Neuroprotective Roles of Vitamin D: Bridging the Gap Between Mechanisms and Clinical Applications in Cognitive Decline
Abstract
1. Introduction
2. Literature Search and Selection Strategy
3. Physiology of Vitamin D
3.1. Vitamin D Synthesis and Metabolism in the Human Body
3.2. Physiological Functions of Vitamin D Beyond Bone Health
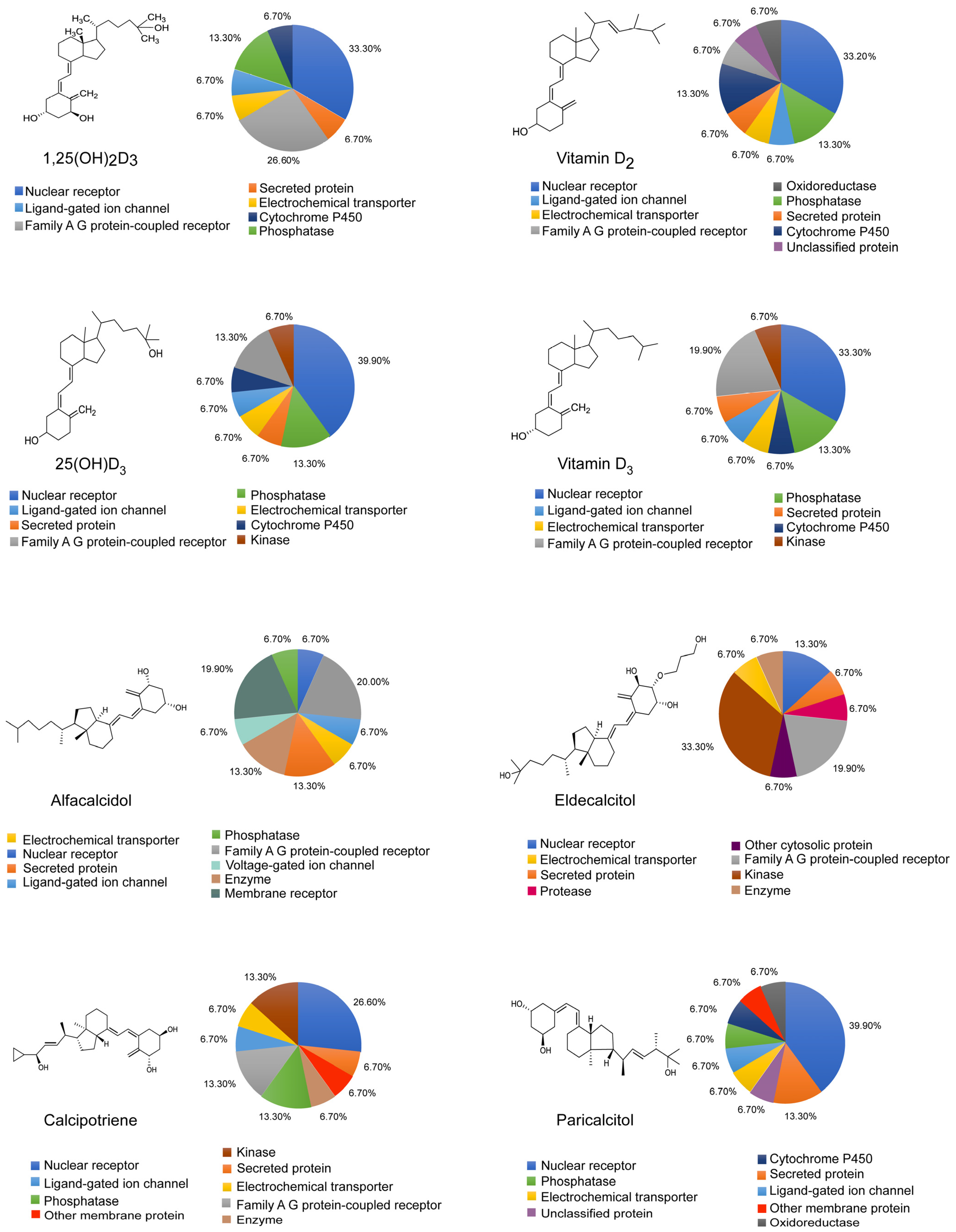
3.3. Widespread Localization and Diverse Functionalities of Vitamin D Receptors
4. Neurobiological Interfaces of Vitamin D with Cognitive Processes
4.1. Neuroanatomical Distribution of Vitamin D Receptors
4.2. Vitamin D and Neurotransmitter Regulation
4.3. The Role of Vitamin D in Neurogenesis and Synaptic Plasticity
5. Clinical and Mechanistic Insights into Vitamin D’s Neuroprotective and Cognitive-Enhancing Roles
5.1. Epidemiological Correlations Between Vitamin D Status and Cognitive Health
5.1.1. Association of Vitamin D Deficiency and Cognitive Decline
5.1.2. Inconclusive Evidence and Variability
5.1.3. Counterproductive Cognitive Effects of Vitamin D Intake
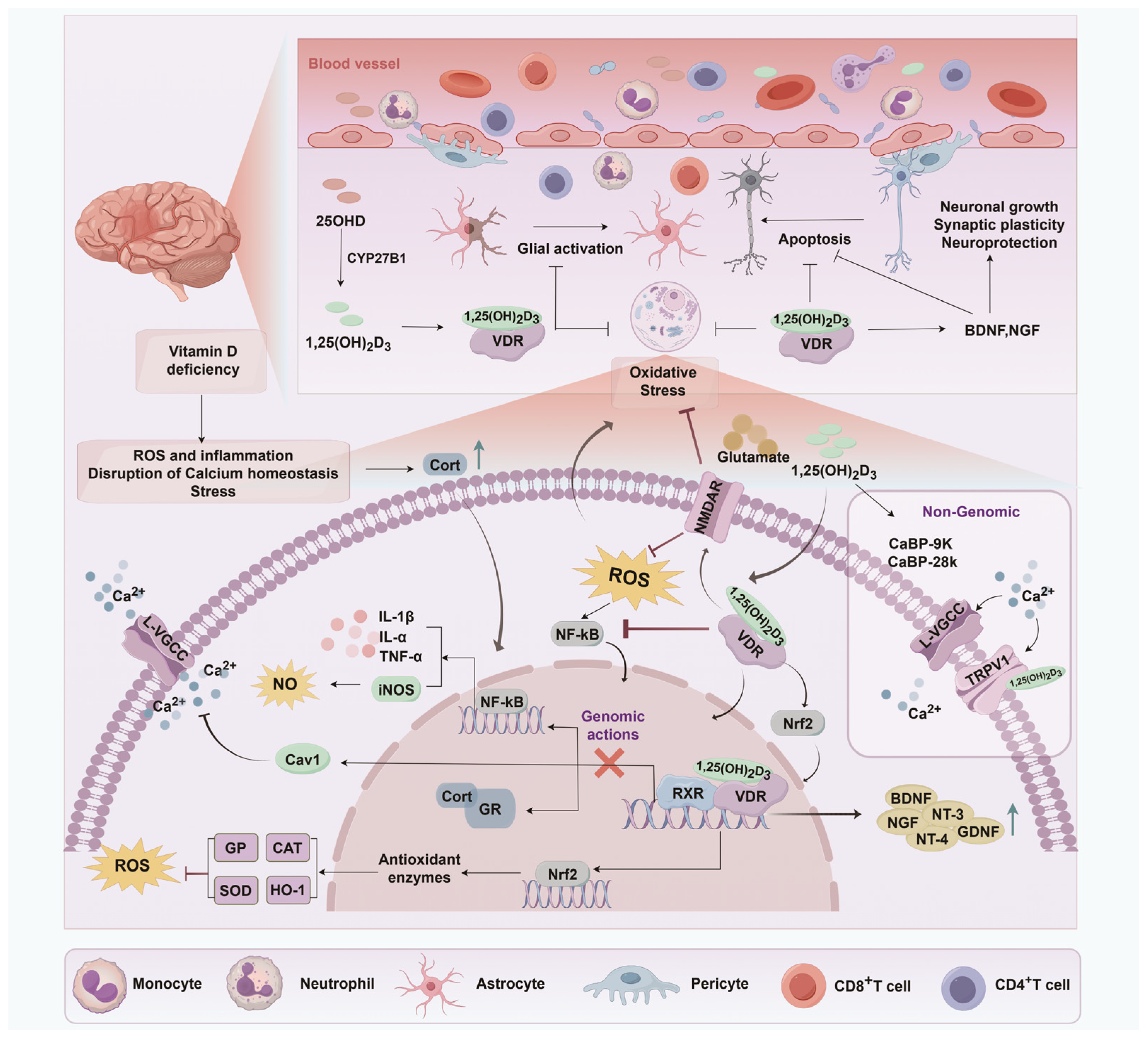
5.2. Potential Mechanisms Underlying the Neuroprotective Roles of Vitamin D
5.2.1. Inhibition of Neuronal Death and Toxicity
5.2.2. Brain Volume Preservation and Vascular Repair
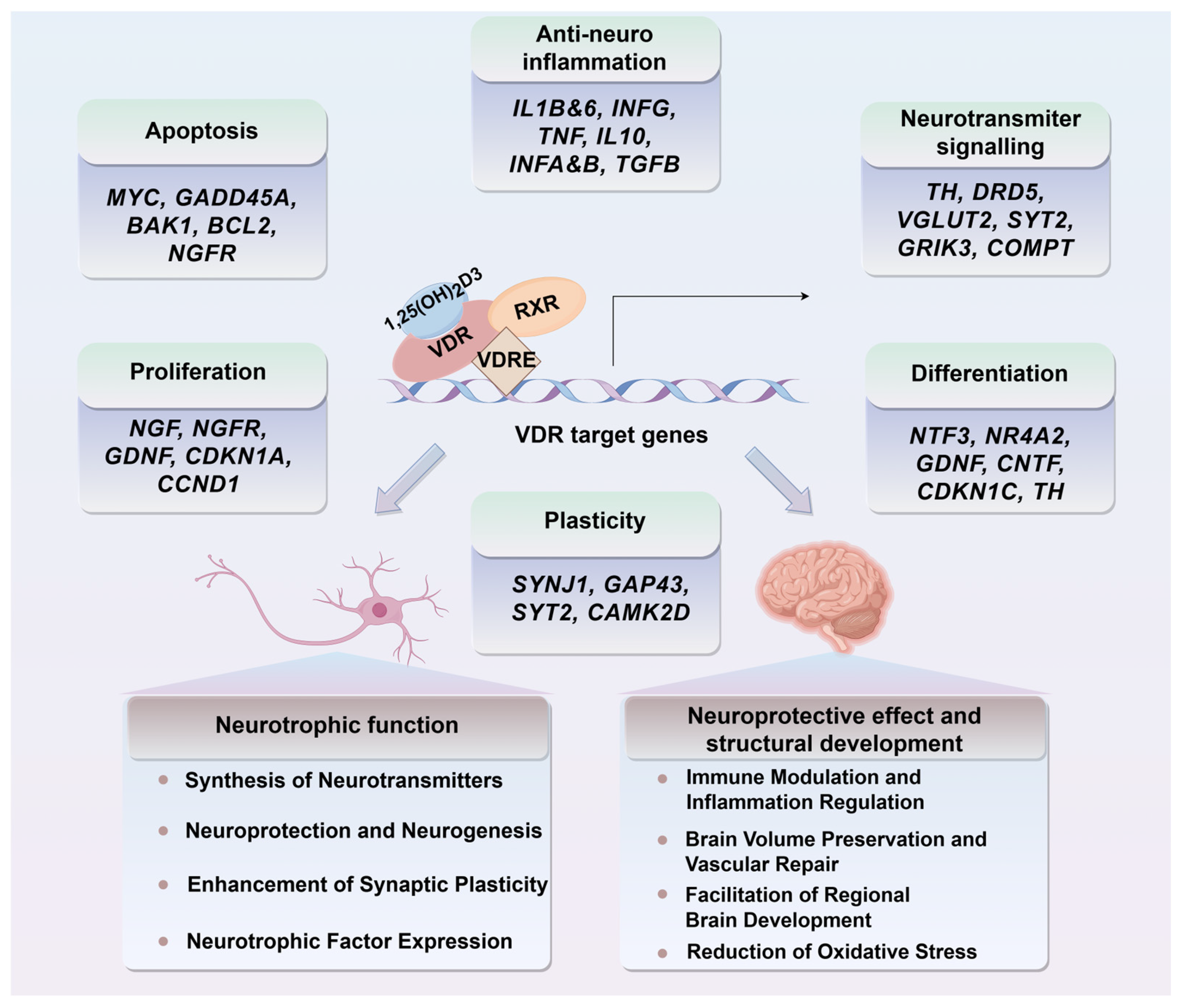
5.2.3. Neurogenesis and Neurotropic Support
5.2.4. Immune Modulation and Inflammation Regulation
5.2.5. Extracellular Vesicle-Mediated Vitamin D Neuroprotection
5.3. Combination Therapies Boosting Cognitive Function: Vitamin D and Neuroprotective Agents
6. Conclusions and Perspectives
6.1. Gaps and Challenges in Current Research
6.1.1. Inconsistency in Research Findings and Clinical Translation
6.1.2. Methodological Challenges and Variability in Vitamin D Measurement
6.1.3. Complexity of Vitamin D’s Role and Its Mechanisms
6.2. Future Directions and Research Priorities
6.2.1. Integration of Vitamin D Assessment in Cognitive Health Screenings
6.2.2. Emerging Research on Vitamin D Analogs and Cognitive Function
6.2.3. Personalized Medicine Approaches to Vitamin D Therapies
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Z.; Hotterbeex, P.; Marent, P.J.; Cerin, E.; Thomis, M.; van Uffelen, J. Physical activity, sedentary behaviour, and cognitive function among older adults: A bibliometric analysis from 2004 to 2024. Ageing Res. Rev. 2024, 97, 102283. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; Barger, K.; Dawson-Hughes, B.; Leurgans, S.E.; Fu, X.; James, B.D.; Holland, T.M.; Agarwal, P.; Wang, J.; Matuszek, G.; et al. Brain vitamin D forms, cognitive decline, and neuropathology in community-dwelling older adults. Alzheimers Dement. 2023, 19, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Shaw, B.C.; Anders, V.R.; Tinkey, R.A.; Habean, M.L.; Brock, O.D.; Frostino, B.J.; Williams, J.L. Immunity impacts cognitive deficits across neurological disorders. J. Neurochem. 2024, 168, 3512–3535. [Google Scholar] [CrossRef] [PubMed]
- Chanti-Ketterl, M.; Pieper, C.F.; Yaffe, K.; Plassman, B.L. Associations Between Traumatic Brain Injury and Cognitive Decline Among Older Male Veterans: A Twin Study. Neurology 2023, 101, e1761–e1770. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Lu, Q.; Farrell, M.; Lappin, J.M.; Shi, J.; Lu, L.; Bao, Y. Global prevalence and burden of HIV-associated neurocognitive disorder: A meta-analysis. Neurology 2020, 95, e2610–e2621. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Rossor, M.N.; Fox, N.C.; Mummery, C.J.; Schott, J.M.; Warren, J.D. The diagnosis of young-onset dementia. Lancet Neurol. 2010, 9, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.H.; Hsu, Y.Y.; Shie, F.S.; Huang, C.C.; Chen, M.H.; Juang, J.L. Non-genomic rewiring of vitamin D receptor to p53 as a key to Alzheimer’s disease. Aging Cell 2021, 20, e13509. [Google Scholar] [CrossRef] [PubMed]
- Fujishiro, H. Vitamin D3 as a potentially modifiable factor in mild cognitive impairment. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1236. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Takacs, I.; Boyanov, M.; Belaya, Z.; Diaconu, C.C.; Mokhort, T.; Zherdova, N.; Rasa, I.; Payer, J.; Pilz, S. Clinical Practice in the Prevention, Diagnosis and Treatment of Vitamin D Deficiency: A Central and Eastern European Expert Consensus Statement. Nutrients 2022, 14, 1483. [Google Scholar] [CrossRef] [PubMed]
- Harse, J.D.; Marriott, R.J.; Zhu, K.; Murray, K.; Bucks, R.S. Vitamin D status and cognitive performance in community-dwelling adults: A dose-response meta-analysis of observational studies. Front. Neuroendocrinol. 2023, 70, 101080. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Cheng, Y.C.; Chiu, C.C.; Liu, H.C.; Huang, M.C.; Tu, Y.K.; Kuo, P.H. Effects of Vitamin D Supplementation on Cognitive Outcomes: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2024, 34, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Cortese, F.; Costantino, M.F.; Luzi, G.; Di Marino, S.; Giordano, P.; Monitillo, F. Vitamin D and cardiovascular disease risk. A literature overview. Mol. Biol. Rep. 2022, 49, 8925–8942. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Bikle, D. Vitamin D Metabolism Revised: Fall of Dogmas. J. Bone Min. Miner. Res. 2019, 34, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, C.; Ix, J.H. New Insights into Vitamin D Metabolism in Kidney Disease and Transplant. Am. J. Kidney Dis. 2024, 84, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Bolland, M.J.; Grey, A. Effects of vitamin D supplements on bone mineral density: A systematic review and meta-analysis. Lancet 2014, 383, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D: Newer Concepts of Its Metabolism and Function at the Basic and Clinical Level. J. Endocr. Soc. 2020, 4, bvz038. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Hou, H.; Song, B.; Xia, Z.; Xu, Y. Vitamin D and ischemic stroke—Association, mechanisms, and therapeutics. Ageing Res. Rev. 2024, 96, 102244. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. The metabolism and mechanism of action of 1,25-dihydroxyvitamin D3. Kidney Int. 1986, 30, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Meng, X.; Tian, Q.; Cao, W.; Fan, X.; Wu, L.; Song, M.; Meng, Q.; Wang, W.; Wang, Y. Vitamin D and Multiple Health Outcomes: An Umbrella Review of Observational Studies, Randomized Controlled Trials, and Mendelian Randomization Studies. Adv. Nutr. 2022, 13, 1044–1062. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Tebben, P.J.; Singh, R.J.; Kumar, R. Vitamin D-Mediated Hypercalcemia: Mechanisms, Diagnosis, and Treatment. Endocr. Rev. 2016, 37, 521–547. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689s–1696s. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Bauerle, K.T.; Bernal-Mizrachi, C. Non-classical Vitamin D Actions for Renal Protection. Front. Med. 2021, 8, 790513. [Google Scholar] [CrossRef] [PubMed]
