It Is Time to Consider the Lost Battle of Microdamaged Piezo2 in the Context of E. coli and Early-Onset Colorectal Cancer
Abstract
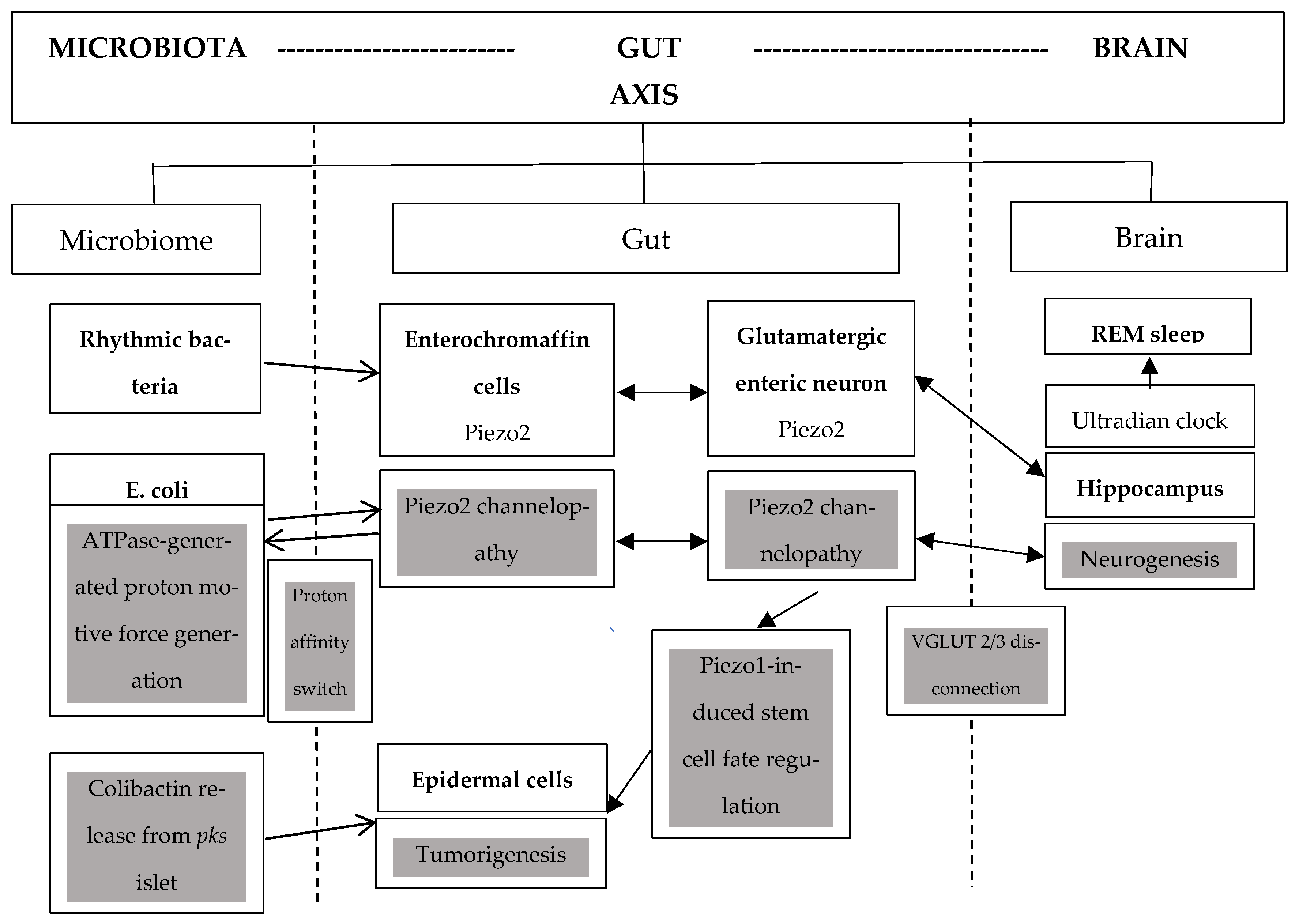
1. Introduction
2. Piezo2 Channelopathy, Dysbiosis, and Circadian Regulation
3. Colibactin, Escherichia coli, and Piezo2
4. Blue Light Link to Colorectal Cancer
5. Nerve Growth Factor and Colorectal Cancer
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diaz-Gay, M.; Dos Santos, W.; Moody, S.; Kazachkova, M.; Abbasi, A.; Steele, C.D.; Vangara, R.; Senkin, S.; Wang, J.; Fitzgerald, S.; et al. Geographic and age variations in mutational processes in colorectal cancer. Nature 2025, 643, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.K.; Fortin, B.M.; Fellows, R.C.; Habowski, A.N.; Verlande, A.; Song, W.A.; Mahieu, A.L.; Lefebvre, A.; Sterrenberg, J.N.; Velez, L.M.; et al. Disruption of the circadian clock drives Apc loss of heterozygosity to accelerate colorectal cancer. Sci. Adv. 2022, 8, eabo2389. [Google Scholar] [CrossRef] [PubMed]
- Adnan, D.; Trinh, J.Q.; Sharma, D.; Alsayid, M.; Bishehsari, F. Early-onset Colon Cancer Shows a Distinct Intestinal Microbiome and a Host-Microbe Interaction. Cancer Prev. Res. 2024, 17, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Moossavi, S.; Bishehsari, F. Microbes: Possible link between modern lifestyle transition and the rise of metabolic syndrome. Obes. Rev. 2019, 20, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Bishehsari, F.; Mahdavinia, M.; Vacca, M.; Malekzadeh, R.; Mariani-Costantini, R. Epidemiological transition of colorectal cancer in developing countries: Environmental factors, molecular pathways, and opportunities for prevention. World J. Gastroenterol. 2014, 20, 6055–6072. [Google Scholar] [CrossRef] [PubMed]
- Ugai, T.; Sasamoto, N.; Lee, H.Y.; Ando, M.; Song, M.; Tamimi, R.M.; Kawachi, I.; Campbell, P.T.; Giovannucci, E.L.; Weiderpass, E.; et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat. Rev. Clin. Oncol. 2022, 19, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.H.; Lukacs, V.; de Nooij, J.C.; Zaytseva, D.; Criddle, C.R.; Francisco, A.; Jessell, T.M.; Wilkinson, K.A.; Patapoutian, A. Piezo2 is the principal mechanotransduction channel for proprioception. Nat. Neurosci. 2015, 18, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Sonkodi, B. Acquired Piezo2 Channelopathy is One Principal Gateway to Pathophysiology. Front. Biosci. (Landmark Ed.) 2025, 30, 33389. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Cai, S.; Ye, C.; Li, T.; Tian, Y.; Liu, E.; Cai, J.; Yuan, X.; Yang, H.; Liang, Q.; et al. Neural influences in colorectal cancer progression and therapeutic strategies. Int. J. Colorectal Dis. 2025, 40, 120. [Google Scholar] [CrossRef] [PubMed]
- Sonkodi, B. Is acquired Piezo2 channelopathy the critical impairment of the brain axes and dysbiosis? OSF Prepr. 2025. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Leung, V.H.; Seradj, S.H.; Sonmez, U.; Servin-Vences, M.R.; Xiao, S.; Ren, X.; Wang, L.; Mishkanian, S.A.; et al. A key role of PIEZO2 mechanosensitive ion channel in adipose sensory innervation. Cell Metab. 2025, 37, 1001–1011 e1007. [Google Scholar] [CrossRef] [PubMed]
