Analysis of Phosphodiesterase-5 (PDE5) Inhibitors in Modulating Inflammatory Markers in Humans: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Identification of Studies
2.2. Population
2.3. Intervention
2.4. Comparisons
2.5. Outcomes
2.6. Research Design
2.7. Data Extraction
2.8. Quality Analysis
2.9. Data Synthesis
3. Results
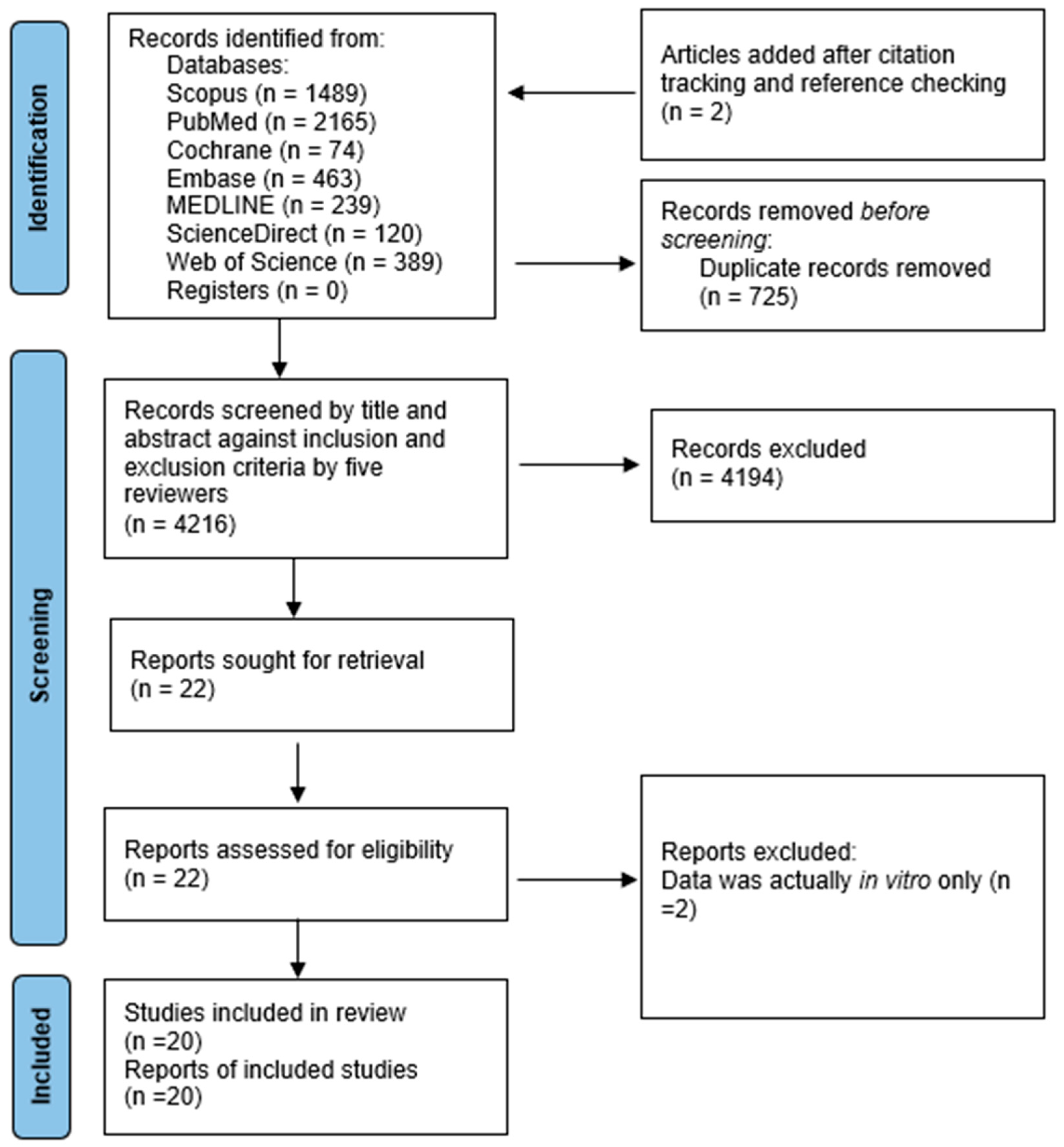
3.1. Yield
3.2. Characteristics of Included Studies
3.3. Effect of PDE5 Inhibitors on Inflammatory Markers
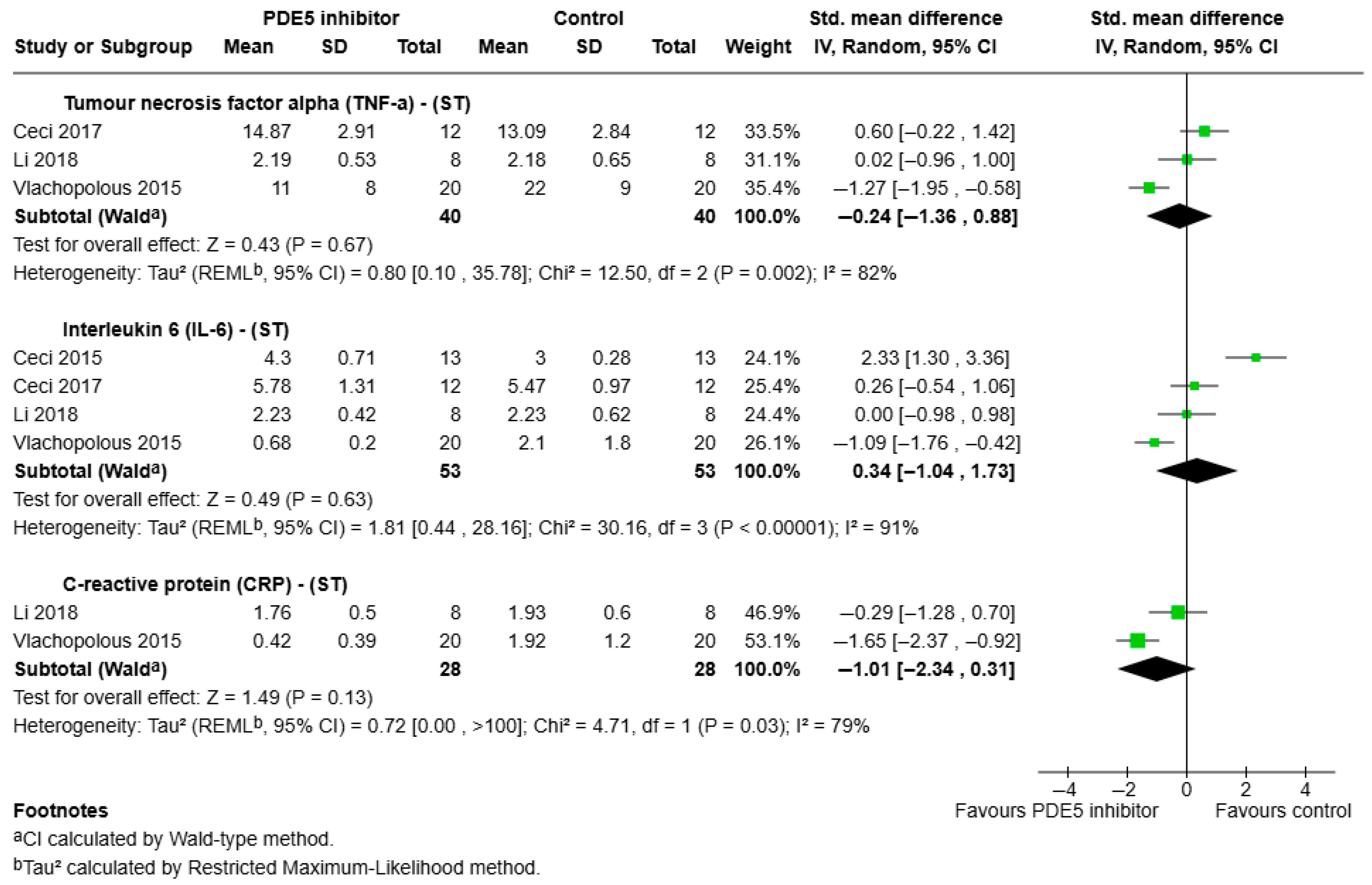
3.3.1. Short-Term Effects of PDE5 Inhibitors on Pro-Inflammatory Outcomes
- a.
- TNF-α:
- b.
- IL-6:
- c.
- IL-8:
- d.
- CRP:
- e.
