Lupeol Attenuates Oxysterol-Induced Dendritic Cell Activation Through NRF2-Mediated Antioxidant and Anti-Inflammatory Effects
Abstract
1. Introduction
2. Results
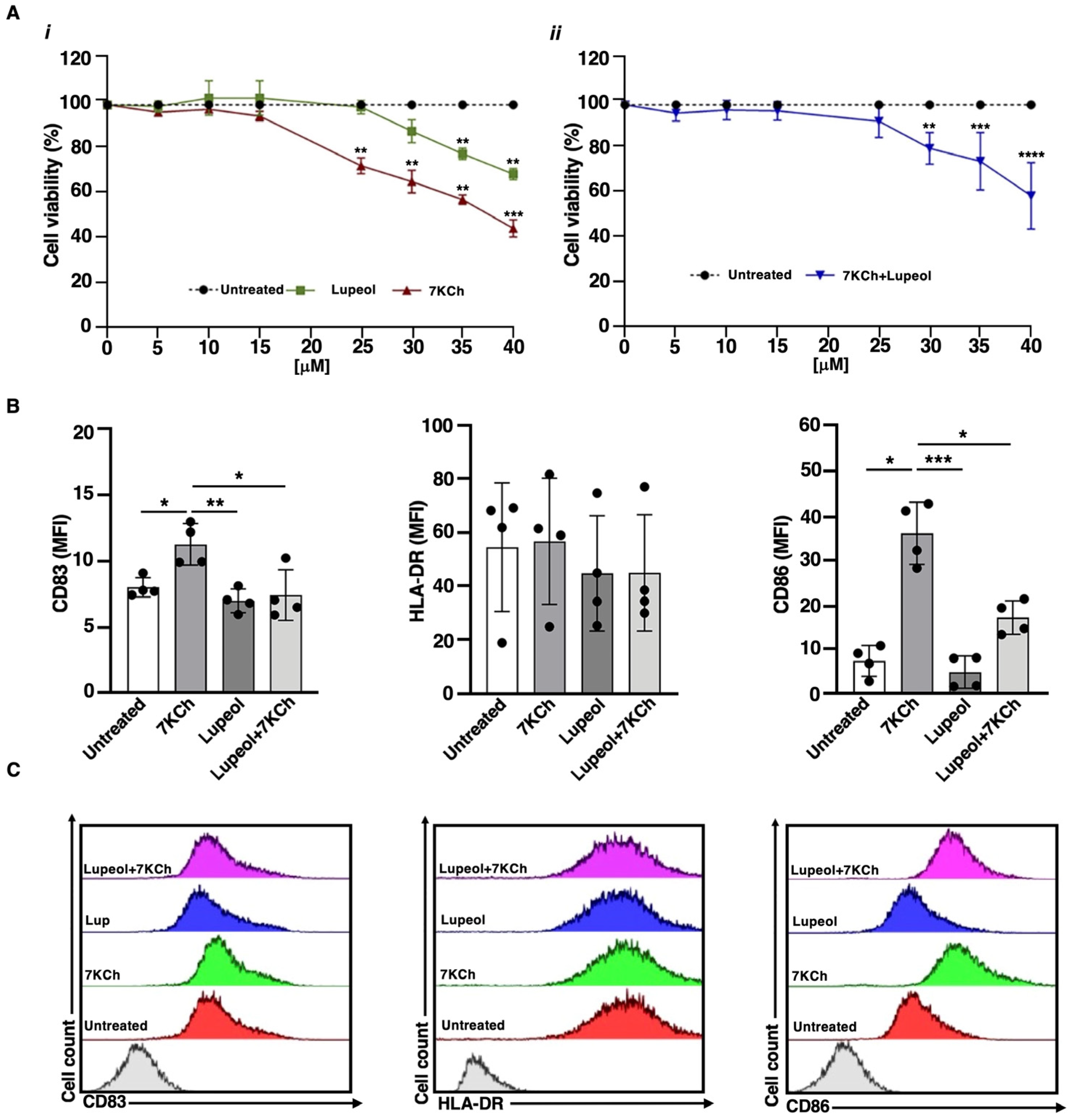
2.1. Lupeol Induces Tolerogenic DCs and Counteracts the 7KCh-Inflammatory Activity
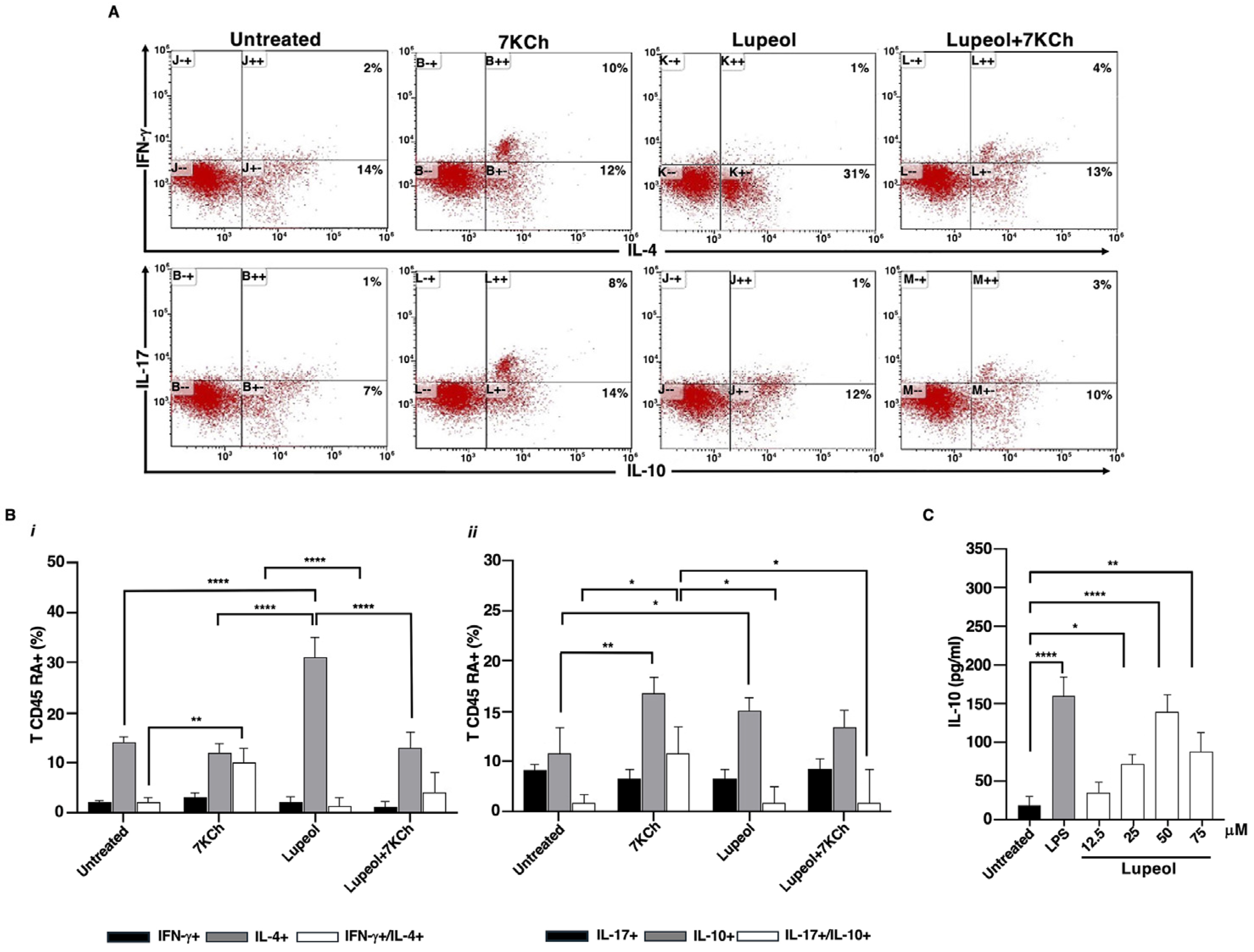
2.2. Lupeol-Treated iDCs Prime Naïve T-Cells into Anti-Inflammatory and Regulatory Cells
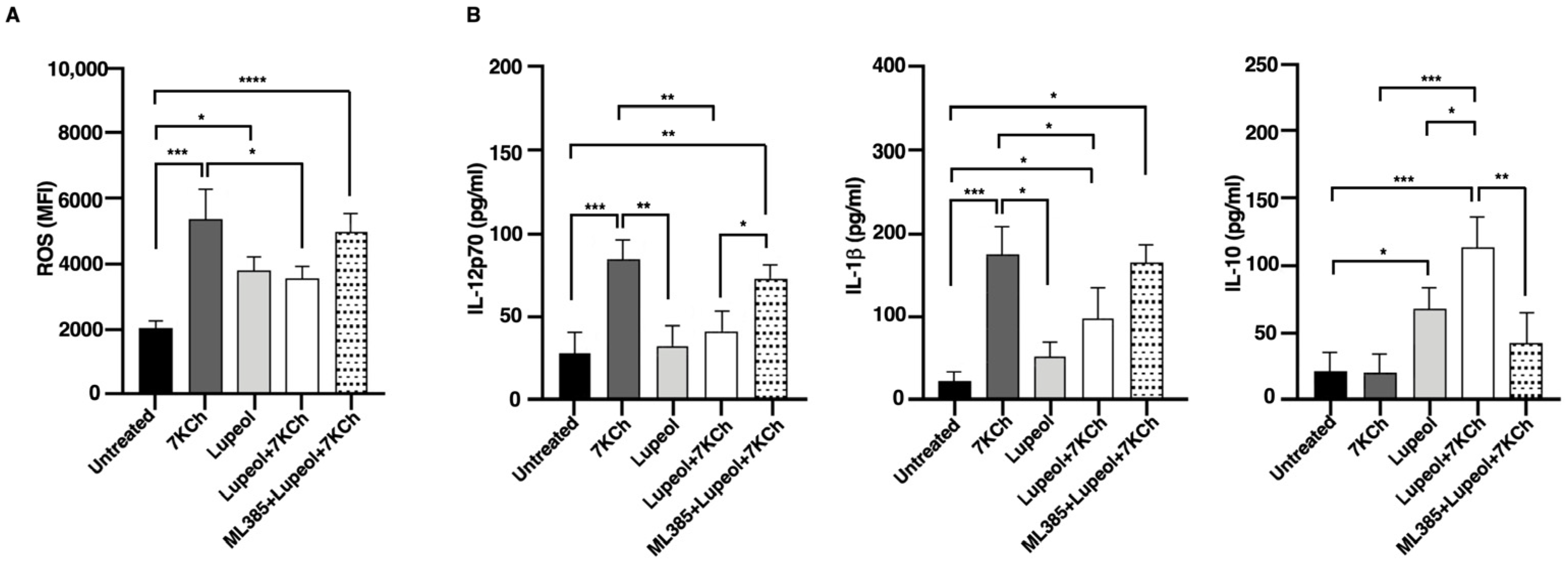
2.3. Lupeol Modulates Oxidative Stress and Cytokine Production in Dendritic Cells via NRF2 Activation
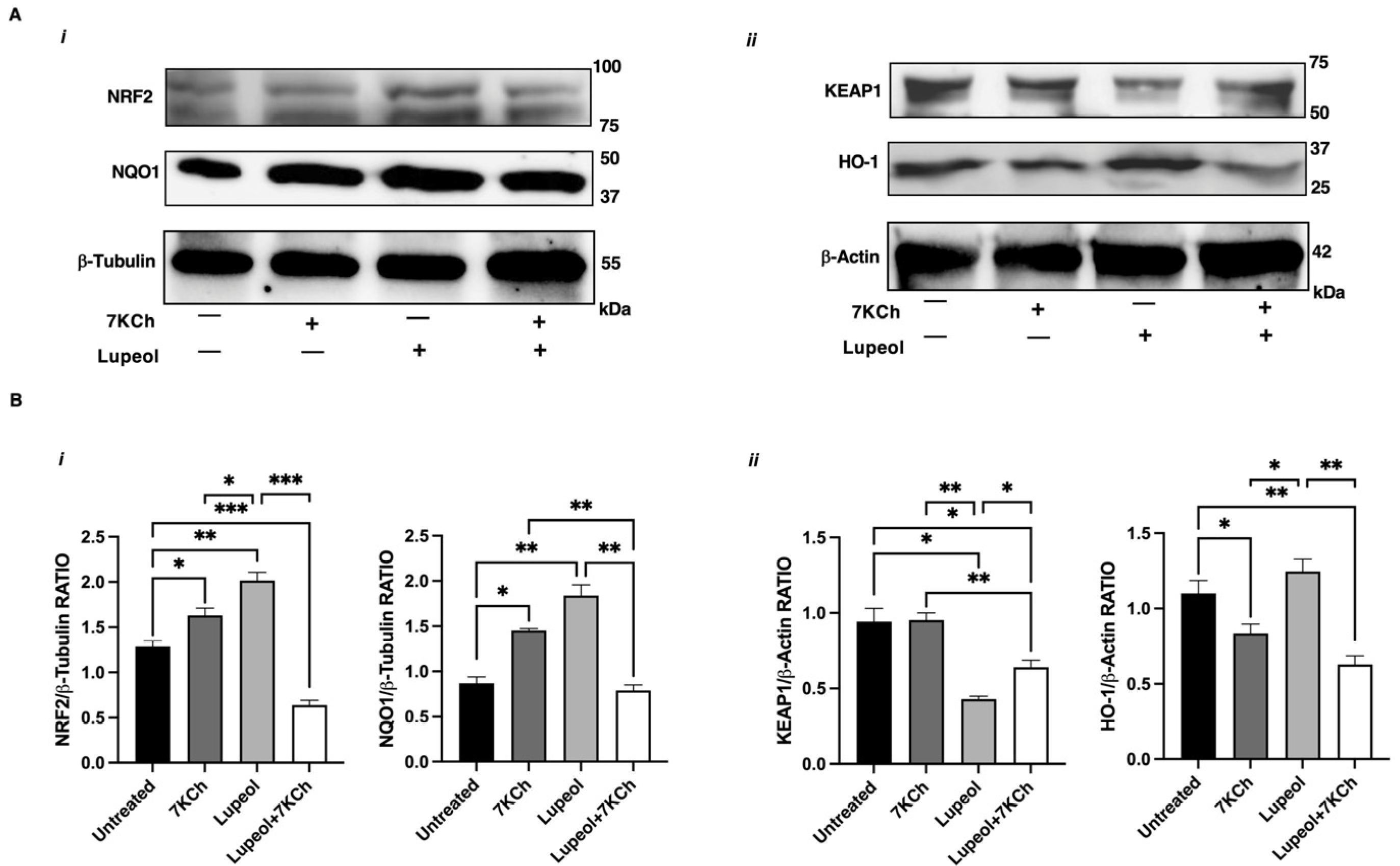
2.4. NRF2 Activation Underlies Lupeol Modulation of DC Maturation
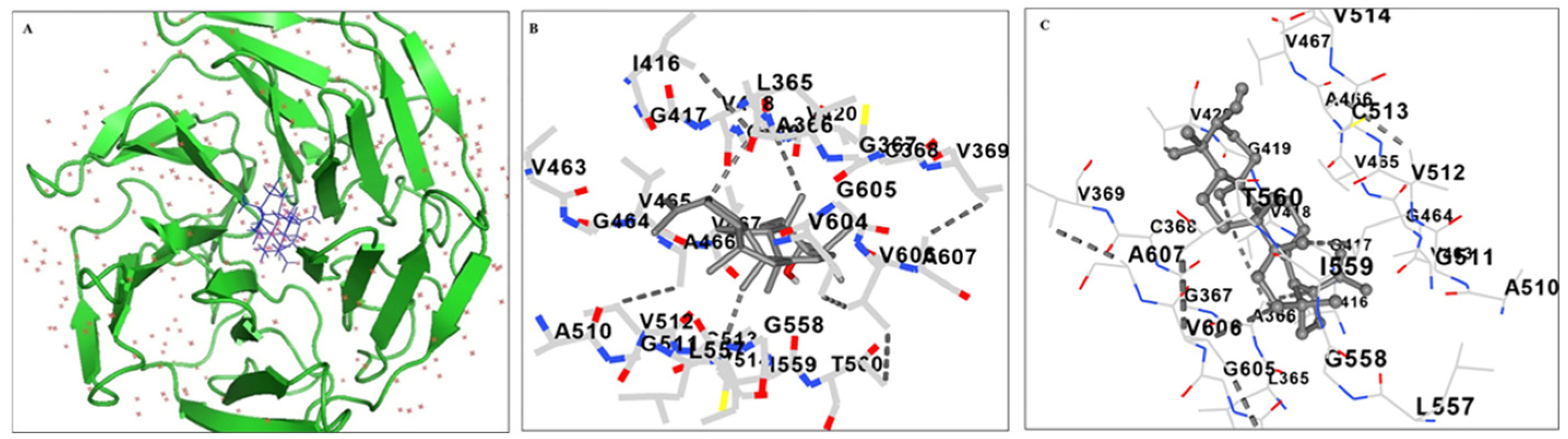
2.5. Molecular Docking Analysis of Lupeol with Kelch Domain of Keap1 Protein
3. Discussion
4. Materials and Methods
4.1. In Vitro Culture of Human Monocyte-Derived Dendritic Cells, T Lymphocytes and Treatments
4.2. Flow Cytometric Analysis of Phenotypic DC Maturation
4.3. Flow Cytometric Analysis of Reactive Oxygen Species (ROS) Production
4.4. T-Cell Priming Assay
4.5. Cytokine Secretion Analysis in Dendritic Cell Culture Supernatants
4.6. Evaluation of NRF2/NQO1/HO-1 Pathway
4.6.1. Indirect Immunofluorescence Labeling
4.6.2. Western Blot Analysis of Dendritic Cell Lysates
4.7. Molecular Docking Studies
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| APC | Antigen-Presenting Cell |
| CD | Cluster of Differentiation |
| DC | Dendritic Cell |
| FACS | Fluorescence-Activated Cell Sorting |
| H2DCF-DA | 2’,7’-Dichlorodihydrofluorescein Diacetate |
| HLA-DR | Human Leukocyte Antigen—DR isotype |
| HO-1 | Heme Oxygenase-1 |
| iDC | Immature Dendritic Cell |
| IFN-γ | Interferon Gamma |
| IL | Interleukin |
| KEAP1 | Kelch-like ECH-associated Protein 1 |
| LPS | Lipopolysaccharide |
| Lup | Lupeol |
| MFI | Mean Fluorescence Intensity |
| ML385 | NRF2 Inhibitor (specific small molecule inhibitor of NRF2 activity) |
| mTOR | Mechanistic Target of Rapamycin |
| NRF2 | Nuclear Factor Erythroid 2–Related Factor 2 |
