A Simultaneous Determination of the B1 and B6 Vitamers Reveals Their Loss During a Single Peritoneal Dialysis Session: Chromatographic and Chemometric Approach
Abstract
1. Introduction
2. Results and Discussion
2.1. Participant Characteristics
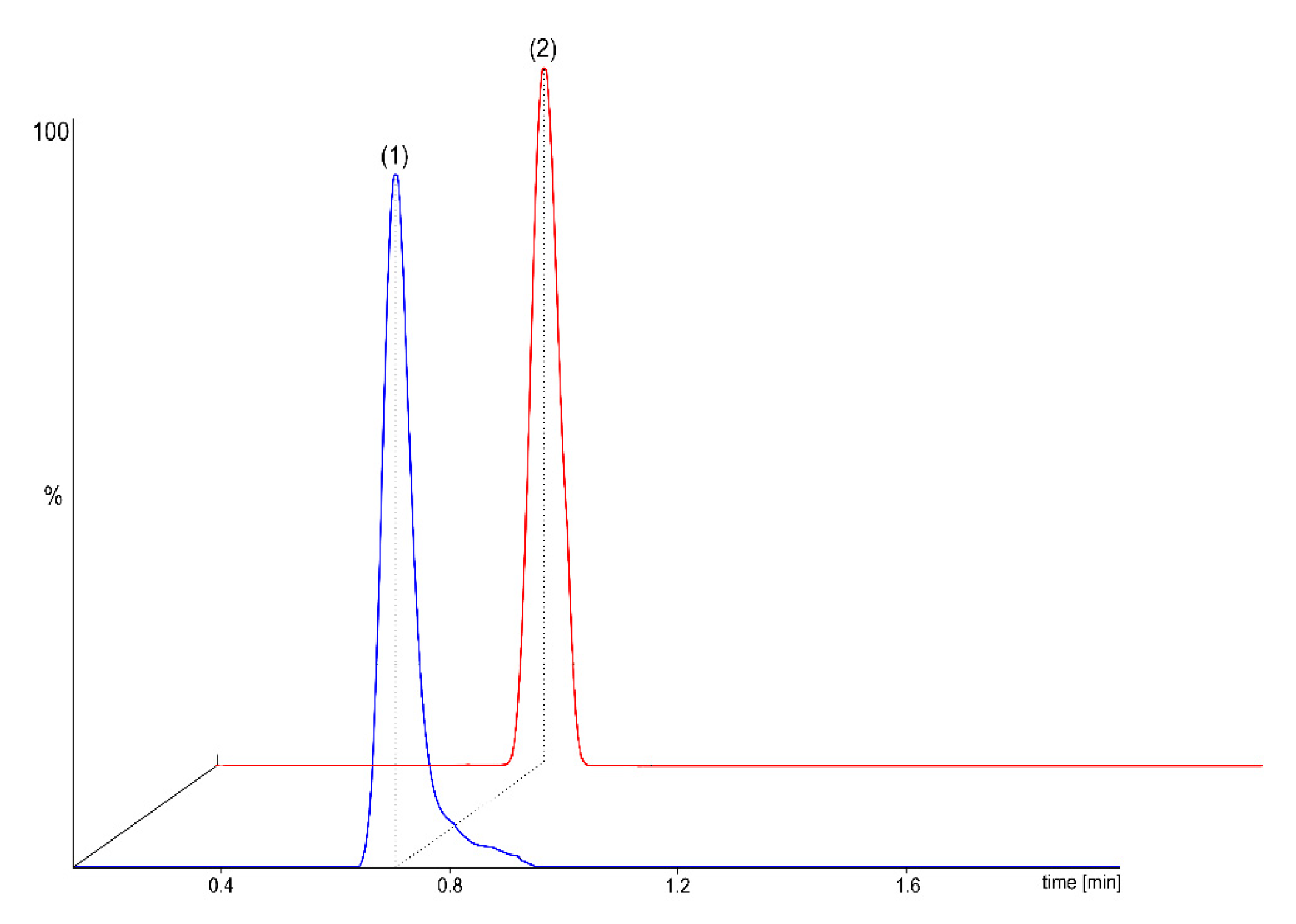
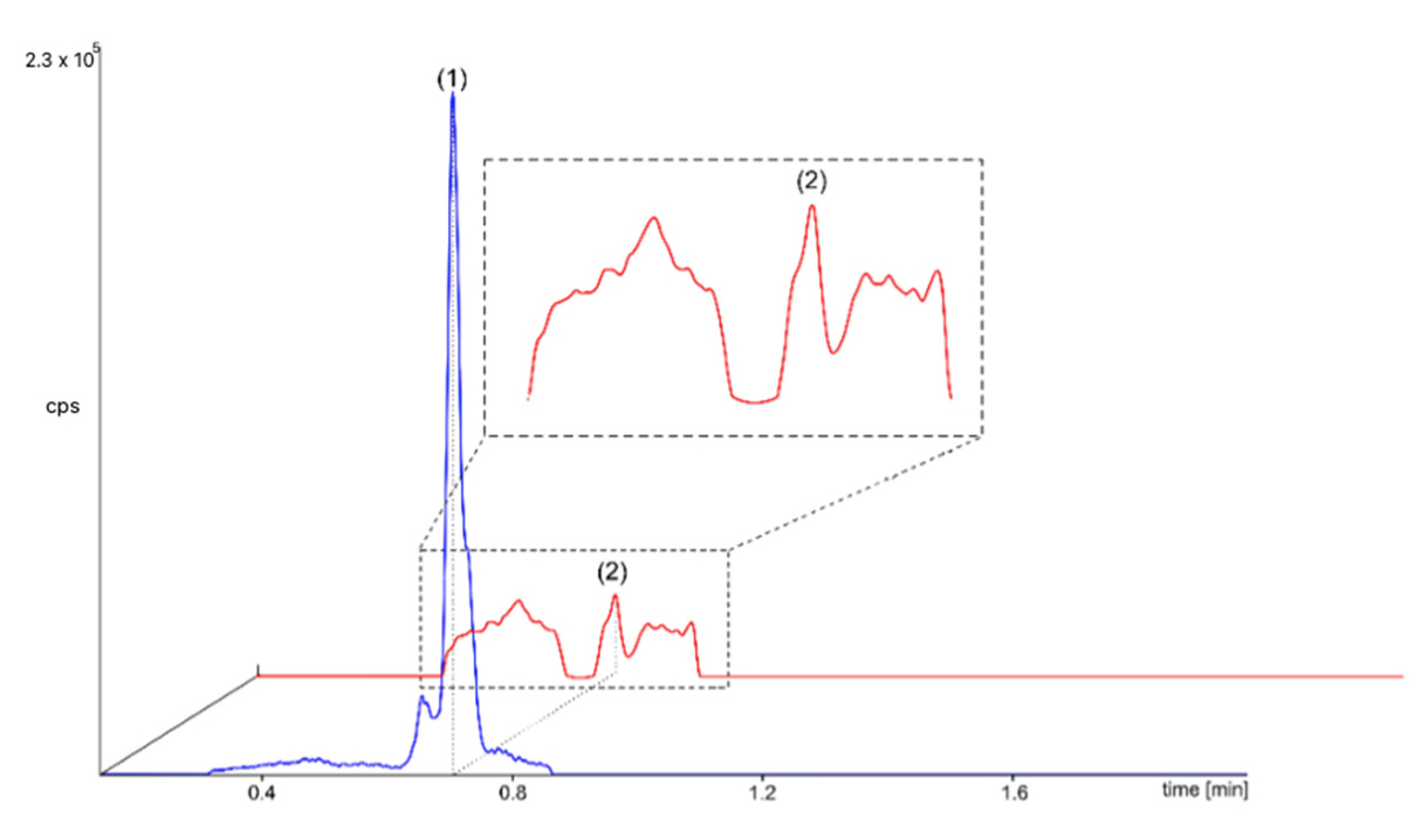
2.2. MS/MS Detection
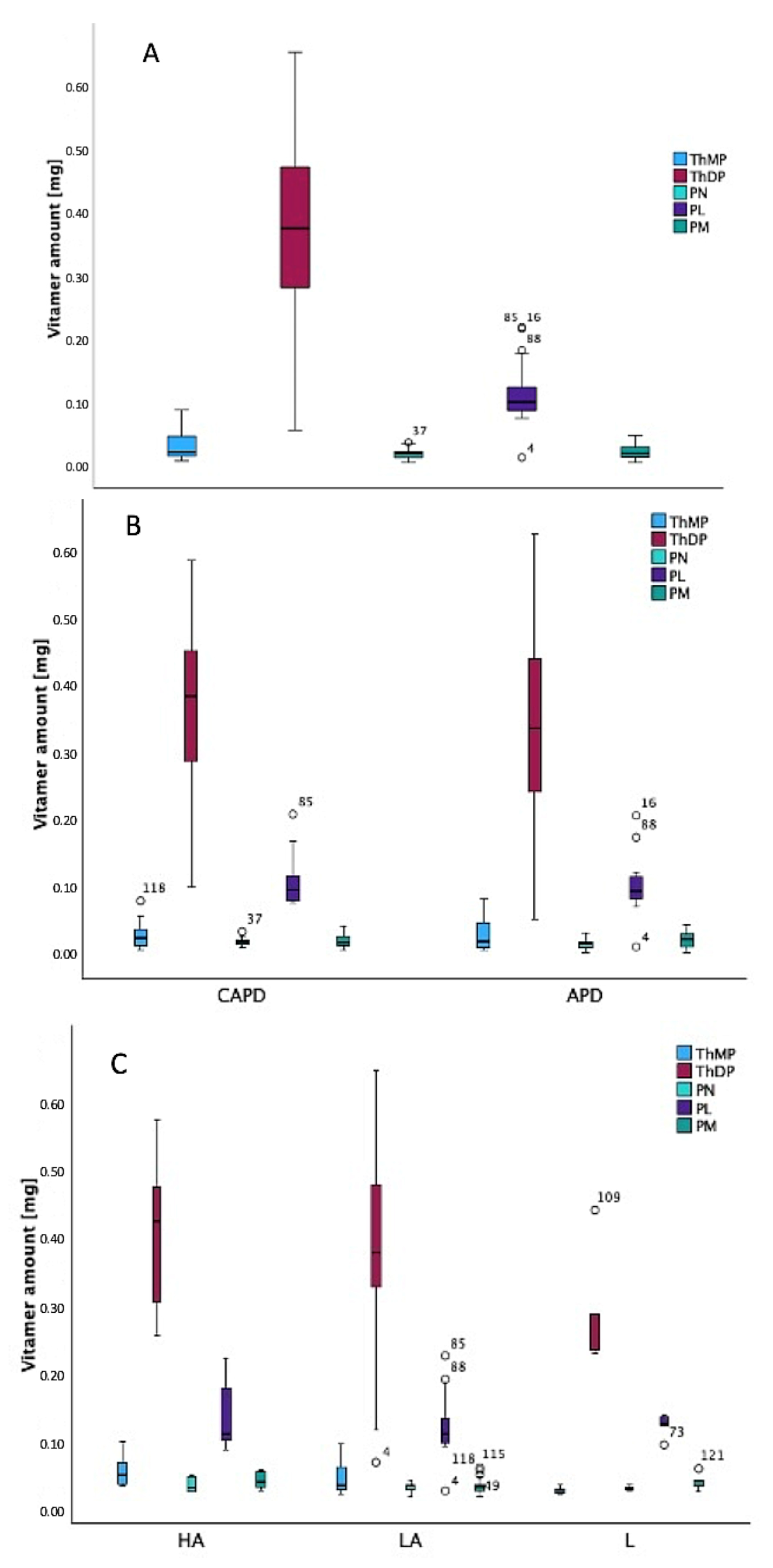
2.3. HPLC/FL Detection
2.3.1. Vitamer Standards Analysis Using Proposed HPLC/FL Assays
2.3.2. Dialysis Effluent Samples Analysis Using Proposed HPLC/FL Assays
2.4. Chemometric Analysis
3. Materials and Methods
3.1. Reagents and Standard Solutions
3.2. Ethical Statements
3.3. Participant Characteristics
3.4. Sample Preparation
3.5. Micro LC-MS/MS Conditions
3.6. HPLC/FL Conditions
3.7. HPLC/FL Method Validation
3.8. Statistical Analysis
4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APD | Automated peritoneal dialysis |
| CAPD | Continuous ambulatory peritoneal dialysis |
| HPLC/FL | High-performance liquid chromatography with fluorescence detection |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MRA | Multiple regression analysis |
| PET | Peritoneal equilibration test |
| PD | Peritoneal dialysis |
| PL | Pyridoxal |
| PLP | Pyridoxal 5-phosphate |
| PN | Pyridoxine |
| PM | Pyridoxamine |
| ThMP | Thiamine monophosphate |
| ThDP | Thiamine diphosphate |
References
- Daugirdas, J.T.; Blake, P.G.; Ing, T.S. Podręcznik Dializoterapii; Czelej: Lublin, Poland, 2008. [Google Scholar]
- Gokal, R.; Mallick, N.P. Peritoneal dialysis. Lancet 1999, 353, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Twardowski, Z.J. Peritoneal equilibration test. Perit. Dial. Bull. 1987, 7, 138–147. [Google Scholar]
- Wang, Y.M.; Afsar, R.E.; Sussman-Dabach, E.J.; White, J.A.; MacLaughlin, H.; Ikizler, T.A. Vitamin supplement use in patients with CKD: Worth the pill burden? Am. J. Kidney Dis. 2024, 83, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Tylicki, A.; Łotowski, Z.; Siemieniuk, M.; Ratkiewicz, A. Thiamine and selected thiamine antivitamins-biological activity and methods of synthesis. Biosci. Rep. 2018, 38, BRS20171148. