Induced Microglial-like Cells Derived from Familial and Sporadic Alzheimer’s Disease Peripheral Blood Monocytes Show Abnormal Phagocytosis and Inflammatory Response to PSEN1 E280A Cholinergic-like Neurons
Abstract
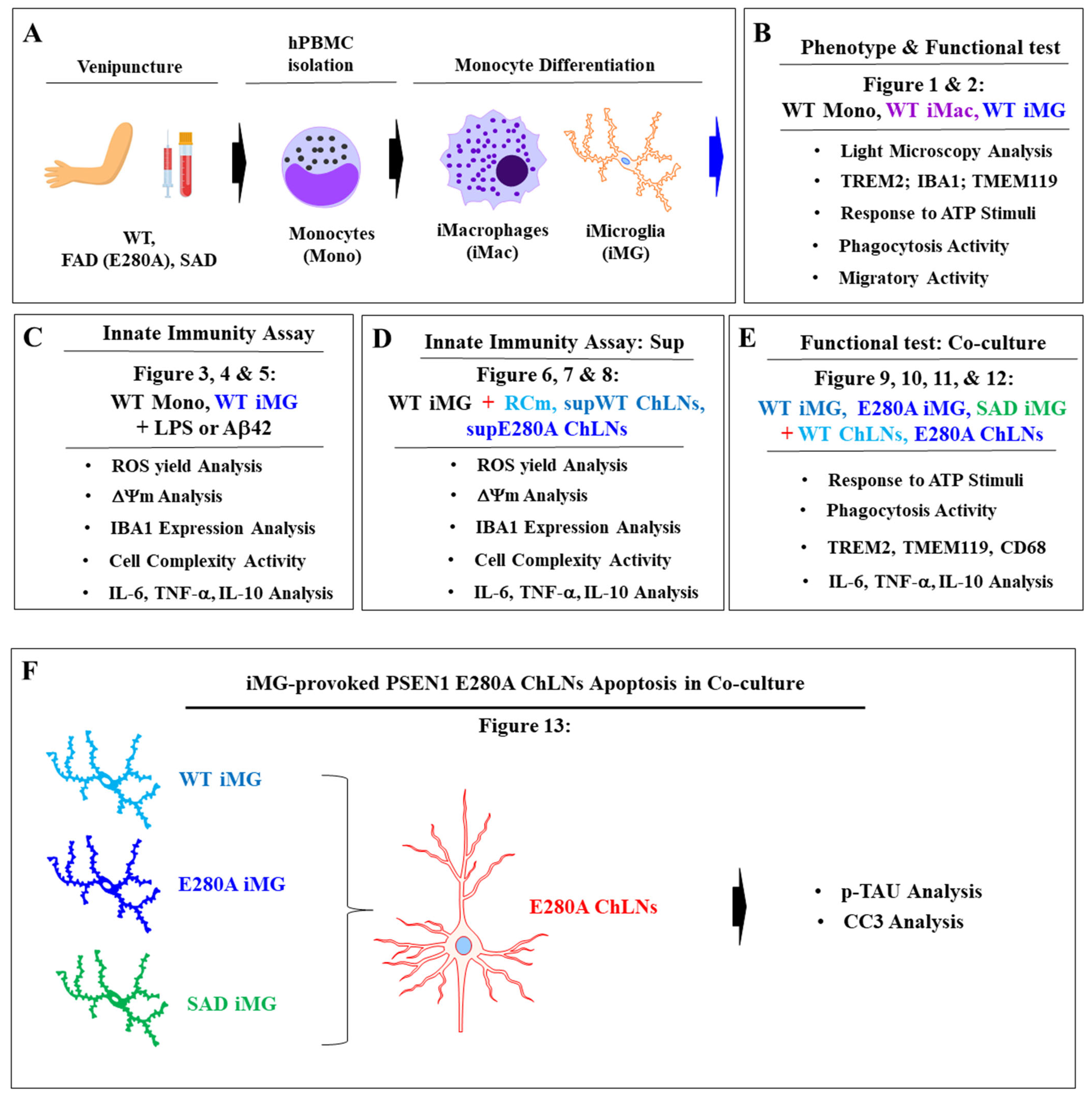
1. Introduction
2. Results
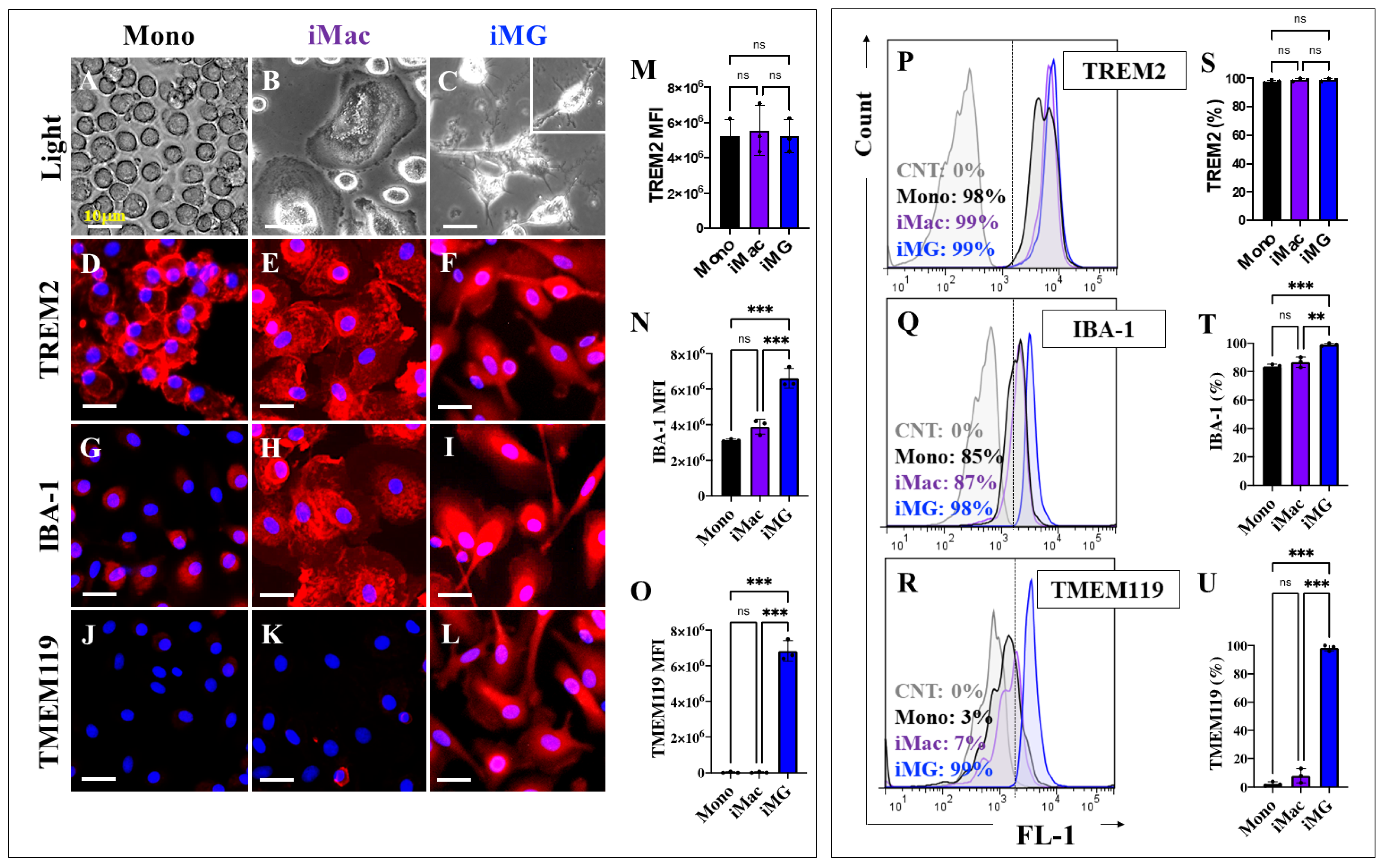
2.1. Induced Microglia-like Cells (iMG) from Human Peripheral Blood Mononuclear Cells (hPBMCs) Exhibit a Typical Microglial Phenotype
2.2. Induced Microglia-like Cells (iMG) Cells, but Not Monocytes, Respond to ATP-Induced Transient Intracellular Ca2+ Influx
2.3. Induced Microglia-like Cells (iMG) Show Higher Phagocytic Activity than Monocytes Exposed to pHrodo™ Red E. coli BioParticles™ Conjugate
2.4. Scratch Wound Induces Higher Migration Activity in Induced Microglia-like Cells (iMG) Rather than in Monocytes (Mono)
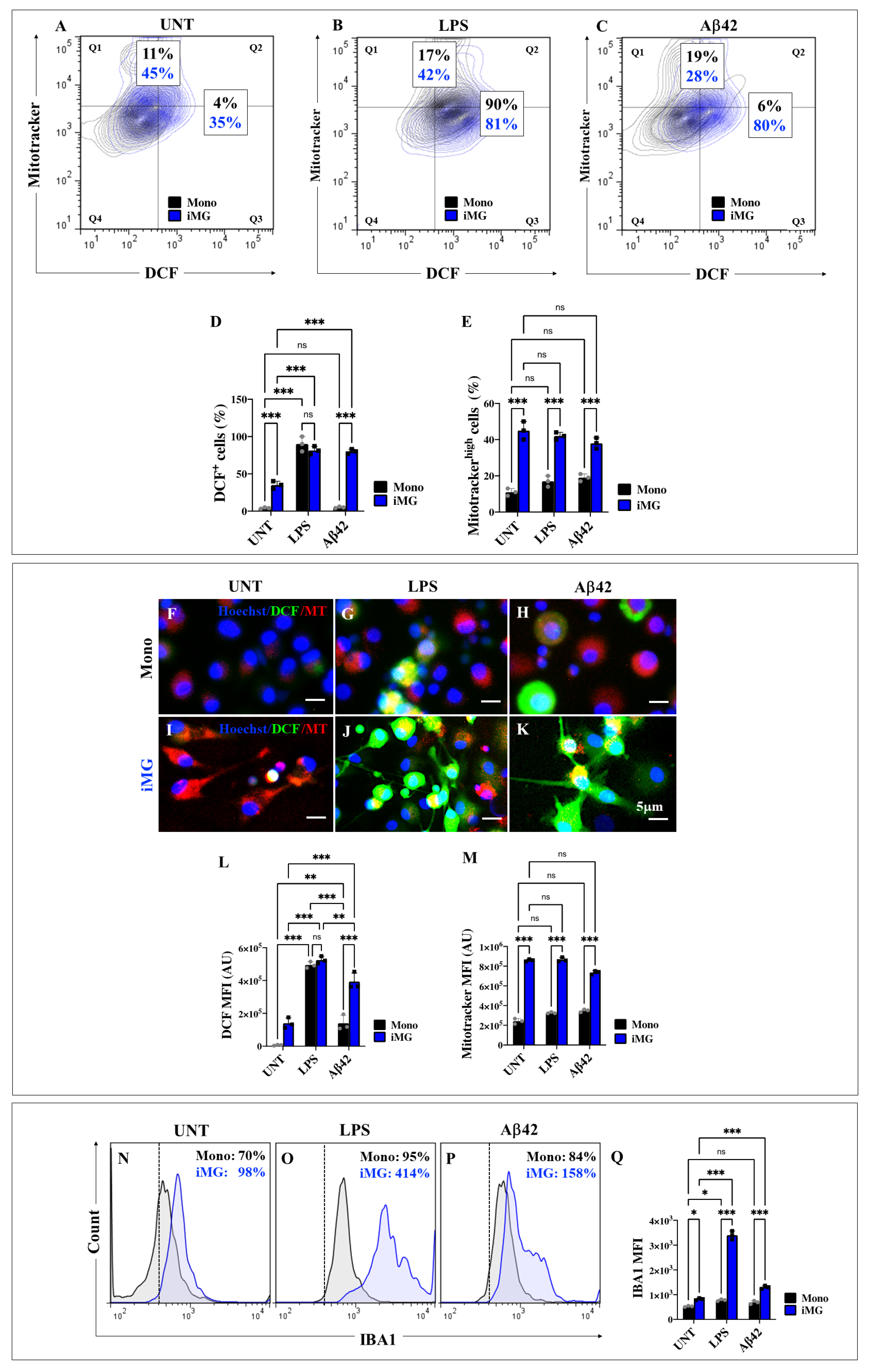
2.5. Induced Microglia-like Cells (iMG) Exposed to Lipopolysaccharide (LPS) and Amyloid Beta 42 (Aβ42) Stimuli Generate High Levels of Intracellular Reactive Oxygen Species (ROS) and Increase Mitochondrial Membrane Potential (∆Ψm)
2.6. Lipopolysaccharide (LPS) or Amyloid Beta 42 (Aβ42) Peptide Stimuli Increase the Expression Levels of Ionized Calcium-Binding Adapter Molecule 1 (IBA1) in Induced Microglia-like Cells (iMG) Rather than in Monocytes (Mono)
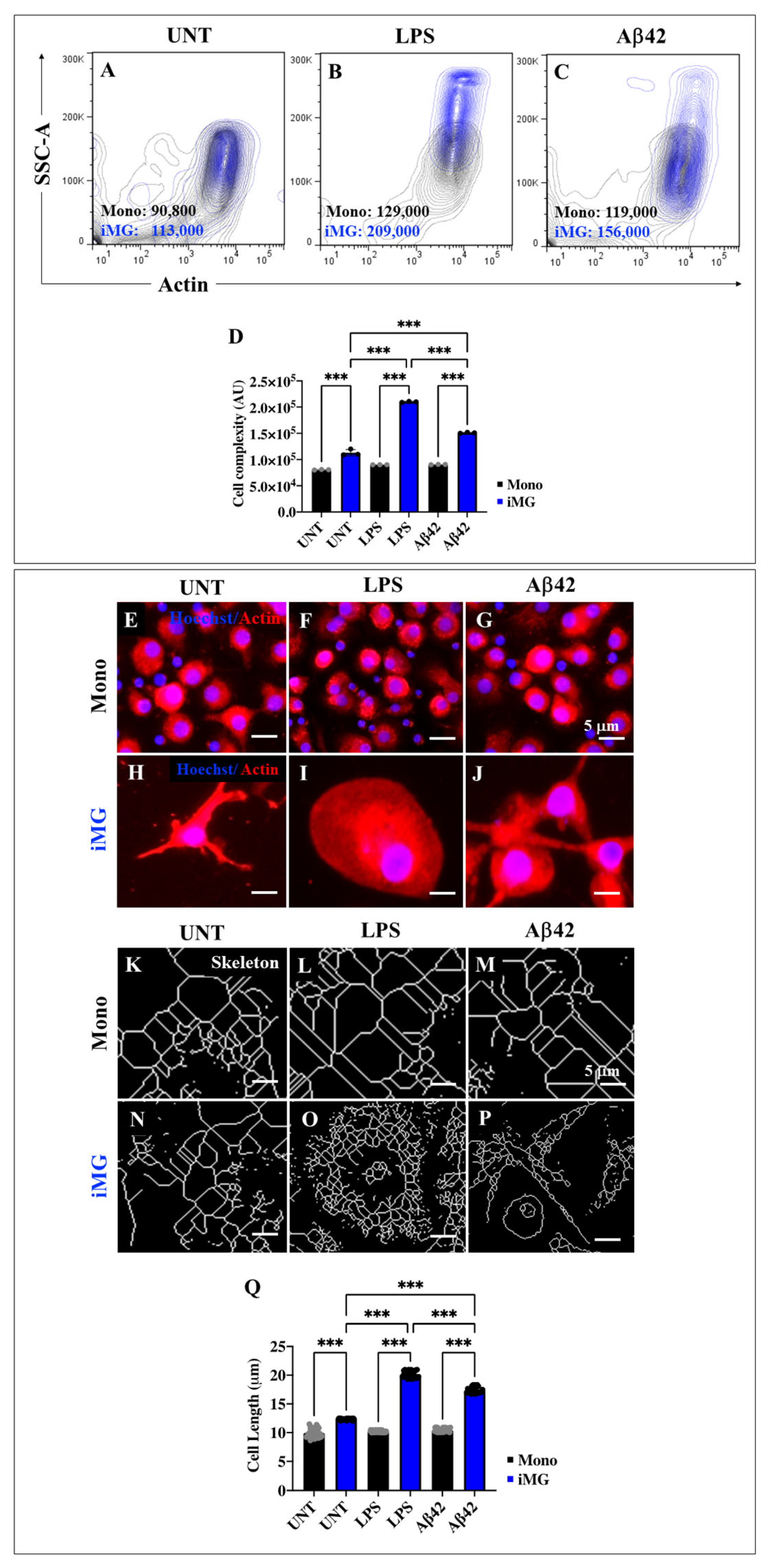
2.7. Lipopolysaccharide (LPS) or Amyloid Beta 42 (Aβ42) Peptide Stimuli Increase the Internal Granularity of Cells (Area) and Expression Levels of Actin in Induced Microglia-like Cells (iMG)
2.8. Lipopolysaccharide (LPS) or Amyloid Beta 42 (Aβ42) Stimuli Induce the Release of Pro-Inflammatory Cytokines Interleukin-6 (IL-6) and Tumor Necrosis Factor Alpha (TNF-α), but Anti-Inflammatory IL-10 Is Absent in Induced Microglia-like Cells (iMG)
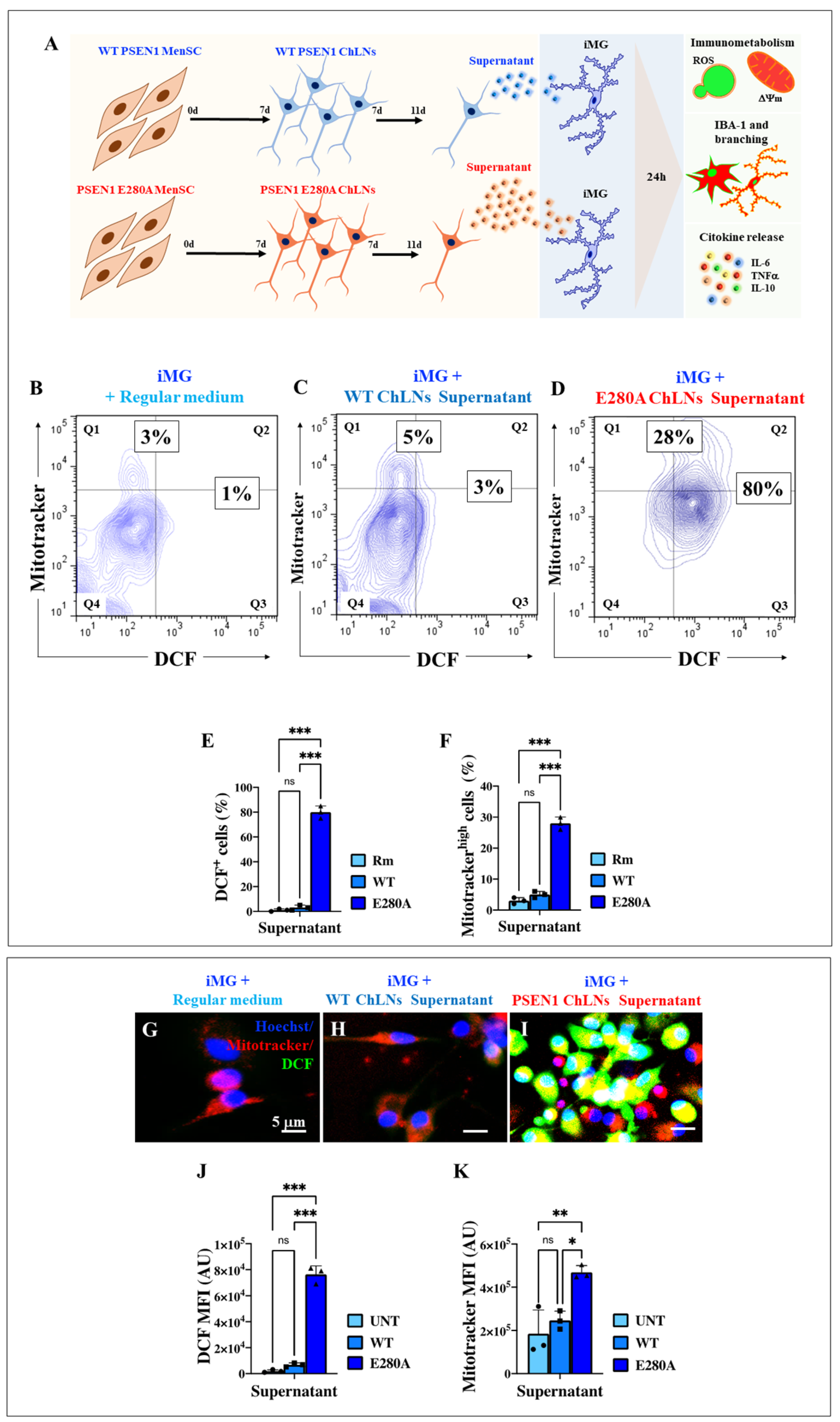
2.9. Presenilin 1 (PSEN1) E280A Cholinergic-like Neuron (ChLN) Culture Supernatant Induces High Intracellular Levels of Reactive Oxygen Species (ROS) and Increases Mitochondrial Membrane Potential (∆Ψm) in Wild Type (WT) Induced Microglia-like Cells (iMG)
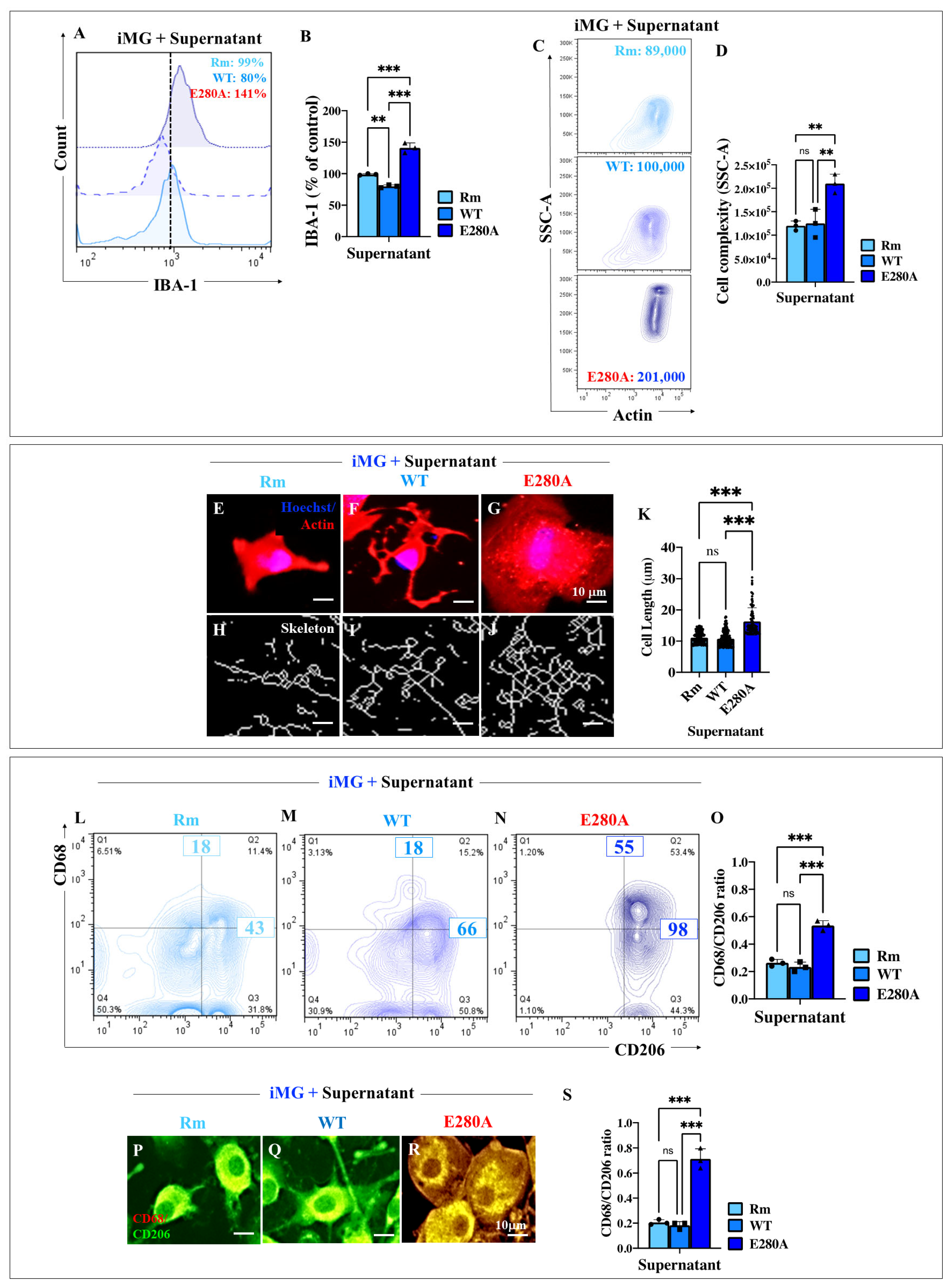
2.10. Presenilin 1 (PSEN1) E280A Cholinergic-like Neuron (ChLN) Culture Supernatant Stimulates High Levels of Ionized Calcium-Binding Adapter Molecule 1 (IBA1) Expression and Increases the Elongation Process in Wild Type (WT) Induced Microglia-like Cells (iMG)
2.11. Presenilin 1 (PSEN1) E280A Cholinergic-like Neuron (ChLN) Culture Supernatant Stimulates High Expression of Pro-Inflammatory Cluster of Differentiation 68 (CD68) in Wild Type (WT) Induced Microglia-like Cells (iMG)
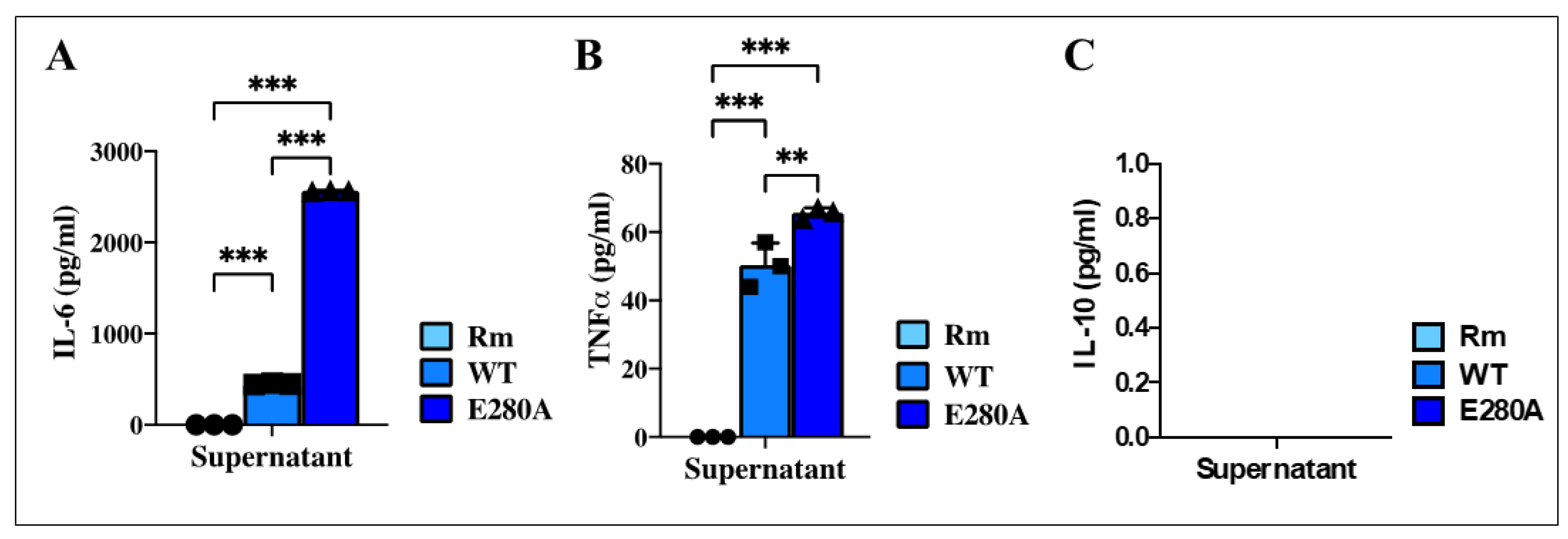
2.12. Presenilin 1 (PSEN1) E280A Cholinergic-like Neuron (ChLN) Culture Supernatant Induces the Release of Cytokine Interleukin-6 (IL-6) and Tumor Necrosis Factor Alpha (TNF-α) in Induced Microglia-like Cells (iMG)
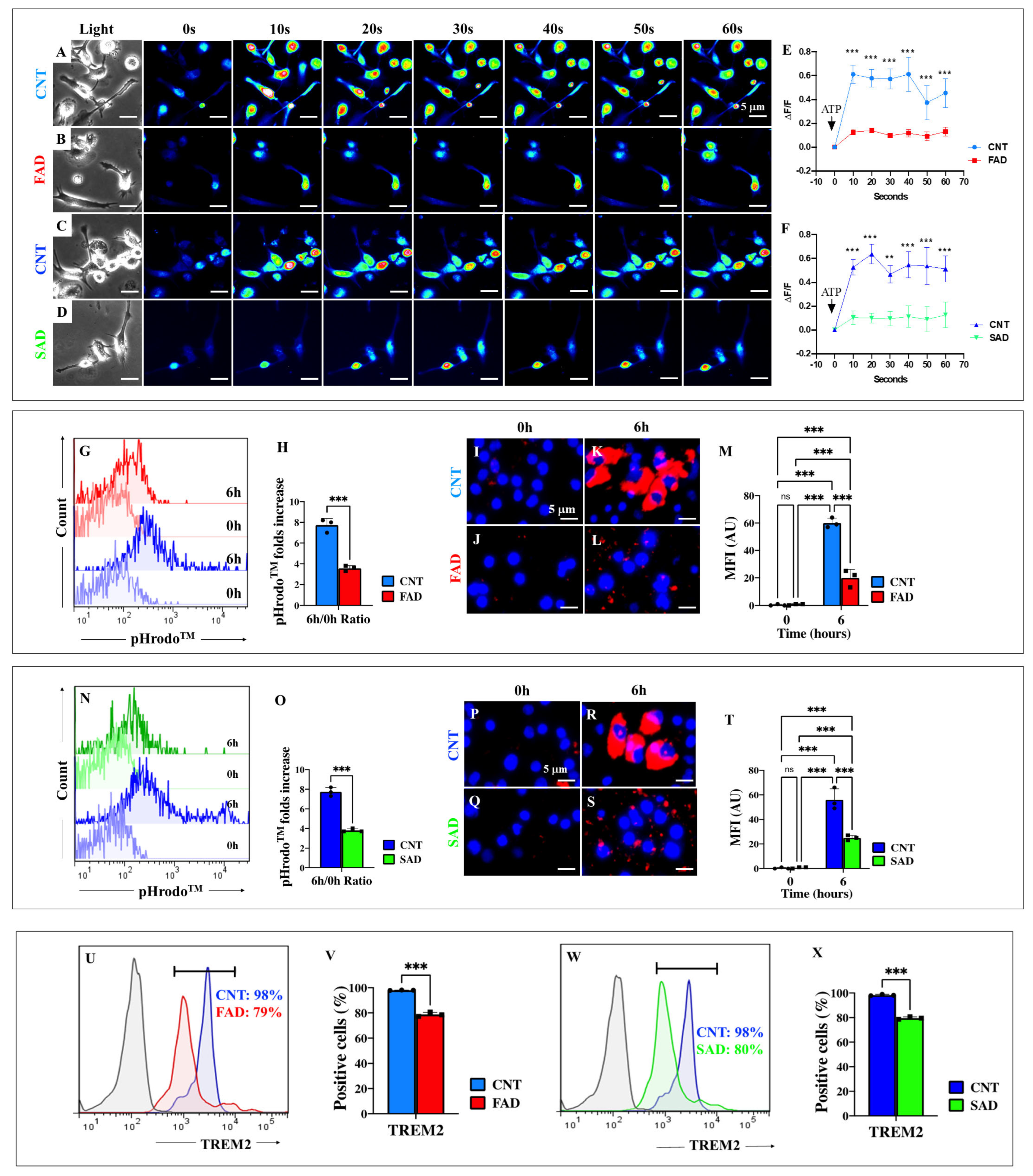
2.13. Presenilin 1 (PSEN1) E280A and Sporadic AD (SAD) Induced Microglia-like Cells (iMG) Are Unresponsive to ATP Stimulation
2.14. Presenilin 1 (PSEN1) E280A and Sporadic AD (SAD) Induced Microglia-like Cells (iMG) Show Defective Phagocytosis Due to Diminished Expression of Triggering Receptor Expressed on Myeloid Cells 2 (TREM2)
2.15. Presenilin 1 (PSEN1) E280A Cholinergic-like Neurons (ChLNs) in Co-Culture with Wild Type (WT) Induced Microglia-like Cells (iMG) Induce High Levels of Microglial Lineage Marker Transmembrane Protein 119 (TMEM119) and Cellular Complexity
