galectin-9 reduces IL-17 level and accelerates Klebsiella pneumoniae infection. Cell. Immunol. 2011, 269, 22–28. [Google Scholar] [CrossRef]
- Lujan, A.L.; Croci, D.O.; Gambarte Tudela, J.A.; Losinno, A.D.; Cagnoni, A.J.; Mariño, K.V.; Damiani, M.T.; Rabinovich, G.A. Glycosylation-dependent galectin-receptor interactions promote Chlamydia trachomatis infection. Proc. Natl. Acad. Sci. USA 2018, 115, e6000–e6009. [Google Scholar] [CrossRef]
- Tamai, R.; Kobayashi-Sakamoto, M.; Kiyoura, Y. Extracellular galectin-1 enhances adhesion to and invasion of oral epithelial cells by Porphyromonas gingivalis. Can. J. Microbiol. 2018, 64, 465–471. [Google Scholar] [CrossRef]
- Davicino, R.C.; Méndez-Huergo, S.P.; Eliçabe, R.J.; Stupirski, J.C.; Autenrieth, I.; Di Genaro, M.S.; Rabinovich, G.A. Galectin-1-Driven Tolerogenic Programs Aggravate Yersinia enterocolitica Infection by Repressing Antibacterial Immunity. J. Immunol. 2017, 199, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.; Meyer, T.F. The Human Gastric Pathogen Helicobacter pylori and Its Association with Gastric Cancer and Ulcer Disease. Ulcers 2011, 2011, 340157. [Google Scholar] [CrossRef]
- Reyes, V.E. Helicobacter pylori Immune Response in Children Versus Adults. Med. Res. Arch. 2022, 10, 3370. [Google Scholar] [CrossRef] [PubMed]
- Keilberg, D.; Ottemann, K.M. How Helicobacter pylori senses, targets and interacts with the gastric epithelium. Environ. Microbiol. 2016, 18, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Almarmouri, C.; El-Gamal, M.I.; Haider, M.; Hamad, M.; Qumar, S.; Sebastian, M.; Ghemrawi, R.; Muhammad, J.S.; Burucoa, C.; Khoder, G. Anti-urease therapy: A targeted approach to mitigating antibiotic resistance in Helicobacter pylori while preserving the gut microflora. Gut Pathog. 2025, 17, 37. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Primers 2023, 9, 19. [Google Scholar] [CrossRef]
- Salvatori, S.; Marafini, I.; Laudisi, F.; Monteleone, G.; Stolfi, C. Helicobacter pylori and Gastric Cancer: Pathogenetic Mechanisms. Int. J. Mol. Sci. 2023, 24, 2895. [Google Scholar] [CrossRef]
- Sasaki, T.; Saito, R.; Oyama, M.; Takeuchi, T.; Tanaka, T.; Natsume, H.; Tamura, M.; Arata, Y.; Hatanaka, T. Galectin-2 Has Bactericidal Effects against Helicobacter pylori in a β-galactoside-Dependent Manner. Int. J. Mol. Sci. 2020, 21, 2697. [Google Scholar] [CrossRef]
- Fan, J.; Zhu, J.; Xu, H. Strategies of Helicobacter pylori in evading host innate and adaptive immunity: Insights and prospects for therapeutic targeting. Front. Cell. Infect. Microbiol. 2024, 14, 1342913. [Google Scholar] [CrossRef]
- Sijmons, D.; Guy, A.J.; Walduck, A.K.; Ramsland, P.A. Helicobacter pylori and the Role of Lipopolysaccharide Variation in Innate Immune Evasion. Front. Immunol. 2022, 13, 868225. [Google Scholar] [CrossRef]
- Robinson, B.; Arthur, C.; Kamili, N.; Stowell, S. Galectin Regulation of Host Microbial Interactions. Trends Glycosci. Glycotechnol. 2018, 30, SE185–SE198. [Google Scholar] [CrossRef]
- Ansari, S.; Yamaoka, Y. Survival of Helicobacter pylori in gastric acidic territory. Helicobacter 2017, 22, e12386. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.Y.; Sheu, B.S.; Wu, J.J. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed. J. 2016, 39, 14–23. [Google Scholar] [CrossRef]
- Murray, E.; Khamri, W.; Walker, M.M.; Eggleton, P.; Moran, A.P.; Ferris, J.A.; Knapp, S.; Karim, Q.N.; Worku, M.; Strong, P.; et al. Expression of surfactant protein D in the human gastric mucosa and during Helicobacter pylori infection. Infect. Immun. 2002, 70, 1481–1487. [Google Scholar] [CrossRef]
- Shamim, A.; Abdul Aziz, M.; Saeed, F.; Kumari, R.; Mary Joseph, A.; Ponnachan, P.; Kishore, U.; Masmoudi, K. Revisiting surfactant protein D: An immune surveillance molecule bridging innate and adaptive immunity. Front. Immunol. 2024, 15, 1491175. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Q.L.; Cheng, D.D.; Xu, W.T.; Lu, N.H. Adhesion and Invasion of Gastric Mucosa Epithelial Cells by Helicobacter pylori. Front. Cell. Infect. Microbiol. 2016, 6, 159. [Google Scholar] [CrossRef]
- Lee, H.; Wang, P.; Hoshino, H.; Ito, Y.; Kobayashi, M.; Nakayama, J.; Seeberger, P.H.; Fukuda, M. Alpha1,4GlcNAc-capped mucin-type O-glycan inhibits cholesterol alpha-glucosyltransferase from Helicobacter pylori and suppresses H. pylori growth. Glycobiology 2008, 18, 549–558. [Google Scholar] [CrossRef]
- Alzahrani, S.; Lina, T.T.; Gonzalez, J.; Pinchuk, I.V.; Beswick, E.J.; Reyes, V.E. Effect of Helicobacter pylori on gastric epithelial cells. World J. Gastroenterol. 2014, 20, 12767–12780. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liao, T.; Debowski, A.W.; Tang, H.; Nilsson, H.-O.; Stubbs, K.A.; Marshall, B.J.; Benghezal, M. Lipopolysaccharide Structure and Biosynthesis in Helicobacter pylori. Helicobacter 2016, 21, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Lina, T.T.; Alzahrani, S.; Gonzalez, J.; Pinchuk, I.V.; Beswick, E.J.; Reyes, V.E. Immune evasion strategies used by Helicobacter pylori. World J. Gastroenterol. 2014, 20, 12753–12766. [Google Scholar] [CrossRef]
- Fowler, M.; Thomas, R.J.; Atherton, J.; Roberts, I.S.; High, N.J. Galectin-3 binds to Helicobacter pylori O-antigen: It is upregulated and rapidly secreted by gastric epithelial cells in response to H. pylori adhesion. Cell. Microbiol. 2006, 8, 44–54. [Google Scholar] [CrossRef]
- Baj, J.; Forma, A.; Sitarz, M.; Portincasa, P.; Garruti, G.; Krasowska, D.; Maciejewski, R. Helicobacter pylori Virulence Factors-Mechanisms of Bacterial Pathogenicity in the Gastric Microenvironment. Cells 2020, 10, 27. [Google Scholar] [CrossRef]