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.C.; Hung, S.H.; Tarng, D.C.; Yang, W.C.; Chen, T.W.; Huang, T.P. Thiamine deficiency and unexplained encephalopathy in hemodialysis and peritoneal dialysis patients. Am. J. Kidney Dis. 2001, 38, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Rudnicki-Velasquez, P.B.; Storoniak, H.; Jagiełło, K.; Kreczko-Kurzawa, J.; Jankowska, M.; Krzymiński, K.J. Comparative studies on vitamin B1 deficiency in whole blood of chronically haemodialysed patients: Chromatographic, fluorimetric and PCA study. J. Chromatogr. B 2021, 1180, 122880. [Google Scholar] [CrossRef] [PubMed]
- Kosmadakis, G.; da Costa Correia, E.; Carceles, O.; Somda, F.; Aguilera, D. Vitamins in dialysis: Who, when and how much? Ren. Fail. 2014, 36, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, M.; Pichette, V.; Geadah, D.; Ouimet, D. Folic acid and pyridoxal-5′-phosphate losses during high-efficiency hemodialysis in patients without hydrosoluble vitamin supplementation. J. Ren. Nutr. 2000, 10, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Kasama, R.; Koch, T.; Canals-Navas, C.; Pitone, J.M. Vitamin B6 and hemodialysis: The impact of high-flux/high-efficiency dialysis and review of the literature. Am. J. Kidney Dis. 1996, 27, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Voelker, A.L.; Taylor, L.S.; Mauer, L.J. Effect of pH and concentration on the chemical stability and reaction kinetics of thiamine mononitrate and thiamine chloride hydrochloride in solution. BMC Chem. 2021, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F. The Vitamins: Fundamental Aspects in Nutrition and Health, 3rd ed.; Academic Press: Burlington, MA, USA, 2008. [Google Scholar]
- Gregory, J.F., 3rd; Kirk, J.R. The bioavailability of vitamin B6 in foods. Nutr. Rev. 1981, 39, 1–8. [Google Scholar] [PubMed]
- Bohrer, D.; do Nascimento, P.C.; Ramirez, A.G.; Mendonça, J.K.A.; de Carvalho, L.M.; Pomblum, S.C.G. Determination of thiamine in blood serum and urine by high-performance liquid chromatography with direct injection and post-column derivatization. Microchem. J. 2004, 8, 71–76. [Google Scholar] [CrossRef]
