Cardioprotective Role of Captopril: From Basic to Applied Investigations
Abstract
1. Introduction
2. Effects of Captopril on Cardiac Remodeling and Heart Function
2.1. Animal Models
| Experimental Model | Dosage | Duration of Treatment (Follow-Up) | Effects | Suggested Mechanism | References |
|---|---|---|---|---|---|
| Rat model of myocardial infarction by coronary artery ligation | 30 mg/kg/d i.p. | 30 d | Lessening of the end-diastolic pressure and the muscle mass biventricularly | Reduction of the locally generated Ang II | [38] |
| Rat model of myocardial infarction by coronary artery ligation | 2 g/L | 3 m | Preservation of maximal forward output, lessens the ventricular dilation | Preload and afterload-reducing properties | [40] |
| Rat model of myocardial infarction by coronary artery ligation | 2 g/L | 3 w | Reduction in ventricle weight; shortening of the prolongation of the time to peak tension in papillary muscle; no changes in developed tension or passive stiffness; slight enhancement in muscle function | Improvement in loading conditions | [42] |
| Rat model of myocardial infarction by coronary artery ligation | 2 g/L | 5–6 w | Unchanged LV weight; decreased RV weight; reduction in LVED pressure and LVED volume | Decrease in blood volume and increase in venous compliance, combined with afterload reduction | [45] |
| Rat model of myocardial infarction by coronary artery ligation | 2 g/L | 1 w | Enhanced the contractile performance of spared myocytes; insignificant reduction in heart weight | Prevention of myocyte length and width increase | [46] |
| Dog model of coronary artery occlusion | 0.25 mg/kg i.v. bolus +0.25 mg/kg/h | 6 h | Reduced infarct size and area at risk | Improvement in collateral flow; reduction in afterload | [49] |
| Rat model of myocardial infarction by coronary artery ligation | 2 g/L | 5 w | Preserved contractility; limited systolic dysfunction; markedly lowered LV filling pressures; normalized the restrictive LV diastolic filling pattern | Decreased preload; reduction in RV-LV interaction; cardiac interstitium alteration | [62] |
2.2. Human Investigations
3. Role of Oxidative Stress and Circulating Molecules in Captopril-Induced Cardioprotection
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| BW | Body weight |
| CI | Cardiac index |
| CO | Cardiac output |
| EF | Ejection fraction |
| eNOS | Endothelial nitric oxide synthase |
| GSH | Glutathione |
| GSH-Px | Glutathione peroxidase |
| GSH-Rd | Glutathione reductase |
| H2O2 | Hydrogen peroxide |
| HF | Heart failure |
| HOCl | Hypochlorous acid |
| HR | Heart rate |
| HW | Heart weight |
| IL | Interleukin |
| I/R | Ischemia-reperfusion |
| LDH | Lactate dehydrogenase |
| LV | Left ventricle/Left ventricular |
| LVED | Left ventricular end-diastolic |
| LVH | Left ventricular hypertrophy |
| MAP | Mean arterial pressure |
| MCF | Maximal coronary flof |
| MDA | Malonaldehyde |
| MMP | Matrix Metalloproteinase |
| MPAP | Mean pulmonary artery pressure |
| MRAP | Mean right atrial pressure |
| MRPP | Maximal rate/pressure product |
| MPCWP | Mean pulmonary-capillary wedge pressure |
| MTT | Pulmonary mean transit time |
| MWT | Maximal working time |
| MWS | Maximal workload sustained |
| NOS | Nitric oxide synthase |
| NS | Non-significant |
| O2− | Superoxide anion |
| OH· | Hydroxyl radical |
| OCl· | Hypohalite radical |
| PAH | Pulmonary arterial hypertension |
| PAP | Pulmonary arterial pressure |
| PCW | Pulmonary-wedge pressure |
| PRA | Plasma renin activity |
| PVR | Pulmonary vascular resistance |
| RAP | Right atrial pressure |
| RAS | renin-angiotensin system |
| RAAS | renin-angiotensin-aldosterone system |
| ROS | Reactive oxygen species |
| RS· | Thiyl radicals |
| RV | Right ventricle/Right ventricular |
| RVH | Right ventricular hypertrophy |
| RVHT | Renovascular hypertension |
| SH | Sulphydryl |
| SOD | Superoxide dismutase |
| SV | Stroke volume |
| SVI | Stroke volume index |
| SVR | Systemic vascular resistance |
| SW | Stroke work |
| SWI | Stroke work index |
| TAC | Thoracic aorta constriction |
| TBARS | Thiobarbituric acid reactive substances |
| TGF | Transforming Growth Factor |
| TNF | Tumor Necrosis Factor |
| TPR | Total peripheral resistance |
| TPuR | Total pulmonary resistance |
| VF | Ventricular fibrillation |
| VPB | Ventricular premature beats |
| VT | Ventricular tachycardia |
| VW | Ventricular weight |
| 1O2 | Singlet oxygen |
References
- Ferreira, S.H. A bradykinin-potentiating factor (bpf) present in the venom of Bothrops jararaca. Br. J. Pharmacol. Chemother. 1965, 24, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Bakhle, Y.S. Conversion of Angiotensin I to Angiotensin II by Cell-free Extracts of Dog Lung. Nature 1968, 220, 919–921. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.H.; Greene, L.J.; Alabaster, V.A.; Bakhle, Y.S.; Vane, J.R. Activity of Various Fractions of Bradykinin Potentiating Factor against Angiotensin I Converting Enzyme. Nature 1970, 225, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Cushman, D.W.; Ondetti, M.A. History of the design of captopril and related inhibitors of angiotensin converting enzyme. Hypertension 1991, 17, 589–592. [Google Scholar] [CrossRef]
- Thind, G.S. Angiotensin converting enzyme inhibitors: Comparative structure, pharmacokinetics, and pharmacodynamics. Cardiovasc. Drugs Ther. 1990, 4, 199–206. [Google Scholar] [CrossRef]
- Duchin, K.L.; McKinstry, D.N.; Cohen, A.I.; Migdalof, B.H. Pharmacokinetics of Captopril in Healthy Subjects and in Patients with Cardiovascular Diseases. Clin. Pharmacokinet. 1988, 14, 241–259. [Google Scholar] [CrossRef]
- Marte, F.; Sankar, P.; Patel, P.; Cassagnol, M. Captopril; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Martin, M.F.; Surrall, K.E.; McKenna, F.; Dixon, J.S.; Bird, H.A.; Wright, V. Captopril: A new treatment for rheumatoid arthritis? Lancet 1984, 1, 1325–1328. [Google Scholar] [CrossRef]
- Herlitz, H.; Tarkowski, A.; Svalander, C.; Volkmann, R.; Westberg, G. Beneficial effect of captopril on systemic lupus erythematosus-like disease in MRL lpr/lpr mice. Int. Arch. Allergy Appl. Immunol. 1988, 85, 272–277. [Google Scholar] [CrossRef]
- Nocito, C.; Lubinsky, C.; Hand, M.; Khan, S.; Patel, T.; Seliga, A.; Winfield, M.; Zuluaga-Ramirez, V.; Fernandes, N.; Shi, X.; et al. Centrally Acting Angiotensin-Converting Enzyme Inhibitor Suppresses Type I Interferon Responses and Decreases Inflammation in the Periphery and the CNS in Lupus-Prone Mice. Front. Immunol. 2020, 11, 573677. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.A.; Azad, A.; Erfani, A.; Shahriarirad, R.; Azarpira, N. Promising results of captopril in improving knee arthrofibrosis and cartilage status: An animal model study. J. Exp. Orthop. 2022, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Han, D.S.; Ha, S.K.; Choi, K.H.; Lee, H.Y. Effect of captopril on heavy proteinuria in patients with various glomerular diseases. Yonsei. Med. J. 1992, 33, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Uesugi, T.; Froh, M.; Gäbele, E.; Isayama, F.; Bradford, B.U.; Ikai, I.; Yamaoka, Y.; Arteel, G.E. Contribution of angiotensin II to alcohol-induced pancreatic fibrosis in rats. J. Pharmacol. Exp. Ther. 2004, 311, 921–928. [Google Scholar] [CrossRef]
- Yu, Q.-H.; Guo, J.-F.; Chen, Y.; Guo, X.-R.; Du, Y.-Q.; Li, Z.-S. Captopril pretreatment protects the lung against severe acute pancreatitis induced injury via inhibiting angiotensin II production and suppressing Rho/ROCK pathway. Kaohsiung J. Med. Sci. 2016, 32, 439–445. [Google Scholar] [CrossRef]
- Uhal, B.D.; Wang, R.; Laukka, J.; Zhuang, J.; Soledad-Conrad, V.; Filippatos, G. Inhibition of amiodarone-induced lung fibrosis but not alveolitis by angiotensin system antagonists. Pharmacol. Toxicol. 2003, 92, 81–87. [Google Scholar] [CrossRef]
- Alpert, M.A.; Pressly, T.A.; Mukerji, V.; Lambert, C.R.; Mukerji, B. Short- and long-term hemodynamic effects of captopril in patients with pulmonary hypertension and selected connective tissue disease. Chest 1992, 102, 1407–1412. [Google Scholar] [CrossRef]
- Volpert, O.V.; Ward, W.F.; Lingen, M.W.; Chesler, L.; Solt, D.B.; Johnson, M.D.; Molteni, A.; Polverini, P.J.; Bouck, N.P. Captopril inhibits angiogenesis and slows the growth of experimental tumors in rats. J. Clin. Investg. 1996, 98, 671–679. [Google Scholar] [CrossRef]
- Lewis, E.J.; Hunsicker, L.G.; Bain, R.P.; Rohde, R.D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 1993, 329, 1456–1462. [Google Scholar] [CrossRef]
- Gao, X.; Liu, K.; Hu, C.; Chen, K.; Jiang, Z. Captopril alleviates oxidative damage in diabetic retinopathy. Life Sci. 2022, 290, 120246. [Google Scholar] [CrossRef]
- Zandifar, E.; Sohrabi Beheshti, S.; Zandifar, A.; Haghjooy Javanmard, S. The Effect of Captopril on Impaired Wound Healing in Experimental Diabetes. Int. J. Endocrinol. 2012, 2012, 785247. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, J.P.T.; Queiroz, L.A.D.; Menikdiwela, K.R.; Pereira, N.; Ramalho, T.; Jancar, S.; Moustaid-Moussa, N.; Martins, J.O. The role of captopril in leukotriene deficient type 1 diabetic mice. Sci. Rep. 2023, 13, 22105. [Google Scholar] [CrossRef] [PubMed]
- Godsel, L.M.; Leon, J.S.; Wang, K.; Fornek, J.L.; Molteni, A.; Engman, D.M. Captopril prevents experimental autoimmune myocarditis. J. Immunol. 2003, 171, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Crouchet, E.; Li, S.; Sojoodi, M.; Bandiera, S.; Fujiwara, N.; Saghire, H.E.; Zhu, S.; Qian, T.; Rasha, F.A.; Zompo, F.D.; et al. Hepatocellular carcinoma chemoprevention by targeting the angiotensin-converting enzyme and EGFR transactivation. JCI Insight 2022, 7, e159254. [Google Scholar] [CrossRef]
- Jonsson, J.R.; Clouston, A.D.; Ando, Y.; Kelemen, L.I.; Horn, M.J.; Adamson, M.D.; Purdie, D.M.; Powell, E.E. Angiotensin-converting enzyme inhibition attenuates the progression of rat hepatic fibrosis. Gastroenterology 2001, 121, 148–155. [Google Scholar] [CrossRef]
- Barshishat-Kupper, M.; Mungunsukh, O.; Tipton, A.J.; McCart, E.A.; Panganiban, R.A.M.; Davis, T.A.; Landauer, M.R.; Day, R.M. Captopril modulates hypoxia-inducible factors and erythropoietin responses in a murine model of total body irradiation. Exp. Hematol. 2011, 39, 293–304. [Google Scholar] [CrossRef]
- Ohrui, T.; Tomita, N.; Sato-Nakagawa, T.; Matsui, T.; Maruyama, M.; Niwa, K.; Arai, H.; Sasaki, H. Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology 2004, 63, 1324–1325. [Google Scholar] [CrossRef]
- Schindler, R.; Dinarello, C.A.; Koch, K.M. Angiotensin-converting-enzyme inhibitors suppress synthesis of tumour necrosis factor and interleukin 1 by human peripheral blood mononuclear cells. Cytokine 1995, 7, 526–533. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Goodman, D.B.; Ventura, E.S. Captopril and lisinopril suppress production of interleukin-12 by human peripheral blood mononuclear cells. Immunol. Lett. 1998, 62, 25–31. [Google Scholar] [CrossRef]
- Mungunsukh, O.; George, J.; McCart, E.A.; Snow, A.L.; Mattapallil, J.J.; Mog, S.R.; Panganiban, R.A.M.; Bolduc, D.L.; Rittase, W.B.; Bouten, R.M.; et al. Captopril reduces lung inflammation and accelerated senescence in response to thoracic radiation in mice. J. Radiat. Res. 2021, 62, 236–248. [Google Scholar] [CrossRef]
- Wengrower, D.; Zanninelli, G.; Pappo, O.; Latella, G.; Sestieri, M.; Villanova, A.; Faitelson, Y.; Pines, M.; Goldin, E. Prevention of fibrosis in experimental colitis by captopril: The role of tgf-beta1. Inflamm. Bowel Dis. 2004, 10, 536–545. [Google Scholar] [CrossRef]
- Wadhwa, S.; Mumper, R.J. D-penicillamine and other low molecular weight thiols: Review of anticancer effects and related mechanisms. Cancer Lett. 2013, 337, 8–21. [Google Scholar] [CrossRef]
- Egan, B.M.; Scharf, A.; Pohl, F.; Kornfeld, K. Control of aging by the renin–angiotensin system: A review of C. elegans, Drosophila, and mammals. Front. Pharmacol. 2022, 13, 938650. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.M.; Pohl, F.; Anderson, X.; Williams, S.C.; Gregory Adodo, I.; Hunt, P.; Wang, Z.; Chiu, C.-H.; Scharf, A.; Mosley, M.; et al. The ACE inhibitor captopril inhibits ACN-1 to control dauer formation and aging. Development 2024, 151, dev202146. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N.; Ferrari, R.; Sharpe, N. Cardiac remodeling—Concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. J. Am. Coll. Cardiol. 2000, 35, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Leancă, S.A.; Crișu, D.; Petriș, A.O.; Afrăsânie, I.; Genes, A.; Costache, A.D.; Tesloianu, D.N.; Costache, I.I. Left Ventricular Remodeling after Myocardial Infarction: From Physiopathology to Treatment. Life 2022, 12, 1111. [Google Scholar] [CrossRef]
- Mill, J.G.; Milanez Mda, C.; Busatto, V.C.; de Moraes, A.C.; Gomes Mda, G. Activation of the cardiac angiotensin-converting enzyme after myocardial infarction and its role in ventricular remodeling. Arq. Bras. Cardiol. 1997, 69, 101–110. [Google Scholar] [CrossRef][Green Version]
- Gay, R.G. Early and late effects of captopril treatment after large myocardial infarction in rats. J. Am. Coll. Cardiol. 1990, 16, 967–977. [Google Scholar] [CrossRef]
- Pfeffer, J.M.; Pfeffer, M.A.; Braunwald, E. Influence of chronic captopril therapy on the infarcted left ventricle of the rat. Circ. Res. 1985, 57, 84–95. [Google Scholar] [CrossRef]
- Litwin, S.E.; Litwin, C.M.; Raya, T.E.; Warner, A.L.; Goldman, S. Contractility and stiffness of noninfarcted myocardium after coronary ligation in rats. Effects of chronic angiotensin converting enzyme inhibition. Circulation 1991, 83, 1028–1037. [Google Scholar] [CrossRef]
- Litwin, S.E.; Raya, T.E.; Warner, A.; Litwin, C.M.; Goldman, S. Effects of captopril on contractility after myocardial infarction: Experimental observations. Am. J. Cardiol. 1991, 68, 26–34. [Google Scholar] [CrossRef]
