Abstract
Growth hormone (GH) signaling has been implicated in tumor progression and therapy resistance across multiple cancer types, yet its role in bladder cancer remains largely unexplored. In this study, we investigated the impact of GH and its receptor (GHR) on therapy resistance and disease progression in urothelial carcinoma (UC) through integrated transcriptomic and in vitro analyses. Transcriptomic profiling of The Cancer Genome Atlas bladder cancer cohort revealed that high tumoral GHR expression was associated with differential upregulation of genes involved in drug efflux, epithelial-to-mesenchymal transition (EMT), and extracellular matrix (ECM) remodeling. Notably, elevated GHR levels correlated with significantly reduced overall survival in patients with UC. In parallel, in vitro experiments demonstrated that GH promotes chemoresistance in UC cell lines via upregulation of ATP-binding cassette-containing (ABC) transporters and activation of EMT. GH also modulated ECM-remodeling-associated genes in a chemotherapy-dependent manner, including matrix metalloproteinases and tissue inhibitors of metalloproteinases. Importantly, these effects were abrogated by Pegvisomant, a GHR antagonist, indicating the functional relevance of GH/GHR signaling in the mediation of these phenotypes. Collectively, our findings support a mechanistic role for GH signaling in driving therapy resistance and tumor aggressiveness in bladder cancer and suggest GHR antagonism as a potential therapeutic strategy to improve treatment outcomes.
1. Introduction
Growth hormone (GH) is a peptide hormone secreted centrally from the anterior pituitary gland, which regulates longitudinal growth, organ development, and whole-body metabolism and promotes diseases such as diabetes and cancer. Additionally, GH is produced peripherally by many tissues, including tumors [1,2,3]. Individuals with acromegaly, characterized by excess GH secretion, have an increased risk for cancer [4,5,6], while individuals with Laron Syndrome (LS), characterized by resistance to GH, are protected against cancer [7,8,9]. Remarkably, in over 30 years of studying these individuals, only a single case of cancer has been documented [8]. This phenomenon is mirrored in mouse models. For example, mice that are transgenic for GH have increased incidence of cancer and tumor burden [10], while mice with an inactivating disruption in the GHR gene have decreased incidence of cancer and tumor burden [11].
GH has been linked to cancer development and progression since 1950 [12], with elevated levels of circulating GH reported in patients with cancer over the past 40 years [13,14]. Additionally, while high levels of GH and GHR expression are associated with poorer prognosis in several cancer types [15,16,17,18], this relationship is not consistent across all cancers—for example, it is not observed in liver cancer or renal clear cell carcinoma [19]. Nevertheless, evaluating this relationship can help to identify cancers that may benefit from treatment strategies that include targeting GH action.
Recent studies have begun to shed light on the specific molecular mechanisms by which GH contributes to cancer development and therapy resistance [15,17,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. The role of GH in cancer therapy resistance involves facilitating active drug efflux, induction of phenotypic plasticity via epithelial-to-mesenchymal transition (EMT), modulation of the tumor microenvironment (TME), and enabling evasion of apoptosis [19,26,28,29,30,34,35,38,39]. Research performed by our laboratory has demonstrated that GH action upregulates expression of ATP-binding cassette-containing (ABC) transporters in cancer, contributing to heightened resistance to anticancer therapies in both in vitro and in vivo models [20,21,23,24,33]. Furthermore, our group and others have identified a critical role of GH in promoting EMT across multiple cancer types [17,18,24,27,34,37]. Although these GH-driven oncogenic processes have been extensively characterized in cancers of the breast, liver, colorectum, endometrium, and prostate, such studies have not been conducted in the context of bladder cancer [19]. Collectively, these findings highlight the potential of targeting GH action as a therapeutic strategy in cancer treatment.
Bladder cancer is one of the most common malignancies of the urinary system, with urothelial carcinoma (UC) comprising nearly 94% of cases. In 2025, an estimated 84,870 new cases are expected to be diagnosed in the United States [40]. While the five-year survival rate for early-stage bladder cancer is as high as 78.4%, it plummets to a mere 8.8% for patients with metastatic disease due to high recurrence rates and limited treatment efficacy [41]. The most common chemotherapeutic regimen for UC is a combination of cisplatin and gemcitabine, although cisplatin-based therapies reportedly have a high rate of failure [42,43]. The challenges in managing advanced UC stem from its tumoral heterogeneity, immune evasion, and resistance to standard therapies [44,45,46,47]. Advancing research into novel treatment strategies is critical for improving patient outcomes and long-term survival.
Previous research has implicated GH in bladder carcinogenesis in rats, with all tested animals developing UC [48]. More recently, GHR has been identified as a potential diagnostic marker for UC [49]. However, beyond these studies, the direct role of GH in UC remains largely unexplored. Downstream signaling molecules in the GH cascade, including STAT3/5 and MEK, have been associated with therapy resistance in UC [50,51], while insulin-like growth factor 1 (IGF-1), the primary downstream effector of GH, has also been linked to both therapy resistance and metastasis in UC [52,53]. Therapy resistance in UC arises from various mechanisms, such as evasion of apoptosis, drug efflux, metabolic reprogramming, and EMT [42,45,54,55,56,57]. Notably, many of these mechanisms intersect with those that are regulated by the action of GH [25].
In this study, we aimed to characterize the expression of GHR and its correlation with patient survival, therapy resistance, and disease progression in UC in silico. We used transcriptomic data from patients with UC in The Cancer Genome Atlas (TCGA) database to evaluate the expression level of GHR in UC tumors in comparison to noncancerous bladder tissue. To identify if bladder cancer may benefit from a treatment strategy that includes targeting GH action, we examined the relationship between GHR expression and patient survival. Additionally, we explored how GHR expression correlates with that of genes linked to therapy resistance and disease progression. We also aimed to begin the exploration of the role of GH in therapy resistance in UC. We first confirmed expression of GHR in UC cells and assessed their response to GH stimulation and its inhibition by Pegvisomant, an FDA-approved GHR antagonist, used for the treatment of acromegaly. Subsequently, we examined how GH and Pegvisomant affect the expression of drug efflux pumps, markers of EMT, matrix metalloproteinases (MMPs), and tissue inhibitors of metalloproteinases (TIMPs) in the presence and absence of the chemotherapeutic agents cisplatin and gemcitabine, which are standard treatments for UC [58,59,60]. Additionally, we assayed the effect of GH and Pegvisomant on cellular migration and invasion. Therefore, we sought to provide a foundational insight into the impact of GH on UC progression and therapy resistance, laying the groundwork for potential avenues for therapeutic intervention.
2. Results
2.1. GHR Is Expressed in UC In Silico and Correlates with Poor Overall Survival
Using transcriptomic data archived in TCGA obtained from 404 patients with bladder cancer [61], we found that, overall, GHR was not expressed as highly in bladder tumors as it was in normal bladder tissue taken from the same patients (Figure 1a). However, upon further analysis of the correlation between GHR expression and tumor stage, we found that GHR expression is significantly positively correlated with increasing tumor stage (rho = 0.144, p = 0.00359) (Figure 1b).
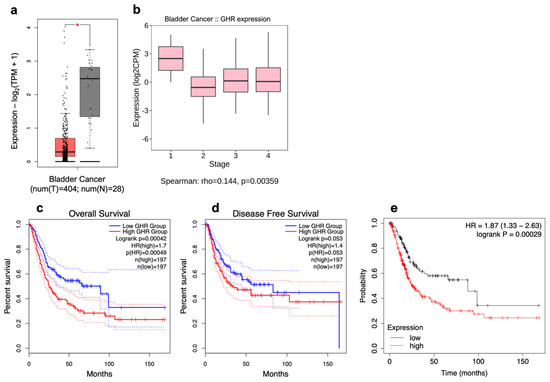
Figure 1.
GHR is expressed in urothelial carcinoma. (a) Tumoral (left; red box) expression of GHR is significantly (* = p < 0.05) less than that in noncancerous urinary bladders (right; grey box) and (b) exhibits a weak but positive correlation with increasing clinical stages of UC. GHR expression is (c) significantly inversely correlated with overall survival (blue line = below median expression of GHR; red line = above median expression of GHR; dashed lines = 95% confidence interval) and (d) trends toward an inverse correlation with disease-free survival but does not reach statistical significance (blue line = below median expression of GHR; red line = above median expression of GHR; dashed lines = 95% confidence interval). (e) Tumoral expression of GHR and PRLR is significantly inversely correlated with patient survival (black line = below median expression of GHR and PRLR; red line = above median expression of GHR and PRLR).
From transcriptomic data archived in TCGA database obtained from 394 patients with bladder cancer, a significant decrease in overall survival (hazard ratio (HR) = 1.7, p = 0.00042) and a trend toward a reduction in disease-free survival (HR = 1.5, p = 0.053) were observed in patients whose tumors express high (above median) levels of GHR in comparison with patients whose tumors express low (below median) levels of GHR (Figure 1c,d). While GHR levels may be lower in bladder tumors relative to normal tissue, higher expression of GHR within a tumor is still correlated with worse patient outcomes. This distinction highlights the importance of relative GHR expression specifically within cancerous tissue as a potential prognostic marker.
Often considered an extension of the GH gene family, the prolactin (PRL) gene has 83.4% sequence identity with human GH-N at the nucleotide level [62]. While the sequence identity of the GHR and the PRL receptor (PRLR) is much lower at only ~28%, their structures are extremely similar [63]. Uniquely, in humans, GH can bind to and activate PRLR in addition to its own cognate receptor. However, PRL can bind only to its own receptor [64]. Tumoral GHR and PRLR expression are significantly inversely correlated with the overall survival of patients with bladder cancer (HR = 1.87, p = 0.00029), as shown in Figure 1e.
For further validation, Kaplan–Meier survival plots with GHR, GH1, IGF-1 receptor (IGF1R), or IGF1 as the reference gene were generated from TCGA database, including 405 patients with bladder cancer. Here, transcriptomic data from patients with bladder cancer were again parsed as above and below median mRNA expression levels, and correlation with overall survival was plotted with respect to sex, stage, and race.
In both female and male sexes as well as the combination of both sexes, GHR expression was significantly inversely correlated with survival (respectively, HR = 2.71, 2.06, 2.12; p = 0.0071, 7.7 × 10−5, 2.3 × 10−5). GHR expression in clinical stage 4 was also inversely correlated with survival in female and male patients alone, as well as the combination of female and male patients (respectively, HR = 3.21, 2.86, 3.15; p = 0.0017, 0.019, 8.5 × 10−5). GHR expression in clinical tumor stage 3 was inversely correlated with survival in male patients alone and the combination of female and male patients (respectively, HR = 3.34, 3.40; p = 0.016, 0.0027). No significant correlation was observed between GHR expression in clinical stage 3 in female patients and survival. However, GHR expression in clinical stage 2 only trended toward an inverse correlation with survival in male patients but did not reach statistical significance (HR = 2.06, p = 0.058). GHR expression in clinical stage 2 in female patients alone and the combination of female and male patients was positively correlated with survival (respectively, HR = 0.15, 0.49; p = 0.0092, 0.035). Correlation between GHR expression in clinical stage 1 and survival was unable to be analyzed due to the insufficient number of patients for which data were available. Lastly, GHR expression in white patients was also significantly inversely correlated with survival (HR = 2.08; p = 2.7 × 10−5) and trended toward an inverse correlation in Black patients without reaching statistical significance (HR = 3.61, p = 0.077), while the opposite was true for Asian patients (HR = 0.22, p = 0.034) (Figure 2 and Figure S1).
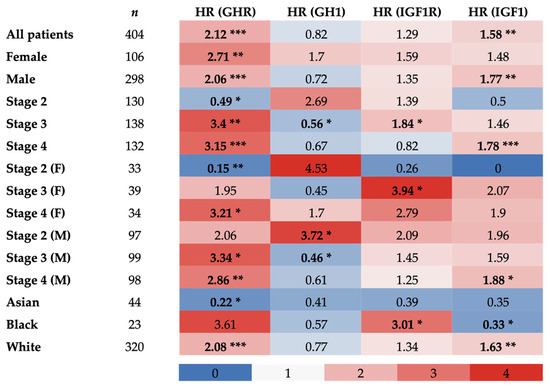
Figure 2.
Hazard ratios of overall survival of patients with UC in TCGA database correlated with tumoral GHR, GH1, IGF1R, and IGF1 expression. Bold values indicate a statistically significant correlation with survival (* = p < 0.05; ** = p < 0.01; *** = p < 0.001).