- Passini, F.S.; Bornstein, B.; Rubin, S.; Kuperman, Y.; Krief, S.; Masschelein, E.; Mehlman, T.; Brandis, A.; Addadi, Y.; Shalom, S.H.; et al. Piezo2 in sensory neurons regulates systemic and adipose tissue metabolism. Cell Metab. 2025, 37, 987–1000.e1006. [Google Scholar] [CrossRef] [PubMed]
- Elek, D.; Toth, M.; Sonkodi, B.; Acs, P.; Kovacs, G.L.; Tardi, P.; Melczer, C. The Efficacy of Soleus Push-Up in Individuals with Prediabetes: A Pilot Study. Sports 2025, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Rothenberg, M.E.; Sun, X.; Bachert, C.; Artis, D.; Zaheer, R.; Deniz, Y.; Rowe, P.; Cyr, S. Neuroimmune interplay during type 2 inflammation: Symptoms, mechanisms, and therapeutic targets in atopic diseases. J. Allergy Clin. Immunol. 2024, 153, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Buzás, A.; Sonkodi, B.; Dér, A. Principal Connection Between Typical Heart-Rate-Variability Parameters as Revealed by a Comparative Analysis of Their Heart-Rate- and Age-Dependence. Preprints 2025. [Google Scholar] [CrossRef]
- McDowell, R.; Perrott, S.; Murchie, P.; Cardwell, C.; Hughes, C.; Samuel, L. Oral antibiotic use and early-onset colorectal cancer: Findings from a case-control study using a national clinical database. Br. J. Cancer 2022, 126, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wanggou, S.; Bodalia, A.; Zhu, M.; Dong, W.; Fan, J.J.; Yin, W.C.; Min, H.K.; Hu, M.; Draghici, D.; et al. A Feedforward Mechanism Mediated by Mechanosensitive Ion Channel PIEZO1 and Tissue Mechanics Promotes Glioma Aggression. Neuron 2018, 100, 799–815 e797. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Reitmeier, S.; Kiessling, S.; Clavel, T.; List, M.; Almeida, E.L.; Ghosh, T.S.; Neuhaus, K.; Grallert, H.; Linseisen, J.; Skurk, T.; et al. Arrhythmic Gut Microbiome Signatures Predict Risk of Type 2 Diabetes. Cell Host Microbe 2020, 28, 258–272 e256. [Google Scholar] [CrossRef] [PubMed]
- Ubilla, P.K.; Ferrada, E.; Marquet, P.A. Rhythmic Bacteria as Biomarkers for Circadian-Related Diseases. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Cao, Q.; Zhao, M.; Su, Y.; Liu, S.; Lin, Y.; Da, H.; Yue, C.; Liu, Y.; Jing, D.; Zhao, Q.; et al. Chronic Stress Dampens Lactobacillus Johnsonii-Mediated Tumor Suppression to Enhance Colorectal Cancer Progression. Cancer Res. 2024, 84, 771–784. [Google Scholar] [CrossRef] [PubMed]
- McCollum, S.E.; Shah, Y.M. Stressing Out Cancer: Chronic Stress Induces Dysbiosis and Enhances Colon Cancer Growth. Cancer Res. 2024, 84, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, J.; Yang, X.; Zhou, G.; Wang, L.; Xiao, B. Tethering Piezo channels to the actin cytoskeleton for mechanogating via the cadherin-beta-catenin mechanotransduction complex. Cell Rep. 2022, 38, 110342. [Google Scholar] [CrossRef] [PubMed]
- Sonkodi, B.; Resch, M.D.; Hortobagyi, T. Is the Sex Difference a Clue to the Pathomechanism of Dry Eye Disease? Watch out for the NGF-TrkA-Piezo2 Signaling Axis and the Piezo2 Channelopathy. J. Mol. Neurosci. 2022, 72, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Sonkodi, B. Progressive Irreversible Proprioceptive Piezo2 Channelopathy-Induced Lost Forced Peripheral Oscillatory Synchronization to the Hippocampal Oscillator May Explain the Onset of Amyotrophic Lateral Sclerosis Pathomechanism. Cells 2024, 13, 492. [Google Scholar] [CrossRef] [PubMed]