- Neuper, T.; Frauenlob, T.; Posselt, G.; Horejs-Hoeck, J. Beyond the gastric epithelium—The paradox of Helicobacter pylori -induced immune responses. Curr. Opin. Immunol. 2022, 76, 102208. [Google Scholar] [CrossRef]
- Cadamuro, A.C.; Rossi, A.F.; Maniezzo, N.M.; Silva, A.E. Helicobacter pylori infection: Host immune response, implications on gene expression and microRNAs. World J. Gastroenterol. 2014, 20, 1424–1437. [Google Scholar] [CrossRef]
- Sun, H.; Yuan, H.; Tan, R.; Li, B.; Guo, G.; Zhang, J.; Jing, H.; Qin, Y.; Zhao, Z.; Zou, Q.; et al. Immunodominant antigens that induce Th1 and Th17 responses protect mice against Helicobacter pylori infection. Oncotarget 2018, 9, 12050–12063. [Google Scholar] [CrossRef]
- Hitzler, I.; Kohler, E.; Engler, D.B.; Yazgan, A.S.; Müller, A. The role of Th cell subsets in the control of Helicobacter infections and in T cell-driven gastric immunopathology. Front. Immunol. 2012, 3, 142. [Google Scholar] [CrossRef]
- Larussa, T.; Leone, I.; Suraci, E.; Imeneo, M.; Luzza, F. Helicobacter pylori and T Helper Cells: Mechanisms of Immune Escape and Tolerance. J. Immunol. Res. 2015, 2015, 981328. [Google Scholar] [CrossRef]
- Taylor, J.M.; Ziman, M.E.; Canfield, D.R.; Vajdy, M.; Solnick, J.V. Effects of a Th1- versus a Th2-biased immune response in protection against Helicobacter pylori challenge in mice. Microb. Pathog. 2008, 44, 20–27. [Google Scholar] [CrossRef]
- Nagata, M.; Ikuse, T.; Tokushima, K.; Arai, N.; Jimbo, K.; Kudo, T.; Shimizu, T. High galectin expression in Helicobacter pylori -infected gastric mucosa in childhood. Pediatr. Neonatol. 2025, 66, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Salama, N.R.; Hartung, M.L.; Müller, A. Life in the human stomach: Persistence strategies of the bacterial pathogen Helicobacter pylori. Nat. Rev. Microbiol. 2013, 11, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.R.; Whitmire, J.M.; Merrell, D.S. A Tale of Two Toxins: Helicobacter Pylori CagA and VacA Modulate Host Pathways that Impact Disease. Front. Microbiol. 2010, 1, 115. [Google Scholar] [CrossRef]
- Lina, T.T.; Pinchuk, I.V.; House, J.; Yamaoka, Y.; Graham, D.Y.; Beswick, E.J.; Reyes, V.E. CagA-dependent downregulation of B7-H2 expression on gastric mucosa and inhibition of Th17 responses during Helicobacter pylori infection. J. Immunol. 2013, 191, 3838–3846. [Google Scholar] [CrossRef]
- Altobelli, A.; Bauer, M.; Velez, K.; Cover, T.L.; Müller, A. Helicobacter pylori VacA Targets Myeloid Cells in the Gastric Lamina Propria To Promote Peripherally Induced Regulatory T-Cell Differentiation and Persistent Infection. mBio 2019, 10, e00388-19. [Google Scholar] [CrossRef]
- Subhash, V.V.; Ho, B. Galectin 3 acts as an enhancer of survival responses in H. pylori-infected gastric cancer cells. Cell Biol. Toxicol. 2016, 32, 23–35. [Google Scholar] [CrossRef]
- Santacroce, L.; Topi, S.; Cafiero, C.; Palmirotta, R.; Jirillo, E. The Role of the Immune Response to Helicobacter pylori Antigens and Its Relevance in Gastric Disorders. Gastrointest. Disord. 2025, 7, 6. [Google Scholar] [CrossRef]
- Skokowski, J.; Vashist, Y.; Girnyi, S.; Cwalinski, T.; Mocarski, P.; Antropoli, C.; Brillantino, A.; Boccardi, V.; Goyal, A.; Ciarleglio, F.A.; et al. The Aging Stomach: Clinical Implications of H. pylori Infection in Older Adults—Challenges and Strategies for Improved Management. Int. J. Mol. Sci. 2024, 25, 12826. [Google Scholar] [CrossRef]
- Jorge, Y.C.; Mataruco, M.M.; Araújo, L.P.; Rossi, A.F.; de Oliveira, J.G.; Valsechi, M.C.; Caetano, A.; Miyazaki, K.; Fazzio, C.S.; Thomé, J.A.; et al. Expression of annexin-A1 and galectin-1 anti-inflammatory proteins and mRNA in chronic gastritis and gastric cancer. Mediat. Inflamm 2013, 2013, 152860. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Cheng, Z.; Yang, S.; Chu, H.; Fan, Y.; Li, C.; Wong, B.H.-Y.; Zheng, S.; Zhu, Y.; et al. Functional variants regulating LGALS1 (Galectin 1) expression affect human susceptibility to influenza A(H7N9). Sci. Rep. 2015, 5, 8517. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Qian, J.; Ding, L.; Yin, S.; Zhou, L.; Zheng, S. Galectin-1: A Traditionally Immunosuppressive Protein Displays Context-Dependent Capacities. Int. J. Mol. Sci. 2023, 24, 6501. [Google Scholar] [CrossRef]
- He, J.; Baum, L.G. Presentation of galectin-1 by extracellular matrix triggers T cell death. J. Biol. Chem. 2004, 279, 4705–4712. [Google Scholar] [CrossRef]
- Sedlář, A.; Trávníčková, M.; Bojarová, P.; Vlachová, M.; Slámová, K.; Křen, V.; Bačáková, L. Interaction between Galectin-3 and Integrins Mediates Cell-Matrix Adhesion in Endothelial Cells and Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 5144. [Google Scholar] [CrossRef]
- Camby, I.; Le Mercier, M.; Lefranc, F.; Kiss, R. Galectin-1: A small protein with major functions. Glycobiology 2006, 16, 137R–157R. [Google Scholar] [CrossRef]
- Novák, J.; Takács, T.; Tilajka, Á.; László, L.; Oravecz, O.; Farkas, E.; Than, N.G.; Buday, L.; Balogh, A.; Vas, V. The sweet and the bitter sides of galectin-1 in immunity: Its role in immune cell functions, apoptosis, and immunotherapies for cancer with a focus on T cells. Semin. Immunopathol. 2025, 47, 24. [Google Scholar] [CrossRef]
- Grabowski, B.; Schmidt, M.A.; Rüter, C. Immunomodulatory Yersinia outer proteins (Yops)-useful tools for bacteria and humans alike. Virulence 2017, 8, 1124–1147. [Google Scholar] [CrossRef]
- Ayona, D.; Zarza, S.M.; Landemarre, L.; Roubinet, B.; Decloquement, P.; Raoult, D.; Fournier, P.E.; Desnues, B. Human galectin-1 and galectin-3 promote Tropheryma whipplei infection. Gut Microbes. 2021, 13, 1–15. [Google Scholar] [CrossRef]
- Suryawanshi, A.; Cao, Z.; Thitiprasert, T.; Zaidi, T.S.; Panjwani, N. Galectin-1-mediated suppression of Pseudomonas aeruginosa-induced corneal immunopathology. J. Immunol. 2013, 190, 6397–6409. [Google Scholar] [CrossRef] [PubMed]
