Molecular Determinants of Bone Plasticity Regeneration After Trauma: Forensic Consequences
Abstract
1. Introduction
2. Molecular Mechanisms Involved in Bone Regeneration and Plasticity
2.1. Master Transcription Factors Involved in Osteogenic Differentiation
2.2. Bone Morphogenic Protein Signalling
2.3. Growth Factors and Cytokines
2.4. Mechanotransduction and Osteocyte Function
2.5. Matrix Metalloproteinases and Extracellular Matrix Remodeling
2.6. Mineralization and Matrix Formation
3. Practical Forensic Applications of the Molecular Mechanisms of Bone Regeneration
3.1. Establishing Forensically Relevant Time Intervals
3.2. Species and Individual Identification
3.3. Trauma Mechanism Reconstruction
3.4. Taphonomic Assessment
Limitations of This Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACP | Amorphous Calcium Phosphate |
| AP-1 | Activator Protein-1 |
| BMP | Bone Morphogenetic Protein |
| BMP-2 | Bone Morphogenetic Protein-2 |
| COL1A2 | Collagen Type 1 Alpha 2 |
| Col1a1 | Collagen Type 1 Alpha 1 gene |
| CXCL1 | C-X-C Motif Chemokine Ligand 1 |
| CCL2 | C-C Motif Chemokine Ligand 2 |
| FGF | Fibroblast Growth Factor |
| FGF2 | Fibroblast Growth Factor 2 |
| Fgfr2 | Fibroblast Growth Factor Receptor 2 gene |
| Fgfr3 | Fibroblast Growth Factor Receptor 3 gene |
| Fn1 | Fibronectin 1 gene |
| Ibsp | Integrin Binding Sialoprotein gene (bone sialoprotein) |
| IL-1β | Interleukin-1 Beta |
| MAPK | Mitogen-Activated Protein Kinase |
| MMP | Matrix Metalloproteinase |
| MMP-2 | Matrix Metalloproteinase-2 |
| MMP-3 | Matrix Metalloproteinase-3 |
| MMP-9 | Matrix Metalloproteinase-9 |
| MMP-12 | Matrix Metalloproteinase-12 |
| MMP-13 | Matrix Metalloproteinase-13 |
| MSCs | Mesenchymal Stem Cells |
| MSICs | Mechanically Sensitive Ion Channels |
| NFATc1 | Nuclear Factor of Activated T-cells Cytoplasmic 1 |
| NF-κB | Nuclear Factor κB |
| OPG | Osteoprotegerin |
| PKA | Protein Kinase A |
| PKP | Protein Kinase P |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| PTH | Parathyroid Hormone |
| PTHrP | Parathyroid Hormone-related Protein |
| RANK | Receptor Activator of Nuclear Factor κB |
| RANKL | Receptor Activator of Nuclear Factor κB Ligand |
| Runx2 | Runt-related Transcription Factor 2 |
| Runx3 | Runt-related Transcription Factor 3 |
| SMAD | Mothers Against Decapentaplegic homolog proteins |
| SOST | Sclerostin gene |
| Sp7 | Specificity Protein 7 (Osterix) |
| Spp1 | Secreted Phosphoprotein 1 gene (osteopontin) |
| TGF-β | Transforming Growth Factor-β |
| TGF-β1 | Transforming Growth Factor-β1 |
| TGF-β2 | Transforming Growth Factor-β2 |
| TGF-β3 | Transforming Growth Factor-β3 |
| TRAF6 | TNF Receptor Associated Factor 6 |
| VEGF | Vascular Endothelial Growth Factor |
| β-CTX | β-CrossLaps (C-terminal telopeptide of type I collagen) |
| Wnt | Wingless-related integration site signaling pathway |
| Wnt5a | Wingless-related integration site 5a |
References
- Raggatt, L.J.; Partridge, N.C. Cellular and Molecular Mechanisms of Bone Remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, Y.; Pacios, S.; Li, S.; Graves, D.T. Cellular and Molecular Aspects of Bone Remodeling. Front. Oral Biol. 2016, 18, 9–16. [Google Scholar] [PubMed]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of Bone Development and Repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Teti, A. Bone Development: Overview of Bone Cells and Signaling. Curr. Osteoporos. Rep. 2011, 9, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-L.; Ishikawa, T.; Quan, L.; Li, D.-R.; Zhao, D.; Michiue, T.; Maeda, H. Evaluation of Postmortem Serum Calcium and Magnesium Levels in Relation to the Causes of Death in Forensic Autopsy. Forensic Sci. Int. 2005, 155, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Papachristou, D.J.; Papachroni, K.K.; Basdra, E.K.; Papavassiliou, A.G. Signaling Networks and Transcription Factors Regulating Mechanotransduction in Bone. Bioessays 2009, 31, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Guan, J.; Zhang, C. Mesenchymal Stem Cells: Mechanisms and Role in Bone Regeneration. Postgrad. Med. J. 2014, 90, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Roffi, A.; Di Martino, A.; Hamdan, M.; De Pasqual, L.; Merli, M.L.; Marcacci, M. Bone Regeneration with Mesenchymal Stem Cells. Clin. Cases Miner. Bone Metab. 2012, 9, 24–27. [Google Scholar] [PubMed]
- Shin, R.L.-Y.; Lee, C.-W.; Shen, O.Y.-J.; Xu, H.; Lee, O.K.-S. The Crosstalk between Mesenchymal Stem Cells and Macrophages in Bone Regeneration: A Systematic Review. Stem Cells Int. 2021, 2021, 8835156. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, R.; Katagiri, W.; Endo, S.; Kobayashi, T. Exosomes from Conditioned Media of Bone Marrow-Derived Mesenchymal Stem Cells Promote Bone Regeneration by Enhancing Angiogenesis. PLoS ONE 2019, 14, e0225472. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Fonticoli, L.; Trubiani, O.; Rajan, T.S.; Marconi, G.D.; Bramanti, P.; Mazzon, E.; Pizzicannella, J.; Diomede, F. Oral Bone Tissue Regeneration: Mesenchymal Stem Cells, Secretome, and Biomaterials. Int. J. Mol. Sci. 2021, 22, 5236. [Google Scholar] [CrossRef] [PubMed]