In females, GH1 expression trended toward an inverse correlation with survival (HR = 1.7; p = 0.083), while the opposite was true in males (HR = 0.72, p = 0.069), with neither reaching statistical significance, and no correlation was observed in the combination of both sexes (HR = 0.82; p = 0.2). GH1 expression in clinical stage 2 in male patients was inversely correlated with survival, trended toward an inverse correlation in the combination of female and male patients without reaching statistical significance, and was not correlated with survival in female patients (respectively, HR = 3.72, 2.69, 4.53; p = 0.021, 0.053, 0.13). Contrarily, a positive correlation between GH1 expression and survival was also observed in clinical stage 3 in male patients and the combination of female and male patients (respectively, HR = 0.46, 0.56; p = 0.025, 0.044), and a trend toward a positive correlation was observed in female patients but statistical significance was not reached (HR = 0.45; p = 0.09). While there were no significant correlations between GH1 expression in clinical stage 4 for any patients and survival, both male patients and the combination of female and male patients trended toward a positive correlation (respectively, HR = 0.61, 0.67, p = 0.056, 0.084). Correlation between GH1 expression in clinical stage 1 and survival was unable to be analyzed due to the insufficient number of patients for which data were available. Additionally, no significant correlation was observed between GH1 expression and survival for Asian, Black, or white patients with UC (Figure 2 and Figure S2).
A significant inverse correlation between tumoral IGF1R expression and survival was observed in clinical stage 3 for female patients and the combination of female and male patients (respectively, HR = 3.94, 1.84; p = 0.019, 0.042). A trend toward an inverse correlation was observed in clinical stage 4 for female patients (HR = 2.79; p = 0.087) and clinical stage 2 for male patients (HR = 2.09; p = 0.057), while, conversely, a trend toward a positive correlation was observed between tumoral IGF1R in clinical stage 2 for female patients and survival (HR = 0.26; p = 0.077), but statistical significance was not reached in any case. Additionally, a significant inverse correlation was found between tumoral IGF1R expression and survival in Black patients (HR = 3.01; p = 0.047). No significant correlations were detected between tumoral IGF1R expression and survival in the following categories: all patients combined, female patients, male patients, clinical stages 2 and 4 for all patients, clinical stages 3 and 4 for male patients, Asian patients, and white patients (Figure 2 and Figure S3).
In both male patients and the combination of female and male patients, tumoral IGF1 expression was significantly inversely correlated with survival (respectively, HR = 1.77, 1.58; p = 0.0023, 0.0025). This trend was also observed in clinical stage 4 for the same patients (respectively, HR = 1.88, 1.78; p = 0.026, 0.009). A trend toward an inverse correlation between IGF1 and survival was present in clinical stage 2 for male patients (HR = 1.96; p = 0.074), whereas the opposite was true in clinical stage 2 for female patients (HR = 0; p = 0.058), but statistical significance was not reached in either case. Additionally, a significant inverse correlation between tumoral IGF1 and survival was seen in white patients, with the opposite observed in Black patients (respectively, HR = 1.63, 0.33; p = 0.0029, 0.047). No significant correlations were detected between tumoral IGF1 expression and survival in female patients, clinical stages 2 and 3 for all patients, clinical stages 3 and 4 for female patients, clinical stage 3 for male patients, and Asian patients (Figure 2 and Figure S4).
2.2. Genes Correlated with GHR Indicate a Therapy Resistance Gene Expression Pattern in UC
Using data from patients with bladder cancer in TCGA database, correlation analyses of differentially expressed mRNAs with respect to GHR mRNA expression in tumor samples were performed. Specific gene modules were queried on the basis of both their roles in driving therapy resistance and past reports of GH-regulated changes in their expression. In the GH–IGF axis module [65,66,67], significantly (False Detection Rate (FDR) 0.05) upregulated genes in both sexes included several genes known to be under the control of GH, such as IGF1 and IGF-binding protein (IGFBP)-5, -7, and -2. We also observed significant upregulation in both sexes of downstream signaling molecules in the GH signaling cascade: RASGRF, Janus kinase (JAK)1/2, signal transducer and activator of transcription (STAT)3/5, phosphatidylinositol-3 kinase (PI3K), the SRC family kinase FYN, protein kinase B (AKT), and mitogen-activated protein kinase (MAPK). In addition, the upregulation in both sexes of suppressors of cytokine signaling (SOCS3, SOCS5, PTPNs) demonstrates active GH signaling in bladder cancer tumors (Figure 3a).
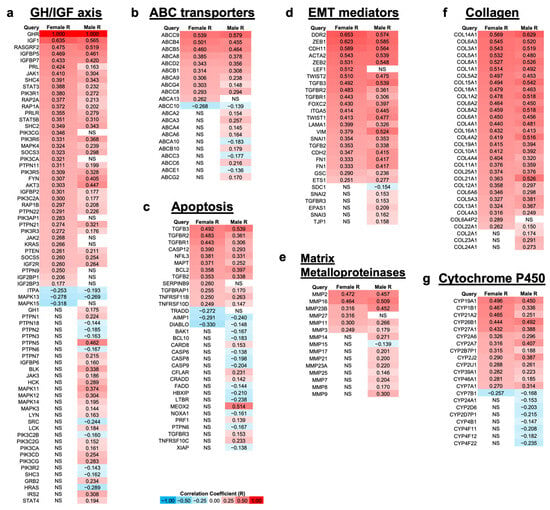
Figure 3.
Genes involved in processes related to therapy resistance are differentially up- and downregulated in female (n = 108) and male (n = 304) patients with UC that have a relatively high mRNA expression level of GHR (FDR 0.05) via TCGA. (a) Genes involved with GH signaling; (b) ATP-binding cassette (ABC) transporters, which are nonspecific drug efflux pumps powered by ATP that have been shown to play a role in cancer drug resistance; (c) genes involved with apoptosis; (d) genes involved in epithelial-to-mesenchymal transition (EMT), which is the beginning of the metastatic process, and it occurs when cells lose epithelial morphology and gain mesenchymal morphology; (e) matrix metalloproteases, which create space for tumors to grow; (f) collagens; (g) cytochrome P450s. NS = not significantly correlated with GHR expression.
In the ABC transporters module [68,69,70], we observed significant upregulation of multidrug efflux pumps that have previously been reported to be upregulated by GH in other cancers [33]: ABCB5 and ABCB1 in both sexes and ABCG2 in males only. In addition, many ABCA family transporters were significantly upregulated in male patients—ABCA2, ABCA3, ABCA4, and ABCA6—with ABCA13 upregulated only in female patients and ABCA8 and ABCA9 significantly upregulated in both sexes (Figure 3b).
In the apoptosis module [71,72,73,74,75], multiple pro-apoptotic genes, such as Bcl-2 homologous antagonist killer (BAK), NADPH oxidase activator 1 (NOXA1), and caspase-6, -8, and -9, were significantly downregulated in male patients, as well as DIABLO in both sexes. This was accompanied by upregulation of anti-apoptotic genes, such as nuclear factor, interleukin-3 regulated (NFIL3), B-cell lymphoma 2 (BCL2), and tumor necrosis factor receptor superfamily (TNFRSF)-11B and -10D in both sexes (Figure 3c).
In the EMT mediators module [76,77,78], all genes, with the exception of syndecan 1 (SDC1), were significantly upregulated in at least one sex in patients with high GHR expression. The EMT-promoting transcription factors zinc finger E-box binding homeobox (ZEB)1, ZEB2, TWIST1, TWIST2, SNAI1, FOXC2, goosecoid (GSC), and ETS1 were significantly upregulated in both sexes. SNAI2 and SNAI3 were upregulated in male patients, and LEF1 was upregulated in female patients. Additionally, the mesenchymal markers cadherin (CDH)2, CDH11, smooth muscle α-actin 2 (ACTA2), α5β1 integrin (ITGA5), vimentin (VIM), and fibronectin (FN1) were all significantly upregulated in both sexes. Many genes related to transforming growth factor beta (TGF-β) signaling were significantly upregulated: TGFB2, TGFB3, TGFBR1, and TGFBR2 in both sexes, with the addition of TGFBR3 in males (Figure 3d).
Similar to the EMT mediators module, in the MMPs module [79,80,81,82], all genes, with the exception of MMP15, were significantly upregulated in at least one sex. The MMPs with the highest correlation with GHR expression in both female and male patients with bladder cancer were MMP2 (rho = 0.472 (F), 0.457 (M)), MMP16 (rho = 0.464 (F), 0.509 (M)), and MMP23B (rho = 0.316 (F), 0.452 (M)) (Figure 3e).
Collagens are a major component of the extracellular matrix (ECM) that is digested by MMPs. Additionally, similar to both the EMT mediators and MMPs modules, all genes in the collagen module [83,84] were significantly upregulated with increasing GHR expression in at least one sex. Most of the collagens in this module are ECM constituent collagens, while only a few are constituents of the basement membrane (Figure 3f).
In the cytochrome P450 (CYP) module [85,86,87], the genes with the highest correlation with GHR expression were CYP19A1 (rho = 0.496 (F), 0.450 (M)), CYP1B1 (rho = 0.467 (F), 0.336 (M)), and CYP21A2 (rho = 0.465 (F), 0.251 (M)). Many CYPs were also significantly inversely correlated with GHR expression in male patients with bladder cancer, and CYP7B1 was downregulated with increased GHR expression in both sexes (Figure 3g).
2.3. GHR Is Expressed in UC Cells
To evaluate mRNA levels of genes involved in GH signaling, cells were starved in FBS-free growth media for 6 h. In human UC cells, GH1, IGF1R, and IGFBP3 were highly expressed, while GHR, IGF1, PRL, and insulin receptor (INSR) were expressed at intermediate levels. In contrast, PRLR and insulin (INS) were expressed at relatively low levels (Figure 4a). In murine UC cells, Ghr, Igf1r, and Insr were highly expressed, whereas Gh1, Igf1, Igfbp3, and Ins exhibited low-to-intermediate expression (Figure 4b). Since GH only signals through the prolactin receptor in humans, mRNA expression levels of prolactin and its receptor were not evaluated in murine cells. Additionally, protein analysis confirmed high expression of GHR in both human and mouse UC cells (Figure 4c,d).
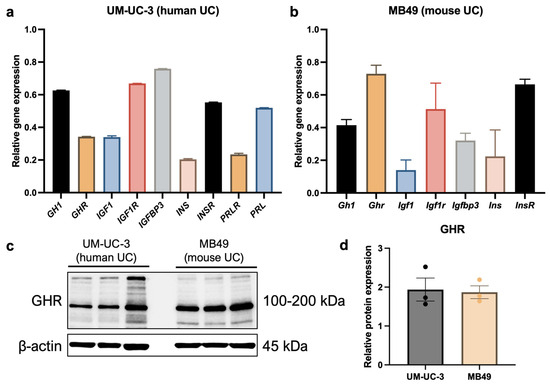
Figure 4.
Human and mouse UC cells express GHR. (a) The human UC cell line UM-UC-3 expresses GHR mRNA, confirmed by qPCR. This cell line also exhibits relatively high expression of other genes involved in GH signaling, including GH1, IGF1R, and IGFBP3. (b) The murine UC cell line MB49 expresses Ghr mRNA, confirmed by qPCR. This cell line also exhibits relatively high levels of Igf1r and Insr. (c,d) High expression of GHR at the protein level was also confirmed by western blot in human and mouse UC cells.
2.4. UC Cells Are Responsive to GH
To assess the response of UC cells to GH and Pegvisomant, cells were serum-starved in FBS-free growth media for 6 h before being treated with GH and Pegvisomant for 12 min. Upon GH stimulation, human UC cells exhibited increased phosphorylation of STAT5, STAT3, AKT, and SRC. Treatment with Pegvisomant both alone and in combination with GH resulted in decreased phosphorylation of STAT5, STAT3, AKT, and SRC. However, p42/44 mitogen-activated protein kinase (MAPK) phosphorylation remained unaffected under all tested conditions, as expected due to the fact that UM-UC-3 cells are positive for the gain-of-function KRASG12C mutation (Figure 5a–f) [88].
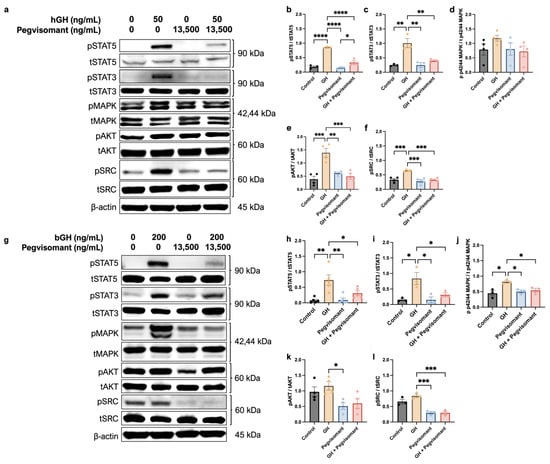
Figure 5.
UC cells are responsive to GH. (a) Western blots show that GH stimulation to human UC cells increases phosphorylation of (b) STAT5, (c) STAT3, (e) AKT, and (f) SRC, which is reversed by the addition of Pegvisomant. (d) Phosphorylation of MAPK was unaffected by GH or Pegvisomant. (g) Western blots show that GH stimulation increases phosphorylation of (h) STAT5, (i) STAT3, and (j) MAPK, which is reversed by the addition of Pegvisomant. While GH did not increase phosphorylation of (k) AKT or (l) SRC, Pegvisomant decreased it. (* = p < 0.05; ** = p < 0.01; *** = p < 0.001 **** = p < 0.0001).
