What Is Similar, What Is Different? Characterization of Mitoferrin-like Proteins from Arabidopsis thaliana and Cucumis sativus
Abstract
1. Introduction
2. Results
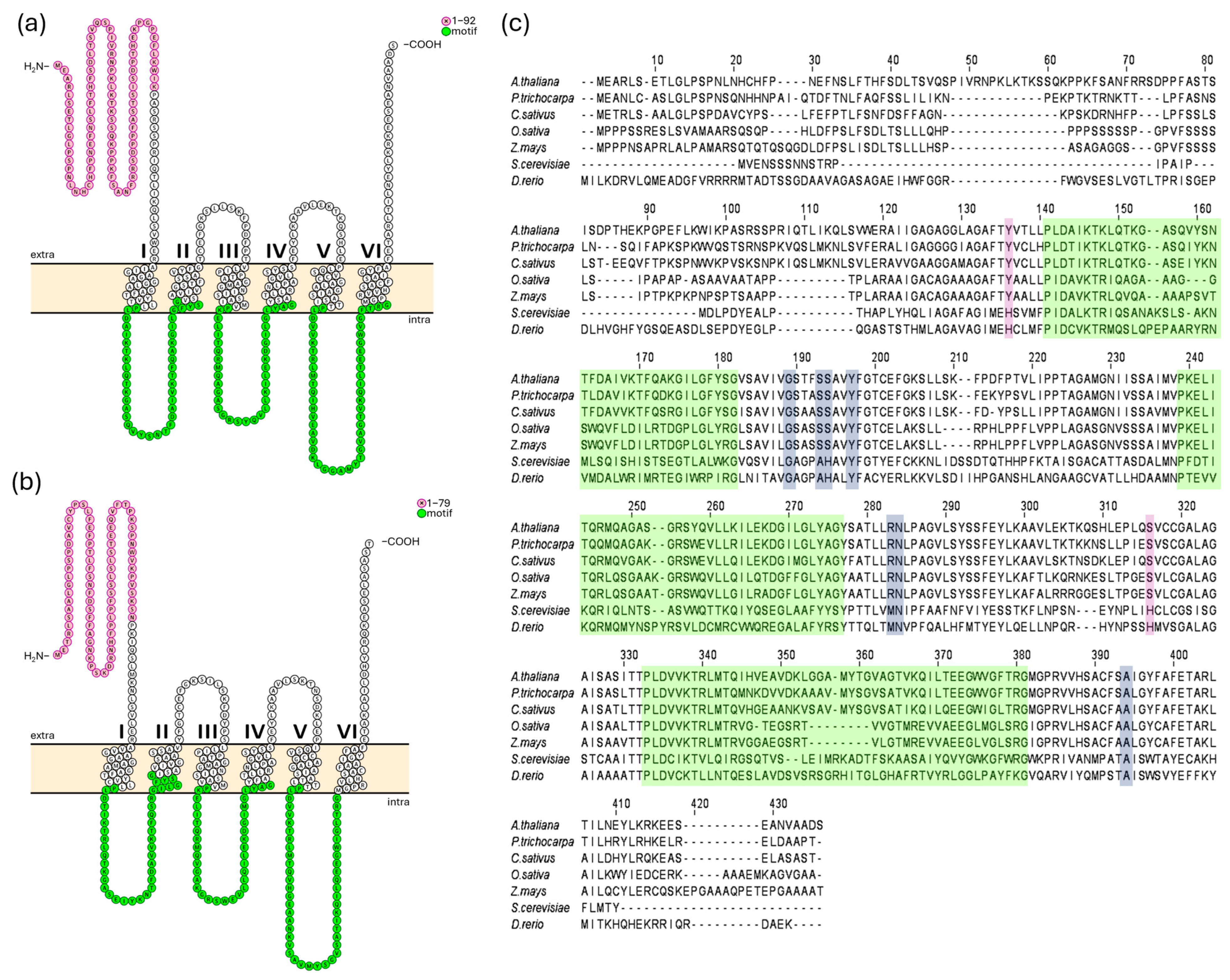
2.1. Identification of CsMFL1 in Cucumis sativus Genome and Characteristic Features of Plant MFL Proteins
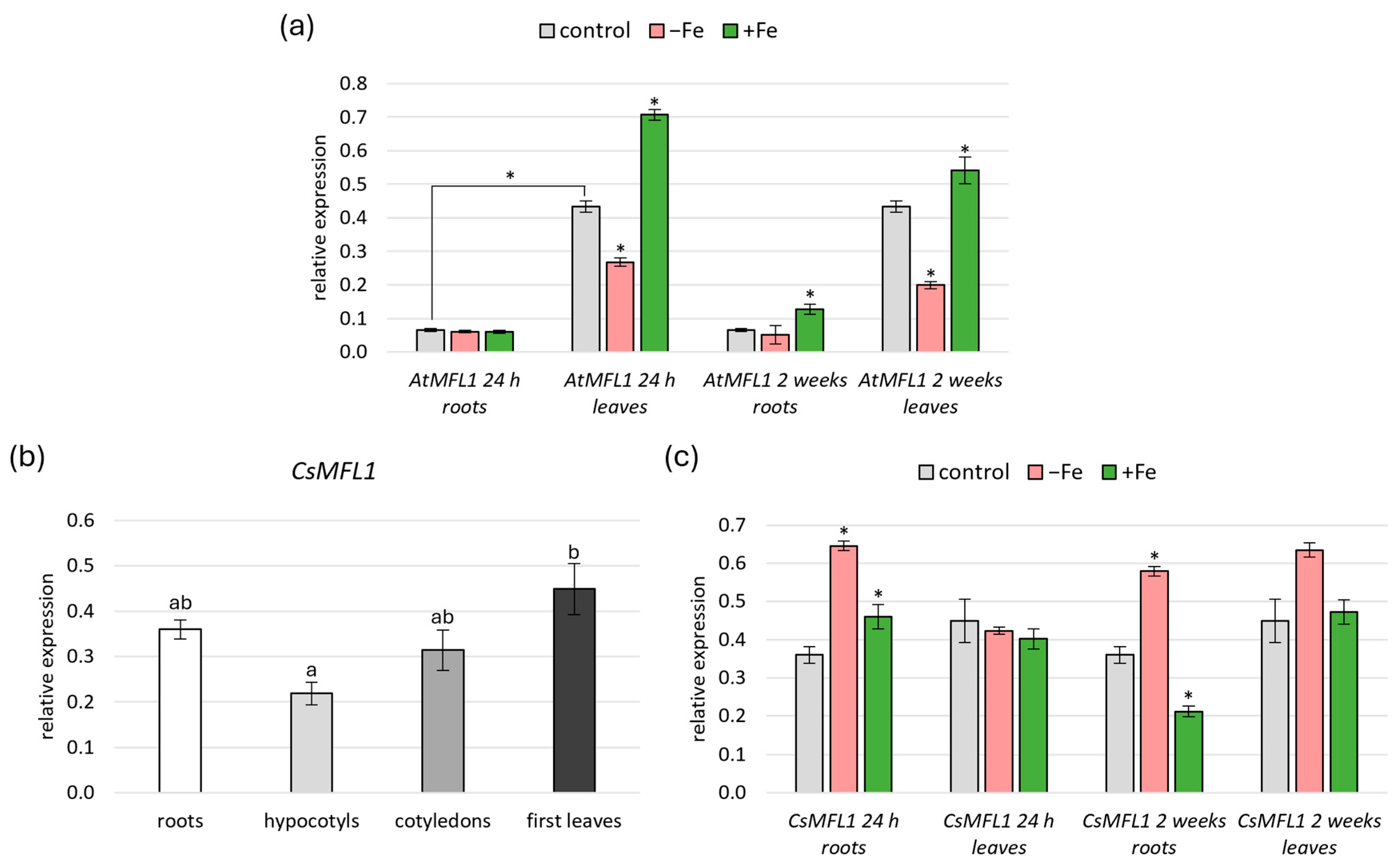
2.2. Expression Analysis of AtMFL1 and CsMFL1
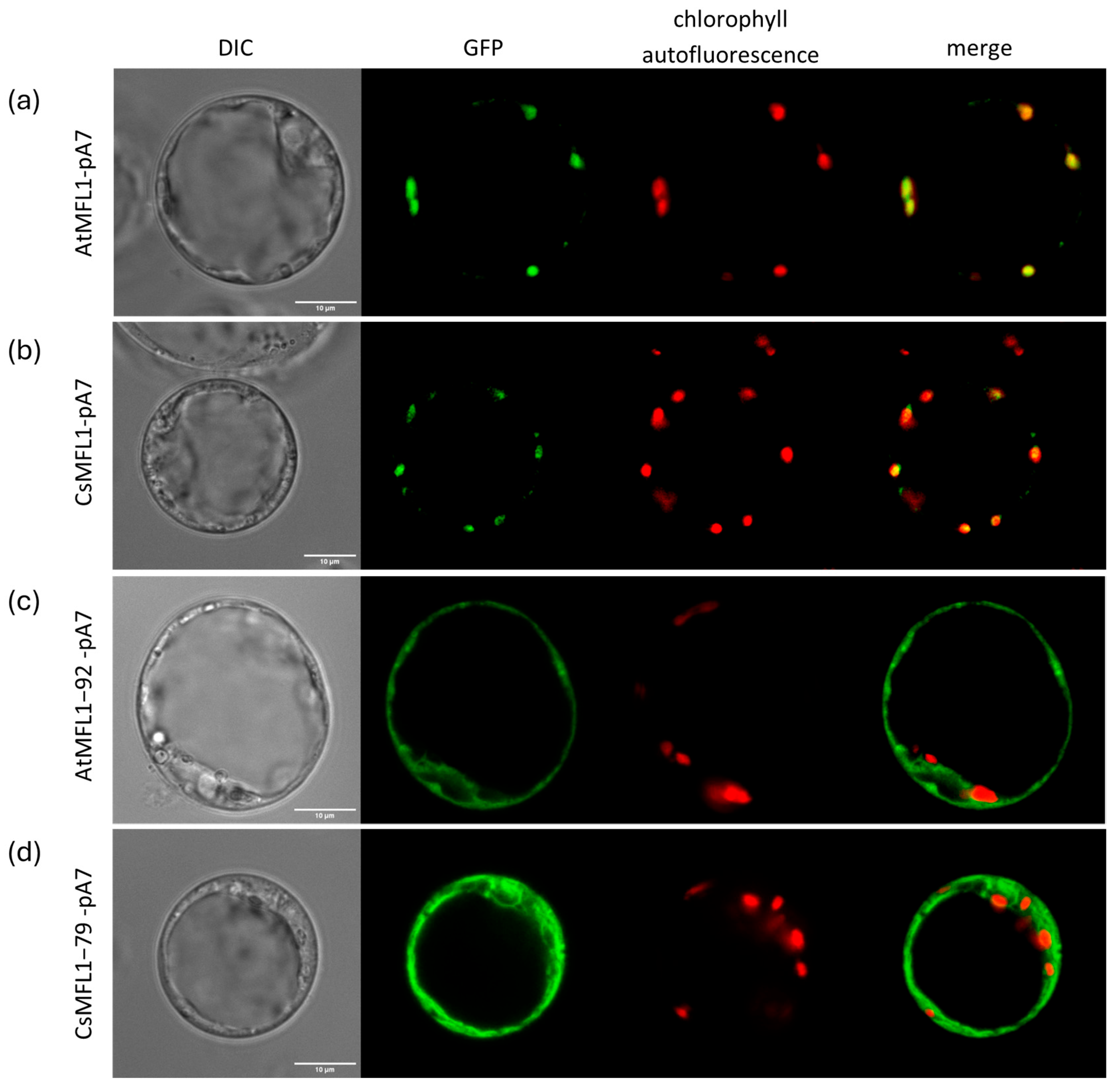
2.3. Effect of a Loss of N-Terminal cTP on MFL Localization
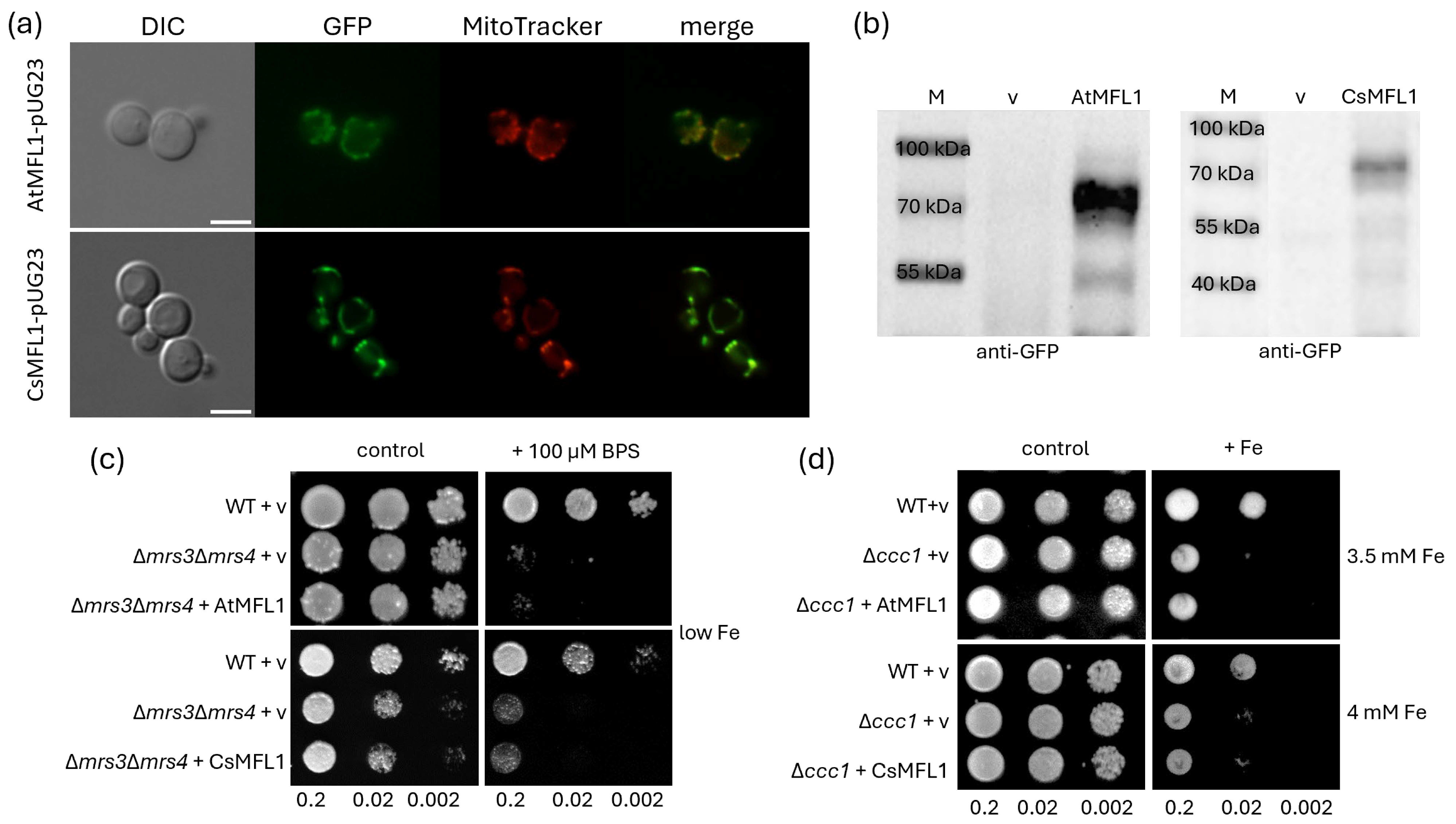
2.4. Functional Characterization of MFL Proteins in Yeasts
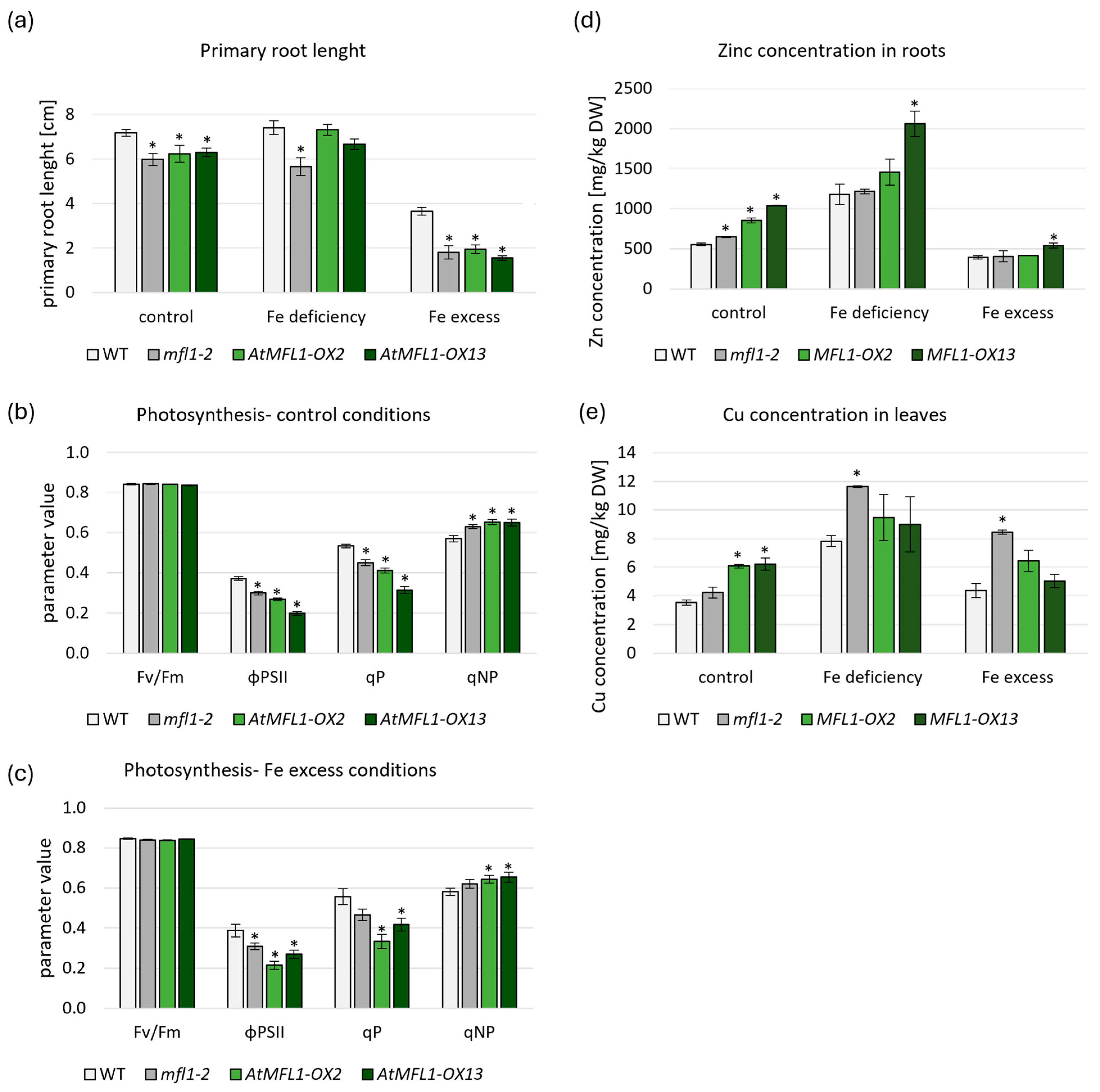
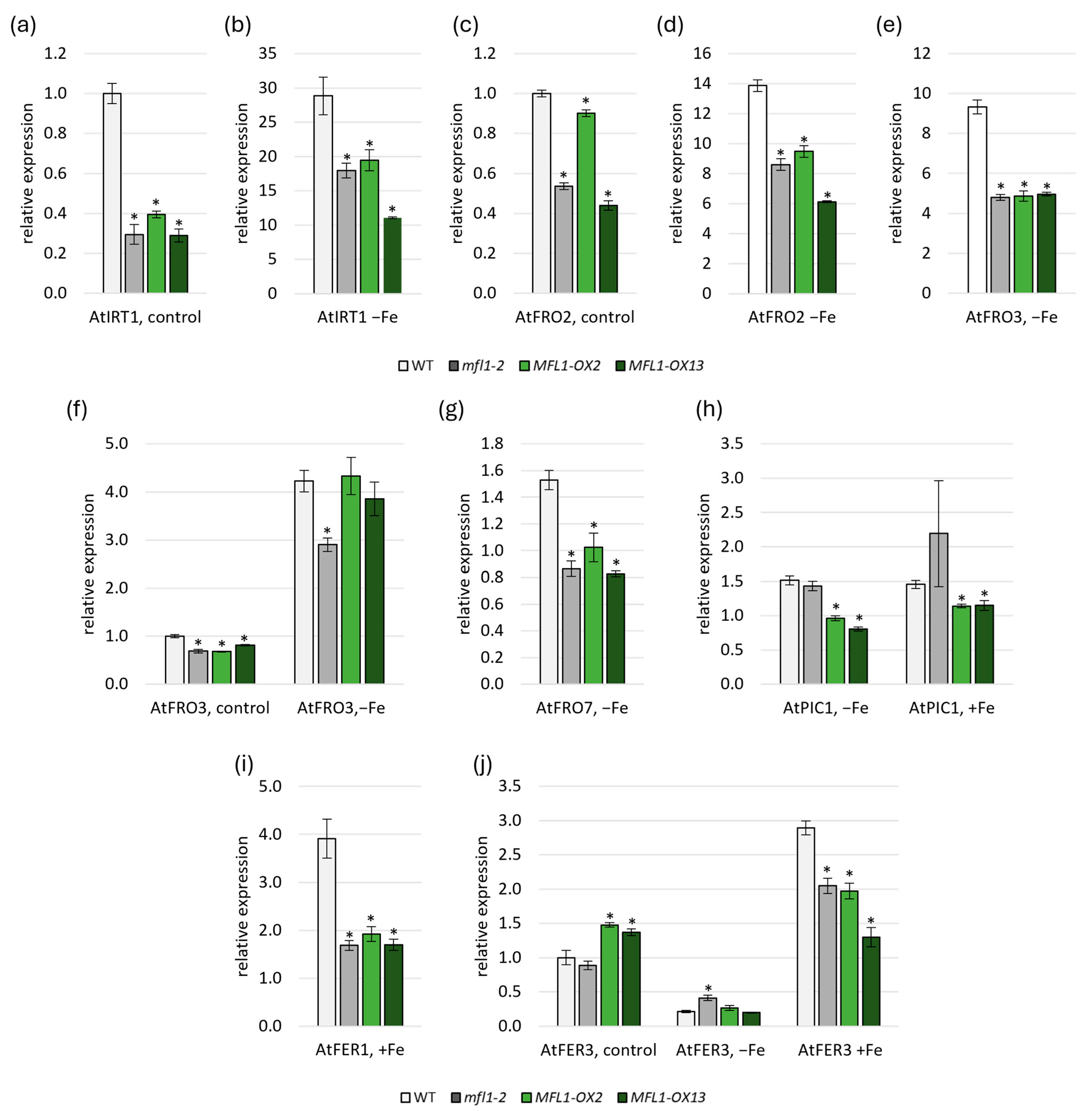
2.5. Effects of AtMFL1 Overexpression on Arabidopsis Plants
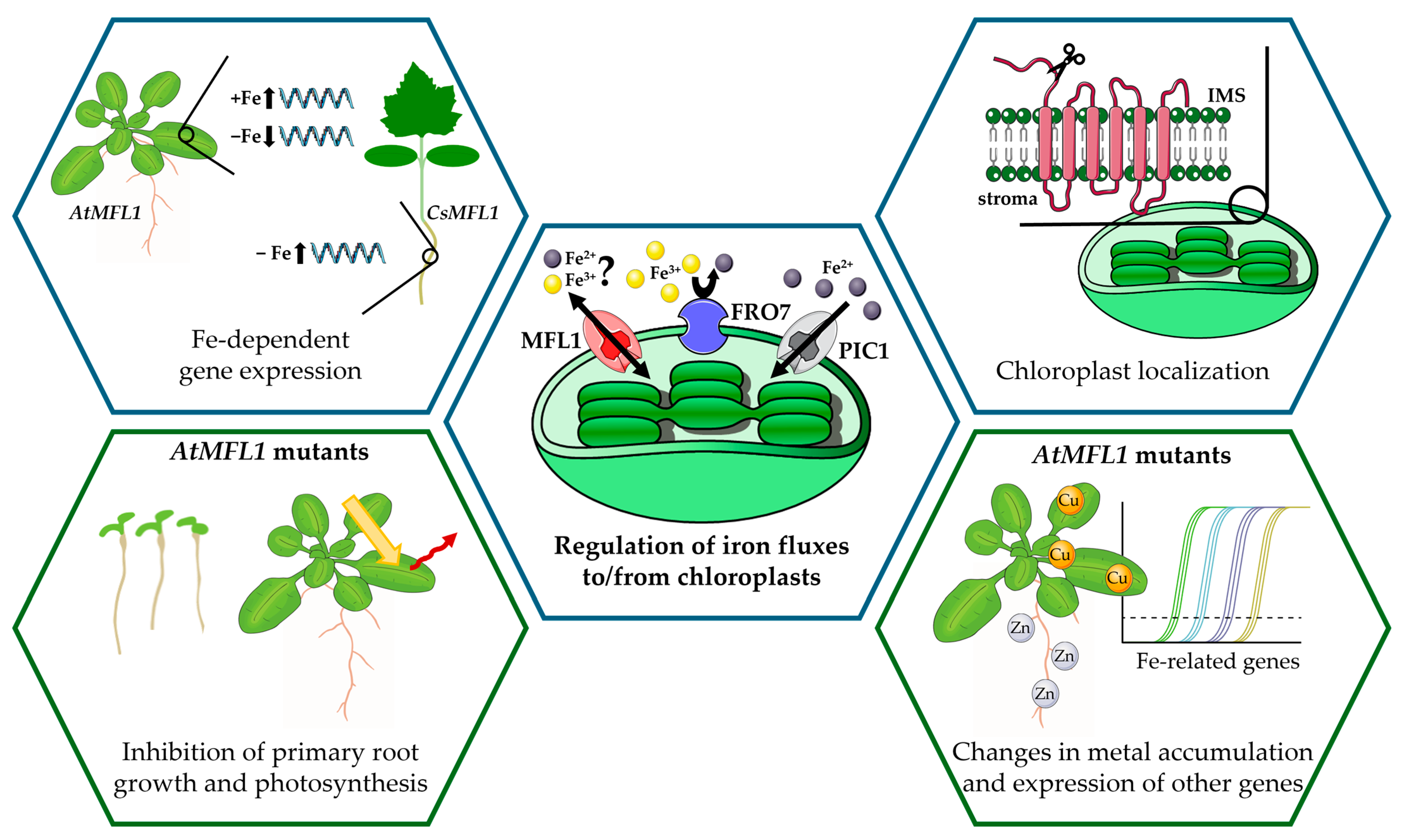
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions
4.2. Arabidopsis Primary Root Measurements
4.3. Complementation Assays and Fluorescent Imaging in Yeast
4.4. Gene Expression Analysis
4.5. Cloning into Expression Vectors
4.6. Protoplast Transformation and Confocal Imaging
4.7. Isolation of Subcellular Fractions from Yeast and Plants
4.8. Protein Determination and Immunoblotting
4.9. Measurements of Photosynthesis Parameters
4.10. Determination of Metal Content in Plants
4.11. Obtaining AtMFL1 Overexpressing Lines
4.12. Bioinformatics and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gould, S.B.; Waller, R.F.; McFadden, G.I. Plastid Evolution. Annu. Rev. Plant Biol. 2008, 59, 491–517. [Google Scholar] [CrossRef] [PubMed]
- Yruela, I. Transition Metals in Plant Photosynthesis. Metallomics 2013, 5, 1090–1109. [Google Scholar] [CrossRef] [PubMed]
- Terry, N.; Low, G. Leaf Chlorophyll Content and Its Relation to the Intracellular Localization of Iron. J. Plant Nutr. 1982, 5, 301–310. [Google Scholar] [CrossRef]
- Moseley, J.L.; Allinger, T.; Herzog, S.; Hoerth, P.; Wehinger, E.; Merchant, S.; Hippler, M. Adaptation to Fe-Deficiency Requires Remodeling of the Photosynthetic Apparatus. EMBO J. 2002, 21, 6709–6720. [Google Scholar] [CrossRef] [PubMed]
- Mubarakshina, M.M.; Ivanov, B.N.; Naydov, I.A.; Hillier, W.; Badger, M.R.; Krieger-Liszkay, A. Production and Diffusion of Chloroplastic H2O2 and Its Implication to Signalling. J. Exp. Bot. 2010, 61, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Trofimov, K.; Mankotia, S.; Ngigi, M.; Baby, D.; Satbhai, S.B.; Bauer, P. Shedding Light on Iron Nutrition: Exploring Intersections of Transcription Factor Cascades in Light and Iron Deficiency Signaling. J. Exp. Bot. 2025, 76, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Solti, Á.; Kovács, K.; Basa, B.; Vértes, A.; Sárvári, É.; Fodor, F. Uptake and Incorporation of Iron in Sugar Beet Chloroplasts. Plant Physiol. Biochem. 