- Losa, R.; Sierra, M.I.; Fernandez, A.; Blanco, D.; Buesa, J.M. Determination of thiamine and its phosphorylated forms in human plasma, erythrocytes and urine by HPLC and fluorescence detection: A preliminary study on cancer patients. J. Pharm. Biomed. Anal. 2005, 37, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Talwar, D.; Davidson, H.; Cooney, J.; O’Reilly, D.S.J. Vitamin B1 status assessed by direct measurement of thiamin pyrophosphate in erythrocytes or whole blood by HPLC: Comparison with transketolase activation assay. Clin. Chem. 2000, 46, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Medina, A.; Fernández-de Córdoba, M.L.; Molina-Díaz, A. Flow injection–solid phase spectrofluorimetric determination of pyridoxine in presence of group B-vitamins. Fresenius J. Anal. Chem. 1999, 363, 265–269. [Google Scholar] [CrossRef]
- Viñas, P.; Balsalobre, N.; López-Erroz, C.; Hernández-Córdoba, M. Determination of vitamin B6 compounds in foods using liquid chromatography with post-column derivatization fluorescence detection. Chromatographia 2004, 59, 381–386. [Google Scholar] [CrossRef]
- Mascher, H. Determination of total pyridoxal in human plasma following oral administration of vitamin B6 by high-performance liquid chromatography with post-column derivatization. J. Pharm. Sci. 1993, 82, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Ismalinda, N.; Misfadhila, Z.; Misfadhila, S. Overview of vitamin B1 analysis methods during 2000–2020. World J. Pharm. Pharm. Sci. 2020, 9, 175–190. [Google Scholar]
- Holler, U.; Brodhag, C.; Knobel, A.; Hofmann, P.; Spitzer, V. Automated determination of selected water-soluble vitamins in tablets using a bench-top robotic system coupled to reversed-phase (RP-18) HPLC with UV detection. J. Pharm. Biomed. Anal. 2003, 31, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.S.J.; Fernandez-Ruiz, V.; Camara, M.; Sanchez-Mata, M.C. Simultaneous determination of vitamin B1 and B2 in complex cereal foods by reversed-phase isocratic HPLC-UV. J. Cereal Sci. 2012, 55, 293–299. [Google Scholar] [CrossRef]