Similarly, stimulation of mouse UC cells with GH resulted in increased phosphorylation of STAT5, STAT3, and MAPK, which was reversed when Pegvisomant was administered alone. Administration of Pegvisomant also decreased the phosphorylation of AKT and SRC. However, when GH and Pegvisomant were combined, MAPK phosphorylation remained elevated, indicating that Pegvisomant failed to inhibit this effect. When the combination of GH and Pegvisomant was administered, phosphorylation of STAT5, STAT3, and SRC was decreased (Figure 5g–l).
2.5. GHR Antagonism Decreases Expression of ABC Transporters in UC Cells
It is well known that GH regulates the expression and activity of ABC transporters in tumor cells [15,20,23,24,33]. ABC transporters function as multidrug efflux pumps, and they play a critical role in actively pumping out chemotherapeutic agents from cancer cells, thereby contributing to the development of chemoresistance [19,89,90]. Here, we examined the effect of GH, Pegvisomant, and the chemotherapies cisplatin and gemcitabine in various combinations on the protein levels of ABCB1, ABCC1, ABCC2, and ABCG2, as they have been reported to mediate GH-induced chemoresistance [20,33].
In mouse UC cells, ABC transporter expression remained unchanged in the absence of chemotherapeutic agents. Upon cisplatin treatment, GH stimulation significantly upregulated ABCB1 and ABCC1 expression compared with GH treatment alone. Pegvisomant successfully reversed this effect, both alone and in combination with GH (Figure 6a–c). Furthermore, Pegvisomant alone reduced expression of ABCB1 in the presence of gemcitabine (Figure 6a,b). ABCC2 expression was suppressed by Pegvisomant in gemcitabine-treated cells regardless of the presence of GH, whereas, in the presence of cisplatin, Pegvisomant failed to lower ABCC2 expression in GH-treated cells (Figure 6a,d). No significant changes were detected in ABCG2 expression under any condition (Figure 6a,e).
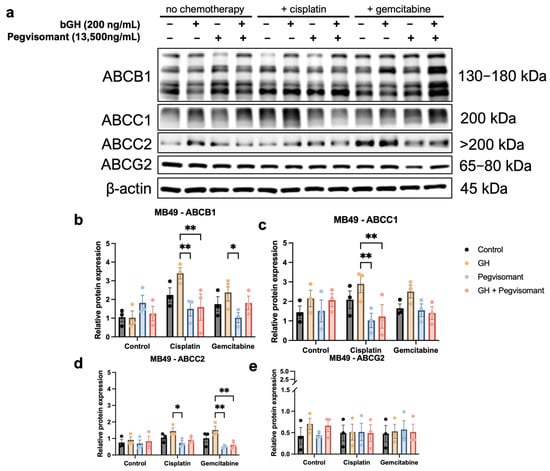
Figure 6.
GH increases expression of ABC transporters in murine UC cells when treated with cisplatin or gemcitabine and GH. (a) Western blots and (b–e) quantification show increased expression of ABCB1, ABCC2, and ABCC2 in the presence of cisplatin and/or gemcitabine when exposed to GH. Pegvisomant is able to reverse this upregulation. (* = p < 0.05; ** = p < 0.01).
2.6. GHR Antagonism Inhibits Cellular Migration and Invasion in UC Cells
GH action is a key regulator of tumor cell migration and invasion [28,36,91]. To assess the impact of GH action on the migratory potential of UC cells, we utilized a wound-healing migration assay [92]. Cells were treated with GH, Pegvisomant, or the combination of GH and Pegvisomant, and cellular migration was assessed by measuring the change in cell-free area before and after treatment. In human UC cells, stimulation with GH led to a significant reduction in cell-free area, demonstrating an increase in cellular migration. This effect was reversed when Pegvisomant was added to cells both alone and in combination with GH, suggesting an inhibitory role in GH-induced migration (Figure 7a,b). Likewise, in mouse UC cells, GH treatment promoted enhanced cellular migration, as evidenced by a decrease in cell-free area. This effect was effectively counteracted by Pegvisomant, both in the presence and absence of GH (Figure 7c,d).
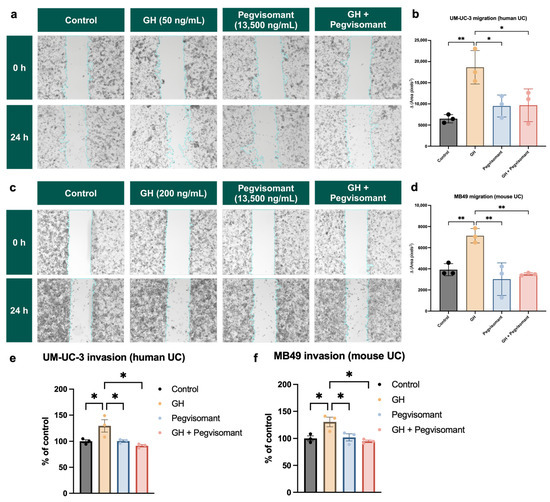
Figure 7.
GHR antagonism inhibits cellular migration and invasion in UC cells. Wound-healing migration assay in (a,b) human and (c,d) mouse UC cells shows that GH treatment significantly reduces cell-free area, indicating increased cellular migration, while Pegvisomant reverses this effect. Fluorometric invasion assay in (e) human and (f) mouse UC cells demonstrates that GH stimulation increases basement membrane invasion, which is blocked by Pegvisomant. (* = p < 0.05; ** = p < 0.01).
Using a fluorometric assay, we evaluated the impact of GH and Pegvisomant on the basement membrane invasion capacity of UC cells. Stimulation with GH enhanced cellular invasion of human UC cells. This effect was reversed by Pegvisomant, both alone and in combination with GH (Figure 7e). A similar pattern was observed in murine UC cells, where GH stimulation led to a significant increase in basement membrane invasion capacity. This phenomenon was effectively reversed when Pegvisomant was administered, both in the presence and absence of GH, indicating that GH-driven invasion is inhibited by Pegvisomant (Figure 7f).
2.7. GH Modulates Expression of Markers of EMT and ECM Remodeling in UC Cells
One of the most well-characterized processes that drives cancer metastasis and is considered a key initiator of tumoral dissemination is EMT [78]. Previous research from our lab has identified GH as a key regulator of EMT both in vitro and in vivo [24,27,33]. Additionally, GH has been shown to upregulate expression of MMPs, which are potent pro-EMT factors that degrade the ECM, enabling tumor growth, invasion, and eventual metastasis [31,32,79,93]. To investigate the role of GH in EMT and ECM remodeling, we assessed protein expression levels of EMT markers, pro-EMT transcription factors, TGF-β, MMPs, and their natural inhibitors, TIMPs, under the previously described treatment conditions.
In mouse UC cells, we observed no significant changes in the expression levels of E-cadherin, Snail, Slug, Twist1, MMP9, MMP14, TIMP1, or TIMP2 in the absence of chemotherapy. However, expression of N-cadherin and Zeb1 was increased by the addition of GH. While Pegvisomant successfully decreased expression of N-cadherin in the presence of GH, it was unable to decrease the Zeb1 expression level. When exposed to cisplatin, GH increased the expression of Snail, Twist1, and MMP14, but no changes were observed in the expression of E-cadherin, N-cadherin, Zeb1, Slug, TIMP1, or TIMP2 with the addition of GH to cisplatin. The addition of Pegvisomant to cisplatin in the absence of GH increased expression of E-cadherin, TIMP1, and TIMP2 while decreasing expression of N-cadherin, Zeb1, Snail, Slug, Twist1, and MMP14. While co-administration of GH and Pegvisomant in the presence of cisplatin was able to successfully increase expression of E-cadherin and decrease that of N-cadherin, Zeb1, Snail, Twist1, and MMP14, it was unable to increase expression of TIMP1 and TIMP2 or decrease expression of Slug and Twist1. No significant changes in MMP9 expression were observed in the presence of cisplatin. When exposed to gemcitabine, GH increased expression of Slug and Twist1, but no changes in expression were observed in E-cadherin, N-cadherin, Zeb1, MMP9, or TIMP1. The addition of Pegvisomant alone to gemcitabine significantly increased expression of E-cadherin and TIMP1, while decreasing expression of N-cadherin, Zeb1, Slug, Twist1, MMP9, and MMP14. In the presence of GH and gemcitabine, Pegvisomant was still able to successfully increase expression of E-cadherin and TIMP1 and decrease expression of N-cadherin, but was not able to decrease expression of Zeb1, Slug, Twist1, or MMP14. No significant changes in expression of Snail or TIMP2 were observed in the presence of gemcitabine. Additionally, expression levels of Vimentin, TGF-β, and MMP2 were unaffected by all treatment conditions (Figure 8). Taken together, these findings suggest that GH promotes EMT in mouse UC cells, particularly in the presence of chemotherapy, while Pegvisomant inhibits many of these effects, though some markers remain unaffected.
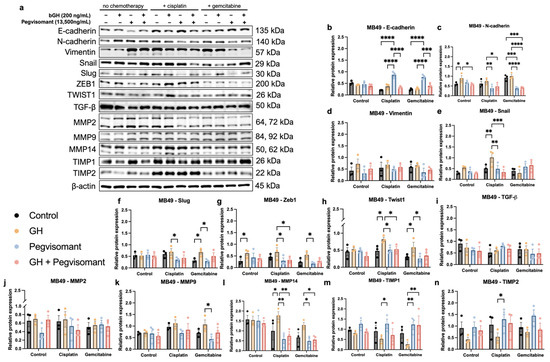
Figure 8.
GH modulates expression of markers of EMT and ECM remodeling in mouse UC cells. (a) Western blots with (b–n) quantification. (* = p < 0.05; ** = p < 0.01; *** = p < 0.001; **** = p < 0.0001).
3. Discussion
This study integrated transcriptomic analysis and in vitro assays to investigate the role of GH signaling in UC. We found that GHR expression correlated with advanced disease stage and reduced survival, despite being lower in UC tumors than in noncancerous bladder tissue. This pattern is consistent in many cancers where targeting GH action has shown therapeutic promise, including melanoma [20,21,22,24,33], pancreatic cancer [23,34,94], breast cancer [13,15,19,26,35,95,96,97,98], and prostate cancer [99,100,101,102,103,104], among others. In these types of cancers, GH plays a key role in creating a tumor microenvironment conducive to cancer progression, despite the fact that GHR is not overexpressed. Notably, GH promoted drug resistance, cellular migration, invasion, and expression of EMT and ECM remodeling markers in vitro, which was reversed with the addition of Pegvisomant.
While endocrine GH secretion by the anterior pituitary gland decreases with age through the process of somatopause [105], peripheral GH secretion, including that by tumor cells, does not, and can even increase [106,107]. Additionally, GHR is expressed by many types of cells in the tumor microenvironment, including tumor cells [108], cancer-associated adipocytes, cancer-associated fibroblasts, immune cells, stromal cells [29], and endothelial cells [109]. This enables highly concentrated, locally produced GH to drive tumor-supportive actions [29,109,110,111,112]. The intricate crosstalk between GH action and the tumor microenvironment underscores the importance of exploring the relationship between GHR expression and patient survival in UC.
GH action is associated with poor prognosis in terms of overall survival and disease-free survival in many cancers, including breast cancer [15,113], liver cancer [16,114,115], colorectal cancer [116], and gastric cancer [117], among others [19]. However, this relationship has never been explored in the context of UC. Despite the evidence implicating GH in promoting tumor progression and therapy resistance in some cancers, other studies suggest that GH itself may not directly cause cancer. Clinical data from patients receiving GH replacement therapy for GH deficiency have not consistently demonstrated an increased risk of cancer development [118,119,120]. Long-term follow-up with patients with acromegaly, characterized by chronic elevation of circulating GH and IGF-1, have reported an association with certain cancers, but this remains controversial, as confounding factors such as age, comorbidities, and genetic predispositions may be causative, rather than increased GH and IGF-1 [5,121,122]. Overall, while GH may contribute to tumor progression through its effects on proliferation, survival, and therapy resistance, current evidence does not definitively support the notion that GH alone is a primary driver of cancer development.
Sex- and stage-specific analyses revealed further complexity. GHR and IGF1 were inversely correlated with survival in male and combined cohorts, particularly at later stages. This could potentially be due to the fact that sex hormones regulate transcription of GH1 differently [123,124,125]. Additionally, female patients represented only approximately one-third of male patients at each clinical stage, a disparity that reflects the greater prevalence of UC in males and may influence the observed data trends. Interestingly, GH1 displayed opposite correlations depending on tumor stage and patient sex. In addition to the pronounced gender disparity in UC prevalence, there is also a significant racial disparity. UC is most frequently diagnosed in Caucasians, where tumoral GHR and IGF1 were inversely correlated with survival. Although UC is less prevalent in Black individuals, they experience disproportionately higher mortality [126,127]. In our study, IGF1R expression was inversely correlated with survival in Black patients, and GHR showed a similar trend that did not reach statistical significance, which may be one of many factors that contribute to their increased mortality rate. Notably, in Asian patients, who have the highest global UC prevalence [128] we observed a positive correlation between GHR expression and survival. These findings highlight the complex interplay between GH signaling and demographic factors in UC, emphasizing the importance of population-specific analyses.
