Extraction of Chitin, Chitosan, and Calcium Acetate from Mussel Shells for Sustainable Waste Management
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation Optimization Results
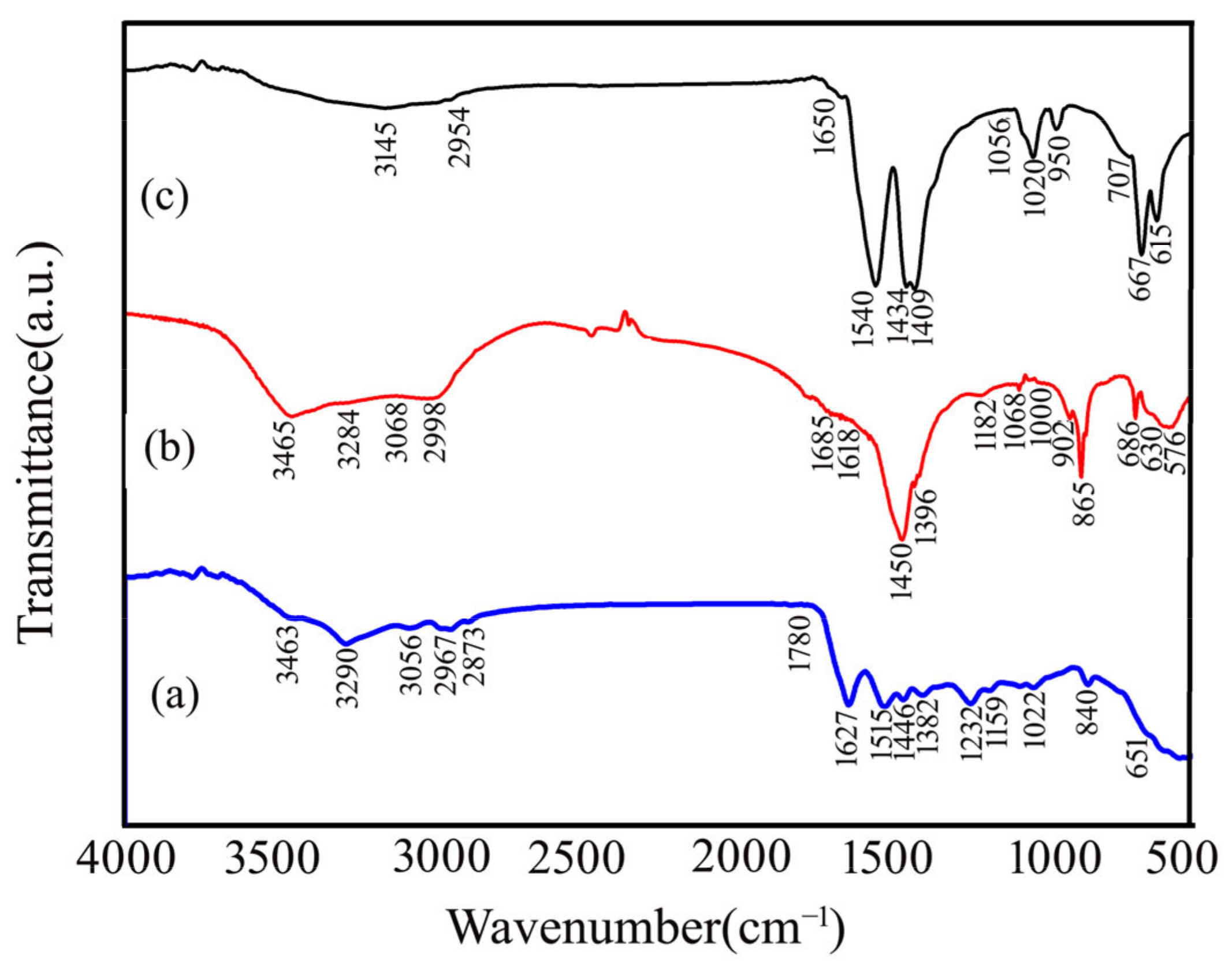
2.2. The Fourier-Transform Infrared (FTIR) Results
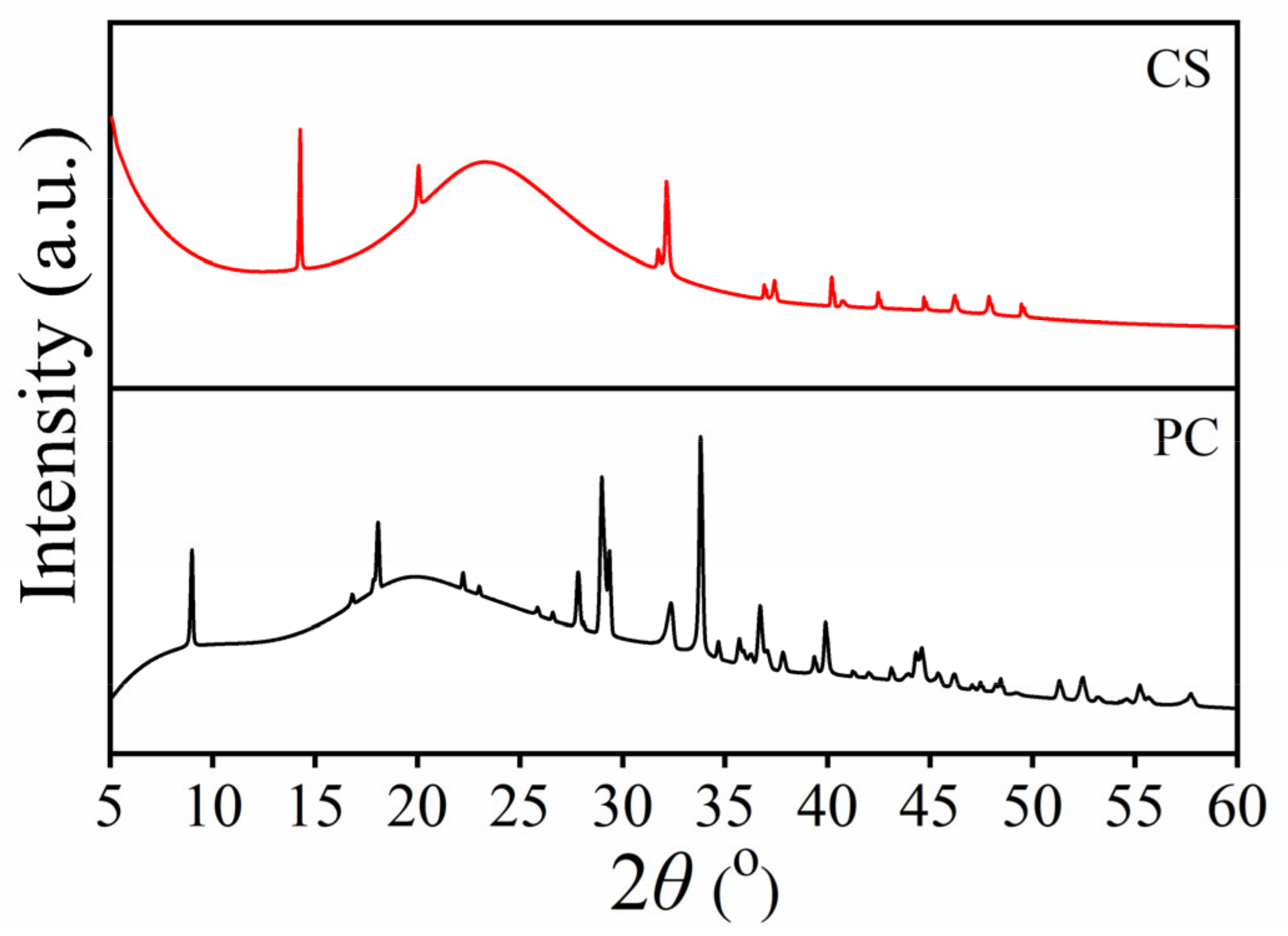
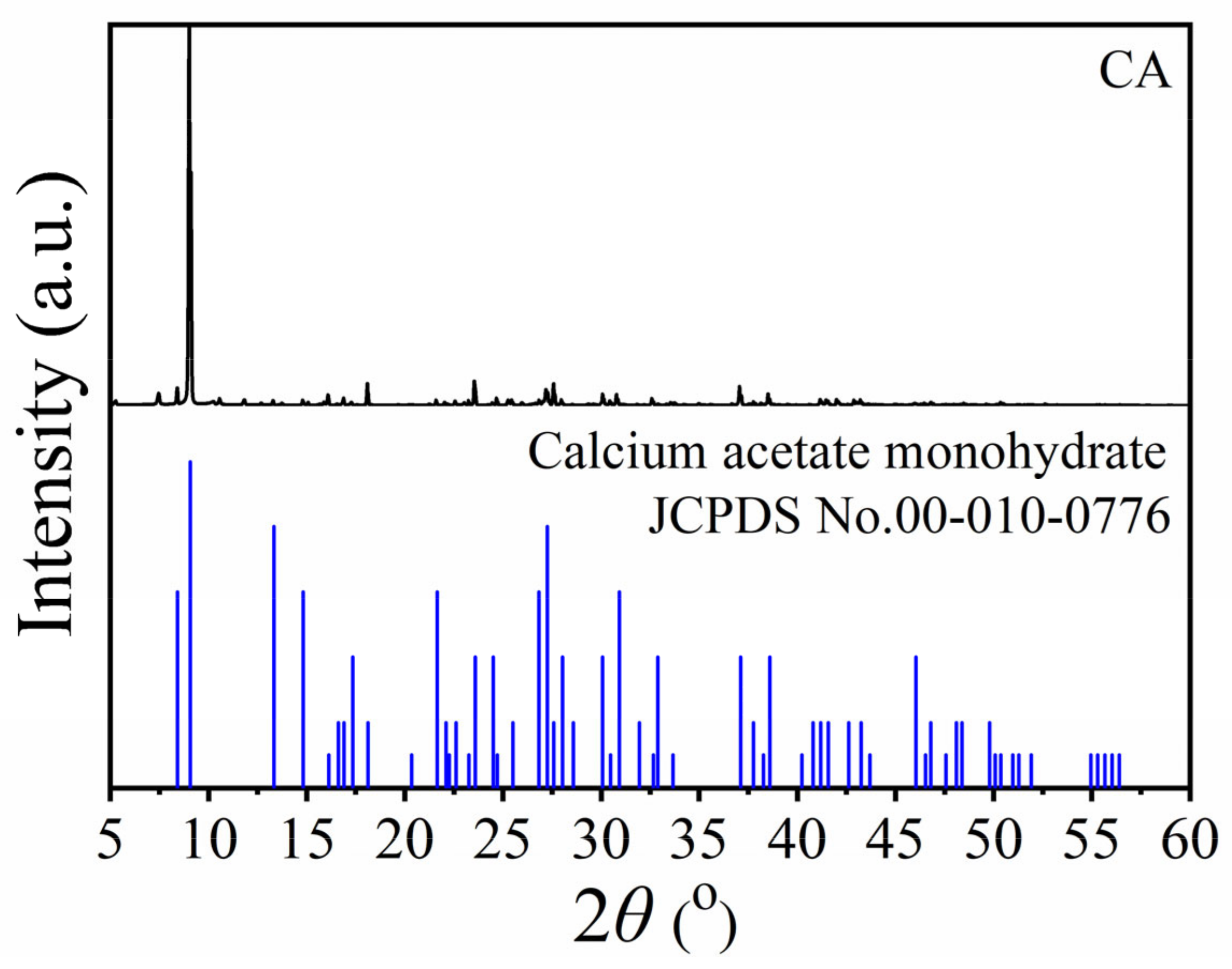
2.3. X-Ray Diffraction (XRD) Results
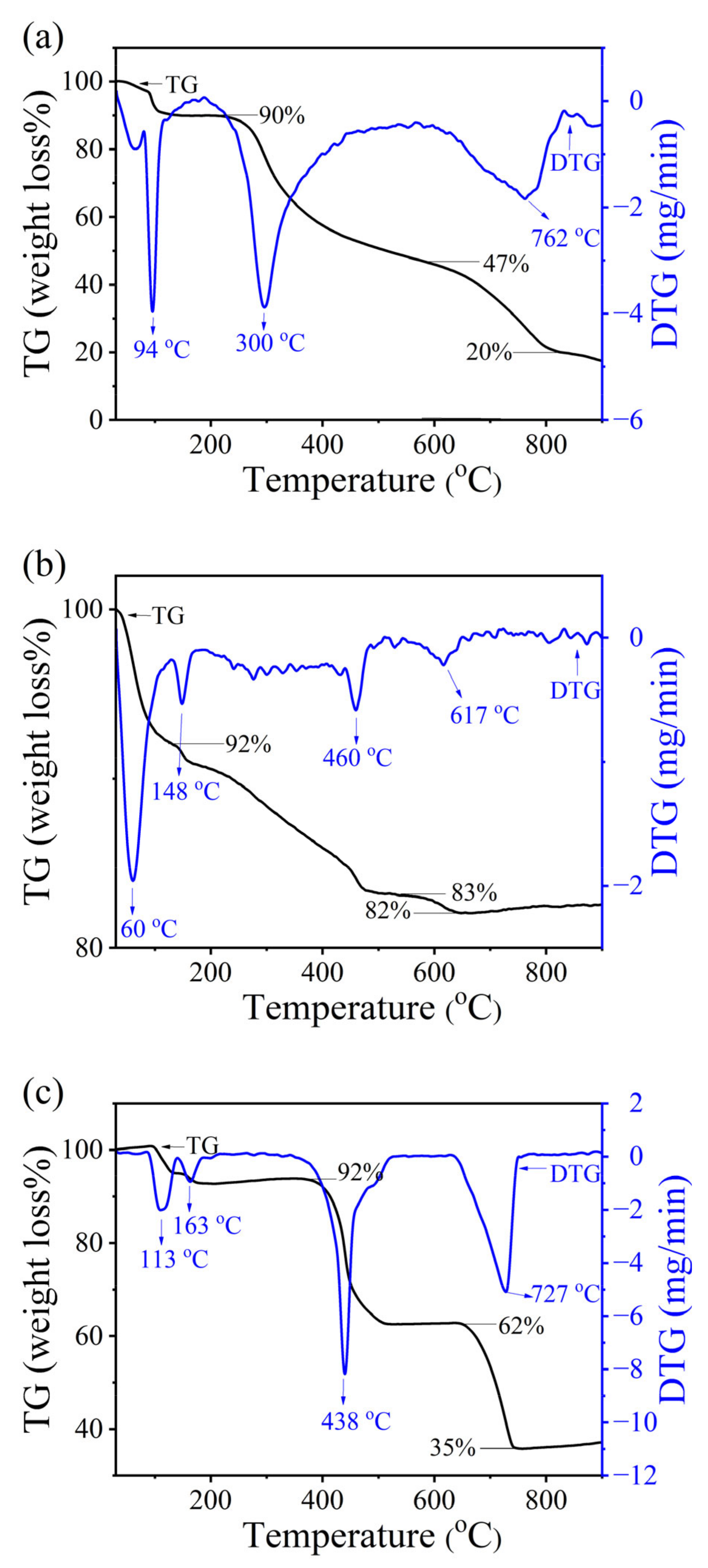
2.4. Thermogravimetric Analysis (TGA) Results
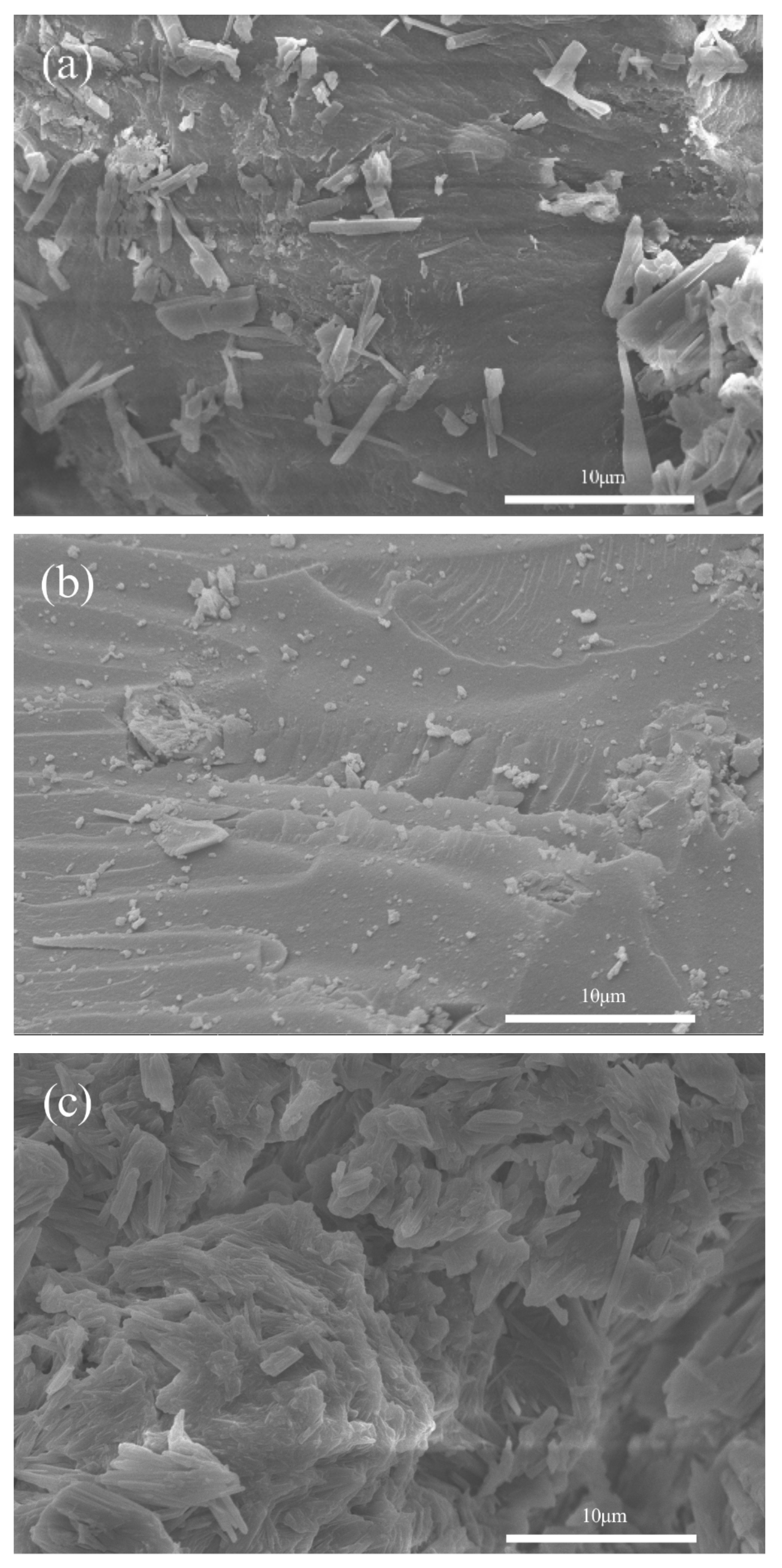
2.5. Scanning Electron Microscope (SEM) Results
3. Materials and Methods
3.1. Materials and Reagents
3.2. Chitin Extraction by Demineralization