- Marszałł, M.L.; Lebiedzińska, A.; Czarnowski, W.; Szefer, P. High-performance liquid chromatography method for the simultaneous determination of thiamine hydrochloride, pyridoxine hydrochloride and cyanocobalamin in pharmaceutical formulations using coulometric electrochemical and ultraviolet detection. J. Chromatogr. A 2005, 1094, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Lebiedzińska, A.; Marszałł, M.L.; Kuta, J.; Szefer, P. Reversed-phase high-performance liquid chromatography method with coulometric electrochemical and ultraviolet detection for the quantification of vitamins B1 (thiamine), B6 (pyridoxamine, pyridoxal and pyridoxine) and B12 in animal and plant foods. J. Chromatogr. A 2007, 1173, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruiz, T.; Martinez-Lozano, C.; Garcia-Martinez, M.D. Simultaneous determination of thiamine and its phosphate esters by a liquid chromatographic method based on post-column photolysis and chemiluminescence detection. J. Pharm. Biomed. Anal. 2009, 50, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Puts, J.; de Groot, M.; Haex, M.; Jakobs, B. Simultaneous determination of underivatized vitamin B1 and B6 in whole blood by reversed phase ultra-high performance liquid chromatography tandem mass spectrometry. PLoS ONE 2015, 10, e0132018. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.S.; Yu, H.S.; Yen, C.H.; Liaw, E.T. Multiple-reaction monitoring tandem mass method for determination of phenolics and water-soluble vitamins in Eccoilopus formosanus. Molecules 2020, 25, 3632. [Google Scholar] [CrossRef] [PubMed]
- Kahoun, D.; Fojtikova, P.; Vacha, F.; Cizkova, M.; Vodicka, R.; Novakova, E.; Hypsa, V. Development and validation of an LC-MS/MS method for determination of B vitamins and some of their derivatives in whole blood. PLoS ONE 2022, 