- Sonkodi, B.; Hortobágyi, T. Amyotrophic lateral sclerosis and delayed onset muscle soreness in light of the impaired blink and stretch reflexes – watch out for Piezo2. Open Med. 2022, 17, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Kaci, G.; Goudercourt, D.; Dennin, V.; Pot, B.; Dore, J.; Ehrlich, S.D.; Renault, P.; Blottiere, H.M.; Daniel, C.; Delorme, C. Anti-inflammatory properties of Streptococcus salivarius, a commensal bacterium of the oral cavity and digestive tract. Appl. Environ. Microbiol. 2014, 80, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Saus, E.; Iraola-Guzman, S.; Willis, J.R.; Brunet-Vega, A.; Gabaldon, T. Microbiome and colorectal cancer: Roles in carcinogenesis and clinical potential. Mol. Asp. Med. 2019, 69, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Sonkodi, B. Miswired Proprioception in Amyotrophic Lateral Sclerosis in Relation to Pain Sensation (and in Delayed Onset Muscle Soreness)-Is Piezo2 Channelopathy a Principal Transcription Activator in Proprioceptive Terminals Besides Being the Potential Primary Damage? Life 2023, 13, 657. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, D. The synthesis of the novel Escherichia coli toxin-colibactin and its mechanisms of tumorigenesis of colorectal cancer. Front. Microbiol. 2024, 15, 1501973. [Google Scholar] [CrossRef] [PubMed]
- Gevorgyan, H.; Baghdasaryan, L.; Trchounian, K. Regulation of metabolism and proton motive force generation during mixed carbon fermentation by an Escherichia coli strain lacking the F(O)F(1)-ATPase. Biochim. Biophys. Acta Bioenerg. 2024, 1865, 149034. [Google Scholar] [CrossRef] [PubMed]
- Homburg, S.; Oswald, E.; Hacker, J.; Dobrindt, U. Expression analysis of the colibactin gene cluster coding for a novel polyketide in Escherichia coli. FEMS Microbiol. Lett. 2007, 275, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Iyadorai, T.; Mariappan, V.; Vellasamy, K.M.; Wanyiri, J.W.; Roslani, A.C.; Lee, G.K.; Sears, C.; Vadivelu, J. Prevalence and association of pks+ Escherichia coli with colorectal cancer in patients at the University Malaya Medical Centre, Malaysia. PLoS ONE 2020, 15, e0228217. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.C.; Perez-Chanona, E.; Muhlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Buc, E.; Dubois, D.; Sauvanet, P.; Raisch, J.; Delmas, J.; Darfeuille-Michaud, A.; Pezet, D.; Bonnet, R. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS ONE 2013, 8, e56964. [Google Scholar] [CrossRef] [PubMed]
- Dejea, C.M.; Fathi, P.; Craig, J.M.; Boleij, A.; Taddese, R.; Geis, A.L.; Wu, X.; DeStefano Shields, C.E.; Hechenbleikner, E.M.; Huso, D.L.; et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 2018, 359, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Desplat, A.; Penalba, V.; Gros, E.; Parpaite, T.; Coste, B.; Delmas, P. Piezo1-Pannexin1 complex couples force detection to ATP secretion in cholangiocytes. J. Gen. Physiol. 2021, 153, e202112871. [Google Scholar] [CrossRef] [PubMed]