2.16. Presenilin 1 (PSEN1) E280A Cholinergic-like Neurons (ChLNs) Induce High Surface Expression of Pro-Inflammatory Cluster of Differentiation 68 (CD68) and Secretion of Cytokine Interleukin-6 (IL-6) and Tumor Necrosis Factor Alpha (TNF-α), but Reduce the Amounts of Secreted Anti-Inflammatory Cytokine IL-10 in Wild Type (WT) Induced Microglia-like Cells (iMG)
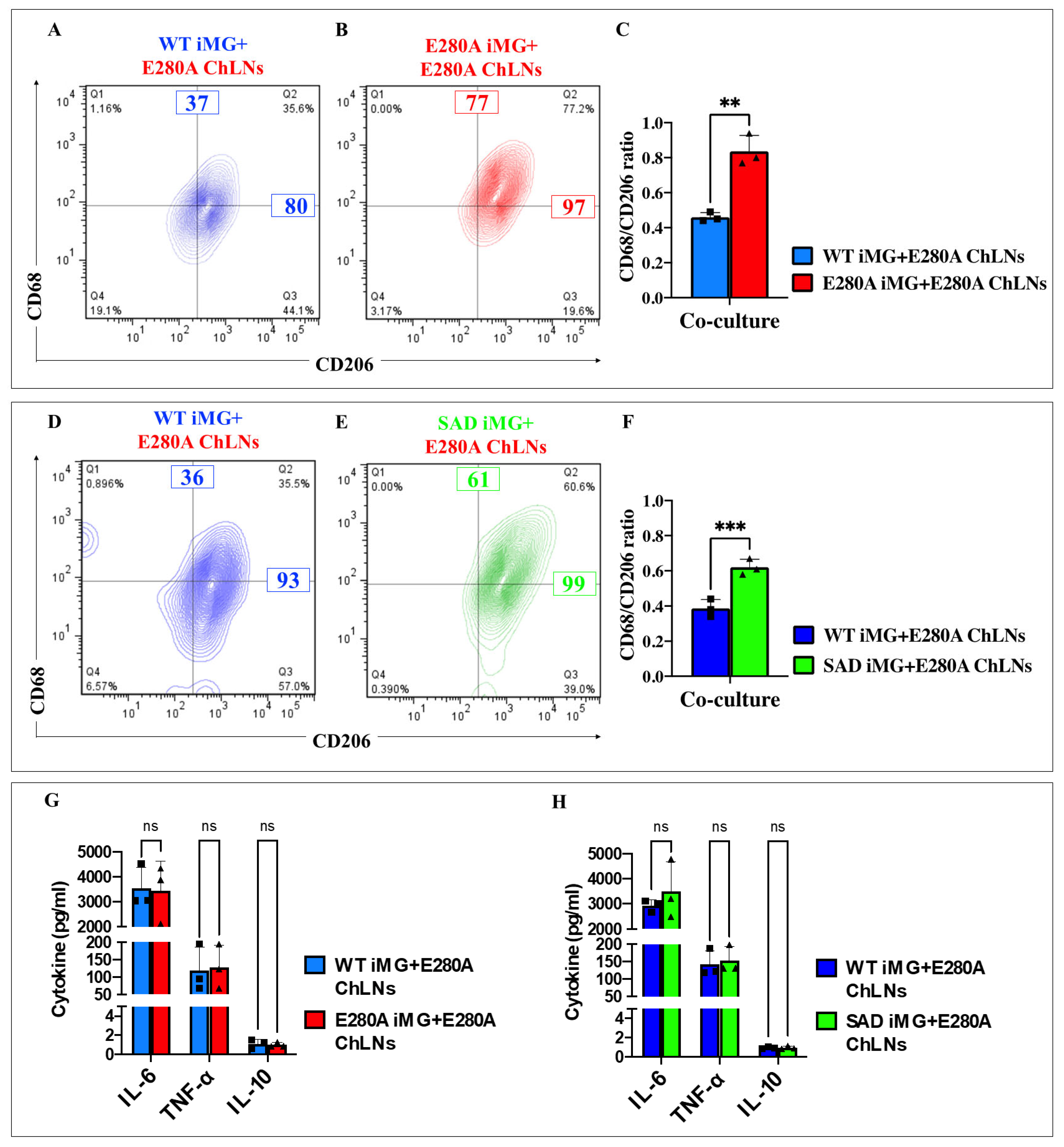
2.17. Presenilin 1 (PSEN1) E280A Cholinergic-like Neurons (ChLNs) Induce a Significant Increase of Pro-Inflammatory M1 Cells (Cluster of Differentiation 68 (CD68)/CD206 Ratio) in PSEN1 E280A and Sporadic AD (SAD) Induced Microglia-like Cells (iMG)
2.18. Presenilin 1 (PSEN1) E280A Cholinergic-like Neurons (ChLNs) Induce a Significant Increase in the Pro-Inflammatory Cytokines Interleukin-6 (IL-6) and Tumor Necrosis Factor Alpha (TNF-α) but Reduce the Anti-Inflammatory IL-10 in Sporadic AD (SAD) and PSEN1 E280A Induced Microglia-like Cells (iMG)
2.19. Presenilin 1 (PSEN1) E280A and Sporadic AD (SAD) Induced Microglia-like Cells (iMG) Induce Abnormal Phosphorylation of Protein TAU and Cleaved Caspase (CC3) in PSEN1 E280A Cholinergic-like Neurons (ChLNs)
3. Discussion
4. Materials and Methods
4.1. Generation of Induced Microglia-like Cells (iMG Cells) from Human Monocytes
4.2. Evaluation of Cell Morphology
4.3. Flow Cytometry Analysis of Microglia Markers
4.4. Immunofluorescence Analysis of Microglia Markers
4.5. Assessment of Microglial Function by Calcium Activity
4.6. Scratch Wound Migration Assay
4.7. Live Flow Cytometry and Imaging Phagocytic Assay with pHrodoTM
4.8. Human Menstrual Stromal Cells (MenSCs)
4.9. Cholinergic-like Neuron (ChLN) Differentiation
4.10. Evaluation of Intracellular Hydrogen Peroxide (H2O2) and Mitochondrial Membrane Potential (ΔΨm) by Fluorescent Microscopy
4.11. Evaluation of Intracellular Hydrogen Peroxide (H2O2) and Mitochondrial Membrane Potential (ΔΨm) by Flow Cytometry
4.12. Microglia Process Extension Analysis
4.13. Cytokine Release Measurement
4.14. Co-Culture of iMG and Cholinergic-like Neuron (ChLNs)
4.15. Flow Cytometry Analysis of Microglia Inflammatory Phenotype
4.16. Immunofluorescence Analysis of Tau and Cleaved Caspase-3 (CC3)
4.17. Apolipoprotein E Genotype
4.18. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendez, M.F. Early-Onset Alzheimer Disease. Neurol. Clin. 2017, 35, 263–281. [Google Scholar] [CrossRef]
- Madnani, R.S. Alzheimer’s Disease: A Mini-Review for the Clinician. Front. Neurol. 2023, 14, 1178588. [Google Scholar] [CrossRef] [PubMed]
- Doher, N.; Davoudi, V.; Magaki, S.; Townley, R.A.; Haeri, M.; Vinters, H.V. Illustrated Neuropathologic Diagnosis of Alzheimer’s Disease. Neurol. Int. 2023, 15, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, T.W.; Nathan Spreng, R. Basal Forebrain Degeneration Precedes and Predicts the Cortical Spread of Alzheimer’s Pathology. Nat. Commun. 2016, 7, 13249. [Google Scholar] [CrossRef]
- Fernández-Cabello, S.; Kronbichler, M.; van Dijk, K.R.A.; Goodman, J.A.; Nathan Spreng, R.; Schmitz, T.W.; On Behalf of the Alzheimer’s Disease Neuroimaging Initiative. Basal Forebrain Volume Reliably Predicts the Cortical Spread of Alzheimer’s Degeneration. Brain 2020, 143, 993–1009. [Google Scholar] [CrossRef]
