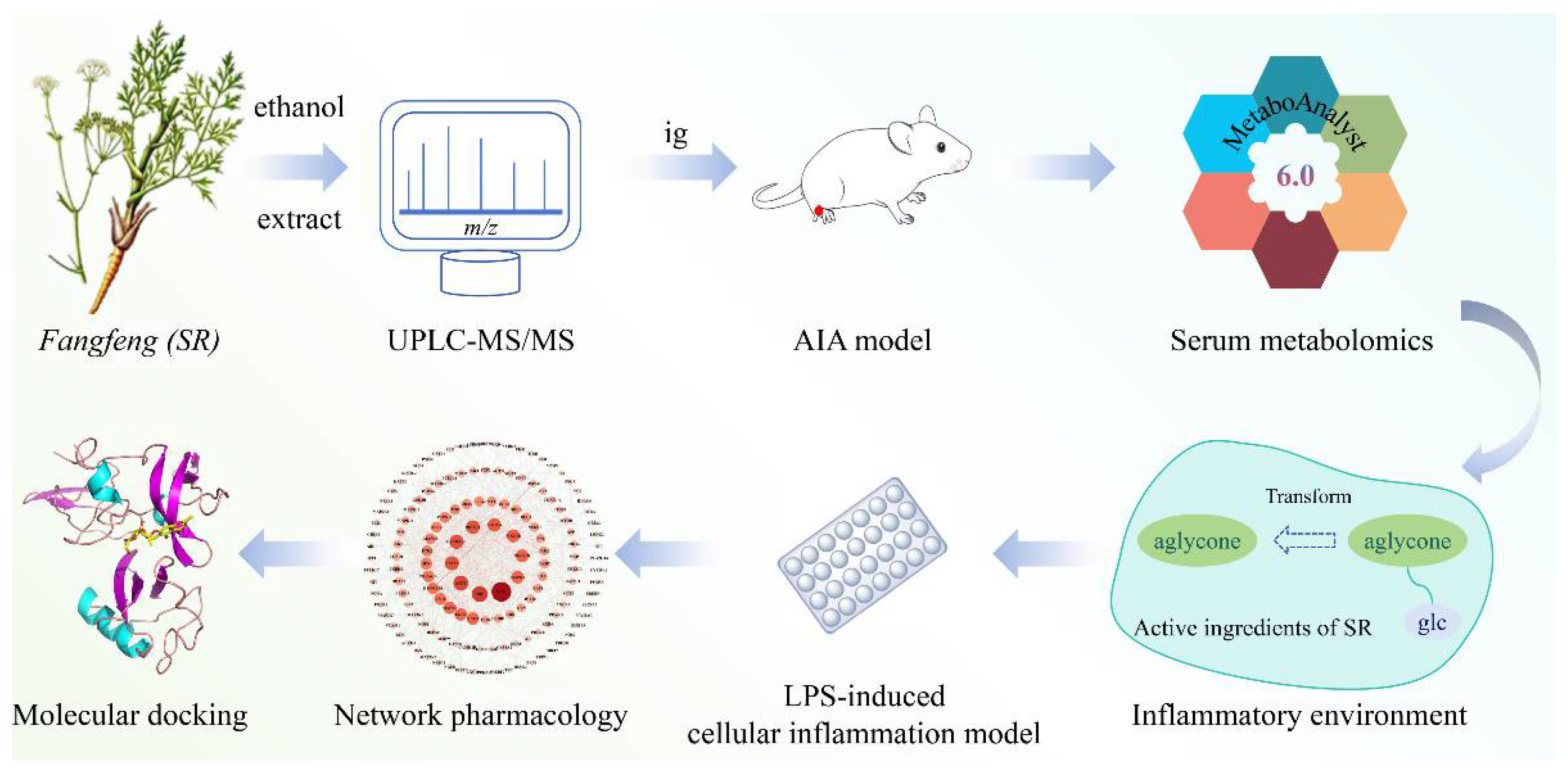
Anti-Inflammatory Pharmacological Mechanism Mediated by the Conversion of Glycosides to Aglycones in Fangfeng (Saposhnikoviae Radix) in Rheumatoid Arthritis Models Based on Serum Metabolomics, Network