- Malhan, D.; Schmidt-Bleek, K.; Duda, G.N.; El Khassawna, T. Landscape of Well-Coordinated Fracture Healing in a Mouse Model Using Molecular and Cellular Analysis. Int. J. Mol. Sci. 2023, 24, 3569. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.E.; Moore-Lotridge, S.N.; Hysong, A.A.; Posey, S.L.; Robinette, J.P.; Blum, D.M.; Benvenuti, M.A.; Cole, H.A.; Egawa, S.; Okawa, A.; et al. Bone Fracture Acute Phase Response-A Unifying Theory of Fracture Repair: Clinical and Scientific Implications. Clin. Rev. Bone Miner. Metab. 2018, 16, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Einhorn, T.A. The Biology of Fracture Healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Nauta, S.; Greven, J.; Hofman, M.; Mohren, R.; Meesters, D.M.; Möckel, D.; Lammers, T.; Hildebrand, F.; Siegel, T.P.; Cuypers, E.; et al. Mass Spectrometry Reveals Molecular Effects of Citrulline Supplementation during Bone Fracture Healing in a Rat Model. J. Am. Soc. Mass Spectrom. 2024, 35, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ding, J.; Yang, G.; Sun, W.; Guo, H.; Zhao, Y. Study on the Mechanism of Qigu Capsule in Upregulating NF-κB/HIF-1α Pathway to Improve the Quality of Bone Callus in Mice at Different Stages of Osteoporotic Fracture Healing. Evid. Based Complement. Altern. Med. 2021, 2021, 9943692. [Google Scholar] [CrossRef] [PubMed]
- Marzona, L.; Pavolini, B. Play and Players in Bone Fracture Healing Match. Clin. Cases Miner. Bone Metab. 2009, 6, 159–162. [Google Scholar] [PubMed]
- Rodríguez-Merchán, E.C. A Review of Recent Developments in the Molecular Mechanisms of Bone Healing. Int. J. Mol. Sci. 2021, 22, 767. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Whole Aspect of Runx2 Functions in Skeletal Development. Int. J. Mol. Sci. 2022, 23, 5776. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of Skeletal Development and Maintenance by Runx2 and Sp7. Int. J. Mol. Sci. 2024, 25, 10102. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Jiang, Q.; Komori, H.; Sakane, C.; Fukuyama, R.; Matsuo, Y.; Ito, K.; Miyazaki, T.; Komori, T. Runt-Related Transcription Factor-2 (Runx2) Is Required for Bone Matrix Protein Gene Expression in Committed Osteoblasts in Mice. J. Bone Miner. Res. 2021, 36, 2081–2095. [Google Scholar] [CrossRef] [PubMed]
- Dalle Carbonare, L.; Innamorati, G.; Valenti, M.T. Transcription Factor Runx2 and Its Application to Bone Tissue Engineering. Stem Cell Rev. 2012, 8, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fukushima, Y.; Nozaki, K.; Nakanishi, H.; Deng, J.; Wakabayashi, N.; Itaka, K. Enhancement of Bone Regeneration by Coadministration of Angiogenic and Osteogenic Factors Using Messenger RNA. Inflamm. Regen. 2023, 43, 32. [Google Scholar] [CrossRef] [PubMed]
- Gargalionis, A.N.; Adamopoulos, C.; Vottis, C.T.; Papavassiliou, A.G.; Basdra, E.K. Runx2 and Polycystins in Bone Mechanotransduction: Challenges for Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 5291. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [PubMed]
- Gomathi, K.; Akshaya, N.; Srinaath, N.; Moorthi, A.; Selvamurugan, N. Regulation of Runx2 by Post-Translational Modifications in Osteoblast Differentiation. Life Sci. 2020, 245, 117389. [Google Scholar] [CrossRef]
- Pakkath Narayanan, A.; Iyyappan, S.; Nagarajan, S. RUNX2 Regulation in Osteoblast Differentiation: A Possible Therapeutic Function of the lncRNA and miRNA-Mediated Network. Differentiation 2024, 140, 100803. [Google Scholar]
- Franceschi, R.T.; Xiao, G.; Jiang, D.; Gopalakrishnan, R.; Yang, S.; Reith, E. Multiple Signaling Pathways Converge on the Cbfa1/Runx2 Transcription Factor to Regulate Osteoblast Differentiation. Connect. Tissue Res. 2003, 44, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Morii, E.; Komori, T.; Kawahata, H.; Sugimoto, M.; Terai, K.; Shimizu, H.; Yasui, T.; Ogihara, H.; Yasui, N.; et al. Transcriptional Regulation of Osteopontin Gene in Vivo by PEBP2alphaA/CBFA1 and ETS1 in the Skeletal Tissues. Oncogene 1998, 17, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Tagashira, S.; Fujiwara, M.; Ogawa, S.; Katsumata, T.; Yamaguchi, A.; Komori, T.; Nakatsuka, M. Cbfa1 Isoforms Exert Functional Differences in Osteoblast Differentiation. J. Biol. Chem. 1999, 274, 6972–6978. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, C.; McCabe, L.R.; Choi, J.Y.; Hiebert, S.W.; Stein, J.L.; Stein, G.S.; Lian, J.B. Runt Homology Domain Proteins in Osteoblast Differentiation: AML3/CBFA1 Is a Major Component of a Bone-Specific Complex. J. Cell. Biochem. 1997, 66, 1–8. [Google Scholar] [CrossRef]
- Kawane, T.; Qin, X.; Jiang, Q.; Miyazaki, T.; Komori, H.; Yoshida, C.A.; Matsuura-Kawata, V.K.D.S.; Sakane, C.; Matsuo, Y.; Nagai, K.; et al. Runx2 Is Required for the Proliferation of Osteoblast Progenitors and Induces Proliferation by Regulating Fgfr2 and Fgfr3. Sci. Rep. 2018, 8, 13551. [Google Scholar] [CrossRef] [PubMed]
