Dysfunction of Autophagy in Adipose Tissue Macrophages Regulated via FoxO1 in Obesity-Related Severe Acute Pancreatitis
Abstract
1. Introduction
2. Results
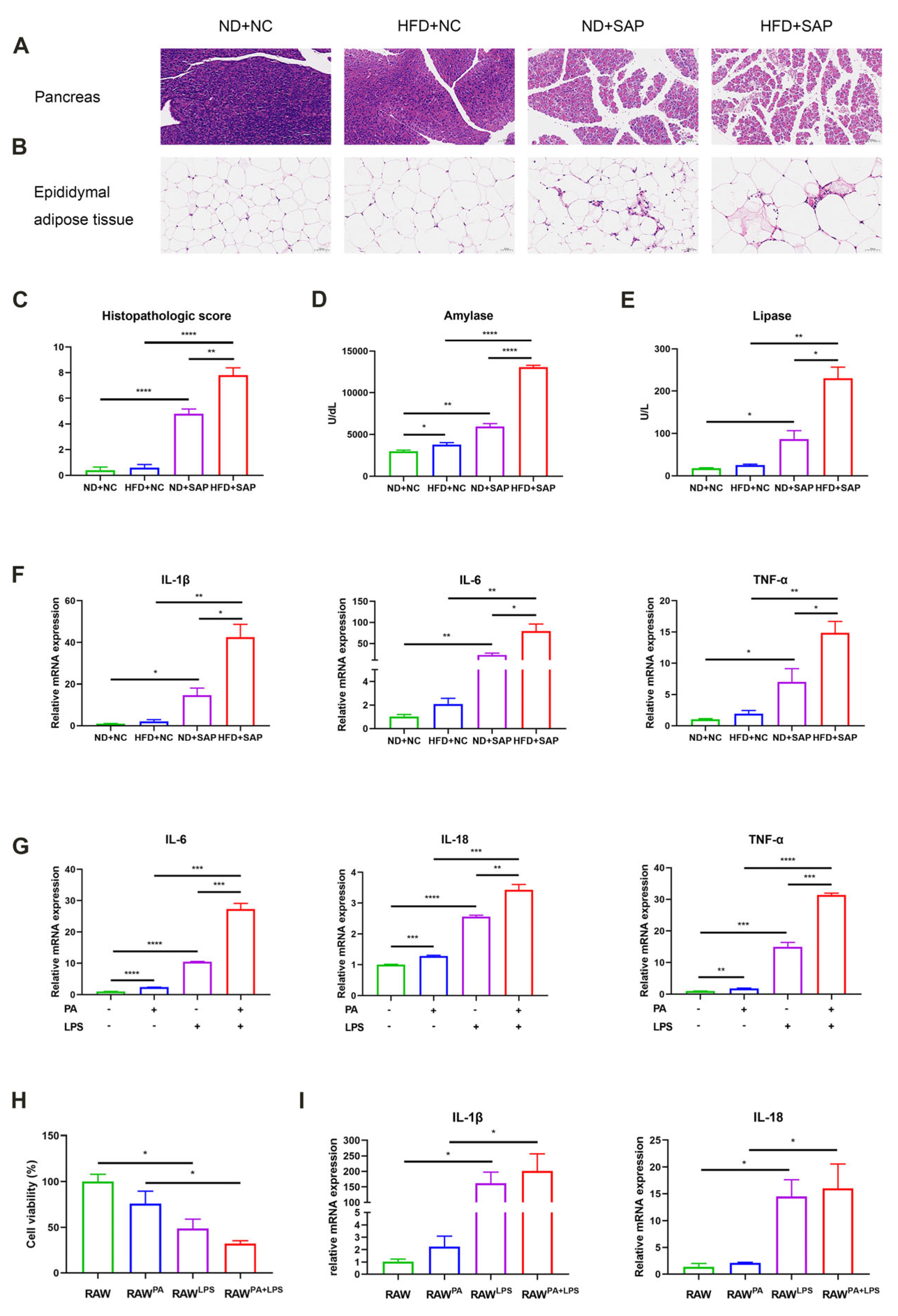
2.1. ATMs Participated in the Aggravated Inflammation of SAP Caused by Obesity
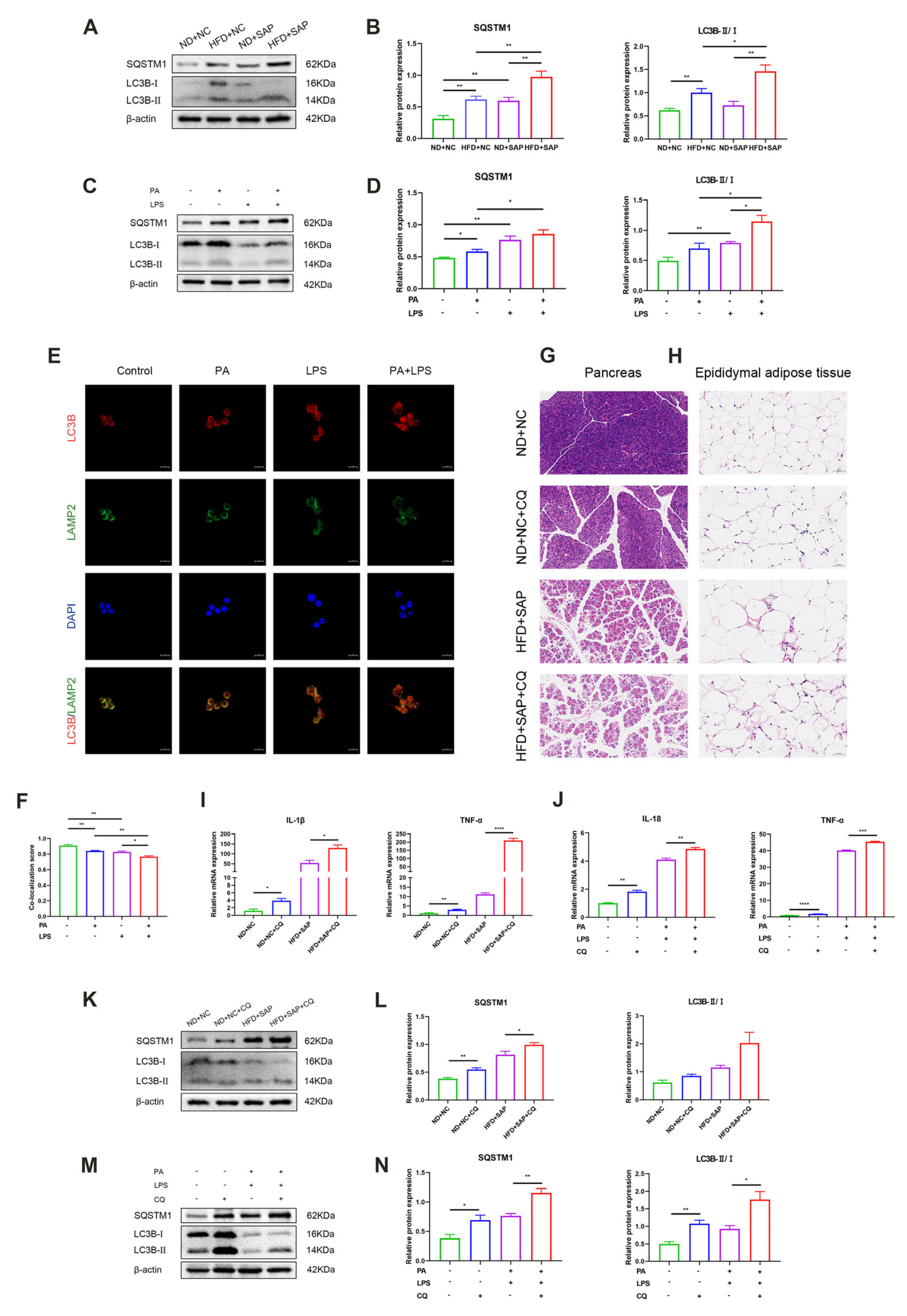
2.2. The Autophagic Flux Was Impaired in ATMs After the Induction of Obesity-Related SAP
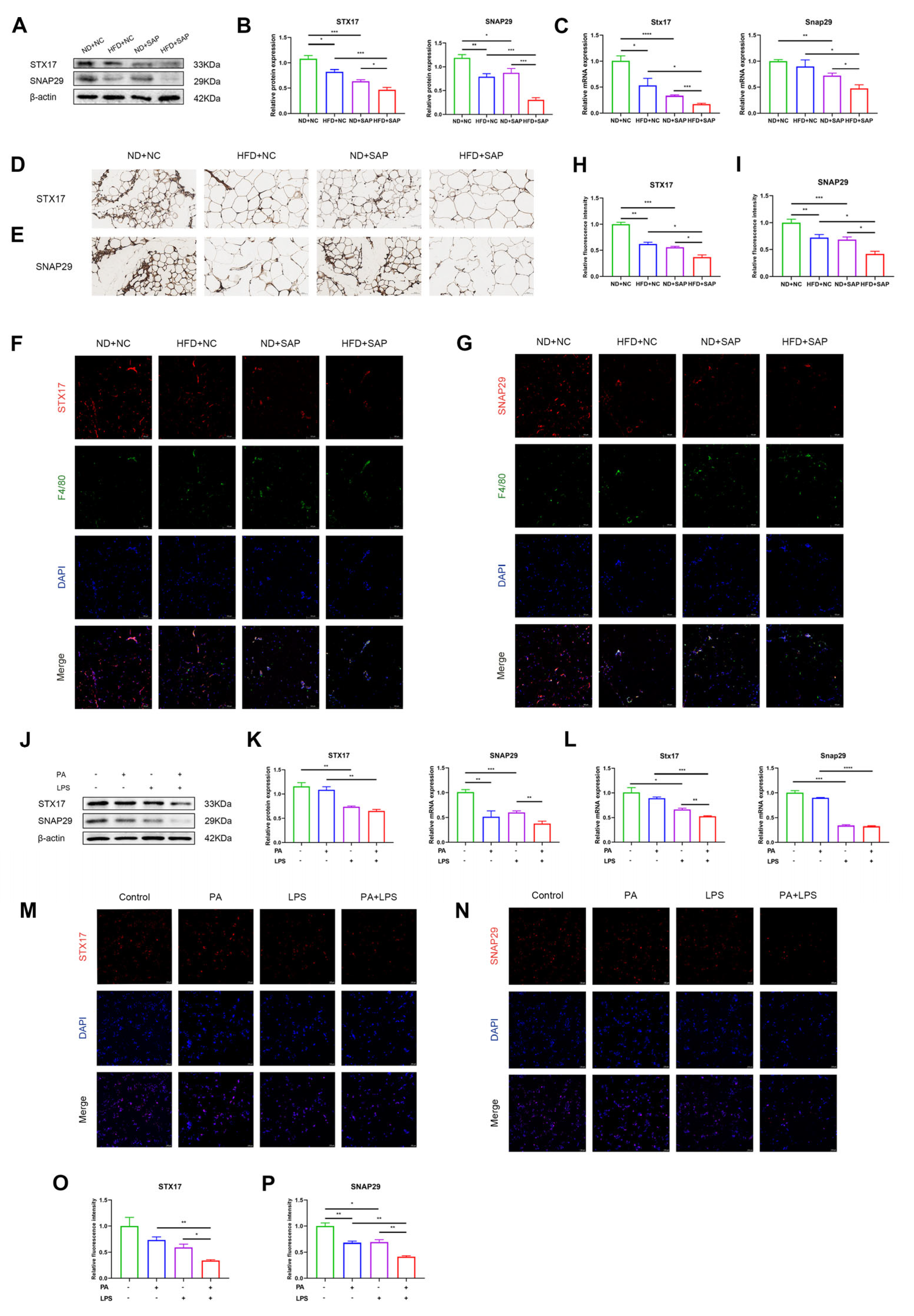
2.3. Expression of Autophagosome-Localized SNARE Proteins Decreased in ATMs of Obesity-Related SAP
2.4. Expression and Transcriptional Activity of FoxO1 Decreased in Obesity-Related SAP
2.5. FoxO1 Regulated the Expression of Autophagosome-Localized SNARE Proteins in ATMs
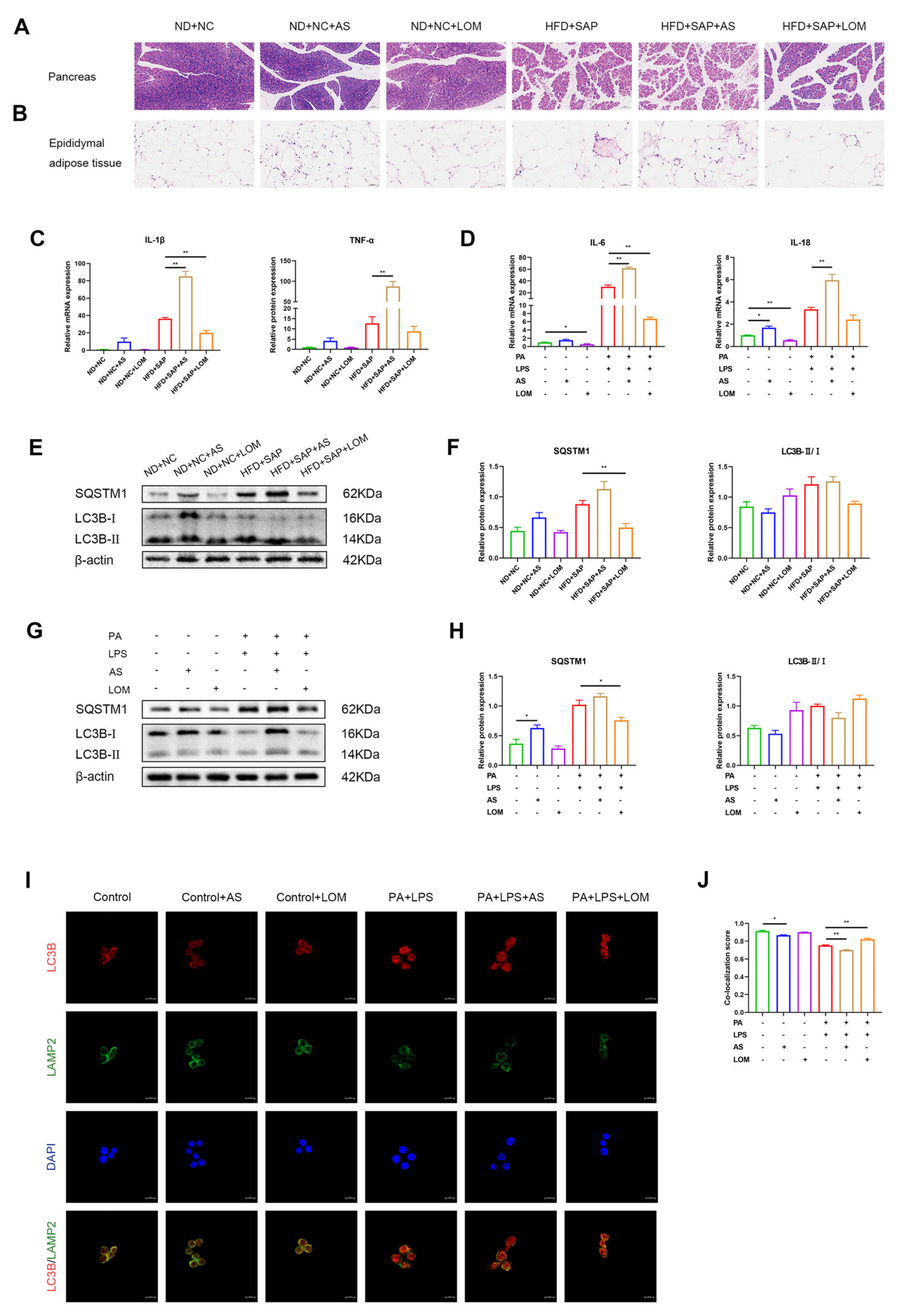
2.6. FoxO1 Regulated the Inflammation and Autophagic Flux in ATMs
3. Discussion
4. Materials and Methods
4.1. Animal Grouping and Processing
4.2. Histopathologic Assessment
4.3. Biochemical Assays
4.4. Cell Culture of RAW264.7 Macrophages and 266-6 Pancreatic Acinar Cells
4.5. Cell Viability Assay with Cell Counting Kit-8
4.6. RNA Analysis
4.7. Western Blot
4.8. Immunofluorescence
4.9. Immunohistochemistry
4.10. RNA-Sequencing
4.11. Graphical Depiction and Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tenner, S.; Baillie, J.; DeWitt, J.; Vege, S.S. American College of Gastroenterology guideline: Management of acute pancreatitis. Am. J. Gastroenterol. 2013, 108, 1400–1415, 1416. [Google Scholar] [CrossRef]
- Zerem, E. Treatment of severe acute pancreatitis and its complications. World J. Gastroenterol. 2014, 20, 13879–13892. [Google Scholar] [CrossRef]