- Sonkodi, B. Delayed-Onset Muscle Soreness Begins with a Transient Neural Switch. Int. J. Mol. Sci. 2025, 26, 2319. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Meng, L.; Gao, J.; Lu, M.; Guo, C.; Li, Y.; Rong, Z.; Ye, Y. PHB2 promotes colorectal cancer cell proliferation and tumorigenesis through NDUFS1-mediated oxidative phosphorylation. Cell Death Dis. 2023, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Aisu, Y.; Oshima, N.; Hyodo, F.; Elhelaly, A.E.; Masuo, A.; Okada, T.; Hisamori, S.; Tsunoda, S.; Hida, K.; Morimoto, T.; et al. Dual inhibition of oxidative phosphorylation and glycolysis exerts a synergistic antitumor effect on colorectal and gastric cancer by creating energy depletion and preventing metabolic switch. PLoS ONE 2024, 19, e0309700. [Google Scholar] [CrossRef] [PubMed]
- Ewald, J.; He, Z.; Dimitriew, W.; Schuster, S. Including glutamine in a resource allocation model of energy metabolism in cancer and yeast cells. NPJ Syst. Biol. Appl. 2024, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Xu, A.; Yan, H.; Xu, D.; Zhang, J.; Fang, X. PIEZO2 promotes cell proliferation and metastasis in colon carcinoma through the SLIT2/ROBO1/VEGFC pathway. Adv. Clin. Exp. Med. 2023, 32, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Liddle, R.A. Mechanosensing Piezo channels in gastrointestinal disorders. J. Clin. Investig. 2023, 133, e171955. [Google Scholar] [CrossRef] [PubMed]
- Vicente, C.M.; Ricci, R.; Nader, H.B.; Toma, L. Syndecan-2 is upregulated in colorectal cancer cells through interactions with extracellular matrix produced by stromal fibroblasts. BMC Cell Biol. 2013, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Song, H.K.; Hwang, E.S.; Lee, A.R.; Han, D.S.; Kim, S.E.; Oh, E.S. Up-regulation of syndecan-2 in proximal colon correlates with acute inflammation. FASEB J. 2019, 33, 11381–11395. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Choi, Y.; Jun, E.; Kim, I.S.; Kim, S.E.; Jung, S.A.; Oh, E.S. Shed syndecan-2 enhances tumorigenic activities of colon cancer cells. Oncotarget 2015, 6, 3874–3886. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Song, H.K.; Hwang, J.; Lee, S.; Park, E.; Oh, A.; Hwang, E.S.; Sung, J.Y.; Kim, Y.N.; Park, K.; et al. Shed syndecan-2 enhances colon cancer progression by increasing cooperative angiogenesis in the tumor microenvironment. Matrix Biol. 2022, 107, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Provencio, I.; Jiang, G.; De Grip, W.J.; Hayes, W.P.; Rollag, M.D. Melanopsin: An opsin in melanophores, brain, and eye. Proc. Natl. Acad. Sci. USA 1998, 95, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.; Engelhardt, M.; Schaupp, P.; Lappe, C.; Ivanov, I.V. The inner clock-Blue light sets the human rhythm. J. Biophotonics 2019, 12, e201900102. [Google Scholar] [CrossRef] [PubMed]
- Bevan, R.J.; Hughes, T.R.; Williams, P.A.; Good, M.A.; Morgan, B.P.; Morgan, J.E. Retinal ganglion cell degeneration correlates with hippocampal spine loss in experimental Alzheimer’s disease. Acta Neuropathol. Commun. 2020, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Morozumi, W.; Inagaki, S.; Iwata, Y.; Nakamura, S.; Hara, H.; Shimazawa, M. Piezo channel plays a part in retinal ganglion cell damage. Exp. Eye Res. 2020, 191, 107900. [Google Scholar] [CrossRef] [PubMed]