- Hamza, F.N.; Daher, S.; Fakhoury, H.M.A.; Grant, W.B.; Kvietys, P.R.; Al-Kattan, K. Immunomodulatory Properties of Vitamin D in the Intestinal and Respiratory Systems. Nutrients 2023, 15, 1696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Qian, F.; Wan, Z.; Chen, X.; Pan, A.; Liu, G. Vitamin D and major chronic diseases. Trends Endocrinol. Metab. 2024, 35, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Kubis, A.M.; Piwowar, A. The new insight on the regulatory role of the vitamin D3 in metabolic pathways characteristic for cancerogenesis and neurodegenerative diseases. Ageing Res. Rev. 2015, 24, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Wolpowitz, D.; Gilchrest, B.A. The vitamin D questions: How much do you need and how should you get it? J. Am. Acad. Dermatol. 2006, 54, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Al Mheid, I.; Quyyumi, A.A. Vitamin D and Cardiovascular Disease: Controversy Unresolved. J. Am. Coll. Cardiol. 2017, 70, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Delrue, C.; Speeckaert, M.M. Vitamin D and Vitamin D-Binding Protein in Health and Disease. Int. J. Mol. Sci. 2023, 24, 4642. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: Evolutionary, physiological and health perspectives. Curr. Drug Targets 2011, 12, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Battault, S.; Whiting, S.J.; Peltier, S.L.; Sadrin, S.; Gerber, G.; Maixent, J.M. Vitamin D metabolism, functions and needs: From science to health claims. Eur. J. Nutr. 2013, 52, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Ramagopalan, S.V.; Heger, A.; Berlanga, A.J.; Maugeri, N.J.; Lincoln, M.R.; Burrell, A.; Handunnetthi, L.; Handel, A.E.; Disanto, G.; Orton, S.M.; et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Res. 2010, 20, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Argano, C.; Mirarchi, L.; Amodeo, S.; Orlando, V.; Torres, A.; Corrao, S. The Role of Vitamin D and Its Molecular Bases in Insulin Resistance, Diabetes, Metabolic Syndrome, and Cardiovascular Disease: State of the Art. Int. J. Mol. Sci. 2023, 24, 15485. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.N.; Cruzat, V.F.; Calton, E.K.; Hart, P.H.; Soares, M.J.; Newsholme, P.; Yovich, J.L. Molecular actions of vitamin D in reproductive cell biology. Reproduction 2017, 153, R29–R42. [Google Scholar] [CrossRef] [PubMed]
- Gáll, Z.; Székely, O. Role of Vitamin D in Cognitive Dysfunction: New Molecular Concepts and Discrepancies between Animal and Human Findings. Nutrients 2021, 13, 3672. [Google Scholar] [CrossRef] [PubMed]
- Ikura, T.; Ito, N. Crystal Structure of the Vitamin D Receptor Ligand-Binding Domain with Lithocholic Acids. Vitam. Horm. 2016, 100, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.P.; Sharma, N.; An, S.S.A. Role of Calcitriol and Vitamin D Receptor (VDR) Gene Polymorphisms in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 4806. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W.; Mizwicki, M.T.; Okamura, W.H. Ligand structure-function relationships in the vitamin D endocrine system from the perspective of drug development (including cancer treatment). In Vitamin D Analogs in Cancer Prevention and Therapy; Reichrath, J., Tilgen, W., Friedrich, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 164, pp. 55–82. [Google Scholar] [CrossRef]
- Chen, R.; Yao, F.; Deng, X.; Yuan, X.; Wei, N.; Xiao, D.; Yu, B. Neuroprotective effect of 1,25-dihydroxyvitamin D3 against hyperoxia-induced brain injury in premature rats. Acta Neurobiol. Exp. 2023, 83, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, Y.G. Vitamin D Receptor Influences Intestinal Barriers in Health and Disease. Cells 2022, 11, 1129. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Campbell, M.J. Vitamin D receptor signaling mechanisms: Integrated actions of a well-defined transcription factor. Steroids 2013, 78, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Kochupillai, N. The physiology of vitamin D : Current concepts. Indian J. Med. Res. 2008, 127, 256–262. [Google Scholar] [PubMed]
- Liu, H.; He, Y.; Beck, J.; da Silva Teixeira, S.; Harrison, K.; Xu, Y.; Sisley, S. Defining vitamin D receptor expression in the brain using a novel VDRCre mouse. J. Comp. Neurol. 2021, 529, 2362–2375. [Google Scholar] [CrossRef] [PubMed]
- Pakpahan, C.; Wungu, C.; Agustinus, A.; Darmadi, D. Do Vitamin D receptor gene polymorphisms affect bone mass density in men?: A meta-analysis of observational studies. Ageing Res. Rev. 2022, 75, 101571. [Google Scholar] [CrossRef] [PubMed]
- Ogunkolade, B.W.; Boucher, B.J.; Prahl, J.M.; Bustin, S.A.; Burrin, J.M.; Noonan, K.; North, B.V.; Mannan, N.; McDermott, M.F.; DeLuca, H.F.; et al. Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. Diabetes 2002, 51, 2294–2300. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)(2)vitamin D(3): Genomic and non-genomic mechanisms. Best. Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Dursun, E.; Gezen-Ak, D. Vitamin D receptor is present on the neuronal plasma membrane and is co-localized with amyloid precursor protein, ADAM10 or Nicastrin. PLoS ONE 2017, 12, e0188605. [Google Scholar] [CrossRef] [PubMed]
- Lalunio, H.; Parker, L.; Hanson, E.D.; Gregorevic, P.; Levinger, I.; Hayes, A.; Goodman, C.A. Detecting the vitamin D receptor (VDR) protein in mouse and human skeletal muscle: Strain-specific, species-specific and inter-individual variation. Mol. Cell. Endocrinol. 2023, 578, 112050. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Meyer, M.B.; Lee, S.M.; Onal, M.; Benkusky, N.A. The vitamin D receptor: Contemporary genomic approaches reveal new basic and translational insights. J. Clin. Investig. 2017, 127, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Pertile, R.A.N.; Brigden, R.; Raman, V.; Cui, X.; Du, Z.; Eyles, D. Vitamin D: A potent regulator of dopaminergic neuron differentiation and function. J. Neurochem. 2023, 166, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Kaviani, M.; Nikooyeh, B.; Zand, H.; Yaghmaei, P.; Neyestani, T.R. Effects of vitamin D supplementation on depression and some involved neurotransmitters. J. Affect. Disord. 2020, 269, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Park, M.; Kim, T. Vitamin D Reduces GABA-Positive Astrocytes in the 5xFAD Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2024, 97, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Anjum, I.; Jaffery, S.S.; Fayyaz, M.; Samoo, Z.; Anjum, S. The Role of Vitamin D in Brain Health: A Mini Literature Review. Cureus 2018, 10, e2960. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.A.; Mesgari-Abbasi, M.; Nameni, G.; Hajiluian, G.; Shahabi, P. The effects of vitamin D administration on brain inflammatory markers in high fat diet induced obese rats. BMC Neurosci. 2017, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Kemény, L.V.; Robinson, K.C.; Hermann, A.L.; Walker, D.M.; Regan, S.; Yew, Y.W.; Lai, Y.C.; Theodosakis, N.; Rivera, P.D.; Ding, W.; et al. Vitamin D deficiency exacerbates UV/endorphin and opioid addiction. Sci. Adv. 2021, 7, eabe4577. [Google Scholar] [CrossRef] [PubMed]
- Luan, W.; Hammond, L.A.; Vuillermot, S.; Meyer, U.; Eyles, D.W. Maternal Vitamin D Prevents Abnormal Dopaminergic Development and Function in a Mouse Model of Prenatal Immune Activation. Sci. Rep. 2018, 8, 9741. [Google Scholar] [CrossRef] [PubMed]
- Pertile, R.A.N.; Cui, X.; Hammond, L.; Eyles, D.W. Vitamin D regulation of GDNF/Ret signaling in dopaminergic neurons. FASEB J. 2018, 32, 819–828. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Feron, F.; Eyles, D.; Mackay-Sim, A. Vitamin D: The neglected neurosteroid? Trends Neurosci. 2001, 24, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Burne, T.H.; McGrath, J.J. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front. Neuroendocrinol. 2013, 34, 47–64. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.C.; Ames, B.N. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008, 22, 982–1001. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.J.; Eyles, D.W.; Pedersen, C.B.; Anderson, C.; Ko, P.; Burne, T.H.; Norgaard-Pedersen, B.; Hougaard, D.M.; Mortensen, P.B. Neonatal vitamin D status and risk of schizophrenia: A population-based case-control study. Arch. Gen. Psychiatry 2010, 67, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Rastegar-Moghaddam, S.H.; Hosseini, M.; Alipour, F.; Rajabian, A.; Ebrahimzadeh Bideskan, A. The effects of vitamin D on learning and memory of hypothyroid juvenile rats and brain tissue acetylcholinesterase activity and oxidative stress indicators. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Vyas, C.M.; Okereke, O.I.; Ogata, S.; Albert, M.; Lee, I.M.; D’Agostino, D.; Buring, J.E.; Cook, N.R.; Grodstein, F.; et al. Effect of vitamin D on cognitive decline: Results from two ancillary studies of the VITAL randomized trial. Sci. Rep. 2021, 11, 23253. [Google Scholar] [CrossRef] [PubMed]