2012, 52, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Solti, Á.; Kovács, K.; Müller, B.; Vázquez, S.; Hamar, É.; Pham, H.D.; Tóth, B.; Abadía, J.; Fodor, F. Does a Voltage-Sensitive Outer Envelope Transport Mechanism Contributes to the Chloroplast Iron Uptake? Planta 2016, 244, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Kovács, K.; Pham, H.D.; Kavak, Y.; Pechoušek, J.; Machala, L.; Zbořil, R.; Szenthe, K.; Abadía, J.; Fodor, F.; et al. Chloroplasts Preferentially Take up Ferric–Citrate over Iron–Nicotianamine Complexes in Brassica Napus. Planta 2019, 249, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Duy, D.; Soll, J.; Philippar, K. Solute Channels of the Outer Membrane: From Bacteria to Chloroplasts. Biol. Chem. 2007, 388, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Duy, D.; Wanner, G.; Meda, A.R.; von Wireén, N.; Soll, J.; Philippar, K. PIC1, an Ancient Permease in Arabidopsis Chloroplasts, Mediates Iron Transport. Plant Cell 2007, 19, 986–1006. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Guo, C.; Terachi, T.; Cai, H.; Yu, D. Tobacco PIC1 Mediates Iron Transport and Regulates Chloroplast Development. Plant Mol. Biol. Rep. 2015, 33, 401–413. [Google Scholar] [CrossRef]
- Jeong, J.; Cohu, C.; Kerkeb, L.; Pilon, M.; Connolly, E.L.; Guerinot, M.L. Chloroplast Fe(III) Chelate Reductase Activity Is Essential for Seedling Viability under Iron Limiting Conditions. Proc. Natl. Acad. Sci. USA 2008, 105, 10619–10624. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, D.; Morandini, P.; Ramirez, L.; Soave, C.; Murgia, I. Identification of an Arabidopsis Mitoferrinlike Carrier Protein Involved in Fe Metabolism. Plant Physiol. Biochem. 2011, 49, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Nury, H.; Dahout-Gonzalez, C.; Trézéguet, V.; Lauquin, G.J.M.; Brandolin, G.; Pebay-Peyroula, E. Relations Between Structure and Function of the Mitochondrial ADP/ATP Carrier. Annu. Rev. Biochem. 2006, 75, 713–741. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F.; Pierri, C.L.; De Grassi, A.; Nunes-Nesi, A.; Fernie, A.R. Evolution, Structure and Function of Mitochondrial Carriers: A Review with New Insights. Plant J. 2011, 66, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Haferkamp, I.; Schmitz-Esser, S. The Plant Mitochondrial Carrier Family: Functional and Evolutionary Aspects. Front. Plant Sci. 2012, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Kunji, E.R.S.; Robinson, A.J. The Conserved Substrate Binding Site of Mitochondrial Carriers. BBA Bioenerg. 2006, 1757, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Cavalcanti, J.H.F.; Nunes-Nesi, A. Metabolic Roles of Plant Mitochondrial Carriers. Biomolecules 2020, 10, 1013. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.; Ishimaru, Y.; Shimo, H.; Nagasaka, S.; Fujimoto, M.; Takanashi, H.; Tsutsumi, N.; An, G.; Nakanishi, H.; Nishizawa, N.K. The Rice Mitochondrial Iron Transporter Is Essential for Plant Growth. Nat. Commun. 2011, 2, 322. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Dashner, Z.S.; Connolly, E.L. Mitochondrial Iron Transporters (MIT1 and MIT2) Are Essential for Iron Homeostasis and Embryogenesis in Arabidopsis Thaliana. Front. Plant Sci. 2019, 10, 1449. [Google Scholar] [CrossRef] [PubMed]