3.3. Chitin Purification
- − Deproteinization
- − Decolorization
3.4. Deacetylation
3.5. Calcium Acetate Production
3.6. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medina Uzcátegui, L.U.; Vergara, K.; Martínez Bordes, G. Sustainable alternatives for by products derived from industrial mussel processing: A critical review. Waste Manag. Res. 2021, 40, 123–138. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Jaisan, C. Physicochemical Properties of Chitosan from Green Mussel Shells (Perna viridis): A Comparative Study. Polymers 2023, 15, 2816. [Google Scholar] [CrossRef]
- Srichanachaichok, W.; Pissuwan, D. Micro/Nano Structural Investigation and Characterization of Mussel Shell Waste in Thailand as a Feasible Bioresource of CaO. Materials 2023, 16, 805. [Google Scholar] [CrossRef]
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nat. News 2015, 524, 155–157. [Google Scholar] [CrossRef]
- Sowmya, R.; Rathinaraj, K.; Sachindra, N.M. An Autolytic Process for Recovery of Antioxidant Activity Rich Carotenoprotein from Shrimp Heads. Mar. Biotechnol. 2011, 13, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Seesanong, S.; Laosinwattana, C.; Boonchom, B. A simple rapid route to synthesize monocalcium phosphate monohydrate using calcium carbonate with different phases derived from green mussel shells. J. Mater. Environ. Sci. 