- Gallego, M.; Espiña, L.; Vegas, L.; Echevarria, E.; Iriarte, M.M.; Casis, O. Spironolactone and captopril attenuates isoproterenol-induced cardiac remodelling in rats. Pharmacol. Res. 2001, 44, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, J.M.; Pfeffer, M.A.; Braunwald, E. Hemodynamic benefits and prolonged survival with long-term captopril therapy in rats with myocardial infarction and heart failure. Circulation 1987, 75, I149–I155. [Google Scholar] [PubMed]
- Raya, T.E.; Gay, R.G.; Aguirre, M.; Goldman, S. Importance of venodilatation in prevention of left ventricular dilatation after chronic large myocardial infarction in rats: A comparison of captopril and hydralazine. Circ. Res. 1989, 64, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Capasso, J.M.; Anversa, P. Mechanical performance of spared myocytes after myocardial infarction in rats: Effects of captopril treatment. Am. J. Physiol. 1992, 263, H841–H849. [Google Scholar] [CrossRef]
- Bélichard, P.; Savard, P.; Cardinal, R.; Nadeau, R.; Gosselin, H.; Paradis, P.; Rouleau, J.L. Markedly different effects on ventricular remodeling result in a decrease in inducibility of ventricular arrhythmias. J. Am. Coll. Cardiol. 1994, 23, 505–513. [Google Scholar] [CrossRef]
- Jugdutt, B.I.; Schwarz-Michorowski, B.L.; Khan, M.I. Effect of long-term captopril therapy on left ventricular remodeling and function during healing of canine myocardial infarction. J. Am. Coll. Cardiol. 1992, 19, 713–721. [Google Scholar] [CrossRef]
- Ertl, G.; Kloner, R.A.; Alexander, R.W.; Braunwald, E. Limitation of experimental infarct size by an angiotensin-converting enzyme inhibitor. Circulation 1982, 65, 40–48. [Google Scholar] [CrossRef]
- Jugdutt, B.I. Effect of captopril and enalapril on left ventricular geometry, function and collagen during healing after anterior and inferior myocardial infarction in a dog model. J. Am. Coll. Cardiol. 1995, 25, 1718–1725. [Google Scholar] [CrossRef]
- Daniell, H.B.; Carson, R.R.; Ballard, K.D.; Thomas, G.R.; Privitera, P.J. Effects of captopril on limiting infarct size in conscious dogs. J. Cardiovasc. Pharmacol. 1984, 6, 1043–1047. [Google Scholar] [CrossRef]
- De Graeff, P.A.; Van Gilst, W.H.; Cees, K.B.; De Langen, D.J.; Kingma, J.H.; Wesseling, H. Concentration-Dependent Protection by Captopril Against Myocardial Damage During Ischemia and Reperfusion in a Closed Chest Pig Model. J. Cardiovasc. Pharmacol. 1987, 9, S37–S42. [Google Scholar] [CrossRef] [PubMed]
- Cushman, D.W.; Wang, F.L.; Fung, W.C.; Grover, G.J.; Harvey, C.M.; Scalese, R.J.; Mitch, S.L.; DeForrest, J.M. Comparisons in vitro, ex vivo, and in vivo of the actions of seven structurally diverse inhibitors of angiotensin converting enzyme (ACE). Br. J. Clin. Pharmacol. 1989, 28 (Suppl. S2), discussion 130S–131S. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.M.; Hutchinson, E.C.; Jones, A.M. Ventricular Weight in Cardiac Hypertrophy. Heart 1952, 14, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Raya, T.E.; Fonken, S.J.; Lee, R.W.; Daugherty, S.; Goldman, S.; Wong, P.C.; Timmermans, P.B.; Morkin, E. Hemodynamic effects of direct angiotensin II blockade compared to converting enzyme inhibition in rat model of heart failure. Am. J. Hypertens. 1991, 4, 334S–340S. [Google Scholar] [CrossRef]
- Capasso, J.M.; Li, P.; Meggs, L.G.; Herman, M.V.; Anversa, P. Efficacy of angiotensin-converting enzyme inhibition and AT1 receptor blockade on cardiac pump performance after myocardial infarction in rats. J. Cardiovasc. Pharmacol. 1994, 23, 584–593. [Google Scholar] [CrossRef]
- Mill, J.G.; Gomes, A.P.; Carrara, A.B.; Gomes, M.G.; Vassallo, D.V. Influence of chronic captopril therapy on the mechanical performance of the infarcted rat heart. Pharmacol. Res. 1994, 29, 77–88. [Google Scholar] [CrossRef]
- Da Conceição Milanez, M.; Da Gloria De Souza Gomes, M.; Vassallo, D.V.; Mill, J. Effects of captopril on interstitial collagen in the myocardium after infarction in rats. J. Card. Fail. 1997, 3, 189–197. [Google Scholar] [CrossRef]
- Yamada, H.; Fabris, B.; Allen, A.M.; Jackson, B.; Johnston, C.I.; Mendelsohn, A.O. Localization of angiotensin converting enzyme in rat heart. Circ. Res. 1991, 68, 141–149. [Google Scholar] [CrossRef]
- Lindpaintner, K.; Jin, M.W.; Niedermaier, N.; Wilhelm, M.J.; Ganten, D. Cardiac angiotensinogen and its local activation in the isolated perfused beating heart. Circ. Res. 1990, 67, 564–573. [Google Scholar] [CrossRef]
- Hirsch, A.T.; Talsness, C.E.; Schunkert, H.; Paul, M.; Dzau, V.J. Tissue-specific activation of cardiac angiotensin converting enzyme in experimental heart failure. Circ. Res. 1991, 69, 475–482. [Google Scholar] [CrossRef]
- Litwin, S.E.; Katz, S.E.; Morgan, J.P.; Douglas, P.S. Long-term captopril treatment improves diastolic filling more than systolic performance in rats with large myocardial infarction. J. Am. Coll. Cardiol. 1996, 28, 773–781. [Google Scholar] [CrossRef]
- Mitręga, K.A.; Spałek, A.M.; Nożyński, J.; Porc, M.; Stankiewicz, M.; Krzemiński, T.F. Cardiomyopathy development protection after myocardial infarction in rats: Successful competition for major dihydropyridines’ common metabolite against captopril. PLoS ONE 2017, 12, e0179633. [Google Scholar] [CrossRef]
- Chiba, S.; Watanabe, H.; Kobayashi, M. Chronotropic and inotropic effects of captopril on the dog heart. Jpn. Heart J. 1983, 24, 269–275. [Google Scholar] [CrossRef]
- Sigurdsson, A.; Swedberg, K. The role of neurohormonal activation in chronic heart failure and postmyocardial infarction. Am. Heart J. 1996, 132, 229–234. [Google Scholar] [CrossRef]
- Michel, J.B.; Lattion, A.L.; Salzmann, J.L.; Cerol, M.L.; Philippe, M.; Camilleri, J.P.; Corvol, P. Hormonal and cardiac effects of converting enzyme inhibition in rat myocardial infarction. Circ. Res. 1988, 62, 641–650. [Google Scholar] [CrossRef]
- Glantz, S.A.; Kernoff, R.S. Muscle stiffness determined from canine left ventricular pressure-volume curves. Circ. Res. 1975, 37, 787–794. [Google Scholar] [CrossRef]
- Anversa, P.; Olivetti, G.; Melissari, M.; Loud, A.V. Stereological measurement of cellular and subcellular hypertrophy and hyperplasia in the papillary muscle of adult rat. J. Mol. Cell. Cardiol. 1980, 12, 781–795. [Google Scholar] [CrossRef]
- Roberts, W.C.; Cohen, L.S. Left ventricular papillary muscles. Description of the normal and a survey of conditions causing them to be abnormal. Circulation 1972, 46, 138–154. [Google Scholar] [CrossRef]
- Eghbali, M.; Weber Karl, T. Collagen and the myocardium: Fibrillar structure, biosynthesis and degradation in relation to hypertrophy and its regression. Mol. Cell. Biochem. 1990, 96, 1–14. [Google Scholar] [CrossRef]
- Weber, K.T.; Sun, Y.; Tyagi, S.C.; Cleutjens, J.P. Collagen network of the myocardium: Function, structural remodeling and regulatory mechanisms. J. Mol. Cell. Cardiol. 1994, 26, 279–292. [Google Scholar] [CrossRef]
- Pelouch, V.; Dixon, I.M.; Golfman, L.; Beamish, R.E.; Dhalla, N.S. Role of extracellular matrix proteins in heart function. Mol. Cell. Biochem. 1993, 129, 101–120. [Google Scholar] [CrossRef]
- Nikolov, A.; Popovski, N. Extracellular Matrix in Heart Disease: Focus on Circulating Collagen Type I and III Derived Peptides as Biomarkers of Myocardial Fibrosis and Their Potential in the Prognosis of Heart Failure: A Concise Review. Metabolites 2022, 12, 297. [Google Scholar] [CrossRef]
- Volders, P.G.A.; Willems, I.E.M.G.; Cleutjens, J.P.M.; Aren, J.-W.; Havenith, M.G.; Daemen, M.J.A.P. Interstitial Collagen is Increased in the Non-infarcted Human Myocardium After Myocardial Infarction. J. Mol. Cell. Cardiol. 1993, 25, 1317–1323. [Google Scholar] [CrossRef]
- Leite, C.M.; Gomes, M.G.; Vassallo, D.V.; Mill, J.G. Changes in collagen content in the residual myocardium surviving after infarction in rats. Influence of propranolol or hydralazine therapy. Arch. Med. Res. 1995, 26, 79–84. [Google Scholar]
- Vankrimpen, C.; Smits, J.; Cleutjens, J.; Debets, J.; Schoemaker, R.; Boudier, H.; Bosman, F.; Daemen, M. DNA synthesis in the non-infarcted cardiac interstitium after left coronary artery ligation in the rat: Effects of captopril. J. Mol. Cell. Cardiol. 1991, 23, 1245–1253. [Google Scholar] [CrossRef]
- Taylor, K.; Patten, R.D.; Smith, J.J.; Aronovitz, M.J.; Wight, J.; Salomon, R.N.; Konstam, M.A. Divergent Effects of Angiotensin-Converting Enzyme Inhibition and Angiotensin II-Receptor Antagonism on Myocardial Cellular Proliferation and Collagen Deposition After Myocardial Infarction in Rats. J. Cardiovasc. Pharmacol. 1998, 31, 654–660. [Google Scholar] [CrossRef]
- van Krimpen, C.; Schoemaker, R.G.; Cleutjens, J.P.; Smits, J.F.; Struyker-Boudier, H.A.; Bosman, F.T.; Daemen, M.J. Angiotensin I converting enzyme inhibitors and cardiac remodeling. Basic Res. Cardiol. 1991, 86 (Suppl. S1), 149–155. [Google Scholar]
- Brilla, C.G.; Zhou, G.; Matsubara, L.; Weber, K.T. Collagen Metabolism in Cultured Adult Rat Cardiac Fibroblasts: Response to Angiotensin II and Aldosterone. J. Mol. Cell. Cardiol. 1994, 26, 809–820. [Google Scholar] [CrossRef]
- Aceto, J.F.; Baker, K.M. [Sar1]angiotensin II receptor-mediated stimulation of protein synthesis in chick heart cells. Am. J. Physiol.-Heart Circ. Physiol. 1990, 258, H806–H813. [Google Scholar] [CrossRef]
- McEwan, P.E.; Gray, G.A.; Sherry, L.; Webb, D.J.; Kenyon, C.J. Differential Effects of Angiotensin II on Cardiac Cell Proliferation and Intramyocardial Perivascular Fibrosis In Vivo. Circulation 1998, 98, 2765–2773. [Google Scholar] [CrossRef]
- Jalil, J.E.; Janicki, J.S.; Pick, R.; Weber, K.T. Coronary Vascular Remodeling and Myocardial Fibrosis in the Rat with Renovascular Hypertension Response to Captopril. Am. J. Hypertens. 1991, 4, 51–55. [Google Scholar] [CrossRef]
- Brilla, C.G.; Pick, R.; Tan, L.B.; Janicki, J.S.; Weber, K.T. Remodeling of the rat right and left ventricles in experimental hypertension. Circ. Res. 1990, 67, 1355–1364. [Google Scholar] [CrossRef]
- Schorb, W.; Booz, G.W.; Dostal, D.E.; Conrad, K.M.; Chang, K.C.; Baker, K.M. Angiotensin II is mitogenic in neonatal rat cardiac fibroblasts. Circ. Res. 1993, 72, 1245–1254. [Google Scholar] [CrossRef]
- Touyz, R.M.; Deng, L.Y.; He, G.; Wu, X.H.; Schiffrin, E.L. Angiotensin II stimulates DNA and protein synthesis in vascular smooth muscle cells from human arteries: Role of extracellular signal-regulated kinases. J. Hypertens. 1999, 17, 907–916. [Google Scholar] [CrossRef]
- Katz, A.M. Angiotensin II: Hemodynamic regulator or growth factor? J. Mol. Cell. Cardiol. 1990, 22, 739–747. [Google Scholar] [CrossRef]
- Tian, G.; Ren, T. Mechanical stress regulates the mechanotransduction and metabolism of cardiac fibroblasts in fibrotic cardiac diseases. Eur. J. Cell Biol. 2023, 102, 151288. [Google Scholar] [CrossRef]
- Gallagher, A.M.; Yu, H.; Printz, M.P. Bradykinin-Induced Reductions in Collagen Gene Expression Involve Prostacyclin. Hypertension 1998, 32, 84–88. [Google Scholar] [CrossRef]
- Harding, P.; LaPointe, M.C. Prostaglandin E2 increases cardiac fibroblast proliferation and increases cyclin D expression via EP1 receptor. Prostaglandins Leukot. Essent. Fat. Acids (PLEFA) 2011, 84, 147–152. [Google Scholar] [CrossRef]
- Tanner, M.A.; Thomas, T.P.; Maitz, C.A.; Grisanti, L.A. β2-Adrenergic Receptors Increase Cardiac Fibroblast Proliferation Through the Gαs/ERK1/2-Dependent Secretion of Interleukin-6. IJMS 2020, 21, 8507. [Google Scholar] [CrossRef]
- Cleutjens, J.P.; Verluyten, M.J.; Smiths, J.F.; Daemen, M.J. Collagen remodeling after myocardial infarction in the rat heart. Am. J. Pathol. 1995, 147, 325–338. [Google Scholar]
- Sun, Y.; Zhang, J.Q.; Zhang, J.; Lamparter, S. Cardiac remodeling by fibrous tissue after infarction in rats. J. Lab. Clin. Med. 2000, 135, 316–323. [Google Scholar] [CrossRef]
- Schoemaker, R. Delayed but not immediate captopril therapy improves cardiac function in conscious rats, following myocardial infarction. J. Mol. Cell. Cardiol. 1991, 23, 187–197. [Google Scholar] [CrossRef]
- Nelissen-Vrancken, H. Early captopril treatment inhibits DNA synthesis in endothelial cells and normalization of maximal coronary flow in infarcted rat hearts. Cardiovasc. Res. 1998, 40, 156–164. [Google Scholar] [CrossRef]
- Caspari, P.G.; Gibson, K.; Harris, P. Changes in myocardial collagen in normal development and after beta blockade. Recent Adv. Stud. Card. Struct. Metab. 1975, 7, 99–104. [Google Scholar]
- Bonnin, C.M.; Sparrow, M.P.; Taylor, R.R. Collagen synthesis and content in right ventricular hypertrophy in the dog. Am. J. Physiol. 1981, 241, H708–H713. [Google Scholar] [CrossRef]
- Oken, D.E.; Boucek, R.J. Quantitation of collagen in human myocardium. Circ. Res. 1957, 5, 357–361. [Google Scholar] [CrossRef]
- Miles, C.; Westaby, J.; Ster, I.C.; Asimaki, A.; Boardman, P.; Joshi, A.; Papadakis, M.; Sharma, S.; Behr, E.R.; Sheppard, M.N. Morphometric characterization of collagen and fat in normal ventricular myocardium. Cardiovasc. Pathol. 2020, 48, 107224. [Google Scholar] [CrossRef]
- Schelbert, E.B.; Fonarow, G.C.; Bonow, R.O.; Butler, J.; Gheorghiade, M. Therapeutic targets in heart failure: Refocusing on the myocardial interstitium. J. Am. Coll. Cardiol. 2014, 63, 2188–2198. [Google Scholar] [CrossRef]
- Fishbein, M.C.; Maclean, D.; Maroko, P.R. Experimental myocardial infarction in the rat: Qualitative and quantitative changes during pathologic evolution. Am. J. Pathol. 1978, 90, 57–70. [Google Scholar]
- Pfeffer, M.A.; Braunwald, E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990, 81, 1161–1172. [Google Scholar] [CrossRef]
- Jin, H.; Yang, R.; Awad, T.A.; Wang, F.; Li, W.; Williams, S.-P.; Ogasawara, A.; Shimada, B.; Williams, P.M.; De Feo, G.; et al. Effects of Early Angiotensin-Converting Enzyme Inhibition on Cardiac Gene Expression After Acute Myocardial Infarction. Circulation 2001, 103, 736–742. [Google Scholar] [CrossRef]
- Chen, S.; Su, J.; Wu, K.; Hu, W.; Gardner, D.G.; Chen, D. Early captopril treatment prevents hypertrophydependent gene expression in hearts of SHR. Am. J. Physiol. 1998, 274, R1511–R1517. [Google Scholar] [CrossRef]