- Kurt, F.; Kurt, B.; Filiz, E.; Yildiz, K.; Akbudak, M.A. Mitochondrial Iron Transporter (MIT) Gene in Potato (Solanum tuberosum): Comparative Bioinformatics, Physiological and Expression Analyses in Response to Drought and Salinity. BioMetals 2022, 35, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Małas, K.; Kabała, K. Identification and Functional Analysis of Two Mitoferrins, CsMIT1 and CsMIT2, Participating in Iron Homeostasis in Cucumber. Int. J. Mol. Sci. 2023, 24, 5050. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F.; Rieder, B.; Ventrella, A.; Blanco, E.; Do, P.T.; Nunes-Nesi, A.; Trauth, A.U.; Fiermonte, G.; Tjaden, J.; Agrimi, G.; et al. Molecular Identification and Functional Characterization of Arabidopsis Thaliana Mitochondrial and Chloroplastic NAD+ Carrier Proteins. J. Biol. Chem. 2009, 284, 31249–31259. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, F.; Linka, N.; Isner, J.-C.; Mutterer, J.; Weber, A.P.M.; Camara, B. Arabidopsis SAMT1 Defines a Plastid Transporter Regulating Plastid Biogenesis and Plant Development. Plant Cell 2006, 18, 3088–3105. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, L.; Arrigoni, R.; Blanco, E.; Carrari, F.; Zanor, M.I.; Studart-Guimaraes, C.; Fernie, A.R.; Palmieri, F. Molecular Identification of an Arabidopsis S-Adenosylmethionine Transporter. Analysis of Organ Distribution, Bacterial Expression, Reconstitution into Liposomes, and Functional Characterization. Plant Physiol. 2006, 142, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Bahaji, A.; Muñoz, F.J.; Ovecka, M.; Baroja-Fernández, E.; Montero, M.; Li, J.; Hidalgo, M.; Almagro, G.; Sesma, M.T.; Ezquer, I.; et al. Specific Delivery of AtBT1 to Mitochondria Complements the Aberrant Growth and Sterility Phenotype of Homozygous Atbt1 Arabidopsis Mutants. Plant J. 2011, 68, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Brazzolotto, X.; Pierrel, F.; Pelosi, L. Three Conserved Histidine Residues Contribute to Mitochondrial Iron Transport through Mitoferrins. Biochem. J. 2014, 460, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Mühlenhoff, U.; Stadler, J.A.; Richhardt, N.; Seubert, A.; Eickhorst, T.; Schweyen, R.J.; Lill, R.; Wiesenberger, G. A Specific Role of the Yeast Mitochondrial Carriers Mrs3/4p in Mitochondrial Iron Acquisition under Iron-Limiting Conditions. J. Biol. Chem. 2003, 278, 40612–40620. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, O.S.; Ward, D.M.; Kaplan, J. CCC1 Is a Transporter That Mediates Vacuolar Iron Storage in Yeast. J. Biol. Chem. 2001, 276, 29515–29519. [Google Scholar] [CrossRef] [PubMed]
- Foury, F.; Roganti, T. Deletion of the Mitochondrial Carrier Genes MRS3 And MRS4 Suppresses Mitochondrial Iron Accumulation in a Yeast Frataxin-Deficient Strain. J. Biol. Chem. 2002, 277, 24475–24483. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.C.; Cope, J.J.; Li, L.; Corson, K.; Hersey, C.; Ackermann, G.E.; Gwynn, B.; Lambert, A.J.; Wingert, R.A.; Traver, D.; et al. Mitoferrin Is Essential for Erythroid Iron Assimilation. Nature 2006, 440, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Metzendorf, C.; Lind, M.I. Drosophila Mitoferrinis Essential for Male Fertility: Evidence for a Role of Mitochondrial Iron Metabolism during Spermatogenesis. BMC Dev. Biol. 2010, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Zybailov, B.; Rutschow, H.; Friso, G.; Rudella, A.; Emanuelsson, O.; Sun, Q.; van Wijk, K.J. Sorting Signals, N-Terminal Modifications and Abundance of the Chloroplast Proteome. PLoS ONE 2008, 3, e1994. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zybailov, B.; Majeran, W.; Friso, G.; Olinares, P.D.B.; van Wijk, K.J. PPDB, the Plant Proteomics Database at Cornell. Nucleic Acids Res. 2009, 37, D969–D974. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Oliva, C.R.; Flor, S.; Griguer, C.E. Mitoferrin, Cellular and Mitochondrial Iron Homeostasis. Cells 2022, 11, 3464. [Google Scholar] [CrossRef] [PubMed]