17, e0271444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, H.J.; Huang, X.L.; Shao, L.J.; Xie, X.L.; Wang, F.; Yang, J.; Pie, P.; Zhang, Z.Q.; Zhai, Y.C.H.; et al. A novel LC-MS/MS assay for vitamin B1, B2 and B6 determination in dried blood spots and its application in children. J. Chromatogr. B 2019, 1112, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Lichodziejewska-Niemierko, M.; Małgorzewicz, S.; Rutkowski, B. Biologically active form of vitamin B1 in human peritoneal effluent. Adv. Clin. Exp. Med. 2017, 26, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Schwotzer, N.; Kanemitsu, M.; Kissling, S.; Darioli, R.; Benghezal, M.; Rezzi, S.; Burnier, M.; Pruijm, M. Water-soluble vitamin levels and supplementation in chronic online hemodiafiltration patients. Kidney Int. Rep. 2020, 5, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D. National Kidney Foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am. J. Kidney Dis. 2001, 37 (Suppl. 1), S66–S70. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Dietary reference values for vitamin B6. EFSA J. 2016, 14, 4485. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Martínez, E.A.; Santillán-Benítez, J.G.; Sandoval-Cabrera, A.; Cervantes-Rebolledo, C. Uric acid is an independent biomarker in the management of a chronic renal disease. Rev. Latinoam. Hipertens. 2021, 16, 329–336. [Google Scholar]
- Sauberlich, H.E. Bioavailability of vitamins. Prog. Food Nutr. Sci. 1985, 9, 1–33. [Google Scholar] [PubMed]
- Institute of Medicine. Dietary Reference Intakes; National Academy Press: Washington, DC, USA, 1998. [Google Scholar]
- Boeschoten, E.W.; Schrijver, J.; Krediet, R.T.; Schreurs, W.H.P.; Arisz, L. Deficiencies of vitamins in CAPD patients: The effect of supplementation. Nephrol. Dial. Transplant. 1988, 2, 187–193. [Google Scholar] [CrossRef]
- Clase, C.M.; Ki, V.; Holden, R.M. Water-Soluble Vitamins in People with Low Glomerular Filtration Rate or on Dialysis: A Review. Semin. Dial. 2013, 26, 546–567. [Google Scholar] [CrossRef] [PubMed]