2019, 10, 113–118. Available online: https://www.jmaterenvironsci.com/Document/vol10/vol10_N2/13-JMES-Seesanong-2019.pdf (accessed on 22 July 2025).
- Mititelu, M.; Stanciu, G.; Drăgănescu, D.; Ioniță, A.C.; Neacșu, S.M.; Dinu, M.; Stefan-van Staden, R.-I.; Moroșan, E. Mussel Shells, a Valuable Calcium Resource for the Pharmaceutical Industry. Mar. Drugs 2021, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Pratama, G.; Munandar, A.; Surilayani, D.; Rizky, J.A.; Hasanah, A.N.; Haryati, S.; Meata, B.A.; Nuryadin, D.F.E.; Aditia, R.P. Characteristics of crab shells and green mussel shells as potential chitosan material from Karangantu, Banten, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2023, 1137, 012033. [Google Scholar] [CrossRef]
- Cadano, J.R.; Jose, M.; Lubi, A.G.; Maling, J.N.; Moraga, J.S.; Shi, Q.Y.; Vegafria, H.M.; VinceCruz-Abeledo, C.C. A comparative study on the raw chitin and chitosan yields of common bio waste from Philippine seafood. Environ. Sci. Pollut. Res. 2020, 28, 11954–11961. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A. Production, Properties, and Some New Applications of Chitin and Its Derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods—A Review. Food Technol. Biotechnol. 2013, 51, 12–25. Available online: https://hrcak.srce.hr/file/146860 (accessed on 13 June 2025).