- Bräutigam, A.; Shrestha, R.P.; Whitten, D.; Wilkerson, C.G.; Carr, K.M.; Froehlich, J.E.; Weber, A.P.M. Low-Coverage Massively Parallel Pyrosequencing of CDNAs Enables Proteomics in Non-Model Species: Comparison of a Species-Specific Database Generated by Pyrosequencing with Databases from Related Species for Proteome Analysis of Pea Chloroplast Envelopes. J. Biotechnol. 2008, 136, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Brautigam, A.; Hoffmann-Benning, S.; Weber, A.P.M. Comparative Proteomics of Chloroplast Envelopes from C3 and C4 Plants Reveals Specific Adaptations of the Plastid Envelope to C4 Photosynthesis and Candidate Proteins Required for Maintaining C4 Metabolite Fluxes. Plant Physiol. 2008, 148, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Bräutigam, A.; Weber, A.P.M. Proteomic Analysis of the Proplastid Envelope Membrane Provides Novel Insights into Small Molecule and Protein Transport across Proplastid Membranes. Mol. Plant 2009, 2, 1247–1261. [Google Scholar] [CrossRef] [PubMed]
- Schleiff, E.; Becker, T. Common Ground for Protein Translocation: Access Control for Mitochondria and Chloroplasts. Nat. Rev. Mol. Cell Biol. 2011, 12, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Heidorn-Czarna, M.; Maziak, A.; Janska, H. Protein Processing in Plant Mitochondria Compared to Yeast and Mammals. Front. Plant Sci. 2022, 13, 824080. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Lee, S.; Lee, J.; Woo, S.; Razzak, M.A.; Vitale, A.; Hwang, I. Molecular Mechanism of the Specificity of Protein Import into Chloroplasts and Mitochondria in Plant Cells. Mol. Plant 2019, 12, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.; Vinegar, B.; Nahal, H.; Ammar, R.; Wilson, G.V.; Provart, N.J. An “Electronic Fluorescent Pictograph” Browser for Exploring and Analyzing Large-Scale Biological Data Sets. PLoS ONE 2007, 2, e718. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.J.; Smyth, G.K. Testing Significance Relative to a Fold-Change Threshold Is a TREAT. Bioinformatics 2009, 25, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Hurt, E.C.; Soltanifar, N.; Goldschmidt-Clermont, M.; Rochaix, J.-D.; Schatz, G. The Cleavable Pre-Sequence of an Imported Chloroplast Protein Directs Attached Polypeptides into Yeast Mitochondria. EMBO J. 1986, 5, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Cleary, S.P.; Tan, F.-C.; Nakrieko, K.-A.; Thompson, S.J.; Mullineaux, P.M.; Creissen, G.P.; von Stedingk, E.; Glaser, E.; Smith, A.G.; Robinson, C. Isolated Plant Mitochondria Import Chloroplast Precursor Proteinsin Vitro with the Same Efficiency as Chloroplasts. J. Biol. Chem. 2002, 277, 5562–5569. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, L.; Jia, X.; Ward, D.M.; Kaplan, J. Genetic and Biochemical Analysis of High Iron Toxicity in Yeast. J. Biol. Chem. 2011, 286, 3851–3862. [Google Scholar] [CrossRef] [PubMed]