- Almkvist, J.; Dahlgren, C.; Leffler, H.; Karlsson, A. Activation of the neutrophil nicotinamide adenine dinucleotide phosphate oxidase by galectin-1. J. Immunol. 2002, 168, 4034–4041. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.C.; Kabeya, L.M.; Azzolini, A.; Cerri, D.G.; Stowell, S.R.; Cummings, R.D.; Lucisano-Valim, Y.M.; Dias-Baruffi, M. Galectin-1 modulation of neutrophil reactive oxygen species production depends on the cell activation state. Mol. Immunol. 2019, 116, 80–89. [Google Scholar] [CrossRef]
- Law, H.L.; Wright, R.D.; Iqbal, A.J.; Norling, L.V.; Cooper, D. A Pro-resolving Role for Galectin-1 in Acute Inflammation. Front. Pharmacol. 2020, 11, 274. [Google Scholar] [CrossRef]
- Toscano, M.A.; Bianco, G.A.; Ilarregui, J.M.; Croci, D.O.; Correale, J.; Hernandez, J.D.; Zwirner, N.W.; Poirier, F.; Riley, E.M.; Baum, L.G.; et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 2007, 8, 825–834. [Google Scholar] [CrossRef] [PubMed]
- van der Leij, J.; van den Berg, A.; Blokzijl, T.; Harms, G.; van Goor, H.; Zwiers, P.; van Weeghel, R.; Poppema, S.; Visser, L. Dimeric galectin-1 induces IL-10 production in T-lymphocytes: An important tool in the regulation of the immune response. J. Pathol. 2004, 204, 511–518. [Google Scholar] [CrossRef]
- La, M.; Cao, T.V.; Cerchiaro, G.; Chilton, K.; Hirabayashi, J.; Kasai, K.; Oliani, S.M.; Chernajovsky, Y.; Perretti, M. A novel biological activity for galectin-1: Inhibition of leukocyte-endothelial cell interactions in experimental inflammation. Am. J. Pathol. 2003, 163, 1505–1515. [Google Scholar] [CrossRef]
- Kato, S.; Nakajima, S.; Nishino, Y.; Ozawa, K.; Minoura, T.; Konno, M.; Maisawa, S.; Toyoda, S.; Yoshimura, N.; Vaid, A.; et al. Association between gastric atrophy and Helicobacter pylori infection in Japanese children: A retrospective multicenter study. Dig. Dis. Sci. 2006, 51, 99–104. [Google Scholar] [CrossRef]
- Estevam, R.B.; da Silva, N.M.J.W.; da Silva, W.; Fonseca, F.M.; Oliveira, A.G.; Nogueira; Pereira, S.A.L.; Pereira, T.L.; Adad, S.J.; Rodrigues, V.J.; et al. Modulation of Galectin-3 and Galectin 9 in gastric mucosa of patients with chronic gastritis and positive Helicobacter pylori infection. Pathol. Res. Pract. 2017, 213, 1276–1281. [Google Scholar] [CrossRef]
- Loser, K.; Sturm, A.; Voskort, M.; Kupas, V.; Balkow, S.; Auriemma, M.; Sternemann, C.; Dignass, A.U.; Luger, T.A.; Beissert, S. Galectin-2 suppresses contact allergy by inducing apoptosis in activated CD8+ T cells. J. Immunol. 2009, 182, 5419–5429. [Google Scholar] [CrossRef]
- Gitt, M.A.; Massa, S.M.; Leffler, H.; Barondes, S.H. Isolation and expression of a gene encoding L-14-II, a new human soluble lactose-binding lectin. J. Biol. Chem. 1992, 267, 10601–10606. [Google Scholar] [CrossRef]
- Tamura, M.; Sato, D.; Nakajima, M.; Saito, M.; Sasaki, T.; Tanaka, T.; Hatanaka, T.; Takeuchi, T.; Arata, Y. Identification of Galectin-2-Mucin Interaction and Possible Formation of a High Molecular Weight Lattice. Biol. Pharm. Bull. 2017, 40, 1789–1795. [Google Scholar] [CrossRef]
- Nio-Kobayashi, J.; Takahashi-Iwanaga, H.; Iwanaga, T. Immunohistochemical localization of six galectin subtypes in the mouse digestive tract. J. Histochem. Cytochem. 2009, 57, 41–50. [Google Scholar] [CrossRef]
- Oka, T.; Murakami, S.; Arata, Y.; Hirabayashi, J.; Kasai, K.; Wada, Y.; Futai, M. Identification and cloning of rat galectin-2: Expression is predominantly in epithelial cells of the stomach. Arch. Biochem. Biophys. 1999, 361, 195–201. [Google Scholar] [CrossRef]
- Thomsen, M.K.; Hansen, G.H.; Danielsen, E.M. Galectin-2 at the enterocyte brush border of the small intestine. Mol. Membr. Biol. 2009, 26, 347–355. [Google Scholar] [CrossRef]
- Charkiewicz, K.; Goscik, J.; Raba, G.; Laudanski, P. Syndecan 4, galectin 2, and death receptor 3 (DR3) as novel proteins in pathophysiology of preeclampsia. J. Matern. Fetal Neonatal Med. 2021, 34, 2965–2970. [Google Scholar] [CrossRef]
- van der Laan, A.M.; Schirmer, S.H.; de Vries, M.R.; Koning, J.J.; Volger, O.L.; Fledderus, J.O.; Bastiaansen, A.J.; Hollander, M.R.; Baggen, J.M.; Koch, K.T.; et al. Galectin-2 expression is dependent on the rs7291467 polymorphism and acts as an inhibitor of arteriogenesis. Eur. Heart J. 2012, 33, 1076–1084. [Google Scholar] [CrossRef]
- Tamura, M.; Tanaka, T.; Fujii, N.; Tanikawa, T.; Oka, S.; Takeuchi, T.; Hatanaka, T.; Kishimoto, S.; Arata, Y. Potential Interaction between Galectin-2 and MUC5AC in Mouse Gastric Mucus. Biol. Pharm. Bull. 2020, 43, 356–360. [Google Scholar] [CrossRef]
- Negedu, M.N.; Duckworth, C.A.; Yu, L.G. Galectin-2 in Health and Diseases. Int. J. Mol. Sci. 2022, 24, 341. [Google Scholar] [CrossRef]
- Paclik, D.; Berndt, U.; Guzy, C.; Dankof, A.; Danese, S.; Holzloehner, P.; Rosewicz, S.; Wiedenmann, B.; Wittig, B.M.; Dignass, A.U.; et al. Galectin-2 induces apoptosis of lamina propria T lymphocytes and ameliorates acute and chronic experimental colitis in mice. J. Mol. Med. 2008, 86, 1395–1406. [Google Scholar] [CrossRef]
- Kamili, N.A.; Arthur, C.M.; Gerner-Smidt, C.; Tafesse, E.; Blenda, A.; Dias-Baruffi, M.; Stowell, S.R. Key regulators of galectin-glycan interactions. Proteomics 2016, 16, 3111–3125. [Google Scholar] [CrossRef]
- Sasaki, T.; Oyama, M.; Kubota, M.; Isshiki, Y.; Takeuchi, T.; Tanaka, T.; Tanikawa, T.; Tamura, M.; Arata, Y.; Hatanaka, T. Galectin-2 Agglutinates Helicobacter pylori via Lipopolysaccharide Containing H Type I Under Weakly Acidic Conditions. Int. J. Mol. Sci. 2024, 25, 8725. [Google Scholar] [CrossRef]