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Jagadish, C.R.; Fabien, S.; Stéphane, S.; Ada, A. Solubility of Chitin: Solvents, Solution Behaviors and Their Related Mechanisms, In Solubility of Polysaccharides; Xhenbo, Z., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 7. [Google Scholar] [CrossRef]
- Singh, S.K. Solubility of lignin and chitin in ionic liquids and their biomedical applications. Int. J. Biol. Macromol. 2019, 132, 265–277. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.D.l.L.; Heras Caballero, A. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.V.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as A Preservative for Fruits and Vegetables: A Review on Chemistry and Antimicrobial Properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Shirvan, A.R.; Shakeri, M.; Bashari, A. Recent advances in application of chitosan and its derivatives in functional finishing of textiles. In The Impact and Prospects of Green Chemistry for Textile Technology; Woodhead Publishing: London, UK, 2019; pp. 107–133. [Google Scholar] [CrossRef]
- Al Manhel, A.J.; Al Hilphy, A.R.S.; Niamah, A.K. Extraction of chitosan, characterisation and its use for water purification. J. Saudi Soc. Agric. Sci. 2018, 17, 186–190. [Google Scholar] [CrossRef]
- Sarode, S.; Upadhyay, P.; Khosa, M.A.; Mak, T.; Shakir, A.; Song, S.; Ullah, A. Overview of wastewater treatment methods with special focus on biopolymer chitin-chitosan. Int. J. Biol. Macromol. 2019, 121, 1086–1100. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S. The versatile biopolymer chitosan: Potential sources, evaluation of extraction methods and applications. Crit. Rev. Microbiol. 2014, 40, 155–175. [Google Scholar] [CrossRef]
- Je, J.-Y.; Kim, S.-K. Antimicrobial action of novel chitin derivative. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2006, 1760, 104–109. [Google Scholar] [CrossRef]
- Jardine, A.; Sayed, S. Valorisation of chitinous biomass for antimicrobial applications. Pure Appl. Chem. 2018, 90, 293–304. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.B.; et al. Characterization of chitin and chitosan derived from Hermetia illucens, a further step in a circular economy process. Sci. Rep. 2022, 12, 6613. [Google Scholar] [CrossRef]
- Al Shaqsi, N.H.K.; Al Hoqani, H.A.S.; Hossain, M.A.; Al Sibani, M.A. Optimization of the demineralization process for the extraction of chitin from Omani Portunidae segnis. Biochem. Biophys. Rep. 2020, 23, 100779. [Google Scholar] [CrossRef] [PubMed]
- N’Tsoukpoe, K.E.; Rammelberg, H.U.; Lele, A.F.; Korhammer, K.; Watts, B.A.; Schmidt, T.; Ruck, W.K.L. A review on the use of calcium chloride in applied thermal engineering. Appl. Therm. Eng. 2015, 75, 513–531. [Google Scholar] [CrossRef]
- Steciak, J.; Levendis, Y.A.; Wise, D.L. Effectiveness of calcium magnesium acetate as dual SO2 NO emission control agent. AIChE J. 1995, 41, 712–722. [Google Scholar] [CrossRef]
- Xu, Y.; Ye, J.; Zhou, D.; Su, L. Research progress on applications of calcium derived from marine organisms. Sci. Rep. 2020, 10, 18425. [Google Scholar] [CrossRef]
- Lu, Y.J.; Carter, E.; Chung, R.A. Use of calcium salts for soybean curd preparation. J. Food Sci. 1980, 45, 32–34. [Google Scholar] [CrossRef]
- Borchert, R. Calcium acetate induces calcium uptake and formation of calcium oxalate crystals in isolated leaflets of Gleditsia triacanthos L. Planta 1986, 168, 571–578. Planta 1986, 168, 571–578. [Google Scholar] [CrossRef]
- Bilton, M.; Brown, A.P.; Milne, S.J. Investigating the optimum conditions for the formation of calcium oxide, used for CO2 sequestration, by thermal decomposition of calcium acetate. J. Phys. Conf. Ser. 2012, 371, 012075. [Google Scholar] [CrossRef]
- Safronova, T.V.; Mukhin, E.A.; Putlyaev, V.I.; Knotko, A.V.; Evdokimov, P.V.; Shatalova, T.B.; Filippov, Y.Y.; Sidorov, A.V.; Karpushkin, E.A. Amorphous calcium phosphate powder synthesized from calcium acetate and polyphosphoric acid for bioceramics application. Ceram. Int. 2017, 43 Pt B, 1310–1317. [Google Scholar] [CrossRef]
- Abbas, M.N.; Ibrahim, S.A.; Abbas, Z.N.; Ibrahim, T.A. Eggshells as a sustainable source for acetone production. J. King Saud Univ. -Eng. Sci. 2022, 34, 381–387. [Google Scholar] [CrossRef]
