Combined Pharmacological Conditioning of Endothelial Cells for Improved Vascular Graft Endothelialization
Abstract
1. Introduction
2. Results
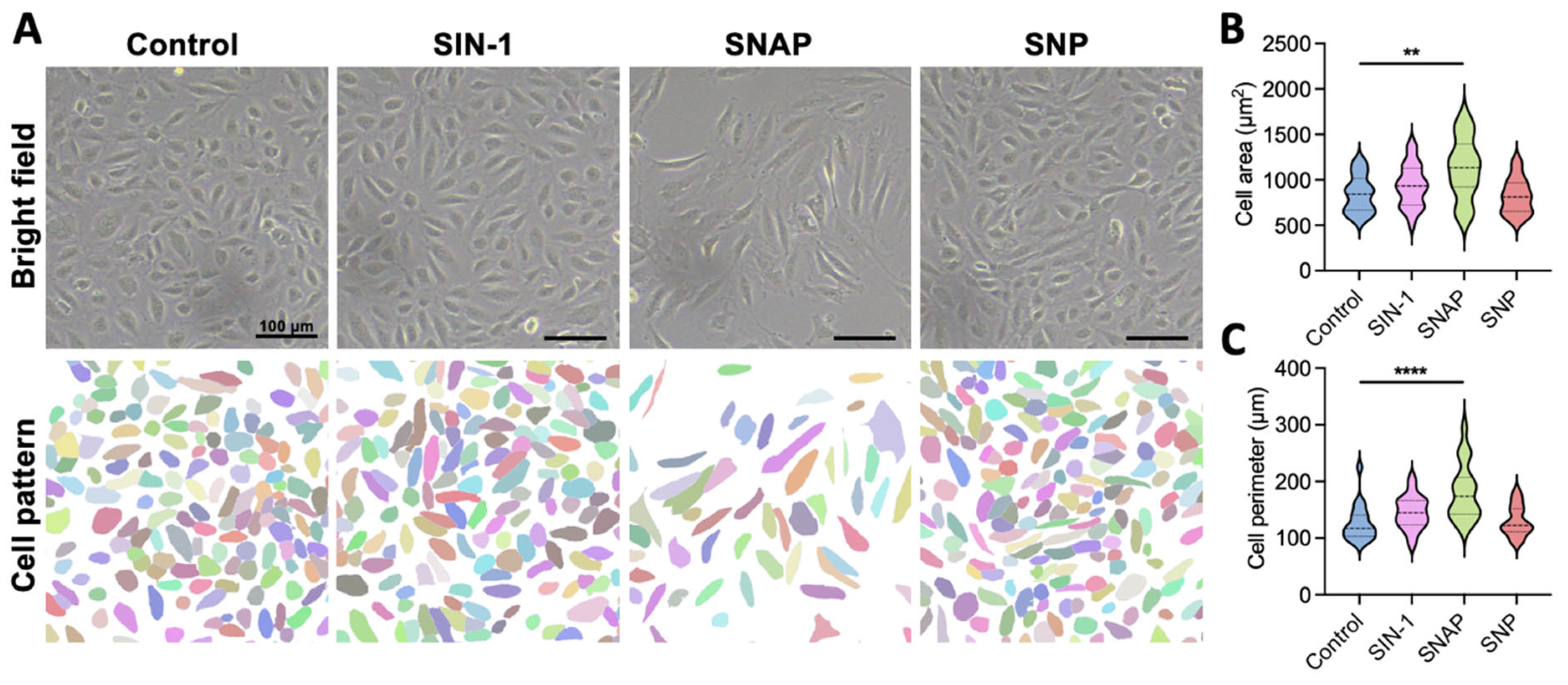
2.1. 3-Morpholinosydnonimine (SIN-1) Promotes Endothelial Cell Growth Through Nitric Oxide (NO) Delivery
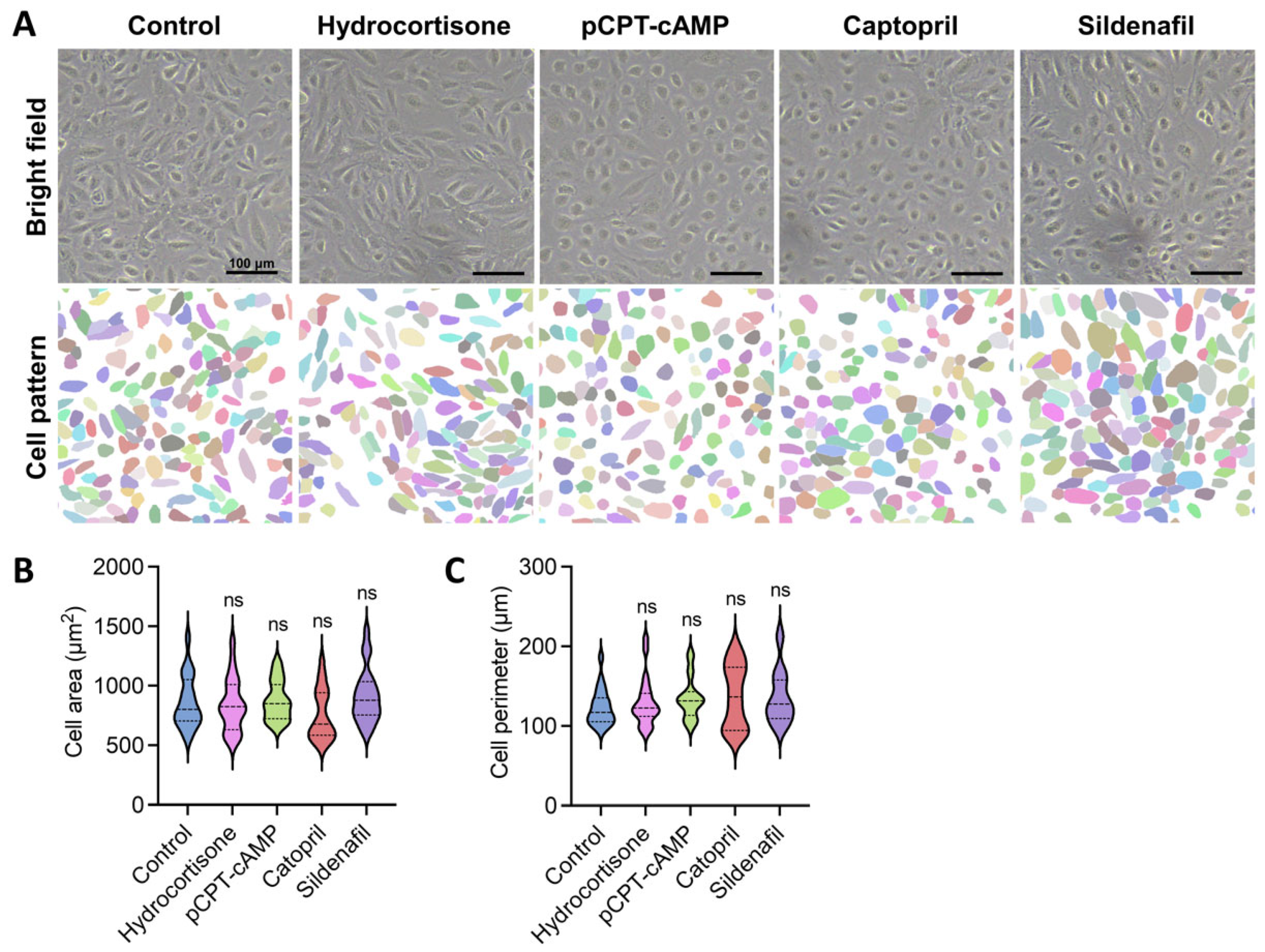
2.2. Pharmacological Modulation of Endothelial Cell Activity Promotes Favorable Morphological Changes
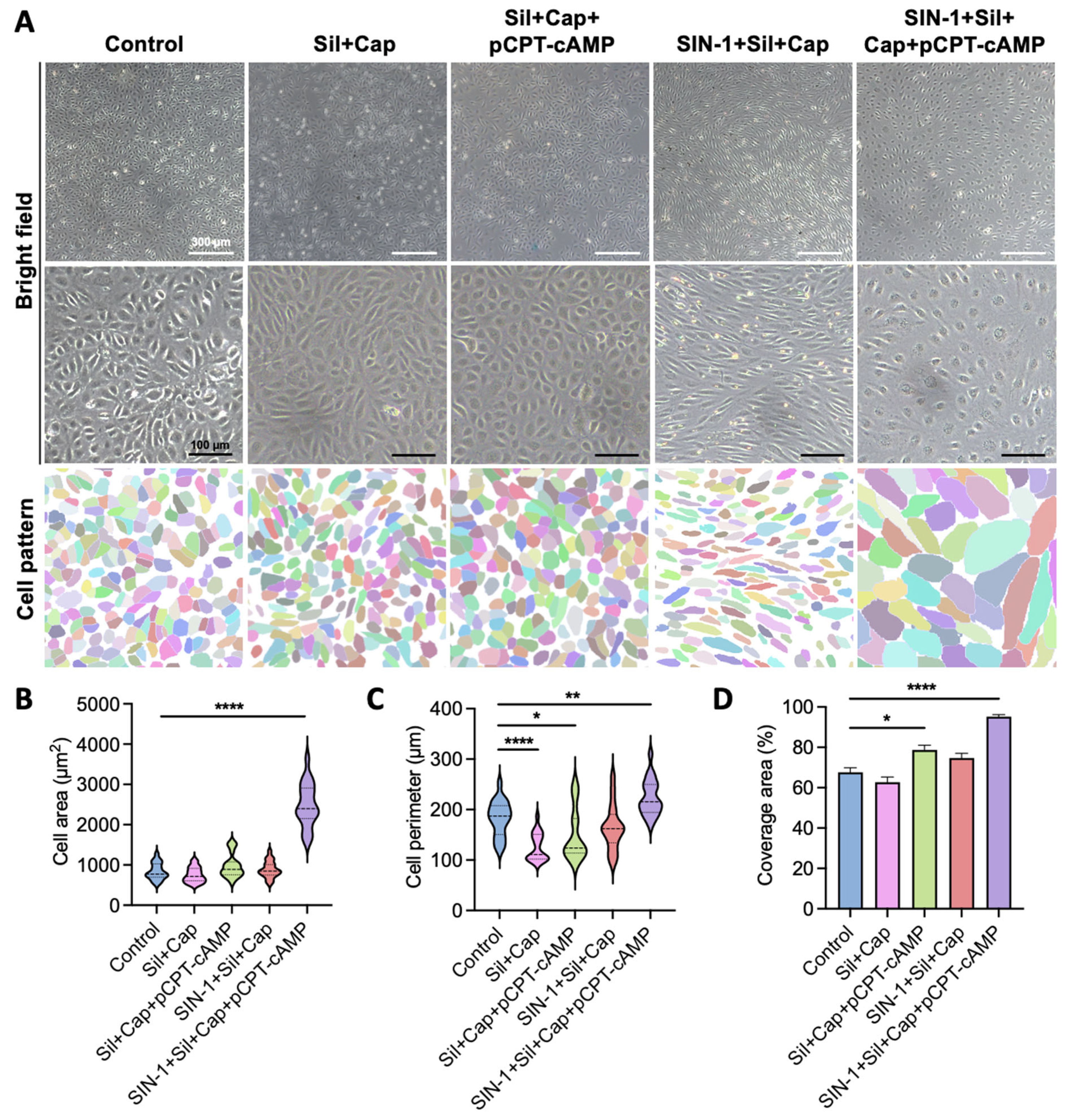
2.3. Synergistic Effects of Agent Combination Enhance Endothelial Cell Spreading
2.4. Agent Combination Enhances Tight Junction Formation Beyond pCPT-cAMP Alone
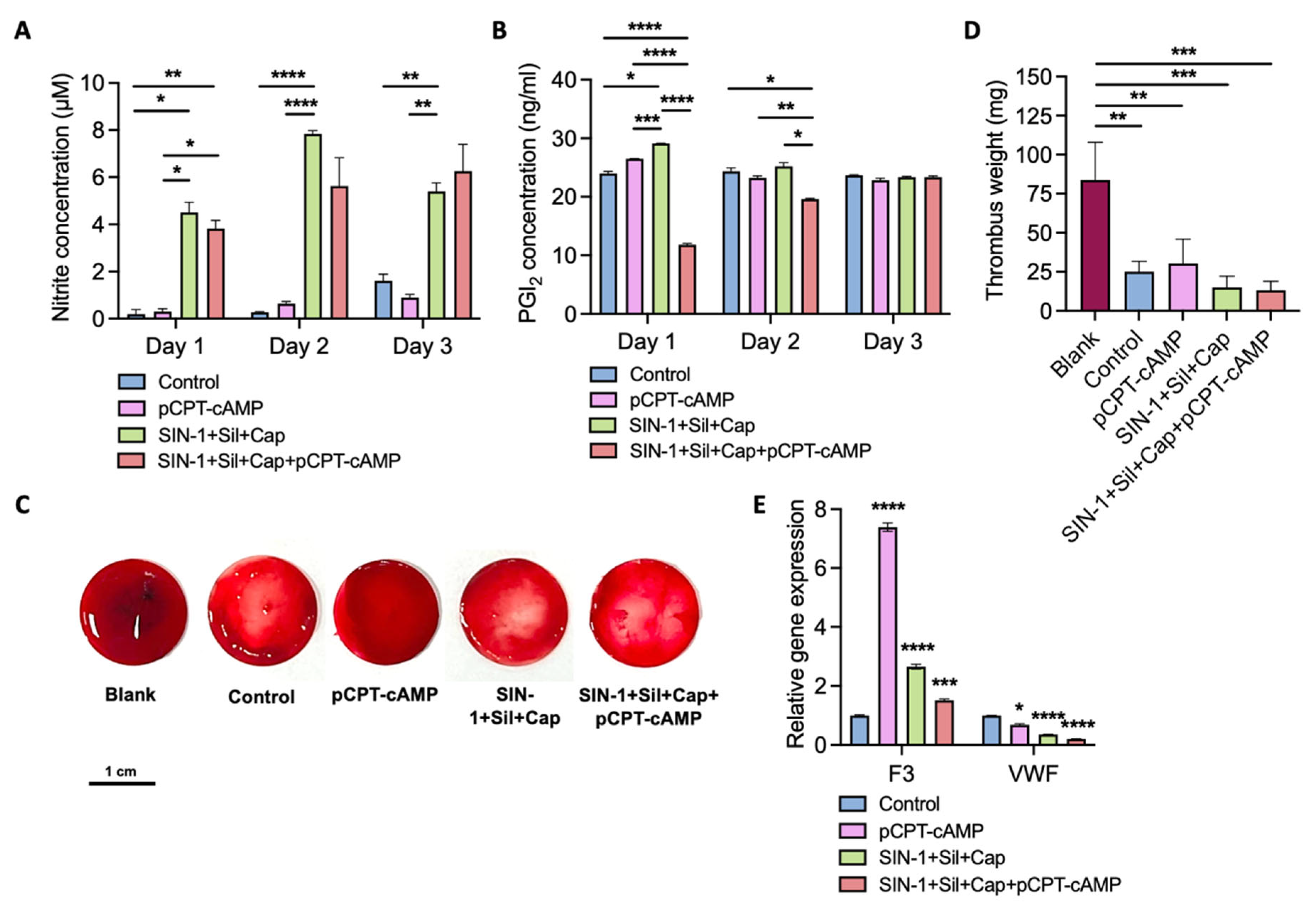
2.5. Endothelial Monolayer Induced by Agent Combination Demonstrates Antithrombogenic Properties
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Cell Toxicity Assay
4.4. Immunofluorescent Staining
4.5. RNA Isolation and qPCR
4.6. Endothelial Permeability Assay
4.7. NO Measurement
4.8. ELISA Test for PGI2
4.9. Anticoagulation Test
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EC | endothelial cell |
| PGI2 | Prostacyclin |
| NO | nitric oxide |
| cAMP | cyclic 3′-5′-adenosine monophosphate |
| PKA | protein kinase A |
| cGMP | cyclic guanosine monophosphate |
| PKG | protein kinase G |
| HUVEC | human umbilical vein endothelial cell |
| SNP | sodium nitroprusside |
| SIN-1 | 3-morpholinosydnonimine |
| SNAP | S-nitroso-N-acetylpenicillamine |
| PBS | phosphate-buffered saline |
| eNOS | endothelial nitric oxide synthase |
| pCPT-cAMP | 8-(4-chlorophenylthio) adenosine 3′,5′-cyclic monophosphate sodium salt |
References
- Ranjan, A.K.; Kumar, U.; Hardikar, A.A.; Poddar, P.; Nair, P.D.; Hardikar, A.A. Human blood vessel-derived endothelial progenitors for endothelialization of small diameter vascular prosthesis. PLoS ONE 2009, 4, e7718. [Google Scholar] [CrossRef] [PubMed]