Pharmacology, and Molecular Docking
Abstract
1. Introduction
2. Results
2.1. Identification of the Constituents in Saposhnikoviae Radix
2.1.1. Furanochromone
2.1.2. Pyranochromone
2.1.3. Coumarin
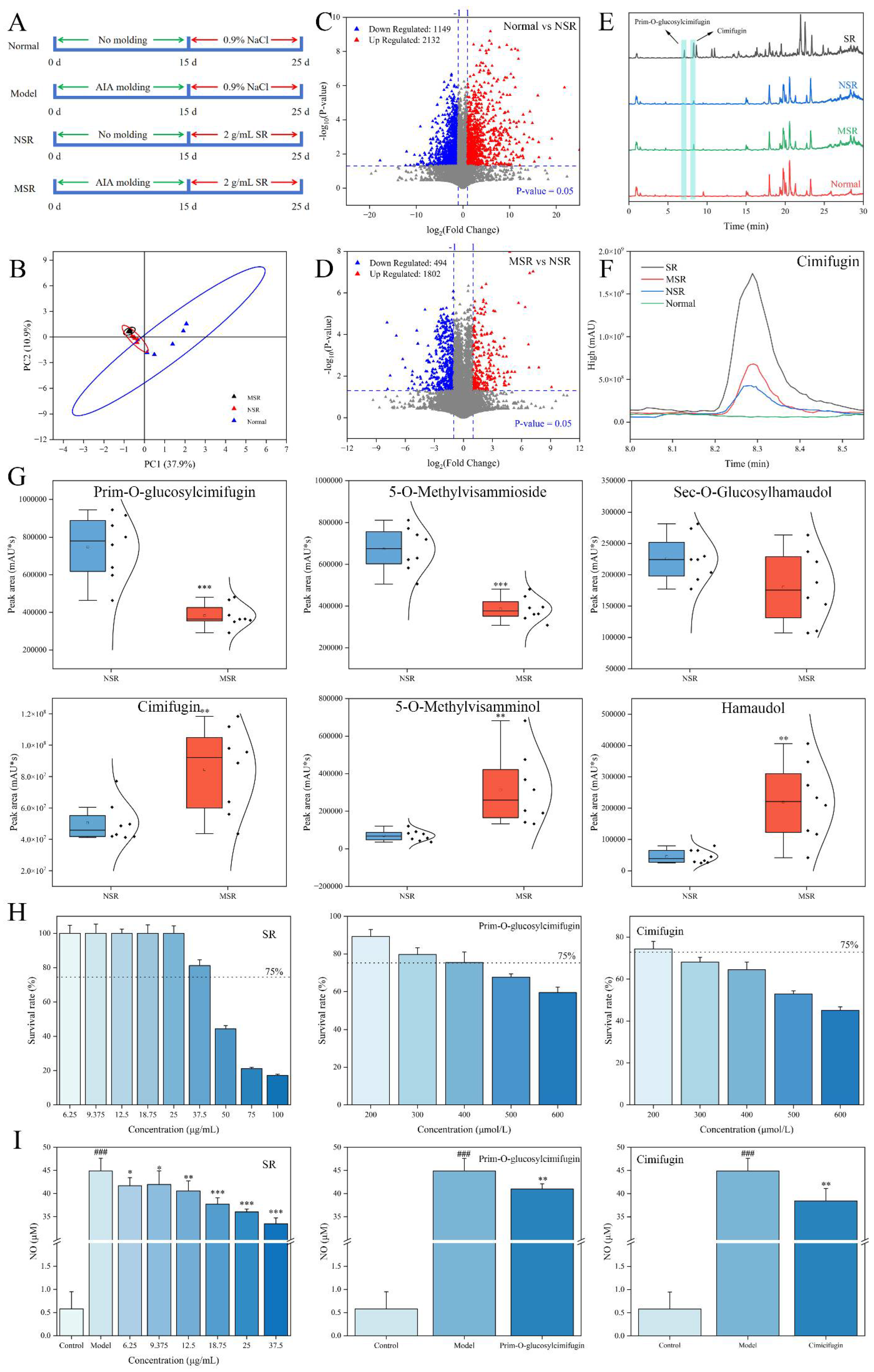