- Mayne, P.E.; Burne, T.H.J. Vitamin D in Synaptic Plasticity, Cognitive Function, and Neuropsychiatric Illness. Trends Neurosci. 2019, 42, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Morello, M.; Pieri, M.; Zenobi, R.; Talamo, A.; Stephan, D.; Landel, V.; Feron, F.; Millet, P. The Influence of Vitamin D on Neurodegeneration and Neurological Disorders: A Rationale for its Physio-pathological Actions. Curr. Pharm. Des. 2020, 26, 2475–2491. [Google Scholar] [CrossRef] [PubMed]
- Lam, V.; Takechi, R.; Mamo, J.C.L. [P4–124]: VITAMIN D, CEREBROCAPILLARY INTEGRITY AND COGNITION IN MURINE MODEL OF ACCELERATED AGEING. Alzheimer’s Dement. 2017, 13, P1304. [Google Scholar] [CrossRef]
- Gómez-Oliva, R.; Geribaldi-Doldán, N.; Domínguez-García, S.; Carrascal, L.; Verástegui, C.; Nunez-Abades, P.; Castro, C. Vitamin D deficiency as a potential risk factor for accelerated aging, impaired hippocampal neurogenesis and cognitive decline: A role for Wnt/β-catenin signaling. Aging 2020, 12, 13824–13844. [Google Scholar] [CrossRef] [PubMed]
- Goodwill, A.M.; Szoeke, C. A Systematic Review and Meta-Analysis of The Effect of Low Vitamin D on Cognition. J. Am. Geriatr. Soc. 2017, 65, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Siracusano, M.; Riccioni, A.; Abate, R.; Benvenuto, A.; Curatolo, P.; Mazzone, L. Vitamin D Deficiency and Autism Spectrum Disorder. Curr. Pharm. Des. 2020, 26, 2460–2474. [Google Scholar] [CrossRef] [PubMed]
- Morello, M.; Landel, V.; Lacassagne, E.; Baranger, K.; Annweiler, C.; Féron, F.; Millet, P. Vitamin D Improves Neurogenesis and Cognition in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6463–6479. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, W.; Hu, P.; Zhang, R.; Dong, X.; Zhang, D. Association of Dietary Vitamin D Intake, Serum 25(OH)D(3), 25(OH)D(2) with Cognitive Performance in the Elderly. Nutrients 2021, 13, 3089. [Google Scholar] [CrossRef] [PubMed]
- Leser, B.; Dalkner, N.; Tmava-Berisha, A.; Fellendorf, F.T.; Unterrainer, H.F.; Stross, T.; Maget, A.; Platzer, M.; Bengesser, S.A.; Häussl, A.; et al. The Influence of Vitamin D Status on Cognitive Ability in Patients with Bipolar Disorder and Healthy Controls. Nutrients 2023, 15, 4111. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Schott, A.M.; Allali, G.; Bridenbaugh, S.A.; Kressig, R.W.; Allain, P.; Herrmann, F.R.; Beauchet, O. Association of vitamin D deficiency with cognitive impairment in older women: Cross-sectional study. Neurology 2010, 74, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Harse, J.D.; Zhu, K.; Bucks, R.S.; Hunter, M.; Lim, E.M.; Cooke, B.R.; Walsh, J.P.; Murray, K. Investigating Potential Dose-Response Relationships between Vitamin D Status and Cognitive Performance: A Cross-Sectional Analysis in Middle- to Older-Aged Adults in the Busselton Healthy Ageing Study. Int. J. Environ. Res. Public Health 2021, 19, 450. [Google Scholar] [CrossRef] [PubMed]
- Buell, J.S.; Scott, T.M.; Dawson-Hughes, B.; Dallal, G.E.; Rosenberg, I.H.; Folstein, M.F.; Tucker, K.L. Vitamin D is associated with cognitive function in elders receiving home health services. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Arosio, B.; Rossi, P.D.; Ferri, E.; Cesari, M.; Vitale, G. Characterization of Vitamin D Status in Older Persons with Cognitive Impairment. Nutrients 2022, 14, 1142. [Google Scholar] [CrossRef] [PubMed]
- Feart, C.; Helmer, C.; Merle, B.; Herrmann, F.R.; Annweiler, C.; Dartigues, J.F.; Delcourt, C.; Samieri, C. Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer’s disease in older adults. Alzheimers Dement. 2017, 13, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Mutua, A.M.; Nampijja, M.; Elliott, A.M.; Pettifor, J.M.; Williams, T.N.; Abubakar, A.; Webb, E.L.; Atkinson, S.H. Vitamin D Status Is Not Associated with Cognitive or Motor Function in Pre-School Ugandan Children. Nutrients 2020, 12, 1662. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Song, Q.; Wang, X.; Li, Y.; Lin, T.; Liang, R.; Jiang, T.; Shu, X.; Ge, N.; Yue, J. Combined associations of vitamin D and cognitive function with all-cause mortality among older adults in Chinese longevity areas: A prospective cohort study. Front. Public Health 2023, 11, 1024341. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, D.J.; Lang, I.A.; Langa, K.M.; Muniz-Terrera, G.; Phillips, C.L.; Cherubini, A.; Ferrucci, L.; Melzer, D. Vitamin D and risk of cognitive decline in elderly persons. Arch. Intern. Med. 2010, 170, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Taneja, S.; Kvestad, I.; Hysing, M.; Bhandari, N.; Strand, T.A. Vitamin D status in early childhood is not associated with cognitive development and linear growth at 6–9 years of age in North Indian children: A cohort study. Nutr. J. 2020, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Dhana, K.; Barnes, L.L.; Agarwal, P.; Liu, X.; Dhana, A.; Desai, P.; Aggarwal, N.; Evans, D.A.; Rajan, K.B. Vitamin D intake and cognitive decline in Blacks and Whites: The role of diet and supplements. Alzheimers Dement. 2023, 19, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Toffanello, E.D.; Coin, A.; Perissinotto, E.; Zambon, S.; Sarti, S.; Veronese, N.; De Rui, M.; Bolzetta, F.; Corti, M.C.; Crepaldi, G.; et al. Vitamin D deficiency predicts cognitive decline in older men and women: The Pro.V.A. Study. Neurology 2014, 83, 2292–2298. [Google Scholar] [CrossRef] [PubMed]
- Duchaine, C.S.; Talbot, D.; Nafti, M.; Giguère, Y.; Dodin, S.; Tourigny, A.; Carmichael, P.-H.; Laurin, D. Vitamin D status, cognitive decline and incident dementia: The Canadian Study of Health and Aging. Can. J. Public Health 2020, 111, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Olsson, E.; Byberg, L.; Karlstrom, B.; Cederholm, T.; Melhus, H.; Sjogren, P.; Kilander, L. Vitamin D is not associated with incident dementia or cognitive impairment: An 18-y follow-up study in community-living old men. Am. J. Clin. Nutr. 2017, 105, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, G.A.; Kritz-Silverstein, D.; Bergstrom, J.; Reas, E.T.; Jassal, S.K.; Barrett-Connor, E.; McEvoy, L.K. Vitamin D Insufficiency and Cognitive Function Trajectories in Older Adults: The Rancho Bernardo Study. J. Alzheimers Dis. 2017, 58, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Specht, I.O.; Janbek, J.; Thorsteinsdottir, F.; Frederiksen, P.; Heitmann, B.L. Neonatal vitamin D levels and cognitive ability in young adulthood. Eur. J. Nutr. 2020, 59, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, K.; Møllegaard Jepsen, J.R.; Sevelsted, A.; Horner, D.; Vinding, R.; Rosenberg, J.B.; Brustad, N.; Eliasen, A.; Mohammadzadeh, P.; Følsgaard, N.; et al. High-dose vitamin D3 supplementation in pregnancy and risk of neurodevelopmental disorders in the children at age 10: A randomized clinical trial. Am. J. Clin. Nutr. 2024, 119, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Castle, M.; Fiedler, N.; Pop, L.C.; Schneider, S.J.; Schlussel, Y.; Sukumar, D.; Hao, L.; Shapses, S.A. Three Doses of Vitamin D and Cognitive Outcomes in Older Women: A Double-Blind Randomized Controlled Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Jia, J.; Zhang, Y.; Miao, R.; Huo, X.; Ma, F. Effects of vitamin D(3) supplementation on cognition and blood lipids: A 12-month randomised, double-blind, placebo-controlled trial. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Hu, J.; Huo, X.; Miao, R.; Zhang, Y.; Ma, F. Effects of vitamin D supplementation on cognitive function and blood Aβ-related biomarkers in older adults with Alzheimer’s disease: A randomised, double-blind, placebo-controlled trial. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Ginde, A.A.; Brown, S.M.; Baughman, A.; Collar, E.M.; Ely, E.W.; Gong, M.N.; Hope, A.A.; Hou, P.C.; Hough, C.L.; et al. Effect of Early High-Dose Vitamin D3 Repletion on Cognitive Outcomes in Critically Ill Adults. Chest 2021, 160, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Cortese, M.; Munger, K.L.; Martínez-Lapiscina, E.H.; Barro, C.; Edan, G.; Freedman, M.S.; Hartung, H.P.; Montalbán, X.; Foley, F.W.; Penner, I.K.; et al. Vitamin D, smoking, EBV, and long-term cognitive performance in MS: 11-year follow-up of BENEFIT. Neurology 2020, 94, e1950–e1960. [Google Scholar] [CrossRef] [PubMed]