- Bette, S.; Eggert, G.; Emmerling, S.; Etter, M.; Schleid, T.; Dinnebier, R.E. Crystal Structure, Polymorphism, and Anisotropic Thermal Expansion of α Ca(CH3COO)2. Cryst. Growth Des. 2020, 20, 5346–5355. [Google Scholar] [CrossRef]
- Seesanong, S.; Seangarun, C.; Boonchom, B.; Laohavisuti, N.; Thompho, S.; Boonmee, W.; Mongkol, S.; Rungrojchaipon, P. Bio-green synthesis of calcium acetate from oyster shell waste at low cost and reducing the emission of greenhouse gases. Sustain. Environ. Res. 2023, 33, 26. [Google Scholar] [CrossRef]
- Seesanong, S.; Seangarun, C.; Boonchom, B.; Ohpasee, N.; Laohavisuti, N.; Boonmee, W.; Rungrojchaipon, P. Green Ca source of cockle shells converted to calcium acetate for environmental sustainability. Heliyon 2024, 10, e32153. [Google Scholar] [CrossRef]
- Nobre, L.C.S.; Santos, S.; Palavra, A.M.F.; Calvete, M.J.F.; de Castro, C.A.N.; Nobre, B.P. Supercritical antisolvent precipitation of calcium acetate from eggshells. J. Supercrit. Fluids 2020, 163, 104862. [Google Scholar] [CrossRef]
- Dumitru, D.-A.; Poplacean, I.-C.; Maskaric, K.; Tămaş, T.; Barbu Tudoran, L.; Cinta Pinzaru, S. Calcium Acetate Drug Produced from Rapana venosa Invasive Gastropod Shells: Green Process Control Assisted by Raman Technology. ACS Omega 2024, 9, 37086–37095. [Google Scholar] [CrossRef]
- Jin, F.; Zhang, G.; Jin, Y.; Watanabe, Y.; Kishita, A.; Enomoto, H. A new process for producing calcium acetate from vegetable wastes for use as an environmentally friendly deicer. Bioresour. Technol. 2010, 101, 7299–7306. [Google Scholar] [CrossRef]
- Singh, A.R.; Rawat, N.S.; Lonibala, R. Solvothermal synthesis, crystal structure of a new Ca(II) coordination polymer [CaII(4–ABA)(CH3COO)(H2O)(DMF)]n and its catalytic epoxidation of cyclohexene. J. Mol. Struct. 2021, 1225, 129074. [Google Scholar] [CrossRef]
- Tolesa, L.D.; Gupta, B.S.; Lee, M.-J. Chitin and chitosan production from shrimp shells using ammonium based ionic liquids. Int. J. Biol. Macromol. 2019, 130, 818–826. [Google Scholar] [CrossRef]
- Liu, T.G.; Li, B.; Huang, W.; Lv, B.; Chen, J.; Zhang, J.X.; Zhu, L.P. Effects and kinetics of a novel temperature cycling treatment on the N deacetylation of chitin in alkaline solution. Carbohydr. Polym. 2009, 77, 110–117. [Google Scholar] [CrossRef]
- Sagheer, F.A.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Kumari, S.; Rath, P.; Sri Hari Kumar, A.; Tiwari, T.N. Extraction and characterization of chitin and chitosan from fishery waste by chemical method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Anand, M.; Kalaivani, R.; Maruthupandy, M.; Kumaraguru, A.; Suresh, S. Extraction and characterization of chitosan from marine crab and squilla collected from the Gulf of Mannar Region, South India. J. Chitin Chitosan Sci. 2014, 2, 280–287. [Google Scholar] [CrossRef]
- Huang, L.; Bi, S.; Pang, J.; Sun, M.; Feng, C.; Chen, X. Preparation and characterization of chitosan from crab shell (Portunus trituberculatus) by NaOH/urea solution freeze thaw pretreatment procedure. Int. J. Biol. Macromol. 2020, 147, 931–936. [Google Scholar] [CrossRef]
- Erdogan, S.; Kaya, M. High similarity in physicochemical properties of chitin and chitosan from nymphs and adults of a grasshopper. Int. J. Biol. Macromol. 2016, 89, 118–126. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P. Preparation and characterization of α chitin from cicada sloughs. Mater. Sci. Eng. C 2010, 30, 357–363. [Google Scholar] [CrossRef]
- Musumeci, A.W.; Frost, R.L.; Wacławik, E.R. A spectroscopic study of the mineral paceite (calcium acetate). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 649–661. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Commons, C.J.; Hudson, T.A.; Sanchez Arlt, R.W. The elusive crystals of calcium acetate hemihydrate: Chiral rods linked by parallel hydrophilic strips. CrystEngComm 2021, 23, 707–713. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Zhao, Y.; Liu, Y. Preparation of Calcium Magnesium Acetate Snow Melting Agent Using Raw Calcium Acetate Rich Made from Eggshells. Waste Biomass Valorization 2020, 11, 6757–6767. [Google Scholar] [CrossRef]
- Baskar, D.; Sampath Kumar, T.S. Effect of deacetylation time on the preparation, properties and swelling behavior of chitosan films. Carbohydr. Polym. 2009, 78, 767–772. [Google Scholar] [CrossRef]
