Cadmium Inhibits Proliferation of Human Bronchial Epithelial BEAS-2B Cells Through Inducing Ferroptosis via Targeted Regulation of the Nrf2/SLC7A11/GPX4 Pathway
Abstract
1. Introduction
2. Results
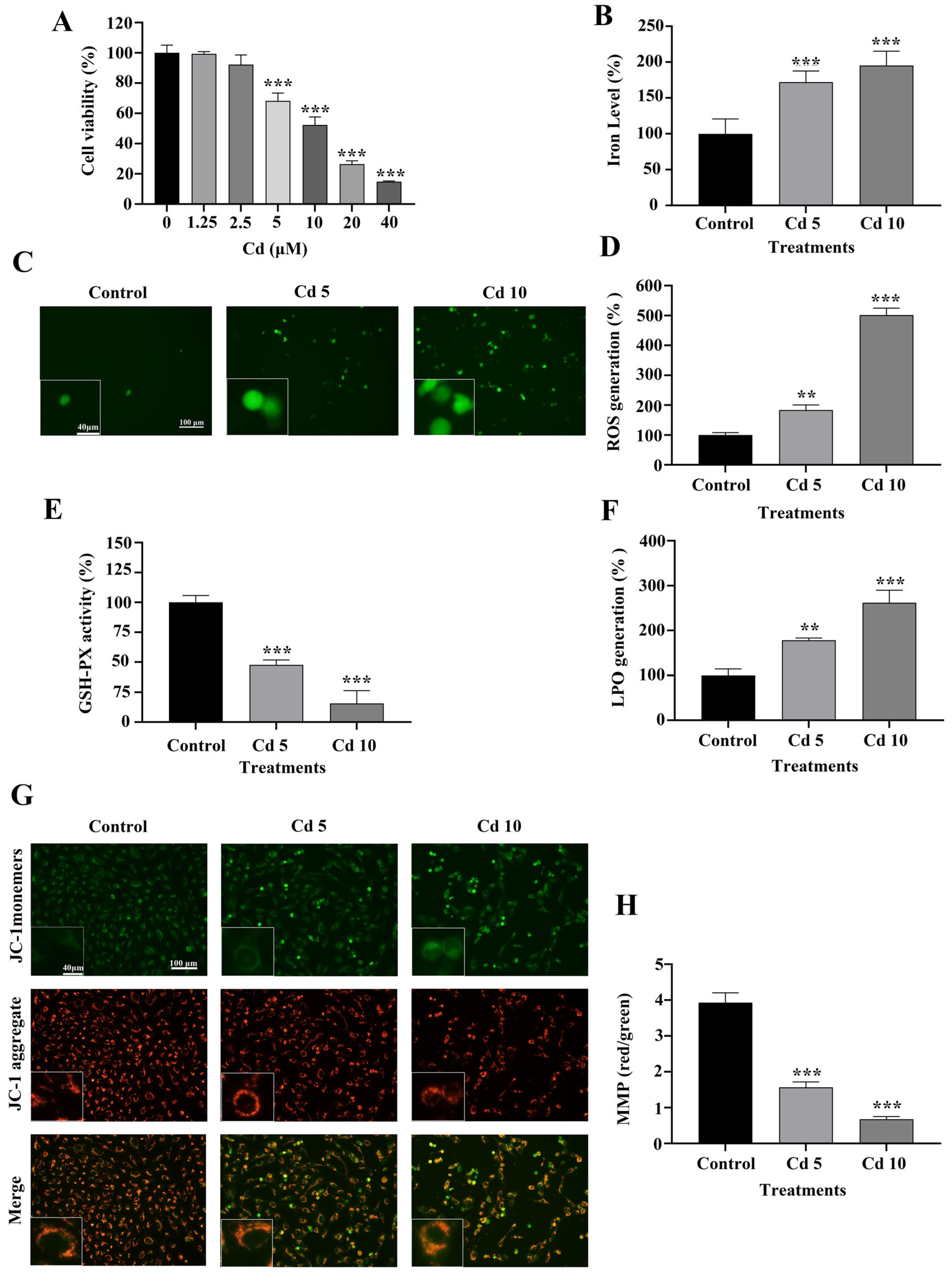
2.1. Cadmium (Cd) Inhibits BEAS-2B Cell Viability
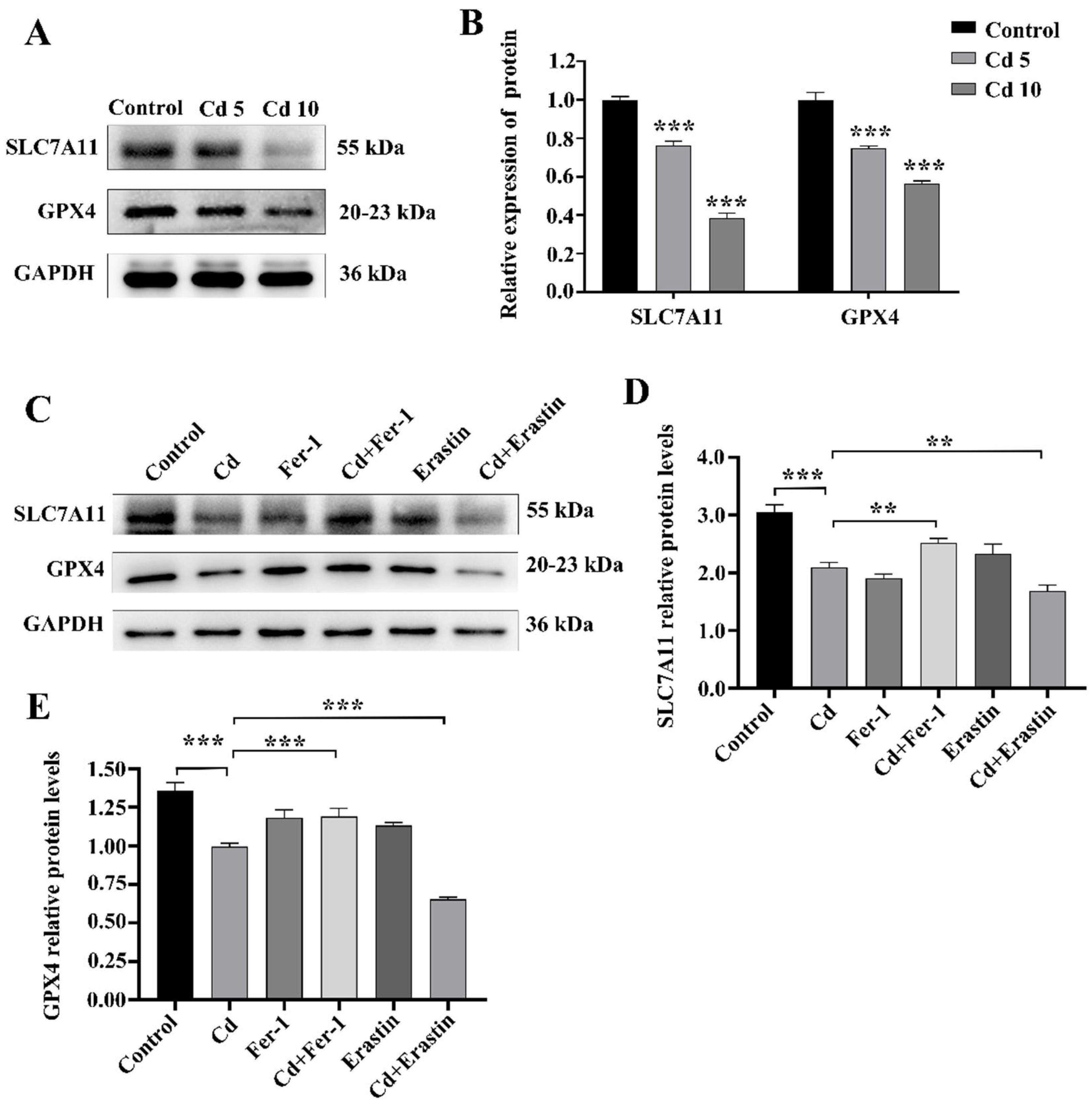
2.2. Cd Induces Ferroptosis by Regulating the Levels of LPO, GSH-PX and MMP in BEAS-2B Cells
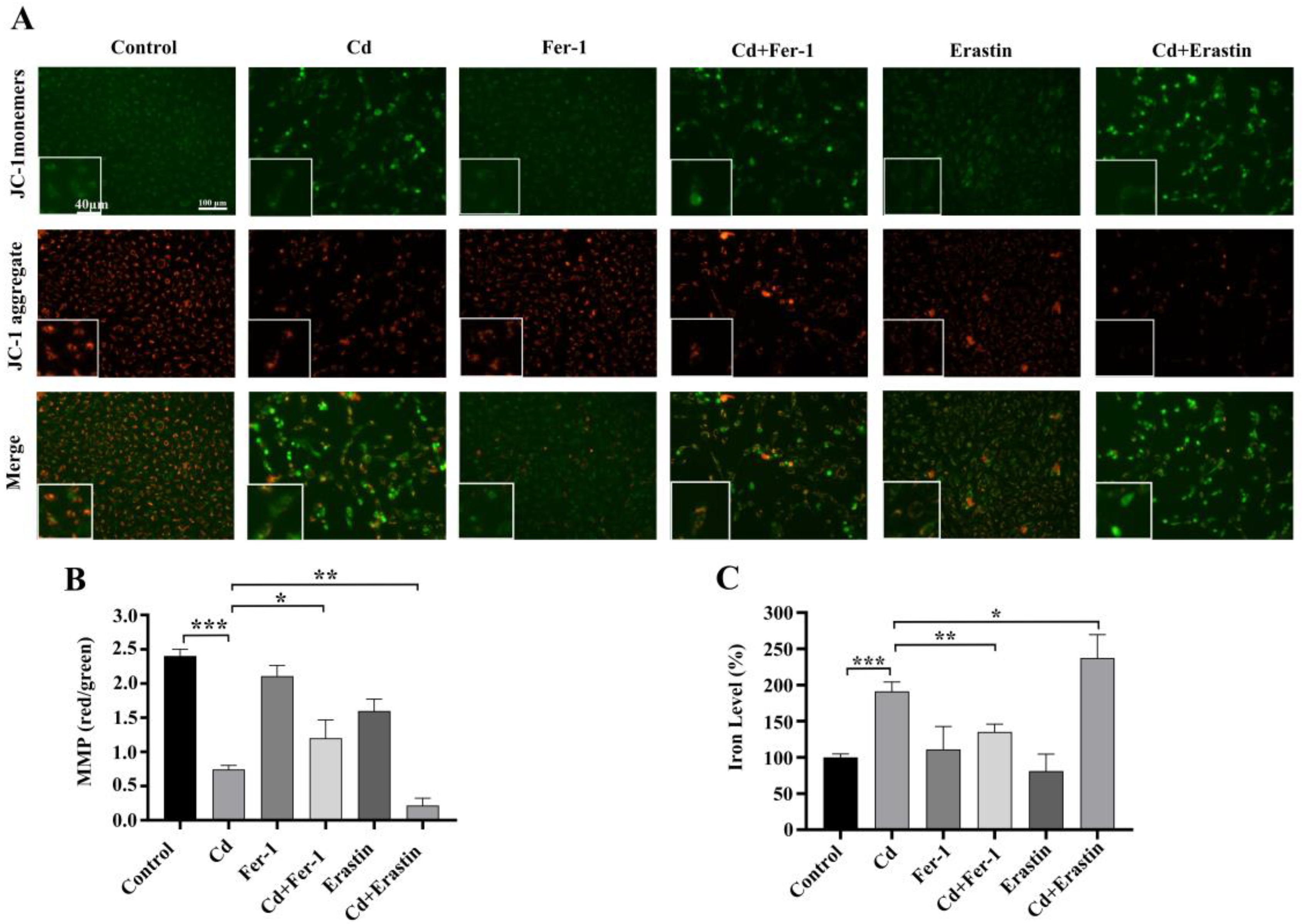
2.3. Fer-1 and Erastin Modulate Cd-Induced Ferroptosis in BEAS-2B Cells
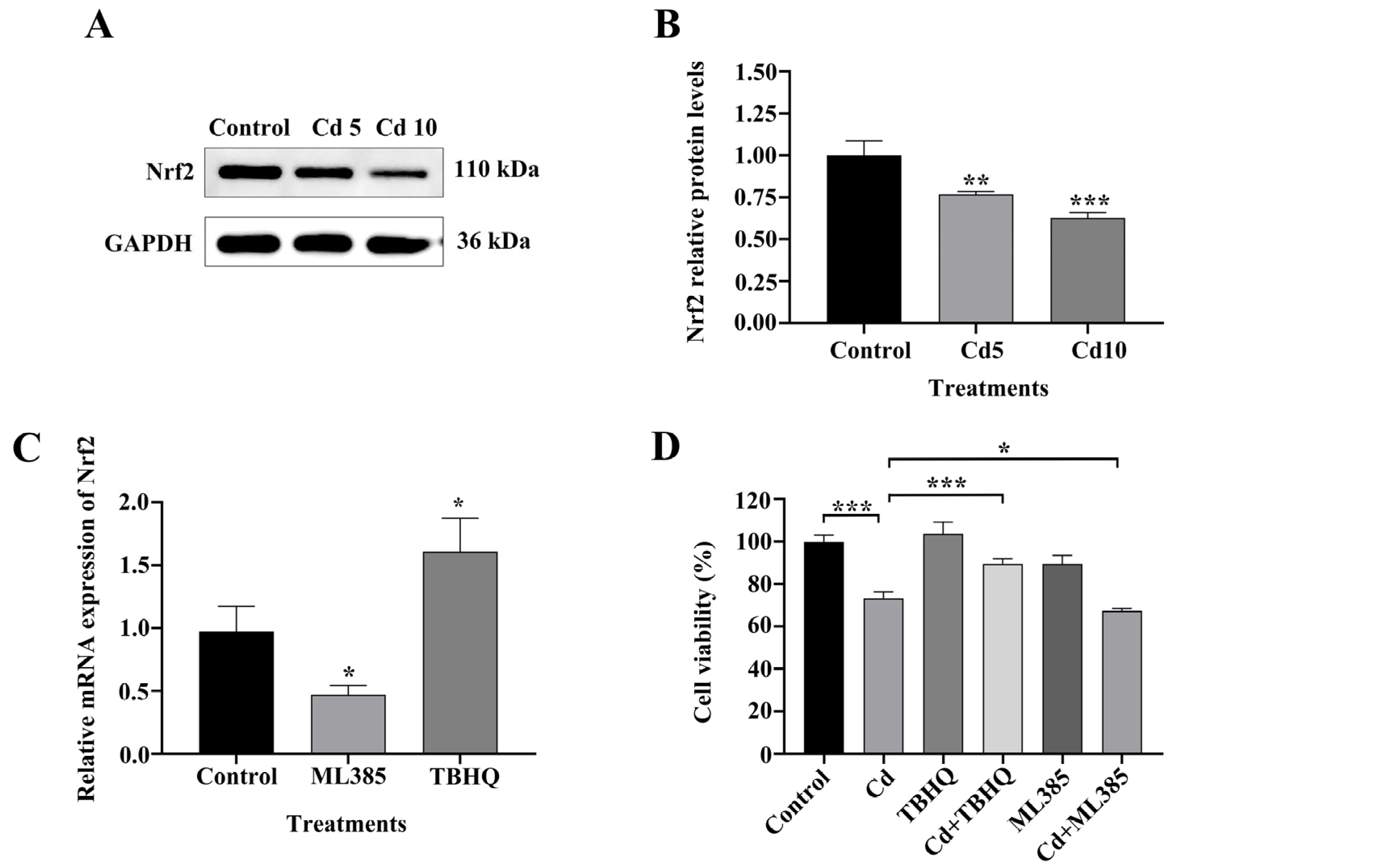
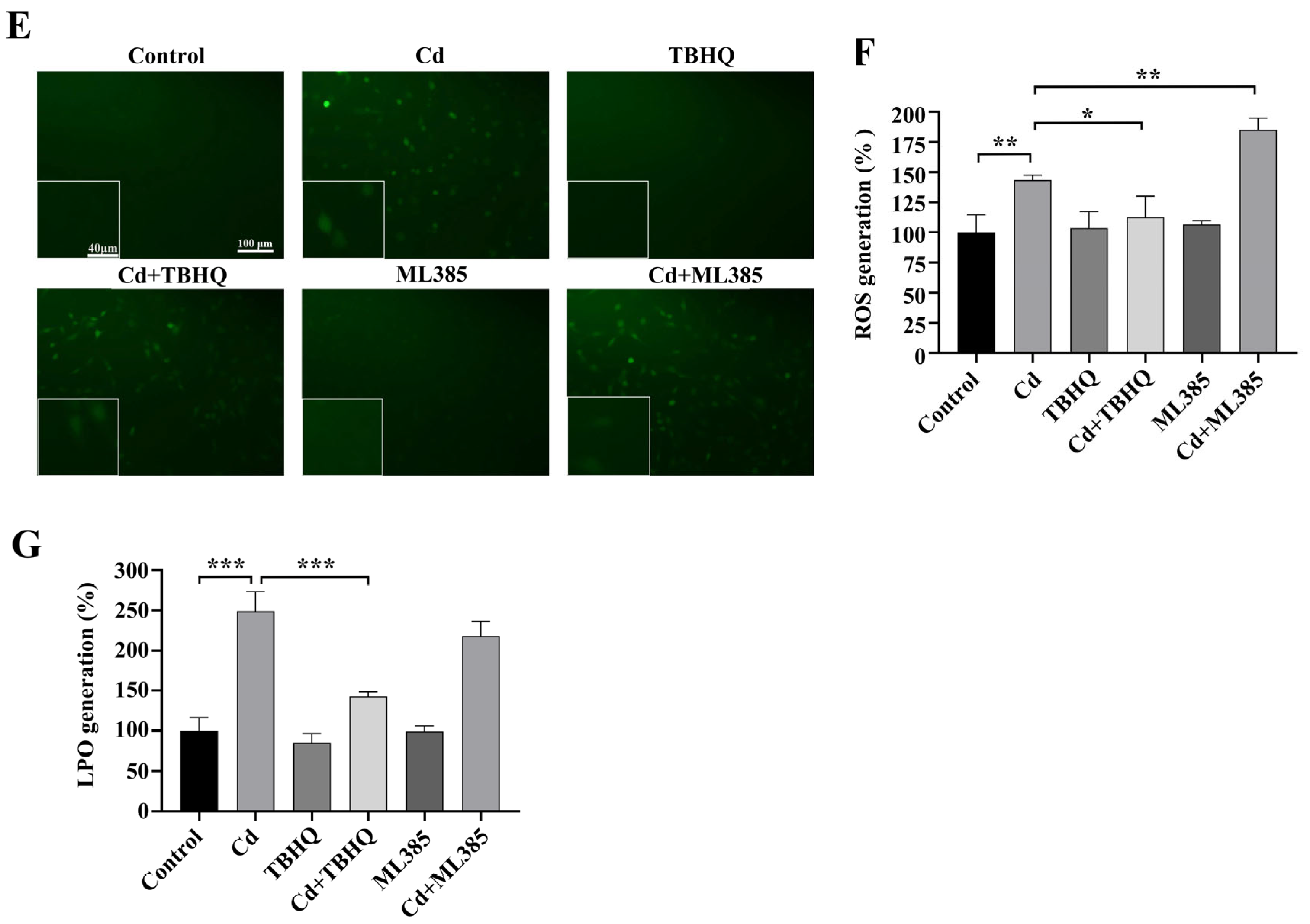
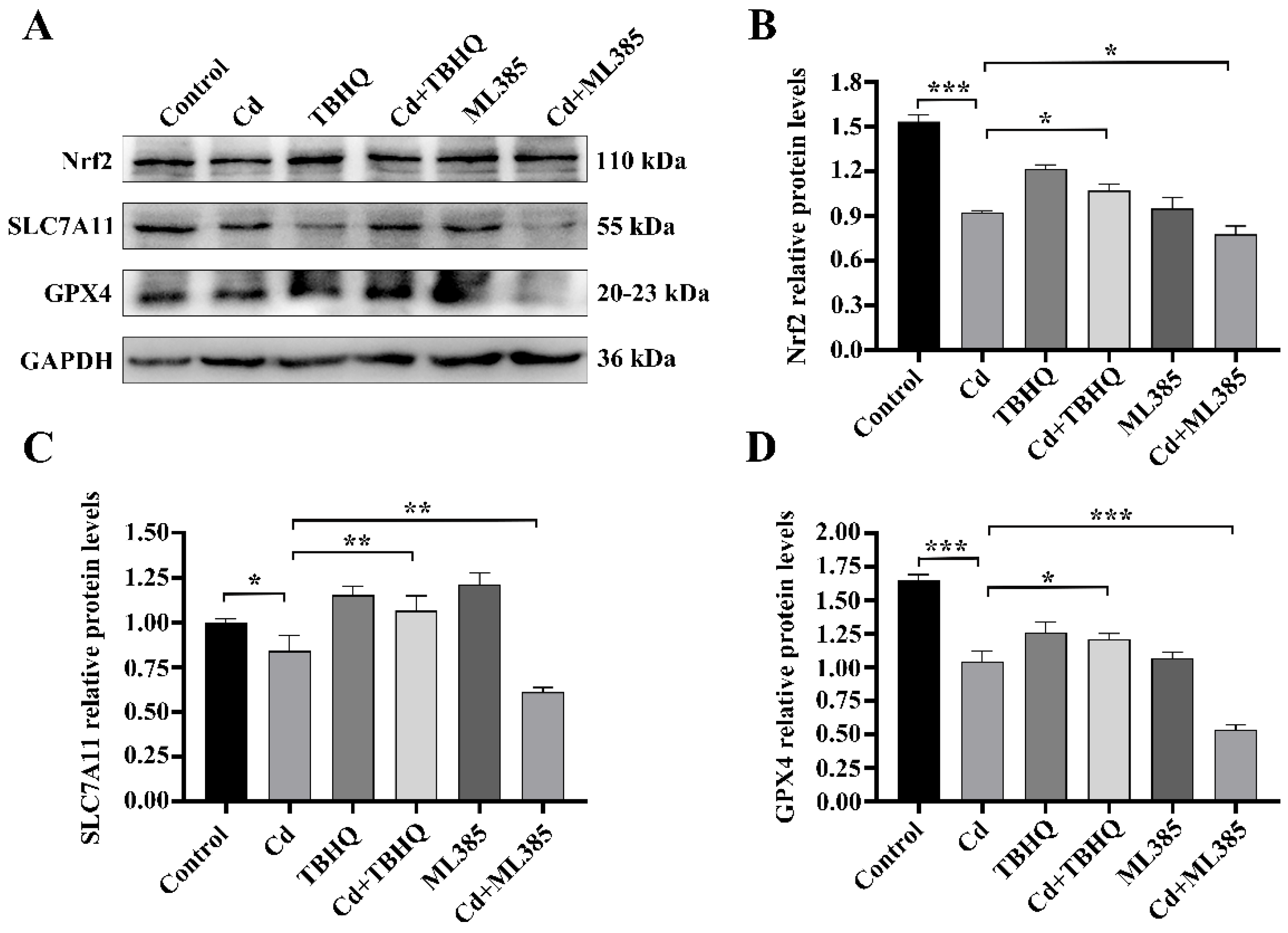
2.4. The Nrf2/SLC7A11/GPX4 Pathway Is Implicated in Cd-Induced Ferroptosis in BEAS-2B Cells
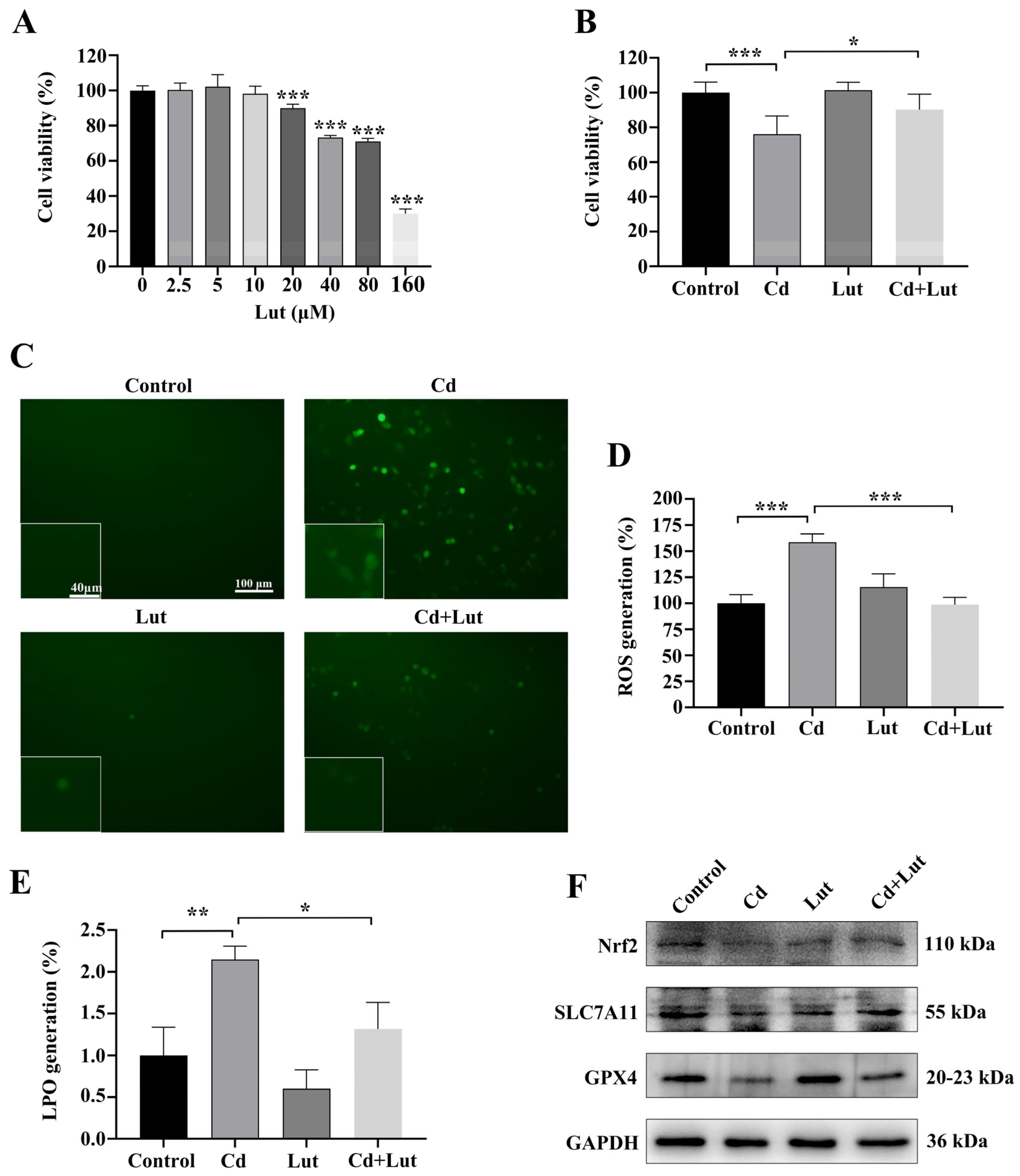
2.5. Luteolin Attenuates Cd-Induced BEAS-2B Cell Injury by Inhibiting Ferroptosis
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reagents and Antibodies
4.3. MTT Assay
4.4. ROS Detection
4.5. Detection of Iron Ions
4.6. Intracellular LPO Detection
4.7. Intracellular GSH-PX Detection
4.8. MMP Assay
4.9. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.10. Western blot
4.11. Statistical Analysis
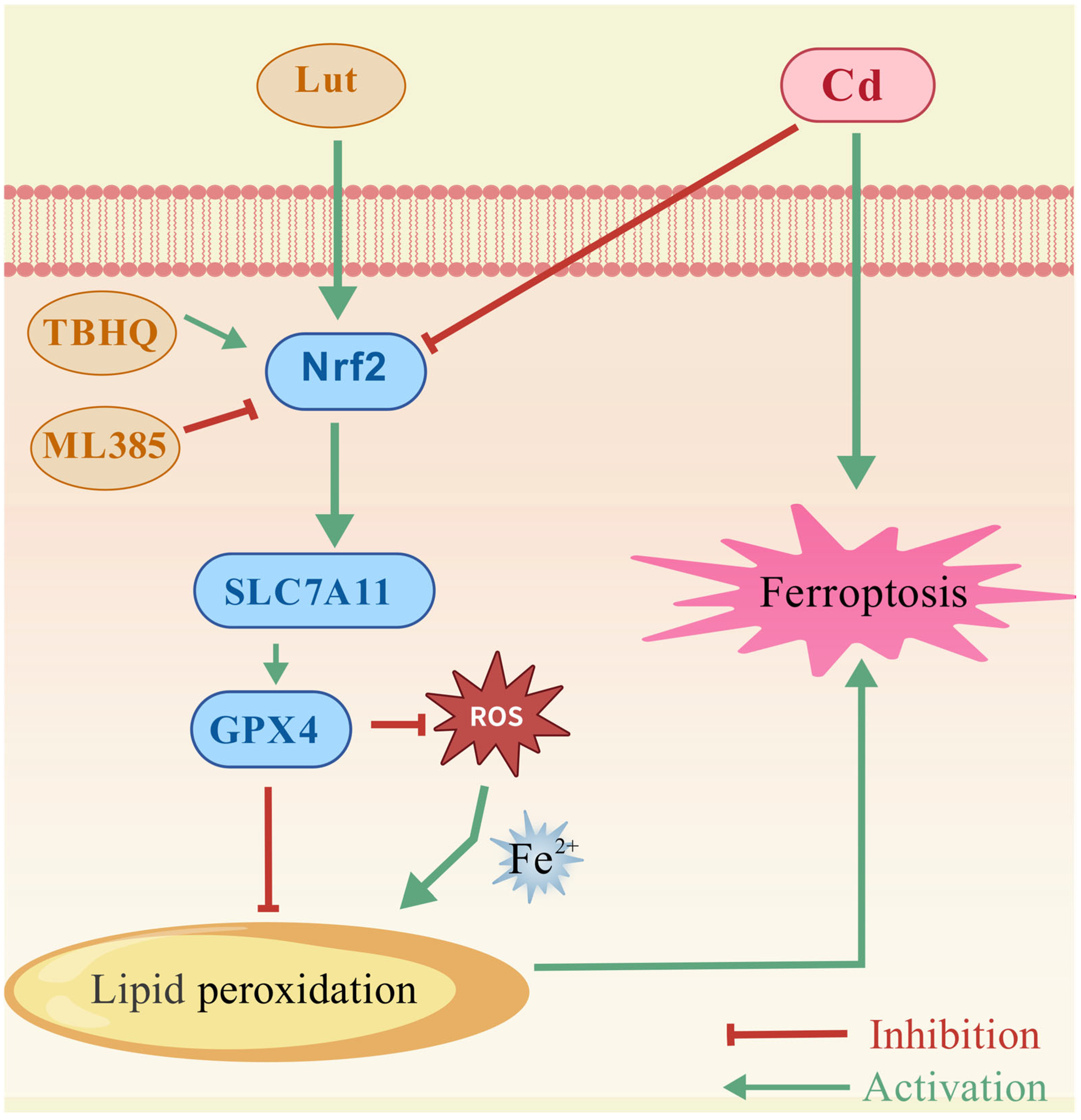
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| Full Name | Abbreviation |
| American Type Culture Collection | ATCC |
| Cadmium | Cd |
| Dimethyl sulfoxide | DMSO |
| Ferrostatin-1 | Fer-1 |
| Glutathione Peroxidase 4 | GPX4 |
| Reduced glutathione | GSH |
| Glutathione Peroxidase | GSH-PX |
| Lipid peroxidation | LPO |
| Luteolin | Lut |
| Mitochondrial membrane potential | MMP |
| Methyl Thiazolyl Tetrazoliun | MTT |
| Nuclear factor erythroid-2 related factor 2 | Nrf2 |
| Phosphate buffered saline | PBS |
| Reactive oxygen | ROS |
| Sodium dodecyl sulphate | SDS |
| Solute carrier family 7 member 11 | SLC7A11 |
| Tert-Butylhydroquinone | TBHQ |