- Lin, C.P.; Frigerio, I.; Boon, B.D.C.; Zhou, Z.; Rozemuller, A.J.M.; Bouwman, F.H.; Schoonheim, M.M.; Van De Berg, W.D.J.; Jonkman, L.E. Structural (Dys)Connectivity Associates with Cholinergic Cell Density in Alzheimer’s Disease. Brain 2022, 145, 2869–2881. [Google Scholar] [CrossRef]
- Mieling, M.; Meier, H.; Bunzeck, N. Structural Degeneration of the Nucleus Basalis of Meynert in Mild Cognitive Impairment and Alzheimer’s Disease–Evidence from an MRI-Based Meta-Analysis. Neurosci. Biobehav. Rev. 2023, 154, 105393. [Google Scholar] [CrossRef]
- Shafiee, N.; Fonov, V.; Dadar, M.; Spreng, R.N.; Collins, D.L. Degeneration in Nucleus Basalis of Meynert Signals Earliest Stage of Alzheimer’s Disease Progression. Neurobiol. Aging 2024, 139, 54–63. [Google Scholar] [CrossRef]
- Liu, A.K.L.; Chang, R.C.C.; Pearce, R.K.B.; Gentleman, S.M. Nucleus Basalis of Meynert Revisited: Anatomy, History and Differential Involvement in Alzheimer’s and Parkinson’s Disease. Acta Neuropathol. 2015, 129, 527–540. [Google Scholar] [CrossRef]
- Pepeu, G.; Grazia Giovannini, M. The Fate of the Brain Cholinergic Neurons in Neurodegenerative Diseases. Brain Res. 2017, 1670, 173–184. [Google Scholar] [CrossRef]
- Deture, M.A.; Dickson, D.W. The Neuropathological Diagnosis of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokop, S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics 2022, 19, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.J.; Renton, A.E.; Fulton-Howard, B.; Podlesny-Drabiniok, A.; Marcora, E.; Goate, A.M. The Complex Genetic Architecture of Alzheimer’s Disease: Novel Insights and Future Directions. EBioMedicine 2023, 90, 104511. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, M.S.; Xia, W.; Ostaszewski, B.L.; Diehl, T.S.; Kimberly, W.T.; Selkoe, D.J. Two Transmembrane Aspartates in Presenilin-1 Required for Presenilin Endoproteolysis and γ-Secretase Activity. Nature 1999, 398, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.Y. γ-Secretase in Alzheimer’s Disease. Exp. Mol. Med. 2022, 54, 433–446. [Google Scholar] [CrossRef]
- Miao, Y.; Wolfe, M.S. Emerging Structures and Dynamic Mechanisms of γ-Secretase for Alzheimer’s Disease. Neural Regen. Res. 2025, 20, 174–180. [Google Scholar] [CrossRef]
- Asik, R.M.; Suganthy, N.; Aarifa, M.A.; Kumar, A.; Szigeti, K.; Mathe, D.; Gulyás, B.; Archunan, G.; Padmanabhan, P. Alzheimer’s Disease: A Molecular View of β-Amyloid Induced Morbific Events. Biomedicines 2021, 9, 1126. [Google Scholar]
- Azargoonjahromi, A. The Duality of Amyloid-β: Its Role in Normal and Alzheimer’s Disease States. Mol. Brain 2024, 17, 44. [Google Scholar] [CrossRef]
- Craft, J.M.; Watterson, D.M.; Van Eldik, L.J. Human Amyloid β-Induced Neuroinflammation Is an Early Event in Neurodegeneration. Glia 2006, 53, 484–490. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Y.; Chen, C.; Chen, H.; Ni, W.; Song, Y.; Lv, B.; Hua, F.; Cui, G.; Zhang, Z. TLR4/Rac1/NLRP3 Pathway Mediates Amyloid-β-Induced Neuroinflammation in Alzheimer’s Disease. J. Alzheimers Dis. 2024, 99, 911–925. [Google Scholar] [CrossRef]
- Merighi, S.; Nigro, M.; Travagli, A.; Gessi, S. Microglia and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12990. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Sun, Y.; Peng, G. Neuroinflammation as a Potential Therapeutic Target in Alzheimer’s Disease. Clin. Interv. Aging 2022, 17, 665–674. [Google Scholar] [CrossRef]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H.K. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 2023, 46, 1–17. [Google Scholar] [CrossRef]