- VCAM:
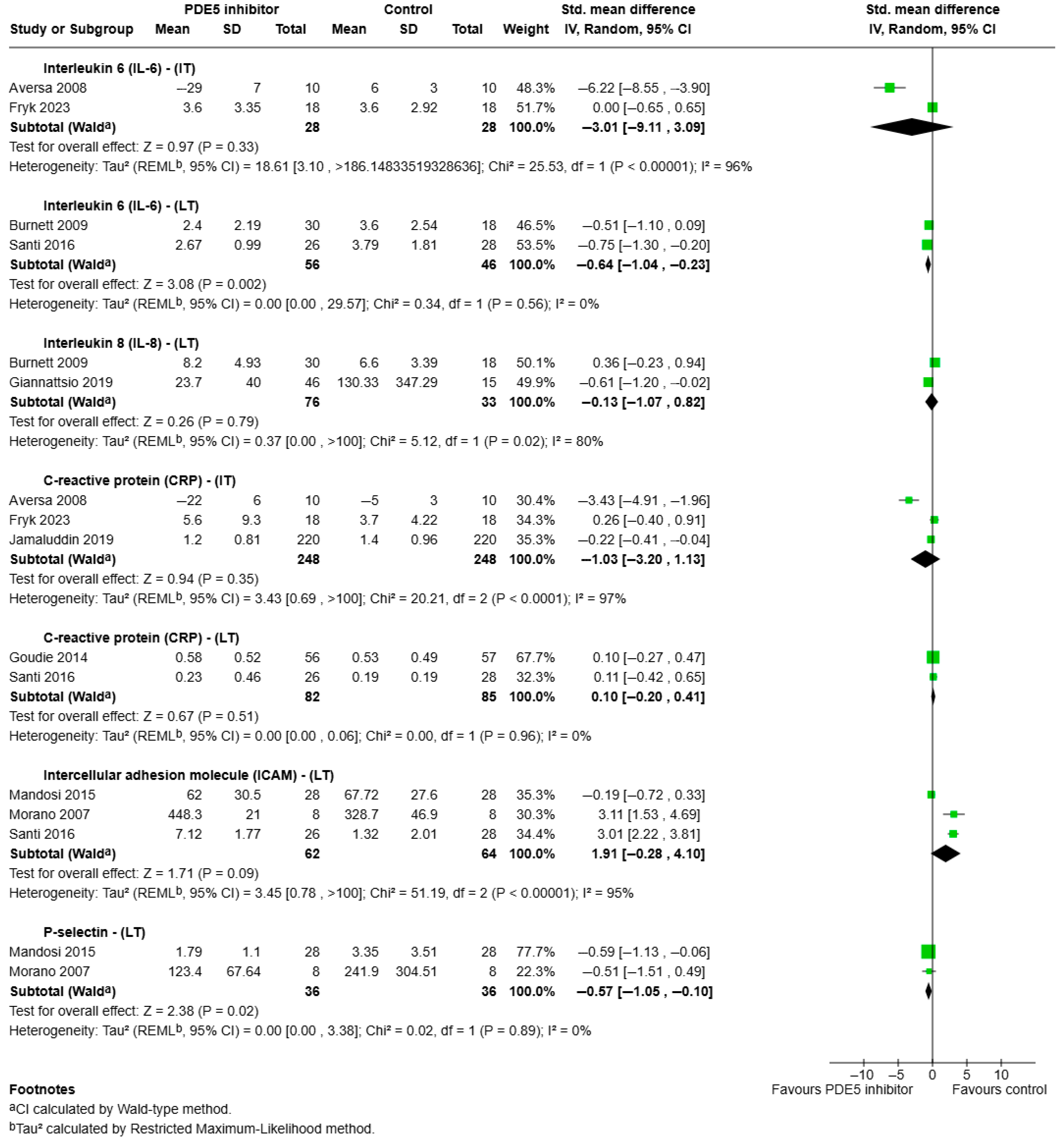
3.3.2. Intermediate—Long Term Effects of PDE5 Inhibitors on Pro-Inflammatory Outcomes
- a.
- IL-6:
- b.
- IL-8:
- c.
- CRP:
- d.
- ICAM:
- e.
- P-selectin:
- f.
- TNF-α:
- g.