- Levi, B.; James, A.W.; Nelson, E.R.; Vistnes, D.; Wu, B.; Lee, M.; Gupta, A.; Longaker, M.T. Human Adipose Derived Stromal Cells Heal Critical Size Mouse Calvarial Defects. PLoS ONE 2010, 5, e11177. [Google Scholar] [CrossRef]
- Li, T.-F.; Dong, Y.; Ionescu, A.M.; Rosier, R.N.; Zuscik, M.J.; Schwarz, E.M.; O’Keefe, R.J.; Drissi, H. Parathyroid Hormone-Related Peptide (PTHrP) Inhibits Runx2 Expression through the PKA Signaling Pathway. Exp. Cell Res. 2004, 299, 128–136. [Google Scholar] [CrossRef]
- Yoshida, C.A.; Komori, H.; Maruyama, Z.; Miyazaki, T.; Kawasaki, K.; Furuichi, T.; Fukuyama, R.; Mori, M.; Yamana, K.; Nakamura, K.; et al. SP7 Inhibits Osteoblast Differentiation at a Late Stage in Mice. PLoS ONE 2012, 7, e32364. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Baek, W.-Y.; Lee, M.-A.; Jung, J.W.; Kim, S.-Y.; Akiyama, H.; de Crombrugghe, B.; Kim, J.-E. Positive Regulation of Adult Bone Formation by Osteoblast-Specific Transcription Factor Osterix. J. Bone Miner. Res. 2009, 24, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Artigas, N.; Ureña, C.; Rodríguez-Carballo, E.; Rosa, J.L.; Ventura, F. Mitogen-Activated Protein Kinase (MAPK)-Regulated Interactions between Osterix and Runx2 Are Critical for the Transcriptional Osteogenic Program. J. Biol. Chem. 2014, 289, 27105–27117. [Google Scholar] [CrossRef]
- Akiyama, T.; Raftery, L.A.; Wharton, K.A. Bone Morphogenetic Protein Signaling: The Pathway and Its Regulation. Genetics 2024, 226, iyad200. [Google Scholar] [CrossRef] [PubMed]
- ten Dijke, P.; Miyazono, K.; Heldin, C.H. Signaling via Hetero-Oligomeric Complexes of Type I and Type II Serine/threonine Kinase Receptors. Curr. Opin. Cell Biol. 1996, 8, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Celeste, A.J.; Thies, R.S.; Song, J.J.; Bernier, S.M.; Goltzman, D.; Lyons, K.M.; Nove, J.; Rosen, V.; Wozney, J.M. A Mammalian Serine/threonine Kinase Receptor Specifically Binds BMP-2 and BMP-4. Biochem. Biophys. Res. Commun. 1994, 205, 1944–1951. [Google Scholar] [CrossRef] [PubMed]
- Hassel, S.; Yakymovych, M.; Hellman, U.; Rönnstrand, L.; Knaus, P.; Souchelnytskyi, S. Interaction and Functional Cooperation between the Serine/threonine Kinase Bone Morphogenetic Protein Type II Receptor with the Tyrosine Kinase Stem Cell Factor Receptor. J. Cell. Physiol. 2006, 206, 457–467. [Google Scholar] [CrossRef]
- Song, B.; Estrada, K.D.; Lyons, K.M. Smad Signaling in Skeletal Development and Regeneration. Cytokine Growth Factor Rev. 2009, 20, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Retting, K.N.; Song, B.; Yoon, B.S.; Lyons, K.M. BMP Canonical Smad Signaling through Smad1 and Smad5 Is Required for Endochondral Bone Formation. Development 2009, 136, 1093–1104. [Google Scholar] [CrossRef]
- Arnold, S.J.; Maretto, S.; Islam, A.; Bikoff, E.K.; Robertson, E.J. Dose-Dependent Smad1, Smad5 and Smad8 Signaling in the Early Mouse Embryo. Dev. Biol. 2006, 296, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, K. Signal Transduction by Bone Morphogenetic Protein Receptors: Functional Roles of Smad Proteins. Bone 1999, 25, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Zwijsen, A.; Verschueren, K.; Huylebroeck, D. New Intracellular Components of Bone Morphogenetic protein/Smad Signaling Cascades. FEBS Lett. 2003, 546, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.-L.; Chen, Z.-H.; Teng, Y.-Y.; Liu, S.-Y.; Jia, Y.; Zhang, K.-W.; Sun, Z.-L.; Wu, J.-J.; Yuan, Z.-D.; Feng, Y.; et al. The Smad Dependent TGF-β and BMP Signaling Pathway in Bone Remodeling and Therapies. Front. Mol. Biosci. 2021, 8, 593310. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Maeda, K.; Takahashi, N. Roles of Wnt Signaling in Bone Formation and Resorption. Jpn. Dent. Sci. Rev. 2008, 44, 76–82. [Google Scholar] [CrossRef]
- Houschyar, K.S.; Tapking, C.; Borrelli, M.R.; Popp, D.; Duscher, D.; Maan, Z.N.; Chelliah, M.P.; Li, J.; Harati, K.; Wallner, C.; et al. Wnt Pathway in Bone Repair and Regeneration—What Do We Know so Far. Front. Cell Dev. Biol. 2018, 6, 170. [Google Scholar] [CrossRef]
- Kamiya, N.; Ye, L.; Kobayashi, T.; Mochida, Y.; Yamauchi, M.; Kronenberg, H.M.; Feng, J.Q.; Mishina, Y. BMP Signaling Negatively Regulates Bone Mass through Sclerostin by Inhibiting the Canonical Wnt Pathway. Development 2008, 135, 3801–3811. [Google Scholar] [CrossRef] [PubMed]
- Minear, S.; Leucht, P.; Jiang, J.; Liu, B.; Zeng, A.; Fuerer, C.; Nusse, R.; Helms, J.A. Wnt Proteins Promote Bone Regeneration. Sci. Transl. Med. 2010, 2, 29ra30. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, A.; Schinke, T.; Bindl, R.; Wehner, T.; Rapp, A.; Haffner-Luntzer, M.; Nemitz, C.; Liedert, A.; Amling, M.; Ignatius, A. The Wnt Serpentine Receptor Frizzled-9 Regulates New Bone Formation in Fracture Healing. PLoS ONE 2013, 8, e84232. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Kunisada, T.; Tsukamoto, H.; Yamazaki, H.; Niwa, H.; Takada, S.; Hayashi, S.I. Wnt Signaling Regulates Hemopoiesis through Stromal Cells. J. Immunol. 2001, 167, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, Q.; Luo, S.; Liu, Z.; Luo, D.; Zhang, B.-Y.; Zhang, D.-D.; Rao, P.; Xiao, J. PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2016, 11, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Takada, I.; Kouzmenko, A.; Kato, S. Wnt and PPARγ Signaling in Osteoblastogenesis and Adipogenesis. Nat. Rev. Rheumatol. 2009, 5, 442–447. [Google Scholar] [CrossRef] [PubMed]