- Kassumeh, S.; Weber, G.R.; Nobl, M.; Priglinger, S.G.; Ohlmann, A. The neuroprotective role of Wnt signaling in the retina. Neural Regen. Res. 2021, 16, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Wahnschaffe, A.; Haedel, S.; Rodenbeck, A.; Stoll, C.; Rudolph, H.; Kozakov, R.; Schoepp, H.; Kunz, D. Out of the lab and into the bathroom: Evening short-term exposure to conventional light suppresses melatonin and increases alertness perception. Int. J. Mol. Sci. 2013, 14, 2573–2589. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Fremeau, R.T., Jr.; Duncan, J.L.; Renteria, R.C.; Yang, H.; Hua, Z.; Liu, X.; LaVail, M.M.; Edwards, R.H.; Copenhagen, D.R. Vesicular glutamate transporter 1 is required for photoreceptor synaptic signaling but not for intrinsic visual functions. J. Neurosci. 2007, 27, 7245–7255. [Google Scholar] [CrossRef] [PubMed]
- Tien, N.W.; Kim, T.; Kerschensteiner, D. Target-Specific Glycinergic Transmission from VGluT3-Expressing Amacrine Cells Shapes Suppressive Contrast Responses in the Retina. Cell Rep. 2016, 15, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, W. A color-coding amacrine cell may provide a blue-off signal in a mammalian retina. Nat. Neurosci. 2012, 15, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Ettaiche, M.; Deval, E.; Cougnon, M.; Lazdunski, M.; Voilley, N. Silencing acid-sensing ion channel 1a alters cone-mediated retinal function. J. Neurosci. 2006, 26, 5800–5809. [Google Scholar] [CrossRef] [PubMed]
- Friedrichsen, K.; Hsiang, J.C.; Lin, C.I.; McCoy, L.; Valkova, K.; Kerschensteiner, D.; Morgan, J.L. Subcellular pathways through VGluT3-expressing mouse amacrine cells provide locally tuned object-motion-selective signals in the retina. Nat. Commun. 2024, 15, 2965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shan, X.; Li, S.; Chang, J.; Zhang, Z.; Dong, Y.; Wang, L.; Liang, F. Retinal light damage: From mechanisms to protective strategies. Surv. Ophthalmol. 2024, 69, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, X.; Zhang, C.; Zhu, H.; Xu, Q.; Bu, Y.; Lei, Y. Redox Imbalance in the Development of Colorectal Cancer. J. Cancer 2017, 8, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, X.; Zhao, D.; Liu, H.; Hu, Y. Calcium homeostasis and cancer: Insights from endoplasmic reticulum-centered organelle communications. Trends Cell Biol. 2023, 33, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Zechini, L.; Camilleri-Brennan, J.; Walsh, J.; Beaven, R.; Moran, O.; Hartley, P.S.; Diaz, M.; Denholm, B. Piezo buffers mechanical stress via modulation of intracellular Ca(2+) handling in the Drosophila heart. Front. Physiol. 2022, 13, 1003999. [Google Scholar] [CrossRef] [PubMed]
- Ventre, S.; Indrieri, A.; Fracassi, C.; Franco, B.; Conte, I.; Cardone, L.; di Bernardo, D. Metabolic regulation of the ultradian oscillator Hes1 by reactive oxygen species. J. Mol. Biol. 2015, 427, 1887–1902. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Yoshiura, S.; Ohtsuka, T.; Bessho, Y.; Harada, T.; Yoshikawa, K.; Kageyama, R. Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science 2002, 298, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Takebayashi, K.; Sasai, Y.; Sakai, Y.; Watanabe, T.; Nakanishi, S.; Kageyama, R. Structure, chromosomal locus, and promoter analysis of the gene encoding the mouse helix-loop-helix factor HES-1. Negative autoregulation through the multiple N box elements. J. Biol. Chem. 1994, 269, 5150–5156. [Google Scholar] [CrossRef] [PubMed]