- Wu, J.N.; Berecek, K.H. Prevention of genetic hypertension by early treatment of spontaneously hypertensive rats with the angiotensin converting enzyme inhibitor captopril. Hypertension 1993, 22, 139–146. [Google Scholar] [CrossRef]
- Lee, R.M.; Berecek, K.H.; Tsoporis, J.; McKenzie, R.; Triggle, C.R. Prevention of hypertension and vascular changes by captopril treatment. Hypertension 1991, 17, 141–150. [Google Scholar] [CrossRef]
- Chen, S.C.; Chen, D.G.; Bao, Y.D.; Jing, X.Q.; Lin, Y.Q.; Wang, H.J. Inhibition of left ventricular hypertrophy and expression of proto-oncogenes c-myc other than c-fos in myocardium by early captopril treatment in SHR rats. Zhongguo Yao Li Xue Bao 1995, 16, 217–222. [Google Scholar]
- Chen, S.C.; Chen, D.G.; Bao, Y.D. Mechanism of inhibition in left ventricular hypertrophy by captopril treatment in spontaneously hypertensive rats. Zhonghua Yi Xue Za Zhi 1995, 75, 74–78, 125. [Google Scholar]
- Rocha, W.A.; Lunz, W.; Baldo, M.P.; Pimentel, E.B.; Dantas, E.M.; Rodrigues, S.L.; Mill, J.G. Kinetics of cardiac and vascular remodeling by spontaneously hypertensive rats after discontinuation of long-term captopril treatment. Braz. J. Med. Biol. Res. 2010, 43, 390–396. [Google Scholar] [CrossRef]
- McDonald, K.M.; Chu, C.; Francis, G.S.; Carlyle, W.; Judd, D.L.; Hauer, K.; Hartman, M.; Cohn, J.N. Effect of delayed intervention with ACE-inhibitor therapy on myocyte hypertrophy and growth of the cardiac interstitium in the rat model of myocardial infarction. J. Mol. Cell. Cardiol. 1997, 29, 3203–3210. [Google Scholar] [CrossRef]
- Gould, K.E.; Taffet, G.E.; Michael, L.H.; Christie, R.M.; Konkol, D.L.; Pocius, J.S.; Zachariah, J.P.; Chaupin, D.F.; Daniel, S.L.; Sandusky, G.E.; et al. Heart failure and greater infarct expansion in middle-aged mice: A relevant model for postinfarction failure. Am. J. Physiol.-Heart Circ. Physiol. 2002, 282, H615–H621. [Google Scholar] [CrossRef]
- Roostalu, U.; Thisted, L.; Skytte, J.L.; Salinas, C.G.; Pedersen, P.J.; Hecksher-Sørensen, J.; Rolin, B.; Hansen, H.H.; MacKrell, J.G.; Christie, R.M.; et al. Effect of captopril on post-infarction remodelling visualized by light sheet microscopy and echocardiography. Sci. Rep. 2021, 11, 5241. [Google Scholar] [CrossRef]
- Spinale, F.G. Myocardial Matrix Remodeling and the Matrix Metalloproteinases: Influence on Cardiac Form and Function. Physiol. Rev. 2007, 87, 1285–1342. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases: Structure, Function, and Biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Sakata, Y.; Yamamoto, K.; Mano, T.; Nishikawa, N.; Yoshida, J.; Hori, M.; Miwa, T.; Masuyama, T. Activation of Matrix Metalloproteinases Precedes Left Ventricular Remodeling in Hypertensive Heart Failure Rats: Its Inhibition as a Primary Effect of Angiotensin-Converting Enzyme Inhibitor. Circulation 2004, 109, 2143–2149. [Google Scholar] [CrossRef]
- Brower, G.L.; Levick, S.P.; Janicki, J.S. Inhibition of matrix metalloproteinase activity by ACE inhibitors prevents left ventricular remodeling in a rat model of heart failure. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H3057–H3064. [Google Scholar] [CrossRef]
- Li, M.; Zhou, T.-F.; Liu, H.-M.; Hua, Y.-M.; Wang, X.-M. Effect of captopril on pulmonary vascular remodeling induced by left-to-right shunt in rats. Sichuan Da Xue Xue Bao Yi Xue Ban 2006, 37, 242–245. [Google Scholar]
- Okada, M.; Kikuzuki, R.; Harada, T.; Hori, Y.; Yamawaki, H.; Hara, Y. Captopril Attenuates Matrix Metalloproteinase-2 and -9 in Monocrotaline-Induced Right Ventricular Hypertrophy in Rats. J. Pharmacol. Sci. 2008, 108, 487–494. [Google Scholar] [CrossRef]
- Wang, X.M.; Shi, K.; Li, J.J.; Chen, T.T.; Guo, Y.H.; Liu, Y.L.; Yang, Y.F.; Yang, S. Effects of angiotensin II intervention on MMP-2, MMP-9, TIMP-1, and collagen expression in rats with pulmonary hypertension. Genet. Mol. Res. 2015, 14, 1707–1717. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, T.; Liu, B.; Wei, L.; Shi, K.; Zhao, S.; Hua, Y.; Liu, H. Changes of MMP-2, 9 and TIMP-1 expressions in rats with pulmonary arterial hypertension after captopril and losartan interventions. Sichuan Da Xue Xue Bao Yi Xue Ban 2009, 40, 255–259. [Google Scholar]
- Cho, Y.K.; Eom, G.H.; Kee, H.J.; Kim, H.-S.; Choi, W.-Y.; Nam, K.-I.; Ma, J.S.; Kook, H. Sodium Valproate, a Histone Deacetylase Inhibitor, but Not Captopril, Prevents Right Ventricular Hypertrophy in Rats. Circ. J. 2010, 74, 760–770. [Google Scholar] [CrossRef]
- Koide, M.; Carabello, B.A.; Conrad, C.C.; Buckley, J.M.; DeFreyte, G.; Barnes, M.; Tomanek, R.J.; Wei, C.C.; Dell’Italia, L.J.; Cooper, G.; et al. Hypertrophic response to hemodynamic overload: Role of load vs. renin-angiotensin system activation. Am. J. Physiol. 1999, 276, H350–H358. [Google Scholar] [CrossRef]
- Okada, M.; Kosaka, N.; Hoshino, Y.; Yamawaki, H.; Hara, Y. Effects of Captopril and Telmisartan on Matrix Metalloproteinase-2 and -9 Expressions and Development of Left Ventricular Fibrosis Induced by Isoprenaline in Rats. Biol. Pharm. Bull. 2010, 33, 1517–1521. [Google Scholar] [CrossRef]
- Campbell, S.E.; Janicki, J.S.; Weber, K.T. Temporal differences in fibroblast proliferation and phenotype expression in response to chronic administration of angiotensin II or aldosterone. J. Mol. Cell. Cardiol. 1995, 27, 1545–1560. [Google Scholar] [CrossRef]
- Brilla, C.G.; Matsubara, L.S.; Weber, K.T. Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J. Mol. Cell. Cardiol. 1993, 25, 563–575. [Google Scholar] [CrossRef]
- Leenen, F.H.H.; White, R.; Yuan, B. Isoproterenol-induced cardiac hypertrophy: Role of circulatory versus cardiac renin-angiotensin system. Am. J. Physiol.-Heart Circ. Physiol. 2001, 281, H2410–H2416. [Google Scholar] [CrossRef]
- Golomb, E.; Abassi, Z.A.; Cuda, G.; Stylianou, M.; Panchal, V.R.; Trachewsky, D.; Keiser, H.R. Angiotensin II maintains, but does not mediate, isoproterenol-induced cardiac hypertrophy in rats. Am. J. Physiol. 1994, 267, H1496–H1506. [Google Scholar] [CrossRef]
- Shimizu, M.; Irimajiri, O.; Nakano, T.; Mizokami, T.; Ogawa, K.; Sanjo, J.; Yamada, H.; Sasaki, H.; Isogai, Y. Effect of captopril on isoproterenol-induced myocardial ornithine decarboxylase activity. J. Mol. Cell. Cardiol. 1991, 23, 665–670. [Google Scholar] [CrossRef]
- Nakano, M.; Kanda, T.; Matsuzaki, S.; Hasegawa, S.; Ma, H.; Imai, S.; Suzuki, T.; Kobayashi, I. Effect of losartan, an AT1 selective angiotensin II receptor antagonist, on isoproterenol-induced cardiac ornithine decarboxylase activity. Res. Commun. Mol. Pathol. Pharmacol. 1995, 88, 21–30. [Google Scholar]
- Shimizu, M.; Sasaki, H.; Sanjo, J.; Ogawa, K.; Mizokami, T.; Yagi, T.; Kato, H.; Hamaya, K.; Namiki, A.; Isogai, Y. Effect of captopril on isoproterenol-induced cardiac hypertrophy and polyamine contents. Jpn. Circ. J. 1992, 56, 1130–1137. [Google Scholar] [CrossRef]
- Laycock, S.K.; Kane, K.A.; McMurray, J.; Parratt, J.R. Captopril and Norepinephrine-Induced Hypertrophy and Haemodynamics in Rats. J. Cardiovasc. Pharmacol. 1996, 27, 667. [Google Scholar] [CrossRef]
- Davis, J.O.; Freeman, R.H. Mechanisms regulating renin release. Physiol. Rev. 1976, 56, 1–56. [Google Scholar] [CrossRef]
- Schrier, R.W. Effects of adrenergic nervous system and catecholamines on systemic and renal hemodynamics, sodium and water excretion and renin secretion. Kidney Int. 1974, 6, 291–306. [Google Scholar] [CrossRef]
- Grimm, D.; Elsner, D.; Schunkert, H.; Pfeifer, M.; Griese, D.; Bruckschlegel, G.; Muders, F.; Riegger, G.A.; Kromer, E.P. Development of heart failure following isoproterenol administration in the rat: Role of the renin-angiotensin system. Cardiovasc. Res. 1998, 37, 91–100. [Google Scholar] [CrossRef]
- Johar, S.; Cave, A.C.; Narayanapanicker, A.; Grieve, D.J.; Shah, A.M. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J. 2006, 20, 1546–1548. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Zheng, R.-H.; Bai, F.; Sturdivant, K.; Wang, N.-P.; James, E.A.; Bose, H.S.; Zhao, Z.-Q. Steroidogenic acute regulatory protein/aldosterone synthase mediates angiotensin II-induced cardiac fibrosis and hypertrophy. Mol. Biol. Rep. 2020, 47, 1207–1222. [Google Scholar] [CrossRef]
- Yasunaga, S.; Yonemochi, H.; Saikawa, T.; Sakata, T. Bradykinin Regulates Captopril-induced Upregulation of β-Adrenergic Receptor in Cultured Neonatal Rat Cardiomyocytes. J. Mol. Cell. Cardiol. 2000, 32, 153–159. [Google Scholar] [CrossRef]
- Hsieh, H.-L.; Yen, M.-H.; Jou, M.-J.; Yang, C.-M. Intracellular signalings underlying bradykinin-induced matrix metalloproteinase-9 expression in rat brain astrocyte-1. Cell. Signal. 2004, 16, 1163–1176. [Google Scholar] [CrossRef]
- Maisel, A.S.; Phillips, C.; Michel, M.C.; Ziegler, M.G.; Carter, S.M. Regulation of cardiac beta-adrenergic receptors by captopril. Implications for congestive heart failure. Circulation 1989, 80, 669–675. [Google Scholar] [CrossRef]
- Resende, M.M.D.; Kriegel, A.J.; Greene, A.S. Combined Effects of Low-Dose Spironolactone and Captopril Therapy in a Rat Model of Genetic Hypertrophic Cardiomyopathy. J. Cardiovasc. Pharmacol. 2006, 48, 265–273. [Google Scholar] [CrossRef]
- Kambara, A.; Holycross, B.J.; Wung, P.; Schanbacher, B.; Ghosh, S.; McCune, S.A.; Bauer, J.A.; Kwiatkowski, P. Combined effects of low-dose oral spironolactone and captopril therapy in a rat model of spontaneous hypertension and heart failure. J. Cardiovasc. Pharmacol. 2003, 41, 830–837. [Google Scholar] [CrossRef]
- Spinale, F.G.; Coker, M.L.; Thomas, C.V.; Walker, J.D.; Mukherjee, R.; Hebbar, L. Time-dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: Relation to ventricular and myocyte function. Circ. Res. 1998, 82, 482–495. [Google Scholar] [CrossRef]
- McElmurray, J.H.; Mukherjee, R.; New, R.B.; Sampson, A.C.; King, M.K.; Hendrick, J.W.; Goldberg, A.; Peterson, T.J.; Hallak, H.; Zile, M.R.; et al. Angiotensin-converting enzyme and matrix metalloproteinase inhibition with developing heart failure: Comparative effects on left ventricular function and geometry. J. Pharmacol. Exp. Ther. 1999, 291, 799–811. [Google Scholar] [CrossRef]
- Spinale, F.G.; Holzgrefe, H.H.; Mukherjee, R.; Webb, M.L.; Hird, R.B.; Cavallo, M.J.; Powell, J.R.; Koster, W.H. Angiotensin-Converting Enzyme Inhibition and Angiotensin II Subtype-1 Receptor Blockade during the Progression of Left Ventricular Dysfunction: Differential Effects on Myocyte Contractile Processes. J. Pharmacol. Exp. Ther. 1997, 283, 1082–1094. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Hang, W.; Shu, H.; Zhou, N. Pirfenidone alleviates cardiac fibrosis induced by pressure overload via inhibiting TGF-β1/Smad3 signalling pathway. J. Cell. Mol. Med. 2022, 26, 4548–4555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Fan, X.; Yang, W.; Yu, B.; Kou, J.; Li, F. Captopril attenuates TAC-induced heart failure via inhibiting Wnt3a/β-catenin and Jak2/Stat3 pathways. Biomed. Pharmacother. 2019, 113, 108780. [Google Scholar] [CrossRef]
- Regan, C.P.; Anderson, P.G.; Bishop, S.P.; Berecek, K.H. Captopril prevents vascular and fibrotic changes but not cardiac hypertrophy in aortic-banded rats. Am. J. Physiol. 1996, 271, H906–H913. [Google Scholar] [CrossRef]
- Rossi, M.A.; Peres, L.C. Effect of captopril on the prevention and regression of myocardial cell hypertrophy and interstitial fibrosis in pressure overload cardiac hypertrophy. Am. Heart J. 1992, 124, 700–709. [Google Scholar] [CrossRef]
- Liu, X.; Sentex, E.; Golfman, L.; Takeda, S.; Osada, M.; Dhalla, N.S. Modification of Cardiac Subcellular Remodeling Due to Pressure Overload by Captopril and Losartan. Clin. Exp. Hypertens. 1999, 21, 145–156. [Google Scholar] [CrossRef]
- Sorbi, D.; Fadly, M.; Hicks, R.; Alexander, S.; Arbeit, L. Captopril inhibits the 72 kDa and 92 kDa matrix metalloproteinases. Kidney Int. 1993, 44, 1266–1272. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Chen, L.; Yin, C.; Chen, P.; Tang, J.; Rong, R.; Li, T.; Hu, L. Captopril inhibits maturation of dendritic cells and maintains their tolerogenic property in atherosclerotic rats. Int. Immunopharmacol. 2015, 28, 715–723. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Haudenschild, C.C.; Nickerson, C.; Drago, R. Antiatherogenic effect of captopril in the Watanabe heritable hyperlipidemic rabbit. Hypertension 1990, 15, 327–331. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhu, B.Q.; Browne, A.E.; Pulukurthy, S.; Chou, T.M.; Sudhir, K.; Glantz, S.A.; Deedwania, P.C.; Chatterjee, K.; Parmley, W.W. Comparative effects of ACE inhibitors and an angiotensin receptor blocker on atherosclerosis and vascular function. J. Cardiovasc. Pharmacol. Ther. 2001, 6, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Kowala, M.C.; Valentine, M.; Recce, R.; Beyer, S.; Goller, N.; Durham, S.; Aberg, G. Enhanced reduction of atherosclerosis in hamsters treated with pravastatin and captopril: ACE in atheromas provides cellular targets for captopril. J. Cardiovasc. Pharmacol. 1998, 32, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Robles, R.G.; Villa, E.; Santirso, R.; Martínez, J.; Ruilope, L.M.; Cuesta, C.; Sancho, J.M. Effects of captopril on sympathetic activity, lipid and carbohydrate metabolism in a model of obesity-induced hypertension in dogs. Am. J. Hypertens. 1993, 6, 1009–1015. [Google Scholar] [CrossRef]
- Lu, Y.; Lei, X.; Di, J.; Huang, S.; Li, J. The effect of captopril and valsartan on preventing the formation of atherosclerotic plaque. Zhonghua Nei Ke Za Zhi 2005, 44, 425–427. [Google Scholar]
- Kowala, M.C.; Grove, R.I.; Aberg, G. Inhibitors of angiotensin converting enzyme decrease early atherosclerosis in hyperlipidemic hamsters. Fosinopril reduces plasma cholesterol and captopril inhibits macrophage-foam cell accumulation independently of blood pressure and plasma lipids. Atherosclerosis 1994, 108, 61–72. [Google Scholar] [CrossRef]
