Neuroplasticity-Based Approaches to Sensory Processing Alterations in Autism Spectrum Disorder
Abstract
1. Introduction
2. Materials and Methods
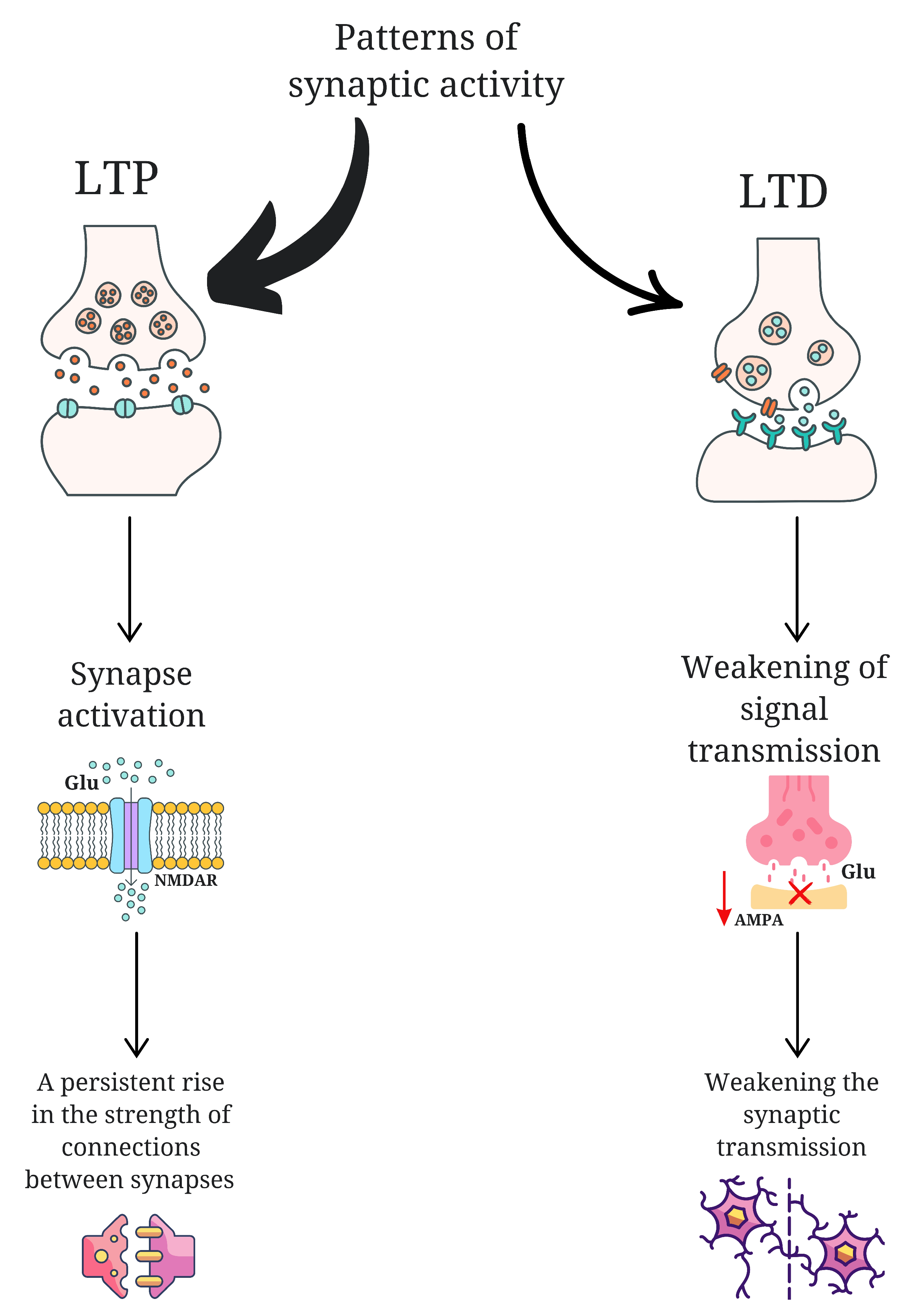
3. Neuroplasticity in ASD
4. Sensory Disturbances and Changes in Brain Anatomy in ASD
4.1. Disturbances in Auditory Stimulus Processing
4.2. Hypersensitivity to Light
4.3. Impaired Processing of Tactile Stimuli
4.4. Incorrect Texture Processing
4.5. Inadequate Pain Response
5. Conventional Sensory Therapies Based on Neuroplasticity
5.1. Ayers Sensory Integration Therapy
5.2. Sensory Integration Therapy
5.3. Snoezelen Therapy
5.4. Animal-Assisted Intervention
5.5. Music Therapy
5.6. Parental Involvement in Therapeutic Interventions for ASD
6. Modern Sensory Integration Therapies
6.1. Virtual Reality/Augmented Reality Technologies
6.2. Cannabinoids
6.3. Bioneurofeedback
6.4. Brain Stimulation Techniques
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lai, M.C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018. MMWR Surveill. Summ. 2021, 70, 1. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Butler, M.G. The Autism Spectrum: Behavioral, Psychiatric and Genetic Associations. Genes 2023, 14, 677. [Google Scholar] [CrossRef] [PubMed]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Hirota, T.; King, B.H. Autism Spectrum Disorder: A Review. JAMA 2023, 329, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, B.; Wu, C.; Wang, J.; Sun, M. Autism Spectrum Disorder: Neurodevelopmental Risk Factors, Biological Mechanism, and Precision Therapy. Int. J. Mol. Sci. 2023, 24, 1819. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, I.; Hatem, S.M.; Montoya, P. Abnormal Pressure Pain, Touch Sensitivity, Proprioception, and Manual Dexterity in Children with Autism Spectrum Disorders. Neural Plast. 2016, 2016, 1723401. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Nakai, N.; Fujima, S.; Choe, K.Y.; Takumi, T. Social circuits and their dysfunction in autism spectrum disorder. Mol. Psychiatry 2023, 28, 3194–3206. [Google Scholar] [CrossRef] [PubMed]
- Pardo, C.A.; Eberhart, C.G. The Neurobiology of Autism. Brain Pathol. 2007, 17, 434. [Google Scholar] [CrossRef] [PubMed]
- Gulyaeva, N.V. Molecular Mechanisms of Neuroplasticity: An Expanding Universe. Biochemistry 2017, 82, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.V.; Ishida, A.; Ishida, W.N.; Matsushita, H.B.; Nishimura, A.; Tsuji, M. Plasticity and injury in the developing brain. Brain Dev. 2009, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.; Spiegler, B.J.; Juranek, J.J.; Bigler, E.D.; Snead, O.C.; Fletcher, J.M. Age, plasticity, and homeostasis in childhood brain disorders. Neurosci. Biobehav. Rev. 2013, 37 Pt 2, 2760–2773. [Google Scholar] [CrossRef] [PubMed]
- Brandes-Aitken, A.; Powers, R.; Wren, J.; Chu, R.; Shapiro, K.A.; Steele, M.; Mukherjee, P.; Marco, E.J. Sensory processing subtypes relate to distinct emotional and behavioral phenotypes in a mixed neurodevelopmental cohort. Sci. Rep. 2024, 14, 29326. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.; Raza, H.; Zaidi, J.; Riaz, S.; Hasan, S.; Syed, N.I. Synapse formation: From cellular and molecular mechanisms to neurodevelopmental and neurodegenerative disorders. J. Neurophysiol. 2019, 121, 1381–1397. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.Y.; Fatemi, A.; Johnston, M.V. Cerebral plasticity: Windows of opportunity in the developing brain. Eur. J. Paediatr. Neurol. 2017, 21, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.J.; Schaaf, R.C. Examining the Neuroscience Evidence for Sensory-Driven Neuroplasticity: Implications for Sensory-Based Occupational Therapy for Children and Adolescents. Am. J. Occup. Ther. 2010, 64, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, J.J.; Fagiolini, M. Autism: A “critical period” disorder? Neural Plast. 2011, 2011, 921680. [Google Scholar] [CrossRef] [PubMed]
- Sears, S.M.S.; Hewett, S.J. Influence of glutamate and GABA transport on brain excitatory/inhibitory balance. Exp. Biol. Med. 2021, 246, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, X.; Zhang, S.; Han, F. Neuroplasticity of children in autism spectrum disorder. Front. Psychiatry 2024, 15, 1362288. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhu, T.; Qu, Y.; Mu, D. Blood Glutamate Levels in Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158688. [Google Scholar] [CrossRef] [PubMed]