- Burkel, W.E. The challenge of small diameter vascular grafts. Med. Prog. Technol. 1988, 14, 165–175. [Google Scholar] [PubMed]
- McGuigan, A.P.; Sefton, M.V. The influence of biomaterials on endothelial cell thrombogenicity. Biomaterials 2007, 28, 2547–2571. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.K.; Thiagarajan, P. Role of endothelium in thrombosis and hemostasis. Annu. Rev. Med. 1996, 47, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Cleaver, O.; Melton, D.A. Endothelial signaling during development. Nat. Med. 2003, 9, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, H.; Esposito, C.; Stern, D.M. Modulation of endothelial hemostatic properties: An active role in the host response. Annu. Rev. Med. 1990, 41, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Obata, T.; Sato, T.; Yamanaka, Y.; Arita, M. NO and cGMP facilitate adenosine production in rat hearts via activation of ecto-5′-nucleotidase. Pflug. Arch. 1998, 436, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Olgasi, C.; Assanelli, S.; Cucci, A.; Follenzi, A. Hemostasis and endothelial functionality: The double face of coagulation factors. Haematologica 2024, 109, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Roest, M.; de Laat, B.; de Groot, P.G.; Huskens, D. Interplay between platelets and coagulation. Blood Rev. 2021, 46, 100733. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, G. Endothelial tight junctions: Permeable barriers of the vessel wall. Thromb. Haemost. 2006, 95, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, G.; Dejana, E. Endothelial cell-to-cell junctions: Molecular organization and role in vascular homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar] [CrossRef] [PubMed]