- van Tienen, F.H.J.; Laeremans, H.; van der Kallen, C.J.H.; Smeets, H.J.M. Wnt5b Stimulates Adipogenesis by Activating PPARgamma, and Inhibiting the Beta-Catenin Dependent Wnt Signaling Pathway Together with Wnt5a. Biochem. Biophys. Res. Commun. 2009, 387, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Darnay, B.G.; Haridas, V.; Ni, J.; Moore, P.A.; Aggarwal, B.B. Characterization of the Intracellular Domain of Receptor Activator of NF-kappaB (RANK). Interaction with Tumor Necrosis Factor Receptor-Associated Factors and Activation of NF-Kappab and c-Jun N-Terminal Kinase. J. Biol. Chem. 1998, 273, 20551–20555. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.-I.; et al. Induction and Activation of the Transcription Factor NFATc1 (NFAT2) Integrate RANKL Signaling in Terminal Differentiation of Osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Yao, Z.; Li, F.; Zhang, Q.; Badell, I.R.; Schwarz, E.M.; Takeshita, S.; Wagner, E.F.; Noda, M.; Matsuo, K.; et al. NF-kappaB p50 and p52 Regulate Receptor Activator of NF-kappaB Ligand (RANKL) and Tumor Necrosis Factor-Induced Osteoclast Precursor Differentiation by Activating c-Fos and NFATc1. J. Biol. Chem. 2007, 282, 18245–18253. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.U.A.; Kijas, A.W.; Lauko, J.; Rowan, A.E. The Mechanosensory Role of Osteocytes and Implications for Bone Health and Disease States. Front. Cell Dev. Biol. 2021, 9, 770143. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R. The Osteocyte: Key Player in Regulating Bone Turnover. RMD Open 2015, 1, e000049. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite-Polymer Biocomposites for Bone Regeneration: A Review of Current Trends. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2046–2057. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F.; Mundy, G.R. Role of Transforming Growth Factor-Beta in Bone Remodeling. Clin. Orthop. Relat. Res. 1990, 250, 261–276. [Google Scholar] [CrossRef]
- Wrana, J.L.; Maeno, M.; Hawrylyshyn, B.; Yao, K.L.; Domenicucci, C.; Sodek, J. Differential Effects of Transforming Growth Factor-Beta on the Synthesis of Extracellular Matrix Proteins by Normal Fetal Rat Calvarial Bone Cell Populations. J. Cell Biol. 1988, 106, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP Signaling in Osteoblast, Skeletal Development, and Bone Formation, Homeostasis and Disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-β/BMP Signaling and Other Molecular Events: Regulation of Osteoblastogenesis and Bone Formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef] [PubMed]
- Balooch, G.; Balooch, M.; Nalla, R.K.; Schilling, S.; Filvaroff, E.H.; Marshall, G.W.; Marshall, S.J.; Ritchie, R.O.; Derynck, R.; Alliston, T. TGF-Beta Regulates the Mechanical Properties and Composition of Bone Matrix. Proc. Natl. Acad. Sci. USA 2005, 102, 18813–18818. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Grafe, I.; Ding, H.; Flores, R.; Munivez, E.; Jiang, M.M.; Dawson, B.; Lee, B.; Ambrose, C.G. Correlations between Bone Mechanical Properties and Bone Composition Parameters in Mouse Models of Dominant and Recessive Osteogenesis Imperfecta and the Response to Anti-TGF-β Treatment. J. Bone Miner. Res. 2017, 32, 347–359. [Google Scholar] [CrossRef]
- Balooch, G. TGF-Beta and Glucocorticoids Regulate Bone Matrix Mechanical Properties and Composition. Ph.D. Thesis, University of California, San Francisco, CA, USA, 2006. [Google Scholar]
- Hu, K.; Olsen, B.R. The Roles of Vascular Endothelial Growth Factor in Bone Repair and Regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Street, J.; Bao, M.; deGuzman, L.; Bunting, S.; Peale, F.V., Jr.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular Endothelial Growth Factor Stimulates Bone Repair by Promoting Angiogenesis and Bone Turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef] [PubMed]
- Grosso, A.; Lunger, A.; Burger, M.G.; Briquez, P.S.; Mai, F.; Hubbell, J.A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. VEGF Dose Controls the Coupling of Angiogenesis and Osteogenesis in Engineered Bone. NPJ Regen. Med. 2023, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Fu, X.; Ma, J.; Lin, M.; Zhang, P.; Wang, Y.; Yan, Q.; Tao, C.; Liu, W.; Tang, B.; et al. Kindlin-2 Mediates Mechanotransduction in Bone by Regulating Expression of Sclerostin in Osteocytes. Commun. Biol. 2021, 4, 402. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Jiang, X.; Dai, Z.; Guo, X.; Weng, T.; Wang, J.; Li, Y.; Feng, G.; Gao, X.; He, L. Sclerostin Mediates Bone Response to Mechanical Unloading through Antagonizing Wnt/beta-Catenin Signaling. J. Bone Miner. Res. 2009, 24, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Galea, G.L.; Lanyon, L.E.; Price, J.S. Sclerostin’s Role in Bone’s Adaptive Response to Mechanical Loading. Bone 2017, 96, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical Stimulation of Bone in Vivo Reduces Osteocyte Expression of Sost/sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef]