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and Differentiation of Microglia. Front. Cell. Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 1–37. [Google Scholar] [CrossRef]
- Mehl, L.C.; Manjally, A.V.; Bouadi, O.; Gibson, E.M.; Tay, T.L. Microglia in Brain Development and Regeneration. Development 2022, 149, dev200425. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Paolicelli, R.C. Microglia-Mediated Synapse Loss in Alzheimer’s Disease. J. Neurosci. 2018, 38, 2911–2919. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zong, S.; Cui, X.; Wang, X.; Wu, S.; Wang, L.; Liu, Y.; Lu, Z. The Effects of Microglia-Associated Neuroinflammation on Alzheimer’s Disease. Front. Immunol. 2023, 14, 1117172. [Google Scholar] [CrossRef]
- Miao, J.; Ma, H.; Yang, Y.; Liao, Y.; Lin, C.; Zheng, J.; Yu, M.; Lan, J. Microglia in Alzheimer’s Disease: Pathogenesis, Mechanisms, and Therapeutic Potentials. Front. Aging Neurosci. 2023, 15, 1201982. [Google Scholar] [CrossRef]
- Lista, S.; Imbimbo, B.P.; Grasso, M.; Fidilio, A.; Emanuele, E.; Minoretti, P.; López-Ortiz, S.; Martín-Hernández, J.; Gabelle, A.; Caruso, G.; et al. Tracking Neuroinflammatory Biomarkers in Alzheimer’s Disease: A Strategy for Individualized Therapeutic Approaches? J. Neuroinflammation 2024, 21, 187. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, J.; Wang, B.; Sun, M.; Yang, H. Microglia in the Neuroinflammatory Pathogenesis of Alzheimer’s Disease and Related Therapeutic Targets. Front. Immunol. 2022, 13, 856376. [Google Scholar] [CrossRef]
- Bernardino, L.; Volonté, C.; Passani, M.B.; Ferreira, R. Editorial: Dual Role of Microglia in Health and Disease: Pushing the Balance Towards Repair. Front. Cell. Neurosci. 2020, 14, 259. [Google Scholar] [CrossRef]
- He, G.; Li, Y.; Deng, H.; Zuo, H. Advances in the Study of Cholinergic Circuits in the Central Nervous System. Ann. Clin. Transl. Neurol. 2023, 10, 2179–2191. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The Cholinergic System in the Pathophysiology and Treatment of Alzheimer’s Disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Gamage, R.; Wagnon, I.; Rossetti, I.; Childs, R.; Niedermayer, G.; Chesworth, R.; Gyengesi, E. Cholinergic Modulation of Glial Function During Aging and Chronic Neuroinflammation. Front. Cell. Neurosci. 2020, 14, 577912. [Google Scholar] [CrossRef] [PubMed]
- Stolero, N.; Frenkel, D. The Dialog between Neurons and Microglia in Alzheimer’s Disease: The Neurotransmitters View. J. Neurochem. 2021, 158, 1412–1424. [Google Scholar] [CrossRef] [PubMed]
- Lemere, C.A.; Lopera, F.; Kosik, K.S.; Lendon, C.L.; Ossa, J.; Saido, T.C.; Yamaguchi, H.; Ruiz, A.; Martinez, A.; Madrigal, L.; et al. The E280A Presenilin 1 Alzheimer Mutation Produces Increased Aβ42 Deposition and Severe Cerebellar Pathology. Nat. Med. 1996, 2, 1146–1150. [Google Scholar] [CrossRef]
- Ghisays, V.; Lopera, F.; Goradia, D.D.; Protas, H.D.; Malek-Ahmadi, M.H.; Chen, Y.; Devadas, V.; Luo, J.; Lee, W.; Baena, A.; et al. PET Evidence of Preclinical Cerebellar Amyloid Plaque Deposition in Autosomal Dominant Alzheimer’s Disease-Causing Presenilin-1 E280A Mutation Carriers. Neuroimage Clin. 2021, 31, 102749. [Google Scholar] [CrossRef]
- Li, N.; Liu, K.; Qiu, Y.; Ren, Z.; Dai, R.; Deng, Y.; Qing, H. Effect of Presenilin Mutations on APP Cleavage; Insights into the Pathogenesis of FAD. Front. Aging Neurosci. 2016, 8, 51. [Google Scholar] [CrossRef]