- Harima, Y.; Imayoshi, I.; Shimojo, H.; Kobayashi, T.; Kageyama, R. The roles and mechanism of ultradian oscillatory expression of the mouse Hes genes. Semin. Cell Dev. Biol. 2014, 34, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Huang, W.; Zhang, Y.; Tang, S.; Zheng, L.; Ma, F.; Wang, Y.; Tang, H.; Li, X. Hes1 promotes cell proliferation and migration by activating Bmi-1 and PTEN/Akt/GSK3beta pathway in human colon cancer. Oncotarget 2015, 6, 38667–38680. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, M.; Zhu, J.; Li, J.; Zhu, X.; Wang, K.; Shen, K.; Yang, K.; Ni, X.; Liu, X.; et al. HES1 promotes aerobic glycolysis and cancer progression of colorectal cancer via IGF2BP2-mediated GLUT1 m6A modification. Cell Death Discov. 2023, 9, 411. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.T.; Tsao, P.N.; Lin, H.L.; Tung, C.C.; Change, M.C.; Chang, Y.T.; Wong, J.M.; Wei, S.C. Hes1 Increases the Invasion Ability of Colorectal Cancer Cells via the STAT3-MMP14 Pathway. PLoS ONE 2015, 10, e0144322. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ke, J.; He, Z.; Chen, Z.; Huang, Q.; Ai, W.; Wang, G.; Wei, Y.; Zou, X.; Zhang, S.; et al. HES1 Promotes Colorectal Cancer Cell Resistance To 5-Fu by Inducing Of EMT and ABC Transporter Proteins. J. Cancer 2017, 8, 2802–2808. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Song, J.W.; Li, W.; Liu, X.; Cao, L.; Wan, L.M.; Tan, Y.X.; Ji, S.P.; Liang, Y.M.; Gong, F. The acid-sensing ion channel, ASIC2, promotes invasion and metastasis of colorectal cancer under acidosis by activating the calcineurin/NFAT1 axis. J. Exp. Clin. Cancer Res. 2017, 36, 130. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, C.L.; Szabo, A.; Torok, B.; Banrevi, K.; Correia, P.; Chaves, T.; Daumas, S.; Zelena, D. A New Player in the Hippocampus: A Review on VGLUT3+ Neurons and Their Role in the Regulation of Hippocampal Activity and Behaviour. Int. J. Mol. Sci. 2022, 23, 790. [Google Scholar] [CrossRef] [PubMed]
- Zygulska, A.L.; Furgala, A.; Krzemieniecki, K.; Wlodarczyk, B.; Thor, P. Autonomic dysregulation in colon cancer patients. Cancer Investig. 2018, 36, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Iida, T.; Ochiai, Y.; Malagola, E.; Zhi, X.; White, R.A.; Qian, J.; Wu, F.; Waterbury, Q.T.; Tu, R.; et al. Neuro-Mesenchymal Interaction Mediated by a beta2-Adrenergic Nerve Growth Factor Feedforward Loop Promotes Colorectal Cancer Progression. Cancer Discov. 2025, 15, 202–226. [Google Scholar] [CrossRef] [PubMed]
- Mizumura, K.; Taguchi, T. Neurochemical mechanism of muscular pain: Insight from the study on delayed onset muscle soreness. J. Physiol. Sci. 2024, 74, 4. [Google Scholar] [CrossRef] [PubMed]
- Langmár, G.; Sümegi, T.; Fülöp, B.; Pozsgai, L.; Mocsai, T.; Tóth, M.; Racz, L.; Kopper, B.; Dér, A.; Búzás, A.; et al. HRV Alterations During Delayed-Onset Muscle Soreness Inducing Exercise—With Piezo2 Interpretation. Preprints 2025. [Google Scholar] [CrossRef]