- Gaudio, A.; Pennisi, P.; Bratengeier, C.; Torrisi, V.; Lindner, B.; Mangiafico, R.A.; Pulvirenti, I.; Hawa, G.; Tringali, G.; Fiore, C.E. Increased Sclerostin Serum Levels Associated with Bone Formation and Resorption Markers in Patients with Immobilization-Induced Bone Loss. J. Clin. Endocrinol. Metab. 2010, 95, 2248–2253. [Google Scholar] [CrossRef] [PubMed]
- Li, M.C.M.; Chow, S.K.H.; Wong, R.M.Y.; Qin, L.; Cheung, W.H. The Role of Osteocytes-Specific Molecular Mechanism in Regulation of Mechanotransduction—A Systematic Review. J. Orthop. Translat. 2021, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Khoswanto, C. Role of Matrix Metalloproteinases in Bone Regeneration: Narrative Review. J. Oral Biol. Craniofac. Res. 2023, 13, 539–543. [Google Scholar] [CrossRef]
- Hardy, E.; Fernandez-Patron, C. Destroy to Rebuild: The Connection between Bone Tissue Remodeling and Matrix Metalloproteinases. Front. Physiol. 2020, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, A.; Zhang, H.; Ekeledo, A.; Lee, S.; Werb, Z.; Plant, G.W.; Noble-Haeusslein, L.J. Deficiency in Matrix Metalloproteinase-2 Results in Long-Term Vascular Instability and Regression in the Injured Mouse Spinal Cord. Exp. Neurol. 2016, 284, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Mosig, R.A.; Martignetti, J.A. Loss of MMP-2 in Murine Osteoblasts Upregulates Osteopontin and Bone Sialoprotein Expression in a Circuit Regulating Bone Homeostasis. Dis. Model. Mech. 2013, 6, 397–403. [Google Scholar] [PubMed]
- Waning, D.L.; Mohammad, K.S.; Reiken, S.; Xie, W.; Andersson, D.C.; John, S.; Chiechi, A.; Wright, L.E.; Umanskaya, A.; Niewolna, M.; et al. Excess TGF-β Mediates Muscle Weakness Associated with Bone Metastases in Mice. Nat. Med. 2015, 21, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.V.; Latour, L.L.; McGavern, D.B. Distinct Myeloid Cell Subsets Promote Meningeal Remodeling and Vascular Repair after Mild Traumatic Brain Injury. Nat. Immunol. 2018, 19, 442–452. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix Metalloproteinases and the Regulation of Tissue Remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Senft, A.P.; Korfhagen, T.R.; Whitsett, J.A.; Shapiro, S.D.; LeVine, A.M. Surfactant Protein-D Regulates Soluble CD14 through Matrix Metalloproteinase-12. J. Immunol. 2005, 174, 4953–4959. [Google Scholar] [CrossRef] [PubMed]
- Barthelemi, S.; Robinet, J.; Garnotel, R.; Antonicelli, F.; Schittly, E.; Hornebeck, W.; Lorimier, S. Mechanical Forces-Induced Human Osteoblasts Differentiation Involves MMP-2/MMP-13/MT1-MMP Proteolytic Cascade. J. Cell. Biochem. 2012, 113, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Herber, R.-P.; Ho, S.P.; Alliston, T. Matrix Metalloproteinase-13 Is Required for Osteocytic Perilacunar Remodeling and Maintains Bone Fracture Resistance. J. Bone Miner. Res. 2012, 27, 1936–1950. [Google Scholar] [CrossRef]
- Shapses, S.A.; Cifuentes, M.; Spevak, L.; Chowdhury, H.; Brittingham, J.; Boskey, A.L.; Denhardt, D.T. Osteopontin Facilitates Bone Resorption, Decreasing Bone Mineral Crystallinity and Content during Calcium Deficiency. Calcif. Tissue Int. 2003, 73, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, E.; Scapolan, M.; Pecolo, M.; Wassermann, B.; Abu-Rumeileh, I.; Balestreri, L.; Borsatti, E.; Tripodo, C.; Colombatti, A.; Spessotto, P. MMP-13 Stimulates Osteoclast Differentiation and Activation in Tumour Breast Bone Metastases. Breast Cancer Res. 2011, 13, R105. [Google Scholar] [CrossRef] [PubMed]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef] [PubMed]
- Lotsari, A.; Rajasekharan, A.K.; Halvarsson, M.; Andersson, M. Transformation of Amorphous Calcium Phosphate to Bone-like Apatite. Nat. Commun. 2018, 9, 4170. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A.; McKittrick, J.; Chen, P.-Y. Structural Biological Materials: Critical Mechanics-Materials Connections. Science 2013, 339, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Fratzl, P.; Gupta, H.S.; Paschalis, E.P.; Roschger, P. Structure and Mechanical Quality of the Collagen–mineral Nano-Composite in Bone. J. Mater. Chem. 2004, 14, 2115–2123. [Google Scholar] [CrossRef]
- Jiang, S.; Jin, W.; Wang, Y.-N.; Pan, H.; Sun, Z.; Tang, R. Effect of the Aggregation State of Amorphous Calcium Phosphate on Hydroxyapatite Nucleation Kinetics. RSC Adv. 2017, 7, 25497–25503. [Google Scholar] [CrossRef]
- Hasegawa, T. Ultrastructure and Biological Function of Matrix Vesicles in Bone Mineralization. Histochem. Cell Biol. 2018, 149, 289–304. [Google Scholar] [CrossRef]