- Froschauer, E.M.; Rietzschel, N.; Hassler, M.R.; Binder, M.; Schweyen, R.J.; Lill, R.; Mühlenhoff, U.; Wiesenberger, G. The Mitochondrial Carrier Rim2 Co-Imports Pyrimidine Nucleotides and Iron. Biochem. J. 2013, 455, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Qian, J. The Function of Transport Protein Mfl1 in Arabidopsis Thaliana. Master’s Thesis, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA, 2023. [Google Scholar]
- Grotz, N.; Guerinot, M.L. Molecular Aspects of Cu, Fe and Zn Homeostasis in Plants. Biochim. Biophys. Acta Mol. Cell Res. 2006, 1763, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Duy, D.; Stübe, R.; Wanner, G.; Philippar, K. The Chloroplast Permease PIC1 Regulates Plant Growth and Development by Directing Homeostasis and Transport of Iron. Plant Physiol. 2011, 155, 1709–1722. [Google Scholar] [CrossRef] [PubMed]
- Briat, J.-F. Metal Lon-Activated Oxidative Stress and Its Control. In Oxidative Stress in Plants; CRC Press: Boca Raton, FL, USA, 2001; pp. 1–24. ISBN 9780429219580. [Google Scholar]
- Shomali, A.; Das, S.; Sarraf, M.; Johnson, R.; Janeeshma, E.; Kumar, V.; Aliniaeifard, S.; Puthur, J.T.; Hasanuzzaman, M. Modulation of Plant Photosynthetic Processes during Metal and Metalloid Stress, and Strategies for Manipulating Photosynthesis-Related Traits. Plant Physiol. Biochem. 2024, 206, 108211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, G.; Wang, M.; Di, D.; Sun, L.; Kronzucker, H.J.; Shi, W. Excess Iron Stress Reduces Root Tip Zone Growth through Nitric Oxide-Mediated Repression of Potassium Homeostasis in Arabidopsis. New Phytol. 2018, 219, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.L.; Fett, J.P.; Guerinot, M.L. Expression of the IRT1 Metal Transporter Is Controlled by Metals at the Levels of Transcript and Protein Accumulation. Plant Cell 2002, 14, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.L.; Campbell, N.H.; Grotz, N.; Prichard, C.L.; Guerinot, M.L. Overexpression of the FRO2 Ferric Chelate Reductase Confers Tolerance to Growth on Low Iron and Uncovers Posttranscriptional Control. Plant Physiol. 2003, 133, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Vert, G.; Grotz, N.; Dédaldéchamp, F.; Gaymard, F.; Guerinot, M.L.; Briat, J.-F.; Curie, C. IRT1, an Arabidopsis Transporter Essential for Iron Uptake from the Soil and for Plant Growth. Plant Cell 2002, 14, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, I.; Campbell, N.H.; Ash, J.S.; Connolly, E.L. Expression Profiling of the Arabidopsis Ferric Chelate Reductase (FRO) Gene Family Reveals Differential Regulation by Iron and Copper. Planta 2006, 223, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Connolly, E.L. Iron Uptake Mechanisms in Plants: Functions of the FRO Family of Ferric Reductases. Plant Sci. 2009, 176, 709–714. [Google Scholar] [CrossRef]
- Kim, L.J.; Tsuyuki, K.M.; Hu, F.; Park, E.Y.; Zhang, J.; Iraheta, J.G.; Chia, J.-C.; Huang, R.; Tucker, A.E.; Clyne, M.; et al. Ferroportin 3 Is a Dual-Targeted Mitochondrial/Chloroplast Iron Exporter Necessary for Iron Homeostasis in Arabidopsis. Plant J. 2021, 107, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Ravet, K.; Touraine, B.; Boucherez, J.; Briat, J.-F.; Gaymard, F.; Cellier, F. Ferritins Control Interaction between Iron Homeostasis and Oxidative Stress in Arabidopsis. Plant J. 2009, 57, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, B.; Bauer, P. FIT, a Regulatory Hub for Iron Deficiency and Stress Signaling in Roots, and FIT-Dependent and -Independent Gene Signatures. J. Exp. Bot. 2020, 71, 1694–1705. [Google Scholar] [CrossRef] [PubMed]