While tumoral GH1 expression can provide some insight into GH action, it may not be as reliable as an indicator of active GH signaling as GHR expression, since GHR levels more directly reflect a tumor’s capacity to respond to circulating GH and activate downstream signaling pathways. Additionally, IGF-1 and its receptor play a critical role in mediating GH signaling and exertion of mitogenic and anti-apoptotic effects in cancer. Given that IGF1 and IGF1R can also be expressed by tumor cells and influence oncogenic processes both in GH-dependent and GH-independent manners [129], their expression levels may further complicate the assessment of the activity of the GH pathway in UC. Therefore, GHR expression, along with IGF1 and IGF1R levels, may provide a more comprehensive understanding of GH signaling in tumor progression.
Beyond its impact on tumor progression and survival, GH action is also a well-established driver of resistance to cancer therapies. An extensive body of research has emerged over the past decade eliciting the molecular mechanisms by which GH influences this process, including but not limited to active drug efflux and cellular detoxification, promotion of EMT and ECM remodeling, and evasion of apoptosis [130]. Additionally, the primary hurdle to overcome in treating UC is that of resistance to therapy [42,43,44,45,47].
Among these mechanisms, the regulation of active drug efflux through the modulation of ABC transporters plays a central role in reducing the intracellular concentration, and thereby efficacy, of chemotherapeutics. ABC transporters are commonly upregulated across cancer types [19,131,132], a phenomenon further exacerbated by both chemotherapy and GH, but reversible by inhibiting GH action [20,22,33]. GH, directly or indirectly via IGF-1, has been shown to increase expression of the ABCB, ABCC, and ABCG subfamilies of ABC transporters in breast cancer [15], melanoma [20,33], ovarian cancer [133,134], colorectal cancer [135], and leukemia [136]. In UC, ABCG2 mediates doxorubicin resistance [137], while lower expression of ABCB1, ABCC2, and ABCG2 correlates with better response to enfortumab vedotin [90]. In our study, tumoral GHR expression correlated positively with multiple ABC transporters (e.g., ABCB1, ABCB4, ABCB5, ABCC8, ABCC9, and ABCG4) in both sexes and with additional transporters (ABCB10, ABCC6, and ABCG2) in male patients. In vitro, GH increased ABCB1 and ABCC2 expression in mouse UC cells treated with cisplatin and gemcitabine, an effect reversed by Pegvisomant. Pegvisomant also reduced ABCC1 expression in the presence of cisplatin. Emerging evidence supports a role for the ABCA subfamily in chemoresistance [138,139,140], with GH upregulating ABCA6 and ABCA8 in pancreatic cancer [23]. Consistently, we observed positive correlations between GHR and ABCA8 and ABCA9 in both sexes, ABCA13 in females, and ABCA6 in males. These findings reinforce GH’s role in ABC-transporter-mediated therapy resistance and highlight Pegvisomant’s potential to counteract this pathway in UC.
In addition to its regulation of ABC transporters, GH also influences drug metabolism through regulation of cytochrome P450 enzymes [141,142,143], which are responsible for metabolizing the majority of clinical drugs, including chemotherapeutics [87]. It is important to recognize that the metabolism of drugs reduces their therapeutic efficacy [144]. In this study, GHR expression correlated with several CYPs (CYP1B1, CYP2A6, CYP2A7, CYP2B7P1, CYP2J2, and CYP2U1) in both male and female patients with UC, suggesting that GH may contribute to altered drug metabolism and reduced chemotherapy efficacy in UC via CYP regulation.
In addition to modulating drug metabolism, GH plays a key role in metastatic progression through regulation of EMT and ECM remodeling [30]. EMT, often described as a “phenotype switch” that signals the onset of metastasis, is more accurately viewed as a sliding scale rather than an abrupt switch. This process involves the loss of epithelial characteristics and acquisition of invasive, mesenchymal traits [78]. In this study, GH significantly enhanced migration and invasion in both human and mouse UC cells, effects that were reversed by Pegvisomant. While EMT is essential in development, it becomes dysregulated in cancer [145], and GH has been shown to induce EMT in both normal and cancerous tissues [19,24,25,27,130,146]. Our transcriptomic analyses identified strong positive correlations between tumoral GHR expression and numerous EMT-related genes (e.g., ZEB1/2, SNAI1, TWIST ½, CDH2, FN1, and TGFB isoforms) in both sexes. In mouse UC cells, GH upregulated N-cadherin, Snail, Slug, and Twist1 in the presence of chemotherapy, while Pegvisomant restored E-cadherin and reduced N-cadherin, Snail, Slug, Zeb1, and Twist1 expression. In the absence of chemotherapy, GH selectively increased N-cadherin and Zeb1, with Pegvisomant reversing N-cadherin upregulation. Vimentin levels were unchanged, suggesting its regulation may be independent of GH signaling in UC. Taken together, these findings support a role for GH in promoting EMT and metastasis, highlighting the potential of Pegvisomant to counteract these processes.
ECM remodeling is a key component of metastasis and is strongly influenced by GH, largely through regulation of MMPs, which digest ECM and promote tumor invasion [24,27,59,147] GH has been shown to upregulate MMP2 and MMP9 in multiple cancers [24,31,32,93], and, in UC, elevated circulating levels of these MMPs are linked to aggressive disease and may serve as biomarkers [148,149,150,151,152]. In this study, GHR expression correlated with several MMPs, including MMP2, MMP3, MMP 11, and MMP16, in both sexes and additional MMPs in male patients. GH increased expression of MMP14 in cisplatin-treated cells, an effect reversed by Pegvisomant, which also reduced expression of MMP9 and MMP14 and increased expression of TIMP1 and TIMP2 in a chemotherapy-dependent manner. In the absence of chemotherapy, no significant changes were observed, and MMP2 and TGF-β expression remained unchanged across all treatment conditions, suggesting selective GH regulation under treatment stress. GH also promotes collagen synthesis and fibrosis, processes known to support tumor progression [153]. All significant correlations between tumoral GHR and collagen genes were positive, reinforcing the role of GH in ECM remodeling and metastatic potential in UC.
Beyond its role in metastasis, GH promotes tumor cell survival and therapy resistance by inhibiting apoptosis [154,155,156]. The mechanism by which GH exerts this action is via upregulation of anti-apoptotic genes, such as BCL2, and downregulation of pro-apoptotic genes, such as p53 and caspases [30]. In this study, tumoral GHR correlated positively with several anti-apoptotic genes (NFIL3, BCL2, TNFRSF11B, and TSFRSF10D), with additional associations observed in a sex-specific manner. Conversely, GHR was negatively correlated with key pro-apoptotic genes, including AIMP1 and DIABLO in both sexes, TRADD in female patients, and CASP6, CASP8, CASP9, BAK1, and FADD in male patients. These findings support the role of GH in fostering a pro-survival TME, thereby reducing the effectiveness of anticancer therapies.
Despite providing valuable insight into the potential role of GH in UC, several limitations should be noted. First, the in silico analyses rely solely on transcriptomic data, which may not reflect protein expression due to post-transcriptional regulation [157]. We sought to address this in part through in vitro validation, but did not perform functional studies to confirm causality. In vivo studies are necessary to determine whether GH directly drives therapy resistance and tumor progression. Additionally, this work focuses on tumor-intrinsic GHR expression and does not account for the complex interactions within the tumor microenvironment, including crosstalk with stromal cells, immune components, and ECM interactions. Since GH signaling is known to affect multiple cell types, a more comprehensive approach incorporating these factors could provide a clearer mechanistic understanding.
Collectively, our findings suggest a potential role for GH action in UC progression, survival outcomes, and resistance to therapy by enhancing drug efflux, EMT, and ECM remodeling. The observed inverse correlation between GHR expression and survival, particularly in advanced clinical stages, supports the notion that GH signaling may contribute to aggressive disease phenotypes. Additionally, the differential expression patterns of GH1, IGF1, and IGF1R across sex and racial groups underscore the complexity of GH action in UC and its potential as a prognostic biomarker. Importantly, Pegvisomant has the ability to reverse GH-driven oncogenic processes, even in the absence of exogenous GH, indicating its ability to block both endocrine and paracrine/autocrine GH signaling. These findings emphasize the need for further investigation into the therapeutic targeting of GH action in UC, which may provide novel strategies to improve patient outcomes, particularly in advanced disease stages. Given the significant overlap between GH signaling and key pathways in cancer progression, future studies should focus on validating these findings in vivo to further assess the feasibility of targeting GH action in UC treatment [44,45,46,47].
4. Materials and Methods
4.1. GHR Expression Analysis
GHR mRNA transcript-level comparison between normal and tumoral tissues of patients with bladder cancer in TCGA database was performed using Gene Expression Profiling Interactive Analysis 2 (GEPIA2) web tool (http://gepia2.cancer-pku.cn/#index; accessed 27 May 2025) [158]. Expression of GHR in different clinical grades and stages of patients with bladder cancer in TCGA database was evaluated using Tumor-Immune System Interaction DataBase (TISIDB) web tool (http://cis.hku.hk/TISIDB/index.php; accessed 27 May 2025) [159].
4.2. Survival Curves
Transcriptomic data archived in TCGA database obtained from patients with bladder cancer were used to perform a multivariate analysis to generate Kaplan–Meier plots for overall survival (OS) with the KM plotter web tool (https://kmplot.com/analysis/; accessed 27 May 2025). With GHR, GH1, IGF1R, or IGF1 as the reference gene, sex-based (all, female, male) stage-based, combinations of sex- and stage-based, and race-based (Asian, Black, white) correlations with patient survival were obtained and OS plots were generated for a total of 405 patients with bladder cancer [160]. OS and disease-free survival (DFS) curves were also generated for 394 patients with bladder cancer in TCGA database with GHR as the reference gene and patients sorted at the median GHR mRNA expression level using GEPIA2 web tool [158].
4.3. Gene Expression Correlation Analyses
Spearman correlation analyses of differentially expressed mRNAs with respect to GHR mRNA expression in tumor samples of patients with bladder cancer in TCGA database were performed using LinkedOmics web tool (http://linkedomics.zhang-lab.org/login.php; accessed 27 May 2025) [161]. The corresponding correlation coefficients, respective p values, and false discovery rates (FDRs) were used to create heat maps in Microsoft Excel. In TCGA dataset, transcriptomic data were available for 108 female and 304 male patients with bladder cancer.
4.4. Cell Culture
One human and one mouse cell line were selected for our studies. UM-UC-3 (human UC; purchased from ATCC #CRL-1749, Manassas, VA, USA) and MB49 (C57BL/6-background murine UC; purchased from Sigma-Aldrich #SCC148, St. Louis, MO, USA) were maintained in EMEM (ThermoFisher #11095098, Waltham, MA, USA) and DMEM growth media (ThermoFisher #11995040), respectively, supplemented with 10% fetal bovine serum (FBS; ThermoFisher #10082147) and 1% penicillin-streptomycin (ThermoFisher #15140122), referred to hereafter as complete growth medium, at 37 °C and 5% CO2. UM-UC-3 is an extremely commonly used cell line to model high-grade UC with nearly 900 citations as of 18 February 2025. Additionally, UM-UC-3 has the highest level of GHR expression among the 26 commercially available cell lines (Figure S5) (proteinatlas.org; accessed 27 May 2025; [162]). We chose MB49 for our studies as we wanted to use a mouse UC cell line that is compatible with C57BL/6J-background mice, and it is the only commercially available one.
4.5. GH, Pegvisomant, and Chemotherapy Treatments
Recombinant active human (R&D Systems #1067-GH, Minneapolis, MN, USA) and bovine (ProSpecBio #CYT-636, Rehovot, HaMerkaz, Israel) GH were administered to human cells at 50 ng/mL and murine cells at 200 ng/mL, respectively. Pegvisomant (Pfizer, New York, NY, USA) was administered to cells at 13,500 ng/mL. Chemotherapies (gemcitabine (Selleck Chemicals #S1714, Houston, TX, USA) and cisplatin (Selleck Chemicals #S1166)) were administered to cells at sub-EC50 concentrations so as not to significantly reduce cell viability. All treatments were performed in growth medium containing 2% FBS or serum-free medium for Western blots of GH signaling intermediates.
4.6. EC50 Determination
Cells were seeded at 50,000 cells/well in 96-well plates in complete growth medium. After 24 h, 10 serial dilutions of chemotherapy drugs made in growth medium supplemented with 2% FBS and 1% penicillin-streptomycin were added to cells and incubated for 48 h at 37 °C. After 48 h, drug dilutions were removed, and cells were incubated with 0.0045% resazurin (ThermoFisher #R12204) solution until solutions in control wells reduced to resorufin and appeared bright pink. Absorbance was read at 570 nm with a reference wavelength of 600 nm. The EC50 values were calculated using Prism 10 software (Figure S6; GraphPad, San Diego, CA, USA). Subsequent chemotherapy treatments were performed at drug concentrations that were 10% lower than their respective EC50 values so as not to significantly reduce cell viability. Using sub-EC50 concentrations of chemotherapies also allowed us to demonstrate that they are efficacious at lower concentrations in the presence of the GHR antagonist Pegvisomant.