- Soto-Mercado, V.; Mendivil-Perez, M.; Velez-Pardo, C.; Jimenez-Del-rio, M. (−)-Epigallocatechin-3-Gallate Diminishes Intra-and Extracellular Amyloid-Induced Cytotoxic Effects on Cholinergic-like Neurons from Familial Alzheimer’s Disease PSEN1 E280A. Biomolecules 2021, 11, 1845. [Google Scholar] [CrossRef]
- Soto-Mercado, V.; Mendivil-Perez, M.; Velez-Pardo, C.; Lopera, F.; Jimenez-Del-Rio, M. Cholinergic-like Neurons Carrying PSEN1 E280A Mutation from Familial Alzheimer’s Disease Reveal Intraneuronal SAPPβ Fragments Accumulation, Hyperphosphorylation of TAU, Oxidative Stress, Apoptosis and Ca2+ Dysregulation: Therapeutic Implications. PLoS ONE 2020, 15, e0221669. [Google Scholar] [CrossRef]
- Sullivan, M.A.; Lane, S.D.; McKenzie, A.D.J.; Ball, S.R.; Sunde, M.; Neely, G.G.; Moreno, C.L.; Maximova, A.; Werry, E.L.; Kassiou, M. IPSC-Derived PSEN2 (N141I) Astrocytes and Microglia Exhibit a Primed Inflammatory Phenotype. J. Neuroinflammation 2024, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.S.; Pálovics, R.; Munson, C.N.; Long, C.; Johansson, P.K.; Yip, O.; Dong, W.; Rawat, E.; West, E.; Schlachetzki, J.C.M.; et al. APOE4/4 Is Linked to Damaging Lipid Droplets in Alzheimer’s Disease Microglia. Nature 2024, 628, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Ohgidani, M.; Kato, T.A.; Setoyama, D.; Sagata, N.; Hashimoto, R.; Shigenobu, K.; Yoshida, T.; Hayakawa, K.; Shimokawa, N.; Miura, D.; et al. Direct Induction of Ramified Microglia-like Cells from Human Monocytes: Dynamic Microglial Dysfunction in Nasu-Hakola Disease. Sci. Rep. 2014, 4, 4957. [Google Scholar] [CrossRef]
- Banerjee, A.; Lu, Y.; Do, K.; Mize, T.; Wu, X.; Chen, X.; Chen, J. Validation of Induced Microglia-Like Cells (IMG Cells) for Future Studies of Brain Diseases. Front. Cell. Neurosci. 2021, 15, 629279. [Google Scholar] [CrossRef]
- Quek, H.; Cuní-López, C.; Stewart, R.; Lim, Y.C.; Roberts, T.L.; White, A.R. A Robust Approach to Differentiate Human Monocyte-Derived Microglia from Peripheral Blood Mononuclear Cells. STAR Protoc. 2022, 3, 101747. [Google Scholar] [CrossRef]
- Colonna, M. The Biology of TREM Receptors. Nat. Rev. Immunol. 2023, 23, 580–594. [Google Scholar] [CrossRef]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-Specific Localisation of a Novel Calcium Binding Protein, Iba1. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Ruan, C.; Elyaman, W. A New Understanding of TMEM119 as a Marker of Microglia. Front. Cell. Neurosci. 2022, 16, 902372. [Google Scholar] [CrossRef]
- Galloway, D.A.; Phillips, A.E.M.; Owen, D.R.J.; Moore, C.S. Phagocytosis in the Brain: Homeostasis and Disease. Front. Immunol. 2019, 10, 790. [Google Scholar] [CrossRef]
- Kim, I.D.; Lee, H.; Jin, Y.C.; Lee, J.K. Osteopontin Peptide Icosamer Containing RGD and SLAYGLR Motifs Enhances the Motility and Phagocytic Activity of Microglia. Exp. Neurobiol. 2017, 26, 339–349. [Google Scholar] [CrossRef]