- Petit, J.M.; Briat, J.F.; Lobréaux, S. Structure and Differential Expression of the Four Members of the Arabidopsis Thaliana Ferritin Gene Family. Biochem. J. 2001, 359, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Vert, G.A.; Briat, J.-F.; Curie, C. Dual Regulation of the Arabidopsis High-Affinity Root Iron Uptake System by Local and Long-Distance Signals. Plant Physiol. 2003, 132, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Barberon, M.; Dubeaux, G.; Kolb, C.; Isono, E.; Zelazny, E.; Vert, G. Polarization of IRON-REGULATED TRANSPORTER 1 (IRT1) to the Plant-Soil Interface Plays Crucial Role in Metal Homeostasis. Proc. Natl. Acad. Sci. USA 2014, 111, 8293–8298. [Google Scholar] [CrossRef] [PubMed]
- Dubeaux, G.; Neveu, J.; Zelazny, E.; Vert, G. Metal Sensing by the IRT1 Transporter-Receptor Orchestrates Its Own Degradation and Plant Metal Nutrition. Mol. Cell 2018, 69, 953–964.e5. [Google Scholar] [CrossRef] [PubMed]
- Gayomba, S.R.; Zhai, Z.; Jung, H.; Vatamaniuk, O.K. Local and Systemic Signaling of Iron Status and Its Interactions with Homeostasis of Other Essential Elements. Front. Plant Sci. 2015, 14, 716. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.; Casero, D.; Singh, V.; Wilson, G.T.; Grande, A.; Yang, H.; Dodani, S.C.; Pellegrini, M.; Huijser, P.; Connolly, E.L.; et al. Transcriptome Sequencing Identifies SPL7-Regulated Copper Acquisition Genes FRO4/FRO5 and the Copper Dependence of Iron Homeostasis in Arabidopsis. Plant Cell 2012, 24, 738–761. [Google Scholar] [CrossRef] [PubMed]
- Araki, R.; Mermod, M.; Yamasaki, H.; Kamiya, T.; Fujiwara, T.; Shikanai, T. SPL7 Locally Regulates Copper-Homeostasis-Related Genes in Arabidopsis. J. Plant Physiol. 2018, 224–225, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ping, H.; Zhao, J.; Li, C.; Li, Y.; Liang, G. IRON MAN Interacts with Cu-DEFICIENCY INDUCED TRANSCRIPTION FACTOR 1 to Maintain Copper Homeostasis. New Phytol. 2024, 242, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Kastoori Ramamurthy, R.; Xiang, Q.; Hsieh, E.-J.; Liu, K.; Zhang, C.; Waters, B.M. New Aspects of Iron–Copper Crosstalk Uncovered by Transcriptomic Characterization of Col-0 and the Copper Uptake Mutant Spl7 in Arabidopsis Thaliana. Metallomics 2018, 10, 1824–1840. [Google Scholar] [CrossRef] [PubMed]
- Wairich, A.; Lima-Melo, Y.; Menguer, P.K.; Ortolan, F.; Ricachenevsky, F.K. Iron, Cold Iron, Is Master of Them All: Iron Crosstalk with Zinc, Copper, Phosphorus, and Nitrogen Homeostasis. J. Exp. Bot. 2025, eraf106. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, R.A.; Menguer, P.K.; Ricachenevsky, F.K. Chapter 2—Molecular Bases of Iron Accumulation Towards the Development of Iron-Enriched Crops. In Plant Micronutrient Use Efficiency; Hossain, M.A., Kamiya, T., Burritt, D.J., Phan Tran, L.-S., Fujiwara, T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 17–54. ISBN 978-0-12-812104-7. [Google Scholar]