- Dimitrijevic Stojanovic, M.; Stojanovic, B.; Radosavljevic, I.; Kovacevic, V.; Jovanovic, I.; Stojanovic, B.S.; Prodanovic, N.; Stankovic, V.; Jocic, M.; Jovanovic, M. Galectin-3’s Complex Interactions in Pancreatic Ductal Adenocarcinoma: From Cellular Signaling to Therapeutic Potential. Biomolecules 2023, 13, 1500. [Google Scholar] [CrossRef]
- Stojanovic, B.S.; Stojanovic, B.; Milovanovic, J.; Arsenijević, A.; Dimitrijevic Stojanovic, M.; Arsenijevic, N.; Milovanovic, M. The Pivotal Role of Galectin-3 in Viral Infection: A Multifaceted Player in Host–Pathogen Interactions. Int. J. Mol. Sci. 2023, 24, 9617. [Google Scholar] [CrossRef]
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta 2006, 1760, 616–635. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Toscano, M.A. Turning ‘sweet’ on immunity: Galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352. [Google Scholar] [CrossRef]
- Milivojcevic Bevc, I.; Tasic-Uros, D.; Stojanovic, B.S.; Jovanovic, I.; Dimitrijevic Stojanovic, M.; Gajovic, N.; Jurisevic, M.; Radosavljevic, G.; Pantic, J.; Stojanovic, B. Redefining Immune Dynamics in Acute Pancreatitis: The Protective Role of Galectin-3 Deletion and Treg Cell Enhancement. Biomolecules 2024, 14, 642. [Google Scholar] [CrossRef]
- Rotshenker, S. Galectin-3 (MAC-2) controls phagocytosis and macropinocytosis through intracellular and extracellular mechanisms. Front. Cell. Neurosci. 2022, 16, 949079. [Google Scholar] [CrossRef]
- Morrison, H.M.; Craft, J.; Rivera-Lugo, R.; Johnson, J.R.; Golovkine, G.R.; Bell, S.L.; Dodd, C.E.; Van Dis, E.; Beatty, W.L.; Margolis, S.R.; et al. Deficiency in Galectin-3, -8, and -9 impairs immunity to chronic Mycobacterium tuberculosis infection but not acute infection with multiple intracellular pathogens. PLoS Pathog. 2023, 19, e1011088. [Google Scholar] [CrossRef]
- Lo, T.H.; Chen, H.L.; Yao, C.I.; Weng, I.C.; Li, C.S.; Huang, C.C.; Chen, N.J.; Lin, C.H.; Liu, F.T. Galectin-3 promotes noncanonical inflammasome activation through intracellular binding to lipopolysaccharide glycans. Proc. Natl. Acad. Sci. USA 2021, 118, e2026246118. [Google Scholar] [CrossRef]
- Park, A.M.; Hagiwara, S.; Hsu, D.K.; Liu, F.T.; Yoshie, O. Galectin-3 Plays an Important Role in Innate Immunity to Gastric Infection by Helicobacter pylori. Infect. Immun. 2016, 84, 1184–1193. [Google Scholar] [CrossRef]
- Reyes, R.E.; González, C.R.; Jiménez, R.C.; Herrera, M.O.; Andrade, A.A.; Karunaratne, D. Mechanisms of O-antigen structural variation of bacterial lipopolysaccharide (LPS). In The Complex World of Polysaccharides; Intechopen: London, UK, 2012; pp. 71–98. [Google Scholar]
- Mey, A.; Leffler, H.; Hmama, Z.; Normier, G.; Revillard, J.P. The animal lectin galectin-3 interacts with bacterial lipopolysaccharides via two independent sites. J. Immunol. 1996, 156, 1572–1577. [Google Scholar] [CrossRef]
- Quattroni, P.; Li, Y.; Lucchesi, D.; Lucas, S.; Hood, D.W.; Herrmann, M.; Gabius, H.J.; Tang, C.M.; Exley, R.M. Galectin-3 binds Neisseria meningitidis and increases interaction with phagocytic cells. Cell. Microbiol. 2012, 14, 1657–1675. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Wu, Y.W.; Kuo, C.F.; Lu, S.L.; Liu, F.T.; Anderson, R.; Lin, C.F.; Liu, Y.L.; Wang, W.Y.; Chen, Y.D.; et al. Galectin-3 Inhibits Galectin-8/Parkin-Mediated Ubiquitination of Group A Streptococcus. mBio 2017, 8, e00899-17. [Google Scholar] [CrossRef]
- Alqahtani, F.; Mahdavi, J.; Wheldon, L.M.; Vassey, M.; Pirinccioglu, N.; Royer, P.J.; Qarani, S.M.; Morroll, S.; Stoof, J.; Holliday, N.D.; et al. Deciphering the complex three-way interaction between the non-integrin laminin receptor, galectin-3 and Neisseria meningitidis. Open Biol. 2014, 4, 140053. [Google Scholar] [CrossRef]
- Altman, E.; Harrison, B.A.; Latta, R.K.; Lee, K.K.; Kelly, J.F.; Thibault, P. Galectin-3-mediated adherence of Proteus mirabilis to Madin-Darby canine kidney cells. Biochem. Cell. Biol. 2001, 79, 783–788. [Google Scholar] [CrossRef]
- Gupta, S.K.; Masinick, S.; Garrett, M.; Hazlett, L.D. Pseudomonas aeruginosa lipopolysaccharide binds galectin-3 and other human corneal epithelial proteins. Infect. Immun. 1997, 65, 2747–2753. [Google Scholar] [CrossRef]
- Subhash, V.V.; Ling, S.S.M.; Ho, B. Extracellular galectin-3 counteracts adhesion and exhibits chemoattraction in Helicobacter pylori -infected gastric cancer cells. Microbiology 2016, 162, 1360–1366. [Google Scholar] [CrossRef]
- Tseng, P.C.; Chen, C.L.; Shan, Y.S.; Lin, C.F. An increase in galectin-3 causes cellular unresponsiveness to IFN-γ-induced signal transduction and growth inhibition in gastric cancer cells. Oncotarget 2016, 7, 15150–15160. [Google Scholar] [CrossRef]
- Edwards, N.J.; Monteiro, M.A.; Faller, G.; Walsh, E.J.; Moran, A.P.; Roberts, I.S.; High, N.J. Lewis X structures in the O antigen side-chain promote adhesion of Helicobacter pylori to the gastric epithelium. Mol. Microbiol. 2000, 35, 1530–1539. [Google Scholar] [CrossRef]
- Moran, A.P. Relevance of fucosylation and Lewis antigen expression in the bacterial gastroduodenal pathogen Helicobacter pylori. Carbohydr. Res. 2008, 343, 1952–1965. [Google Scholar] [CrossRef]
- Figura, N.; Marano, L.; Moretti, E.; Ponzetto, A. Helicobacter pylori infection and gastric carcinoma: Not all the strains and patients are alike. World J. Gastrointest. Oncol. 2016, 8, 40–54. [Google Scholar] [CrossRef]
- Karlsson, A.; Follin, P.; Leffler, H.; Dahlgren, C. Galectin-3 Activates the NADPH-Oxidase in Exudated but not Peripheral Blood Neutrophils. Blood 1998, 91, 3430–3438. [Google Scholar] [CrossRef]
- Yoshii, T.; Fukumori, T.; Honjo, Y.; Inohara, H.; Kim, H.-R.C.; Raz, A. Galectin-3 Phosphorylation Is Required for Its Anti-apoptotic Function and Cell Cycle Arrest. J. Biol. Chem. 2002, 277, 6852–6857. [Google Scholar] [CrossRef]