- VCAM:
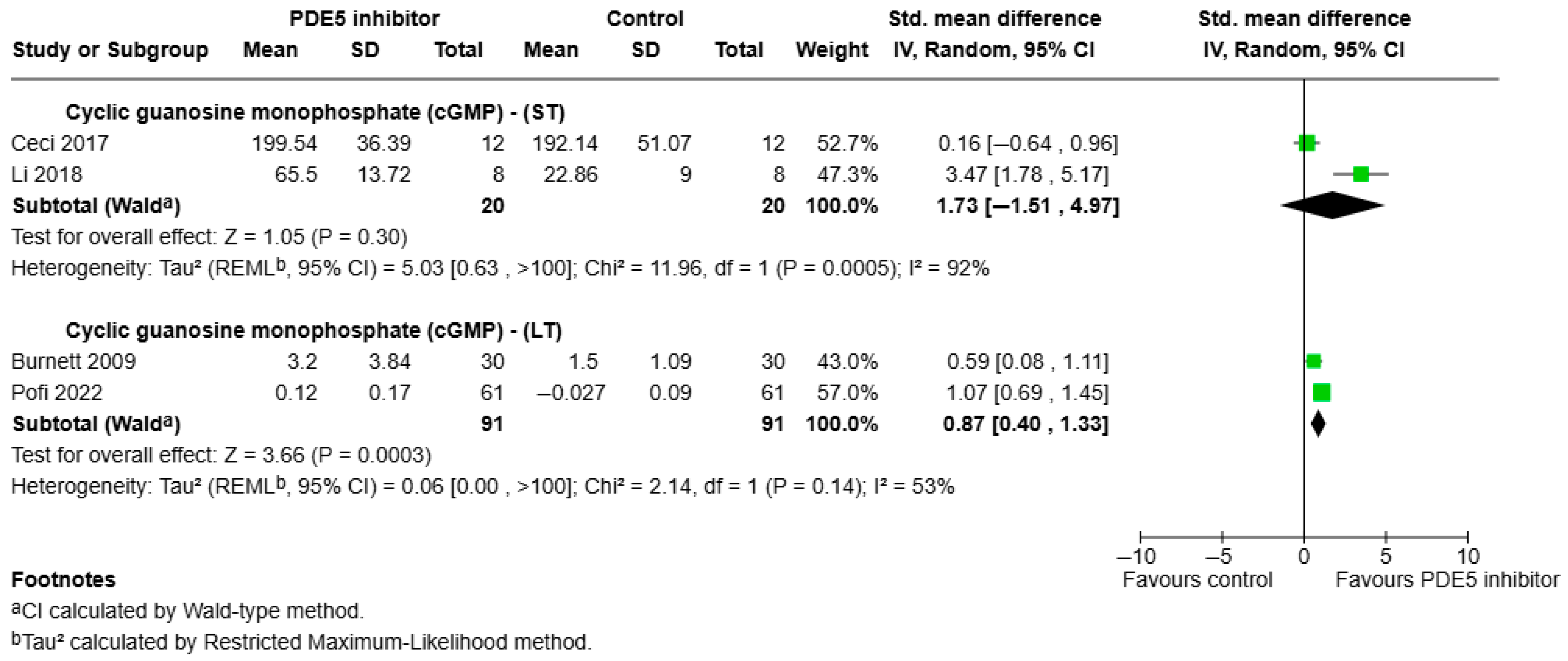
3.3.3. Effects of PDE5 Inhibitors on Anti-Inflammatory Outcomes
- a.
- cGMP:
- b.
- IL-10:
- c.
- NO:
3.3.4. Other Inflammatory Outcomes
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDE5 | Phosphodiesterase 5 |
| TNF-α | Tumour necrosis factor alpha |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-10 | Interleukin 10 |
| NO | Nitric oxide |
| cGMP | Cyclic guanosine monophosphate |
| VCAM | Vascular cell adhesion molecule |
| ICAM | Intracellular adhesion molecule |
| CRP | C-reactive protein |
| ST | Short term |
| IT | Intermediate term |
| LT | Long term |
| CI | Confidence interval |
| SMD | Standardised mean differences |
| SD | Standard deviation |
References
- Crescioli, C.; Paronetto, M.P. The Emerging Role of Phosphodiesterase 5 Inhibition in Neurological Disorders: The State of the Art. Cells 2024, 13, 1720. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. The Ongoing Challenge of Chronic Conditions in Australia; Australian Institute of Health and Welfare: Canberra, Australia, 2024. Available online: https://www.aihw.gov.au/reports/australias-health/chronic-conditions-challenge (accessed on 23 May 2025).
- Australian Institute of Health and Welfare. Chronic Conditions; Australian Institute of Health and Welfare: Canberra, Australia, 2024. Available online: https://www.aihw.gov.au/reports/australias-health/chronic-conditions (accessed on 23 May 2025).