| NQO1 | NAD(P)H Quinone Dehydrogenase 1 |
| ROS | Reactive Oxygen Species |
| SD | Standard Deviation |
| Treg | Regulatory T Cell |
| Th1/Th2/Th17 | T Helper Cell Subtypes 1, 2, and 17 |
| TolDC | Tolerogenic Dendritic Cell |
| t-BHQ | Tertbutylhydroquinone |
| 7KCh | 7-Ketocholesterol |
References
- Hayter, S.M.; Cook, M.C. Updated Assessment of the Prevalence, Spectrum and Case Definition of Autoimmune Disease. Autoimmun. Rev. 2012, 11, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Duc, D.; Vigne, S.; Pot, C. Oxysterols in Autoimmunity. Int. J. Mol. Sci. 2019, 20, 4522. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.M.; Brachova, L.; Higgins, K.; Obermiller, L.; Sevanian, A.; Khandrika, S.; Reaven, P.D. Induction of Monocyte Differentiation and Foam Cell Formation in Vitro by 7-Ketocholesterol. J. Lipid Res. 2002, 43, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Perrin-Cocon, L.; Coutant, F.; Agaugué, S.; Deforges, S.; André, P.; Lotteau, V. Oxidized Low-Density Lipoprotein Promotes Mature Dendritic Cell Transition from Differentiating Monocyte. J. Immunol. 2001, 167, 3785–3791. [Google Scholar] [CrossRef] [PubMed]
- Ates, I.; Yılmaz, A.D.; Buttari, B.; Arese, M.; Saso, L.; Suzen, S. A Review of the Potential of Nuclear Factor [Erythroid-Derived 2]-like 2 Activation in Autoimmune Diseases. Brain Sci. 2023, 13, 1532. [Google Scholar] [CrossRef] [PubMed]
- Lilamand, M.; Kelaiditi, E.; Guyonnet, S.; Antonelli Incalzi, R.; Raynaud-Simon, A.; Vellas, B.; Cesari, M. Flavonoids and Arterial Stiffness: Promising Perspectives. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Roşian, Ş.H.; Boarescu, I.; Boarescu, P.M. Antioxidant and Anti-Inflammatory Effects of Bioactive Compounds in Atherosclerosis. Int. J. Mol. Sci. 2025, 26, 1379. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid-Lowering Nutraceuticals in Clinical Practice: Position Paper from an International Lipid Expert Panel. Nutr. Rev. 2017, 75, 731–767. [Google Scholar] [CrossRef] [PubMed]