- Calabria, A.R.; Weidenfeller, C.; Jones, A.R.; de Vries, H.E.; Shusta, E.V. Puromycin-purified rat brain microvascular endothelial cell cultures exhibit improved barrier properties in response to glucocorticoid induction. J. Neurochem. 2006, 97, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C.E.; Lum, H.; Schaphorst, K.L.; Verin, A.D.; Garcia, J.G. Regulation of endothelial barrier function by the cAMP-dependent protein kinase. Endothelium 2000, 7, 287–308. [Google Scholar] [CrossRef] [PubMed]
- Beese, M.; Wyss, K.; Haubitz, M.; Kirsch, T. Effect of cAMP derivates on assembly and maintenance of tight junctions in human umbilical vein endothelial cells. BMC Cell Biol. 2010, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Kraling, B.M.; Wiederschain, D.G.; Boehm, T.; Rehn, M.; Mulliken, J.B.; Moses, M.A. The role of matrix metalloproteinase activity in the maturation of human capillary endothelial cells in vitro. J. Cell Sci. 1999, 112 Pt 10, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Pratzel, S.; Rode, M.; Kraling, B.M. Induction of endothelial barrier function in vitro. Ann. N. Y. Acad. Sci. 2000, 915, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Augustin, H.G.; Koh, G.Y. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science 2017, 357, eaal2379. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Li, Z.; Dong, J.; Gao, Y.; Chang, Z.; Ding, X.; Li, S.; Li, Y.; Zeng, Y.; Xin, Q.; et al. Heterogeneity in endothelial cells and widespread venous arterialization during early vascular development in mammals. Cell Res. 2022, 32, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Stanley, G.; Sinha, R.; D’Amato, G.; Das, S.; Rhee, S.; Chang, A.H.; Poduri, A.; Raftrey, B.; Dinh, T.T.; et al. Single-cell analysis of early progenitor cells that build coronary arteries. Nature 2018, 559, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.J.; Sun, N.; Lee, H.; Godlewski, B.; Mathys, H.; Galani, K.; Zhou, B.; Jiang, X.; Ng, A.P.; Mantero, J.; et al. Single-cell dissection of the human brain vasculature. Nature 2022, 603, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E.; Hirschi, K.K.; Simons, M. The molecular basis of endothelial cell plasticity. Nat. Commun. 