- Golub, E.E. Role of Matrix Vesicles in Biomineralization. Biochim. Biophys. Acta 2009, 1790, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, P.H.; Blair, H.C.; Beer Stolz, D.; Riazanski, V.; Ray, E.C.; Tourkova, I.L.; Nelson, D.J. Cellular and Extracellular Matrix of Bone, with Principles of Synthesis and Dependency of Mineral Deposition on Cell Membrane Transport. Am. J. Physiol. Cell Physiol. 2020, 318, C111–C124. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.L.; Centi, A.; Smith, S.R.; Gundberg, C. The Role of Osteocalcin in Human Glucose Metabolism: Marker or Mediator? Nat. Rev. Endocrinol. 2013, 9, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Alamdari, S.; Pfaendtner, J. Impact of Glutamate Carboxylation in the Adsorption of the α-1 Domain of Osteocalcin to Hydroxyapatite and Titania. Mol. Syst. Des. Eng. 2020, 5, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, I.M.; Landis, W.J. Local and Systemic Regulation of Mineralization: Role of Coupling Factors, Pyrophosphate, Polyphosphates, Vitamin D, Fetuin, Matrix Gla Protein, and Osteopontin. In Mechanisms of Mineralization of Vertebrate Skeletal and Dental Tissues; Springer International Publishing: Cham, Germany, 2023; pp. 403–444. ISBN 9783031343025. [Google Scholar]
- Xu, Z.; Yang, C.; Wu, F.; Tan, X.; Guo, Y.; Zhang, H.; Wang, H.; Sui, X.; Xu, Z.; Zhao, M.; et al. Triple-Gene Deletion for Osteocalcin Significantly Impairs the Alignment of Hydroxyapatite Crystals and Collagen in Mice. Front. Physiol. 2023, 14, 1136561. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. What Is the Function of Osteocalcin? J. Oral Biosci. 2020, 62, 223–227. [Google Scholar] [CrossRef]
- Millán, J.L. The Role of Phosphatases in the Initiation of Skeletal Mineralization. Calcif. Tissue Int. 2013, 93, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Gericke, A.; Qin, C.; Spevak, L.; Fujimoto, Y.; Butler, W.T.; Sørensen, E.S.; Boskey, A.L. Importance of Phosphorylation for Osteopontin Regulation of Biomineralization. Calcif. Tissue Int. 2005, 77, 45–54. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Ramachandran, A.; Dahl, T.; George, S.; Schultz, D.; Cookson, D.; Veis, A.; George, A. Phosphorylation of Phosphophoryn Is Crucial for Its Function as a Mediator of Biomineralization. J. Biol. Chem. 2005, 280, 33109–33114. [Google Scholar] [CrossRef] [PubMed]
- de Boer, H.H.; van der Merwe, A.E.; Hammer, S.; Steyn, M.; Maat, G.J.R. Assessing Post-Traumatic Time Interval in Human Dry Bone: Assessment of Post-Traumatic Time Interval. Int. J. Osteoarchaeol. 2015, 25, 98–109. [Google Scholar] [CrossRef]
- Cappella, A.; de Boer, H.H.; Cammilli, P.; De Angelis, D.; Messina, C.; Sconfienza, L.M.; Sardanelli, F.; Sforza, C.; Cattaneo, C. Histologic and Radiological Analysis on Bone Fractures: Estimation of Posttraumatic Survival Time in Skeletal Trauma. Forensic Sci. Int. 2019, 302, 109909. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gao, C.; Liu, N.; Zhu, Y.; Zhu, X.; Su, X.; Zhang, Q.; Wu, Y.; Zhang, C.; Liu, A.; et al. The Effect of Traumatic Brain Injury on Bone Healing from a Novel Exosome Centered Perspective in a Mice Model. J. Orthop. Translat. 2021, 30, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Hergemöller, D.F.; Pelegrine, A.A.; Pasquali, P.J.; Scavone de Macedo, L.G.; Teixeira, M.L.; Moy, P.K.; Aloise, A.C. Osteocalcin and Runx2 Expression in Anterior Maxillary Reconstructions Using Bone Xenografts Associated to Bone Marrow Aspirate Concentrate. Contemp. Clin. Dent. 2022, 13, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Gerdesmeyer, L.; Tübel, J.; Obermeier, A.; Harrasser, N.; Glowalla, C.; von Eisenhart-Rothe, R.; Burgkart, R. Extracorporeal Magnetotransduction Therapy as a New Form of Electromagnetic Wave Therapy: From Gene Upregulation to Accelerated Matrix Mineralization in Bone Healing. Biomedicines 2024, 12, 2269. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.B.; Taurozzi, A.J.; Hughes, N.; Pedersen, D.D.; Kontopoulos, I.; Mackie, M.; Collins, M.J. Comparing Biological and Pathological Factors Affecting Osteocalcin Concentrations in Archaeological Skeletal Remains. J. Archaeol. Sci. Rep. 2020, 34, 102573. [Google Scholar] [CrossRef]
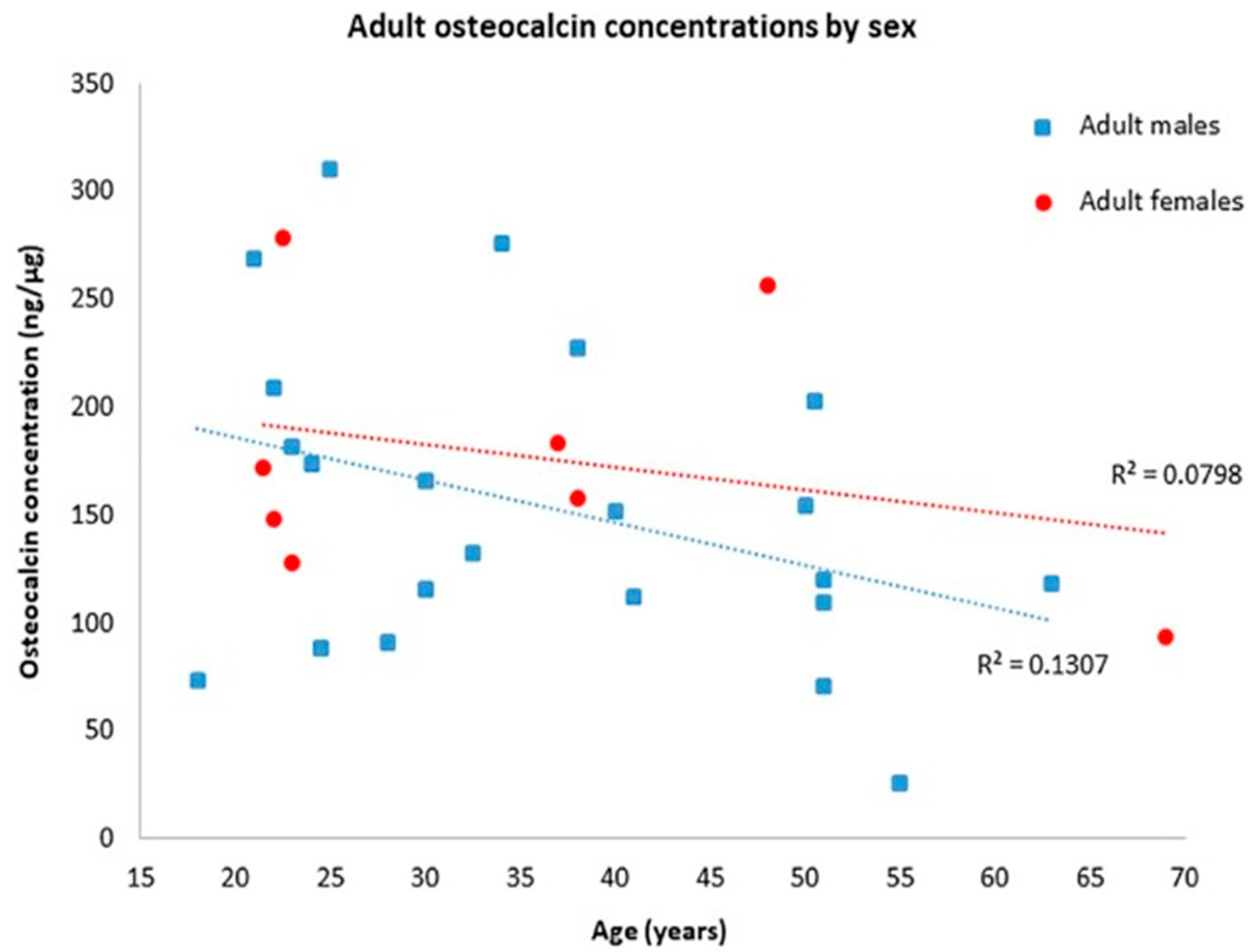
- Ritz, S.; Turzynski, A.; Schütz, H.W.; Hollmann, A.; Rochholz, G. Identification of Osteocalcin as a Permanent Aging Constituent of the Bone Matrix: Basis for an Accurate Age at Death Determination. Forensic Sci. Int. 1996, 77, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Rengachary, S.S. Bone Morphogenetic Proteins: Basic Concepts. Neurosurg. Focus 2002, 13, e2. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-S.; Yang, K.D.; Kuo, Y.-R.; Wang, C.-J.; Sheen-Chen, S.-M.; Huang, H.-C.; Chen, Y.