- De, M.; Serpa, G.; Zuiker, E.; Hisert, K.B.; Liles, W.C.; Manicone, A.M.; Hemann, E.A.; Long, M.E. MEK1/2 Inhibition Decreases pro-Inflammatory Responses in Macrophages from People with Cystic Fibrosis and Mitigates Severity of Illness in Experimental Murine Methicillin-Resistant Staphylococcus Aureus Infection. Front. Cell Infect. Microbiol. 2024, 14, 1275940. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Kushwaha, R.; Molesworth, K.; Mychko, O.; Makarava, N.; Baskakov, I.V. Phagocytic Activities of Reactive Microglia and Astrocytes Associated with Prion Diseases Are Dysregulated in Opposite Directions. Cells 2021, 10, 1728. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In Vitro Scratch Assay: A Convenient and Inexpensive Method for Analysis of Cell Migration in Vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Kenkhuis, B.; Somarakis, A.; Kleindouwel, L.R.T.; van Roon-Mom, W.M.C.; Höllt, T.; van der Weerd, L. Co-Expression Patterns of Microglia Markers Iba1, TMEM119 and P2RY12 in Alzheimer’s Disease. Neurobiol. Dis. 2022, 167, 105684. [Google Scholar] [CrossRef]
- Savage, J.C.; Carrier, M.; Tremblay, M.È. Morphology of Microglia Across Contexts of Health and Disease. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2019; Volume 2034, pp. 13–26. [Google Scholar]
- Torres-Platas, S.G.; Comeau, S.; Rachalski, A.; Bo, G.D.; Cruceanu, C.; Turecki, G.; Giros, B.; Mechawar, N. Morphometric Characterization of Microglial Phenotypes in Human Cerebral Cortex. J. Neuroinflamm. 2014, 11, 12. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef]
- Mendivil-Perez, M.; Velez-Pardo, C.; Lopera, F.; Kosik, K.S.; Jimenez-Del-Rio, M. PSEN1 E280A Cholinergic-like Neurons and Cerebral Spheroids Derived from Mesenchymal Stromal Cells and from Induced Pluripotent Stem Cells Are Neuropathologically Equivalent. Int. J. Mol. Sci. 2023, 24, 8957. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef]
- Satoh, J.; Kino, Y.; Asahina, N.; Takitani, M.; Miyoshi, J.; Ishida, T.; Saito, Y. TMEM119 Marks a Subset of Microglia in the Human Brain. Neuropathology 2016, 36, 39–49. [Google Scholar] [CrossRef]
- Muffat, J.; Li, Y.; Yuan, B.; Mitalipova, M.; Omer, A.; Corcoran, S.; Bakiasi, G.; Tsai, L.-H.; Aubourg, P.; Ransohoff, R.M.; et al. Efficient Derivation of Microglia-like Cells from Human Pluripotent Stem Cells. Nat. Med. 2016, 22, 1358–1367. [Google Scholar] [CrossRef]
- Douvaras, P.; Sun, B.; Wang, M.; Kruglikov, I.; Lallos, G.; Zimmer, M.; Terrenoire, C.; Zhang, B.; Gandy, S.; Schadt, E.; et al. Directed Differentiation of Human Pluripotent Stem Cells to Microglia. Stem Cell Rep. 2017, 8, 1516–1524. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. Ros Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-ΚB by Toll-like Receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Warden, A.S.; Han, C.; Hansen, E.; Trescott, S.; Nguyen, C.; Kim, R.; Schafer, D.; Johnson, A.; Wright, M.; Ramirez, G.; et al. Tools for Studying Human Microglia: In Vitro and in Vivo Strategies. Brain Behav. Immun. 2023, 107, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Farfara, D.; Trudler, D.; Segev-Amzaleg, N.; Galron, R.; Stein, R.; Frenkel, D. γ-Secretase Component Presenilin Is Important for Microglia β-Amyloid Clearance. Ann. Neurol. 2011, 69, 170–180. [Google Scholar] [CrossRef]
- Ledo, J.H.; Liebmann, T.; Zhang, R.; Chang, J.C.; Azevedo, E.P.; Wong, E.; Silva, H.M.; Troyanskaya, O.G.; Bustos, V.; Greengard, P. Presenilin 1 Phosphorylation Regulates Amyloid-β Degradation by Microglia. Mol. Psychiatry 2021, 26, 5620–5635. [Google Scholar] [CrossRef]