2017, 8, 14361. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Acharya, B.R.; Peyret, G.; Fardin, M.A.; Mege, R.M.; Ladoux, B.; Yap, A.S.; Fanning, A.S.; Peifer, M. Remodeling the zonula adherens in response to tension and the role of afadin in this response. J. Cell Biol. 2016, 213, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Hatte, G.; Prigent, C.; Tassan, J.P. Tight junctions negatively regulate mechanical forces applied to adherens junctions in vertebrate epithelial tissue. J. Cell Sci. 2018, 131, jcs208736. [Google Scholar] [CrossRef] [PubMed]
- Skamrahl, M.; Pang, H.; Ferle, M.; Gottwald, J.; Rubeling, A.; Maraspini, R.; Honigmann, A.; Oswald, T.A.; Janshoff, A. Tight Junction ZO Proteins Maintain Tissue Fluidity, Ensuring Efficient Collective Cell Migration. Adv. Sci. 2021, 8, e2100478. [Google Scholar] [CrossRef] [PubMed]
- Dugina, V.B.; Shagieva, G.S.; Shakhov, A.S.; Alieva, I.B. The Cytoplasmic Actins in the Regulation of Endothelial Cell Function. Int. J. Mol. Sci. 2021, 22, 7836. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Zeiher, A.M. Nitric oxide-an endothelial cell survival factor. Cell Death Differ. 1999, 6, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.N. Chemistry of nitric oxide and related species. Methods Enzymol. 2008, 436, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Maeng, H.J.; Bang, Y.J.; Chung, S.J. Functional impairment of P-glycoprotein by sodium nitroprusside pretreatment in mouse brain capillary endothelial cells. Arch. Pharm. Res. 2012, 35, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Buzinari, T.C.; Oishi, J.C.; De Moraes, T.F.; Vatanabe, I.P.; Selistre-de-Araujo, H.S.; Pestana, C.R.; Rodrigues, G.J. Treatment with sodium nitroprusside improves the endothelial function in aortic rings with endothelial dysfunction. Eur. J. Pharm. Sci. 2017, 105, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Freischlag, J.A.; Lesniak, R.; Kelly, H.; Mudaliar, J.H.; Cambria, R.A.; Seabrook, M.D.; Towne, J.B. Endothelial damage due to ischemia and reperfusion is prevented with SIN-1. Cardiovasc. Surg. 1998, 6, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, B.; Subelzu, N.; Calcerrada, P.; Straliotto, M.R.; Piacenza, L.; Cassina, A.; Rocha, J.B.; Radi, R.; de Bem, A.F.; Peluffo, G. Impact of SIN-1-derived peroxynitrite flux on endothelial cell redox homeostasis and bioenergetics: Protective role of diphenyl diselenide via induction of peroxiredoxins. Free Radic. Res. 2015, 49, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Polte, T.; Oberle, S.; Schroder, H. The nitric oxide donor SIN-1 protects endothelial cells from tumor necrosis factor-alpha-mediated cytotoxicity: Possible role for cyclic GMP and heme oxygenase. J. Mol. Cell. Cardiol. 1997, 29, 3305–3310. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Tom, B.; Dendorfer, A.; Danser, A.H. Bradykinin, angiotensin-(1-7), and ACE inhibitors: How do they interact? Int. J. Biochem. Cell Biol. 2003, 35, 792–801. [Google Scholar] [CrossRef] [PubMed]
- El-Ashmawy, N.E.; Khedr, N.F.; El-Bahrawy, H.A.; Hamada, O.B. Anti-inflammatory and Antioxidant Effects of Captopril Compared to Methylprednisolone in L-Arginine-Induced Acute Pancreatitis. Dig. Dis. Sci. 2018, 63, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Caetano, E.S.P.; Mattioli, S.V.; da Silva, M.L.S.; Martins, L.Z.; Almeida, A.A.; da Rocha, A.L.V.; Nunes, P.R.; Grandini, N.A.; Correa, C.R.; Zochio, G.P.; et al. Sildenafil attenuates oxidative stress and endothelial dysfunction in lead-induced hypertension. Basic Clin. Pharmacol. Toxicol. 2023, 133, 142–155. [Google Scholar] [CrossRef] [PubMed]