- Monday, H.R.; Younts, T.J.; Castillo, P.E. Long-Term Plasticity of Neurotransmitter Release: Emerging Mechanisms and Contributions to Brain Function and Disease. Annu. Rev. Neurosci. 2018, 41, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Alkadhi, K.A. NMDA Receptor-Independent LTP in Mammalian Nervous System. Prog. Neurobiol. 2021, 200, 101986. [Google Scholar] [CrossRef] [PubMed]
- Lüscher, C.; Huber, K.M. Group 1 mGluR-dependent synaptic long-term depression (mGluR-LTD): Mechanisms and implications for circuitry & disease. Neuron 2010, 65, 445. [Google Scholar] [CrossRef] [PubMed]
- Kossut, M. Basic mechanism of neuroplasticity. Neuropsychiatr. I Neuropsychol./Neuropsychiatry Neuropsychol. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Nelson, S.B.; Valakh, V. Excitatory/Inhibitory Balance and Circuit Homeostasis in Autism Spectrum Disorders. Neuron 2015, 87, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Mullins, C.; Fishell, G.; Tsien, R.W. Unifying Views of Autism Spectrum Disorders: A Consideration of Autoregulatory Feedback Loops. Neuron 2016, 89, 1131–1156. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, M.; Piccinin, S.; Molinaro, G.; Di Menna, L.; Riozzi, B.; Cannella, M.; Motolese, M.; Vetere, G.; Catania, M.V.; Battaglia, G.; et al. Changes in mGlu5 receptor-dependent synaptic plasticity and coupling to homer proteins in the hippocampus of Ube3A hemizygous mice modeling angelman syndrome. J. Neurosci. 2014, 34, 4558–4566. [Google Scholar] [CrossRef] [PubMed]
- Huguet, G.; Ey, E.; Bourgeron, T. The genetic landscapes of autism spectrum disorders. Annu. Rev. Genomics Hum. Genet. 2013, 14, 191–213. [Google Scholar] [CrossRef] [PubMed]
- Winden, K.D.; Ebrahimi-Fakhari, D.; Sahin, M. Abnormal mTOR Activation in Autism. Annu. Rev. Neurosci. 2018, 41, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Gudsnuk, K.; Kuo, S.H.; Cotrina, M.L.; Rosoklija, G.; Sosunov, A.; Sonders, M.S.; Kanter, E.; Castagna, C.; Yamamoto, A.; et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 2014, 83, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Durand, C.M.; Perroy, J.; Loll, F.; Perrais, D.; Fagni, L.; Bourgeron, T.; Montcouquiol, M.; Sans, N. SHANK3 mutations identified in autism lead to modification of dendritic spine morphology via an actin-dependent mechanism. Mol. Psychiatry 2012, 17, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Leblond, C.S.; Nava, C.; Polge, A.; Gauthier, J.; Huguet, G.; Lumbroso, S.; Giuliano, F.; Stordeur, C.; Depienne, C.; Mouzat, K.; et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: A gradient of severity in cognitive impairments. PLoS Genet. 2014, 10, e100458. [Google Scholar] [CrossRef] [PubMed]
- Caño, C.H.-D.; Varela-Andrés, N.; Cebrián-León, A.; Deogracias, R. Neurotrophins and Their Receptors: BDNF’s Role in GABAergic Neurodevelopment and Disease. Int. J. Mol. Sci. 2024, 25, 8312. [Google Scholar] [CrossRef] [PubMed]
- Robinson-Agramonte, M.D.L.A.; Michalski, B.; Vidal-Martinez, B.; Hernández, L.R.; Santiesteban, M.W.; Fahnestock, M. BDNF, proBDNF and IGF-1 serum levels in naïve and medicated subjects with autism. Sci Rep 2022, 12, 13768. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; You, H.; Mizui, T.; Ishikawa, Y.; Takao, K.; Miyakawa, T.; Li, X.; Bai, T.; Xia, K.; Zhang, L.; et al. Inhibiting proBDNF to mature BDNF conversion leads to ASD-like phenotypes in vivo. Mol. Psychiatry 2024, 29, 3462–3474. [Google Scholar] [CrossRef] [PubMed]
- Kalani, L.; Kim, B.H.; Vincent, J.B.; Ausió, J. MeCP2 ubiquitination and sumoylation, in search of a function. Hum. Mol. Genet. 2024, 33, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Cheng, T.-L.; Li, G.; Sun, S.-B.; Yu, S.-Y.; Zhang, Y.; Du, Y.-S.; Qiu, Z. Identification of Autism-Related MECP2 Mutations by Whole-Exome Sequencing and Functional Validation. Mol. Autism 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Lau, B.Y.B.; Ewall, G.; Huang, Z.J.; Shea, S.D. MECP2 Regulates Cortical Plasticity Underlying a Learned Behaviour in Adult Female Mice. Nat. Commun. 2017, 8, 14077. [Google Scholar] [CrossRef] [PubMed]
- Kádková, A.; Radecke, J.; Sørensen, J.B. The SNAP-25 Protein Family. Neuroscience 2019, 420, 50–71. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, E.; Guerini, F.R.; Carta, A.; Chiappedi, M.; Sotgiu, S.; Mensi, M.M.; Agliardi, C.; Zanzottera, M.; Clerici, M. The Role of SNAP-25 in Autism Spectrum Disorders Onset Patterns. IJMS 2023, 24, 14042. [Google Scholar] [CrossRef] [PubMed]