- Blythe, N.M.; Muraki, K.; Ludlow, M.J.; Stylianidis, V.; Gilbert, H.T.J.; Evans, E.L.; Cuthbertson, K.; Foster, R.; Swift, J.; Li, J.; et al. Mechanically activated Piezo1 channels of cardiac fibroblasts stimulate p38 mitogen-activated protein kinase activity and interleukin-6 secretion. J. Biol. Chem. 2019, 294, 17395–17408. [Google Scholar] [CrossRef] [PubMed]
- Emig, R.; Knodt, W.; Krussig, M.J.; Zgierski-Johnston, C.M.; Gorka, O.; Gross, O.; Kohl, P.; Ravens, U.; Peyronnet, R. Piezo1 Channels Contribute to the Regulation of Human Atrial Fibroblast Mechanical Properties and Matrix Stiffness Sensing. Cells 2021, 10, 663. [Google Scholar] [CrossRef] [PubMed]
- Bersani, G.; Iannitelli, A.; Massoni, E.; Garavini, A.; Grilli, A.; Di Giannantonio, M.; Conti, C.M.; Pancheri, P. Ultradian variation of nerve growth factor plasma levels in healthy and schizophrenic subjects. Int. J. Immunopathol. Pharmacol. 2004, 17, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Pedemonte, M.; Goldstein-Daruech, N.; Velluti, R.A. Temporal correlations between heart rate, medullary units and hippocampal theta rhythm in anesthetized, sleeping and awake guinea pigs. Auton. Neurosci. 2003, 107, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Pedemonte, M.; Velluti, R.A. Sensory processing could be temporally organized by ultradian brain rhythms. Rev. Neurol. 2005, 40, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yamazaki, S.; Cox, K.H.; Huang, Y.L.; Miller, E.W.; Takahashi, J.S. Coupling-dependent metabolic ultradian rhythms in confluent cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2211142119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, H.; Huo, L.; Wang, S.; Yang, Q.; Ye, Z.; Cao, J.; Wu, S.; Ma, C.; Shang, C. Neural mechanism of trigeminal nerve stimulation recovering defensive arousal responses in traumatic brain injury. Theranostics 2025, 15, 2315–2337. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.V., Jr.; Kutiyanawalla, A.; Pillai, A. Age-dependent alterations in nerve growth factor (NGF)-related proteins, sortilin, and learning and memory in rats. Physiol. Behav. 2011, 102, 149–157. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonkodi, B. It Is Time to Consider the Lost Battle of Microdamaged Piezo2 in the Context of E. coli and Early-Onset Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 7160. https://doi.org/10.3390/ijms26157160
Sonkodi B. It Is Time to Consider the Lost Battle of Microdamaged Piezo2 in the Context of E. coli and Early-Onset Colorectal Cancer. International Journal of Molecular Sciences. 2025; 26(15):7160. https://doi.org/10.3390/ijms26157160
Chicago/Turabian StyleSonkodi, Balázs. 2025. "It Is Time to Consider the Lost Battle of Microdamaged Piezo2 in the Context of E. coli and Early-Onset Colorectal Cancer" International Journal of Molecular Sciences 26, no. 15: 7160. https://doi.org/10.3390/ijms26157160
APA StyleSonkodi, B. (2025). It Is Time to Consider the Lost Battle of Microdamaged Piezo2 in the Context of E. coli and Early-Onset Colorectal Cancer. International Journal of Molecular Sciences, 26(15), 7160. https://doi.org/10.3390/ijms26157160