- Schietzel, S.; Fischer, K.; Brugger, P.; Orav, E.J.; Renerts, K.; Gagesch, M.; Freystaetter, G.; Stähelin, H.B.; Egli, A.; Bischoff-Ferrari, H.A. Effect of 2000 IU compared with 800 IU vitamin D on cognitive performance among adults age 60 years and older: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Montero-Odasso, M.; Zou, G.; Speechley, M.; Almeida, Q.J.; Liu-Ambrose, T.; Middleton, L.E.; Camicioli, R.; Bray, N.W.; Li, K.Z.H.; Fraser, S.; et al. Effects of Exercise Alone or Combined With Cognitive Training and Vitamin D Supplementation to Improve Cognition in Adults with Mild Cognitive Impairment: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2324465. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.J.; Bellgrove, M.A.; Hall, T.; Phan, W.M.; Eyles, D.W.; Kvaskoff, D.; McGrath, J.J. Effects of vitamin D supplementation on cognitive and emotional functioning in young adults—A randomised controlled trial. PLoS ONE 2011, 6, e25966. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, H.; Xiong, Y.; Chen, C.; Duan, K.; Jia, J.; Ma, F. Vitamin D Supplementation Improves Cognitive Function Through Reducing Oxidative Stress Regulated by Telomere Length in Older Adults with Mild Cognitive Impairment: A 12-Month Randomized Controlled Trial. J. Alzheimers Dis. 2020, 78, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Littlejohns, T.J.; Henley, W.E.; Lang, I.A.; Annweiler, C.; Beauchet, O.; Chaves, P.H.; Fried, L.; Kestenbaum, B.R.; Kuller, L.H.; Langa, K.M.; et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology 2014, 83, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Aspell, N.; Healy, M.; Mc Partlin, J.; Lawlor, B.A.; O’Sullivan, M. Effects of vitamin D supplementation on cognitive function in healthy, community dwelling older adults: Results from a randomised double-blind placebo-controlled pilot trial. Proc. Nutr. Soc. 2017, 76, E59. [Google Scholar] [CrossRef]
- Rossom, R.C.; Espeland, M.A.; Manson, J.E.; Dysken, M.W.; Johnson, K.C.; Lane, D.S.; LeBlanc, E.S.; Lederle, F.A.; Masaki, K.H.; Margolis, K.L. Calcium and vitamin D supplementation and cognitive impairment in the women’s health initiative. J. Am. Geriatr. Soc. 2012, 60, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Gil Martinez, V.; Avedillo Salas, A.; Santander Ballestin, S. Vitamin Supplementation and Dementia: A Systematic Review. Nutrients 2022, 14, 1033. [Google Scholar] [CrossRef] [PubMed]
- Owusu, J.E.; Islam, S.; Katumuluwa, S.S.; Stolberg, A.R.; Usera, G.L.; Anwarullah, A.A.; Shieh, A.; Dhaliwal, R.; Ragolia, L.; Mikhail, M.B.; et al. Cognition and Vitamin D in Older African-American Women- Physical performance and Osteoporosis prevention with vitamin D in older African Americans Trial and Dementia. J. Am. Geriatr. Soc. 2019, 67, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Maddock, J. Vitamin D, Common Mental Disorders and Cognition: Insights from Genetic and Observational Epidemiology. Ph.D. Thesis, University College London, London, UK, 2014. [Google Scholar]
- Brewer, L.D.; Thibault, V.; Chen, K.C.; Langub, M.C.; Landfield, P.W.; Porter, N.M. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J. Neurosci. 2001, 21, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Prabhakaran, K.; Zhang, X.; Zhang, L.; Liu, H.; Borowitz, J.L.; Isom, G.E. 1Alpha,25-dihydroxyvitamin D3 attenuates cyanide-induced neurotoxicity by inhibiting uncoupling protein-2 up-regulation. J. Neurosci. Res. 2008, 86, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Durk, M.R.; Han, K.; Chow, E.C.Y.; Ahrens, R.; Henderson, J.T.; Fraser, P.E.; Pang, K.S. 1α,25-Dihydroxyvitamin D3 reduces cerebral amyloid-β accumulation and improves cognition in mouse models of Alzheimer’s disease. J. Neurosci. 2014, 34, 7091–7101. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, Y.; Xu, J.; Li, J.; Lv, C.; Gao, Q.; Zhang, C.; Jin, C.; Wang, R.; Jiao, R.; et al. Vitamin D Improves Cognitive Impairment and Alleviates Ferroptosis via the Nrf2 Signaling Pathway in Aging Mice. Int. J. Mol. Sci. 2023, 24, 15315. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Song, S.; Cui, J.; Feng, Y.; Gao, J.; Jiang, P. Vitamin D Receptor Activation Influences NADPH Oxidase (NOX(2)) Activity and Protects against Neurological Deficits and Apoptosis in a Rat Model of Traumatic Brain Injury. Oxidative Med. Cell. Longev. 2017, 2017, 9245702. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yang, X.; Wang, Y.; Zhou, C. Vitamin D Protects against Traumatic Brain Injury via Modulating TLR4/MyD88/NF-κB Pathway-Mediated Microglial Polarization and Neuroinflammation. BioMed Res. Int. 2022, 2022, 3363036. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Eyles, D.W. Vitamin D and the Central Nervous System: Causative and Preventative Mechanisms in Brain Disorders. Nutrients 2022, 14, 4353. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, C.A.; Yannakoulia, M.; Scarmeas, N. Vitamin D and cognition: An update of the current evidence. J. Alzheimers Dis. 2014, 42 (Suppl. S3), S71–S80. [Google Scholar] [CrossRef] [PubMed]
- Rihal, V.; Khan, H.; Kaur, A.; Singh, T.G. Vitamin D as therapeutic modulator in cerebrovascular diseases: A mechanistic perspectives. Crit. Rev. Food Sci. Nutr. 2023, 63, 7772–7794. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.S.; Leisegang, M.S.; Kruse, C.; Vogel, J.; Schürmann, C.; Dehne, N.; Weigert, A.; Herrmann, E.; Brüne, B.; Shah, A.M.; et al. Vitamin D promotes vascular regeneration. Circulation 2014, 130, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, B.; Lökk, J.; Solomon, A.; Mangialasche, F.; Miralbell, J.; Spulber, G.; Annerbo, S.; Andreasen, N.; Winblad, B.; Cedazo-Minguez, A.; et al. Vitamin D in relation to cognitive impairment, cerebrospinal fluid biomarkers, and brain volumes. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- van der Schaft, J.; Koek, H.L.; Dijkstra, E.; Verhaar, H.J.; van der Schouw, Y.T.; Emmelot-Vonk, M.H. The association between vitamin D and cognition: A systematic review. Ageing Res. Rev. 2013, 12, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Marek, K.; Cichoń, N.; Saluk-Bijak, J.; Bijak, M.; Miller, E. The Role of Vitamin D in Stroke Prevention and the Effects of Its Supplementation for Post-Stroke Rehabilitation: A Narrative Review. Nutrients 2022, 14, 2761. [Google Scholar] [CrossRef] [PubMed]