- Boxhoorn, L.; Voermans, R.P.; Bouwense, S.A.; Bruno, M.J.; Verdonk, R.C.; Boermeester, M.A.; van Santvoort, H.C.; Besselink, M.G. Acute pancreatitis. Lancet 2020, 396, 726–734. [Google Scholar] [CrossRef]
- Abu Hilal, M.; Armstrong, T. The impact of obesity on the course and outcome of acute pancreatitis. Obes. Surg. 2008, 18, 326–328. [Google Scholar] [CrossRef]
- Khatua, B.; El-Kurdi, B.; Singh, V.P. Obesity and pancreatitis. Curr. Opin. Gastroenterol. 2017, 33, 374–382. [Google Scholar] [CrossRef]
- Hu, F.; Lou, N.; Jiao, J.; Guo, F.; Xiang, H.; Shang, D. Macrophages in pancreatitis: Mechanisms and therapeutic potential. Biomed. Pharmacother. 2020, 131, 110693. [Google Scholar] [CrossRef]
- Shrivastava, P.; Bhatia, M. Essential role of monocytes and macrophages in the progression of acute pancreatitis. World J. Gastroenterol. 2010, 16, 3995–4002. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Thomas, D.; Apovian, C. Macrophage functions in lean and obese adipose tissue. Metab. Clin. Exp. 2017, 72, 120–143. [Google Scholar] [CrossRef]
- Xu, T.; Sheng, L.; Guo, X.; Ding, Z. Free Fatty Acid Increases the Expression of NLRP3-Caspase1 in Adipose Tissue Macrophages in Obese Severe Acute Pancreatitis. Dig. Dis. Sci. 2022, 67, 2220–2231. [Google Scholar] [CrossRef]
- Gukovskaya, A.S.; Gukovsky, I.; Algül, H.; Habtezion, A. Autophagy, Inflammation, and Immune Dysfunction in the Pathogenesis of Pancreatitis. Gastroenterology 2017, 153, 1212–1226. [Google Scholar] [CrossRef]
- Zhang, T.; Gan, Y.; Zhu, S. Association between autophagy and acute pancreatitis. Front. Genet. 2023, 14, 998035. [Google Scholar] [CrossRef]