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The interplay between cytokines, inflammation, and antioxidants: Mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Ding, L.; Gao, R.Y.; Liu, J.; Shang, J. Interleukin-6 signal transduction and its role in hepatic lipid metabolic disorders. Cytokine 2014, 66, 133–142. [Google Scholar] [CrossRef] [PubMed]
- David, J.M.; Dominguez, C.; Hamilton, D.H.; Palena, C. The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance. Vaccines 2016, 4, 22. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Pacinella, G.; Ciaccio, A.M.; Tuttolomondo, A. Endothelial Dysfunction and Chronic Inflammation: The Cornerstones of Vascular Alterations in Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 15722. [Google Scholar] [CrossRef]
- Anderton, H.; Wicks, I.P.; Silke, J. Cell death in chronic inflammation: Breaking the cycle to treat rheumatic disease. Nat. Rev. Rheumatol. 2020, 16, 496–513. [Google Scholar] [CrossRef]
- Su, W.J.; Hu, T.; Jiang, C.L. Cool the Inflamed Brain: A Novel Anti-inflammatory Strategy for the Treatment of Major Depressive Disorder. Curr. Neuropharmacol. 2024, 22, 810–842. [Google Scholar] [CrossRef]
- Yu, H.; MacIsaac, D.; Wong, J.J.; Sellers, Z.M.; Wren, A.A.; Bensen, R.; Kin, C.; Park, K.T. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment. Pharmacol. Ther. 2018, 47, 364–370. [Google Scholar] [CrossRef]
- Vignozzi, L.; Gacci, M.; Cellai, I.; Morelli, A.; Maneschi, E.; Comeglio, P.; Santi, R.; Filippi, S.; Sebastianelli, A.; Nesi, G.; et al. PDE5 inhibitors blunt inflammation in human BPH: A potential mechanism of action for PDE5 inhibitors in LUTS. Prostate 2013, 73, 1391–1402. [Google Scholar] [CrossRef]
- ElHady, A.K.; El-Gamil, D.S.; Abdel-Halim, M.; Abadi, A.H. Advancements in Phosphodiesterase 5 Inhibitors: Unveiling Present and Future Perspectives. Pharmaceuticals 2023, 16, 1266. [Google Scholar] [CrossRef] [PubMed]
- Paronetto, M.P.; Crescioli, C. Rethinking of phosphodiesterase 5 inhibition: The old, the new and the perspective in human health. Front. Endocrinol. 2024, 15, 1461642. [Google Scholar] [CrossRef] [PubMed]
- Deyoung, L.; Chung, E.; Kovac, J.R.; Romano, W.; Brock, G.B. Daily use of sildenafil improves endothelial function in men with type 2 diabetes. J. Androl. 2012, 33, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yang, J.; Wang, L.; Peng, S.; Yin, J.; Jia, L.; Yang, X.; Yuan, Z.; Wu, C. NF-κB Upregulates Type 5 Phosphodiesterase in N9 Microglial Cells: Inhibition by Sildenafil and Yonkenafil. Mol. Neurobiol. 2016, 53, 2647–2658. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Axell, A.; Turek, I.; Wright, B.; Meehan-Andrews, T.; Irving, H.R. Modulation of Inflammatory Cytokine Production in Human Monocytes by cGMP and IRAK3. Int. J. Mol. Sci. 2022, 23, 2552. [Google Scholar] [CrossRef]
- Balarini, C.M.; Leal, M.A.; Gomes, I.B.; Pereira, T.M.; Gava, A.L.; Meyrelles, S.S.; Vasquez, E.C. Sildenafil restores endothelial function in the apolipoprotein E knockout mouse. J. Transl. Med. 2013, 11, 3. [Google Scholar] [CrossRef]
- Roumeguère, T.; Zouaoui Boudjeltia, K.; Babar, S.; Nuyens, V.; Rousseau, A.; Van Antwerpen, P.; Ducobu, J.; Wespes, E.; Vanhaeverbeek, M. Effects of Phosphodiesterase Inhibitors on the Inflammatory Response of Endothelial Cells Stimulated by Myeloperoxidase-Modified Low-Density Lipoprotein or Tumor Necrosis Factor Alpha. Eur. Urol. 2010, 57, 522–529. [Google Scholar] [CrossRef]
- Koka, S.; Xi, L.; Kukreja, R.C. Chronic inhibition of phosphodiesterase 5 with tadalafil affords cardioprotection in a mouse model of metabolic syndrome: Role of nitric oxide. Mol. Cell. Biochem. 2020, 468, 47–58. [Google Scholar] [CrossRef]
- Kumar, S.; Ashraf, M. Tadalafil, a Phosphodiesterase Inhibitor Protects Stem Cells over Longer Period Against Hypoxia/Reoxygenation Injury Through STAT3/PKG-I Signaling. Stem Cells Dev. 2015, 24, 1332–1341. [Google Scholar] [CrossRef]