- Migocka, M.; Małas, K.; Maciaszczyk-Dziubinska, E.; Posyniak, E.; Migdal, I.; Szczech, P. Cucumber Golgi Protein CsMTP5 Forms a Zn-Transporting Heterodimer with High Molecular Mass Protein CsMTP12. Plant Sci. 2018, 277, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Morel, M.; Crouzet, J.; Gravot, A.; Auroy, P.; Leonhardt, N.; Vavasseur, A.; Richaud, P. AtHMA3, a P1B-ATPase Allowing Cd/Zn/Co/Pb Vacuolar Storage in Arabidopsis. Plant Physiol. 2009, 149, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Gollhofer, J.; Timofeev, R.; Lan, P.; Schmidt, W.; Buckhout, T.J. Vacuolar-Iron-Transporter1-Like Proteins Mediate Iron Homeostasis in Arabidopsis. PLoS ONE 2014, 9, e110468. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kaplan, J. A Mitochondrial-Vacuolar Signaling Pathway in Yeast That Affects Iron and Copper Metabolism. J. Biol. Chem. 2004, 279, 33653–33661. [Google Scholar] [CrossRef] [PubMed]
- Baker Brachmann, C.; Davies, A.; Cost, G.J.; Caputo, E.; Li, J.; Hieter, P.; Boeke, J.D. Designer Deletion Strains Derived from Saccharomyces Cerevisiae S288C: A Useful Set of Strains and Plasmids for PCR-Mediated Gene Disruption and Other Applications. Yeast 1998, 14, 115–132. [Google Scholar] [CrossRef]
- Niedenthal, R.K.; Riles, L.; Johnston, M.; Hegemann, J.H. Green Fluorescent Protein as a Marker for Gene Expression and Subcellular Localization in Budding Yeast. Yeast 1996, 12, 773–786. [Google Scholar] [CrossRef]
- Han, B.; Yang, Z.; Samma, M.K.; Wang, R.; Shen, W. Systematic Validation of Candidate Reference Genes for QRT-PCR Normalization under Iron Deficiency in Arabidopsis. BioMetals 2013, 26, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Czechowski, T.; Stitt, M.; Altmann, T.; Udvardi, M.K.; Scheible, W.-R. Genome-Wide Identification and Testing of Superior Reference Genes for Transcript Normalization in Arabidopsis. Plant Physiol. 2005, 139, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Migocka, M.; Papierniak, A. Identification of Suitable Reference Genes for Studying Gene Expression in Cucumber Plants Subjected to Abiotic Stress and Growth Regulators. Mol. Breed. 2011, 28, 343–357. [Google Scholar] [CrossRef]
- Voelker, C.; Schmidt, D.; Mueller-Roeber, B.; Czempinski, K. Members of the Arabidopsis AtTPK/KCO Family Form Homomeric Vacuolar Channels in Planta. Plant J. 2006, 48, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.D.; Grossniklaus, U. A Gateway Cloning Vector Set for High-Throughput Functional Analysis of Genes in Planta. Plant Physiol. 2003, 133, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Thomine, S.; Lelièvre, F.; Debarbieux, E.; Schroeder, J.I.; Barbier-Brygoo, H. AtNRAMP3, a Multispecific Vacuolar Metal Transporter Involved in Plant Responses to Iron Deficiency. Plant J. 2003, 34, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Gregg, C.; Kyryakov, P.; Titorenko, V.I. Purification of Mitochondria from Yeast Cells. J. Vis. Exp. 2009, 30, e1417. [Google Scholar] [CrossRef] [PubMed]