- Wang, S.; Ni, H.M.; Chao, X.; Wang, H.; Bridges, B.; Kumer, S.; Schmitt, T.; Mareninova, O.; Gukovskaya, A.; De Lisle, R.C.; et al. Impaired TFEB-mediated lysosomal biogenesis promotes the development of pancreatitis in mice and is associated with human pancreatitis. Autophagy 2019, 15, 1954–1969. [Google Scholar] [CrossRef]
- Saitoh, T.; Akira, S. Regulation of inflammasomes by autophagy. J. Allergy Clin. Immunol. 2016, 138, 28–36. [Google Scholar] [CrossRef]
- Huang, H.; Ouyang, Q.; Mei, K.; Liu, T.; Sun, Q.; Liu, W.; Liu, R. Acetylation of SCFD1 regulates SNARE complex formation and autophagosome-lysosome fusion. Autophagy 2023, 19, 189–203. [Google Scholar] [CrossRef]
- Lőrincz, P.; Juhász, G. Autophagosome-Lysosome Fusion. J. Mol. Biol. 2020, 432, 2462–2482. [Google Scholar] [CrossRef]
- Tian, X.; Teng, J.; Chen, J. New insights regarding SNARE proteins in autophagosome-lysosome fusion. Autophagy 2021, 17, 2680–2688. [Google Scholar] [CrossRef]
- Itakura, E.; Kishi-Itakura, C.; Mizushima, N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 2012, 151, 1256–1269. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Cheng, Z. The FoxO-Autophagy Axis in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.Q.; Li, A.; Yang, Y.; Li, X.X.; Zhang, L.N.; Guo, H.C. The regulation of FOXO1 and its role in disease progression. Life Sci. 2018, 193, 124–131. [Google Scholar] [CrossRef]
- Nagashima, T.; Shigematsu, N.; Maruki, R.; Urano, Y.; Tanaka, H.; Shimaya, A.; Shimokawa, T.; Shibasaki, M. Discovery of novel forkhead box O1 inhibitors for treating type 2 diabetes: Improvement of fasting glycemia in diabetic db/db mice. Mol. Pharmacol. 2010, 78, 961–970. [Google Scholar] [CrossRef]