- Tomita, N.; Hotta, Y.; Naiki-Ito, A.; Hirano, K.; Kataoka, T.; Maeda, Y.; Takahashi, S.; Kimura, K. The phosphodiesterase 5 inhibitor tadalafil has renoprotective effects in a rat model of chronic kidney disease. Physiol. Rep. 2020, 8, e14556. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, C.; Di Luigi, L.; Lenzi, A.; Crescioli, C. Phosphodiesterase type 5 inhibitors: Back and forward from cardiac indications. J. Endocrinol. Investig. 2016, 39, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Di Luigi, L.; Corinaldesi, C.; Colletti, M.; Scolletta, S.; Antinozzi, C.; Vannelli, G.B.; Giannetta, E.; Gianfrilli, D.; Isidori, A.M.; Migliaccio, S.; et al. Phosphodiesterase Type 5 Inhibitor Sildenafil Decreases the Proinflammatory Chemokine CXCL10 in Human Cardiomyocytes and in Subjects with Diabetic Cardiomyopathy. Inflammation 2016, 39, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Ioakeimidis, N.; Rokkas, K.; Angelis, A.; Terentes-Printzios, D.; Stefanadis, C.; Tousoulis, D. Acute effect of sildenafil on inflammatory markers/mediators in patients with vasculogenic erectile dysfunction. Int. J. Cardiol. 2015, 182, 98–101. [Google Scholar] [CrossRef]
- Swiecicka, A. The efficacy of PDE5 inhibitors in diabetic patients. Andrology 2023, 11, 245–256. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Aversa, A.; Vitale, C.; Volterrani, M.; Fabbri, A.; Spera, G.; Fini, M.; Rosano, G.M. Chronic administration of Sildenafil improves markers of endothelial function in men with Type 2 diabetes. Diabet. Med. 2008, 25, 37–44. [Google Scholar] [CrossRef]
- Burnett, A.L.; Strong, T.D.; Trock, B.J.; Jin, L.; Bivalacqua, T.J.; Musicki, B. Serum biomarker measurements of endothelial function and oxidative stress after daily dosing of sildenafil in type 2 diabetic men with erectile dysfunction. J. Urol. 2009, 181, 245–251. [Google Scholar] [CrossRef]
- Ceci, R.; Duranti, G.; Sgrò, P.; Sabatini, S.; Di Luigi, L. Acute tadalafil administration increases plasma fatty acids without changes in the inflammatory response in healthy men. Acta Biochim. Pol. 2017, 64, 687–691. [Google Scholar] [CrossRef]
- Ceci, R.; Duranti, G.; Sgrò, P.; Sansone, M.; Guidetti, L.; Baldari, C.; Sabatini, S.; Di Luigi, L. Effects of tadalafil administration on plasma markers of exercise-induced muscle damage, IL6 and antioxidant status capacity. Eur. J. Appl. Physiol. 2015, 115, 531–539. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Ross, R.L.; Abignano, G.; Antinozzi, C.; Marampon, F.; di Luigi, L.; Buch, M.H.; Riccieri, V.; Lenzi, A.; Crescioli, C.; et al. Muscle Damage in Systemic Sclerosis and CXCL10: The Potential Therapeutic Role of PDE5 Inhibition. Int. J. Mol. Sci. 2021, 22, 2894. [Google Scholar] [CrossRef] [PubMed]
- Fryk, E.; Rodrigues Silva, V.R.; Bauzá-Thorbrügge, M.; Schmelz, M.; Gan, L.M.; Strindberg, L.; Jansson, P.A. Feasibility of high-dose tadalafil and effects on insulin resistance in well-controlled patients with type 2 diabetes (MAKROTAD): A single-centre, double-blind, randomised, placebo-controlled, cross-over phase 2 trial. EClinicalMedicine 2023, 59, 101985. [Google Scholar] [CrossRef] [PubMed]
- Giannattasio, S.; Corinaldesi, C.; Colletti, M.; Di Luigi, L.; Antinozzi, C.; Filardi, T.; Scolletta, S.; Basili, S.; Lenzi, A.; Morano, S.; et al. The phosphodiesterase 5 inhibitor sildenafil decreases the proinflammatory chemokine IL-8 in diabetic cardiomyopathy: In vivo and in vitro evidence. J. Endocrinol. Investig. 2019, 42, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Goudie, A.R.; Lipworth, B.J.; Hopkinson, P.J.; Wei, L.; Struthers, A.D. Tadalafil in patients with chronic obstructive pulmonary disease: A randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir. Med. 2014, 2, 293–300. [Google Scholar] [CrossRef]
- Jamaluddin; Bansal, M.; Srivastava, G.K.; Gupta, N.P. Role of Serum High-Sensitivity C-Reactive Protein as a Predictor of Therapeutic Response to Tadalafil in Patients With Erectile Dysfunction: A Prospective Observational Study. J. Sex. Med. 2019, 16, 1912–1921. [Google Scholar] [CrossRef]