- Hatakeyama, M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat. Rev. Cancer 2004, 4, 688–694. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Nakahara, S.; Hogan, V.; Raz, A. Galectin-3 in apoptosis, a novel therapeutic target. J. Bioenerg. Biomembr. 2007, 39, 79–84. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, I.J.; Cheong, T.C.; Lee, S.J.; Lotan, R.; Park, S.H.; Chun, K.H. Galectin-3 increases gastric cancer cell motility by up-regulating fascin-1 expression. Gastroenterology 2010, 138, 1035–1045.e452. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Shin, J.Y.; Lee, K.D.; Bae, Y.K.; Choi, I.J.; Park, S.H.; Chun, K.H. Galectin-3 facilitates cell motility in gastric cancer by up-regulating protease-activated receptor-1 (PAR-1) and matrix metalloproteinase-1 (MMP-1). PLoS ONE 2011, 6, e25103. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Bellis, S.L. Emerging Role of α2,6-Sialic Acid as a Negative Regulator of Galectin Binding and Function. J. Biol. Chem. 2011, 286, 5935–5941. [Google Scholar] [CrossRef]
- Leclaire, C.; Lecointe, K.; Gunning, P.A.; Tribolo, S.; Kavanaugh, D.W.; Wittmann, A.; Latousakis, D.; MacKenzie, D.A.; Kawasaki, N.; Juge, N. Molecular basis for intestinal mucin recognition by galectin-3 and C-type lectins. FASEB J. 2018, 32, 3301–3320. [Google Scholar] [CrossRef]
- Zick, Y. Galectin-8, cytokines, and the storm. Biochem. Soc. Trans. 2022, 50, 135–149. [Google Scholar] [CrossRef]
- Purić, E.; Nilsson, U.J.; Anderluh, M. Galectin-8 inhibition and functions in immune response and tumor biology. Med. Res. Rev. 2024, 44, 2236–2265. [Google Scholar] [CrossRef]
- Wu, S.; Liu, H.; Zhang, H.; Lin, C.; Li, R.; Cao, Y.; He, H.; Li, H.; Shen, Z.; Qin, J.; et al. Galectin-8 is associated with recurrence and survival of patients with non-metastatic gastric cancer after surgery. Tumour Biol. 2016, 37, 12635–12642. [Google Scholar] [CrossRef]
- Diskin, S.; Cao, Z.; Leffler, H.; Panjwani, N. The role of integrin glycosylation in galectin-8-mediated trabecular meshwork cell adhesion and spreading. Glycobiology 2009, 19, 29–37. [Google Scholar] [CrossRef]
- Xia, B.; Lu, Y.-l.; Peng, J.; Liang, J.-w.; Li, F.-q.; Ding, J.-y.; Wan, C.-w.; Le, C.-y.; Dai, J.-l.; Jie, W.; et al. Galactin-8 DNA methylation mediates macrophage autophagy through the MAPK/mTOR pathway to alleviate atherosclerosis. Sci. Rep. 2025, 15, 603. [Google Scholar] [CrossRef]
- Tribulatti, M.V.; Carabelli, J.; Prato, C.A.; Campetella, O. Galectin-8 in the onset of the immune response and inflammation. Glycobiology 2020, 30, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.L.; Lopez, K.L.; Cox, J.S.; Patrick, K.L.; Watson, R.O. Galectin-8 Senses Phagosomal Damage and Recruits Selective Autophagy Adapter TAX1BP1 To Control Mycobacterium tuberculosis Infection in Macrophages. mBio 2021, 12, e0187120. [Google Scholar] [CrossRef]
- Thurston, T.L.; Wandel, M.P.; von Muhlinen, N.; Foeglein, A.; Randow, F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 2012, 482, 414–418. [Google Scholar] [CrossRef]
- Li, F.Y.; Weng, I.C.; Lin, C.H.; Kao, M.C.; Wu, M.S.; Chen, H.Y.; Liu, F.T. Helicobacter pylori induces intracellular galectin-8 aggregation around damaged lysosomes within gastric epithelial cells in a host O-glycan-dependent manner. Glycobiology 2019, 29, 151–162. [Google Scholar] [CrossRef]
- Huff, J.L.; Hansen, L.M.; Solnick, J.V. Gastric transcription profile of Helicobacter pylori infection in the rhesus macaque. Infect. Immun. 2004, 72, 5216–5226. [Google Scholar] [CrossRef]
- Palframan, S.L.; Kwok, T.; Gabriel, K. Vacuolating cytotoxin A (VacA), a key toxin for Helicobacter pylori pathogenesis. Front. Cell. Infect. Microbiol. 2012, 2, 92. [Google Scholar] [CrossRef] [PubMed]
- Carabelli, J.; Quattrocchi, V.; D’Antuono, A.; Zamorano, P.; Tribulatti, M.V.; Campetella, O. Galectin-8 activates dendritic cells and stimulates antigen-specific immune response elicitation. J. Leukoc. Biol. 2017, 102, 1237–1247. [Google Scholar] [CrossRef]
- Moar, P.; Tandon, R. Galectin-9 as a biomarker of disease severity. Cell. Immunol. 2021, 361, 104287. [Google Scholar] [CrossRef]
- Wiersma, V.R.; de Bruyn, M.; Helfrich, W.; Bremer, E. Therapeutic potential of Galectin-9 in human disease. Med. Res. Rev. 2013, 33 (Suppl. S1), E102–E126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, C.; Li, Y.; Li, H.; Zhang, W.; Liu, J.; Wang, L.; Sun, C. Galectin-9 in cancer therapy: From immune checkpoint ligand to promising therapeutic target. Front. Cell Dev. Biol. 2024, 11, 1332205. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Leng, P.; Xu, H.; Li, X. The regulating role of galectin-9 in immune cell populations. Front. Pharmacol. 2024, 15, 1462061. [Google Scholar] [CrossRef]
- Robinson, B.S.; Saeedi, B.; Arthur, C.M.; Owens, J.; Naudin, C.; Ahmed, N.; Luo, L.; Jones, R.; Neish, A.; Stowell, S.R. Galectin-9 Is a Novel Regulator of Epithelial Restitution. Am. J. Pathol. 2020, 190, 1657–1666. [Google Scholar] [CrossRef]
- Chen, W.S.; Cao, Z.; Truong, L.; Sugaya, S.; Panjwani, N. Fingerprinting of galectins in normal, P. aeruginosa-infected, and chemically burned mouse corneas. Investig. Ophthalmol. Vis. Sci. 2015, 56, 515–525. [Google Scholar] [CrossRef]
- Nagahara, K.; Arikawa, T.; Oomizu, S.; Kontani, K.; Nobumoto, A.; Tateno, H.; Watanabe, K.; Niki, T.; Katoh, S.; Miyake, M.; et al. Galectin-9 increases Tim-3+ dendritic cells and CD8+ T cells and enhances antitumor immunity via galectin-9-Tim-3 interactions. J. Immunol. 2008, 181, 7660–7669. [Google Scholar] [CrossRef]