- deGraeff, P.A.; deLangen, C.D.; van Gilst, W.H.; Bel, K.; Scholtens, E.; Kingma, J.H.; Wesseling, H. Protective effects of captopril against ischemia/reperfusion-induced ventricular arrhythmias in vitro and in vivo. Am. J. Med. 1988, 84, 67–74. [Google Scholar] [CrossRef]
- Olmez, E.; Birincioglu, M.; Aksoy, T.; Acet, A. Effects of captopril on ischaemia-reperfusion-induced arrhythmias in an in vivo rat model. Pharmacol. Res. 1995, 32, 37–41. [Google Scholar] [CrossRef]
- Di Napoli, P.; Di Gregorio, G.; De Sanctis, F.; Gallina, S.; Di Girolamo, E.; Trevi, G.P.; Barsotti, A. The myocardial protective effects of cardiac tissue ACE inhibition in experimental ischemia-reperfusion in isolated rat hearts. Cardiologia 1993, 38, 107–112. [Google Scholar]
- Kingma, J.H.; de Graeff, P.A.; van Gilst, W.H.; van Binsbergen, E.; de Langen, C.D.; Wesseling, H. Effects of intravenous captopril on inducible sustained ventricular tachycardia one week after experimental infarction in the anaesthetized pig. Postgrad. Med. J. 1986, 62 (Suppl. S1), 159–163. [Google Scholar]
- de Langen, C.D.; de Graeff, P.A.; van Gilst, W.H.; Bel, K.J.; Kingma, J.H.; Wesseling, H. Effects of angiotensin II and captopril on inducible sustained ventricular tachycardia two weeks after myocardial infarction in the pig. J. Cardiovasc. Pharmacol. 1989, 13, 186–191. [Google Scholar] [CrossRef]
- Van Gilst, W.H.; De Graeff, P.A.; Kingma, J.H.; Wesseling, H.; De Langen, C.D. Captopril reduces purine loss and reperfusion arrhythmias in the rat heart after coronary artery occlusion. Eur. J. Pharmacol. 1984, 100, 113–117. [Google Scholar] [CrossRef] [PubMed]
- van Gilst, W.H.; de Graeff, P.A.; Wesseling, H.; de Langen, C.D. Reduction of reperfusion arrhythmias in the ischemic isolated rat heart by angiotensin converting enzyme inhibitors: A comparison of captopril, enalapril, and HOE 498. J. Cardiovasc. Pharmacol. 1986, 8, 722–728. [Google Scholar] [PubMed]
- Ozer, M.K.; Sahna, E.; Birincioglu, M.; Acet, A. Effects of captopril and losartan on myocardial ischemia-reperfusion induced arrhythmias and necrosis in rats. Pharmacol. Res. 2002, 45, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Sun, Y.; Sievers, R.E.; Browne, A.E.; Pulukurthy, S.; Sudhir, K.; Lee, R.J.; Chou, T.M.; Chatterjee, K.; Parmley, W.W. Comparative effects of pretreatment with captopril and losartan on cardiovascular protection in a rat model of ischemia-reperfusion. J. Am. Coll. Cardiol. 2000, 35, 787–795. [Google Scholar] [CrossRef]
- Rochette, L.; Ribuot, C.; Belichard, P.; Bril, A.; Devissaguet, M. Protective effect of angiotensin converting enzyme inhibitors (CEI): Captopril and perindopril on vulnerability to ventricular fibrillation during myocardial ischemia and reperfusion in rat. Clin. Exp. Hypertens. A 1987, 9, 365–368. [Google Scholar] [CrossRef]
- McKenna, W.J.; Haywood, G.A. The role of ACE inhibitors in the treatment of arrhythmias. Clin. Cardiol. 1990, 13, VII-49–VII-52. [Google Scholar] [CrossRef]
- Rials, S.J.; Wu, Y.; Xu, X.; Filart, R.A.; Marinchak, R.A.; Kowey, P.R. Regression of left ventricular hypertrophy with captopril restores normal ventricular action potential duration, dispersion of refractoriness, and vulnerability to inducible ventricular fibrillation. Circulation 1997, 96, 1330–1336. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Lu, Z.-Y. Effect of captopril on membrane currents of ventricular myocytes. J. Tongji Med. Univ. 1995, 15, 209–211. [Google Scholar] [CrossRef]
- Hemsworth, P.D.; Pallandi, R.T.; Campbell, T.J. Cardiac electrophysiological actions of captopril: Lack of direct antiarrhythmic effects. Br. J. Pharmacol. 1989, 98, 192–196. [Google Scholar] [CrossRef]
- Arad, M.; Shotan, A.; Horowitz, L.; Klein, R.; Rabinowitz, B. Effects of Captopril on Metabolic and Hemodynamic Alterations in Global Ischemia and Reperfusion in the Isolated Working Rat Heart. J. Cardiovasc. Pharmacol. 1992, 19, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Gupta, J.K.; Qureshi, S.S.; Vishwakarma, V.K. Role of cardiac renin angiotensin system in ischemia reperfusion injury and preconditioning of heart. Indian Heart J. 2016, 68, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Dragasevic, N.; Jakovljevic, V.; Zivkovic, V.; Draginic, N.; Andjic, M.; Bolevich, S.; Jovic, S. The role of aldosterone inhibitors in cardiac ischemia–reperfusion injury. Can. J. Physiol. Pharmacol. 2021, 99, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Miura, T.; Ura, N.; Ogawa, T.; Suzuki, K.; Shimamoto, K.; Iimura, O. Captopril potentiates the myocardial infarct size-limiting effect of ischemic preconditioning through bradykinin B2 receptor activation. J. Am. Coll. Cardiol. 1996, 28, 1616–1622. [Google Scholar] [CrossRef]
- Marktanner, R.; Nacke, P.; Feindt, P.; Hohlfeld, T.; Schipke, J.D.; Gams, E. Delayed preconditioning via angiotensin-converting enzyme inhibition: Pros and cons from an experimental study. Clin. Exp. Pharmacol. Physiol. 2006, 33, 787–792. [Google Scholar] [CrossRef]
- Ito, H.; Hayashi, I.; Izumi, T.; Majima, M. Bradykinin inhibits development of myocardial infarction through B2 receptor signalling by increment of regional blood flow around the ischaemic lesions in rats. Br. J. Pharmacol. 2003, 138, 225–233. [Google Scholar] [CrossRef]
- Menasché, P.; Grousset, C.; Peynet, J.; Mouas, C.; Bloch, G.; Piwnica, A. Pretreatment with captopril improves myocardial recovery after cardioplegic arrest. J. Cardiovasc. Pharmacol. 1992, 19, 402–407. [Google Scholar] [CrossRef]
- Liu, F.M.; Xu, S.C. Effect of captopril cardioplegia on renin-angiotensin system, prostaglandins, free radicals and electrolytes in the isolated hypothermic ischemic and reperfusion rabbit hearts. Chin. Med. J. 1993, 106, 903–906. [Google Scholar]
- Gurevitch, J.; Pevni, D.; Frolkis, I.; Matsa, M.; Paz, Y.; Mohr, R.; Yakirevich, V. Captopril in cardioplegia and reperfusion: Protective effects on the ischemic heart. Ann. Thorac. Surg. 1997, 63, 627–633. [Google Scholar] [CrossRef]
- Podesser, B.K.; Schirnhofer, J.; Bernecker, O.Y.; Kröner, A.; Franz, M.; Semsroth, S.; Fellner, B.; Neumüller, J.; Hallström, S.; Wolner, E. Optimizing Ischemia/Reperfusion in the Failing Rat Heart—Improved Myocardial Protection with Acute ACE Inhibition. Circulation 2002, 106, I-277. [Google Scholar] [CrossRef]
- Divisová, J.; Vavrínková, H.; Tutterová, M.; Kazdová, L.; Meschisvili, E. Effect of ACE inhibitor captopril and L-arginine on the metabolism and on ischemia-reperfusion injury of the isolated rat heart. Physiol. Res. 2001, 50, 143–152. [Google Scholar] [CrossRef]
- Huizer, T.; Van Der Meer, P.; De Jong, J.W. Captopril inhibits angiotensin I-induced coronary flow reduction in isolated rat heart but has no effect on contractility or energy metabolism. Eur. Heart J. 1992, 13, 109–114. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Pfeffer, J.M.; Steinberg, C.; Finn, P. Survival after an experimental myocardial infarction: Beneficial effects of long-term therapy with captopril. Circulation 1985, 72, 406–412. [Google Scholar] [CrossRef]
- Bech, O.M.; Sørensen, J.D.; Jensen, M.K.; Diamant, B.; Steiness, E. Effects of long-term coenzyme Q10 and captopril treatment on survival and functional capacity in rats with experimentally induced heart infarction. J. Pharmacol. Exp. Ther. 1990, 255, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Ribner, H.S.; Keung, E.; Sonnenblick, E.H.; LeJemtel, T.H. Treatment of chronic congestive heart failure with captopril, an oral inhibitor of angiotensin-converting enzyme. N. Engl. J. Med. 1979, 301, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Levine, T.B.; Franciosa, J.A.; Cohn, J.N. Acute and long-term response to an oral converting-enzyme inhibitor, captopril, in congestive heart failure. Circulation 1980, 62, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ader, R.; Chatterjee, K.; Ports, T.; Brundage, B.; Hiramatsu, B.; Parmley, W. Immediate and sustained hemodynamic and clinical improvement in chronic heart failure by an oral angiotensin-converting enzyme inhibitor. Circulation 1980, 61, 931–937. [Google Scholar] [CrossRef]
- Faxon, D.P.; Halperin, J.L.; Creager, M.A.; Gavras, H.; Schick, E.C.; Ryan, T.J. Angiotensin inhibition in severe heart failure: Acute central and limb hemodynamic effects of captopril with observations on sustained oral therapy. Am. Heart J. 1981, 101, 548–556. [Google Scholar] [CrossRef]
- Dzau, V.J.; Colucci, W.S.; Williams, G.H.; Curfman, G.; Meggs, L.; Hollenberg, N.K. Sustained effectiveness of converting-enzyme inhibition in patients with severe congestive heart failure. N. Engl. J. Med. 1980, 302, 1373–1379. [Google Scholar] [CrossRef]
- Chatterjee, K.; Rouleau, J.L.; Parmley, W.W. Haemodynamic and myocardial metabolic effects of captopril in chronic heart failure. Br. Heart J. 1982, 47, 233–238. [Google Scholar] [CrossRef]
- Turini, G.A.; Brunner, H.R.; Gribic, M.; Waeber, B.; Gavras, H. Improvement of chronic congestive heart-failure by oral captopril. Lancet 1979, 1, 1213–1215. [Google Scholar] [CrossRef]
- Maslowski, A.H.; Ikram, H.; Nicholls, M.G.; Espiner, E.A. Haemodynamic, hormonal, and electrolyte responses to captopril in resistant heart failure. Lancet 1981, 1, 71–74. [Google Scholar] [CrossRef]
- Awan, N.A.; Evenson, M.K.; Needham, K.E.; Win, A.; Mason, D.T. Efficacy of oral angiotensin-converting enzyme inhibition with captopril therapy in severe chronic normotensive congestive heart failure. Am. Heart J. 1981, 101, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Wenting, G.J.; Man in’t veld, A.J.; Woittiez, A.J.; Boomsma, F.; Laird-Meeter, K.; Simoons, M.L.; Hugenholtz, P.G.; Schalekamp, M.A. Effects of captopril in acute and chronic heart failure. Correlations with plasma levels of noradrenaline, renin, and aldosterone. Br. Heart J. 1983, 49, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, D.N.; Douglas, J.E.; Coxon, R.J.; Long, B. Low-dose captopril in chronic heart failure: Acute haemodynamic effects and long-term treatment. Lancet 1980, 2, 1154–1157. [Google Scholar] [CrossRef] [PubMed]
- Kramer, B.L.; Massie, B.M.; Topic, N. Controlled trial of captopril in chronic heart failure: A rest and exercise hemodynamic study. Circulation 1983, 67, 807–816. [Google Scholar] [CrossRef]
- Massie, B.; Kramer, B.L.; Topic, N.; Henderson, S.G. Hemodynamic and radionuclide effects of acute captopril therapy for heart failure: Changes in left and right ventricular volumes and function at rest and during exercise. Circulation 1982, 65, 1374–1381. [Google Scholar] [CrossRef]
- Franciosa, J.A.; Goldsmith, S.R.; Cohn, J.N. Contrasting immediate and long-term effects of isosorbide dinitrate on exercise capacity in congestive heart failure. Am. J. Med. 1980, 69, 559–566. [Google Scholar] [CrossRef]
- Massie, B.M.; Kramer, B.; Haughom, F. Acute and long-term effects of vasodilator therapy on resting and exercise hemodynamics and exercise tolerance. Circulation 1981, 64, 1218–1226. [Google Scholar] [CrossRef]
- Zelis, R.; Nellis, S.H.; Longhurst, J.; Lee, G.; Mason, D.T. Abnormalities in the regional circulations accompanying congestive heart failure. Prog. Cardiovasc. Dis. 1975, 18, 181–199. [Google Scholar] [CrossRef]
- Levine, T.B.; Olivari, M.T.; Cohn, J.N. Hemodynamic and regional blood flow response to captopril in congestive heart failure. Am. J. Med. 1984, 76, 38–42. [Google Scholar] [CrossRef]
- Creager, M.A.; Halperin, J.L.; Bernard, D.B.; Faxon, D.P.; Melidossian, C.D.; Gavras, H.; Ryan, T.J. Acute regional circulatory and renal hemodynamic effects of converting-enzyme inhibition in patients with congestive heart failure. Circulation 1981, 64, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, B.; Raine, A.E.; Cooper, R.; Ledingham, J.G. Changes in cerebral blood flow in patients with severe congestive cardiac failure before and after captopril treatment. Am. J. Med. 1984, 76, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Faxon, D.P.; Creager, M.A.; Halperin, J.L.; Bernard, D.B.; Ryan, T.J. Redistribution of regional blood flow following angiotensin-converting enzyme inhibition. Comparison of normal subjects and patients with heart failure. Am. J. Med. 1984, 76, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Salah, E.M.; Bastacky, S.I.; Jackson, E.K.; Tofovic, S.P. Captopril Attenuates Cardiovascular and Renal Disease in a Rat Model of Heart Failure with Preserved Ejection Fraction. J. Cardiovasc. Pharmacol. 2018, 71, 205–214. [Google Scholar] [CrossRef]
- Powers, E.R.; Bannerman, K.S.; Stone, J.; Reison, D.S.; Escala, E.L.; Kalischer, A.; Weiss, M.B.; Sciacca, R.R.; Cannon, P.J. The effect of captopril on renal, coronary, and systemic hemodynamics in patients with severe congestive heart failure. Am. Heart J. 1982, 104, 1203–1210. [Google Scholar] [CrossRef]
- Creager, M.A.; Faxon, D.P.; Halperin, J.L.; Melidossian, C.D.; McCabe, C.H.; Schick, E.C.; Ryan, T.J. Determinants of clinical response and survival in patients with congestive heart failure treated with captopril. Am. Heart J. 1982, 104, 1147–1154. [Google Scholar] [CrossRef]
- Fouad, F.M.; Tarazi, R.C.; Bravo, E.L.; Hart, N.J.; Castle, L.W.; Salcedo, E.E. Long-term control of congestive heart failure with captopril. Am. J. Cardiol. 1982, 49, 1489–1496. [Google Scholar] [CrossRef]
- Massie, B.M.; Kramer, B.L.; Topic, N. Lack of relationship between the short-term hemodynamic effects of captopril and subsequent clinical responses. Circulation 1984, 69, 1135–1141. [Google Scholar] [CrossRef]
- Fouad, F.M.; El-Tobgi, S.; Tarazi, R.C.; Bravo, E.L.; Hart, N.J.; Shirey, E.K.; Lim, J. Captopril in congestive heart failure resistant to other vasodilators. Eur. Heart J. 1984, 5, 47–54. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Braunwald, E.; Moyé, L.A.; Basta, L.; Brown, E.J.; Cuddy, T.E.; Davis, B.R.; Geltman, E.M.; Goldman, S.; Flaker, G.C. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N. Engl. J. Med. 1992, 327, 669–677. [Google Scholar] [CrossRef]