| Tris(hydroxymethyl)aminomethane | Tris |
References
- Liu, W.; Zhang, B.; Huang, Z.; Pan, X.; Chen, X.; Hu, C.; Liu, H.; Jiang, Y.; Sun, X.; Peng, Y. Cadmium body burden and gestational diabetes mellitus: A prospective study. Environ. Health Perspect. 2018, 126, 027006. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Järup, L.; Åkesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Silvera, S.A.N.; Rohan, T.E. Trace elements and cancer risk: A review of the epidemiologic evidence. Cancer Causes Control. 2007, 18, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Ou, A.-J.; Jin, L.; Yang, N.-S.-Y.; Deng, P.; Guan, C.-X.; Huang, X.-T.; Duan, J.-X.; Zhou, Y. Cadmium exposure triggers alveolar epithelial cell pyroptosis by inducing mitochondrial oxidative stress and activating the cGAS-STING pathway. Cell Commun. Signal. 2024, 22, 566. [Google Scholar] [CrossRef] [PubMed]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Lv, Z.; Li, S.; Jiang, H.; Han, B.; Zheng, X.; Liu, Y.; Zhang, Z. Chronic arsenic exposure induces ferroptosis via enhancing ferritinophagy in chicken livers. Sci. Total Environ. 2023, 890, 164172. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, C.; Ma, X.; Zhao, J.; Li, S.; Liu, C.; Tang, Y.; Zhou, J.; Chen, J.; Li, X.; Li, W. Oleanolic acid inhibits mercury chloride induced-liver ferroptosis by regulating ROS/iron overload. Ecotoxicol. Environ. Saf. 2023, 258, 114973. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Zhou, B.; Young, J.L.; Wintergerst, K.; Cai, L. Exposure to low-dose cadmium induces testicular ferroptosis. Ecotoxicol. Environ. Saf. 2022, 234, 113373. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Lin, X.; Xu, Y.; Tong, T.; Zhang, J.; He, H.; Yang, L.; Lu, Y.; Zhou, Z. Cadmium induces ferroptosis mediated inflammation by activating Gpx4/Ager/p65 axis in pancreatic β-cells. Sci. Total Environ. 2022, 849, 157819. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Lin, B.; Jin, W.; Tang, L.; Hu, S.; Cai, R. NRF2, a Superstar of Ferroptosis. Antioxidants 2023, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Seibt, T.M.; Proneth, B.; Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. Free. Radic. Biol. Med. 2019, 133, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Goswami, D.; Adiseshaiah, P.P.; Burgan, W.; Yi, M.; Guerin, T.M.; Kozlov, S.V.; Nissley, D.V.; McCormick, F. Undermining glutaminolysis bolsters chemotherapy while NRF2 promotes chemoresistance in KRAS-driven pancreatic cancers. Cancer Res. 2020, 80, 1630–1643. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, C.; Li, Q.; Liu, Y.; Cao, J. HMGA2-mediated glutamine metabolism is required for Cd-induced cell growth and cell migration. Toxicology 2024, 507, 153899. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.-J.; Dong, X.; Zhuang, H.-W.; Pang, F.-X.; Ding, S.-C.; Li, N.; Mai, Y.-X.; Zhou, S.-T.; Wang, J.-Y.; Zhang, J.-F. Baicalin induces ferroptosis in osteosarcomas through a novel Nrf2/xCT/GPX4 regulatory axis. Phytomedicine 2023, 116, 154881. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bao, L.; Pan, Y.; Zhu, X.; Cheng, J.; Zhang, J.; Chu, W. The role of miR-216a-mediated Nrf2 pathway in muscle oxidative stress of Siniperca chuatsi induced by cadmium. Ecotoxicol. Environ. Saf. 2024, 283, 116863. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Sun, Y.; Su, Y.; Guan, W.; Wang, Y.; Han, J.; Wang, S.; Yang, B.; Wang, Q.; Kuang, H. Luteolin: A promising multifunctional natural flavonoid for human diseases. Phytother. Res. 2024, 38, 3417–3443. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yu, H.; Baiyun, R.; Lu, J.; Li, S.; Bing, Q.; Zhang, X.; Zhang, Z. Protective effects of dietary luteolin against mercuric chloride-induced lung injury in mice: Involvement of AKT/Nrf2 and NF-κB pathways. Food Chem. Toxicol. 