- Corradini, I.; Verderio, C.; Sala, M.; Wilson, M.C.; Matteoli, M. SNAP-25 in Neuropsychiatric Disorders. Ann. N. Y. Acad. Sci. 2009, 1152, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Rotschafer, S.E. Auditory Discrimination in Autism Spectrum Disorder. Front. Neurosci. 2021, 15, 651209. [Google Scholar] [CrossRef]
- Kirby, A.V.; Dickie, V.A.; Baranek, G.T. Sensory experiences of children with autism spectrum disorder: In their own words. Autism 2014, 19, 316. [Google Scholar] [CrossRef] [PubMed]
- Lucker, J.R.; Doman, A. Neural Mechanisms Involved in Hypersensitive Hearing: Helping Children with ASD Who Are Overly Sensitive to Sounds. Autism Res. Treat. 2015, 2015, 369035. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Porges, S.W.; Lewis, G.F. The polyvagal hypothesis: Common mechanisms mediating autonomic regulation, vocalizations and listening. Handb. Behav. Neurosci. 2010, 109, 255–264. [Google Scholar] [CrossRef]
- Xie, F.; Pascual, E.; Oakley, T. Functional echolalia in autism speech: Verbal formulae and repeated prior utterances as communicative and cognitive strategies. Front. Psychol. 2023, 14, 1010615. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.; Green, S.A.; Lawrence, K.E.; Inada, M.; Liu, J.; Bookheimer, S.Y.; Dapretto, M. Social Attention in Autism: Neural Sensitivity to Speech Over Background Noise Predicts Encoding of Social Information. Front. Psychiatry 2020, 11, 517323. [Google Scholar] [CrossRef] [PubMed]
- Pérez, P.S.; Nordahl-Hansen, A.; Kaale, A. The Role of Context in Language Development for Children With Autism Spectrum Disorder. Front. Psychol. 2020, 11, 563925. [Google Scholar] [CrossRef] [PubMed]
- Thye, M.D.; Bednarz, H.M.; Herringshaw, A.J.; Sartin, E.B.; Kana, R.K. The impact of atypical sensory processing on social impairments in autism spectrum disorder. Dev. Cogn. Neurosci. 2017, 29, 151. [Google Scholar] [CrossRef] [PubMed]
- Just, M.A.; Cherkassky, V.L.; Keller, T.A.; Minshew, N.J. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain 2004, 127 Pt 8, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Jamal, W.; Cardinaux, A.; Haskins, A.J.; Kjelgaard, M.; Sinha, P. Reduced Sensory Habituation in Autism and Its Correlation with Behavioral Measures. J. Autism Dev. Disord. 2021, 51, 3153–3164. [Google Scholar] [CrossRef] [PubMed]
- Kosslyn, S.M.; Thompson, W.L.; Klm, I.J.; Alpert, N.M. Topographical representations of mental images in primary visual cortex. Nature 1995, 378, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Damarla, S.R.; Keller, T.A.; Kana, R.K.; Cherkassky, V.L.; Williams, D.L.; Minshew, N.J.; Just, M.A. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: Evidence from an fMRI study of an embedded figures task. Autism Res. 2010, 3, 273. [Google Scholar] [CrossRef] [PubMed]
- Manjaly, Z.M.; Bruning, N.; Neufang, S.; Stephan, K.E.; Brieber, S.; Marshall, J.C.; Kamp-Becker, I.; Remschmidt, H.; Herpertz-Dahlmann, B.; Konrad, K.; et al. Neurophysiological correlates of relatively enhanced local visual search in autistic adolescents. Neuroimage 2007, 35, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Grandin, T. An Inside View of Autism. In High-Functioning Individuals with Autism; Springer Nature: Berlin/Heidelberg, Germany, 1992; pp. 105–126. [Google Scholar] [CrossRef]
- Baranek, G.T.; Foster, L.G.; Berkson, G. Tactile defensiveness and stereotyped behaviors. Am. J. Occup. Ther. 1997, 51, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Elwin, M.; Ek, L.; Schröder, A.; Kjellin, L. Autobiographical accounts of sensing in Asperger syndrome and high-functioning autism. Arch. Psychiatr. Nurs. 2012, 26, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Sacrey, L.A.R.; Germani, T.; Bryson, S.E.; Zwaigenbaum, L. Reaching and grasping in autism spectrum disorder: A review of recent literature. Front. Neurol. 2014, 5, 65872. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Mao, X.; Zhu, C.; Zou, X.; Peng, F.; Yang, W.; Li, B.; Li, G.; Ge, T.; Cui, R. GABAergic System Dysfunction in Autism Spectrum Disorders. Front. Cell Dev. Biol. 2022, 9, 781327. [Google Scholar] [CrossRef] [PubMed]