- Michos, E.D.; Gottesman, R.F. Vitamin D for the prevention of stroke incidence and disability: Promising but too early for prime time. Eur. J. Neurol. 2013, 20, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Groves, N.J.; McGrath, J.J.; Burne, T.H. Vitamin D as a neurosteroid affecting the developing and adult brain. Annu. Rev. Nutr. 2014, 34, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Kuznia, S.; Zhu, A.; Akutsu, T.; Buring, J.E.; Camargo, C.A., Jr.; Cook, N.R.; Chen, L.J.; Cheng, T.D.; Hantunen, S.; Lee, I.M.; et al. Efficacy of vitamin D(3) supplementation on cancer mortality: Systematic review and individual patient data meta-analysis of randomised controlled trials. Ageing Res. Rev. 2023, 87, 101923. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, K.S.; Marijnissen, R.M.; van den Brink, R.H.S.; Oude Voshaar, R.C.; Hegeman, J.M. Adverse health outcomes in vitamin D supplementation trials for depression: A systematic review. Ageing Res. Rev. 2021, 71, 101442. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.; Meng, X. Vitamin D and neurodegenerative diseases. Heliyon 2023, 9, e12877. [Google Scholar] [CrossRef] [PubMed]
- Khairy, E.Y.; Attia, M.M. Protective effects of vitamin D on neurophysiologic alterations in brain aging: Role of brain-derived neurotrophic factor (BDNF). Nutr. Neurosci. 2021, 24, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Norlin, M. Effects of vitamin D in the nervous system: Special focus on interaction with steroid hormone signalling and a possible role in the treatment of brain cancer. J. Neuroendocrinol. 2020, 32, e12799. [Google Scholar] [CrossRef] [PubMed]
- Doumit, M.; El-Mallah, C.; El-Makkawi, A.; Obeid, O.; Kobeissy, F.; Darwish, H.; Abou-Kheir, W. Vitamin D Deficiency Does Not Affect Cognition and Neurogenesis in Adult C57Bl/6 Mice. Nutrients 2024, 16, 2938. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and immune function: An overview. Proc. Nutr. Soc. 2012, 71, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Goulart, R.A.; Gasparini, R.G. Associations between inflammatory bowel diseases and vitamin D. Crit. Rev. Food Sci. Nutr. 2019, 59, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Nissou, M.F.; Guttin, A.; Zenga, C.; Berger, F.; Issartel, J.P.; Wion, D. Additional clues for a protective role of vitamin D in neurodegenerative diseases: 1,25-dihydroxyvitamin D3 triggers an anti-inflammatory response in brain pericytes. J. Alzheimers Dis. 2014, 42, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef] [PubMed]
- Koduah, P.; Paul, F.; Dörr, J.M. Vitamin D in the prevention, prediction and treatment of neurodegenerative and neuroinflammatory diseases. EPMA J. 2017, 8, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Shah, N.; Zanetti, B.R.; Maugeri, M.; Silvestre, R.N.; Fatima, F.; Neder, L.; Valadi, H. Extracellular Vesicles and Matrix Remodeling Enzymes: The Emerging Roles in Extracellular Matrix Remodeling, Progression of Diseases and Tissue Repair. Cells 2018, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, L.; Kong, Y.; Xu, D.; Bao, Y.; Zhang, Z.; Liao, Z.; Jiao, J.; Fan, D.; Long, X.; et al. Vitamin D-binding protein in plasma microglia-derived extracellular vesicles as a potential biomarker for major depressive disorder. Genes Dis. 2024, 11, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Kodam, S.P.; Ullah, M. Diagnostic and Therapeutic Potential of Extracellular Vesicles. Technol. Cancer Res. Treat. 2021, 20, 15330338211041203. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Sangha, A.; Quon, M.; Pfeffer, G.; Orton, S.M. The Role of Vitamin D in Neuroprotection in Multiple Sclerosis: An Update. Nutrients 2023, 15, 2978. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Ma, H.; Chen, M.; Bai, J. Vitamin D alleviates neuronal injury in cerebral ischemia-reperfusion via enhancing the Nrf2/HO-1 antioxidant pathway to counteract NLRP3-mediated pyroptosis. J. Neuropathol. Exp. Neurol. 2023, 82, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Alamro, A.A.; Alsulami, E.A.; Almutlaq, M.; Alghamedi, A.; Alokail, M.; Haq, S.H. Therapeutic Potential of Vitamin D and Curcumin in an In Vitro Model of Alzheimer Disease. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520924311. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.D.C.; Medeiros, E.B.; Zabot, G.C.; Pereira, N.S.; do Nascimento, N.B.; Lidio, A.V.; Scheffer, Â.K.; Rempel, L.C.T.; Macarini, B.M.N.; Costa, M.A.; et al. Neuroprotective effects of combined therapy with memantine, donepezil, and vitamin D in ovariectomized female mice subjected to dementia model. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 122, 110653. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.E.; Poles, J.; Shaw, D.P.; Karhu, E.; Khan, S.A.; Lyons, A.E.; Sacco, S.B.; McDaniel, H.R. The effects of twenty-one nutrients and phytonutrients on cognitive function: A narrative review. J. Clin. Transl. Res. 2021, 7, 575–620. [Google Scholar] [PubMed]
- Begum, M.; Saikia, R.; Saikia, S.P. Triple quadrupole liquid chromatography-mass spectrometry-mediated evaluation of vitamin D(2) accumulation potential, antioxidant capacities, and total polyphenol content of white jelly mushroom (Tremella fuciformis Berk.). Mycologia 2024, 116, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Wellington, V.N.A.; Sundaram, V.L.; Singh, S.; Sundaram, U. Dietary Supplementation with Vitamin D, Fish Oil or Resveratrol Modulates the Gut Microbiome in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 23, 206. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, A.F.; Afify, M.A.; Mahfouz, A.M.; Shahzad, N.; Bamagous, G.A.; Al Ghamdi, S.S. Vitamin D enhances antiepileptic and cognitive effects of lamotrigine in pentylenetetrazole-kindled rats. Brain Res. 2017, 1673, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Hua, F.; Wang, J.; Sayeed, I.; Wang, X.; Chen, Z.; Yousuf, S.; Atif, F.; Stein, D.G. Progesterone and vitamin D: Improvement after traumatic brain injury in middle-aged rats. Horm. Behav. 2013, 64, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Joob, B.; Wiwanitkit, V. Vitamin D receptor gene Foki polymorphism and glucocorticoid response. Int. Forum Allergy Rhinol. 2020, 10, 577. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Castrillon, J.L.; Usategui-Martín, R.; Pludowski, P. Treatment of Vitamin D Deficiency with Calcifediol: Efficacy and Safety Profile and Predictability of Efficacy. Nutrients 2022, 14, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; da Silva, J.A.P.; Blauth, M.; Felson, D.T.; McCloskey, E.V.; Watzl, B.; Hofbauer, L.C.; et al. Effect of Vitamin D Supplementation, Omega-3 Fatty Acid Supplementation, or a Strength-Training Exercise Program on Clinical Outcomes in Older Adults: The DO-HEALTH Randomized Clinical Trial. JAMA 2020, 324, 1855–1868. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, U.D.; Kumari Kamalkishore, M.; Vittalrao, A.M.; Kumar Siraganahalli Eshwaraiah, P. Cognition enhancing abilities of vitamin D, epalrestat and their combination in diabetic rats with and without scopolamine induced amnesia. Cogn. Neurodyn. 2022, 16, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Guo, Y.; Cui, L.; Huang, L.; Guo, Q.; Huang, G. Study of Diet Habits and Cognitive Function in the Chinese Middle-Aged and Elderly Population: The Association between Folic Acid, B Vitamins, Vitamin D, Coenzyme Q10 Supplementation and Cognitive Ability. Nutrients 2023, 15, 1243. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Wu, P.F.; Liu, Y.Z.; Jiang, Y.L.; Wan, M.D.; Xiao, X.W.; Yang, Q.J.; Jiao, B.; Liao, X.X.; Wang, J.L.; et al. Association Between Serum Vitamins and the Risk of Alzheimer’s Disease in Chinese Population. J. Alzheimers Dis. 2022, 85, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Stevens, C.M.; Margret, J.J.; Levine, S.N. Alzheimer’s Disease: A Review of Pathology, Current Treatments, and the Potential Therapeutic Effect of Decreasing Oxidative Stress by Combined Vitamin D and l-Cysteine Supplementation. Antioxid. Redox Signal. 