- Nazakat, L.; Ali, S.; Summer, M.; Nazakat, F.; Noor, S.; Riaz, A. Pharmacological Modes of Plant-Derived Compounds for Targeting Inflammation in Rheumatoid Arthritis: A Comprehensive Review on Immunomodulatory Perspective. Inflammopharmacology 2025, 33, 1537–1581. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Singh, G.; Datusalia, A.K. Potential Therapeutic Applications of Phytoconstituents as Immunomodulators: Pre-Clinical and Clinical Evidences. Phyther. Res. 2021, 35, 3702–3731. [Google Scholar] [CrossRef] [PubMed]
- Buttari, B.; Profumo, E.; Segoni, L.; D’Arcangelo, D.; Rossi, S.; Facchiano, F.; Saso, L.; Businaro, R.; Iuliano, L.; Riganò, R. Resveratrol Counteracts Inflammation in Human M1 and M2 Macrophages upon Challenge with 7-Oxo-Cholesterol: Potential Therapeutic Implications in Atherosclerosis. Oxid. Med. Cell. Longev. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Culletta, G.; Buttari, B.; Arese, M.; Brogi, S.; Almerico, A.M.; Saso, L.; Tutone, M. Natural Products as Non-Covalent and Covalent Modulators of the KEAP1/NRF2 Pathway Exerting Antioxidant Effects. Eur. J. Med. Chem. 2024, 270, 116355. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Profumo, E.; Togna, A.R.; Riganò, R.; Saso, L.; Buttari, B. Lupeol Counteracts the Proinflammatory Signalling Triggered in Macrophages by 7-Keto-Cholesterol: New Perspectives in the Therapy of Atherosclerosis. Oxid. Med. Cell. Longev. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Saurin, S.; Meineck, M.; Erkel, G.; Opatz, T.; Weinmann-Menke, J.; Pautz, A. Drug Candidates for Autoimmune Diseases. Pharmaceuticals 2022, 15, 503. [Google Scholar] [CrossRef] [PubMed]
- Umbreen, H.; Zhang, X.; Tang, K.T.; Lin, C.C. Regulation of Myeloid Dendritic Cells by Synthetic and Natural Compounds for the Treatment of Rheumatoid Arthritis. Int. J. Mol. Sci. 2023, 24, 238. [Google Scholar] [CrossRef] [PubMed]