- McRae, M.; LaFratta, L.M.; Nguyen, B.M.; Paris, J.J.; Hauser, K.F.; Conway, D.E. Characterization of cell-cell junction changes associated with the formation of a strong endothelial barrier. Tissue Barriers 2018, 6, e1405774. [Google Scholar] [CrossRef] [PubMed]
- Felinski, E.A.; Cox, A.E.; Phillips, B.E.; Antonetti, D.A. Glucocorticoids induce transactivation of tight junction genes occludin and claudin-5 in retinal endothelial cells via a novel cis-element. Exp. Eye Res. 2008, 86, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Cui, S.; Wang, J.; Xu, T.; Du, H.; Yue, H.; Ye, H.; Guo, J.; Zhang, J.; Li, P.; et al. Early Progression of Abdominal Aortic Aneurysm is Decelerated by Improved Endothelial Barrier Function via ALDH2-LIN28B-ELK3 Signaling. Adv. Sci. 2023, 10, e2302231. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Oh, I.S.; Park, S.M.; Kim, H.G. Sildenafil citrate induces migration of mouse aortic endothelial cells and proteinase secretion. Biotechnol. Bioprocess Eng. 2006, 11, 402–407. [Google Scholar] [CrossRef]
- Mailloux, A.; Deslandes, B.; Vaubourdolle, M.; Baudin, B. Captopril and enalaprilat decrease antioxidant defences in human endothelial cells and are unable to protect against apoptosis. Cell Biol. Int. 2003, 27, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Prasain, N.; Stevens, T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc. Res. 2009, 77, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Shasby, D.M.; Shasby, S.S.; Sullivan, J.M.; Peach, M.J. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ. Res. 1982, 51, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Tornavaca, O.; Chia, M.; Dufton, N.; Almagro, L.O.; Conway, D.E.; Randi, A.M.; Schwartz, M.A.; Matter, K.; Balda, M.S. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J. Cell Biol. 2015, 208, 821–838. [Google Scholar] [CrossRef] [PubMed]