4.7. Real-Time Quantitative PCR (RT-qPCR)
Total RNA was isolated from cultured cells using a commercially available kit (IBI Scientific #IB4730, Dubuque, IA, USA). Reverse-transcription PCR was performed using a commercially available kit (ThermoFisher #4368814). cDNA was quantified using NanoDrop 2000 (ThermoFisher #ND-2000) and diluted to 200 ng/µL in nuclease-free water. We used 500 ng of cDNA with gene- and species-specific forward and reverse primers at 10 µM (Table 1; all manufactured by Sigma-Aldrich, St. Louis, MO, USA) and SYBR green/ROX qPCR mix (ThermoFisher #K0222) to amplify specified genes using the QuantStudio 3 qPCR machine and software (ThermoFisher #A28567). RNA expression was first normalized against two reference genes (ACTB and GAPDH in human cells; Actb and Tubb5 in mouse cells), and expression levels were then quantified using the 2−∆∆Ct method in Microsoft Excel.

Table 1.
Primer sequences (5′ → 3′).
4.8. Western Blot
Total protein was isolated from cells after 12 min of treatment for GH signaling intermediates, as the phosphorylation levels reach a peak between 5 and 30 min following stimulation with GH, or 48 h otherwise, as this incubation time allows for a significant change in protein levels to occur after a stimulus, using RIPA buffer (ThermoFisher #J62524.AE) diluted to 1.5× and supplemented with 1% PMSF (ThermoFisher #36978) and 1% protease-phosphatase inhibitor (ThermoFisher #78442). Protein concentration was determined using the Bradford assay (BioRad #5000006, Hercules, CA, USA). A total of 80 µg of protein for GH signaling intermediates or 20 µg otherwise was boiled with Laemmli buffer (BioRad #1610747) for 8 min before separation by SDS-PAGE. Proteins were transferred to a PVDF membrane overnight at 4 °C. The membrane was blocked in 5% nonfat dry milk in tris-buffered saline with Tween-20 (TBS/T) for 1 h at room temperature before overnight incubation with primary antibody diluted in blocking buffer at 4 °C (Table 2; Cell Signaling Technology (CST), Danvers, MA, USA; ProteinTech, Rosemont, IL, USA; Invitrogen, Waltham, MA, USA; Bioss USA, Woburn, MA, USA). The membrane was then washed with TBS/T, incubated with secondary antibody (CST #7074 1:2000) for 60 min at room temperature, and washed again before application of West Femto Super Signal Chemiluminescence detection reagent (ThermoFisher #34095) and imaging using the Odyssey XF (LI-COR Biosciences, Lincoln, NE, USA) or Azure 300 (Azure Biosystems, Dublin, CA, USA) imaging system. Densitometry analysis was performed using ImageJ software (version 1.51) [163]. Protein expression was normalized to a loading control (β-actin) before analysis.

Table 2.
Primary antibody information.
4.9. Migration Assay
Cells were seeded at 7 105 cells/mL in complete growth medium inside each well of 2-well culture inserts (Ibidi #80209, Fitchburg, WI, USA) inserted into 12-well plates. After 24 h, culture inserts were removed, cells were washed with 1X sterile PBS, and culture medium supplemented with 2% FBS and 1% penicillin-streptomycin was added to cells. Images were captured using a Cytation 3 Imaging Reader (BioTek Instruments, Winooski, VT, USA) and Gen5 software (BioTek Instruments). Culture medium was removed, GH and Pegvisomant treatments were administered, and cells were incubated at 37 °C supplemented with 5% CO2 for 24 h. After 24 h, images were captured again and compared to original images using ImageJ software [163] and a software plugin that uses a computer vision segmentation algorithm to provide an unbiased method to quantify the cell-free region created by the culture insert, correct for any nonzero angle, and assess the average width along with variations [164].
4.10. Invasion Assay
Cells were seeded in 25 cm2 culture flasks in complete growth medium and pre-treated for 1 week with GH and Pegvisomant once every 48 h in growth medium containing 2% FBS. Cells were then enzymatically removed from the culture flasks using 0.25% trypsin-EDTA before being seeded at 0.5 105 (MB49) or 1.0 105 cells/mL (UM-UC-3) in the basement membrane chamber of the invasion assay kit (Cell BioLabs #CBA-112, San Diego, CA, USA) with GH and Pegvisomant treatments in appropriate suspensions. The basement membrane chambers of the invasion assay kit were then carefully placed into the feeder tray of the invasion assay kit, which contained complete culture medium. The full invasion assay plate, which contained both the basement membrane chamber and the feeder tray, was incubated at 37 °C supplemented with 5% CO2 for 24 h. A total of 30 min prior to the end of the 24 h incubation period, cell detachment solution was added to the cell harvesting tray of the invasion assay kit and incubated for 30 min at 37 °C supplemented with 5% CO2. At the end of the 24 h incubation period, cells that had invaded through the basement membrane chamber of the invasion assay plate were dislodged by placing the basement membrane chamber onto the cell harvesting tray and tilting it several times. Lysis buffer containing fluorescent dye was added to all samples before incubation for 20 min at room temperature. After 20 min, the mixture from the cell harvesting tray was transferred to a black-walled 96-well plate and fluorescence was measured at 480 nm/520 nm using a Cytation 3 Imaging Reader (BioTek Instruments) and Gen5 software (BioTek Instruments).
4.11. Statistical Analyses
All experiments were repeated at least thrice. Tests of variance and homogeneity were performed, followed by parametric (Student’s t-test, ANOVA) or non-parametric (Wilcoxon sign-rank test) analyses as appropriate to identify significant differences. Protein expression was normalized to a loading control before analysis. All analyses were performed using Prism 10 software (GraphPad) unless otherwise noted, with significance being defined as p 0.05.
5. Conclusions
Together, this work was directed at testing the hypothesis that inhibiting GH action will reverse therapy resistance and improve disease prognosis in UC. This study investigated the role of GHR expression in UC using transcriptomic data from TCGA. We found that GHR expression is associated with advanced UC stages, reduced survival, and increased resistance to therapy, indicating its potential as a prognostic biomarker and therapeutic target. Additionally, we sought to confirm these findings in vitro. We observed that GH plays a direct role in promoting therapy resistance and metastatic processes in UC by upregulating ABC transporters, inducing EMT, and modulating ECM remodeling. Pegvisomant effectively counteracts these effects, highlighting its potential as a therapeutic strategy to enhance treatment efficacy in UC.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26157113/s1.
Author Contributions
Conceptualization, E.D., R.B., and J.J.K.; methodology, E.D., R.B., and J.J.K.; software, E.D. and R.B.; validation, E.D., H.M., and L.J.C.; formal analysis, E.D.; investigation, E.D., H.M., and L.J.C.; resources, D.E.B. and J.J.K.; data curation, E.D.; writing—original draft preparation, E.D.; writing—review and editing, E.D., H.M., L.J.C., R.B., D.E.B., and J.J.K.; visualization, E.D.; supervision, R.B., D.E.B., and J.J.K.; project administration, J.J.K.; funding acquisition, E.D., D.E.B., and J.J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by a John J. Kopchick Molecular and Cellular Biology / Translational Biomedical Sciences Research Fellowship award (Ohio University, Athens, OH, USA E.D.), the State of Ohio’s Eminent Scholar Program to J.J.K., which includes a gift from Milton and Lawrence Goll, the American Veterans, the Institute For Molecular Medicine and Aging (Ohio University, Athens, OH, USA), and the Molecular and Cellular Biology Program (Ohio University, Athens, OH, USA).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data described in the manuscript are contained in the main text or supporting information. Additional requests are to be addressed to the corresponding author.
Acknowledgments
We thank Jonathan Young, Silvana Duran-Ortiz, Grace Lach, Rebecca Coffey, Meredith Klein, and Arshad Ahmad for their help and support with techniques and data analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GH | Growth hormone |
| LS | Laron syndrome |
| GHR | Growth hormone receptor |
| EMT | Epithelial-to-mesenchymal transition |
| ECM | Extracellular matrix |
| ABC | ATP-binding cassette |
| UC | Urothelial carcinoma |
| IGF-1 | Insulin-like growth factor 1 |
| MMP | Matrix metalloproteinase |
| TIMP | Tissue inhibitor of metalloproteinase |
| TCGA | The Cancer Genome Atlas |
| HR | Hazard ratio |
| PRL | Prolactin |
| PRLR | Prolactin receptor |
| InsR | Insulin receptor |
| Ins | Insulin |
| STAT | Signal transducer and activator of transcription |
| JAK | Janus kinase |
| MAPK | p42/44 mitogen-activated protein kinase |
| AKT | Protein kinase B |
| TGF-β | Transforming growth factor beta |
References
- Brinkman, J.E.; Tariq, M.A.; Leavitt, L.; Sharma, S. Physiology, Growth Hormone; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lobie, P.E.; Waxman, D.J. Growth Hormone (GH). Encycl. Horm. 2003, 208–216. [Google Scholar] [CrossRef]
- Lu, M.; Flanagan, J.U.; Langley, R.J.; Hay, M.P.; Perry, J.K. Targeting Growth Hormone Function: Strategies and Therapeutic Applications. Signal Transduct. Target. Ther. 2019, 4, 3. [Google Scholar] [CrossRef]
- Dagdelen, S.; Cinar, N.; Erbas, T. Increased Thyroid Cancer Risk in Acromegaly. Pituitary 2014, 17, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Dal, J.; Leisner, M.Z.; Hermansen, K.; Farkas, D.K.; Bengtsen, M.; Kistorp, C.; Nielsen, E.H.; Andersen, M.; Feldt-Rasmussen, U.; Dekkers, O.M.; et al. Cancer Incidence in Patients with Acromegaly: A Cohort Study and Meta-Analysis of the Literature. J. Clin. Endocrinol. Metab. 2018, 103, 2182–2188. [Google Scholar] [CrossRef]
- Renehan, A.G.; O’Connell, J.; O’Halloran, D.; Shanahan, F.; Potten, C.S.; O’Dwyer, S.T.; Shalet, S.M. Acromegaly and Colorectal Cancer: A Comprehensive Review of Epidemiology, Biological Mechanisms, and Clinical Implications. Horm. Metab. Res. 2003, 35, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Aguirre, J.; Balasubramanian, P.; Guevara-Aguirre, M.; Wei, M.; Madia, F.; Cheng, C.-W.; Hwang, D.; Martin-Montalvo, A.; Saavedra, J.; Ingles, S.; et al. Growth Hormone Receptor Deficiency Is Associated with a Major Reduction in Pro-Aging Signaling, Cancer and Diabetes in Humans. Sci. Transl. Med. 2011, 3, 70ra13. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Aguirre, J.; Rosenbloom, A.L. Obesity, Diabetes and Cancer: Insight into the Relationship from a Cohort with Growth Hormone Receptor Deficiency. Diabetologia 2015, 58, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Laron, Z.; Kauli, R.; Lapkina, L.; Werner, H. IGF-I Deficiency, Longevity and Cancer Protection of Patients with Laron Syndrome. Mutat. Res. Rev. Mutat. Res. 2017, 772, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Snibson, K.J.; Bhathal, P.S.; Adams, T.E. Overexpressed Growth Hormone (GH) Synergistically Promotes Carcinogen-Initiated Liver Tumour Growth by Promoting Cellular Proliferation in Emerging Hepatocellular Neoplasms in Female and Male GH-Transgenic Mice. Liver 2001, 21, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Ikeno, Y.; Hubbard, G.B.; Lee, S.; Cortez, L.A.; Lew, C.M.; Webb, C.R.; Berryman, D.E.; List, E.O.; Kopchick, J.J.; Bartke, A. Reduced Incidence and Delayed Occurrence of Fatal Neoplastic Diseases in Growth Hormone Receptor/Binding Protein Knockout Mice. J. Gerontol. Ser. A 2009, 64, 522–529. [Google Scholar] [CrossRef]