- Cautain, B.; Castillo, F.; Musso, L.; Ferreira, B.I.; de Pedro, N.; Rodriguez Quesada, L.; Machado, S.; Vicente, F.; Dallavalle, S.; Link, W. Discovery of a Novel, Isothiazolonaphthoquinone-Based Small Molecule Activator of FOXO Nuclear-Cytoplasmic Shuttling. PLoS ONE 2016, 11, e0167491. [Google Scholar] [CrossRef]
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018, 155, 407–417. [Google Scholar] [CrossRef]
- Yu, Q.; Xu, T.; Ding, F.; Ding, Z.; Lin, R. Decreased infiltration of adipose tissue macrophages and amplified inflammation of adipose tissue in obese mice with severe acute pancreatitis. Pancreatology 2021. [Google Scholar] [CrossRef]
- Piplani, H.; Marek-Iannucci, S.; Sin, J.; Hou, J.; Takahashi, T.; Sharma, A.; de Freitas Germano, J.; Waldron, R.T.; Saadaeijahromi, H.; Song, Y.; et al. Simvastatin induces autophagic flux to restore cerulein-impaired phagosome-lysosome fusion in acute pancreatitis. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165530. [Google Scholar] [CrossRef]
- Shen, S.; Li, B.; Dai, J.; Wu, Z.; He, Y.; Wen, L.; Wang, X.; Hu, G. BRD4 Inhibition Protects Against Acute Pancreatitis Through Restoring Impaired Autophagic Flux. Front. Pharmacol. 2020, 11, 618. [Google Scholar] [CrossRef]
- Mizunoe, Y.; Sudo, Y.; Okita, N.; Hiraoka, H.; Mikami, K.; Narahara, T.; Negishi, A.; Yoshida, M.; Higashibata, R.; Watanabe, S.; et al. Involvement of lysosomal dysfunction in autophagosome accumulation and early pathologies in adipose tissue of obese mice. Autophagy 2017, 13, 642–653. [Google Scholar] [CrossRef]