- Balasco, L.; Provenzano, G.; Bozzi, Y. Sensory Abnormalities in Autism Spectrum Disorders: A Focus on the Tactile Domain, From Genetic Mouse Models to the Clinic. Front. Psychiatry 2020, 10, 464344. [Google Scholar] [CrossRef] [PubMed]
- Daniels, N.; Moerkerke, M.; Steyaert, J.; Bamps, A.; Debbaut, E.; Prinsen, J.; Tang, T.; Van der Donck, S.; Boets, B.; Alaerts, K. Effects of multiple-dose intranasal oxytocin administration on social responsiveness in children with autism: A randomized, placebo-controlled trial. Mol. Autism 2023, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Schreck, K.A.; Williams, K. Food preferences and factors influencing food selectivity for children with autism spectrum disorders. Res. Dev. Disabil. 2006, 27, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Choi, S.J.; Kim, Y.; Cho, M.S.; Kim, Y.R.; Oh, J.E. Mealtime Behaviors and Food Preferences of Students with Autism Spectrum Disorder. Foods 2020, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Foss-Feig, J.H.; Heacock, J.L.; Cascio, C.J. Tactile Responsiveness Patterns And Their Association With Core Features In Autism Spectrum Disorders. Res. Autism Spectr. Disord. 2012, 6, 337. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.E.; Ratai, E.M.; Kanwisher, N. Reduced GABAergic Action in the Autistic Brain. Curr. Biol. 2016, 26, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, O.V.; Bogdanov, V.B.; Pizano, A.; Bouvard, M.; Cazalets, J.R.; Mellen, N.; Amestoy, A. The Current View on the Paradox of Pain in Autism Spectrum Disorders. Front. Psychiatry 2022, 13, 910824. [Google Scholar] [CrossRef] [PubMed]
- Allely, C.S. Pain sensitivity and observer perception of pain in individuals with autistic spectrum disorder. Sci. World J. 2013, 2013, 916178. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X. A revisit of the amygdala theory of autism: Twenty years after. Neuropsychologia 2023, 183, 108519. [Google Scholar] [CrossRef] [PubMed]
- Nomi, J.S.; Molnar-Szakacs, I.; Uddin, L.Q. Insular function in autism: Update and future directions in neuroimaging and interventions. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.A.; Jordan, C.J. Dopamine, behavior, and addiction. J. Biomed. Sci. 2021, 28, 83. [Google Scholar] [CrossRef] [PubMed]
- Eden, K.E.; De Vries, P.J.; Moss, J.; Richards, C.; Oliver, C. Self-injury and aggression in tuberous sclerosis complex: Cross syndrome comparison and associated risk markers. J. Neurodev. Disord. 2014, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.J.; Mailloux, Z.; Schoen, S.; Bundy, A.; May-Benson, T.A.; Parham, L.D.; Smith Roley, S.; Schaaf, R.C. Neural Foundations of Ayres Sensory Integration®. Brain Sci. 2019, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.A.; Greenough, W.T. Experience-driven brain plasticity: Beyond the synapse. Neuron Glia Biol. 2004, 1, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.; Lane, S.J.; Richards, L. Using animal models of enriched environments to inform research on sensory integration intervention for the rehabilitation of neurodevelopmental disorders. J. Neurodev. Disord. 2010, 2, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Parham, L.D.; Roley, S.S.; May-Benson, T.A.; Koomar, J.; Brett-Green, B.; Burke, J.P.; Cohn, E.S.; Mailloux, Z.; Miller, L.J.; Schaaf, R.C. Development of a fidelity measure for research on the effectiveness of the Ayres Sensory Integration intervention. Am. J. Occup. Ther. 2011, 65, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, B.A.; Koenig, K.; Kinnealey, M.; Sheppard, M.; Henderson, L. Effectiveness of sensory integration interventions in children with autism spectrum disorders: A pilot study. Am. J. Occup. Ther. 2011, 65, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Raditha, C.; Handryastuti, S.; Pusponegoro, H.D.; Mangunatmadja, I. Positive behavioral effect of sensory integration intervention in young children with autism spectrum disorder. Pediatr. Res. 2023, 93, 1667–1671. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Jang, J.S.; Jeon, A.R.; Kim, G.; Kwon, M.; Cho, B.; Lee, N. Effectiveness of sensory integration therapy in children, focusing on Korean children: A systematic review and meta-analysis. World J. Clin. Cases 2024, 12, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Barton, E.E.; Reichow, B.; Schnitz, A.; Smith, I.C.; Sherlock, D. A systematic review of sensory-based treatments for children with disabilities. Res. Dev. Disabil. 2015, 37, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.M.; Carter, M.; Stephenson, J. Systematic review of sensory integration therapy for individuals with disabilities: Single case design studies. Res. Dev. Disabil. 2015, 47, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.J.; Hinkley, L.B.N.; Hill, S.S.; Nagarajan, S.S. Sensory Processing in Autism: A Review of Neurophysiologic Findings. Pediatr. Res. 2011, 69 Pt 2, 48R. [Google Scholar] [CrossRef] [PubMed]