-J. Temporal and Spatial Expression of Bone Morphogenetic Proteins in Extracorporeal Shock Wave-Promoted Healing of Segmental Defect. Bone 2003, 32, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.J.; Gerstenfeld, L.C.; Einhorn, T.A. Differential Temporal Expression of Members of the Transforming Growth Factor β Superfamily During Murine Fracture Healing. J. Bone Miner. Res. 2002, 17, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Ghazizadeh, M.; Shimizu, H.; Matsumoto, H.; Saito, N.; Yagi, T.; Mashiko, K.; Mashiko, K.; Kawai, M.; Yokota, H. Delayed Expression of Circulating TGF-β1 and BMP-2 Levels in Human Nonunion Long Bone Fracture Healing. J. Nippon Med. Sch. 2017, 84, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Onishi, T.; Ishidou, Y.; Nagamine, T.; Yone, K.; Imamura, T.; Kato, M.; Sampath, T.K.; ten Dijke, P.; Sakou, T. Distinct and Overlapping Patterns of Localization of Bone Morphogenetic Protein (BMP) Family Members and a BMP Type II Receptor during Fracture Healing in Rats. Bone 1998, 22, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Nurrachman, A.S.; Azhari, A.; Epsilawati, L.; Pramanik, F. Temporal Pattern of Micro-CT Angiography Vascular Parameters and VEGF mRNA Expression in Fracture Healing: A Radiograph and Molecular Comparison. Eur. J. Dent. 2023, 17, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Ishida, Y.; Kimura, A.; Takayasu, T.; Eisenmenger, W.; Kondo, T. Forensic Application of VEGF Expression to Skin Wound Age Determination. Int. J. Legal Med. 2004, 118, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Steinbrech, D.S.; Mehrara, B.J.; Saadeh, P.B.; Greenwald, J.A.; Spector, J.A.; Gittes, G.K.; Longaker, M.T. VEGF Expression in an Osteoblast-like Cell Line Is Regulated by a Hypoxia Response Mechanism. Am. J. Physiol. Cell Physiol. 2000, 278, C853–C860. [Google Scholar] [CrossRef] [PubMed]
- Araldi, E.; Schipani, E. Hypoxia, HIFs and Bone Development. Bone 2010, 47, 190–196. [Google Scholar] [CrossRef] [PubMed]
- An, S.S.; Jin, H.L.; Kim, K.N.; Kim, D.S.; Cho, J.; Liu, M.-L.; Oh, J.S.; Yoon, D.H.; Lee, M.H.; Ha, Y. Neuroprotective Effect of Combined Hypoxia-Induced VEGF and Bone Marrow-Derived Mesenchymal Stem Cell Treatment. Childs. Nerv. Syst. 2010, 26, 323–331. [Google Scholar] [CrossRef] [PubMed]
- van Hinsbergh, V.W.; Collen, A.; Koolwijk, P. Role of Fibrin Matrix in Angiogenesis. Ann. N. Y. Acad. Sci. 2001, 936, 426–437. [Google Scholar] [CrossRef]
- Burger, M.G.; Grosso, A.; Briquez, P.S.; Born, G.M.E.; Lunger, A.; Schrenk, F.; Todorov, A.; Sacchi, V.; Hubbell, J.A.; Schaefer, D.J.; et al. Robust Coupling of Angiogenesis and Osteogenesis by VEGF-Decorated Matrices for Bone Regeneration. Acta Biomater. 2022, 149, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Steinbrech, D.S.; Mehrara, B.J.; Rowe, N.M.; Dudziak, M.E.; Luchs, J.S.; Saadeh, P.B.; Gittes, G.K.; Longaker, M.T. Gene Expression of TGF-Beta, TGF-Beta Receptor, and Extracellular Matrix Proteins during Membranous Bone Healing in Rats. Plast. Reconstr. Surg. 2000, 105, 2028–2038. [Google Scholar] [CrossRef] [PubMed]
- Haubruck, P.; Heller, R.; Apitz, P.; Kammerer, A.; Alamouti, A.; Daniel, V.; Schmidmaier, G.; Moghaddam, A. Evaluation of Matrix Metalloproteases as Early Biomarkers for Bone Regeneration during the Applied Masquelet Therapy for Non-Unions. Injury 2018, 49, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Anwar, M.F.; Ahmed, K.; Aftab, M.; Nazim, F.; Bari, M.F.; Mustafa, M.; Vohra, F.; Alrahlah, A.; Mughal, N.; et al. Baseline MMP Expression in Periapical Granuloma and Its Relationship with Periapical Wound Healing after Surgical Endodontic Treatment. BMC Oral Health 2021, 21, 562. [Google Scholar] [CrossRef] [PubMed]
- Niedecker, A.; Huhn, R.; Ritz-Timme, S.; Mayer, F. Complex Challenges of Estimating the Age and Vitality of Muscle Wounds: A Study with Matrix Metalloproteinases and Their Inhibitors on Animal and Human Tissue Samples. Int. J. Legal Med. 2021, 135, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Alibegović, A.; Umek, N.; Pušnik, L.; Zubiavrre Martinez, I. Comparison of the Visual Scoring Method and Semi-Automatic Image Analysis for Evaluating Staining Intensity of Human Cartilage Sections. Image Anal. Stereol. 2024, 43, 131–137. [Google Scholar] [CrossRef]
- Cvetko, M.; Knific, T.; Frangež, R.; Motaln, H.; Rogelj, B.; Alibegović, A.; Gombač, M. Postmortem Chondrocyte Viability in Porcine Articular Cartilage: Influence of Time, Temperature, and Burial under Winter Conditions. J. Forensic Sci. 2024, 69, 1094–1101. [Google Scholar] [CrossRef]
- Alibegović, A.; Balažic, J.; Petrovič, D.; Hribar, G.; Blagus, R.; Drobnič, M. Viability of Human Articular Chondrocytes Harvested Postmortem: Changes with Time and Temperature of in Vitro Culture Conditions. J. Forensic Sci. 2014, 59, 522–528. [Google Scholar] [CrossRef]
- Ubelaker, D.; Wu, Y. Fragment Analysis in Forensic Anthropology. Forensic Sci. Res. 2020, 5, 260–265. [Google Scholar] [CrossRef]
- Garvin, H.; Dunn, R.; Sholts, S.; Litten, M.S.; Mohamed, M.; Kuttickat, N.; Skantz, N. Forensic Tools for Species Identification of Skeletal Remains: Metrics, Statistics, and OsteoID. Biology 2021, 11, 25. [Google Scholar] [CrossRef]
- Taniguchi, K.; Miyaguchi, H. COL1A2 Barcoding: Bone Species Identification via Shotgun Proteomics. J. Proteome Res. 2024, 23, 377–385. [Google Scholar] [CrossRef]