- Moon, H.D.; Simpson, M.E.; Li, C.H.; Evans, H.M. Neoplasms in Rats Treated with Pituitary Growth Hormone; Pulmonary and Lymphatic Tissues. Cancer Res. 1950, 10, 297–308. [Google Scholar] [PubMed]
- Emerman, J.T.; Leahy, M.; Gout, P.W.; Bruchovsky, N. Elevated Growth Hormone Levels in Sera from Breast Cancer Patients. Horm. Metab. Res. 1985, 17, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Giuliani, A.; Bianco, G.; De Cata, A.; Balzanelli, M.; Carella, A.M.; La Viola, M.; Tarquini, R. Decreased Serum Levels of Insulin-like Growth Factor (IGF)-I in Patients with Lung Cancer: Temporal Relationship with Growth Hormone (GH) Levels. Anticancer. Res. 1999, 19, 1397–1399. [Google Scholar] [PubMed]
- Arumugam, A.; Subramani, R.; Nandy, S.B.; Terreros, D.; Dwivedi, A.K.; Saltzstein, E.; Lakshmanaswamy, R. Silencing Growth Hormone Receptor Inhibits Estrogen Receptor Negative Breast Cancer through ATP-Binding Cassette Sub-Family G Member 2. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kaseb, A.O.; Haque, A.; Vishwamitra, D.; Hassan, M.M.; Xiao, L.; George, B.; Sahu, V.; Mohamed, Y.I.; Carmagnani Pestana, R.; Lombardo, J.L.; et al. Blockade of Growth Hormone Receptor Signaling by Using Pegvisomant: A Functional Therapeutic Strategy in Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 986305. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wu, W.; Yuan, Y.; Pandey, V.; Wu, Z.; Lu, X.; Zhang, W.; Chen, Y.; Wu, M.; Zhang, M.; et al. Human Growth Hormone and Human Prolactin Function as Autocrine/Paracrine Promoters of Progression of Hepatocellular Carcinoma. Oncotarget 2016, 7, 29465–29479. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Yang, K.; Wan, Y.; Qian, P.-X.; Perry, J.K.; Chiesa, J.; Mertani, H.C.; Zhu, T.; Lobie, P.E. Tumor Expression of Human Growth Hormone and Human Prolactin Predict a Worse Survival Outcome in Patients with Mammary or Endometrial Carcinoma. J. Clin. Endocrinol. Metab. 2011, 96, E1619–E1629. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Boguszewski, C.L.; Kopchick, J.J. Growth Hormone Action as a Target in Cancer: Significance, Mechanisms and Possible Therapies. Endocr. Rev. 2024, 46, 224–280. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Baumgaertel, N.; Wu, S.; Kopchick, J.J. Growth Hormone Receptor Knockdown Sensitizes Human Melanoma Cells to Chemotherapy by Attenuating Expression of ABC Drug Efflux Pumps. Horm. Cancer 2017, 8, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Kulkarni, P.; Qian, Y.; Walsh, C.; Arora, P.; Davis, E.; Duran-Ortiz, S.; Funk, K.; Ibarra, D.; Kruse, C.; et al. Growth Hormone Upregulates Melanocyte-Inducing Transcription Factor Expression and Activity via JAK2-STAT5 and SRC Signaling in GH Receptor-Positive Human Melanoma. Cancers 2019, 11, 1352. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Qian, Y.; Mathes, S.; Terry, J.; Arnett, N.; Riddell, T.; Stevens, A.; Funk, K.; Bell, S.; Bokal, Z.; et al. Growth Hormone Receptor Antagonism Downregulates ATP-Binding Cassette Transporters Contributing to Improved Drug Efficacy against Melanoma and Hepatocarcinoma in Vivo. Front. Oncol. 2022, 12, 3369. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Kulkarni, P.; Swegan, D.; Duran-Ortiz, S.; Ahmad, A.; Caggiano, L.J.; Davis, E.; Walsh, C.; Brenya, E.; Koshal, A.; et al. Growth Hormone Receptor Antagonist Markedly Improves Gemcitabine Response in a Mouse Xenograft Model of Human Pancreatic Cancer. Int. J. Mol. Sci. 2024, 25, 7438. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Wu, S.; Kopchick, J.J. Targeting Growth Hormone Receptor in Human Melanoma Cells Attenuates Tumor Progression and Epithelial Mesenchymal Transition via Suppression of Multiple Oncogenic Pathways. Oncotarget 2017, 8, 21579–21598. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Kopchick, J.J. The Effects of Growth Hormone on Therapy Resistance in Cancer. Cancer Drug Resist. 2019, 2, 827–846. [Google Scholar] [CrossRef]
- Bougen, N.M.; Steiner, M.; Pertziger, M.; Banerjee, A.; Brunet-Dunand, S.E.; Zhu, T.; Lobie, P.E.; Perry, J.K. Autocrine Human GH Promotes Radioresistance in Mammary and Endometrial Carcinoma Cells. Endocr. Relat. Cancer 2012, 19, 625–644. [Google Scholar] [CrossRef][Green Version]
- Brittain, A.L.; Basu, R.; Qian, Y.; Kopchick, J.J. Growth Hormone and the Epithelial-to-Mesenchymal Transition. J. Clin. Endocrinol. Metab. 2017, 102, 3662–3673. [Google Scholar] [CrossRef]
- Chen, Y.J.; You, M.L.; Chong, Q.Y.; Pandey, V.; Zhuang, Q.S.; Liu, D.X.; Ma, L.; Zhu, T.; Lobie, P.E. Autocrine Human Growth Hormone Promotes Invasive and Cancer Stem Cell-Like Behavior of Hepatocellular Carcinoma Cells by STAT3 Dependent Inhibition of CLAUDIN-1 Expression. Int. J. Mol. Sci. 2017, 18, 1274. [Google Scholar] [CrossRef]
- Chesnokova, V.; Melmed, S. Growth Hormone in the Tumor Microenvironment. Arch. Endocrinol. Metab. 2019, 63, 568–575. [Google Scholar] [CrossRef]
- Kopchick, J.J.; Basu, R.; Berryman, D.E.; Jorgensen, J.O.L.; Johannsson, G.; Puri, V. Covert Actions of Growth Hormone: Fibrosis, Cardiovascular Diseases and Cancer. Nat. Rev. Endocrinol. 2022, 18, 558–573. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Basu, R.; Bonn, T.; Low, B.; Mazurek, N.; Kopchick, J.J. Growth Hormone Upregulates Melanoma Drug Resistance and Migration via Melanoma-Derived Exosomes. Cancers 2024, 16, 2636. [Google Scholar] [CrossRef]
- Mukhina, S.; Mertani, H.C.; Guo, K.; Lee, K.O.; Gluckman, P.D.; Lobie, P.E. Phenotypic Conversion of Human Mammary Carcinoma Cells by Autocrine Human Growth Hormone. Proc. Natl. Acad. Sci. USA 2004, 101, 15166–15171. [Google Scholar] [CrossRef]
- Qian, Y.; Basu, R.; Mathes, S.C.; Arnett, N.A.; Duran-Ortiz, S.; Funk, K.R.; Brittain, A.L.; Kulkarni, P.; Terry, J.C.; Davis, E.; et al. Growth Hormone Upregulates Mediators of Melanoma Drug Efflux and Epithelial-to-Mesenchymal Transition In Vitro and In Vivo. Cancers 2020, 12, 3640. [Google Scholar] [CrossRef]
- Subramani, R.; Lopez-Valdez, R.; Salcido, A.; Boopalan, T.; Arumugam, A.; Nandy, S.; Lakshmanaswamy, R. Growth Hormone Receptor Inhibition Decreases the Growth and Metastasis of Pancreatic Ductal Adenocarcinoma. Exp. Mol. Med. 2014, 46, e117. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Nandy, S.B.; Pedroza, D.A.; Lakshmanaswamy, R. Role of Growth Hormone in Breast Cancer. Endocrinology 2017, 158, 1543–1555. [Google Scholar] [CrossRef]
- Wang, J.-J.; Chong, Q.-Y.; Sun, X.-B.; You, M.-L.; Pandey, V.; Chen, Y.-J.; Zhuang, Q.-S.; Liu, D.-X.; Ma, L.; Wu, Z.-S.; et al. Autocrine hGH Stimulates Oncogenicity, Epithelial-Mesenchymal Transition and Cancer Stem Cell-like Behavior in Human Colorectal Carcinoma. Oncotarget 2017, 8, 103900–103918. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.J.; Conway-Campbell, B.L. The Oncogenic Potential of Autocrine Human Growth Hormone in Breast Cancer. Proc. Natl. Acad. Sci. USA 2004, 101, 14992–14993. [Google Scholar] [CrossRef]
- Ben-Shlomo, A.; Deng, N.; Ding, E.; Yamamoto, M.; Mamelak, A.; Chesnokova, V.; Labadzhyan, A.; Melmed, S. DNA Damage and Growth Hormone Hypersecretion in Pituitary Somatotroph Adenomas. J. Clin. Investig. 2020, 130, 5738–5755. [Google Scholar] [CrossRef]
- Chesnokova, V.; Zhou, C.; Ben-Shlomo, A.; Zonis, S.; Tani, Y.; Ren, S.G.; Melmed, S. Growth Hormone Is a Cellular Senescence Target in Pituitary and Nonpituitary Cells. Proc. Natl. Acad. Sci. USA 2013, 110, E3331–E3339. [Google Scholar] [CrossRef] [PubMed]
- Key Statistics for Bladder Cancer. Available online: https://www.cancer.org/cancer/types/bladder-cancer/about/key-statistics.html (accessed on 30 January 2025).
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Gao, D.; Shi, Y.; Fan, G.; Yu, X.; Yang, E.; Cheng, H.; Tian, J.; Ding, H.; Liu, S.; et al. SRC Enhanced Cisplatin Resistance in Bladder Cancer by Reprogramming Glycolysis and Pentose Phosphate Pathway. Commun. Biol. 2025, 8, 36. [Google Scholar] [CrossRef]
- Li, F.; Zheng, Z.; Chen, W.; Li, D.; Zhang, H.; Zhu, Y.; Mo, Q.; Zhao, X.; Fan, Q.; Deng, F.; et al. Regulation of Cisplatin Resistance in Bladder Cancer by Epigenetic Mechanisms. Drug Resist. Updates. 2023, 68, 100938. [Google Scholar] [CrossRef] [PubMed]
- Dobruch, J.; Oszczudłowski, M. Bladder Cancer: Current Challenges and Future Directions. Medicina 2021, 57, 749. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.M.; Khafaga, A.F.; Abdellatif, A.M. Bladder Cancer: Therapeutic Challenges and Role of 3D Cell Culture Systems in the Screening of Novel Cancer Therapeutics. Cancer Cell Int. 2023, 23, 251. [Google Scholar] [CrossRef]
- Hemenway, G.; Anker, J.F.; Riviere, P.; Rose, B.S.; Galsky, M.D.; Ghatalia, P. Advancements in Urothelial Cancer Care: Optimizing Treatment for Your Patient. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e432054. [Google Scholar] [CrossRef]
- Soares, A.; Bourlon, M.T.; Wong, A.; Joshi, A.; Jardim, D.; Korbenfeld, E.; Karak, F.E.; Orlandi, F.; Sze, H.; Ansari, J.; et al. Management of Metastatic Urothelial Carcinoma in Emerging Markets (EM): An Expert Opinion. Clin. Genitourin. Cancer 2024, 22, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Akaza, H.; Matsuki, K.; Matsushima, H.; Koiso, K.; Aso, Y. Stimulatory Effects of Growth Hormone on Rat Bladder Carcinogenesis. Cancer 1991, 68, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hua, S.; Jiang, Q.; Xie, Z.; Wu, L.; Wang, X.; Shi, F.; Dong, S.; Jiang, J. Identification of Feature Genes of a Novel Neural Network Model for Bladder Cancer. Front. Genet. 2022, 13, 912171. [Google Scholar] [CrossRef] [PubMed]
- Hindupur, S.V.; Schmid, S.C.; Koch, J.A.; Youssef, A.; Baur, E.M.; Wang, D.; Horn, T.; Slotta-Huspenina, J.; Gschwend, J.E.; Holm, P.S.; et al. STAT3/5 Inhibitors Suppress Proliferation in Bladder Cancer and Enhance Oncolytic Adenovirus Therapy. Int. J. Mol. Sci. 2020, 21, 1106. [Google Scholar] [CrossRef]
- Merrill, N.M.; Vandecan, N.M.; Day, K.C.; Palmbos, P.L.; Day, M.L.; Udager, A.M.; Merajver, S.D.; Soellner, M.B. MEK Is a Promising Target in the Basal Subtype of Bladder Cancer. Oncotarget 2020, 11, 3921. [Google Scholar] [CrossRef]
- Sun, H.Z.; Wu, S.F.; Tu, Z.H. Blockage of IGF-1R Signaling Sensitizes Urinary Bladder Cancer Cells to Mitomycin-Mediated Cytotoxicity. Cell Res. 2001, 11, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Metalli, D.; Lovat, F.; Tripodi, F.; Genua, M.; Xu, S.Q.; Spinelli, M.; Alberghina, L.; Vanoni, M.; Baffa, R.; Gomella, L.G.; et al. The Insulin-Like Growth Factor Receptor I Promotes Motility and Invasion of Bladder Cancer Cells through Akt- and Mitogen-Activated Protein Kinase-Dependent Activation of Paxillin. Am. J. Pathol. 2010, 176, 2997–3006. [Google Scholar] [CrossRef]
- Du, Y.; Miao, W.; Jiang, X.; Cao, J.; Wang, B.; Wang, Y.; Yu, J.; Wang, X.; Liu, H. The Epithelial to Mesenchymal Transition Related Gene Calumenin Is an Adverse Prognostic Factor of Bladder Cancer Correlated with Tumor Microenvironment Remodeling, Gene Mutation, and Ferroptosis. Front. Oncol. 2021, 11, 683951. [Google Scholar] [CrossRef] [PubMed]