- Green, S.A.; Rudie, J.D.; Colich, N.L.; Wood, J.J.; Shirinyan, D.; Hernandez, L.; Tottenham, N.; Dapretto, M.; Bookheimer, S.Y. Overreactive brain responses to sensory stimuli in youth with autism spectrum disorders. J. Am. Acad. Child. Adolesc. Psychiatry 2013, 52, 1158–1172. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Gratiot, M.; Owen, J.P.; Brandes-Aitken, A.; Desai, S.S.; Hill, S.S.; Arnett, A.B.; Harris, J.; Marco, E.J.; Mukherjee, P. White Matter Microstructure is Associated with Auditory and Tactile Processing in Children with and without Sensory Processing Disorder. Front. Neuroanat. 2016, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Fava, L.; Strauss, K. Multi-sensory rooms: Comparing effects of the Snoezelen and the Stimulus Preference environment on the behavior of adults with profound mental retardation. Res. Dev. Disabil. 2010, 31, 160–171. [Google Scholar] [CrossRef] [PubMed]
- De Domenico, C.; Di Cara, M.; Piccolo, A.; Settimo, C.; Leonardi, S.; Giuffrè, G.; De Cola, M.C.; Cucinotta, F.; Tripodi, E.; Impallomeni, C.; et al. Exploring the Usefulness of a Multi-Sensory Environment on Sensory Behaviors in Children with Autism Spectrum Disorder. J. Clin. Med. 2024, 13, 4162. [Google Scholar] [CrossRef] [PubMed]
- Ludvigh, C.L.; Jerzy, R.; Zuzana, B. Analysis of Psychological and Physiological Responses to Snoezelen Multisensory Stimulation. J. Neurosci. Neurol. Disord. 2024, 8, 115–125. [Google Scholar] [CrossRef]
- Xiao, N.; Shinwari, K.; Kiselev, S.; Huang, X.; Li, B.; Qi, J. Effects of Equine-Assisted Activities and Therapies for Individuals with Autism Spectrum Disorder: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public. Health 2023, 20, 2630. [Google Scholar] [CrossRef] [PubMed]
- Esposito, L.; Mccune, S.; Griffin, J.A.; Maholmes, V. Directions in Human–Animal Interaction Research: Child Development, Health, and Therapeutic Interventions. Child. Dev. Perspect. 2011, 5, 205–211. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, S.; You, Y.; Wang, Y.; Zhang, Y. Effects of a Therapeutic Horseback Riding Program on Social Interaction and Communication in Children with Autism. Int. J. Environ. Res. Public. Health 2021, 18, 2656. [Google Scholar] [CrossRef] [PubMed]
- Katz-Nave, G.; Adini, Y.; Hetzroni, O.E.; Bonneh, Y.S. Sequence Learning in Minimally Verbal Children With ASD and the Beneficial Effect of Vestibular Stimulation. Autism Res. 2020, 13, 320–337. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.S.N.; Li, W.H.C.; Ho, L.L.K.; Phiri, L.; Choi, K.C. Nature-Based Interventions for Autistic Children: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, E2346715. [Google Scholar] [CrossRef] [PubMed]
- Ghasemtabar, S.; Hosseini, M.; Fayyaz, I.; Arab, S.; Naghashian, H.; Poudineh, Z. Music therapy: An effective approach in improving social skills of children with autism. Adv. Biomed. Res. 2015, 4, 157. [Google Scholar] [CrossRef] [PubMed]
- Caria, A.; Venuti, P.; De Falco, S. Functional and dysfunctional brain circuits underlying emotional processing of music in autism spectrum disorders. Cereb. Cortex 2011, 21, 2838–2849. [Google Scholar] [CrossRef] [PubMed]
- Gassner, L.; Geretsegger, M.; Mayer-Ferbas, J. Effectiveness of music therapy for autism spectrum disorder, dementia, depression, insomnia and schizophrenia: Update of systematic reviews. Eur. J. Public. Health 2022, 32, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Zaatar, M.T.; Alhakim, K.; Enayeh, M.; Tamer, R. The transformative power of music: Insights into neuroplasticity, health, and disease. Brain Behav. Immun. Health 2023, 35, 100716. [Google Scholar] [CrossRef] [PubMed]