- Rutherford, J.D.; Pfeffer, M.A.; Moyé, L.A.; Davis, B.R.; Flaker, G.C.; Kowey, P.R.; Lamas, G.A.; Miller, H.S.; Packer, M.; Rouleau, J.L. Effects of captopril on ischemic events after myocardial infarction. Results of the Survival and Ventricular Enlargement trial. SAVE Investigators. Circulation 1994, 90, 1731–1738. [Google Scholar] [CrossRef]
- Søgaard, P.; Gøtzsche, C.O.; Ravkilde, J.; Thygesen, K. Effects of captopril on ischemia and dysfunction of the left ventricle after myocardial infarction. Circulation 1993, 87, 1093–1099. [Google Scholar] [CrossRef]
- Gøtzsche, C.O.; Søgaard, P.; Ravkilde, J.; Thygesen, K. Effects of captopril on left ventricular systolic and diastolic function after acute myocardial infarction. Am. J. Cardiol. 1992, 70, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, N.; Murphy, J.; Smith, H.; Hannan, S. Treatment of patients with symptomless left ventricular dysfunction after myocardial infarction. Lancet 1988, 1, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, N.; Murphy, J.; Smith, H.; Hannan, S. Preventive treatment of asymptomatic left ventricular dysfunction following myocardial infarction. Eur. Heart J. 1990, 11 (Suppl. B), 147–156. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, N.; Smith, H.; Murphy, J.; Greaves, S.; Hart, H.; Gamble, G. Early prevention of left ventricular dysfunction after myocardial infarction with angiotensin-converting-enzyme inhibition. Lancet 1991, 337, 872–876. [Google Scholar] [CrossRef]
- Foy, S.G.; Crozier, I.G.; Turner, J.G.; Richards, A.M.; Frampton, C.M.; Nicholls, M.G.; Ikram, H. Comparison of enalapril versus captopril on left ventricular function and survival three months after acute myocardial infarction (the “PRACTICAL” study). Am. J. Cardiol. 1994, 73, 1180–1186. [Google Scholar] [CrossRef]
- French, J.K.; Amos, D.J.; Williams, B.F.; Cross, D.B.; Elliott, J.M.; Hart, H.H.; Williams, M.G.; Norris, R.M.; Ashton, N.G.; Whitlock, R.M.; et al. Effects of early captopril administration after thrombolysis on regional wall motion in relation to infarct artery blood flow. J. Am. Coll. Cardiol. 1999, 33, 139–145. [Google Scholar] [CrossRef]
- Nabel, E.G.; Topol, E.J.; Galeana, A.; Ellis, S.G.; Bates, E.R.; Werns, S.W.; Walton, J.A.; Muller, D.W.; Schwaiger, M.; Pitt, B. A randomized placebo-controlled trial of combined early intravenous captopril and recombinant tissue-type plasminogen activator therapy in acute myocardial infarction. J. Am. Coll. Cardiol. 1991, 17, 467–473. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Lamas, G.A.; Vaughan, D.E.; Parisi, A.F.; Braunwald, E. Effect of captopril on progressive ventricular dilatation after anterior myocardial infarction. N. Engl. J. Med. 1988, 319, 80–86. [Google Scholar] [CrossRef]
- Kleber, F.X.; Niemöller, L.; Doering, W. Impact of converting enzyme inhibition on progression of chronic heart failure: Results of the Munich Mild Heart Failure Trial. Br. Heart J. 1992, 67, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Captopril Multicenter Research Group. A placebo-controlled trial of captopril in refractory chronic congestive heart failure. J. Am. Coll. Cardiol. 1983, 2, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Silberbauer, K.; Punzengruber, C.; Sinzinger, H. Endogenous Prostaglandin E2 Metabolite Levels, Renin-Angiotensin System and Catecholamines versus Acute Hemodynamic Response to Captopril in Chronic Congestive Heart Failure. Cardiology 1983, 70, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.D.; Antman, E.M.; Lorell, B.H.; Barry, W.H.; Smith, T.W. Potentiation of the cardiovascular effects of nitroglycerin by N-acetylcysteine. Circulation 1983, 68, 1247–1253. [Google Scholar] [CrossRef]
- van Gilst, W.H.; de Graeff, P.A.; Scholtens, E.; de Langen, C.D.; Wesseling, H. Potentiation of isosorbide dinitrate-induced coronary dilatation by captopril. J. Cardiovasc. Pharmacol. 1987, 9, 254–255. [Google Scholar] [CrossRef]
- ACE Inhibitor Myocardial Infarction Collaborative Group. Indications for ACE inhibitors in the early treatment of acute myocardial infarction: Systematic overview of individual data from 100,000 patients in randomized trials. Circulation 1998, 97, 2202–2212. [Google Scholar] [CrossRef]
- Flather, M.D.; Yusuf, S.; Køber, L.; Pfeffer, M.; Hall, A.; Murray, G.; Torp-Pedersen, C.; Ball, S.; Pogue, J.; Moyé, L.; et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: A systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group. Lancet 2000, 355, 1575–1581. [Google Scholar] [CrossRef]
- Bussmann, W.D.; Störger, H.; Hadler, D.; Reifart, N.; Fassbinder, W.; Jungmann, E.; Kaltenbach, M. Long-term treatment of severe chronic heart failure with captopril: A double-blind, randomized, placebo-controlled, long-term study. J. Cardiovasc. Pharmacol. 1987, 9 (Suppl. S2), S50–S60. [Google Scholar] [CrossRef]
- Jiang, M. The changes in renin-angiotensin-aldosterone system in acute myocardial infarction and the effects of converting enzyme inhibitor-captopril. Zhonghua Xin Xue Guan Bing Za Zhi 1991, 19, 342–344, 396. [Google Scholar]
- Lijnen, P.; Fagard, R.; Staessen, J.; Verschueren, L.J.; Amery, A. Dose response in captopril therapy of hypertension. Clin. Pharmacol. Ther. 1980, 28, 310–315. [Google Scholar] [CrossRef]
- Lijnen, P.; Staessen, J.; Fagard, R.; Amery, A. Increase in plasma aldosterone during prolonged captopril treatment. Am. J. Cardiol. 1982, 49, 1561–1563. [Google Scholar] [CrossRef]
- Struthers, A.D. The clinical implications of aldosterone escape in congestive heart failure. Eur. J. Heart Fail. 2004, 6, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.; Dargie, H.J.; Hodsman, G.P.; Ball, S.G.; Robertson, J.I.; Morton, J.J.; East, B.W.; Robertson, I.; Murray, G.D.; Gillen, G. Captopril in heart failure. A double blind controlled trial. Br. Heart J. 1984, 52, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, M.G.; Ikram, H.; Espiner, E.A.; Maslowski, A.H.; Scandrett, M.S.; Penman, T. Hemodynamic and hormonal responses during captopril therapy for heart failure: Acute, chronic and withdrawal studies. Am. J. Cardiol. 1982, 49, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- van Kats, J.P.; Danser, A.H.; van Meegen, J.R.; Sassen, L.M.; Verdouw, P.D.; Schalekamp, M.A. Angiotensin production by the heart: A quantitative study in pigs with the use of radiolabeled angiotensin infusions. Circulation 1998, 98, 73–81. [Google Scholar] [CrossRef]
- Wolny, A.; Clozel, J.P.; Rein, J.; Mory, P.; Vogt, P.; Turino, M.; Kiowski, W.; Fischli, W. Functional and biochemical analysis of angiotensin II-forming pathways in the human heart. Circ. Res. 1997, 80, 219–227. [Google Scholar] [CrossRef]
- Pitt, B.; Poole-Wilson, P.A.; Segal, R.; Martinez, F.A.; Dickstein, K.; Camm, A.J.; Konstam, M.A.; Riegger, G.; Klinger, G.H.; Neaton, J.; et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: Randomised trial--the Losartan Heart Failure Survival Study ELITE II. Lancet 2000, 355, 1582–1587. [Google Scholar] [CrossRef]
- Dickstein, K.; Kjekshus, J. OPTIMAAL Steering Committee of the OPTIMAAL Study Group. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: The OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet 2002, 360, 752–760. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; McMurray, J.J.V.; Velazquez, E.J.; Rouleau, J.-L.; Køber, L.; Maggioni, A.P.; Solomon, S.D.; Swedberg, K.; Van de Werf, F.; White, H.; et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N. Engl. J. Med. 2003, 349, 1893–1906. [Google Scholar] [CrossRef]
- Hansson, L.; Lindholm, L.H.; Niskanen, L.; Lanke, J.; Hedner, T.; Niklason, A.; Luomanmäki, K.; Dahlöf, B.; De Faire, U.; Mörlin, C.; et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: The Captopril Prevention Project (CAPPP) randomised trial. Lancet 1999, 353, 611–616. [Google Scholar] [CrossRef]
- Hansen, M.L.; Gislason, G.H.; Køber, L.; Schramm, T.K.; Folke, F.; Buch, P.; Abildstrom, S.Z.; Madsen, M.; Rasmussen, S.; Torp-Pedersen, C. Different angiotensin-converting enzyme inhibitors have similar clinical efficacy after myocardial infarction. Br. J. Clin. Pharmacol. 2007, 65, 217. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, A.F.; van Gilst, W.H.; van Veldhuisen, D.J.; de Vries, R.J.; Dunselman, P.H.; Kingma, J.H. Long-term anti-ischemic effects of angiotensin-converting enzyme inhibition in patients after myocardial infarction. The Captopril and Thrombolysis Study (CATS) Investigators. J. Am. Coll. Cardiol. 1997, 30, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Strozzi, C.; Cocco, G.; Portaluppi, F.; Urso, L.; Alfiero, R.; Tasini, M.T.; Montanari, L.; Al Yassini, K.; Rizzo, A. Effects of Captopril on the Physical Work Capacity of Normotensive Patients with Stable-Effort Angina pectoris. Cardiology 1987, 74, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Gemici, K.; Kazazoğlu, A.; Yeşilbursa, D.; Serdar, A.; Ener, S.; Aydnlar, A.; Büyükuysal, L.; Çobanoğlu, N. The effects of sublingual administration of captopril on parameters of exercise test and neurohormonal activation in patients with stable angina pectoris. Int. J. Angiol. 2011, 7, 238–243. [Google Scholar] [CrossRef]
- Dhawan, S.; Soni, D.; Chandra, N.; Dviwedi, S.; Agarwal, A.; Puri, V.K. Use of captopril as an isolated agent for the management of stable angina pectoris--a double blind randomised trial. Indian Heart J. 1992, 44, 151–154. [Google Scholar]
- Akhras, F.; Jackson, G. The role of captopril as single therapy in hypertension and angina pectoris. Int. J. Cardiol. 1991, 33, 259–266. [Google Scholar] [CrossRef]
- Metelitsa, V.I.; Martsevich, S.Y.; Kozyreva, M.P.; Slastnikova, I.D. Enhancement of the efficacy of isosorbide dinitrate by captopril in stable angina pectoris. Am. J. Cardiol. 1992, 69, 291–296. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Henderson, E.; McLenachan, J.; Findlay, I.N.; Dargie, H.J. Effect of Captopril, an angiotensin-converting enzyme inhibitor, in patients with angina pectoris and heart failure. J. Am. Coll. Cardiol. 1991, 17, 733–739. [Google Scholar] [CrossRef]
- Chiladakis, J.A.; Karapanos, G.; Agelopoulos, G.; Alexopoulos, D.; Manolis, A.S. Effects of early captopril therapy after myocardial infarction on the incidence of late potentials. Clin. Cardiol. 2000, 23, 96–102. [Google Scholar] [CrossRef]
- Pipilis, A.; Flather, M.; Collins, R.; Hargreaves, A.; Kolettis, T.; Boon, N.; Foster, C.; Appleby, P.; Sleight, P. Effects on ventricular arrhythmias of oral captopril and of oral mononitrate started early in acute myocardial infarction: Results of a randomised placebo controlled trial. Br. Heart J. 1993, 69, 161–165. [Google Scholar] [CrossRef]
- Budaj, A.; Cybulski, J.; Cedro, K.; Karczmarewicz, S.; Maciejewicz, J.; Wiśniewski, M.; Ceremuzyński, L. Effects of captopril on ventricular arrhythmias in the early and late phase of suspected acute myocardial infarction. Randomized, placebo-controlled substudy of ISIS-4. Eur. Heart J. 1996, 17, 1506–1510. [Google Scholar] [CrossRef]
- Søgaard, P.; Gøtzsche, C.O.; Ravkilde, J.; Nørgaard, A.; Thygesen, K. Ventricular arrhythmias in the acute and chronic phases after acute myocardial infarction. Effect of intervention with captopril. Circulation 1994, 90, 101–107. [Google Scholar] [CrossRef]
- Pasquale, P.D.; Paterna, S.; Cannizzaro, S.; Bucca, V. Does captopril treatment before thrombolysis in acute myocardial infarction attenuate reperfusion damage? Short-term and long-term effects. Int. J. Cardiol. 1994, 43, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, P.; Paterna, S.; Cannizzaro, S.; Albano, V.; Valdes, L.; Licata, G.; Barone, G. Captopril and glutathione before thrombolysis in acute myocardial infarction: A pilot study. Drugs Exp. Clin. Res. 1992, 18, 401–406. [Google Scholar] [PubMed]
- Kingma, J.H.; van Gilst, W.H.; Peels, C.H.; Dambrink, J.H.; Verheugt, F.W.; Wielenga, R.P. Acute intervention with captopril during thrombolysis in patients with first anterior myocardial infarction. Results from the Captopril and Thrombolysis Study (CATS). Eur. Heart. J. 1994, 15, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, P.; Paterna, S.; Bucca, V.; Maringhini, G.; Magatti, M. Effects of the administration of captopril, metoprolol and of the captopril-metoprolol combination as adjuvant therapy during thrombolysis in acute myocardial infarction. Int. J. Cardiol. 1994, 46, 107–112. [Google Scholar] [CrossRef]
- Cohn, J. Comparative Effects of Therapy with Captopril and Digoxin in Patients with Mild to Moderate Heart Failure. JAMA 1988, 259, 539. [Google Scholar] [CrossRef]
- Das, D.K.; Maulik, N.; Engelman, R.M. Redox regulation of angiotensin II signaling in the heart. J. Cell. Mol. Med. 2004, 8, 144–152. [Google Scholar] [CrossRef]
- Morris, S.D.; Yellon, D.M. Angiotensin-Converting Enzyme Inhibitors Potentiate Preconditioning Through Bradykinin B2Receptor Activation in Human Heart. J. Am. Coll. Cardiol. 1997, 29, 1599–1606. [Google Scholar] [CrossRef]
- Pasquale, P.D.; Paterna, S.; Valenza, M.; Valdes, L.; Albano, V.; Trombino, G.; Vitrano, G.; Cannizzaro, S.; Licata, G.; Albiero, R. Effects of captopril on myocardial protection during cardioplegia. Int. J. Cardiol. 1993, 42, 225–230. [Google Scholar] [CrossRef]
- Reinhardt, D. Cardiac remodelling in end stage heart failure: Upregulation of matrix metalloproteinase (MMP) irrespective of the underlying disease, and evidence for a direct inhibitory effect of ACE inhibitors on MMP. Heart 2002, 88, 525–530. [Google Scholar] [CrossRef]
- Kuntze, L.B.; Antonio, R.C.; Izidoro-Toledo, T.C.; Meschiari, C.A.; Tanus-Santos, J.E.; Gerlach, R.F. Captopril and Lisinopril Only Inhibit Matrix Metalloproteinase-2 (MMP-2) Activity at Millimolar Concentrations. Basic Clin. Pharma. Tox. 2014, 114, 233–239. [Google Scholar] [CrossRef]
- Yamamoto, D.; Takai, S.; Hirahara, I.; Kusano, E. Captopril directly inhibits matrix metalloproteinase-2 activity in continuous ambulatory peritoneal dialysis therapy. Clin. Chim. Acta 2010, 411, 762–764. [Google Scholar] [CrossRef]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxidative Med. Cell. Longev. 2020, 2020, e5732956. [Google Scholar] [CrossRef]