- del Cornò, M.; Scazzocchio, B.; Masella, R.; Gessani, S. Regulation of Dendritic Cell Function by Dietary Polyphenols. Crit. Rev. Food Sci. Nutr. 2016, 56, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Aldahlawi, A. Modulation of Dendritic Cell Immune Functions by Plant Components. J. Microsc. Ultrastruct. 2016, 4, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of Dendritic Cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Kleijwegt, F.S.; Laban, S.; Duinkerken, G.; Joosten, A.M.; Koeleman, B.P.C.; Nikolic, T.; Roep, B.O. Transfer of Regulatory Properties from Tolerogenic to Proinflammatory Dendritic Cells via Induced Autoreactive Regulatory T Cells. J. Immunol. 2011, 187, 6357–6364. [Google Scholar] [CrossRef] [PubMed]
- Hubo, M.; Trinschek, B.; Kryczanowsky, F.; Tuettenberg, A.; Steinbrink, K.; Jonuleit, H. Costimulatory Molecules on Immunogenic versus Tolerogenic Human Dendritic Cells. Front. Immunol. 2013, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Rutella, S.; Danese, S.; Leone, G. Tolerogenic Dendritic Cells: Cytokine Modulation Comes of Age. Blood 2006, 108, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- García-González, P.; Ubilla-Olguín, G.; Catalán, D.; Schinnerling, K.; Aguillón, J.C. Tolerogenic Dendritic Cells for Reprogramming of Lymphocyte Responses in Autoimmune Diseases. Autoimmun. Rev. 2016, 15, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.J.; Ahl, P.J.; Connolly, J.E. Metabolism Is Central to Tolerogenic Dendritic Cell Function. Mediat. Inflamm. 2016, 2016, 2636701. [Google Scholar] [CrossRef] [PubMed]
- Sozzani, S.; Del Prete, A.; Bosisio, D. Dendritic Cell Recruitment and Activation in Autoimmunity. J. Autoimmun. 2017, 85, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.T.; Frazee, C.S.; Herman, P.J.; Chen, S.; Chen, A.; Kuo, Y.; Ellebrecht, C.T. Current Advancements in Cellular Immunotherapy for Autoimmune Disease. Semin. Immunopathol. 2025, 47, 7. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.K.; Fitzgerald, H.K.; Fletcher, J.M.; Dunne, A. Plant-Derived Polyphenols Modulate Human Dendritic Cell Metabolism and Immune Function via AMPK-Dependent Induction of Heme Oxygenase-1. Front. Immunol. 