- Wu, C.; Thalhamer, T.; Franca, R.F.; Xiao, S.; Wang, C.; Hotta, C.; Zhu, C.; Hirashima, M.; Anderson, A.C.; Kuchroo, V.K. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity 2014, 41, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Freire de Melo, F.; Rocha, A.M.; Rocha, G.A.; Pedroso, S.H.; de Assis Batista, S.; Fonseca de Castro, L.P.; Carvalho, S.D.; Bittencourt, P.F.; de Oliveira, C.A.; Corrêa-Oliveira, R.; et al. A regulatory instead of an IL-17 T response predominates in Helicobacter pylori -associated gastritis in children. Microbes Infect. 2012, 14, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Schlichtner, S.; Meyer, N.H.; Yasinska, I.M.; Aliu, N.; Berger, S.M.; Gibbs, B.F.; Fasler-Kan, E.; Sumbayev, V.V. Functional role of galectin-9 in directing human innate immune reactions to Gram-negative bacteria and T cell apoptosis. Int. Immunopharmacol. 2021, 100, 108155. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Structural classification of galectins and their modes of glycan-mediated interactions. Galectins (Gal) are grouped into three structural types: proto-type galectins (e.g., Gal-1) contain a single carbohydrate recognition domain (CRD) and typically form non-covalent homodimers; tandem-repeat galectins (e.g., Gal-8) possess two CRDs connected by a linker peptide; and chimera-type galectins (e.g., Gal-3) have one CRD and an N-terminal domain that enables oligomerization into pentamers. The lower panel shows how each galectin type mediates biological functions: proto-type galectins support cell–extracellular matrix (ECM) interactions; tandem-repeat galectins facilitate intercellular cross-talk; and chimera-type galectins form multivalent lattices that organize membrane receptors and assemble signaling platforms.
Figure 1.
Structural classification of galectins and their modes of glycan-mediated interactions. Galectins (Gal) are grouped into three structural types: proto-type galectins (e.g., Gal-1) contain a single carbohydrate recognition domain (CRD) and typically form non-covalent homodimers; tandem-repeat galectins (e.g., Gal-8) possess two CRDs connected by a linker peptide; and chimera-type galectins (e.g., Gal-3) have one CRD and an N-terminal domain that enables oligomerization into pentamers. The lower panel shows how each galectin type mediates biological functions: proto-type galectins support cell–extracellular matrix (ECM) interactions; tandem-repeat galectins facilitate intercellular cross-talk; and chimera-type galectins form multivalent lattices that organize membrane receptors and assemble signaling platforms.
Figure 2.
Molecular pathways of Helicobacter pylori-induced immune activation, immune evasion, and modulation by galectins within the gastric mucosa. H. pylori colonization begins with bacterial motility mediated by flagella, enabling the bacterium to penetrate the gastric mucus layer and adhere to epithelial cells via outer membrane proteins and lipopolysaccharides. The bacterium deploys the type IV secretion system to translocate the CagA effector protein into host epithelial cells, where CagA activates NF-κB signaling. This leads to the upregulation of IL-8 production, driving neutrophil recruitment to the infection site, which exacerbates mucosal inflammation and tissue injury. Simultaneously, VacA toxin enters epithelial cells through endocytosis, where it disrupts mitochondrial membranes, inducing apoptosis of epithelial cells, contributing to mucosal damage. VacA also targets lysosomal membranes, leading to lysosomal dysfunction and oxidative stress, which impairs intracellular pathogen clearance. The immune system responds via Th1 and Th17 polarization, producing IFN-γ and IL-17, respectively, which drive chronic inflammation but are insufficient for bacterial eradication due to immune evasion strategies. H. pylori counters these defenses by promoting the expansion of regulatory T cells (Tregs), partly facilitated by VacA- and CagA-mediated suppression of co-stimulatory signals, which collectively inhibit Th1/Th17 responses and promote immune tolerance within the mucosa. Red arrows mean inhibition, green and black arrows mean activation.
Figure 2.
Molecular pathways of Helicobacter pylori-induced immune activation, immune evasion, and modulation by galectins within the gastric mucosa. H. pylori colonization begins with bacterial motility mediated by flagella, enabling the bacterium to penetrate the gastric mucus layer and adhere to epithelial cells via outer membrane proteins and lipopolysaccharides. The bacterium deploys the type IV secretion system to translocate the CagA effector protein into host epithelial cells, where CagA activates NF-κB signaling. This leads to the upregulation of IL-8 production, driving neutrophil recruitment to the infection site, which exacerbates mucosal inflammation and tissue injury. Simultaneously, VacA toxin enters epithelial cells through endocytosis, where it disrupts mitochondrial membranes, inducing apoptosis of epithelial cells, contributing to mucosal damage. VacA also targets lysosomal membranes, leading to lysosomal dysfunction and oxidative stress, which impairs intracellular pathogen clearance. The immune system responds via Th1 and Th17 polarization, producing IFN-γ and IL-17, respectively, which drive chronic inflammation but are insufficient for bacterial eradication due to immune evasion strategies. H. pylori counters these defenses by promoting the expansion of regulatory T cells (Tregs), partly facilitated by VacA- and CagA-mediated suppression of co-stimulatory signals, which collectively inhibit Th1/Th17 responses and promote immune tolerance within the mucosa. Red arrows mean inhibition, green and black arrows mean activation.
![Ijms 26 07216 g002]()
Figure 3.
Flowchart of study selection process.
Figure 3.
Flowchart of study selection process.
Table 1.
Comparative overview of galectin family members in innate and adaptive immunity and their roles in bacterial infections.
Table 1.