- Fortunato, F.; Bürgers, H.; Bergmann, F.; Rieger, P.; Büchler, M.W.; Kroemer, G.; Werner, J. Impaired autolysosome formation correlates with Lamp-2 depletion: Role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology 2009, 137, 350–360.e5. [Google Scholar] [CrossRef]
- Mareninova, O.A.; Sendler, M.; Malla, S.R.; Yakubov, I.; French, S.W.; Tokhtaeva, E.; Vagin, O.; Oorschot, V.; Lüllmann-Rauch, R.; Blanz, J.; et al. Lysosome associated membrane proteins maintain pancreatic acinar cell homeostasis: LAMP-2 deficient mice develop pancreatitis. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 678–694. [Google Scholar] [CrossRef]
- Diao, J.; Liu, R.; Rong, Y.; Zhao, M.; Zhang, J.; Lai, Y.; Zhou, Q.; Wilz, L.M.; Li, J.; Vivona, S.; et al. ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature 2015, 520, 563–566. [Google Scholar] [CrossRef]
- Cheng, Z.; White, M.F. Targeting Forkhead box O1 from the concept to metabolic diseases: Lessons from mouse models. Antioxid. Redox Signal. 2011, 14, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.X.; Heine, M.; Schlein, C.; Ramakrishnan, R.; Liu, J.; Belnavis, G.; Haimi, I.; Fischer, A.W.; Ginsberg, H.N.; Heeren, J.; et al. FoxO transcription factors are required for hepatic HDL cholesterol clearance. J. Clin. Investig. 2018, 128, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Tao, R.; DePinho, R.A.; Dong, X.C. The autophagy-related gene 14 (Atg14) is regulated by forkhead box O transcription factors and circadian rhythms and plays a critical role in hepatic autophagy and lipid metabolism. J. Biol. Chem. 2012, 287, 39107–39114. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, Q.; Sun, Y.Y.; Xing, Y.F.; Wang, Y.B.; Lu, X.T.; Bai, W.W.; Liu, X.Q.; Zhao, Y.X. Resveratrol-enhanced autophagic flux ameliorates myocardial oxidative stress injury in diabetic mice. J. Cell Mol. Med. 2014, 18, 1599–1611. [Google Scholar] [CrossRef]
- Wang, S.; Xia, P.; Huang, G.; Zhu, P.; Liu, J.; Ye, B.; Du, Y.; Fan, Z. FoxO1-mediated autophagy is required for NK cell development and innate immunity. Nat. Commun. 2016, 7, 11023. [Google Scholar] [CrossRef]
- Navina, S.; Acharya, C.; DeLany, J.P.; Orlichenko, L.S.; Baty, C.J.; Shiva, S.S.; Durgampudi, C.; Karlsson, J.M.; Lee, K.; Bae, K.T.; et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci. Transl. Med. 2011, 3, 107ra110. [Google Scholar] [CrossRef]
- Patel, K.; Trivedi, R.N.; Durgampudi, C.; Noel, P.; Cline, R.A.; DeLany, J.P.; Navina, S.; Singh, V.P. Lipolysis of visceral adipocyte triglyceride by pancreatic lipases converts mild acute pancreatitis to severe pancreatitis independent of necrosis and inflammation. Am. J. Pathol. 2015, 185, 808–819. [Google Scholar] [CrossRef]
- Xu, S.; Lu, F.; Gao, J.; Yuan, Y. Inflammation-mediated metabolic regulation in adipose tissue. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2024, 25, e13724. [Google Scholar] [CrossRef]
- Gordy, C.; He, Y.W. The crosstalk between autophagy and apoptosis: Where does this lead? Protein Cell 2012, 3, 17–27. [Google Scholar] [CrossRef]
- Guo, R.; Wang, H.; Cui, N. Autophagy Regulation on Pyroptosis: Mechanism and Medical Implication in Sepsis. Mediat. Inflamm. 2021, 2021, 9925059. [Google Scholar] [CrossRef]