2019, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Verna, G.; Liso, M.; Salviati, E.; Esposito, T.; Carbone, D.; Pecoraro, C.; Chieppa, M.; Campiglia, P. Hop-Derived Fraction Rich in Beta Acids and Prenylflavonoids Regulates the Inflammatory Response in Dendritic Cells Differently from Quercetin: Unveiling Metabolic Changes by Mass Spectrometry-Based Metabolomics. Food Funct. 2021, 12, 12800–12811. [Google Scholar] [CrossRef] [PubMed]
- Alba, G.; Dakhaoui, H.; Santa-Maria, C.; Palomares, F.; Cejudo-Guillen, M.; Geniz, I.; Sobrino, F.; Montserrat-de la Paz, S.; Lopez-Enriquez, S. Nutraceuticals as Potential Therapeutic Modulators in Immunometabolism. Nutrients 2023, 15, 411. [Google Scholar] [CrossRef] [PubMed]
- Rao, Q.; Ma, G.-C.; Wu, H.; Li, M.; Xu, W.; Wang, G.-J.; Wang, D.; Zhang, C.-E.; Ma, Z.-J.; Zhang, Z.-T. Dendritic Cell Combination Therapy Reduces the Toxicity of Triptolide and Ameliorates Colitis in Murine Models. Drug Deliv. 2022, 29, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Min, S.Y.; Yan, M.; Kim, S.B.; Ravikumar, S.; Kwon, S.R.; Vanarsa, K.; Kim, H.Y.; Davis, L.S.; Mohan, C. Green Tea Epigallocatechin-3-Gallate Suppresses Autoimmune Arthritis through Indoleamine-2,3-Dioxygenase Expressing Dendritic Cells and the Nuclear Factor, Erythroid 2-like 2 Antioxidant Pathway. J. Inflamm. 2015, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Kumar Das, S.; Ghosh, N.; Sinha, K.; Sil, P.C. Lupeol: A Dietary and Medicinal Triterpene with Therapeutic Potential. Biochem. Pharmacol. 2024, 229, 116545. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Khan, A.; Lee, H.J.; Rehman, I.U.; Khan, I.; Alam, S.I.; Kim, M.O. Lupeol, a Plant-Derived Triterpenoid, Protects Mice Brains against Aβ-Induced Oxidative Stress and Neurodegeneration. Biomedicines 2020, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Kim, S.M.; Lee, S.A.; Eo, S.K.; Kim, K. Oxysterols Induce Transition of Monocytic Cells to Phenotypically Mature Dendritic Cell-like Cells. Biochem. Biophys. Res. Commun. 2013, 438, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Vigne, S.; Chalmin, F.; Duc, D.; Clottu, A.S.; Apetoh, L.; Lobaccaro, J.M.A.; Christen, I.; Zhang, J.; Pot, C. IL-27-Induced Type 1 Regulatory T-Cells Produce Oxysterols That Constrain IL-10 Production. Front. Immunol. 2017, 8, 1184. [Google Scholar] [CrossRef] [PubMed]