- Maezawa, I.; Zimin, P.I.; Wulff, H.; Jin, L.W. Amyloid-β Protein Oligomer at Low Nanomolar Concentrations Activates Microglia and Induces Microglial Neurotoxicity. J. Biol. Chem. 2011, 286, 3693–3706. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, J.; Xiong, L.; She, L.; Li, L.; Tang, H.; Zeng, Y.; Chen, F.; Han, X.; Ye, S.; et al. β-Amyloid Binds to Microglia Dectin-1 to Induce Inflammatory Response in the Pathogenesis of Alzheimer’s Disease. Int. J. Biol. Sci. 2023, 19, 3249–3265. [Google Scholar] [CrossRef]
- Guadagno, J.; Xu, X.; Karajgikar, M.; Brown, A.; Cregan, S.P. Microglia-Derived TNFα Induces Apoptosis in Neural Precursor Cells via Transcriptional Activation of the Bcl-2 Family Member Puma. Cell Death Dis. 2013, 4, e538. [Google Scholar] [CrossRef]
- Chen, Y.; Strickland, M.R.; Soranno, A.; Holtzman, D.M. Apolipoprotein E: Structural Insights and Links to Alzheimer Disease Pathogenesis. Neuron 2021, 109, 205–221. [Google Scholar] [CrossRef]
- Li, Z.; Martens, Y.A.; Ren, Y.; Jin, Y.; Sekiya, H.; Doss, S.V.; Kouri, N.; Castanedes-Casey, M.; Christensen, T.A.; Miller Nevalainen, L.B.; et al. APOE Genotype Determines Cell-Type-Specific Pathological Landscape of Alzheimer’s Disease. Neuron 2025, 113, 1380–1397.e7. [Google Scholar] [CrossRef]
- Acosta-Baena, N.; Sepulveda-Falla, D.; Lopera-Gómez, C.M.; Jaramillo-Elorza, M.C.; Moreno, S.; Aguirre-Acevedo, D.C.; Saldarriaga, A.; Lopera, F. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer’s disease: A retrospective cohort study. Lancet Neurol. 2011, 10, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kyuragi, S.; Inamine, S.; Ohgidani, M.; Kato, T.A. Engineering of Human Blood-Induced Microglia-like Cells for Reverse-Translational Brain Research. J. Vis. Exp. 2024, 211, e66819. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, T.J.; Mousseau, D.D. Brain organoids engineered to give rise to glia and neural networks after 90 days in culture exhibit human-specific proteoforms. Front. Cell. Neurosci. 2024, 18, 1383688. [Google Scholar] [CrossRef] [PubMed]
- Sharo, C.; Zhai, T.; Huang, Z. Investigation of Potential Drug Targets Involved in Inflammation Contributing to Alzheimer’s Disease Progression. Pharmaceuticals 2024, 17, 137. [Google Scholar] [CrossRef]
- Sharo, C.; Zhang, J.; Zhai, T.; Bao, J.; Garcia-Epelboim, A.; Mamourian, E.; Shen, L.; Huang, Z. Repurposing FDA-Approved Drugs Against Potential Drug Targets Involved in Brain Inflammation Contributing to Alzheimer’s Disease. Targets 2024, 2, 446–469. [Google Scholar] [CrossRef]
- Hübschmann, V.; Korkut-Demirbaş, M.; Siegert, S. Assessing Human IPSC-Derived Microglia Identity and Function by Immunostaining, Phagocytosis, Calcium Activity, and Inflammation Assay. STAR Protoc. 2022, 3, 101866. [Google Scholar] [CrossRef]
- Lively, S.; Schlichter, L.C. The Microglial Activation State Regulates Migration and Roles of Matrix-Dissolving Enzymes for Invasion. J. Neuroinflamm. 2013, 10, 843–875. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Figueroa, F.T.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An Image J Plugin for the High Throughput Image Analysis of in Vitro Scratch Wound Healing Assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]
- Soto-Mercado, V.; Mendivil-Perez, M.; Velez-Pardo, C.; Jimenez-Del-Rio, M. Neuroprotective Effect of Combined Treatment with Epigallocatechin 3-Gallate and Melatonin on Familial Alzheimer’s Disease PSEN1 E280A Cerebral Spheroids Derived from Menstrual Mesenchymal Stromal Cells. J. Alzheimers Dis. 2024, 99, S51–S66. [Google Scholar] [CrossRef]