2024, 40, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Badaeva, A.; Danilov, A.; Kosareva, A.; Lepshina, M.; Novikov, V.; Vorobyeva, Y.; Danilov, A. Neuronutritional Approach to Fibromyalgia Management: A Narrative Review. Pain Ther. 2024, 13, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P.; Formenti, A.M.; Adler, R.A.; Binkley, N.; Bouillon, R.; Lazaretti-Castro, M.; Marcocci, C.; Napoli, N.; Rizzoli, R.; Giustina, A. Vitamin D: Dosing, levels, form, and route of administration: Does one approach fit all? Rev. Endocr. Metab. Disord. 2021, 22, 1201–1218. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Allali, G.; Allain, P.; Bridenbaugh, S.; Schott, A.M.; Kressig, R.W.; Beauchet, O. Vitamin D and cognitive performance in adults: A systematic review. Eur. J. Neurol. 2009, 16, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Schlogl, M.; Holick, M.F. Vitamin D and neurocognitive function. Clin. Interv. Aging 2014, 9, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Bassuk, S.S. Vitamin D research and clinical practice: At a crossroads. JAMA 2015, 313, 1311–1312. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.; Waterhouse, M.; Rahman, S.; Baxter, C.; Romero, B.D.; McLeod, D.S.A.; Armstrong, B.K.; Ebeling, P.R.; English, D.R.; Hartel, G.; et al. Vitamin D supplementation and cognition-Results from analyses of the D-Health trial. J. Am. Geriatr. Soc. 2023, 71, 1773–1784. [Google Scholar] [CrossRef] [PubMed]
- Latimer, C.S.; Brewer, L.D.; Searcy, J.L.; Chen, K.C.; Popović, J.; Kraner, S.D.; Thibault, O.; Blalock, E.M.; Landfield, P.W.; Porter, N.M. Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc. Natl. Acad. Sci. USA 2014, 111, E4359–E4366. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; van den Heuvel, E.G.; Schoemaker, R.J.; Prévéraud, D.P.; Macdonald, H.M.; Arcot, J. 25-Hydroxyvitamin D as a Biomarker of Vitamin D Status and Its Modeling to Inform Strategies for Prevention of Vitamin D Deficiency within the Population. Adv. Nutr. 2017, 8, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Aspell, N.; Lawlor, B.; O’Sullivan, M.J.T.P.o.t.N.S. Is there a role for vitamin D in supporting cognitive function as we age? Proc. Nutr. Soc. 2018, 77, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Balion, C.; Griffith, L.; Strifler, L.; Henderson, M.; Patterson, C.; Heckman, G.; Llewellyn, D.; Raina, P. Vitamin D, cognition, and dementia: A systematic review and meta-analysis. Neurology 2012, 79, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, C.; Verlinden, L.; Verstuyf, A. The future of vitamin D analogs. Front. Physiol. 2014, 5, 122. [Google Scholar] [CrossRef] [PubMed]
- Pierides, A.M. Pharmacology and therapeutic use of vitamin D and its analogues. Drugs 1981, 21, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, S.; Lu, J.; Boehm, M.F. Vitamin D analogs: Mechanism of action and therapeutic applications. Curr. Med. Chem. 2001, 8, 1661–1679. [Google Scholar] [CrossRef] [PubMed]
- Thiel, A.; Hermanns, C.; Lauer, A.A.; Reichrath, J.; Erhardt, T.; Hartmann, T.; Grimm, M.O.W.; Grimm, H.S. Vitamin D and Its Analogues: From Differences in Molecular Mechanisms to Potential Benefits of Adapted Use in the Treatment of Alzheimer’s Disease. Nutrients 2023, 15, 1684. [Google Scholar] [CrossRef] [PubMed]
- Briones, T.L.; Darwish, H. Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. J. Neuroinflamm. 2012, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Di Sanzo, M.; Cipolloni, L.; Borro, M.; La Russa, R.; Santurro, A.; Scopetti, M.; Simmaco, M.; Frati, P. Clinical Applications of Personalized Medicine: A New Paradigm and Challenge. Curr. Pharm. Biotechnol. 2017, 18, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Allahyari, E.; Hanachi, P.; Ariakia, F.; Kashfi, T.E.; Ferns, G.A.; Bahrami, A.; Mobarhan, M.G. The relationship between neuropsychological function and responsiveness to vitamin D supplementation using artificial neural networks. Nutr. Health 2020, 26, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Kueider, A.M.; Tanaka, T.; An, Y.; Kitner-Triolo, M.H.; Palchamy, E.; Ferrucci, L.; Thambisetty, M. State- and trait-dependent associations of vitamin-D with brain function during aging. Neurobiol. Aging 2016, 39, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Dudley, J.T.; Listgarten, J.; Stegle, O.; Brenner, S.E.; Parts, L. Personalized medicine: From genotypes, molecular phenotypes and the quantified self, towards improved medicine. Pac. Symp. Biocomput. 2015, 342–346. [Google Scholar]
- Jukic, M.; Milosavljević, F.; Molden, E.; Ingelman-Sundberg, M. Pharmacogenomics in treatment of depression and psychosis: An update. Trends Pharmacol. Sci. 2022, 43, 1055–1069. [Google Scholar] [CrossRef] [PubMed]

| Neurotransmitter | Regulation by Vitamin D | Effects | Reference |
|---|---|---|---|
| DA a | TH b ↑, DAT c ↑, VMAT2 d ↑ | ↑ DA synthesis and release | [55] |
| 5-HT e | TPH2 f ↑, SERT g mod | ↑ 5-HT production | [56] |
| GABA h | GAD i ↑ | ↑ GABA synthesis | [57] |
| Glu j | NMDAR k ↑, AMPAR l ↑ | Modulates excitatory signaling | [58] |
| Ach m | Neuroprotection and inflammation modulation. | Indirect via neuroprotection | [59] |
| Endorphins | POMC n ↑ | ↑ β-endorphin levels | [60] |
| Study Method | Participants | Results | Analysis | Country | References |
|---|---|---|---|---|---|
| Cross-Sectional study | 2425 participants for dietary VD intake, 2735 participants for serum 25(OH)D, aged 60+ | Increased dietary vitamin D intake and higher serum concentrations of total 25(OH)D and 25(OH)D3 were linked to improved cognitive performance (OR a and 95% CI b for highest vs. lowest intake group). | Dietary VD intake and serum 25(OH)D3 concentration are positively associated with better cognitive performance. | USA | [77] |
| Cross-Sectional study | 86 outpatients with bipolar disorder and 93 healthy controls | No significant correlation was observed between 25(OH)D, 24,25(OH)2D, VMR, and cognitive functions (attention, memory, executive function). | There is no evidence to suggest that vitamin D metabolism modulates cognitive function in euthymic patients with bipolar disorder (BD). | Austria | [78] |
| Cross-Sectional study | 752 women aged ≥ 75 years | 25(OH)D deficiency (<10 ng/mL): 129/752 (17.2%), Mean SPMSQ c score: 25(OH)D deficiency = 8.56, 25(OH)D sufficient = 9.05, OR for cognitive impairment: 2.08 (p = 0.007). | 25(OH)D deficiency was associated with cognitive impairment in older women. | France | [79] |
| Cross-Sectional study | 4872 middle- to older-aged adults (2678 females) | Women: Improvement in global cognition plateaued around levels of 80 nM/L. Men: Better attention accuracy with increasing levels, but poorer semantic verbal fluency and global cognition. | The associations between serum 25(OH)D levels and cognitive performance might indicate early dose-response relationships, especially in female subjects. | Australia | [80] |
| Cross-Sectional study | 1080 elders (65–99 years) | 25(OH)D > 20 ng/mL associated with better executive function. | Low 25(OH)D linked to executive dysfunction. | USA | [81] |
| Cross-Sectional study | 509 community-dwelling older adults (64–92 years) | Vitamin D levels and MMSE d score: positive association (β = 0.16, p < 0.001), Median vitamin D levels, MCI e = 17 ng/mL, AD = 12 ng/mL, MD = 10 ng/mL, Controls = 12 ng/mL. | Patients with dementia (AD and MD) had the lowest vitamin D levels, whereas patients with MCI had higher levels compared to the other groups. | Italy | [82] |
| Prospective cohort study | 916 participants aged 65+, nondemented at baseline, followed for up to 12 years | 25(OH)D deficiency: 218/916 (24%), HR f for cognitive decline: 2.85 (95% CI 1.37–5.97). | Sufficient levels of vitamin D in the elderly might decelerate mental deterioration and stave off or avert dementia, with a special focus on Alzheimer’s disease. | France | [83] |