2018, 113, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.-O.; Pratheeshkumar, P.; Wang, Y.; Kim, D.; Zhang, Z.; Shi, X. Protection from Cr (VI)-induced malignant cell transformation and tumorigenesis of Cr (VI)-transformed cells by luteolin through Nrf2 signaling. Toxicol. Appl. Pharmacol. 2017, 331, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Chu, N.; Zhang, X.; Chen, S.; Zhen, Q.; Wang, Y. Luteolin has a significant protective effect against cadmium-induced injury in lung epithelial BEAS-2B cells. Nan Fang Yi Ke Da Xue Xue Bao J. South. Med. Univ. 2021, 41, 729–735. [Google Scholar]
- Hu, W.; Xia, M.; Zhang, C.; Song, B.; Xia, Z.; Guo, C.; Cui, Y.; Jiang, W.; Zhang, S.; Xu, D. Chronic cadmium exposure induces epithelial mesenchymal transition in prostate cancer cells through a TGF-β-independent, endoplasmic reticulum stress induced pathway. Toxicol. Lett. 2021, 353, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rodríguez, H.F.; Graniel-Amador, M.A.; Cruz-Camacho, C.J.; Cantú-Martínez, A.A.; Martínez-Martínez, A.; Petricevich, V.L.; Montes, S.; Castañeda-Corral, G.; Jiménez-Andrade, J.M. Characterization of pain-related behaviors, changes in bone microarchitecture and sensory innervation induced by chronic cadmium exposure in adult mice. Neurotoxicology 2022, 89, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Sun, J.; Xia, L.; Shi, Q.; Hou, Y.; Zhang, L.; Li, M.; Fan, C.; Sun, B. Ferroptosis mechanism and Alzheimer’s disease. Neural Regen. Res. 2024, 19, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Liu, L.; Shi, X.; Liu, Y.; Wang, J.; Fang, X.; Chen, Z.; Ai, D.; Zhu, Y.; Zhang, X. Alox15/15-HpETE aggravates myocardial ischemia-reperfusion injury by promoting cardiomyocyte ferroptosis. Circulation 2023, 147, 1444–1460. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhu, C.; Tang, D.; Dou, Q.P.; Shen, J.; Chen, X. The role of ferroptosis in lung cancer. Biomark. Res. 2021, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Kurmangaliyeva, S.; Baktikulova, K.; Tkachenko, V.; Seitkhanova, B.; Shapambayev, N.; Rakhimzhanova, F.; Almagambetova, A.; Kurmangaliyev, K. An Overview of Hexavalent Chromium-Induced Necroptosis, Pyroptosis, and Ferroptosis. Biol. Trace Elem. Res. 2025, 203, 2619–2635. [Google Scholar] [CrossRef] [PubMed]
- Aschner, M.; Skalny, A.V.; Martins, A.C.; Sinitskii, A.I.; Farina, M.; Lu, R.; Barbosa, F., Jr.; Gluhcheva, Y.G.; Santamaria, A.; Tinkov, A.A. Ferroptosis as a mechanism of non-ferrous metal toxicity. Arch. Toxicol. 2022, 96, 2391–2417. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; You, L.; Zhang, J.; Chang, Y.-Z.; Yu, P. Brain iron metabolism, redox balance and neurological diseases. Antioxidants 2023, 12, 1289. [Google Scholar] [CrossRef] [PubMed]
- Luan, P.; Sun, Y.; Zhu, Y.; Qiao, S.; Hu, G.; Liu, Q.; Zhang, Z. Cadmium exposure promotes activation of cerebrum and cerebellum ferroptosis and necrosis in swine. Ecotoxicol. Environ. Saf. 2021, 224, 112650. [Google Scholar] [CrossRef] [PubMed]
- Capelletti, M.M.; Manceau, H.; Puy, H.; Peoc’h, K. Ferroptosis in liver diseases: An overview. Int. J. Mol. Sci. 2020, 21, 4908. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liu, J.; Piao, H.; Zhu, Z.; Wei, R.; Liu, K. ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis. Front. Immunol. 2022, 13, 1039241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Q.; Chang, M.; Pan, Y.; Yahaya, B.H.; Liu, Y.; Lin, J. Chemotherapy impairs ovarian function through excessive ROS-induced ferroptosis. Cell Death Dis. 2023, 14, 340. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Otasevic, V.; Vucetic, M.; Grigorov, I.; Martinovic, V.; Stancic, A. Ferroptosis in different pathological contexts seen through the eyes of mitochondria. Oxidative Med. Cell. Longev. 2021, 2021, 5537330. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, R.; Zhang, L.; Xue, Q.; Wang, R.; Xu, J.; Wang, C.; Meng, C.; Lu, R.; Yin, F.; Guo, L. Ferritinophagy is involved in hexavalent chromium-induced ferroptosis in Sertoli cells. Toxicol. Appl. Pharmacol. 2024, 492, 117139. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jin, Y.; Liu, J.; Liu, Q.; Shen, Y.; Zuo, S.; Yu, Y. EP1 activation inhibits doxorubicin-cardiomyocyte ferroptosis via Nrf2. Redox Biol. 2023, 65, 102825. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Duan, Y.; Yang, Y.; Chen, E.; Huang, H.; Wang, X.; Zhou, J. Sodium aescinate induces renal toxicity by promoting Nrf2/GPX4-mediated ferroptosis. Chem.-Biol. Interact. 2024, 391, 110892. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Wu, H.; Zhu, L.; Gao, J.; Deng, G. Triptolide Promotes Ferroptosis in Cervical Cancer Cell via NRF2/xCT/GPX4. Phytother. Res. 2025, 39, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.-T.; Ding, W.-Y.; Lu, X.-X.; Zhang, Q.-H.; Du, J.-X.; Wang, L.-J.; Yang, M.-N.; Yin, Y.; Liu, F.-J. Pharmacological and mechanistic aspects of quercetin in osteoporosis. Front. Pharmacol. 2024, 15, 1338951. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhong, H.; Huang, Z.; Chen, X.; You, J.; Zou, T. A critical review of kaempferol in intestinal health and diseases. Antioxidants 2023, 12, 1642. [Google Scholar] [CrossRef] [PubMed]
- Rakoczy, K.; Kaczor, J.; Sołtyk, A.; Szymańska, N.; Stecko, J.; Sleziak, J.; Kulbacka, J.; Baczyńska, D. Application of luteolin in neoplasms and nonneoplastic diseases. Int. J. Mol. Sci. 2023, 24, 15995. [Google Scholar] [CrossRef] [PubMed]
- Can, B.; Sanlier, N. Alzheimer, Parkinson, dementia, and phytochemicals: Insight review. Crit. Rev. Food Sci. Nutr. 2025, 65, 1706–1728. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ding, Y.; Wu, D.; Liu, P. Rutin, puerarin and silymarin regulated aluminum-induced imbalance of neurotransmitters and metal elements in brain of rats. Biol. Trace Elem. Res. 2024, 202, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Sharma, M.; Singh, S.K.; Gulati, M.; Chellappan, D.K.; Zacconi, F.; De Rubis, G.; Gupta, G.; Sharifi-Rad, J.; Cho, W.C. Luteolin: A flavonoid with a multifaceted anticancer potential. Cancer Cell Int. 2022, 22, 386. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Chen, P.-J.; Chang, S.-H.; Weng, Y.-T.; Chang, F.-R.; Chang, K.-Y.; Chen, C.-Y.; Kao, T.-I.; Hwang, T.-L. Luteolin attenuates neutrophilic oxidative stress and inflammatory arthritis by inhibiting Raf1 activity. Biochem. Pharmacol. 2018, 154, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Yang, S.; Chen, L.; Yu, W.; Xia, Y.; Li, B.; Zhou, X.; Cheng, F. Luteolin alleviated calcium oxalate crystal induced kidney injury by inhibiting Nr4a1-mediated ferroptosis. Phytomedicine 2025, 136, 156302. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.-C.; Lin, J.-H.; Lee, W.-S.; Liu, C.-H.; Lin, T.-Y.; Yang, K.-T. Baicalein and luteolin inhibit ischemia/reperfusion-induced ferroptosis in rat cardiomyocytes. Int. J. Cardiol. 2023, 375, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Lin, B.; Wang, F.; Xu, P.; Wang, N. Mangiferin attenuates osteoporosis by inhibiting osteoblastic ferroptosis through Keap1/Nrf2/SLC7A11/GPX4 pathway. Phytomedicine 2024, 124, 155282. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Han, J.; Yan, Z.; Li, K. Paeonol attenuates LPS-induced inflammatory injury in mastitis by regulating the MAPK and Nrf2 signaling pathways. Res. Vet. Sci. 2025, 182, 105481. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wang, C.; Jin, Y.; Meng, Q.; Wu, J.; Sun, H. Kaempferol-induced GPER upregulation attenuates atherosclerosis via the PI3K/AKT/Nrf2 pathway. Pharm. Biol. 2021, 59, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Agyin, J.K.; Santhamma, B.; Nair, H.B.; Roy, S.S.; Tekmal, R.R. BU-32: A novel proteasome inhibitor for breast cancer. Breast Cancer Res. 2009, 11, R74. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, J.; Zhao, T.; Sun, M.; Xu, M.; Che, S.; Pan, Z.; Wu, C.; Shen, L. Polystyrene nanoplastics lead to ferroptosis in the lungs. J. Adv. Res. 2024, 56, 31–41. [Google Scholar] [CrossRef] [PubMed]


| Name | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | CGCTCTCTGCTCCTCCTGTT | CCATGGTGTCTGAGCGATGT |
| NRF2 | AGGACATGGAGCAAGTTTGG | TCTGTCAGTGTGGCTTCTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Qiu, Z.; Chen, L.; Zhang, T.; Wei, D.; Chen, X.; Wang, Y. Cadmium Inhibits Proliferation of Human Bronchial Epithelial BEAS-2B Cells Through Inducing Ferroptosis via Targeted Regulation of the Nrf2/SLC7A11/GPX4 Pathway. Int. J. Mol. Sci. 2025, 26, 7204. https://doi.org/10.3390/ijms26157204
Li H, Qiu Z, Chen L, Zhang T, Wei D, Chen X, Wang Y. Cadmium Inhibits Proliferation of Human Bronchial Epithelial BEAS-2B Cells Through Inducing Ferroptosis via Targeted Regulation of the Nrf2/SLC7A11/GPX4 Pathway. International Journal of Molecular Sciences. 2025; 26(15):7204. https://doi.org/10.3390/ijms26157204
Chicago/Turabian StyleLi, Huan, Zixin Qiu, Long Chen, Tianbao Zhang, Diandian Wei, Xue Chen, and Yun Wang. 2025. "Cadmium Inhibits Proliferation of Human Bronchial Epithelial BEAS-2B Cells Through Inducing Ferroptosis via Targeted Regulation of the Nrf2/SLC7A11/GPX4 Pathway" International Journal of Molecular Sciences 26, no. 15: 7204. https://doi.org/10.3390/ijms26157204
APA StyleLi, H., Qiu, Z., Chen, L., Zhang, T., Wei, D., Chen, X., & Wang, Y. (2025). Cadmium Inhibits Proliferation of Human Bronchial Epithelial BEAS-2B Cells Through Inducing Ferroptosis via Targeted Regulation of the Nrf2/SLC7A11/GPX4 Pathway. International Journal of Molecular Sciences, 26(15), 7204. https://doi.org/10.3390/ijms26157204