- Weissman, D.; Maack, C. Redox signaling in heart failure and therapeutic implications. Free Radic. Biol. Med. 2021, 171, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Liu, S.; Sun, Y.; Chen, C.; Yang, S.; Lin, M.; Long, J.; Yao, J.; Lin, Y.; Yi, F.; et al. Targeting oxidative stress as a preventive and therapeutic approach for cardiovascular disease. J. Transl. Med. 2023, 21, 519. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.A.; Robinson, P.A. Copper chelates of antirheumatic and anti-inflammatory agents: Their superoxide dismutase-like activity and stability. Br. J. Rheumatol. 1985, 24, 128–136. [Google Scholar] [CrossRef]
- Chopra, M.; Scott, N.; McMurray, J.; McLay, J.; Bridges, A.; Smith, W.E.; Belch, J.J. Captopril: A free radical scavenger. Br. J. Clin. Pharmacol. 1989, 27, 396–399. [Google Scholar] [CrossRef]
- Bagchi, D.; Prasad, R.; Das, D.K. Direct scavenging of free radicals by captopril, an angiotensin converting enzyme inhibitor. Biochem. Biophys. Res. Commun. 1989, 158, 52–57. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Tomlinson, B. Antioxidant power of angiotensin-converting enzyme inhibitors in vitro. Brit. J. Clin. Pharma. 1998, 45, 168–169. [Google Scholar] [CrossRef]
- Lapenna, D.; De Gioia, S.; Ciofani, G.; Daniele, F.; Cuccurullo, F. Captopril has no significant scavenging antioxidant activity in human plasma in vitro or in vivo. Br. J. Clin. Pharmacol. 1996, 42, 451–456. [Google Scholar] [CrossRef]
- Suzuki, S.; Sato, H.; Shimada, H.; Takashima, N.; Arakawa, M. Comparative free radical scavenging action of angiotensin-converting enzyme inhibitors with and without the sulfhydryl radical. Pharmacology 1993, 47, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Westlin, W.; Mullane, K. Does captopril attenuate reperfusion-induced myocardial dysfunction by scavenging free radicals? Circulation 1988, 77, I30–I39. [Google Scholar] [PubMed]
- Kükürt, A.; Gelen, V.; Başer, Ö.F.; Deveci, H.A.; Karapehlivan, M.; Kükürt, A.; Gelen, V.; Başer, Ö.F.; Deveci, H.A.; Karapehlivan, M. Thiols: Role in Oxidative Stress-Related Disorders. In Accenting Lipid Peroxidation; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Grover, G.J.; Sleph, P.G.; Dzwonczyk, S.; Wang, P.; Fung, W.; Tobias, D.; Cushman, D.W. Effects of different angiotensin-converting enzyme (ACE) inhibitors on ischemic isolated rat hearts: Relationship between cardiac ACE inhibition and cardioprotection. J. Pharmacol. Exp. Ther. 1991, 257, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Engelman, R.M.; Rousou, J.A.; Cordis, G.A.; Das, D.K. Attenuation of myocardial reperfusion injury by sulfhydryl-containing angiotensin converting enzyme inhibitors. Cardiovasc. Drugs Ther. 1992, 6, 437–443. [Google Scholar] [CrossRef]
- Chopra, M.; McMurray, J.; Stewart, J.; Dargie, H.J.; Smith, W.E. Free radical scavenging: A potentially beneficial action of thiol-containing angiotensin converting enzyme inhibitors. Biochem. Soc. Trans. 1990, 18, 1184–1185. [Google Scholar] [CrossRef]
- Chopra, M.; Beswick, H.; Clapperton, M.; Dargie, H.J.; Smith, W.E.; McMurray, J. Antioxidant effects of angiotensin-converting enzyme (ACE) inhibitors: Free radical and oxidant scavenging are sulfhydryl dependent, but lipid peroxidation is inhibited by both sulfhydryl- and nonsulfhydryl-containing ACE inhibitors. J. Cardiovasc. Pharmacol. 1992, 19, 330–340. [Google Scholar] [CrossRef]
- Kukreja, R.C.; Kontos, H.A.; Hess, M.L. Captopril and enalaprilat do not scavenge the superoxide anion. Am. J. Cardiol. 1990, 65, 24I–27I. [Google Scholar] [CrossRef]
- Mehta, J.L.; Nicolini, F.A.; Lawson, D.L. Sulfhydryl Group in Angiotensin Converting Enzyme Inhibitors and Superoxide Radical Formation. J. Cardiovasc. Pharmacol. 1990, 16, 847–849. [Google Scholar] [CrossRef]
- Engelman, R.M.; Rousou, J.A.; Iyengar, J.; Das, D.K. Captopril, an ACE inhibitor, for optimizing reperfusion after acute myocardial infarction. Ann. Thorac. Surg. 1991, 52, 918–926. [Google Scholar] [CrossRef]
- Bagchi, D.; Iyengar, J.; Stockwell, P.; Das, D.K. Enhanced prostaglandin production in the ischemic-reperfused myocardium by captopril linked with its free radical scavenging action. Prostaglandins Leukot Essent Fat. Acids 1989, 38, 145–150. [Google Scholar] [CrossRef]
- Altuntaş, Y.; Güven, M.; Ince, E.; Açbay, O.; Caner, M.; Kanigür-Sultuybek, G. The in vitro effects of captopril on the levels of lipid peroxidation and glutathione of erythrocytes in type II diabetes. J. Basic Clin. Physiol. Pharmacol. 1995, 6, 281–288. [Google Scholar] [CrossRef]
- Kaufman, M.J. Comparison of the Free Radical-scavenging Ability of Captopril and Ascorbic Acid in an In-vitro Model of Lipid Oxidation. Implications for Reperfusion Injury and ACE Inhibitor Therapy. J. Pharm. Pharmacol. 2011, 46, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Akanmu, D.; Cecchini, R.; Halliwell, B. Evaluation of the ability of the angiotensin-converting enzyme inhibitor captopril to scavenge reactive oxygen species. Chem. Biol. Interact. 1991, 77, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Misík, V.; Mak, I.T.; Stafford, R.E.; Weglicki, W.B. Reactions of captopril and epicaptopril with transition metal ions and hydroxyl radicals: An EPR spectroscopy study. Free Radic. Biol. Med. 1993, 15, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, D.; De Gioia, S.; Ciofani, G.; Cuccurullo, F. Captopril Induces Iron Release From Ferritin and Oxidative Stress. J. Pharm. Pharmacol. 2011, 47, 59–61. [Google Scholar] [CrossRef]
- Tien, M.; Bucher, J.R.; Aust, S.D. Thiol-dependent lipid peroxidation. Biochem. Biophys. Res. Commun. 1982, 107, 279–285. [Google Scholar] [CrossRef]
- Chaudiere, J.; Wilhelmsen, E.C.; Tappel, A.L. Mechanism of selenium-glutathione peroxidase and its inhibition by mercaptocarboxylic acids and other mercaptans. J. Biol. Chem. 1984, 259, 1043–1050. [Google Scholar] [CrossRef]
- Schöneich, C.; Asmus, K.-D.; Dillinger, U.; Bruchhausen, F.V. Thiyl radical attack on polyunsaturated fatty acids: A possible route to lipid peroxidation. Biochem. Biophys. Res. Commun. 1989, 161, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Forni, L.G.; Hilton, P.J.; Willson, R.L.; Cheeseman, K.H. Free radical reactions involving the angiotensin converting enzyme inhibitor captopril. Redox Rep. 1996, 2, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Isbir, C.S.; Dogan, R.; Farsak, B.; Ayd1n, M. The effect of captopril on membrane bound enzymes in ischemia-reperfusion injury. Cardiovasc. Surg. 2000, 8, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Goliik, A.; Weissgarten, J.; Evans, S.; Cohen, N.; Averbukh, Z.; Zaidenstein, R.; Cotariu, D.; Modai, D. Erythrocyte Na+, K+ and Ca2+, Mg2+-ATPase activities in hypertensives on angiotensin-converting enzyme inhibitors. Clin. Biochem. 1996, 29, 249–254. [Google Scholar] [CrossRef]
- Doğan, R.; Sarigül, A.; Isbir, S.; Farsak, B.; Tuncer, M.; Kilinç, K.; Demircin, M. Beneficial effect of captopril against ischaemia-reperfusion injury in isolated guinea pig hearts. Scand. J. Clin. Lab. Investig. 1998, 58, 119–126. [Google Scholar] [CrossRef]
- Li, K.; Chen, X. Protective effects of captopril and enalapril on myocardial ischemia and reperfusion damage of rat. J. Mol. Cell. Cardiol. 1987, 19, 909–915. [Google Scholar] [CrossRef]
- van Gilst, W.H.; van Wijngaarden, J.; Scholtens, E.; de Graeff, P.A.; de Langen, C.D.; Wesseling, H. Captopril-induced increase in coronary flow: An SH-dependent effect on arachidonic acid metabolism? J. Cardiovasc. Pharmacol. 1987, 9 (Suppl. S2), S31–S36. [Google Scholar] [CrossRef]
- Düsing, R.; Scherhag, R.; Landsberg, G.; Glänzer, K.; Kramer, H.J. The converting enzyme inhibitor captopril stimulates prostacyclin synthesis by isolated rat aorta. Eur. J. Pharmacol. 1983, 91, 501–504. [Google Scholar] [CrossRef]
- Pi, X. Captopril and ramiprilat protect against free radical injury in isolated working rat hearts. J. Mol. Cell. Cardiol. 1989, 21, 1261–1271. [Google Scholar] [CrossRef]
- Fantone, J.C.; Kinnes, D.A. Prostaglandin E1 and prostaglandin I2 modulation of superoxide production by human neutrophils. Biochem. Biophys Res. Commun. 1983, 113, 506–512. [Google Scholar] [CrossRef]
- Arroyo, C.M.; Wade, J.V.; Ichimori, K.; Nakazawa, H. The scavenging of hydroxyl radical(•OH) by a prostacyclin analogue, taprostene. Chem.-Biol. Interact. 1994, 91, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-C.; Huang, C.-J.; Tam, K.; Chen, S.-F.; Tan, K.T.; Tsai, M.-S.; Lin, T.-N.; Shyue, S.-K. Stabilization of hypoxia-inducible factor-1{alpha} by prostacyclin under prolonged hypoxia via reducing reactive oxygen species level in endothelial cells. J. Biol. Chem. 2005, 280, 36567–36574. [Google Scholar] [CrossRef] [PubMed]
- Swartz, S.L.; Williams, G.H.; Hollenberg, N.K.; Crantz, F.R.; Levine, L.; Moore, T.J.; Dluhy, R.G. Increase in Prostaglandins during Converting Enzyme Inhibition. Clin. Sci. 1980, 59, 133s–135s. [Google Scholar] [CrossRef]
- Moore, T.J.; Crantz, F.R.; Hollenberg, N.K.; Koletsky, R.J.; Leboff, M.S.; Swartz, S.L.; Levine, L.; Podolsky, S.; Dluhy, R.G.; Williams, G.H. Contribution of prostaglandins to the antihypertensive action of captopril in essential hypertension. Hypertension 1981, 3, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Swartz, S.L.; Williams, G.H. Angiotensin-converting enzyme inhibition and prostaglandins. Am. J. Cardiol. 1982, 49, 1405–1409. [Google Scholar] [CrossRef]
- Abe, K.; Ito, T.; Sato, M.; Haruyama, T.; Sato, K.; Omata, K.; Hiwatari, M.; Sakurai, Y.; Imai, Y.; Yoshinaga, K. Role of Prostaglandin in the Antihypertensive Mechanism of Captopril in Low Renin Hypertension. Clin. Sci. 1980, 59, 141s–144s. [Google Scholar] [CrossRef]
- Goldstone, R.; Martin, K.; Zipser, R.; Horton, R. Evidence for a dual action of converting enzyme inhibitor on blood pressure in normal man. Prostaglandins 1981, 22, 587–598. [Google Scholar] [CrossRef]
- Covi, G.; Minuz, P.; Capuzzo, G.; Lechi, C.; Delva, P.; Lechi, A. Reduction of the antihypertensive effect of captopril induced by prostaglandin synthetase inhibition. Int. J. Clin. Pharmacol. Res. 1984, 4, 47–52. [Google Scholar]
- Witzgall, H.; Hirsch, F.; Scherer, B.; Weber, P.C. Acute haemodynamic and hormonal effects of captopril are diminished by indomethacin. Clin. Sci. 1982, 62, 611–615. [Google Scholar] [CrossRef]
- Mezei, Z.; Kis, B.; Gecse, A.; Telegdy, G.; Abrahám, G.; Sonkodi, S. Platelet eicosanoids and the effect of captopril in blood pressure regulation. Eur. J. Pharmacol. 1997, 340, 67–73. [Google Scholar] [CrossRef]
- Bolterman, R.J.; Manriquez, M.C.; Ruiz, M.C.O.; Juncos, L.A.; Romero, J.C. Effects of Captopril on the Renin Angiotensin System, Oxidative Stress, and Endothelin in Normal and Hypertensive Rats. Hypertension 2005, 46, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Pechánová, O. Contribution of captopril thiol group to the prevention of spontaneous hypertension. Physiol. Res. 2007, 56 (Suppl. S2), S41–S48. [Google Scholar] [CrossRef] [PubMed]
- Bernátová, I.; Pechánová, O.; Simko, F. Captopril prevents NO-deficient hypertension and left ventricular hypertrophy without affecting nitric oxide synthase activity in rats. Physiol. Res. 1996, 45, 311–316. [Google Scholar] [PubMed]
- Ikeda, U.; Shimada, K. Nitric oxide release from rat aortic smooth muscle cells is not attenuated by angiotensin converting enzyme inhibitors. Eur. J. Pharmacol. 1994, 269, 319–323. [Google Scholar] [CrossRef]
- Pechanova, O.; Matuskova, J.; Capikova, D.; Jendekova, L.; Paulis, L.; Simko, F. Effect of spironolactone and captopril on nitric oxide and S-nitrosothiol formation in kidney of L-NAME-treated rats. Kidney Int. 2006, 70, 170–176. [Google Scholar] [CrossRef]
- Zicha, J.; Dobešová, Z.; Kuneš, J. Antihypertensive Mechanisms of Chronic Captopril or N-Acetylcysteine Treatment in L-NAME Hypertensive Rats. Hypertens. Res. 2006, 29, 1021–1027. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Satoh, N.; Ueda, S. Possible impact of nitric oxide on the antihypertensive effect of captopril and zaprinast. Adv. Therapy 2003, 20, 143–148. [Google Scholar] [CrossRef]
- de Cavanagh, E.M.V.; Inserra, F.; Ferder, L.; Fraga, C.G. Enalapril and captopril enhance glutathione-dependent antioxidant defenses in mouse tissues. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2000, 278, R572–R577. [Google Scholar] [CrossRef]
- Mak, I.T.; Freedman, A.M.; Dickens, B.F.; Weglicki, W.B. Protective effects of sulfhydryl-containing angiotensin converting enzyme inhibitors against free radical injury in endothelial cells. Biochem. Pharmacol. 1990, 40, 2169–2175. [Google Scholar] [CrossRef]
- Mittra, S.; Singh, M. Possible mechanism of captopril induced endothelium-dependent relaxation in isolated rabbit aorta. Mol. Cell. Biochem. 1998, 183, 63–67. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.; Kim, Y.H.; Chung, W.-S.; Suh, J.K.; Kim, S.J. Antioxidant Effect of Captopril and Enalapril on Reactive Oxygen Species-Induced Endothelial Dysfunction in the Rabbit Abdominal Aorta. Korean J. Thorac. Cardiovasc. Surg. 2013, 46, 14–21. [Google Scholar] [CrossRef]
- Fu, Y.; Xiong, Y.; Fu, S. Captopril restores endothelium-dependent relaxation of rat aortic rings after exposure to homocysteine. J. Cardiovasc. Pharmacol. 2003, 42, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, L.; Sun, X.; Liu, Y.; Song, T. Captopril restores endothelium-dependent relaxation induced by advanced oxidation protein products in rat aorta. J. Cardiovasc. Pharmacol. 2005, 46, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-F.; Xiong, Y.; Guo, Z. A reduction of endogenous asymmetric dimethylarginine contributes to the effect of captopril on endothelial dysfunction induced by homocysteine in rats. Eur. J. Pharmacol. 2005, 508, 167–175. [Google Scholar] [CrossRef]