- Stadhouders, R.; Lubberts, E.; Hendriks, R.W. A Cellular and Molecular View of T Helper 17 Cell Plasticity in Autoimmunity. J. Autoimmun. 2018, 87, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Maggi, L.; Liotta, F.; Cosmi, L.; Annunziato, F. Biological and Clinical Significance of T Helper 17 Cell Plasticity. Immunology 2019, 158, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Soroosh, P.; Wu, J.; Xue, X.; Song, J.; Sutton, S.W.; Sablad, M.; Yu, J.; Nelen, M.I.; Liu, X.; Castro, G.; et al. Oxysterols Are Agonist Ligands of RORγt and Drive Th17 Cell Differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 12163–12168. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Ikado, K.; Shindo, K.; Koizumi, H.; Fujiwara, D. Identification of 14-Dehydroergosterol as a Novel Anti-Inflammatory Compound Inducing Tolerogenic Dendritic Cells. Sci. Rep. 2017, 7, 13903. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the Immune System. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.B.; Kleijwegt, F.S.; Waelkens, E.; Lage, K.; Nikolic, T.; Hansen, D.A.; Workman, C.T.; Roep, B.O.; Overbergh, L.; Mathieu, C. Differential Protein Pathways in 1,25-Dihydroxyvitamin D 3 and Dexamethasone Modulated Tolerogenic Human Dendritic Cells. J. Proteome Res. 2012, 11, 941–971. [Google Scholar] [CrossRef] [PubMed]
- Švajger, U.; Rožman, P.J. Recent Discoveries in Dendritic Cell Tolerance-Inducing Pharmacological Molecules. Int. Immunopharmacol. 2020, 81, 106275. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Wu, Z.; Xing, C.; Miao, Z. Small Molecules Inhibiting Keap1-Nrf2 Protein-Protein Interactions: A Novel Approach to Activate Nrf2 Function. Medchemcomm 2017, 8, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.H.; Chen, H.A.; Gau, R.J.; Yen, J.H.; Suen, J.L. Heme Oxygenase-1-Expressing Dendritic Cells Promote Foxp3+ Regulatory T Cell Differentiation and Induce Less Severe Airway Inflammation in Murine Models. PLoS ONE 2016, 11, e0168919. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xu, Z.; Fan, R.; Wang, C.; Ji, W.; Ma, Y.; Cai, W.; Zhang, Y.; Yang, N.; Zou, S.; et al. HO-1 Alleviates Cholesterol-Induced Oxidative Stress through Activation of Nrf2/ERK and Inhibition of PI3K/AKT Pathways in Endothelial Cells. Mol. Med. Rep. 2017, 16, 3519–3527. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yu, Y.; Sang, R.; Li, J.; Ge, B.; Zhang, X. Protective Effects of Taraxasterol against Ethanol-Induced Liver Injury by Regulating CYP2E1/Nrf2/HO-1 and NF-ΚB Signaling Pathways in Mice. Oxid. Med. Cell. Longev. 2018, 2018, 8284107. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Dai, Z.; Tang, W.; Liu, C.; Tang, B.; Sase, A. Astragaloside IV Alleviates Cerebral Ischemia-Reperfusion Injury Through NLRP3 Inflammasome-Mediated Pyroptosis Inhibition via Activating Nrf2. Oxid. Med. Cell. Longev. 2021, 2021, 9925561. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yin, R.; Wu, R.; Ramirez, C.N.; Sargsyan, D.; Li, S.; Wang, L.; Cheng, D.; Wang, C.; Hudlikar, R.; et al. DNA Methylome and Transcriptome Alterations and Cancer Prevention by Triterpenoid Ursolic Acid in UVB-Induced Skin Tumor in Mice. Mol. Carcinog. 2019, 58, 1738–1753. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Mackensen, A.; Mougiakakos, D. CAR T-Cell Therapy in Autoimmune Diseases. Lancet 2023, 402, 2034–2044. [Google Scholar] [CrossRef] [PubMed]
- Buttari, B.; Profumo, E.; Mattei, V.; Siracusano, A.; Ortona, E.; Margutti, P.; Salvati, B.; Sorice, M.; Rigano, R. Oxidized Beta(2)-Glycoprotein I Induces Human Dendritic Cell Maturation and Promotes a T Helper Type 1 Response. Blood 2005, 106, 3880–3887. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Avogadro, G.R.H. An Advanced Semantic Chemical Editor Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Delano, W.L. PyMOL: An Open-Source Molecular Graphics Tool. Available online: https://legacy.ccp4.ac.uk/newsletters/newsletter40/11_pymol.pdf (accessed on 22 July 2025).