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Physicochemical characterization of chitin and chitosan from crab shells. Int. J. Biol. Macromol. 2019, 75, 15–21. [Google Scholar] [CrossRef]
- Suneeta, K.; Rath, P.; Annamareddy, A. Chitosan from shrimp shell (Crangon crangon) and fish scales (Labeo rohita): Extraction and characterization. Afr. J. Biotechnol. 2016, 15, 1258–1268. [Google Scholar] [CrossRef]
- Basosidik, S.; Pankaew, P.; Hoonnivathana, E.; Limsuwan, P.; Naemchanthara, K. Characterization of Chitin Extracted from Waste Sources via XRD, FTIR, and TGA Techniques. Adv. Mater. Res. 2012, 488–489, 1404–1408. [Google Scholar] [CrossRef]
- Kittur, F.S.; Prashanth, K.V.H.; Udaya Sankar, K. Tharanathan Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr. Polym. 2002, 49, 185–193. [Google Scholar] [CrossRef]
- Nieto, J.M.; Peniche-Covas, C.; Padro’n, G. Characterization of chitosan by pyrolysis mass spectrometry, thermal analysis and differential scanning calorimetry. Thermochim. Acta 1991, 176, 63–68. [Google Scholar] [CrossRef]
- López, F.A.; Mercê, A.L.R.; Alguacil, F.J.; López Delgado, A. A kinetic study on the thermal behaviour of chitosan. J. Therm. Anal. Calorim. 2008, 91, 633–639. [Google Scholar] [CrossRef]
- Barbosa, H.F.G.; Francisco, D.S.; Ferreira, A.P.G.; Cavalheiro, É.T.G. A new look towards the thermal decomposition of chitins and chitosans with different degrees of deacetylation by coupled TG FTIR. Carbohydr. Polym. 2019, 225, 115232. [Google Scholar] [CrossRef]
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm chrysalides. Carbohydr. Polym. 2006, 64, 98–103. [Google Scholar] [CrossRef]
- Qu, X.; Wirsén, A.; Albertsson, A.C. Effect of lactic/glycolic acid side chains on the thermal degradation kinetics of chitosan derivatives. Polymer 2000, 41, 4841–4847. [Google Scholar] [CrossRef]
- Seesanong, S.; Seangarun, C.; Boonchom, B.; Laohavisuti, N.; Boonmee, W.; Thompho, S.; Rungrojchaipon, P. Low Cost and Eco Friendly Calcium Oxide Prepared via Thermal Decompositions of Calcium Carbonate and Calcium Acetate Precursors Derived from Waste Oyster Shells. Materials 2024, 17, 3875. [Google Scholar] [CrossRef] [PubMed]
- Thongkam, M.; Saelim, J.; Boonchom, B.; Seesanong, S.; Chaiseeda, K.; Laohavisuti, N.; Bunya-Atichart, K.; Boonmee, W.; Taemchuay, D. Simple and Rapid Synthesis of Calcium Acetate from Scallop Shells to Reduce Environmental Issues. Adsorpt. Sci. Technol. 2021, 2021, 6450289. [Google Scholar] [CrossRef]
- Lagat, M.K.; Were, S.; Ndwigah, F.; Kemboi, V.J.; Kipkoech, C.; Tanga, C.M. Antimicrobial Activity of Chemically and Biologically Treated Chitosan Prepared from Black Soldier Fly (Hermetia illucens) Pupal Shell Waste. Microorganisms 2021, 9, 2417. [Google Scholar] [CrossRef]
- Greene, B.H.C.; Robertson, K.N.; Young, J.C.O.C.; Clyburne, J.A.C. Lactic acid demineralization of green crab (Carcinus maenas) shells: Effect of reaction conditions and isolation of an unusual calcium complex. Green Chem. Lett. Rev. 2016, 9, 1–11. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seangarun, C.; Seesanong, S.; Boonchom, B.; Laohavisuti, N.; Rungrojchaipon, P.; Boonmee, W.; Punthipayanon, S.; Thongkam, M. Extraction of Chitin, Chitosan, and Calcium Acetate from Mussel Shells for Sustainable Waste Management. Int. J. Mol. Sci. 2025, 26, 7107. https://doi.org/10.3390/ijms26157107
Seangarun C, Seesanong S, Boonchom B, Laohavisuti N, Rungrojchaipon P, Boonmee W, Punthipayanon S, Thongkam M. Extraction of Chitin, Chitosan, and Calcium Acetate from Mussel Shells for Sustainable Waste Management. International Journal of Molecular Sciences. 2025; 26(15):7107. https://doi.org/10.3390/ijms26157107
Chicago/Turabian StyleSeangarun, Chaowared, Somkiat Seesanong, Banjong Boonchom, Nongnuch Laohavisuti, Pesak Rungrojchaipon, Wimonmat Boonmee, Sirichet Punthipayanon, and Montree Thongkam. 2025. "Extraction of Chitin, Chitosan, and Calcium Acetate from Mussel Shells for Sustainable Waste Management" International Journal of Molecular Sciences 26, no. 15: 7107. https://doi.org/10.3390/ijms26157107
APA StyleSeangarun, C., Seesanong, S., Boonchom, B., Laohavisuti, N., Rungrojchaipon, P., Boonmee, W., Punthipayanon, S., & Thongkam, M. (2025). Extraction of Chitin, Chitosan, and Calcium Acetate from Mussel Shells for Sustainable Waste Management. International Journal of Molecular Sciences, 26(15), 7107. https://doi.org/10.3390/ijms26157107