- Martins-Lima, C.; Chianese, U.; Benedetti, R.; Altucci, L.; Jerónimo, C.; Correia, M.P. Tumor Microenvironment and Epithelial-Mesenchymal Transition in Bladder Cancer: Cytokines in the Game? Front. Mol. Biosci. 2023, 9, 1070383. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Santoni, M.; Ciccarese, C.; Brunelli, M.; Conti, A.; Santini, D.; Montironi, R.; Cascinu, S.; Tortora, G. Emerging Concepts on Drug Resistance in Bladder Cancer: Implications for Future Strategies. Crit. Rev. Oncol. Hematol. 2015, 96, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Junge, J.A.; Delfarah, A.; Lu, Y.-T.; Arnesano, C.; Iqbal, M.; Delijani, K.; Hsieh, T.-C.; Hodara, E.; Mehta, H.H.; et al. Bladder Cancer Cells Shift Rapidly and Spontaneously to Cisplatin-Resistant Oxidative Phosphorylation That Is Trackable in Real Time. Sci. Rep. 2022, 12, 5518. [Google Scholar] [CrossRef] [PubMed]
- Dart, A. EMT in Chemoresistance. Nat. Rev. Cancer 2023, 23, 349. [Google Scholar] [CrossRef] [PubMed]
- Tune, B.X.J.; Sim, M.S.; Poh, C.L.; Guad, R.M.; Woon, C.K.; Hazarika, I.; Das, A.; Gopinath, S.C.B.; Rajan, M.; Sekar, M.; et al. Matrix Metalloproteinases in Chemoresistance: Regulatory Roles, Molecular Interactions, and Potential Inhibitors. J. Oncol. 2022, 2022, 3249766. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, Y.; Dai, H.; Han, B. Epithelial–Mesenchymal Transition-Mediated Tumor Therapeutic Resistance. Molecules 2022, 27, 4750. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A Greedy Algorithm for Aligning DNA Sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Somers, W.; Ultsch, M.; De Vos, A.M.; Kossiakoff, A.A. The X-Ray Structure of a Growth Hormone-Prolactin Receptor Complex. Nature 1994, 372, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, D.; Jiang, J.; Deng, L.; Zhang, Y.; Yu, H.; Bahl, D.; Langenheim, J.F.; Chen, W.Y.; Fuchs, S.Y.; et al. The Role of Prolactin Receptor in GH Signaling in Breast Cancer Cells. Mol. Endocrinol. 2013, 27, 266–279. [Google Scholar] [CrossRef]
- Carter-Su, C.; Schwartz, J.; Argetsinger, L.S. Growth Hormone Signaling Pathways. Growth Horm. IGF Res. 2016, 28, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Dehkhoda, F.; Lee, C.M.M.; Medina, J.; Brooks, A.J. The Growth Hormone Receptor: Mechanism of Receptor Activation, Cell Signaling, and Physiological Aspects. Front. Endocrinol. 2018, 9, 35. [Google Scholar] [CrossRef]
- Herrington, J.; Carter-Su, C. Signaling Pathways Activated by the Growth Hormone Receptor. Trends Endocrinol. Metab. 2001, 12, 252–257. [Google Scholar] [CrossRef]
- Dean, M. The Human ATP-Binding Cassette (ABC) Transporter Superfamily; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2002. [Google Scholar]
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC Transporters: The Power to Change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Vasiliou, V.; Vasiliou, K.; Nebert, D.W. Human ATP-Binding Cassette (ABC) Transporter Family. Hum. Genom. 2009, 3, 281. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, P.; Ahluwalia, M.; Mondal, A.K.; Sahajpal, N.; Kota, V.; Rojiani, M.V.; Rojiani, A.M.; Kolhe, R. Immunogenomic Gene Signature of Cell-Death Associated Genes with Prognostic Implications in Lung Cancer. Cancers 2021, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, F.; Bokhari, S.R.A. Apoptosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Kiraz, Y.; Adan, A.; Kartal Yandim, M.; Baran, Y. Major Apoptotic Mechanisms and Genes Involved in Apoptosis. Tumor Biol. 2016, 37, 8471–8486. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.A.; Kirby, R. Apoptosis: A Review of Pro-apoptotic and Anti-apoptotic Pathways and Dysregulation in Disease. J. Vet. Emergency Crit. Care 2008, 18, 572–585. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Vasaikar, S.V.; Deshmukh, A.P.; den Hollander, P.; Addanki, S.; Kuburich, N.A.; Kudaravalli, S.; Joseph, R.; Chang, J.T.; Soundararajan, R.; Mani, S.A. EMTome: A Resource for Pan-Cancer Analysis of Epithelial-Mesenchymal Transition Genes and Signatures. Br. J. Cancer 2021, 124, 259–269. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and Definitions for Research on Epithelial–Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New Functions for the Matrix Metalloproteinases in Cancer Progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and Function of Matrix Metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Kukacka, J.; Krizkova, S.; Huska, D.; Adam, V.; Masarik, M.; Prusa, R.; Kizek, R. Matrix Metalloproteinases. Curr. Med. Chem. 2010, 17, 3751–3768. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens--Structure, Function, and Biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Gilani, B.; Cassagnol, M. Biochemistry, Cytochrome P450. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef]
- Santha, S.; Ling, X.; Aljahdali, I.A.M.; Rasam, S.S.; Wang, X.; Liao, J.; Wang, J.; Fountzilas, C.; Li, Q.; Qu, J.; et al. Mutant Kras as a Biomarker Plays a Favorable Role in FL118-Induced Apoptosis, Reactive Oxygen Species (ROS) Production and Modulation of Survivin, Mcl-1 and XIAP in Human Bladder Cancer. Cancers 2020, 12, 3413. [Google Scholar] [CrossRef]
- Hour, T.C.; Chen, J.; Huang, C.Y.; Guan, J.Y.; Lu, S.H.; Hsieh, C.Y.; Pu, Y.S. Characterization of Chemoresistance Mechanisms in a Series of Cisplatin-Resistant Transitional Carcinoma Cell Lines. Anticancer Res. 2000, 20, 3221–3225. [Google Scholar] [PubMed]
- Kijima, T.; Takada-Owada, A.; Shimoda, H.; Kokubun, H.; Uematsu, T.; Takei, K.; Betsunoh, H.; Yashi, M.; Ishida, K.; Kamai, T. Predictive Role of ABC Transporters in the Efficacy of Enfortumab Vedotin for Urothelial Carcinoma. BJUI Compass 2025, 6, e488. [Google Scholar] [CrossRef] [PubMed]
- Vouyovitch, C.M.; Perry, J.K.; Liu, D.X.; Bezin, L.; Vilain, E.; Diaz, J.J.; Lobie, P.E.; Mertani, H.C. WNT4 Mediates the Autocrine Effects of Growth Hormone in Mammary Carcinoma Cells. Endocr. Relat. Cancer 2016, 23, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Luty, M.; Szydlak, R.; Pabijan, J.; Øvreeide, I.H.; Prot, V.E.; Zemła, J.; Stokke, B.T.; Lekka, M. Migration, Proliferation, and Elasticity of Bladder Cancer Cells on Lectin-Coated Surfaces 2024. bioRxiv 2024. [Google Scholar] [CrossRef]
- Pandey, V.; Perry, J.K.; Mohankumar, K.M.; Kong, X.-J.; Liu, S.-M.; Wu, Z.-S.; Mitchell, M.D.; Zhu, T.; Lobie, P.E. Autocrine Human Growth Hormone Stimulates Oncogenicity of Endometrial Carcinoma Cells. Endocrinology 2008, 149, 3909–3919. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.; Ezrin, C.; Yamashita, S.; Melmed, S. Recurrent Acromegaly Resulting from Ectopic Growth Hormone Gene Expression by a Metastatic Pancreatic Tumor. Cancer 1993, 71, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Gebre-Medhin, M.; Kindblom, L.-G.; Wennbo, H.; Törnell, J.; Meis-Kindblom, J.M. Growth Hormone Receptor Is Expressed in Human Breast Cancer. Am. J. Pathol. 2001, 158, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.K.; Mohankumar, K.M.; Emerald, B.S.; Mertani, H.C.; Lobie, P.E. The Contribution of Growth Hormone to Mammary Neoplasia. J. Mammary Gland. Biol. Neoplasia 2008, 13, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jamieson, S.M.F.; Perry, J.K. Targeting Growth Hormone in Cancer: Future Perspectives. Endocr. Relat. Cancer 2023, 30, e230033. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, Y.; Xu, G.; Fu, C. Growth Hormone Receptor Promotes Breast Cancer Progression via the BRAF/MEK/ERK Signaling Pathway. FEBS Open Bio 2020, 10, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Bidosee, M.; Karry, R.; Weiss-Messer, E.; Barkey, R.J. Regulation of Growth Hormone Receptors in Human Prostate Cancer Cell Lines. Mol. Cell. Endocrinol. 2009, 309, 82–92. [Google Scholar] [CrossRef]
- Recouvreux, M.V.; Wu, J.B.; Gao, A.C.; Zonis, S.; Chesnokova, V.; Bhowmick, N.; Chung, L.W.; Melmed, S. Androgen Receptor Regulation of Local Growth Hormone in Prostate Cancer Cells. Endocrinology 2017, 158, 2255–2268. [Google Scholar] [CrossRef] [PubMed]
- Unterberger, C.J.; Maklakova, V.I.; Lazar, M.; Arneson, P.D.; Mcilwain, S.J.; Tsourkas, P.K.; Hu, R.; Kopchick, J.J.; Swanson, S.M.; Marker, P.C. GH Action in Prostate Cancer Cells Promotes Proliferation, Limits Apoptosis, and Regulates Cancer-Related Gene Expression. Endocrinology 2022, 163, bqac031. [Google Scholar] [CrossRef]
- Wang, Z.; Prins, G.S.; Coschigano, K.T.; Kopchick, J.J.; Green, J.E.; Ray, V.H.; Hedayat, S.; Christov, K.T.; Unterman, T.G.; Swanson, S.M. Disruption of Growth Hormone Signaling Retards Early Stages of Prostate Carcinogenesis in the C3(1)/T Antigen Mouse. Endocrinology 2005, 146, 5188–5196. [Google Scholar] [CrossRef] [PubMed]
- Weiss-Messer, E.; Merom, O.; Adi, A.; Karry, R.; Bidosee, M.; Ber, R.; Kaploun, A.; Stein, A.; Barkey, R.J. Growth Hormone (GH) Receptors in Prostate Cancer: Gene Expression in Human Tissues and Cell Lines and Characterization, GH Signaling and Androgen Receptor Regulation in LNCaP Cells. Mol. Cell. Endocrinol. 2004, 220, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-L.; Kyprianou, N. Androgen Receptor and Growth Factor Signaling Cross-Talk in Prostate Cancer Cells. Endocr. Relat. Cancer 2008, 15, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, S.A.; Hoffman, A.R. The Somatopause: Should Growth Hormone Deficiency in Older People Be Treated? Clin. Geriatr. Med. 1997, 13, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Chesnokova, V.; Zonis, S.; Apostolou, A.; Estrada, H.Q.; Knott, S.; Wawrowsky, K.; Michelsen, K.; Ben-Shlomo, A.; Barrett, R.; Gorbunova, V.; et al. Local Non-Pituitary Growth Hormone Is Induced with Aging and Facilitates Epithelial Damage. Cell Rep. 2021, 37, 110068. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.; Arámburo, C.; Sanders, E.J. Extrapituitary Production of Anterior Pituitary Hormones: An Overview. Endocrine 2012, 41, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Qian, Y.; Kopchick, J.J. Lessons from Growth Hormone Receptor Gene-Disrupted Mice: Are There Benefits of Endocrine Defects? Eur. J. Endocrinol. 2018, 178, R155–R181. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, D.T.; Singal, P.K.; Al-Banaw, A. Growth Hormone in Vascular Pathology: Neovascularization and Expression of Receptors Is Associated with Cellular Proliferation. Anticancer Res. 2007, 27, 4201–4218. [Google Scholar] [PubMed]
- Dos Santos Reis, M.D.; Dos Santos, Y.M.O.; de Menezes, C.A.; Borbely, K.S.C.; Smaniotto, S. Resident Murine Macrophage Migration and Phagocytosis Are Modulated by Growth Hormone. Cell Biol. Int. 2018, 42, 615–623. [Google Scholar] [CrossRef]
- Kopchick, J.J.; Berryman, D.E.; Puri, V.; Lee, K.Y.; Jorgensen, J.O.L. The Effects of Growth Hormone on Adipose Tissue: Old Observations, New Mechanisms. Nat. Rev. Endocrinol. 2020, 16, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, S.H.; Kim, J.Y.; Lee, Y. The Effect of Growth Hormone on Fibroblast Proliferation and Keratinocyte Migration. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2010, 63, E364–E369. [Google Scholar] [CrossRef] [PubMed]
- Lantvit, D.D.; Unterberger, C.J.; Lazar, M.; Arneson, P.D.; Longhurst, C.A.; Swanson, S.M.; Marker, P.C. Mammary Tumors Growing in the Absence of Growth Hormone Are More Sensitive to Doxorubicin Than Wild-Type Tumors. Endocrinology 2021, 162, bqab013. [Google Scholar] [CrossRef]