- Kilic, M.; Erkan, A.; Zengin, S.; Dundar, G.; Boyaci, C. Inflammatory biomarkers may predict response to phosphodiesterase type 5 inhibitor treatment in patients with erectile dysfunction. Investig. Clin. Urol. 2023, 64, 404–411. [Google Scholar] [CrossRef]
- Kumar, T.; Aujla, H.; Woźniak, M.; Dott, W.; Sullo, N.; Joel-David, L.; Pais, P.; Smallwood, D.; Miller, D.; Eagle-Hemming, B.; et al. Intravenous sildenafil citrate and post-cardiac surgery acute kidney injury: A double-blind, randomised, placebo-controlled trial. Br. J. Anaesth. 2020, 124, 693–701. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Xiang, L.; Dong, J.; Liu, M.; Xiang, G. Sildenafil induces browning of subcutaneous white adipose tissue in overweight adults. Metabolism 2018, 78, 106–117. [Google Scholar] [CrossRef]
- Mandosi, E.; Giannetta, E.; Filardi, T.; Lococo, M.; Bertolini, C.; Fallarino, M.; Gianfrilli, D.; Venneri, M.A.; Lenti, L.; Lenzi, A.; et al. Endothelial dysfunction markers as a therapeutic target for Sildenafil treatment and effects on metabolic control in type 2 diabetes. Expert Opin. Ther. Targets 2015, 19, 1617–1622. [Google Scholar] [CrossRef]
- Morano, S.; Mandosi, E.; Fallarino, M.; Gatti, A.; Tiberti, C.; Sensi, M.; Gandini, L.; Buchetti, B.; Lenti, L.; Jannini, E.A.; et al. Antioxidant treatment associated with sildenafil reduces monocyte activation and markers of endothelial damage in patients with diabetic erectile dysfunction: A double-blind, placebo-controlled study. Eur. Urol. 2007, 52, 1768–1774. [Google Scholar] [CrossRef]
- Pofi, R.; Giannetta, E.; Feola, T.; Galea, N.; Barbagallo, F.; Campolo, F.; Badagliacca, R.; Barbano, B.; Ciolina, F.; Defeudis, G.; et al. Sex-specific effects of daily tadalafil on diabetic heart kinetics in RECOGITO, a randomized, double-blind, placebo-controlled trial. Sci. Transl. Med. 2022, 14, eabl8503. [Google Scholar] [CrossRef]
- Santi, D.; Granata, A.R.; Guidi, A.; Pignatti, E.; Trenti, T.; Roli, L.; Bozic, R.; Zaza, S.; Pacchioni, C.; Romano, S.; et al. Six months of daily treatment with vardenafil improves parameters of endothelial inflammation and of hypogonadism in male patients with type 2 diabetes and erectile dysfunction: A randomized, double-blind, prospective trial. Eur. J. Endocrinol. 2016, 174, 513–522. [Google Scholar] [CrossRef]
- Semen, K.; Yelisyeyeva, O.; Jarocka-Karpowicz, I.; Kaminskyy, D.; Solovey, L.; Skrzydlewska, E.; Yavorskyi, O. Sildenafil reduces signs of oxidative stress in pulmonary arterial hypertension: Evaluation by fatty acid composition, level of hydroxynonenal and heart rate variability. Redox Biol. 2016, 7, 48–57. [Google Scholar] [CrossRef]
- Taylor-Cousar, J.L.; Wiley, C.; Felton, L.A.; St Clair, C.; Jones, M.; Curran-Everett, D.; Poch, K.; Nichols, D.P.; Solomon, G.M.; Saavedra, M.T.; et al. Pharmacokinetics and tolerability of oral sildenafil in adults with cystic fibrosis lung disease. J. Cyst. Fibros. 2015, 14, 228–236. [Google Scholar] [CrossRef]
- Francis, S.H.; Busch, J.L.; Corbin, J.D. cGMP-Dependent Protein Kinases and cGMP Phosphodiesterases in Nitric Oxide and cGMP Action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Gough, P.; Myles, I.A. Tumor Necrosis Factor Receptors: Pleiotropic Signaling Complexes and Their Differential Effects. Front. Immunol. 2020, 11, 585880. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Goyal, A.; Patel, B.C. C-Reactive Protein: Clinical Relevance and Interpretation. In StatPearls; StatPearls Publishing Copyright © 2025; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ganjali, S.; Gotto, A.M., Jr.; Ruscica, M.; Atkin, S.L.; Butler, A.E.; Banach, M.; Sahebkar, A. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J. Cell. Physiol. 2018, 233, 9237–9246. [Google Scholar] [CrossRef] [PubMed]
- Australian Medicines Handbook, Australian Medicines Handbook Online, 2024 ed. Available online: https://amhonline.amh.net.au/ (accessed on 6 June 2025).