- Sharda, M.; Tuerk, C.; Chowdhury, R.; Jamey, K.; Foster, N.; Custo-Blanch, M.; Tan, M.; Nadig, A.; Hyde, K. Music improves social communication and auditory–motor connectivity in children with autism. Transl. Psychiatry 2018, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Kučikienė, D.; Praninskienė, R. The impact of music on the bioelectrical oscillations of the brain. Acta Med. Litu. 2018, 25, 101. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, L.; Mas-Herrero, E.; Zatorre, R.J.; Ripollés, P.; Gomez-Andres, A.; Alicart, H.; Olivé, G.; Marco-Pallarés, J.; Antonijoan, R.M.; Valle, M.; et al. Dopamine modulates the reward experiences elicited by music. Proc. Natl. Acad. Sci. USA 2019, 116, 3793–3798. [Google Scholar] [CrossRef] [PubMed]
- Bearss, K.; Johnson, C.; Smith, T.; Lecavalier, L.; Swiezy, N.; Aman, M.; McAdam, D.B.; Butter, E.; Stillitano, C.; Minshawi, N.; et al. Effect of parent training vs parent education on behavioral problems in children with autism spectrum disorder: A randomized clinical trial. JAMA J. Am. Med. Assoc. 2015, 313, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Charman, T.; McConachie, H.; Aldred, C.; Slonims, V.; Howlin, P.; Le Couteur, A.; Leadbitter, K.; Hudry, K.; Byford, S.; et al. Parent-mediated communication-focused treatment in children with autism (PACT): A randomised controlled trial. Lancet 2010, 375, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G.; Jones, E.J.; Merkle, K.; Venema, K.; Lowy, R.; Faja, S.; Kamara, D.; Murias, M.; Greenson, J.; Winter, J.; et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J. Am. Acad. Child. Adolesc. Psychiatry 2012, 51, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.; Zagoory-Sharon, O.; Weisman, O.; Schneiderman, I.; Gordon, I.; Maoz, R.; Shalev, I.; Ebstein, R.P. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol. Psychiatry 2012, 72, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, B.; Mazurek, J.; Rutkowski, S.; Kiper, P.; Turolla, A.; Szczepańska-Gieracha, J. Virtual reality in psychiatric disorders: A systematic review of reviews. Complement Ther. Med. 2020, 52, 102480. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Shen, L.; Ma, C.; Chen, J.; Tian, Y.; Zhang, C.; Gong, Z.; Li, M.; Wang, C.; Pan, L.; et al. Effects of a Nonwearable Digital Therapeutic Intervention on Preschoolers With Autism Spectrum Disorder in China: Open-Label Randomized Controlled Trial. J. Med. Internet Res. 2023, 25, e45836. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Armas, J.; Flores-Cortes, M.; Pineda-Galan, C.; Luque-Suarez, A.; La Touche, R. Role of Immersive Virtual Reality in Motor Behaviour Decision-Making in Chronic Pain Patients. Brain Sci. 2023, 13, 617. [Google Scholar] [CrossRef] [PubMed]
- Katona, I.; Rancz, E.A.; Acsády, L.; Ledent, C.; Mackie, K.; Hájos, N.; Freund, T.F. Distribution of CB1 Cannabinoid Receptors in the Amygdala and their Role in the Control of GABAergic Transmission. J. Neurosci. 2001, 21, 9506. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Kross, E. Sensory emotion regulation. Trends Cogn. Sci. 2023, 27, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U. Cannabinoids: Role in Neurological Diseases and Psychiatric Disorders. Int. J. Mol. Sci. 2024, 26, 152. [Google Scholar] [CrossRef] [PubMed]
- Irving, A.J.; Rae, M.G.; Coutts, A.A. Cannabinoids on the brain. Sci. World J. 2002, 2, 632–648. [Google Scholar] [CrossRef] [PubMed]
- Silva Junior, E.A.D.; Medeiros, W.M.B.; Torro, N.; Sousa, J.M.M.D.; Almeida, I.B.C.M.D.; Costa, F.B.D.; Pontes, K.M.; Nunes, E.L.G.; Rosa, M.D.D.; Albuquerque, K.L.G.D.D. Cannabis and cannabinoid use in autism spectrum disorder: A systematic review. Trends Psychiatry Psychother. 2022, 44, e20200149. [Google Scholar] [CrossRef] [PubMed]
- Ros, T.; Baars, B.J.; Lanius, R.A.; Vuilleumier, P. Tuning pathological brain oscillations with neurofeedback: A systems neuroscience framework. Front. Hum. Neurosci. 2014, 8, 1008. [Google Scholar] [CrossRef] [PubMed]
- Bagdasaryan, J.; Le Van Quyen, M. Experiencing your brain: Neurofeedback as a new bridge between neuroscience and phenomenology. Front. Hum. Neurosci. 2013, 7, 680. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.; Mizrachi, L.; Granovsky, Y.; Issachar, G.; Yuval-Greenberg, S.; Bar-Shalita, T. Neurofeedback Therapy for Sensory Over-Responsiveness-A Feasibility Study. Sensors 2022, 22, 1845. [Google Scholar] [CrossRef] [PubMed]
- Mayaud, L.; Wu, H.; Barthélemy, Q.; Favennec, P.; Delpierre, Y.; Congedo, M.; Dupeyron, A.; Ritz, M. Alpha-phase synchrony EEG training for multi-resistant chronic low back pain patients: An open-label pilot study. Eur. Spine J. 2019, 28, 2487–2501. [Google Scholar] [CrossRef] [PubMed]