- Fanning, A.S.; Jameson, B.J.; Jesaitis, L.A.; Anderson, J.M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998, 273, 29745–29753. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, W.J.; Choi, Y.K.; Song, H.S.; Son, M.J.; Gelman, I.H.; Kim, Y.J.; Kim, K.W. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat. Med. 2003, 9, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, Y.; Li, Y.; Niu, Y.; Hu, Z.; Deng, C.; Chen, Y.; Hu, B.; Huang, Y.; Deng, X. Delayed Administration of IGFBP7 Improved Bone Defect Healing via ZO-1 Dependent Vessel Stabilization. Adv. Sci. 2025, 12, e2406965. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Tsukita, S.; Yamazaki, Y.; Sugimoto, H. Rho GTP exchange factor ARHGEF11 regulates the integrity of epithelial junctions by connecting ZO-1 and RhoA-myosin II signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 9905–9910. [Google Scholar] [CrossRef] [PubMed]
- Paik, N.Y.; Neethling, J.; Anwar, M.; Gupta, P.; Sanborn, M.A.; Shen, Z.; Bandara, T.; Hyun, J.; Naiche, L.A.; Kitajewski, J.K.; et al. Notch transcriptional target tmtc1 maintains vascular homeostasis. Cell Mol. Life Sci. 2024, 81, 370. [Google Scholar] [CrossRef] [PubMed]
- Kural, M.H.; Wang, J.; Gui, L.; Yuan, Y.; Li, G.; Leiby, K.L.; Quijano, E.; Tellides, G.; Saltzman, W.M.; Niklason, L.E. Fas ligand and nitric oxide combination to control smooth muscle growth while sparing endothelium. Biomaterials 2019, 212, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Sakai, S.; Dewachter, L.; Dewachter, C.; Rondelet, B.; Naeije, R.; Ieda, M. Prostacyclin receptor agonists induce DUSP1 to inhibit pulmonary artery smooth muscle cell proliferation. Life Sci. 2023, 315, 121372. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.; Savion, N.; Gospodarowicz, D.; Shuman, M.A. Effect of cell density on thrombin binding to a specific site on bovine vascular endothelial cells. J. Cell Biol. 1981, 90, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Benz, P.M.; Blume, C.; Moebius, J.; Oschatz, C.; Schuh, K.; Sickmann, A.; Walter, U.; Feller, S.M.; Renne, T. Cytoskeleton assembly at endothelial cell-cell contacts is regulated by alphaII-spectrin-VASP complexes. J. Cell Biol. 2008, 180, 205–219. [Google Scholar] [CrossRef] [PubMed]