- Nichols, D.J.; Muirhead, G.J.; Harness, J.A. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: Absolute bioavailability, food effects and dose proportionality. Br. J. Clin. Pharmacol. 2002, 53 (Suppl. 1), 5s–12s. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.S.; Geethakumari, A.M.; Biswas, K.H. Phosphodiesterase 5 (PDE5): Structure-function regulation and therapeutic applications of inhibitors. Biomed. Pharmacother. 2021, 134, 111128. [Google Scholar] [CrossRef]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2020, 33, 127–148. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Kong, D.H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, R.K.; Bhardwaj, T.R. Therapeutic role of nitric oxide as emerging molecule. Biomed. Pharmacother. 2017, 85, 182–201. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An evolving chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Ye, Z.; Zhong, L.; Zhu, S.; Wang, Y.; Zheng, J.; Wang, S.; Zhang, J.; Huang, R. The P-selectin and PSGL-1 axis accelerates atherosclerosis via activation of dendritic cells by the TLR4 signaling pathway. Cell Death Dis. 2019, 10, 507. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Criteria |
|---|
| Must contain the concept of inflammation, regulation, or intervention of inflammation. |
| Must contain the concept of Phosphodiesterase-5 inhibitors. |
| Must contain primary or secondary outcome markers of inflammation. |
| Must be human studies. |
| Must be adults over 18 years of age. |
| Must not contain in vitro or in vivo (animal) studies. |
| Must not contain review articles. |
| Must be peer-reviewed studies. |
| Must be in English. |
| Duration of Outcome Measurement After Intervention | Comparison Groups | Outcome Measures |
|---|---|---|
| Short term (ST)—Less than 1 week | Pre and post intervention | TNF-α, IL-6, CRP, cGMP |
| Intermediate term (IT)—4–6 weeks | Pre and post intervention | IL-6, CRP |
| Long term (LT)—Equal to or more than 12 weeks | Pre and post intervention | IL-6, IL-8, CRP, cGMP, ICAM, P-selectin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cianciarulo, C.; Nguyen, T.H.; Zacharias, A.; Standen, N.; Tucci, J.; Irving, H. Analysis of Phosphodiesterase-5 (PDE5) Inhibitors in Modulating Inflammatory Markers in Humans: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 7155. https://doi.org/10.3390/ijms26157155
Cianciarulo C, Nguyen TH, Zacharias A, Standen N, Tucci J, Irving H. Analysis of Phosphodiesterase-5 (PDE5) Inhibitors in Modulating Inflammatory Markers in Humans: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2025; 26(15):7155. https://doi.org/10.3390/ijms26157155
Chicago/Turabian StyleCianciarulo, Cassandra, Trang H. Nguyen, Anita Zacharias, Nick Standen, Joseph Tucci, and Helen Irving. 2025. "Analysis of Phosphodiesterase-5 (PDE5) Inhibitors in Modulating Inflammatory Markers in Humans: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 26, no. 15: 7155. https://doi.org/10.3390/ijms26157155
APA StyleCianciarulo, C., Nguyen, T. H., Zacharias, A., Standen, N., Tucci, J., & Irving, H. (2025). Analysis of Phosphodiesterase-5 (PDE5) Inhibitors in Modulating Inflammatory Markers in Humans: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 26(15), 7155. https://doi.org/10.3390/ijms26157155