| Prospective cohort study | 302 Ugandan children, aged below 5 years | 8 (2.7%) had 25(OH)D levels below 50 nmol/L, 105 (35.8%) had levels between 50 and 75 nmol/L, and 189 (62.6%) had levels exceeding 75 nmol/L. | There is no indication that earlier vitamin D levels are related to cognitive or motor outcomes in 5-year-old children from Uganda. | Uganda | [84] |
| Prospective cohort study | 1673 community-dwelling adults ≥ 65 years | 899 deaths (53.7%) over 3.8 (1.9) years follow-up, 25(OH)D concentration < 50 nmol/L associated with higher risk of cognitive impairment (OR 3.17, 95% CI: 2.06 to 5.00). | Reduced levels of plasma 25(OH)D and cognitive impairment were linked to elevated risks of all-cause mortality. When both conditions coexisted, the risk was even greater. | China | [85] |
| Prospective cohort study | 858 adults aged 65 years or older | Severely 25(OH)D deficient had 1.60 times higher risk of substantial cognitive decline compared to those with sufficient 25(OH)D levels. | Low levels of vitamin D were associated with substantial cognitive decline over a 6-year period. | England | [86] |
| Prospective cohort study | 791 North Indian children, aged 6–9 years | 45.8% of the participants had vitamin D deficiency, 32.7% had inadequate levels, and 21.5% had sufficient levels. | The vitamin D levels during early childhood do not appear to be linked to cognitive development or linear growth when children reach school age. | India | [87] |
| Prospective cohort study | 2061 Black and 1329 White individuals | Vitamin D intake was linked to a slower rate of cognitive decline in Black individuals but not in White individuals. The highest tertile of dietary vitamin D intake was associated with a 0.017 units/year slower cognitive decline in Black individuals. | Dietary vitamin D might contribute to slowing cognitive decline in Black individuals, regardless of supplementation. | USA | [88] |
| Prospective cohort study | 1927 elderly subjects (mean age 73.9 years) | 25(OH)D deficiency (<50 nmol/L) or insufficiency (50–75 nmol/L) associated with higher risk of cognitive decline, Relative risk (95% CI) for cognitive dysfunction: 1.36 (1.04–1.80) for deficiency, 1.29 (1.00–1.76) for insufficiency. | Reduced levels of 25(OH)D are linked to a higher risk of cognitive decline in older adults. | Italy | [89] |
| Prospective cohort study | 661 subjects aged 65+ years | Average follow-up: 5.4 years. 141 cases of dementia (100 AD). No significant link between 25(OH)D and cognitive decline, dementia, or AD. | Vitamin D status showed no significant protective effect on cognitive function. Higher levels of 25(OH)D were linked to an increased risk of dementia and AD in women. | Canada | [90] |
| Prospective cohort study | 1182 Swedish men aged 71+ years | Mean follow-up of 18 years, 116 developed AD, 64 vascular dementia, 250 all-cause dementia; no association between vitamin D status and cognitive outcomes. | No association between baseline vitamin D status and long-term risk of developing dementia or cognitive impairment. | Sweden | [91] |
| Longitudinal cohort study | 1058 adults, median age 75, 62% women | 14% of the participants had vitamin D insufficiency (25OHD < 30 ng/mL), which was linked to worse baseline performance on the MMSE, Trails B, Category Fluency, and Long-Term Retrieval tests. | Moderately low levels of vitamin D were linked to worse performance across various cognitive domains, yet did not forecast cognitive decline over a 12-year period. | USA | [92] |
| Longitudinal cohort study | 290 decedents from the Rush Memory and Aging Project, aged 65+ | Higher concentrations of 25(OH)D3 in the brain were associated with a 25–33% reduction in the odds of dementia or MCI at the final visit before death (p ≤ 0.031). | Higher brain 25(OH)D3 concentrations associated with better cognitive function prior to death but not with post-mortem neuropathology. | USA | [2] |
| Nested cohort study | 818 newborns, followed up to young adulthood (mean age 19.4 years) | Median neonatal 25(OH)D3 levels: 26.2 nmol/L, BPP IQ scores: 3rd quintile = 101.0, 4th quintile = 101.2, 1st quintile = 97.6. | No significant relationship between neonatal 25(OH)D3 levels and BPP IQ scores overall, slightly higher BPP IQ scores in 3rd and 4th quintiles compared to 1st quintile. | Denmark | [93] |
| Randomized Controlled Trial | 623 pregnant women, 551 children evaluated (277 high-dose, 274 standard dose) | No significant impact on motor milestones (β = 0.08, 95% CI, -0.26 to 0.43; p = 0.64), cognitive development (score difference: 0.34, 95% CI, −1.32 to 1.99; p = 0.70), or language development at 2 years (median [IQR], 232 [113–346] words vs. 253 [149–382.5] words; p = 0.02). | Pregnancy supplementation with high-dose vitamin D3 did not enhance neurodevelopmental outcomes in children up to 6 years of age, with the exception of a reduction in language development at 2 years in the high-dose group. | Denmark | [94] |
| Randomized Controlled Trial | 58 postmenopausal women (58 ± 6 years), BMI 30.0 ± 3.5 kg/m2 | 25(OH)D levels increased to 30.2, 36.0, and 40.8 ng/mL in 600, 2000, and 4000 IU/d groups, respectively, 2000 IU/day group showed superior performance in learning and memory assessments (p < 0.05). | Higher doses of vitamin D (2000 IU/d) showed positive effects on learning and memory, 4000 IU/d group had slower reaction time compared to 600 IU/d group. | USA | [95] |
| Randomized Controlled Trial | 3424 participants (VITAL-Cog), 794 participants (CTSC-Cog), aged 60+ years | The pooled mean difference in the annual decline rate for vitamin D3 vs. placebo was 0.01 (95% CI −0.01, 0.02; p = 0.39). Black participants showed better cognitive maintenance with vitamin D3 (MD = 0.04, 95% CI 0.01, 0.08). | Supplementation with Vitamin D3 (2000 IU/day) did not show an association with cognitive decline over a 2–3 year period in community-dwelling older individuals, yet it might offer modest cognitive advantages for older Black adults. | USA | [69] |
| Randomized, Double-Blind, Placebo-Controlled Trial | 93 intervention, 88 control, elderly subjects with MCI | Significant improvements in WAIS-RC scores, FIQ g (mean difference: 3.45 points at 6 months, 7.31 points at 12 months), VIQ h, PIQ i, and decreased TG j, TC k, HDL-C l, LDL-C m in intervention group compared to control. | Vitamin D3 supplementation (400 IU/day) for 12 months significantly enhanced cognitive function and blood lipids in elderly individuals with MCI. | China | [96] |
| Randomized, Double-Blind, Placebo-Controlled Trial | 210 patients with AD (105 intervention, 105 control) | Significant improvements in plasma Aβ42 n (decreased by 11.31% in intervention group vs. 0.27% in control), APP o, BACE1 p, APPmRNA q, BACE1mRNA r levels, and cognitive scores in intervention group over control group. | Vitamin D supplementation (800 IU/day) for 12 months may enhance cognitive function and reduce Aβ-related biomarkers in elderly individuals with AD. | China | [97] |
| Multicenter, blinded, randomized clinical trial | 95 critically ill adults (47 vitamin D3 treatment, 48 placebo) | Adjusted median RBANS s score: Vitamin D3 = 79.6, Placebo = 82.1, Adjusted median executive function composite scores: Vitamin D3 = 8.1, Placebo = 8.7. | High doses of vitamin D3 failed to enhance long-term global cognition or executive function in critically ill adults. | USA | [98] |