- Martín-del-Campo, F.; Batis-Ruvalcaba, C.; González-Espinoza, L.; Rojas-Campos, E.; Ángel, J.R.; Ruiz, N.; González, J.; Pazarín, L.; Cueto-Manzano, A.M. Dietary micronutrient intake in peritoneal dialysis patients: Relationship with nutrition and inflammation status. Perit. Dial. Int. 2012, 32, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 7th ed.; Pearson Education: Upper Saddle River, NJ, USA, 2014. [Google Scholar]
- Jankowska, M.; Rudnicki-Velasquez, P.; Storoniak, H.; Rutkowski, P.; Rutkowski, B.; Krzymiński, K.; Dębska-Ślizień, A. Thiamine diphosphate status and dialysis-related losses in end-stage kidney disease patients treated with hemodialysis. Blood Purif. 2017, 44, 293–300. [Google Scholar] [CrossRef] [PubMed]
- General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Coll Dent. 2014, 81, 14–18. [Google Scholar]
- Mitsikaris, P.D.; Kokokiris, L.; Pritsa, A.; Papadopoulos, A.N.; Kalogiouri, N.P. Investigating the tocopherol contents of walnut seed oils produced in different European countries analyzed by HPLC-UV: A comparative study on the basis of geographical origin. Foods 2022, 11, 3719. [Google Scholar] [CrossRef] [PubMed]
- Kutner, M.H.; Nachtsheim, C.J.; Neter, J.; Li, W. Applied Linear Statistical Models, 5th ed.; McGraw-Hill: Boston, MA, USA, 2005. [Google Scholar]
- Breusch, T.S.; Pagan, A.R. A simple test for heteroscedasticity and random coefficient variation. Econometrica 1979, 47, 1287–1294. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- O’Brien, R.M. A caution regarding rules of thumb for variance inflation factors. Qual. Quant. 2007, 41, 673–690. [Google Scholar] [CrossRef]
- Belsley, D.A.; Kuh, E.; Welsch, R.E. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity; Wiley: New York, NY, USA, 1980. [Google Scholar]
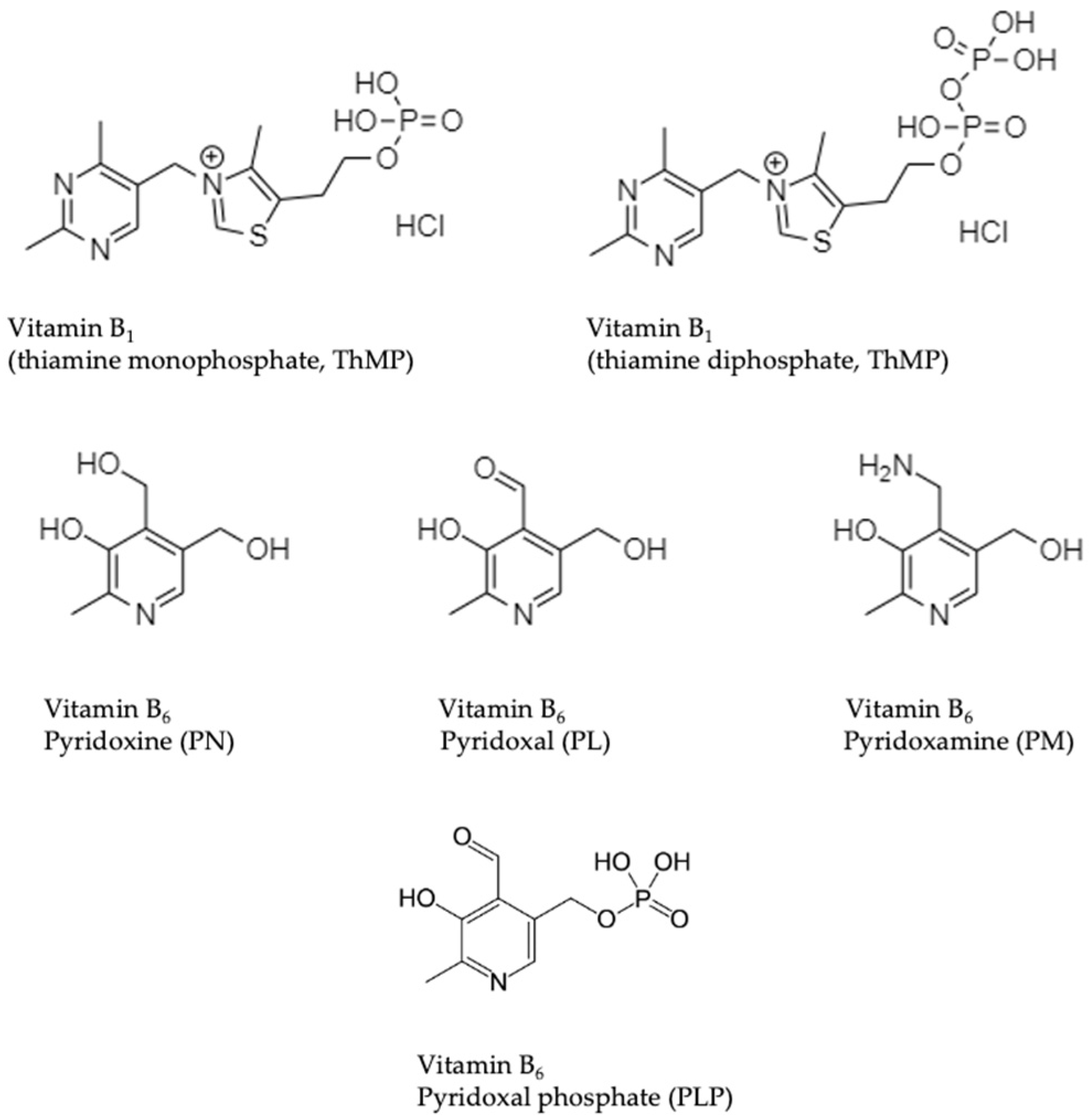

| Characteristic (Parameter) | Range |
|---|---|
| Age [years] | 20–88 |
| Weight [kg] | 45.5–106.0 |
| Height [cm] | 150–195 |
| Volume of dialysis fluid [mL] | 1650–15.800 |
| Urine output [mL/24 h] | 0–2550 |
| Kt/V | 1.25–4.69 |
| nPCR [g/kg/24 h] | 0.67–2.25 |
| BUN—after 4 h [mg/dL] | 0.1–13.5 |
| BUN—after 24 h [mg/dL] | 3.04–219.40 |