- Liu, Y.-H.; You, Y.; Song, T.; Wu, S.-J.; Liu, L.-Y. Impairment of Endothelium-Dependent Relaxation of Rat Aortas by Homocysteine Thiolactone and Attenuation by Captopril. J. Cardiovasc. Pharmacol. 2007, 50, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Liu, L.-Y.; Wu, J.-X.; Chen, S.-X.; Sun, Y.-X. Comparison of captopril and enalapril to study the role of the sulfhydryl-group in improvement of endothelial dysfunction with ACE inhibitors in high dieted methionine mice. J. Cardiovasc. Pharmacol. 2006, 47, 82–88. [Google Scholar] [CrossRef]
- Chen, S.-X.; Song, T.; Zhou, S.-H.; Liu, Y.-H.; Wu, S.-J.; Liu, L.-Y. Protective effects of ACE inhibitors on vascular endothelial dysfunction induced by exogenous advanced oxidation protein products in rats. Eur. J. Pharmacol. 2008, 584, 368–375. [Google Scholar] [CrossRef]
- Mailloux, A.; Deslandes, B.; Vaubourdolle, M.; Baudin, B. Captopril and enalaprilat decrease antioxidant defences in human endothelial cells and are unable to protect against apoptosis. Cell Biol. Int. 2003, 27, 825–830. [Google Scholar] [CrossRef]
- Andreoli, S.P. Captopril scavenges hydrogen peroxide and reduces, but does not eliminate, oxidant-induced cell injury. Am. J. Physiol. 1993, 264, F120–F127. [Google Scholar] [CrossRef]
- Keaton, A.K.; White, C.R.; Berecek, K.H. Captopril treatment and its withdrawal prevents impairment of endothelium-dependent responses in the spontaneously hypertensive rat. Clin. Exp. Hypertens 1998, 20, 847–866. [Google Scholar] [CrossRef]
- Dyer, S.M.; Frewin, D.B.; Head, R.J. The influence of chronic captopril treatment and its withdrawal on endothelium-dependent relaxation. Blood Press 1992, 1, 247–253. [Google Scholar] [CrossRef]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Tamba, M.; Torreggiani, A. Free radical scavenging and copper chelation: A potentially beneficial action of captopril. Free Radic. Res. 2000, 32, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Reguli, J.; Miŝík, V. Superoxide Scavenging by Thiol/Copper Complex of Captopril—An Epr Spectroscopy Study. Free Radic. Res. 1995, 22, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Jay, D.; Cuéllar, A.; Zamorano, R.; Muñoz, E.; Gleason, R. Captopril does not scavenge superoxide: Captopril prevents O2-. production by chelating copper. Arch. Biochem. Biophys. 1991, 290, 463–467. [Google Scholar] [CrossRef]
- Jay, D.; Cuella, A.; Jay, E. Superoxide dismutase activity of the captopril-iron complex. Mol. Cell. Biochem. 1995, 146, 45–47. [Google Scholar] [CrossRef]
- Ondetti, M.A. Structural relationships of angiotensin converting-enzyme inhibitors to pharmacologic activity. Circulation 1988, 77, I74–I78. [Google Scholar]
- Russell, J.; Spickett, C.M.; Reglinski, J.; Smith, W.E.; McMurray, J.; Abdullah, I.B. Alteration of the erythrocyte glutathione redox balance by N -acetylcysteine, captopril and exogenous glutathione. FEBS Lett. 1994, 347, 215–220. [Google Scholar] [CrossRef]
- Miguel-Carrasco, J.L.; Monserrat, M.T.; Mate, A.; Vázquez, C.M. Comparative effects of captopril and l-carnitine on blood pressure and antioxidant enzyme gene expression in the heart of spontaneously hypertensive rats. Eur. J. Pharmacol. 2010, 632, 65–72. [Google Scholar] [CrossRef]
- Mozaffari, M.S.; Liu, J.Y.; Abebe, W.; Baban, B. Mechanisms of load dependency of myocardial ischemia reperfusion injury. Am. J. Cardiovasc. Dis. 2013, 3, 180. [Google Scholar]
- Yanagishita, T.; Tomita, M.; Itoh, S.; Mukae, S.; Arata, H.; Ishioka, H.; Geshi, E.; Konno, N.; Katagiri, T. Protective effect of captopril on ischemic myocardium. Jpn. Circ. J. 1997, 61, 161–169. [Google Scholar] [CrossRef]
- Takeo, S.; Nasa, Y.; Tanonaka, K.; Yamaguchi, F.; Yabe, K.; Hayashi, H.; Dhalla, N.S. Role of cardiac renin-angiotensin system in sarcoplasmic reticulum function and gene expression in the ischemic-reperfused heart. Mol. Cell. Biochem. 2000, 212, 227–235. [Google Scholar] [CrossRef]
- Menshikova, E.V.; Salama, G. Cardiac ischemia oxidizes regulatory thiols on ryanodine receptors: Captopril acts as a reducing agent to improve Ca2+ uptake by ischemic sarcoplasmic reticulum. J. Cardiovasc. Pharmacol. 2000, 36, 656–668. [Google Scholar] [CrossRef]
- Shao, Q.; Ren, B.; Zarain-Herzberg, A.; Ganguly, P.K.; Dhalla, N.S. Captopril Treatment Improves the Sarcoplasmic Reticular Ca2+Transport in Heart Failure Due to Myocardial Infarction. J. Mol. Cell. Cardiol. 1999, 31, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, F.; Sanbe, A.; Takeo, S. Effects of long-term treatment with trandolapril on sarcoplasmic reticulum function of cardiac muscle in rats with chronic heart failure following myocardial infarction. Br. J. Pharmacol. 1998, 123, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Ren, B.; Saini, H.K.; Netticadan, T.; Takeda, N.; Dhalla, N.S. Sarcoplasmic reticulum Ca2+ transport and gene expression in congestive heart failure are modified by imidapril treatment. Am. J. Physiol.-Heart Circ. Physiol. 2005, 288, H1674–H1682. [Google Scholar] [CrossRef]
- Schneider, R.; Iscovitz, H.; Ilan, Z.; Bernstein, K.; Gros, M.; Iaina, A. Oxygen free radical scavenger system intermediates in essential hypertensive patients before and immediately after sublingual captopril administration. Isr. J. Med. Sci. 1990, 26, 491–495. [Google Scholar]
- Tkaczewski, W.; Kedziora, J.; Buczyński, A.; Dziekański, S.; Ryniec, A. Effect of captopril on superoxide dismutase (SOD-1) activity and malondialdehyde (MDA) level in blood platelets in patients with arterial hypertension. Kardiol. Pol. 1989, 32, 138–141. [Google Scholar]
- de Cavanagh, E.M.V.; Inserra, F.; Ferder, L.; Romano, L.; Ercole, L.; Fraga, C.G. Superoxide dismutase and glutathione peroxidase activities are increased by enalapril and captopril in mouse liver. FEBS Lett. 1995, 361, 22–24. [Google Scholar] [CrossRef]
- de Cavanagh, E.M.; Fraga, C.G.; Ferder, L.; Inserra, F. Enalapril and captopril enhance antioxidant defenses in mouse tissues. Am. J. Physiol. 1997, 272, R514–R518. [Google Scholar] [CrossRef]
- Helliwell, T.R.; Yeung, J.H.; Park, B.K. Hepatic necrosis and glutathione depletion in captopril-treated mice. Br. J. Exp. Pathol. 1985, 66, 67–78. [Google Scholar]
- Yeung, J.H.K.; Breckenridge, A.M.; Park, B.K. Drug protein conjugates—VI. Role of glutathione in the metabolism of captopril and captopril plasma protein conjugates. Biochem. Pharmacol. 1983, 32, 3619–3625. [Google Scholar] [CrossRef]
- Maulik, S.K.; Kumari, R.; Maulik, M.; Manchanda, S.C.; Gupta, S.K. Captopril and its time of administration in myocardial ischaemic-reperfusion injury. Pharmacol. Res. 2001, 44, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.; Khaper, N.; Dhalla, A.K.; Singal, P.K. Anti-free radical mechanisms in captopril protection against reperfusion injury in isolated rat hearts. Can. J. Cardiol. 1996, 12, 1099–1104. [Google Scholar] [PubMed]
- Cabell, K.S.; Ma, L.; Johnson, P. Effects of antihypertensive drugs on rat tissue antioxidant enzyme activities and lipid peroxidation levels. Biochem. Pharmacol. 1997, 54, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, Z.; Oron-Herman, M.; Rosenthal, T.; Pappo, O.; Link, G.; Sela, B.-A.; Grozovski, M. Effects of Amlodipine, Captopril, and Bezafibrate on Oxidative Milieu in Rats with Fatty Liver. Dig. Dis. Sci. 2008, 53, 777–784. [Google Scholar] [CrossRef]
- Djordjević, V.B.; Grubor-Lajsić, G.; Jovanovic-Galović, A.; Pavlović, D.; Cvetković, T.; Pejović, M.; Lecić, N. Selenium-dependent GSH-Px in erythrocytes of patients with hypertension treated with ACE inhibitors. J. Environ. Pathol. Toxicol. Oncol. 1998, 17, 277–280. [Google Scholar]
- Djordjević, V.B.; Pavlović, D.; Pejović, M.; Cvetković, T.; Lecić, N.; Deljanin-Ilić, M. Changes of lipid peroxides and antioxidative factors levels in blood of patients treated with ACE inhibitors. Clin. Nephrol. 1997, 47, 243–247. [Google Scholar]
- Bashara, M. Effects of atenolol, captopril, and their combination on oxidative stress in hypertensive patients. Med. J. Babylon 2009, 6, 6. [Google Scholar]
- Asdaq, S.M.; Inamdar, M.N. Potential of garlic and its active constituent, S-allyl cysteine, as antihypertensive and cardioprotective in presence of captopril. Phytomedicine 2010, 17, 1016–1026. [Google Scholar] [CrossRef]
- Maneesai, P.; Prasarttong, P.; Bunbupha, S.; Kukongviriyapan, U.; Kukongviriyapan, V.; Tangsucharit, P.; Prachaney, P.; Pakdeechote, P. Synergistic Antihypertensive Effect of Carthamus tinctorius L. Extract and Captopril in l-NAME-Induced Hypertensive Rats via Restoration of eNOS and AT1R Expression. Nutrients 2016, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.A.D.; Liu, H.; Baldi, S.; Wang, Y.; Chen, P.; Lu, F.; Liu, S. Antihypertensive, antioxidant, and renal protective impact of integrated GJD with captopril in spontaneously hypertensive rats. Sci. Rep. 2023, 13, 10944. [Google Scholar] [CrossRef] [PubMed]
- Paseban, M.; Niazmand, S. The Comparison of Antioxidant Effect of Aspirin, Metformin, Atorvastatin and Captopril Co-administration in the Heart and Kidney Tissues of Diabetic Rats. IJPR 2021, 20, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, D.; De Gioia, S.; Mezzetti, A.; Ciofani, G.; Di Ilio, C.; Cuccurullo, F. The prooxidant properties of captopril. Biochem. Pharmacol. 1995, 50, 27–32. [Google Scholar] [CrossRef]
- Bartosz, M.; Kedziora, J.; Bartosz, G. Antioxidant and Prooxidant Properties of Captopril and Enalapril. Free Radic. Biol. Med. 1997, 23, 729–735. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Wei, J.; Zheng, H.; Zhou, N.; Xu, X.; Deng, X.; Liu, T.; Zou, Y. The immune system in cardiovascular diseases: From basic mechanisms to therapeutic implications. Sig. Transduct. Target Ther. 2025, 10, 166. [Google Scholar] [CrossRef]
- Zhao, S.-P.; Xie, X.-M. Captopril inhibits the production of tumor necrosis factor-α by human mononuclear cells in patients with congestive heart failure. Clin. Chim. Acta 2001, 304, 85–90. [Google Scholar] [CrossRef]
- Sheikhi, A.; Jaberi, Y.; Esmaeilzadeh, A.; Khani, M.; Moosaeefard, M.; Shafaqatian, M. The effect of cardiovascular drugs on pro-inflammatory cytokine secretion and natural killer activity of peripheral blood mononuclear cells of patients with chronic heart failure in vitro. Pak. J. Biol. Sci. 2007, 10, 1580–1587. [Google Scholar] [CrossRef]
- Rezkalla, S.; Kloner, R.A.; Khatib, G.; Khatib, R. Beneficial effects of captopril in acute coxsackievirus B3 murine myocarditis. Circulation 1990, 81, 1039–1046. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.A.; Metwally, M.E.; El-khawanki, M.M.; Hashim, A.M. Protective effect of captopril against clozapine-induced myocarditis in rats: Role of oxidative stress, proinflammatory cytokines and DNA damage. Chem.-Biol. Interact. 2014, 216, 43–52. [Google Scholar] [CrossRef]
- Miguel-Carrasco, J.L.; Zambrano, S.; Blanca, A.J.; Mate, A.; Vázquez, C.M. Captopril reduces cardiac inflammatory markers in spontaneously hypertensive rats by inactivation of NF-kB. J. Inflamm. 2010, 7, 21. [Google Scholar] [CrossRef]
- El-Sayed, S.F.; Abdelhamid, A.M.; ZeinElabdeen, S.G.; El-wafaey, D.I.; Moursi, S.M.M. Melatonin enhances captopril mediated cardioprotective effects and improves mitochondrial dynamics in male Wistar rats with chronic heart failure. Sci. Rep. 2024, 14, 575. [Google Scholar] [CrossRef]
- Sepehri, Z.; Masoumi, M.; Ebrahimi, N.; Kiani, Z.; Nasiri, A.A.; Kohan, F.; Sheikh Fathollahi, M.; Kazemi Arababadi, M.; Asadikaram, G. Atorvastatin, Losartan and Captopril Lead to Upregulation of TGF-β, and Downregulation of IL-6 in Coronary Artery Disease and Hypertension. PLoS ONE 2016, 11, e0168312. [Google Scholar] [CrossRef]
- Akbari, H.; Asadikaram, G.; Jafari, A.; Nazari-Robati, M.; Ebrahimi, G.; Ebrahimi, N.; Masoumi, M. Atorvastatin, losartan and captopril may upregulate IL-22 in hypertension and coronary artery disease; the role of gene polymorphism. Life Sci. 2018, 207, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.J.; Jurado-Flores, M.; Hammoud, R.; Feng, V.; Gonzales, K.; Onstead-Haas, L.; Mooradian, A.D. The Effects of Known Cardioprotective Drugs on Proinflammatory Cytokine Secretion From Human Coronary Artery Endothelial Cells. Am. J. Ther. 2019, 26, e321–e332. [Google Scholar] [CrossRef] [PubMed]
- Andersson, P.; Cederholm, T.; Johansson, A.-S.; Palmblad, J. Captopril-impaired production of tumor necrosis factor-alpha-induced interleukin-1beta in human monocytes is associated with altered intracellular distribution of nuclear factor-kappaB. J. Lab. Clin. Med. 2002, 140, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, D.; Nihei, J.; Cardillo, F.; Singh, R. The ACE inhibitors enalapril and captopril modulate cytokine responses in Balb/c and C57Bl/6 normal mice and increase CD4(+)CD103(+)CD25(negative) splenic T cell numbers. Cell. Immunol. 2010, 260, 92–97. [Google Scholar] [CrossRef]
- Lapteva, N.; Ide, K.; Nieda, M.; Ando, Y.; Hatta-Ohashi, Y.; Minami, M.; Dymshits, G.; Egawa, K.; Juji, T.; Tokunaga, K. Activation and suppression of renin–angiotensin system in human dendritic cells. Biochem. Biophys. Res. Commun. 2002, 296, 194–200. [Google Scholar] [CrossRef]
- Peeters, A.C.; Netea, M.G.; Kullberg, B.J.; Thien, T.; van der Meer, J.W. The effect of renin-angiotensin system inhibitors on pro- and anti-inflammatory cytokine production. Immunology 1998, 94, 376–379. [Google Scholar] [CrossRef]
- Fukuzawa, M.; Satoh, J.; Sagara, M.; Muto, G.; Muto, Y.; Nishimura, S.; Miyaguchi, S.; Qiang, X.L.; Sakata, Y.; Nakazawa, T.; et al. Angiotensin converting enzyme inhibitors suppress production of tumor necrosis factor-α in vitro and in vivo. Immunopharmacology 1997, 36, 49–55. [Google Scholar] [CrossRef]
- Amirfakhryan, H.; New, K.J. The role of myeloperoxidase as a biomarker in atherosclerotic cardiovascular disease. Cardiol. Plus 2024, 9, 195. [Google Scholar] [CrossRef]