| Reagent or Antibody | Source | Identifier |
|---|---|---|
| SNP | KeyGen BioTECH (Nanjing, China) | Cat#KGR0017 |
| SIN-1 | Cayman Chemical (Ann Arbor, MI, USA) | Cat#82220 |
| SNAP | Cayman Chemical (Ann Arbor, MI, USA) | Cat#82250 |
| Hydrocortisone | MCE (Monmouth Junction, NJ, USA) | Cat#HY-N0583 |
| Captopril | Sigma-Aldrich (St. Louis, MO, USA) | Cat#C8856 |
| Sildenafil | Solarbio (Beijing, China) | Cat#6070 |
| pCPT-cAMP | Sigma-Aldrich (St. Louis, MO, USA) | Cat#C3912 |
| Dylight-554 Phalloidin | CST (Danvers, MA, USA) | Cat#13054S |
| ZO-1 antibody | Abcam (Cambridge, MA, USA) | Cat#ab221547 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Zhou, X.; Liu, X.; Liu, C.; Zhang, J.; Dong, L. Combined Pharmacological Conditioning of Endothelial Cells for Improved Vascular Graft Endothelialization. Int. J. Mol. Sci. 2025, 26, 7183. https://doi.org/10.3390/ijms26157183
Lu Z, Zhou X, Liu X, Liu C, Zhang J, Dong L. Combined Pharmacological Conditioning of Endothelial Cells for Improved Vascular Graft Endothelialization. International Journal of Molecular Sciences. 2025; 26(15):7183. https://doi.org/10.3390/ijms26157183
Chicago/Turabian StyleLu, Zhiyao, Xuqian Zhou, Xiaowen Liu, Chunyan Liu, Junfeng Zhang, and Lei Dong. 2025. "Combined Pharmacological Conditioning of Endothelial Cells for Improved Vascular Graft Endothelialization" International Journal of Molecular Sciences 26, no. 15: 7183. https://doi.org/10.3390/ijms26157183
APA StyleLu, Z., Zhou, X., Liu, X., Liu, C., Zhang, J., & Dong, L. (2025). Combined Pharmacological Conditioning of Endothelial Cells for Improved Vascular Graft Endothelialization. International Journal of Molecular Sciences, 26(15), 7183. https://doi.org/10.3390/ijms26157183