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W.; et al. Calculating Structures and Free Energies of Complex Molecules: Combining Molecular Mechanics and Continuum Models. Acc. Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef] [PubMed]

| Energy (kcal/mol) | Kelch Domain-Lupeol |
|---|---|
| ΔEELE | −197.52 ± 11.48 |
| ΔEVDW | −134.43 ± 9.44 |
| ΔEGAS | −331.95 ± 14.62 |
| ΔGNPS | −25.10 ± 5.30 |
| ΔGPS | 301.04 ± 13.53 |
| ΔGSOL | 275.94 ± 16.42 |
| ΔGbinding | −56.01 ± 8.04 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, S.; Capozzi, A.; Profumo, E.; Alessandri, C.; Sorice, M.; Saso, L.; Buttari, B. Lupeol Attenuates Oxysterol-Induced Dendritic Cell Activation Through NRF2-Mediated Antioxidant and Anti-Inflammatory Effects. Int. J. Mol. Sci. 2025, 26, 7179. https://doi.org/10.3390/ijms26157179
Saha S, Capozzi A, Profumo E, Alessandri C, Sorice M, Saso L, Buttari B. Lupeol Attenuates Oxysterol-Induced Dendritic Cell Activation Through NRF2-Mediated Antioxidant and Anti-Inflammatory Effects. International Journal of Molecular Sciences. 2025; 26(15):7179. https://doi.org/10.3390/ijms26157179
Chicago/Turabian StyleSaha, Sarmistha, Antonella Capozzi, Elisabetta Profumo, Cristiano Alessandri, Maurizio Sorice, Luciano Saso, and Brigitta Buttari. 2025. "Lupeol Attenuates Oxysterol-Induced Dendritic Cell Activation Through NRF2-Mediated Antioxidant and Anti-Inflammatory Effects" International Journal of Molecular Sciences 26, no. 15: 7179. https://doi.org/10.3390/ijms26157179
APA StyleSaha, S., Capozzi, A., Profumo, E., Alessandri, C., Sorice, M., Saso, L., & Buttari, B. (2025). Lupeol Attenuates Oxysterol-Induced Dendritic Cell Activation Through NRF2-Mediated Antioxidant and Anti-Inflammatory Effects. International Journal of Molecular Sciences, 26(15), 7179. https://doi.org/10.3390/ijms26157179