| Creatinine—after 4 h [mg/dL] | 0.10–2.20 |
| Creatinine—after 24 h [mg/dL] | 0.80–37.80 |
| Haemoglobin [g/L] | 7.3–14.0 |
| Haematocrit [%] | 23.4–42.5 |
| Protein peritoneal loss [g/ 24 h] | 0.0–1.74 |
| White blood cells [×109/L] | 2.84–12.43 |
| Lymphocytes [×109/L] | 0.44–2.95 |
| Neutrocytes [×109/L] | 1.01–8.29 |
| ThMP | ThDP | PN | PL | PM | |
|---|---|---|---|---|---|
| Tested Parameter | Value | Value | Value | Value | Value |
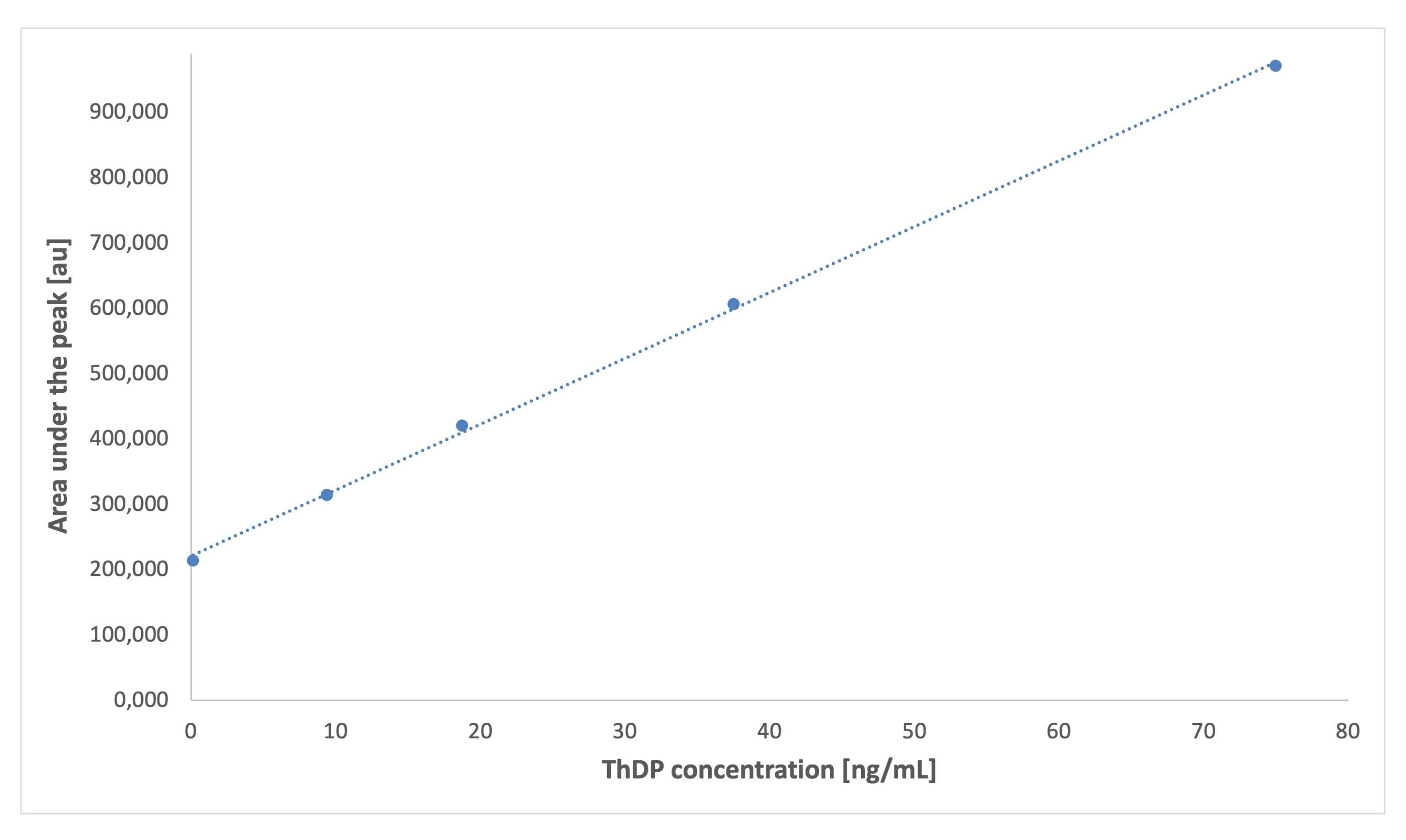
| Equation of the calibration graph (c—vitamer’s concentration, [ng/mL] | 99,856 c + 5650 | 96,250 c + 6096 | 8350 c + 106 | 6971 c + 196 | 7338 c + 217 |
| Linearity range [ng/mL] | 0.12–75.0 | 0.15–75.0 | 1.50–50.0 | 1.50–50.0 | 1.50–50.0 |
| Correlation coefficient (R) | 0.9968 | 0.9983 | 0.9928 | 0.9973 | 0.9951 |
| Coefficient of variation (CV), [%] | 3.12 | 4.16 | 2.68 | 3.94 | 1.76 |
| LOD [ng/mL] | 0.08 | 0.02 | 0.03 | 0.01 | 0.03 |
| LOQ [ng/mL] | 0.21 | 0.07 | 0.09 | 0.05 | 0.08 |
| Range [ng/mL] | |||||
|---|---|---|---|---|---|
| Probe | ThMP | ThDP | PN | PL | PM |
| A | 0.53–6.88 | 5.12–50.24 | 0.65–2.61 | 5.83–17.26 | 0.46–3.64 |
| B | 0.53–1.66 | 0.45–31.16 | 0.08–1.56 | 0.09–16.63 | 0.08–1.33 |
| C | 0.43–3.72 | 2.17–40.14 | 0.30–1.81 | 1.54–16.20 | 0.23–2.67 |
| Predictor | B | SE B | β (Beta) | t | p-Value | 95% CI for B |
|---|---|---|---|---|---|---|
| (Constant) | −0.121 | 0.242 | − | −0.500 | 0.623 | [−0.628, 0.386] |
| Weight | 0.007 | 0.002 | 0.771 | 4.413 | <0.001 | [0.004, 0.010] |
| Haemoglobin (Hb) | 0.255 | 0.103 | 0.255 | 2.472 | 0.023 | [0.039, 0.470] |
| Hematocrit (Hct) | 0.078 | 0.036 | 0.275 | 2.189 | 0.041 | [0.003, 0.152] |
| KD0 | 0.087 | 0.049 | 0.284 | 1.778 | 0.091 | [−0.015, 0.189] |
| ||||||
| Ion Transition Monitored Parameters | ||||||||
| Compound | Quantitative [Q] qualitative [q] parent ion > fragment ion * | DP (V) | EP (V) | CXP (V) | CE (V) | MRM ratio | ||
| PLP | Q 248 > 150 q 248 > 134 | 46 | 10 | 16 8 | 19 25 | 1.4 | ||
| PL | Q 170 > 152 q 170 > 134 | 46 | 10 | 16 8 | 19 25 | 1.4 | ||