- Jiang, C.; Zhu, S.; Zhan, W.; Lou, L.; Li, A.; Cai, J. Comparative Analysis of Bone Turnover Markers in Bone Marrow and Peripheral Blood: Implications for Osteoporosis. J. Orthop. Surg. Res. 2024, 19, 163. [Google Scholar] [CrossRef] [PubMed]
- Bonicelli, A.; Mickleburgh, H.L.; Chighine, A.; Locci, E.; Wescott, D.J.; Procopio, N. The “ForensOMICS” Approach to Forensic Post-Mortem Interval Estimation: Combining Metabolomics, Lipidomics and Proteomics for the Analysis of Human Bone. bioRxiv 2022. [Google Scholar]
- Smit, T.H. Closing the Osteon: Do Osteocytes Sense Strain Rate rather than Fluid Flow? Bioessays 2021, 43, e2000327. [Google Scholar] [CrossRef]
- Schwab, N.; Galtés, I.; Winter-Buchwalder, M.; Ortega-Sánchez, M.; Jordana, X. Osteonal Microcracking Pattern: A Potential Vitality Marker in Human Bone Trauma. Biology 2023, 12, 399. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B.; Martin, R.B.; Schaffler, M.B.; Radin, E.L. Bone Remodeling in Response to in Vivo Fatigue Microdamage. J. Biomech. 1985, 18, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H.; Pavalko, F.M. Mechanotransduction and Functional Response of the Skeleton to Physical Stress: The Mechanisms and Mechanics of Bone Adaptation. J. Orthop. Sci. 1998, 3, 346–355. [Google Scholar] [CrossRef]
- You, L.-D.; Weinbaum, S.; Cowin, S.C.; Schaffler, M.B. Ultrastructure of the Osteocyte Process and Its Pericellular Matrix. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2004, 278, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Cambré, I.; Gaublomme, D.; Burssens, A.; Jacques, P.; Schryvers, N.; De Muynck, A.; Meuris, L.; Lambrecht, S.; Carter, S.; de Bleser, P.; et al. Mechanical Strain Determines the Site-Specific Localization of Inflammation and Tissue Damage in Arthritis. Nat. Commun. 2018, 9, 4613. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.M.; Fink, D.; Smith, H.; Olsen, Z.; Jacobs, C.; Anderson, D. The Relationship between Intra-Articular Fracture Energy and a Patient’s Inflammatory Response. J. Orthop. Trauma 2024, 38, e225–e229. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, P.V.; Macauley, K.; Ash, R.D.; Shoup, B.; Scannella, J.B. Taphonomic and Diagenetic Pathways to Protein Preservation, Part I: The Case of Tyrannosaurus Rex Specimen MOR 1125. Biology 2021, 10, 1193. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, P.V.; Ash, R.D.; Scannella, J.B. Taphonomic and Diagenetic Pathways to Protein Preservation, Part II: The Case of Brachylophosaurus Canadensis Specimen MOR 2598. Biology 2022, 11, 1177. [Google Scholar] [CrossRef] [PubMed]
- Procopio, N.; Mein, C.A.; Starace, S.; Bonicelli, A.; Williams, A. Bone Diagenesis in Short Timescales: Insights from an Exploratory Proteomic Analysis. Biology 2021, 10, 460. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Bonete, G.; Pérez-Cárceles, M.D.; Maurandi-López, A.; Pérez-Martínez, C.; Luna, A. Association between Protein Profile and Postmortem Interval in Human Bone Remains. J. Proteom. 2019, 192, 54–63. [Google Scholar] [CrossRef]
- Pérez-Martínez, C.; Pérez-Cárceles, M.D.; Legaz, I.; Prieto-Bonete, G.; Luna, A. Quantification of Nitrogenous Bases, DNA and Collagen Type I for the Estimation of the Postmortem Interval in Bone Remains. Forensic Sci. Int. 2017, 281, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, H.; Hathway, B.; Procopio, N. Aquatic Decomposition of Mammalian Corpses: A Forensic Proteomic Approach. J. Proteome Res. 2020, 19, 2122–2135. [Google Scholar] [CrossRef]
- Baldock, J.A.; Skjemstad, J.O. Role of the Soil Matrix and Minerals in Protecting Natural Organic Materials against Biological Attack. Org. Geochem. 2000, 31, 697–710. [Google Scholar] [CrossRef]
- Kleber, M.; Bourg, I.C.; Coward, E.K.; Hansel, C.M.; Myneni, S.C.B.; Nunan, N. Dynamic Interactions at the Mineral–organic Matter Interface. Nat. Rev. Earth Environ. 2021, 2, 402–421. [Google Scholar] [CrossRef]
- Barker, W.W.; Banfield, J.F. Zones of Chemical and Physical Interaction at Interfaces between Microbial Communities and Minerals: A Model. Geomicrobiol. J. 1998, 15, 223–244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hostiuc, S.; Negoi, I.; Costescu, M.; Siserman, C. Molecular Determinants of Bone Plasticity Regeneration After Trauma: Forensic Consequences. Int. J. Mol. Sci. 2025, 26, 7184. https://doi.org/10.3390/ijms26157184
Hostiuc S, Negoi I, Costescu M, Siserman C. Molecular Determinants of Bone Plasticity Regeneration After Trauma: Forensic Consequences. International Journal of Molecular Sciences. 2025; 26(15):7184. https://doi.org/10.3390/ijms26157184
Chicago/Turabian StyleHostiuc, Sorin, Ionut Negoi, Mihnea Costescu, and Costel Siserman. 2025. "Molecular Determinants of Bone Plasticity Regeneration After Trauma: Forensic Consequences" International Journal of Molecular Sciences 26, no. 15: 7184. https://doi.org/10.3390/ijms26157184
APA StyleHostiuc, S., Negoi, I., Costescu, M., & Siserman, C. (2025). Molecular Determinants of Bone Plasticity Regeneration After Trauma: Forensic Consequences. International Journal of Molecular Sciences, 26(15), 7184. https://doi.org/10.3390/ijms26157184