| Multicenter double-blind phase 3 clinical trial with 11-year follow-up | 278 patients with clinically isolated syndrome, part of BENEFIT trial | Higher vitamin D levels were linked to better cognitive performance, while smoking was associated with worse outcomes. A 50-nmol/L increase in mean 25(OH)D reduced the odds of poorer PASAT t performance by 65% at year 11. | In patients with MS, lower vitamin D levels and smoking following clinical onset were associated with poorer long-term cognitive function and neuronal integrity. | Global (multicenter) | [99] |
| Randomized Controlled Trial | 273 community-dwelling older adults aged ≥ 60 y | There was no significant difference in cognitive performance between the groups receiving 2000 IU and 800 IU of vitamin D3 per day. | Over a 24-month period, 2000 IU of vitamin D3 per day does not offer superior cognitive benefits compared to 800 IU per day. | Switzerland | [100] |
| Randomized Controlled Trial | 175 adults aged 65–84 with MCI | Multidomain intervention (exercise + cognitive training) improved ADAS-Cog-13 u score compared to control (mean difference −2.64 points; p = 0.005). | Multidomain intervention of aerobic-resistance exercises with cognitive training improves cognition in adults with MCI more than exercise alone. | Canada | [101] |
| Randomized Controlled Trial | 128 participants, aged 18–30 years | No significant changes observed in working memory (F = 1.09, p = 0.30), response inhibition (F = 0.82, p = 0.37), or cognitive flexibility (F = 1.37, p = 0.24). | Although there were significant improvements in vitamin D levels in the active group, no notable changes were observed in cognitive or emotional functioning. | Australia | [102] |
| Randomized Controlled Trial | 183 subjects with MCI | A 12-month course of vitamin D supplementation led to improvements in FSIQ v and reductions in markers of oxidative stress. | Vitamin D enhances cognition and reduces oxidative stress in MCI. | China | [103] |
| Combined Therapy | Description of Action | Target Mechanism(s) | Neurological Conditions Addressed | Reference |
|---|---|---|---|---|
| Vitamin D with Antioxidants Nrf2 a and HO-1 b | Elevation of Nrf2 and HO-1 expression, leading to increased antioxidant enzyme levels. | Oxidative stress reduction, inflammation mitigation. | General (targets oxidative stress and inflammation) | [142] |
| Vitamin D with Alpha Lipoic Acid (ALA) and Curcumin | Effectively treats brain aging by targeting astrocytes under oxidative stress conditions. | Oxidative stress reduction, astrocyte protection. | Alzheimer’s disease, Parkinson’s disease | [143] |
| Vitamin D with Antioxidant Enzymes | Promotes the expression of neuroprotective factors and antioxidant enzymes to prevent neurodegeneration. | Neuroprotection, antioxidant enzyme expression. | Neurodegenerative Diseases | [141] |
| Vitamin D with Memantine and Donepezil | Demonstrated significant neuroprotective effects in a dementia model. | Neuroprotection, potential enhancement of cognitive function. | Dementia (modeled in ovariectomized female mice) | [144] |
| Vitamin D with Ginkgo Biloba | Improves cerebral blood flow and cognitive function. | Enhanced cerebral circulation, antioxidant activity. | Cognitive impairment, vascular dementia | [145] |
| Vitamin D with Polyphenols (e.g., from green tea or fruits) | The antioxidant and anti-inflammatory properties may improve cognitive function. | Oxidative stress reduction, inflammation mitigation, cognitive enhancement. | General cognitive health, neuroprotection | [146] |
| Vitamin D with Resveratrol | Exhibits antioxidant and anti-inflammatory effects. | Inflammation reduction, neuronal cell protection. | Aging-related cognitive decline, neuroinflammation | [147] |
| Vitamin D with Lamotrigine | Synergistic neuroprotective effect. | Neuronal stability, oxidative stress reduction. | Neurological disorders with cognitive aspects | [148] |
| Vitamin D with Progesterone | Combined therapy may provide neuroprotection and improve cognitive function. | Modulation of neurosteroid levels, enhancement of neuroprotective pathways, reduction of neuronal damage. | Cognitive decline, neurodegenerative diseases, and potential neuroprotection in brain injury | [149] |
| Vitamin D with Glucocorticoids | Synergistic anti-inflammatory effects of vitamin D and glucocorticoids. | Anti-inflammatory, immunomodulation. | Multiple sclerosis, neuroinflammatory diseases | [150] |
| Vitamin D with Low-Dose Calcitriol | Significantly reduced acute relapse rates in patients with multiple sclerosis. | Disease-modifying effect in multiple sclerosis, reduction of relapse rates. | Relapsing-remitting multiple sclerosis | [151] |
| Vitamin D and Cathelicidin Antimicrobial Peptide (CAMP) | VDR activation leads to upregulation of CAMP gene for enhanced innate immune responses. | Innate immune response enhancement, antimicrobial peptide upregulation. | Innate immune response against nervous system infections | [152] |
| Vitamin D with Omega-3 Fatty Acids | Combination of omega-3 fatty acids (DHA, EPA) with vitamin D to support cognitive health and reduce inflammation. | Anti-inflammatory, neuroprotection, improved blood flow in the brain. | Cognitive decline, Alzheimer’s disease, multiple sclerosis | [153] |
| Vitamin D with Epalrestat | Vitamin D and epalrestat co-administered for enhanced neuroprotection. | Antioxidant enhancement, aldose reductase inhibition, acetylcholinesterase reduction. | Neurological complications in diabetes, cognitive decline | [154] |
| Vitamin D with Coenzyme Q10 | Antioxidant coenzyme Q10 supports mitochondrial function and energy production in conjunction with vitamin D. | Mitochondrial function support, energy metabolism, reduction of oxidative stress. | Neurodegenerative diseases, cognitive impairment. | [155] |
| Vitamin D with B Vitamins (B6, B9, B12) | Essential for brain health and may reduce homocysteine levels. | Homocysteine metabolism regulation, cognitive function support. | Cognitive decline, vascular health | [156] |
| Vitamin D with L-Cysteine | Co-supplementation of vitamin D and L-Cysteine, a precursor to glutathione (GSH), may improve GSH levels and upregulate vitamin D-metabolizing genes. | Upregulation of GSH and vitamin D-metabolizing genes, reduction of oxidative stress, enhancement of vitamin D bioavailability. | Oxidative stress and chronic diseases such as dementia, diabetes, and heart disease | [157] |
| Vitamin D with Acetyl-L-Carnitine | Supports energy metabolism and has neuroprotective properties. | Energy metabolism support, neuronal membrane protection. | Cognitive decline, mitochondrial dysfunction | [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhong, Z.; Xie, J.; Ni, B.; Wu, Y. Neuroprotective Roles of Vitamin D: Bridging the Gap Between Mechanisms and Clinical Applications in Cognitive Decline. Int. J. Mol. Sci. 2025, 26, 7146. https://doi.org/10.3390/ijms26157146
Liu Y, Zhong Z, Xie J, Ni B, Wu Y. Neuroprotective Roles of Vitamin D: Bridging the Gap Between Mechanisms and Clinical Applications in Cognitive Decline. International Journal of Molecular Sciences. 2025; 26(15):7146. https://doi.org/10.3390/ijms26157146
Chicago/Turabian StyleLiu, Yaoyuan, Zhifeng Zhong, Jiaxin Xie, Bing Ni, and Yu Wu. 2025. "Neuroprotective Roles of Vitamin D: Bridging the Gap Between Mechanisms and Clinical Applications in Cognitive Decline" International Journal of Molecular Sciences 26, no. 15: 7146. https://doi.org/10.3390/ijms26157146
APA StyleLiu, Y., Zhong, Z., Xie, J., Ni, B., & Wu, Y. (2025). Neuroprotective Roles of Vitamin D: Bridging the Gap Between Mechanisms and Clinical Applications in Cognitive Decline. International Journal of Molecular Sciences, 26(15), 7146. https://doi.org/10.3390/ijms26157146