- Tosti, B.; Corrado, S.; Mancone, S.; Di Libero, T.; Carissimo, C.; Cerro, G.; Rodio, A.; da Silva, V.F.; Coimbra, D.R.; Andrade, A.; et al. Neurofeedback Training Protocols in Sports: A Systematic Review of Recent Advances in Performance, Anxiety, and Emotional Regulation. Brain Sci. 2024, 14, 1036. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985, 1, 1106–1107. [Google Scholar] [CrossRef] [PubMed]
- Jannati, A.; Ryan, M.A.; Kaye, H.L.; Tsuboyama, M.; Rotenberg, A. Biomarkers obtained by transcranial magnetic stimulation in neurodevelopmental disorders. J. Clin. Neurophysiol. 2022, 39, 135. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, M.; Chen, J.; Cai, G.; Liu, Q.; Zhao, Y.; Huang, Z.; Lan, Y. Non-invasive brain stimulation effectively improves post-stroke sensory impairment: A systematic review and meta-analysis. J. Neural Transm. 2023, 130, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, A.; Zarafshan, H.; Vand, S.R.; Mohammadi, M.R. Effects of Non-invasive Neurostimulation on Autism Spectrum Disorder: A Systematic Review. Clin. Psychopharmacol. Neurosci. 2020, 18, 527–552. [Google Scholar] [CrossRef] [PubMed]
- Lauro, L.J.R.; Rosanova, M.; Mattavelli, G.; Convento, S.; Pisoni, A.; Opitz, A.; Bolognini, N.; Vallar, G. TDCS increases cortical excitability: Direct evidence from TMS-EEG. Cortex 2014, 58, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.J.; Morais, S.; Sayal, A.; Pereira, J.; Meneses, S.; Areias, G.; Direito, B.; Macedo, A.; Castelo-Branco, M. Neurofeedback training of executive function in autism spectrum disorder: Distinct effects on brain activity levels and compensatory connectivity changes. J. Neurodev. Disord. 2024, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, L.; He, R.; Song, P.; Xu, P.; Wang, Y.; Li, F. Repetitive transcranial magnetic stimulation modulates long-range functional connectivity in autism spectrum disorder. J. Psychiatr. Res. 2023, 160, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.J.; Tommerdahl, M.; McGonigle, D.J. Stimulating somatosensory psychophysics: A double-blind, sham-controlled study of the neurobiological mechanisms of tDCS. Front. Cell Neurosci. 2015, 9, 400. [Google Scholar] [CrossRef] [PubMed]
- Omairi, C.; Mailloux, Z.; Antoniuk, S.A.; Schaaf, R. Occupational Therapy Using Ayres Sensory Integration®: A Randomized Controlled Trial in Brazil. Am. J. Occup. Ther. 2022, 76, 7604205160. [Google Scholar] [CrossRef] [PubMed]
- O’Haire, M.E.; McKenzie, S.J.; Beck, A.M.; Slaughter, V. Social behaviors increase in children with autism in the presence of animals compared to toys. PLoS ONE 2013, 8, e57010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kasari, C.; Gulsrud, A.C.; Wong, C.; Kwon, S.; Locke, J. Randomized controlled caregiver mediated joint engagement intervention for toddlers with autism. J. Autism. Dev. Disord. 2010, 40, 1045–1056. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kouijzer, M.; van Schie, H.; de Moor, J.M.H.; Gerrits, B.J.; Buitelaar, J.K. Neurofeedback treatment in autism: Preliminary findings in behavioral, cognitive, and neurophysiological functioning. Res. Aut. Spectr. Disord. 2010, 4, 386–399. [Google Scholar] [CrossRef]
- Yuan, L.X.; Wang, X.K.; Yang, C.; Zhang, Q.R.; Ma, S.Z.; Zang, Y.F.; Dong, W.Q. A systematic review of transcranial magnetic stimulation treatment for autism spectrum disorder. Heliyon 2024, 10, e32251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Neuroanatomical and Neurotransmitter Abnormalities in Sensory Processing Disorders in ASD | |
|---|---|
| Incorrect processing of auditory stimuli | Abnormalities in the limbic system [44] Abnormal vagus nerve response [45] |
| Hypersensitivity to light | Abnormalities in the visual cortex [50] Abnormalities in the primary cortex (V1) and the extrastriate cortex [52] Decreased activity in the frontal regions [52] Hyperactivation of the occipital regions [44,53] |
| Incorrect processing of tactile stimuli | Insufficient GABA inhibition [59] Abnormal oxytocin level [61] |
| Incorrect texture processing | Increased reactivity of the sensory cortex [62] Impaired communication between the sensory cortex and other brain regions, including the limbic system [43] Decreased level of the inhibitory neurotransmitter GABA [65] |