| ThMP | Q 345 > 122 q 345 > 224 | 41 | 10 | 14 10 | 23 23 | 4.8 | ||
| ThDP | Q 425 > 122 q 425 > 304 | 31 | 10 | 22 22 | 27 23 | 2.1 | ||
| MS/MS Operation Parameters | ||||||||
| Compound | Quantitative [Q] qualitative [q] parent ion > fragment ion * | CUR (psi) | IS (V) | T (°C) | GS1 (psi) | GS2 (psi) | ||
| PLP | Q 248 > 150 q 248 > 134 | 30 | 5500 | 200 | 20 | 20 | ||
| PL | Q 170 > 152 q 170 > 134 | 30 | 5500 | 200 | 20 | 20 | ||
| ThMP | Q 345 > 122 q 345 > 224 | 20 | 5500 | 200 | 20 | 20 | ||
| ThDP | Q 425 > 122 q 425 > 304 | 20 | 5500 | 300 | 30 | 20 | ||
| Chosen parameters | 20 | 5500 | 200 | 20 | 20 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudnicki-Velasquez, P.; Krzymiński, K.; Jankowska, M.; Baraniak, A.; Czaplewska, P. A Simultaneous Determination of the B1 and B6 Vitamers Reveals Their Loss During a Single Peritoneal Dialysis Session: Chromatographic and Chemometric Approach. Int. J. Mol. Sci. 2025, 26, 7177. https://doi.org/10.3390/ijms26157177
Rudnicki-Velasquez P, Krzymiński K, Jankowska M, Baraniak A, Czaplewska P. A Simultaneous Determination of the B1 and B6 Vitamers Reveals Their Loss During a Single Peritoneal Dialysis Session: Chromatographic and Chemometric Approach. International Journal of Molecular Sciences. 2025; 26(15):7177. https://doi.org/10.3390/ijms26157177
Chicago/Turabian StyleRudnicki-Velasquez, Paweł, Karol Krzymiński, Magdalena Jankowska, Anna Baraniak, and Paulina Czaplewska. 2025. "A Simultaneous Determination of the B1 and B6 Vitamers Reveals Their Loss During a Single Peritoneal Dialysis Session: Chromatographic and Chemometric Approach" International Journal of Molecular Sciences 26, no. 15: 7177. https://doi.org/10.3390/ijms26157177
APA StyleRudnicki-Velasquez, P., Krzymiński, K., Jankowska, M., Baraniak, A., & Czaplewska, P. (2025). A Simultaneous Determination of the B1 and B6 Vitamers Reveals Their Loss During a Single Peritoneal Dialysis Session: Chromatographic and Chemometric Approach. International Journal of Molecular Sciences, 26(15), 7177. https://doi.org/10.3390/ijms26157177