- Bacigalupo, M.L.; Piazza, V.G.; Cicconi, N.S.; Carabias, P.; Bartke, A.; Fang, Y.; Sotelo, A.I.; Rabinovich, G.A.; Troncoso, M.F.; Miquet, J.G. Growth Hormone Upregulates the Pro-Tumorigenic Galectin 1 in Mouse Liver. Endocr. Connect. 2019, 8, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Sahu, V.; Lombardo, J.L.; Xiao, L.; George, B.; Wolff, R.A.; Morris, J.S.; Rashid, A.; Kopchick, J.J.; Kaseb, A.O.; et al. Disruption of Growth Hormone Receptor Signaling Abrogates Hepatocellular Carcinoma Development. J. Hepatocell. Carcinoma 2022, 9, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Yang, J.; Huang, W.-D.; Wang, J.; Zhang, Q. siRNA-Targeted Inhibition of Growth Hormone Receptor in Human Colon Cancer SW480 Cells. World J. Gastroenterol. 2013, 19, 8108–8113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ji, Z.; Li, J.; Zhang, S.; Wu, C.; Zhang, R.; Guo, Z. Growth Hormone Associated with Treatment Efficacy of Immune Checkpoint Inhibitors in Gastric Cancer Patients. Front. Oncol. 2022, 12, 917313. [Google Scholar] [CrossRef] [PubMed]
- Boguszewski, M.C.S.; Boguszewski, C.L.; Chemaitilly, W.; Cohen, L.E.; Gebauer, J.; Higham, C.; Hoffman, A.R.; Polak, M.; Yuen, K.C.J.; Alos, N.; et al. Safety of Growth Hormone Replacement in Survivors of Cancer and Intracranial and Pituitary Tumours: A Consensus Statement. Eur. J. Endocrinol. 2022, 186, P35–P52. [Google Scholar] [CrossRef] [PubMed]
- Ogilvy-Stuart, A.L.; Gleeson, H. Cancer Risk Following Growth Hormone Use in Childhood: Implications for Current Practice. Drug Saf. 2004, 27, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Sävendahl, L.; Cooke, R.; Tidblad, A.; Beckers, D.; Butler, G.; Cianfarani, S.; Clayton, P.; Coste, J.; Hokken-Koelega, A.C.S.; Kiess, W.; et al. Long-Term Mortality after Childhood Growth Hormone Treatment: The SAGhE Cohort Study. Lancet Diabetes Endocrinol. 2020, 8, 683–692. [Google Scholar] [CrossRef]
- Danilowicz, K.; Sosa, S. Acromegaly and Cancer: An Update. Arch. Med. Res. 2023, 54, 102914. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Xiao, P.; Wang, Y.; Fang, C.; Li, Y. Risk of Cancer in Acromegaly Patients: An Updated Meta-Analysis and Systematic Review. PLoS ONE 2023, 18, e0285335. [Google Scholar] [CrossRef]
- Zhang, Y. Understanding the Gender Disparity in Bladder Cancer Risk: The Impact of Sex Hormones and Liver on Bladder Susceptibility to Carcinogens. J. Environ. Sci. Health Part C 2013, 31, 287–304. [Google Scholar] [CrossRef]
- Avtanski, D.; Novaira, H.J.; Wu, S.; Romero, C.J.; Kineman, R.; Luque, R.M.; Wondisford, F.; Radovick, S. Both Estrogen Receptor α and β Stimulate Pituitary GH Gene Expression. Mol. Endocrinol. 2014, 28, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.-C.; Johannsson, G.; Leong, G.M.; Ho, K.K.Y. Estrogen Regulation of Growth Hormone Action. Endocr. Rev. 2004, 25, 693–721. [Google Scholar] [CrossRef] [PubMed]
- Rosiello, G.; Palumbo, C.; Deuker, M.; Stolzenbach, L.F.; Martin, T.; Tian, Z.; Gallina, A.; Montorsi, F.; Black, P.; Kassouf, W.; et al. Racial Differences in the Distribution of Bladder Cancer Metastases: A Population-Based Analysis. Cent. Eur. J. Urol. 2020, 73, 407–415. [Google Scholar] [CrossRef]
- Puri, D.; Pandit, K.; Choi, N.; Rose, B.S.; McKay, R.R.; Bagrodia, A. Striving for Equity: Examining Health Disparities in Urologic Oncology. Cancers 2024, 16, 3559. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lupu, F.; Terwilliger, J.D.; Lee, K.; Segre, G.V.; Efstratiadis, A. Roles of Growth Hormone and Insulin-like Growth Factor 1 in Mouse Postnatal Growth. Dev. Biol. 2001, 229, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Kopchick, J.J. GH and IGF1 in Cancer Therapy Resistance. Endocr. Relat. Cancer 2023, 30, e220414. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shi, T.; Zhang, L.; Zhu, P.; Deng, M.; Huang, C.; Hu, T.; Jiang, L.; Li, J. Mammalian Drug Efflux Transporters of the ATP Binding Cassette (ABC) Family in Multidrug Resistance: A Review of the Past Decade. Cancer Lett. 2016, 370, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Yu, A.-M. ABC Transporters in Multidrug Resistance and Pharmacokinetics, and Strategies for Drug Development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Benabbou, N.; Mirshahi, P.; Cadillon, M.; Soria, J.; Therwath, A.; Mirshahi, M. Hospicells Promote Upregulation of the ATP-Binding Cassette Genes by Insulin-like Growth Factor-I via the JAK2/STAT3 Signaling Pathway in an Ovarian Cancer Cell Line. Int. J. Oncol. 2013, 43, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Shi, H.; Ren, F.; Wang, J.; Wu, Q.; Li, X.; Zhang, R. Inhibition of the IGF Signaling Pathway Reverses Cisplatin Resistance in Ovarian Cancer Cells. BMC Cancer 2017, 17, 851. [Google Scholar] [CrossRef]
- Shen, K.; Cui, D.; Sun, L.; Lu, Y.; Han, M.; Liu, J. Inhibition of IGF-IR Increases Chemosensitivity in Human Colorectal Cancer Cells through MRP-2 Promoter Suppression. J. Cell. Biochem. 2012, 113, 2086–2097. [Google Scholar] [CrossRef]
- Benabbou, N.; Mirshahi, P.; Bordu, C.; Faussat, A.-M.; Tang, R.; Therwath, A.; Soria, J.; Marie, J.-P.; Mirshahi, M. A Subset of Bone Marrow Stromal Cells Regulate ATP-Binding Cassette Gene Expression via Insulin-like Growth Factor-I in a Leukemia Cell Line. Int. J. Oncol. 2014, 45, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.G.; Mun, M.H.; Jeong, M.S.; Kim, W.T.; Lee, S.R.; Chung, J.W.; Kim, S.I.; Kim, T.N.; Nam, J.K.; Leem, S.H. Drug Resistance of Bladder Cancer Cells through Activation of ABCG2 by FOXM1. BMB Rep. 2018, 51, 98. [Google Scholar] [CrossRef] [PubMed]
- Pasello, M.; Giudice, A.M.; Scotlandi, K. The ABC Subfamily A Transporters: Multifaceted Players with Incipient Potentialities in Cancer. Semin. Cancer Biol. 2020, 60, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yuan, H.; Gu, J.; Xu, D.; Wang, M.; Qiao, J.; Yang, X.; Zhang, J.; Yao, M.; Gu, J.; et al. ABCA8-Mediated Efflux of Taurocholic Acid Contributes to Gemcitabine Insensitivity in Human Pancreatic Cancer via the S1PR2-ERK Pathway. Cell Death Discov. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhou, G.; Zhang, M.; Zhang, N. ABCA8 Elevation Predicts the Prognosis and Exerts the Anti-Oncogenic Effects on the Malignancy of Non-Small Cell Lung Cancer via TCF21-Mediated Inactivation of PI3K/AKT. Mol. Biotechnol. 2025, 67, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.W.; Liddle, C.; Coverdale, S.; Lou, J.C.; Boyages, S.C. Growth Hormone Treatment Increases Cytochrome P450-Mediated Antipyrine Clearance in Man. J. Clin. Endocrinol. Metab. 1996, 81, 1999–2001. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Banerjee, S.; Shapiro, B.H. Growth Hormone—A Newly Identified Developmental Organizer. J. Endocrinol. 2017, 232, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Waxman, D.J.; O’Connor, C. Growth Hormone Regulation of Sex-Dependent Liver Gene Expression. Mol. Endocrinol. 2006, 20, 2613–2629. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Huang, J.; Moorthy, B.; Wang, C.; Hu, M.; Gao, S.; Ghose, R. Role of Drug Metabolizing Enzymes in Chemotherapy-Induced Gastrointestinal Toxicity and Hepatotoxicity. Expert Opin. Drug Metab. Toxicol. 2020, 16, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- Hay, E.D. An Overview of Epithelio-Mesenchymal Transformation. Acta Anat. 1995, 154, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R. The Matrix in Cancer. Nat. Rev. Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2022, 23, 146. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Ali-Labib, R.; Swellam, M.; Bassiony, M.; Tash, F.; El-Zayat, T.M. Noninvasive Diagnosis of Bladder Cancer by Detection of Matrix Metalloproteinases (MMP-2 and MMP-9) and Their Inhibitor (TIMP-2) in Urine. Eur. Urol. 2007, 52, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Kader, A.K.; Liu, J.; Shao, L.; Dinney, C.P.; Lin, J.; Wang, Y.; Gu, J.; Grossman, H.B.; Wu, X. Matrix Metalloproteinase Polymorphisms Are Associated with Bladder Cancer Invasiveness. Clin. Cancer Res. 2007, 13, 2614–2620. [Google Scholar] [CrossRef] [PubMed]
- Kudelski, J.; Tokarzewicz, A.; Gudowska-Sawczuk, M.; Mroczko, B.; Chłosta, P.; Bruczko-Goralewska, M.; Mitura, P.; Młynarczyk, G. The Significance of Matrix Metalloproteinase 9 (MMP-9) and Metalloproteinase 2 (MMP-2) in Urinary Bladder Cancer. Biomedicines 2023, 11, 956. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Liang, C.; Zhu, J.; Xu, A.; Zhao, K.; Hua, Y.; Zhang, J.; Chen, W.; Suo, C.; Zhang, C.; et al. Prognostic Role of Matrix Metalloproteinases in Bladder Carcinoma: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 32309–32321. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Faba, O.; Palou-Redorta, J.; Fernández-Gómez, J.M.; Algaba, F.; Eiró, N.; Villavicencio, H.; Vizoso, F.J. Matrix Metalloproteinases and Bladder Cancer: What Is New? ISRN Urol. 2012, 2012, 581539. [Google Scholar] [CrossRef]
- Doessing, S.; Heinemeier, K.M.; Holm, L.; Mackey, A.L.; Schjerling, P.; Rennie, M.; Smith, K.; Reitelseder, S.; Kappelgaard, A.-M.; Rasmussen, M.H.; et al. Growth Hormone Stimulates the Collagen Synthesis in Human Tendon and Skeletal Muscle without Affecting Myofibrillar Protein Synthesis. J. Physiol. 2010, 588, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Bogazzi, F.; Ultimieri, F.; Raggi, F.; Russo, D.; Vanacore, R.; Guida, C.; Brogioni, S.; Cosci, C.; Gasperi, M.; Bartalena, L.; et al. Growth Hormone Inhibits Apoptosis in Human Colonic Cancer Cell Lines: Antagonistic Effects of Peroxisome Proliferator Activated Receptor-γ Ligands. Endocrinology 2004, 145, 3353–3362. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Luo, S.; Fan, P.; Zhu, H.; Li, Y.; Huang, W. Growth Hormone Activates PI3K/Akt Signaling and Inhibits ROS Accumulation and Apoptosis in Granulosa Cells of Patients with Polycystic Ovary Syndrome. Reprod. Biol. Endocrinol. 2020, 18, 121. [Google Scholar] [CrossRef]
- Jeay, S.; Sonenshein, G.E.; Postel-Vinay, M.C.; Baixeras, E. Growth Hormone Prevents Apoptosis through Activation of Nuclear Factor-kappaB in Interleukin-3-Dependent Ba/F3 Cell Line. Mol. Endocrinol. 2000, 14, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Buccitelli, C.; Selbach, M. mRNAs, Proteins and the Emerging Principles of Gene Expression Control. Nat. Rev. Genet. 2020, 21, 630–644. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An Enhanced Web Server for Large-Scale Expression Profiling and Interactive Analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed]
- Ru, B.; Wong, C.N.; Tong, Y.; Zhong, J.Y.; Zhong, S.S.W.; Wu, W.C.; Chu, K.C.; Wong, C.Y.; Lau, C.Y.; Chen, I.; et al. TISIDB: An Integrated Repository Portal for Tumor-Immune System Interactions. Bioinformatics 2019, 35, 4200–4202. [Google Scholar] [CrossRef] [PubMed]
- Györffy, B. Integrated Analysis of Public Datasets for the Discovery and Validation of Survival-Associated Genes in Solid Tumors. Innovation 2024, 5, 100625. [Google Scholar] [CrossRef] [PubMed]
- Vasaikar, S.V.; Straub, P.; Wang, J.; Zhang, B. LinkedOmics: Analyzing Multi-Omics Data within and across 32 Cancer Types. Nucleic Acids Res. 2018, 46, D956–D963. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An Image J Plugin for the High Throughput Image Analysis of In Vitro Scratch Wound Healing Assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).