- Jairaman, A.; McQuade, A.; Granzotto, A.; Kang, Y.J.; Chadarevian, J.P.; Gandhi, S.; Parker, I.; Smith, I.; Cho, H.; Sensi, S.L.; et al. TREM2 Regulates Purinergic Receptor-Mediated Calcium Signaling and Motility in Human IPSC-Derived Microglia. eLlife 2022, 11, e73021. [Google Scholar] [CrossRef] [PubMed]
- Lazic, S.E.; Clarke-Williams, C.J.; Munafò, M.R. What Exactly Is ‘N’ in Cell Culture and Animal Experiments? PLoS Biol. 2018, 16, e2005282. [Google Scholar] [CrossRef] [PubMed]
- Connor, S.M.; Rashid, M.; Ryan, K.J.; Patel, K.; Boyd, J.D.; Smith, J.; Elyaman, W.; Bennett, D.A.; Bradshaw, E.M. GW5074 Increases Microglial Phagocytic Activities: Potential Therapeutic Direction for Alzheimer’s Disease. Front. Cell Neurosci. 2022, 16, 894601. [Google Scholar] [CrossRef]
- Plantone, D.; Pardini, M.; Righi, D.; Manco, C.; Colombo, B.M.; De Stefano, N. The Role of TNF-α in Alzheimer’s Disease: A Narrative Review. Cells 2024, 13, 54. [Google Scholar] [CrossRef]
- Karunaweera, N.; Raju, R.; Gyengesi, E.; Munch, G. Plant Polyphenols as Inhibitors of Nf-Kb Induced Cytokine Production—A Potential Anti-Inflammatory Treatment for Alzheimer’s Disease? Front. Mol. Neurosci. 2015, 8, 24. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; So, K.F.; Jiang, W.; Chiu, K. Targeting Microglia in Alzheimer’s Disease: Pathogenesis and Potential Therapeutic Strategies. Biomolecules 2024, 14, 833. [Google Scholar] [CrossRef]





| Sample | TBC# | Cell Type | Differentiated Cell Type | Age | Sex | APOE*/*Genotype |
|---|---|---|---|---|---|---|
| WT (CNT) | PBMC0001 | PBMC | WT iMG | 39 | Male | 3/3 |
| FAD E280 | PBMC1117 | PBMC | E280A iMG | 45 | Male | 3/3 |
| WT (CNT) | PBMC0002 | PBMC | WT iMG | 82 | Male | 3/3 |
| SAD | PBMC21513 | PBMC | SAD iMG | 75 | Male | 3/4 |
| WT MenSC | MSC-MB0001 | MenSC | WT ChLNs | 23 | Female | 3/3 |
| MenSC E280A | MSC-MB0002 | MenSC | E280AChLNs | 25 | Female | 3/4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Mercado, V.; Mendivil-Perez, M.; Velez-Pardo, C.; Jimenez-Del-Rio, M. Induced Microglial-like Cells Derived from Familial and Sporadic Alzheimer’s Disease Peripheral Blood Monocytes Show Abnormal Phagocytosis and Inflammatory Response to PSEN1 E280A Cholinergic-like Neurons. Int. J. Mol. Sci. 2025, 26, 7162. https://doi.org/10.3390/ijms26157162
Soto-Mercado V, Mendivil-Perez M, Velez-Pardo C, Jimenez-Del-Rio M. Induced Microglial-like Cells Derived from Familial and Sporadic Alzheimer’s Disease Peripheral Blood Monocytes Show Abnormal Phagocytosis and Inflammatory Response to PSEN1 E280A Cholinergic-like Neurons. International Journal of Molecular Sciences. 2025; 26(15):7162. https://doi.org/10.3390/ijms26157162
Chicago/Turabian StyleSoto-Mercado, Viviana, Miguel Mendivil-Perez, Carlos Velez-Pardo, and Marlene Jimenez-Del-Rio. 2025. "Induced Microglial-like Cells Derived from Familial and Sporadic Alzheimer’s Disease Peripheral Blood Monocytes Show Abnormal Phagocytosis and Inflammatory Response to PSEN1 E280A Cholinergic-like Neurons" International Journal of Molecular Sciences 26, no. 15: 7162. https://doi.org/10.3390/ijms26157162
APA StyleSoto-Mercado, V., Mendivil-Perez, M., Velez-Pardo, C., & Jimenez-Del-Rio, M. (2025). Induced Microglial-like Cells Derived from Familial and Sporadic Alzheimer’s Disease Peripheral Blood Monocytes Show Abnormal Phagocytosis and Inflammatory Response to PSEN1 E280A Cholinergic-like Neurons. International Journal of Molecular Sciences, 26(15), 7162. https://doi.org/10.3390/ijms26157162