Comparative overview of galectin family members in innate and adaptive immunity and their roles in bacterial infections.
| Galectin | Innate Immunity | Adaptive Immunity | Bacterial Infections |
|---|
| Galectin-1 | - Induces immune tolerance | - Promotes T cell apoptosis (effector cells) | - Facilitates bacterial invasion via bridging host and bacterial glycoproteins (e.g., Chlamydia trachomatis, P. gingivalis) |
| - Modulates cytokine production in dendritic cells | - Regulates T cell homeostasis (prevents autoimmunity) | - Induced by bacterial effectors via MAPK signaling |
| - Suppresses neutrophil recruitment in skin inflammation | - Modulates B cell responses via glycan recognition |
| Galectin-3 | - Acts as DAMP activating innate immune cells | - Elevates TCR activation threshold by enhancing TCR glycosylation | - Binds LPS and outer membrane of Gram-negative bacteria (Salmonella, Neisseria) |
| - Enhances NLRP3 inflammasome activation (↑ IL-1β, IL-18) | - Downregulates TCR-CD8 co-localization | - Context-dependent inflammation modulation |
| - Promotes M2 macrophage polarization | - Prevents T cell apoptosis (via BCL-2 interaction) | - Suppresses autophagy (favors bacterial persistence, e.g., Listeria, Group A Streptococcus) |
| - Enhances phagocytosis (actin rearrangement) | - Inhibits plasma cell differentiation from B1 B cells |
| - Modulates neutrophil and eosinophil recruitment context-dependently |
| Galectin-4 | Tandem-repeat structure supports flexible glycan binding (limited specific roles in innate immunity defined) | - Modulates B cell responses (less characterized than Gal-1, -3, -9) | - Limited evidence |
| Galectin-7 | - Suppresses neutrophil recruitment in psoriasis and skin inflammation | - Limited evidence | - Limited evidence |
| Galectin-8 | - Promotes autophagy by recognizing damaged vacuoles | - Induces T cell apoptosis | - Promotes antibacterial autophagy |
| - Affects B cell responses via glycan interactions |
| Galectin-9 | - Attenuates NLRP3 inflammasome via autophagy of NLRP3 | - Enhances IL-17 production in CD4+ T cells (promotes Th17 differentiation) | - Induces apoptosis of pro-inflammatory Th1/Th17 cells (e.g., in Klebsiella pneumonia infection) |
| - Promotes M1 macrophage polarization | - Facilitates IgA production | - Immunosuppressive in bacterial infections |
| - Enhances phagocytosis in dendritic cells | - Induces apoptosis of effector T cells |
| - Dampens CD8+ T cell cytotoxicity |
| Galectin-12 | - Promotes M1 macrophage polarization (pro-inflammatory) | - Limited evidence | - Limited evidence |
Table 2.
Immunomodulatory functions and spatial expression of Galectin-1 during Helicobacter pylori infection, with insights into clinical relevance and potential therapeutic strategies.
Table 2.
Immunomodulatory functions and spatial expression of Galectin-1 during Helicobacter pylori infection, with insights into clinical relevance and potential therapeutic strategies.
| Function/Observation | Detail | Clinical Significance/Therapeutic Implication |
|---|
| Suppression of pro-inflammatory T cells | Induces apoptosis of Th1 and Th17 cells | Therapeutic inhibition of Gal-1 could restore Th1/Th17 responses and enhance bacterial clearance in chronic H. pylori infection |
| Cytokine modulation | ↓ IL-12, ↓ IFN-γ, ↓ TNF-α; ↑ IL-10 | Modulating Gal-1 activity may rebalance cytokine milieu to limit immune suppression |
| Innate immune modulation | Inhibits neutrophil infiltration and arachidonic acid release from LPS-activated macrophages | Potential target to mitigate immune tolerance that permits persistent colonization |
| Suppression of iNOS expression | Reduces nitric oxide synthesis in macrophages | Gal-1 inhibition might enhance macrophage bactericidal activity |
| Role in pediatric gastritis | Promotes tolerance, facilitates persistent colonization, and limits tissue damage | Explains higher H. pylori persistence in children; modulation could improve pediatric treatment strategies |
| Regional expression in gastric mucosa (children) | ↑ Gal-1 in corpus epithelium; ↑ in corpus stroma upon infection; no change in antrum | Regional targeting of Gal-1 could prevent corpus-protective, antrum-predominant gastritis patterns |
| Regional expression in gastric mucosa (adults) | No significant infection-induced change in Gal-1 expression | Gal-1 modulation may still aid in reactivating immune clearance |
| Contribution to gastritis pattern | May mitigate corpus inflammation, contributing to antral-predominant gastritis in children | Understanding Gal-1 spatial expression could guide precision therapeutics in gastritis management |
Table 3.
Antibacterial mechanisms and expression patterns of Galectin-2 in Helicobacter pylori infection alongside clinical significance and therapeutic implications.
Table 3.
Antibacterial mechanisms and expression patterns of Galectin-2 in Helicobacter pylori infection alongside clinical significance and therapeutic implications.
| Function/Observation | Detail | Clinical Significance/Therapeutic Implication |
|---|
| Antibacterial role | Directly kills H. pylori and aggregates bacteria via β-galactoside interactions | Gal-2-based therapeutic strategies could reinforce mucosal defenses and limit H. pylori colonization |
| Target specificity | Binds to H type I blood group antigen within H. pylori LPS O-antigen | Potential for glycan-targeted therapies enhancing Gal-2-mediated recognition |
| pH dependency | Binding is optimal at pH 6.0, simulating gastric microenvironment | Pharmacological stabilization of Gal-2 activity at gastric pH could optimize antibacterial effects |
| Aggregation mechanism | Homodimerizes via CRDs and crosslinks neighboring H. pylori cells | Enhancing aggregation properties may restrict bacterial motility and spread |
| Bactericidal effect | Causes bacterial death in central regions of aggregates via membrane disruption | Gal-2 mimetics could offer direct bactericidal interventions |
| Concentration dependency | Aggregation and killing are dose-dependent; inhibited by lactose | Local delivery of Gal-2 or analogs might achieve effective concentrations in gastric mucosa |
| Host expression pattern | Gal-2 expression is downregulated in gastric tissue during H. pylori infection | Therapies upregulating Gal-2 may counteract bacterial suppression mechanisms |
| Immunity-independent function | Acts without opsonins or immune cells; part of innate epithelial defense | Promotes epithelial-autonomous defense; potential adjunctive approach to antibiotic therapy |
Table 4.
Dual role of Galectin-3 in Helicobacter pylori infection and gastric carcinogenesis, including clinical significance and potential for therapeutic targeting.
Table 4.
Dual role of Galectin-3 in Helicobacter pylori infection and gastric carcinogenesis, including clinical significance and potential for therapeutic targeting.
| Function/Observation | Detail | Clinical Significance/Therapeutic Implication |
|---|
| Mucosal defense (extracellular) | Secreted by gastric epithelial cells into mucus; entraps H. pylori and limits colonization | Targeting Gal-3 may reinforce mucosal defenses to reduce bacterial burden |
| Effect on bacterial adhesion | Recombinant Gal-3 reduces adhesion of H. pylori to epithelial cells | Therapeutic supplementation of recombinant Gal-3 might prevent early colonization |
| Role in phagocyte function | Enhances killing of phagocytosed H. pylori by macrophages | Boosting Gal-3 expression in immune cells could improve intracellular pathogen clearance |
| Chemotactic activity | Promotes recruitment of monocytes and neutrophils; stimulates neutrophil respiratory burst | Exploiting Gal-3’s chemotactic properties could enhance innate immune cell infiltration to infection sites |
| Regulation by bacterial factors | Upregulated by H. pylori via CagA-dependent ERK/MAPK signaling | Blocking Gal-3 upregulation may mitigate downstream pro-oncogenic signaling |
| Interaction with bacterial LPS | Binds polymeric Lewis X motifs via CRD; promotes bacterial clustering and retention | Targeting Gal-3–LPS interactions may reduce mucosal bacterial retention and persistence |
| Intracellular anti-apoptotic activity | Inhibits apoptosis in infected epithelial cells; modulates NF-κB and IL-8 signaling | Gal-3 inhibition may restore apoptosis and reduce hyperproliferation, lowering cancer risk |
| Cellular localization dynamics | Shifts from nucleus to cytoplasm via CRM1-mediated export after infection | Pharmacologic modulation of Gal-3 localization might control its pro-survival effects |
| Role in immune evasion and transformation | Promotes epithelial cell survival and hyperproliferation; contributes to tumor microenvironment | Gal-3 could serve as a biomarker for early neoplastic changes and a target for chemopreventive interventions |
| Link to gastric cancer | Associated with early carcinogenic changes and immune resistance in gastric mucosa | Targeting Gal-3 may prevent progression from chronic gastritis to gastric cancer |
Table 5.