- Schmidt, J.; Rattner, D.W.; Lewandrowski, K.; Compton, C.C.; Mandavilli, U.; Knoefel, W.T.; Warshaw, A.L. A better model of acute pancreatitis for evaluating therapy. Ann. Surg. 1992, 215, 44–56. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.H.; Kang, H.; Lee, S.; Park, S.Y.; Cho, Y.; Lim, Y.M.; Ahn, J.W.; Kim, Y.H.; Chung, S.; et al. TFEB-GDF15 axis protects against obesity and insulin resistance as a lysosomal stress response. Nat. Metab. 2021, 3, 410–427. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, X.; Zhang, Z.; Lin, L.; Guo, X.; Ding, Z. Dysfunction of Autophagy in Adipose Tissue Macrophages Regulated via FoxO1 in Obesity-Related Severe Acute Pancreatitis. Int. J. Mol. Sci. 2025, 26, 7206. https://doi.org/10.3390/ijms26157206
Ling X, Zhang Z, Lin L, Guo X, Ding Z. Dysfunction of Autophagy in Adipose Tissue Macrophages Regulated via FoxO1 in Obesity-Related Severe Acute Pancreatitis. International Journal of Molecular Sciences. 2025; 26(15):7206. https://doi.org/10.3390/ijms26157206
Chicago/Turabian StyleLing, Xin, Zewen Zhang, Lihui Lin, Xianwen Guo, and Zhen Ding. 2025. "Dysfunction of Autophagy in Adipose Tissue Macrophages Regulated via FoxO1 in Obesity-Related Severe Acute Pancreatitis" International Journal of Molecular Sciences 26, no. 15: 7206. https://doi.org/10.3390/ijms26157206
APA StyleLing, X., Zhang, Z., Lin, L., Guo, X., & Ding, Z. (2025). Dysfunction of Autophagy in Adipose Tissue Macrophages Regulated via FoxO1 in Obesity-Related Severe Acute Pancreatitis. International Journal of Molecular Sciences, 26(15), 7206. https://doi.org/10.3390/ijms26157206


_Kim.png)