- Abd Al Haleem, E.N.; Ahmed, S.F.; Temraz, A.; El-Tantawy, W.H. Evaluation of the cardioprotective effect of Casuarina suberosa extract in rats. Drug Chem. Toxicol. 2022, 45, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Kerımoğlu, O.; Şahbaz, S.; Şehırlı, Ö.; Ozdemır, Z.N.; Çetınel, Ş.; Dortunç, B.; Şener, G. Pharmacodynamical evaluation of matrix type transdermal therapeutic systems containing captopril. Acta Pol. Pharm. 2015, 72, 799–806. [Google Scholar] [PubMed]
- Delfraissy, J.F.; Galanaud, P.; Balavoine, J.F.; Wallon, C.; Dormont, J. Captopril and immune regulation. Kidney Int. 1984, 25, 925–929. [Google Scholar] [CrossRef]
- Bryniarski, P.; Nazimek, K.; Marcinkiewicz, J. Captopril Combined with Furosemide or Hydrochlorothiazide Affects Macrophage Functions in Mouse Contact Hypersensitivity Response. Int. J. Mol. Sci. 2021, 23, 74. [Google Scholar] [CrossRef]
- Bryniarski, P.; Nazimek, K.; Marcinkiewicz, J. Anti-Inflammatory Activities of Captopril and Diuretics on Macrophage Activity in Mouse Humoral Immune Response. Int. J. Mol. Sci. 2021, 22, 11374. [Google Scholar] [CrossRef]
- Vallejo Ardila, D.L.; Walsh, K.A.; Fifis, T.; Paolini, R.; Kastrappis, G.; Christophi, C.; Perini, M.V. Immunomodulatory effects of renin-angiotensin system inhibitors on T lymphocytes in mice with colorectal liver metastases. J. Immunother. Cancer 2020, 8, e000487. [Google Scholar] [CrossRef]
- Peng, H.; Carretero, O.A.; Vuljaj, N.; Liao, T.-D.; Motivala, A.; Peterson, E.L.; Rhaleb, N.-E. Angiotensin-converting enzyme inhibitors: A new mechanism of action. Circulation 2005, 112, 2436–2445. [Google Scholar] [CrossRef]
- Kumar, N.; Yin, C. The anti-inflammatory peptide Ac-SDKP: Synthesis, role in ACE inhibition, and its therapeutic potential in hypertension and cardiovascular diseases. Pharmacol. Res. 2018, 134, 268–279. [Google Scholar] [CrossRef]
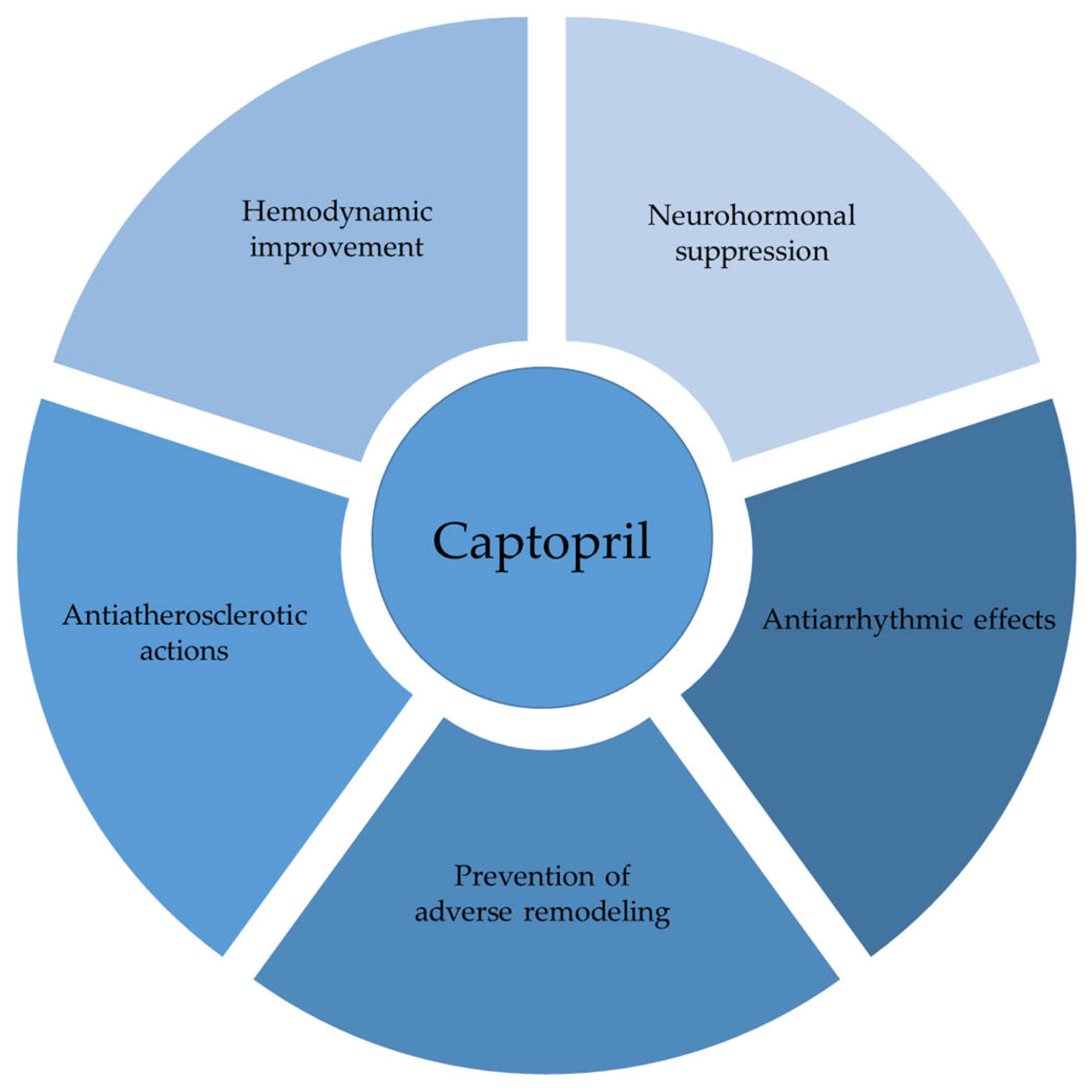
| References | Timepoint of Administration | Duration of Treatment | Dosage | Results | Comment |
|---|---|---|---|---|---|
| [39] | 2 h after operation and 21 d after infarction | 4 m | 2 g/L | Improved hemodynamic function; reduction in the mass of both ventricles and LV dilatation | NS between two timepoints |
| [56] | Immediately postoperatively | 7 d | 2 g/L | Unloading systolic and diastolic blood pressure of ventricles; No regression of cardiac hypertrophy | Improved but not restored cardiac pump performance |
| [57] | On the day of surgery | 4–5 w | 30 mg/kg i.p. | Reduction in left filling pressures; no change in RV systolic and end-diastolic pressures; reduction in hypertrophic growth in right but not left heart chambers | Minor improvement in systolic LV performance |
| [58] | After infarction | 6 w captopril | 30 mg/kg i.p. | Prevention of RVH; reduction in LV weight | Lesser effects of treatment in only early and late therapy |
| Early (3 w) | First 3 w captopril, Last 3 w saline | Partial prevention of LVH and no effect on RV weight | |||
| Late (last 3 w) | First 3 w Saline, Last 3 w captopril | Regression of hypertrophy in both ventricles | |||
| [93] | 2nd day of infarction | 3 w | 500 µg/kg/h s.c. | Reduction in HW; unaltered CO; increased HR and decreased SV and SW | Early therapy had unfavorable effects, yet delayed therapy improved cardiac function |
| 21st day of infarction | 2 w | 100 or 500 µg/kg/h s.c. | Decrease in MAP, TPR no reduction in the ventricles’ weight; increased CO through SV | ||
| 5 w from infarction | One-time | 10 mg/kg i.v.bolus | Reduced afterload; no change in cardiac performance | ||
| [102] | 2nd day of infarction | 8 w | 2 g/L | Prevention of hypertrophy; improved CI and SV index, reduced SVR | Normalized the myocardial infarction-induced genes expression |
| [109] | 28th day of infarction | 28 days | 2 g/L | Prevention of progressive myocyte cell lengthening; modest reduction in myocyte cell volume | Even late treatment may attenuate myocyte remodeling |
| [111] | 7th day of infarction | 4 w | 0.2 g/L | Regression of hypertrophy; preserved or improved EF; reduced ventricular dilatation; improved vascularization in the infarction border zone; no reduction in infarct size and LV anterior wall thickness | Delayed effects are notable |
| Role-In Group | Daily Dose | Hemodynamic Changes | Effects | Correlation Between PRA and Hemodynamic Changes | Hormonal Changes | Comment | References |
|---|---|---|---|---|---|---|---|
| NYHA III-IV; n = 10 | 25–150 mg | CI ↑, MAP ↓, MPAP ↓, MRAP ↓, SVR ↓, MPCWP ↓, HR ↓ * | SV↑, left ventricle filling pressure ↓ | No correlation found | Not evaluated | Increased treadmill performance after 4 w | [186] |
| NYHA III-IV; n = 11 | 25–275 mg | CI ↑, MAP ↓, RAP ↓, SVR ↓, PVR ↓, PCW ↓, HR↓ * | Arterial and venous vasodilatory effects; | Confirmed for ↓ MAP, SVR, PVR, PCW and ↑CI | Improved treadmill-exercise tolerance after ≥2 m | [187] | |
| NYHA III-IV; n = 10 | 25–100 mg | CO ↑, SV ↑, SWI ↑, RAP ↓, PAP ↓ SVR ↓, PCW ↓, HR↓ | Improved LV function | Not evaluated | Improved exercise duration on the treadmill and bicycle after 8 w | [188] | |
| NYHA III-IV; n = 10 | 25–125 mg | CI ↑, SVI ↑, SWI ↔, MAP ↓, MPAP ↓, MPCWP ↓, MRAP ↓, SVR ↓, TPuR ↓, HR ↔ | Enhanced cardiac performance | Pretreatment PRA correlated with the CI | Aldosterone ↓, Norepinephrine ↑ | Increased treadmill-exercise time after 1 m | [189] |
| NYHA III-IV; n = 5 | 150–450 mg | CO ↑, MAP ↓, PAP ↓, PCW ↓, RAP ↔ | Improved circulatory dynamics | Confirmed for MAP, PAP | Aldosterone ↓, Cortisol ↔, Noradrenaline ↔, Adrenaline ↔ | Improved exercise tolerance on subsequent mobilization in the hospital | [193] |
| Role-In Group | Daily Dose and Follow-Up | Hemodynamic Changes | Effects | Exercise Capacity | Neurohormonal Changes | Comment | References |
|---|---|---|---|---|---|---|---|
| NYHA III-IV; n = 18 | 12.5–50 mg TID; 1–3 m | CI ↑, SVI ↑, MAP ↓, MPCWP ↓, SVR ↓, HR ↔ | Improved symptoms and NYHA class | Treadmill-exercise duration improved | Not evaluated | Sustained hemodynamic changes occurred during acute phase | [196] |
| NYHA III-IV; n = 11 | 75–200 mg; 2–10 m | CI ↑, MAP ↓, TPR ↓, MTT ↓, HR ↔ | Not evaluated | PRA ↑, Aldosterone ↓, Catecholamines ↓ * | No correlation between PRA and hemodynamic changes | [211] | |
| NYHA III-IV; n = 9 | 37.5–300 mg; 3 m | CI ↑, MAP ↓, TPR ↓, PAP ↓, PCW ↓ | symptomatic improvement | Not evaluated during the long-term course | Acute-phase hemodynamic improvements were maintained | [195] | |
| NYHA III-IV; n = 36 | 75–300 mg; up to 36 m | Evaluated during acute phase only | Improved symptoms and NYHA class | Improved at early follow-up (2–5 m), sustained in late follow-up (11–18 m) | Pre- and post-treatment SWI, post-treatment CI and SVI predicted long-term clinical effects | [208] | |
| NYHA III-IV; n = 19 | 75–300 mg; 6 m | CO ↑, MTT↓; HR ↓ | Clinical improvement | Not evaluated | PRA ↑, Aldosterone ↓, Catecholamines ↓ * | Normalization of MTT best correlated with clinical progress | [209] |
| Tissue Homogenates | Enzymes and Lipid Peroxidation Levels | Observed Drugs, Dosage, Route of Administration and Duration of Treatment | |||
|---|---|---|---|---|---|
| Cap 50 mg/kg/d; via Drinking Water; 12 w [357] Hyd 15 mg/kg/d; Ter 45 mg/kg/d | |||||
| WKY | SHR | ||||
| Myocardium | Cu/Zn-SOD | Cap, Hyd, Ter ↔ | Cap, Hyd, Ter ↑ | ||
| Mn-SOD | Cap, Hyd, Ter ↔ | Cap, Hyd ↔, Ter ↓ | |||
| GSH-Px | Cap ↔, Hyd, Ter ↓ | Cap, Hyd, Ter ↓ | |||
| Catalase | Cap ↑, Hyd, Ter ↔ | Cap ↔, Hyd, Ter ↓ | |||
| TBARS | Cap, Hyd, Ter ↓ | Cap ↔, Hyd, Ter ↓ | |||
| Liver | Cu/Zn-SOD | Cap, Hyd ↔, Ter ↑ | Cap ↓, Hyd, Ter ↑ | ||
| Mn-SOD | Cap, Hyd, Ter ↔ | Cap ↓, Hyd ↔, Ter↓ | |||
| GSH-Px | Cap, Hyd ↓, Ter ↑ | Cap, Hyd ↓, Ter ↑ | |||
| Catalase | Cap ↓, Hyd ↔, Ter ↑ | Cap ↓, Hyd, Ter ↔ | |||
| TBARS | Cap ↓, Hyd, Ter ↔ | Cap, Hyd ↔, Ter ↑ | |||
| Skeletal muscle | Cu/Zn-SOD | Cap, Hyd, Ter ↔ | Cap, Hyd, Ter ↔ | ||
| Mn-SOD | Cap, Hyd, Ter ↔ | Cap, Hyd ↔, Ter ↓ | |||
| GSH-Px | Cap, Hyd, Ter ↔ | Cap, Hyd, Ter ↔ | |||
| Catalase | Cap, Hyd, Ter ↓ | Cap, Hyd ↔, Ter ↑ | |||
| TBARS | Cap, Hyd, Ter ↔ | Cap, Hyd, Ter ↔ | |||
| Tissue Homogenates | Enzymes and Lipid Peroxidation Levels | Observed Drugs, Dosage, and Duration of Treatment | |||
| Cap 50 mg/L; Enap 20 mg/L via Drinking Water 4–11 w and 11 w | |||||
| CF1-Mice | |||||
| 4–11 w [351] | 11 w [352] | 11 w [320] | |||
| Myocardium | Cu/Zn-SOD | Not evaluated | Cap, Enap ↑ | Not evaluated | |
| Mn-SOD | Cap, Enap ↔ | ||||
| Se-GSH-Px | Cap ↔ | Cap, Enap ↔ | |||
| Catalase | Cap, Enap ↔ | Not evaluated | |||
| TBARS | Not evaluated | ||||
| GSSG+GSH | Cap, Enap ↔ | ||||
| GSSG-Rd | Cap, Enap ↔ | ||||
| Liver | Cu/Zn-SOD | Cap, Enap ↑ | Not evaluated | Not evaluated | |
| Mn-SOD | Cap, Enap ↑ | ||||
| Se-GSH-Px | Cap, Enap ↑ | Cap, Enap ↑ | |||
| Catalase | Cap, Enap ↔ | Not evaluated | |||
| TBARS | Not evaluated | Cap ↓, Enap ↔ | |||
| GSSG+GSH | Not evaluated | Cap, Enap ↔ | |||
| GSSG-Rd | Cap, Enap↑ | ||||
| Kidney | Cortex | Cu/Zn-SOD | Not evaluated | Cap, Enap ↔ | Not evaluated |
| Mn-SOD | Cap, Enap ↔ | ||||
| Se-GSH-Px | Cap ↔ | Cap, Enap↑ | |||
| Catalase | Cap, Enap ↔ | Not evaluated | |||
| TBARS | Not evaluated | ||||
| GSSG+GSH | Cap, Enap ↔ | ||||
| GSSG-Rd | Cap ↔, Enap ↑ | ||||
| Medulla | Cu/Zn-SOD | Cap, Enap ↑ | Not evaluated | ||
| Mn-SOD | Cap, Enap ↔ | ||||
| Se-GSH-Px | Cap ↔, Enap ↑ | Cap ↔, Enap ↑ | |||
| Catalase | Cap, Enap ↔ | Not evaluated | |||
| TBARS | Not evaluated | ||||
| GSSG+GSH | Cap, Enap ↔ | ||||
| GSSG-Rd | Cap, Enap ↔ | ||||
| Brain | Cu/Zn-SOD | Cap, Enap ↔ | Not evaluated | ||
| Mn-SOD | Cap, Enap ↔ | ||||
| Se-GSH-Px | Cap ↔ | Cap, Enap ↔ | |||
| Catalase | Cap, Enap ↔ | Not evaluated | |||
| TBARS | Not evaluated | ||||
| GSSG+GSH | Cap, Enap↑ | ||||
| GSSG-Rd | Cap, Enap↑ | ||||
| Erythrocytes | Cu/Zn-SOD | Cap ↔, Enap ↑ | Not evaluated | ||
| Mn-SOD | Cap, Enap ↔ | ||||
| Se-GSH-Px | Cap ↔ | Cap, Enap ↔ | |||
| Catalase | Cap, Enap ↔ | Not evaluated | |||
| TBARS | Not evaluated | Cap, Enap ↓ | |||
| GSSG+GSH | Cap, Enap↑ | ||||
| GSSG-Rd | Cap, Enap↑ | ||||
| Lungs | Cu/Zn-SOD | Not evaluated | |||
| Mn-SOD | |||||
| Se-GSH-Px | Cap, Enap ↔ | ||||
| Catalase | Not evaluated | ||||
| TBARS | |||||
| GSSG+GSH | Cap ↔, Enap↑ | ||||
| GSSG-Rd | Cap, Enap ↔ | ||||
| Antioxidative Properties [270,271,272,273,275,276,279,280,281,283,288,295,296,298,300,301,320,321,324,325,326,327,329,339,341,345,349,350,358,361,362,363,364,365] | Pro-Oxidative Properties [288,289,290,366,367] | ||
|---|---|---|---|
| Mechanism | Effects | Mechanism | Effects |
| Free scavenging activity | Direct neutralization of O2, OH·; OCL·; HOCL; H2O2; 1O2 | Fe3+-dependent pro-oxidant activity | Induction of lipid peroxidation |
| Free recyclable scavenger of glutathione | Maintains and regenerates intracellular GSH levels | Generation of OH· and RS· | |
| Restoring Na+ K+/Mg2+ ATPase and Ca2+ Mg2+/ATPase levels | Stabilizes ion transport and reduces oxidative stress caused by ionic imbalance | Captopril-mediated iron release from ferritin | Generation of TBA reactive deoxyribose degradation products |
| Inhibition of enhanced lipid peroxidation | Decreases formation of malondialdehyde (MDA) and TBARS | Cu2+-dependent pro-oxidant activity | |
| Induction of PGI2 synthesis | PGI2 exerts antioxidant effects | ||
| Sustained NO bioavailability | Increases NO synthetase activity and NO production | ||
| Increases enzymatic endogenous antioxidants | Enhances the activity of SOD, GSH-Px, GSH-Rd, CuZn-SOD, and Mn-SOD | ||
| Enhances non-enzymatic antioxidant defense | Protection against oxidative damage | ||
| Preservation of exogenous antioxidants | Maintains α-Tocopherol levels | ||
| Reverses sulfhydryl oxidation of RyRs | Facilitates Ca2+ reuptake | ||
| Potentiation of antioxidative effects | Augmented antioxidative capacity of captopril when concomitantly administered with other substances and drugs | ||
| Effect | Molecular Mediator | References |
|---|---|---|
| Reduction | TNF-α | [29,369,370,372,374,380,382] |
| IL-1α | [29,380] | |
| IL-1β | [373,377,378] | |
| IL-6 | [151,373,374,375,377,388] | |
| TGF-β | [390] | |
| Immune-complex deposition (C3, IgG) | [12] | |
| IFNα | [12] | |
| IL-8 | [377] | |
| IL-10 | [380] | |
| IL-12 | [30,151,380,387] | |
| IL-18 | [380] | |
| MMP-2 | [115,117,118,149,263,265] | |
| MMP-9 | [117,118,149,263] | |
| MPO | [384,385] | |
| Unaltered | Synthesis of C3 component | [29] |
| TGF-β1 | [388] | |
| Increase | IL-10 | [151,372,373,374,379] |
| Ac-SDKP | [390] | |
| IL-2 | [379] | |
| TGF-β | [151,375] | |
| IL-22 | [376] | |
| PGE2 | [225] | |
| 6-keto PGF1α, TxB2 | [285] | |
| PGI2 | [298,300] | |
| PGD2 | [312] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoiljkovic, M.; Jakovljevic, V.; Milosavljevic, J.; Bolevich, S.; Jeremic, N.; Canovic, P.; Fisenko, V.P.; Tikhonov, D.A.; Krylova, I.N.; Bolevich, S.; et al. Cardioprotective Role of Captopril: From Basic to Applied Investigations. Int. J. Mol. Sci. 2025, 26, 7215. https://doi.org/10.3390/ijms26157215
Stoiljkovic M, Jakovljevic V, Milosavljevic J, Bolevich S, Jeremic N, Canovic P, Fisenko VP, Tikhonov DA, Krylova IN, Bolevich S, et al. Cardioprotective Role of Captopril: From Basic to Applied Investigations. International Journal of Molecular Sciences. 2025; 26(15):7215. https://doi.org/10.3390/ijms26157215
Chicago/Turabian StyleStoiljkovic, Marko, Vladimir Jakovljevic, Jovan Milosavljevic, Sergey Bolevich, Nevena Jeremic, Petar Canovic, Vladimir Petrovich Fisenko, Dmitriy Alexandrovich Tikhonov, Irina Nikolaevna Krylova, Stefani Bolevich, and et al. 2025. "Cardioprotective Role of Captopril: From Basic to Applied Investigations" International Journal of Molecular Sciences 26, no. 15: 7215. https://doi.org/10.3390/ijms26157215
APA StyleStoiljkovic, M., Jakovljevic, V., Milosavljevic, J., Bolevich, S., Jeremic, N., Canovic, P., Fisenko, V. P., Tikhonov, D. A., Krylova, I. N., Bolevich, S., Chichkova, N. V., & Zivkovic, V. (2025). Cardioprotective Role of Captopril: From Basic to Applied Investigations. International Journal of Molecular Sciences, 26(15), 7215. https://doi.org/10.3390/ijms26157215