- Besagni, C.; Piller, L.E.; Bréhélin, C. Preparation of Plastoglobules from Arabidopsis Plastids for Proteomic Analysis and Other Studies. In Chloroplast Research in Arabidopsis: Methods and Protocols, Volume II; Jarvis, R.P., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 223–239. ISBN 978-1-61779-237-3. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Burzyński, M.; Żurek, A. Effects of Copper and Cadmium on Photosynthesis in Cucumber Cotyledons. Photosynthetica 2007, 45, 239–244. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral Dip: A Simplified Method for -Mediated Transformation Of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.J.; Mott, E.K.; Parsley, K.; Aspinall, S.; Gray, J.C.; Cottage, A. A Rapid and Robust Method of Identifying Transformed Arabidopsis Thaliana Seedlings Following Floral Dip Transformation. Plant Methods 2006, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Dobson, L.; Reményi, I.; Tusnády, G.E. CCTOP: A Consensus Constrained TOPology Prediction Web Server. Nucleic Acids Res. 2015, 43, W408–W412. [Google Scholar] [CrossRef] [PubMed]
- Omasits, U.; Ahrens, C.H.; Müller, S.; Wollscheid, B. Protter: Interactive Protein Feature Visualization and Integration with Experimental Proteomic Data. Bioinformatics 2014, 30, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Emanuelsson, O.; Nielsen, H.; Von Heijne, G. ChloroP, a Neural Network-Based Method for Predicting Chloroplast Transit Peptides and Their Cleavage Sites. Protein Sci. 1999, 8, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, H.; Ai, Q.; Liang, G.; Yu, D. Two BHLH Transcription Factors, BHLH34 and BHLH104, Regulate Iron Homeostasis in Arabidopsis Thaliana. Plant Physiol. 2016, 170, 2478–2493. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; An, F.; Zhang, S.; Ji, Z.; Ling, H.-Q.; Zuo, J. Light-Regulated, Tissue-Specific, and Cell Differentiation-Specific Expression of the Arabidopsis Fe(III)-Chelate Reductase Gene AtFRO6. Plant Physiol. 2006, 140, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Duc, C.; Cellier, F.; Lobréaux, S.; Briat, J.-F.; Gaymard, F. Regulation of Iron Homeostasis in Arabidopsis Thaliana by the Clock Regulator Time for Coffee. J. Biol. Chem. 2009, 284, 36271–36281. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małas, K.; Polechońska, L.; Kabała, K. What Is Similar, What Is Different? Characterization of Mitoferrin-like Proteins from Arabidopsis thaliana and Cucumis sativus. Int. J. Mol. Sci. 2025, 26, 7103. https://doi.org/10.3390/ijms26157103
Małas K, Polechońska L, Kabała K. What Is Similar, What Is Different? Characterization of Mitoferrin-like Proteins from Arabidopsis thaliana and Cucumis sativus. International Journal of Molecular Sciences. 2025; 26(15):7103. https://doi.org/10.3390/ijms26157103
Chicago/Turabian StyleMałas, Karolina, Ludmiła Polechońska, and Katarzyna Kabała. 2025. "What Is Similar, What Is Different? Characterization of Mitoferrin-like Proteins from Arabidopsis thaliana and Cucumis sativus" International Journal of Molecular Sciences 26, no. 15: 7103. https://doi.org/10.3390/ijms26157103
APA StyleMałas, K., Polechońska, L., & Kabała, K. (2025). What Is Similar, What Is Different? Characterization of Mitoferrin-like Proteins from Arabidopsis thaliana and Cucumis sativus. International Journal of Molecular Sciences, 26(15), 7103. https://doi.org/10.3390/ijms26157103