Functional roles of Galectin-8 during Helicobacter pylori infection, highlighting intracellular immune defense mechanisms and clinical/therapeutic implications.
Table 5.
Functional roles of Galectin-8 during Helicobacter pylori infection, highlighting intracellular immune defense mechanisms and clinical/therapeutic implications.
| Function/Observation | Detail | Clinical Significance/Therapeutic Implication |
|---|
| Expression pattern | Upregulated in gastric tissue during H. pylori infection (human and macaque models) | Gal-8 expression could serve as a biomarker for epithelial stress and infection severity |
| Intracellular accumulation | Aggregates in response to lysosomal membrane damage | Therapeutic enhancement of Gal-8 activity may improve lysosomal integrity and resistance to bacterial persistence |
| Glycan recognition | Binds exposed O-glycans on damaged lysosomes; O-glycosylation essential for recruitment | Modulating host glycosylation patterns could optimize Gal-8 recruitment and function |
| Role in selective autophagy | Recruits autophagy adaptor NDP52; colocalizes with autophagosomes | Augmenting Gal-8 function may restore defective autophagy in H. pylori-infected cells, reducing bacterial survival |
| Effect of Gal-8 depletion | Reduces autophagic flux in infected cells | Gal-8 or mimetic therapies could enhance autophagic clearance in chronic H. pylori infection |
| VacA-dependent enhancement | VacA toxin amplifies lysosomal damage and promotes Gal-8 accumulation | Gal-8 upregulation could counteract VacA-mediated cellular injury, limiting pathogen-induced damage |
| Mechanisms of VacA action | (1) Pore formation in lysosomes, (2) Mitophagy-induced oxidative stress, (3) Autophagy modulation | Understanding VacA-Gal-8 dynamics may inform combinatorial therapies that target both bacterial toxins and host defenses |
| Extracellular role | Secreted Gal-8 activates dendritic cells and enhances proinflammatory cytokine production | Extracellular Gal-8 could be harnessed to boost mucosal immune activation in settings of immune evasion |
| Dual function | Intracellular damage sensor and extracellular immune modulator | |
Table 6.
Immunoregulatory functions of Galectin-9 in Helicobacter pylori infection, with clinical significance and potential therapeutic implications.
Table 6.
Immunoregulatory functions of Galectin-9 in Helicobacter pylori infection, with clinical significance and potential therapeutic implications.
| Function/Observation | Detail | Clinical Significance/Therapeutic Implication |
|---|
| Expression pattern | Upregulated in pediatric gastric mucosa during H. pylori infection; not significantly induced in adults | Modulation of Gal-9 could enhance immune clearance in children |
| Cellular localization | Primarily localized in gastric epithelium; limited expression in stromal compartments | Spatial targeting of Gal-9 may enable compartment-specific therapeutic strategies |
| Interaction with immune checkpoints | Binds Tim-3 receptor on Th1 and Th17 cells → induces apoptosis via calcium signaling | Gal-9/Tim-3 pathway inhibition may restore Th1/Th17 responses to strengthen bacterial clearance |
| Treg cell induction | Enhances TGF-β receptor I and CD44 co-localization → promotes inducible Treg differentiation | Targeting Gal-9 may rebalance Treg/effector T cell ratios to limit immune suppression |
| Immune profile modulation | Suppresses Th1/Th17 responses; expands FoxP3+ Tregs → promotes immune tolerance | Therapeutic modulation may prevent excessive tolerance and chronic bacterial persistence |
| Age-dependent effect | Stronger immunosuppressive effect and Treg dominance in children compared to adults | Justifies developing age-adapted interventions focusing on Gal-9 inhibition in pediatric gastritis |
| Effect on inflammation | Correlates with reduced mucosal inflammation and limited immune cell recruitment in children | Fine-tuning Gal-9 levels may optimize the balance between inflammation control and effective immunity |
| Role in bacterial containment | Binds bacterial LPS; may enhance macrophage recognition and antimicrobial activity | Potential for Gal-9-based adjuvants to enhance innate immune recognition while avoiding tissue damage |
| Clinical implication | Facilitates chronic colonization with minimal tissue damage in pediatric patients | Inhibiting Gal-9 may improve H. pylori eradication rates and reduce long-term complications like gastritis or cancer |
Table 7.
Summary of structural features, immunomodulatory functions, and roles of individual galectins in H. pylori infection and gastric pathology.
Table 7.
Summary of structural features, immunomodulatory functions, and roles of individual galectins in H. pylori infection and gastric pathology.
| Galectin | Structural Type | Key Immune Functions | Role in H. pylori Infection | Effect on Bacterial Persistence | Contribution to Gastric Pathology | References |
|---|
| Galectin-1 | Proto-type (homodimer) | Promotes immune tolerance; induces Th1/Th17 apoptosis; suppresses neutrophil infiltration | Suppresses Th1/Th17-driven inflammation; favors immune tolerance in pediatric gastritis | Facilitates bacterial persistence via immunosuppression | Limits tissue damage in pediatric mucosa; potential role in immune evasion | [89,110,111,112,113,114] |
| Galectin-2 | Proto-type (homodimer) | Stabilizes epithelial junctions; crosslinks mucins; induces bacterial aggregation | Directly binds H. pylori LPS; promotes aggregation and bacterial death | Contributes to bacterial clearance via aggregation and bactericidal activity | Reinforces mucosal barrier; may counteract infection-induced damage | [68,117,123,127] |
| Galectin-3 | Chimera-type (pentamerization via N-terminal domain) | Acts as DAMP; enhances phagocytosis; modulates inflammasome activation; anti-apoptotic in T cells | Binds H. pylori LPS; enhances macrophage bacterial killing; regulates monocyte/neutrophil chemotaxis | Both limits and facilitates persistence: antimicrobial extracellularly but impairs autophagy intracellularly | Promotes epithelial survival and proliferation; implicated in gastric carcinogenesis | [11,81,94,114,136,144,145,146,147,150,151,152] |
| Galectin-8 | Tandem-repeat (two CRDs) | Senses damaged lysosomes; recruits autophagy adaptors (NDP52); stimulates dendritic cells | Marks lysosomal damage induced by H. pylori; promotes selective autophagy | Enhances bacterial clearance via autophagy | Contributes to inflammation and tissue remodeling via cytokine induction | [165,166,168] |
| Galectin-9 | Tandem-repeat (two CRDs) | Suppresses Th1/Th17; enhances Treg differentiation; induces T cell apoptosis | Induces immune tolerance; dampens pro-inflammatory responses | Facilitates persistence by limiting Th1/Th17 responses | Facilitates persistence by limiting Th1/Th17 responses | [42,89,177,178] |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).