| Inadequate pain response | Overactive or underactive amygdala [68] Insula reduced activity [69] Increased endorphin levels [71] |
| Therapy | Description | Therapeutic Effects | Limitations |
|---|---|---|---|
| Ayers Sensory Integration (ASI) | Therapy based on individualized sensorimotor activities through play [72] | Improvement in self-care, social and communication skills; increased participation in daily activities [74,75,124] | Requires high treatment fidelity; therapist must be trained in ASI; limited availability [16] |
| Sensory Integration Therapy (SIT) | Based on the theory of sensory processing; targets multiple sensory modalities, including visual, tactile, and vestibular input [78] | Improvements in sensory, social, and adaptive functioning [78] | Inconsistent implementation across studies; efficacy remains controversial [79,80] |
| Snoezelen Therapy (Multisensory Environment) | Controlled multisensory environment using light, sound, and touch [84] | Reduces challenging behaviors, improves attention, eye contact, and social interaction [85] | Limited number of studies; subjective outcome measures [86] |
| Animal-Assisted Intervention (AAI) | Use of animals as part of therapy [87] | Enhances cognitive functions, motor control, social engagement; improves child–parent interaction [88,89,91] | High cost; access issues; requires further controlled studies [125] |
| Parental involvement | Training and involving parents directly in delivering therapeutic activities at home or during sessions [100] | Improved communication, social reciprocity, and generalization of skills across settings [99,100] | Requires high parental commitment; variation in fidelity; outcomes depend on parent–therapist alliance [126] |
| Music Therapy (MT) | Use of music listening and participation as therapeutic medium [92] | Improves social interaction, brain connectivity, family quality of life [92] | No improvement in core symptoms; few high-quality studies [94] |
| Virtual/Augmented Reality (VR/AR) | Use of immersive digital environments for sensory stimulation and feedback [103] | Improves motor and sensory functions; objective assessment of responses [104] | Expensive equipment; limited standardization; early-phase research [104] |
| Cannabinoids | Modulation of neurotransmission via CB1/CB2 receptors [106] | Reduces hyperactivity, aggression, sleep disturbances; improves attention and communication [110] | Lack of randomized trials; potential side effects; preliminary data [110] |
| Bioneurofeedback (EEG-NF) | Self-regulation of brain oscillatory activity using EEG feedback [111] | Improved frontal cortex activation and quality of life in sensory hyperactivation [113] | No significant change in alpha waves; requires more research into EEG patterns [127] |
| Non-invasive Brain Stimulation (TMS/tDCS) | Magnetic/electrical stimulation to influence neural activity and plasticity [116] | Improvement in executive function, social behavior, and repetitive behaviors [119] | Still experimental in ASD; needs protocol standardization and more trials [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suprunowicz, M.; Bogucka, J.; Szczerbińska, N.; Modzelewski, S.; Oracz, A.J.; Konarzewska, B.; Waszkiewicz, N. Neuroplasticity-Based Approaches to Sensory Processing Alterations in Autism Spectrum Disorder. Int. J. Mol. Sci. 2025, 26, 7102. https://doi.org/10.3390/ijms26157102
Suprunowicz M, Bogucka J, Szczerbińska N, Modzelewski S, Oracz AJ, Konarzewska B, Waszkiewicz N. Neuroplasticity-Based Approaches to Sensory Processing Alterations in Autism Spectrum Disorder. International Journal of Molecular Sciences. 2025; 26(15):7102. https://doi.org/10.3390/ijms26157102
Chicago/Turabian StyleSuprunowicz, Maria, Julia Bogucka, Natalia Szczerbińska, Stefan Modzelewski, Aleksandra Julia Oracz, Beata Konarzewska, and Napoleon Waszkiewicz. 2025. "Neuroplasticity-Based Approaches to Sensory Processing Alterations in Autism Spectrum Disorder" International Journal of Molecular Sciences 26, no. 15: 7102. https://doi.org/10.3390/ijms26157102
APA StyleSuprunowicz, M., Bogucka, J., Szczerbińska, N., Modzelewski, S., Oracz, A. J., Konarzewska, B., & Waszkiewicz, N. (2025). Neuroplasticity-Based Approaches to Sensory Processing Alterations in Autism Spectrum Disorder. International Journal of Molecular Sciences, 26(15), 7102. https://doi.org/10.3390/ijms26157102