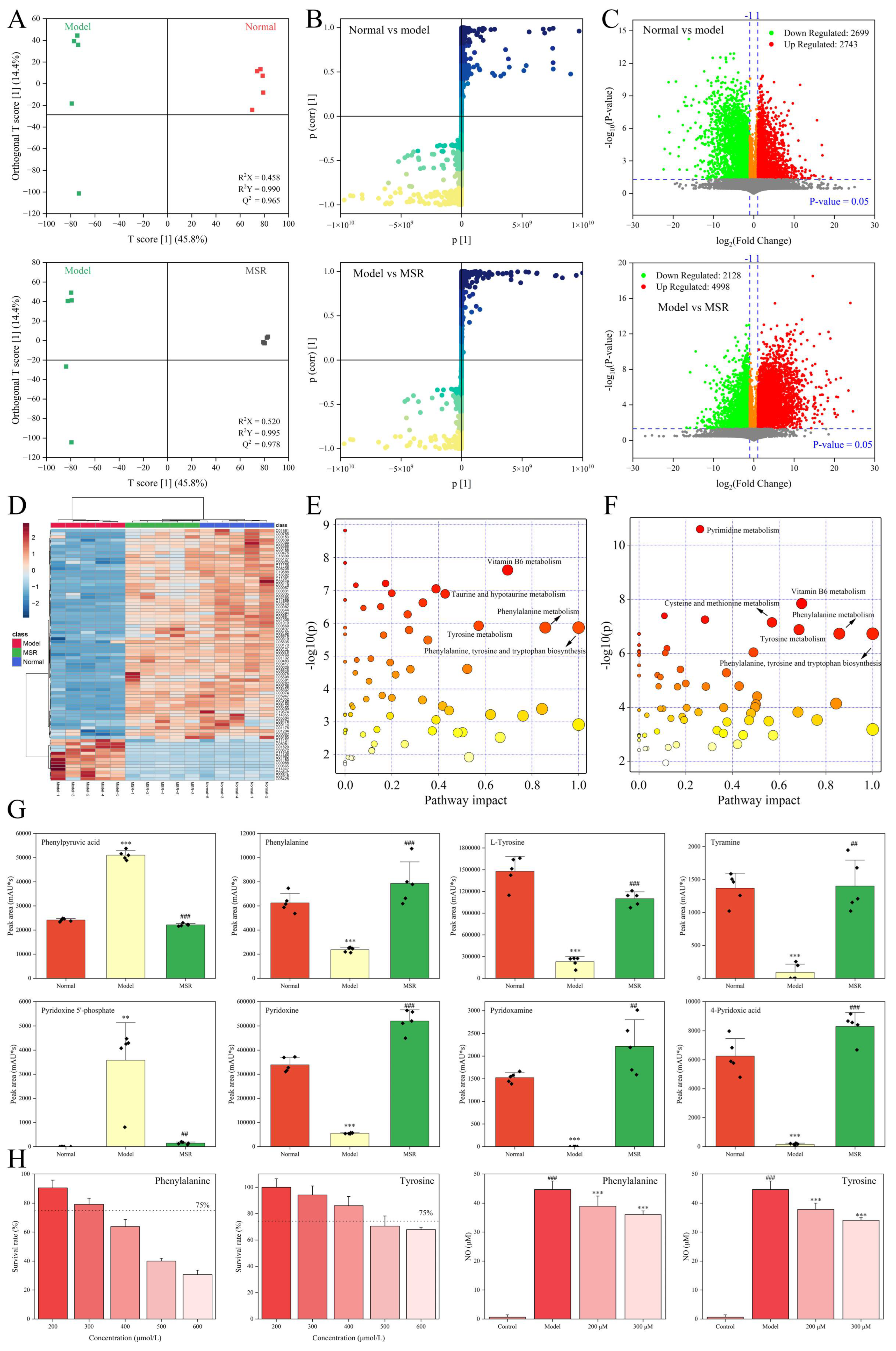
2.2. Identification of Metabolites In Vivo of Saposhnikoviae Radix
2.2.1. Exogenous Metabolites Analysis
2.2.2. Endogenous Metabolites Analysis
2.3. Anti-Inflammatory Mechanism of Saposhnikoviae Radix
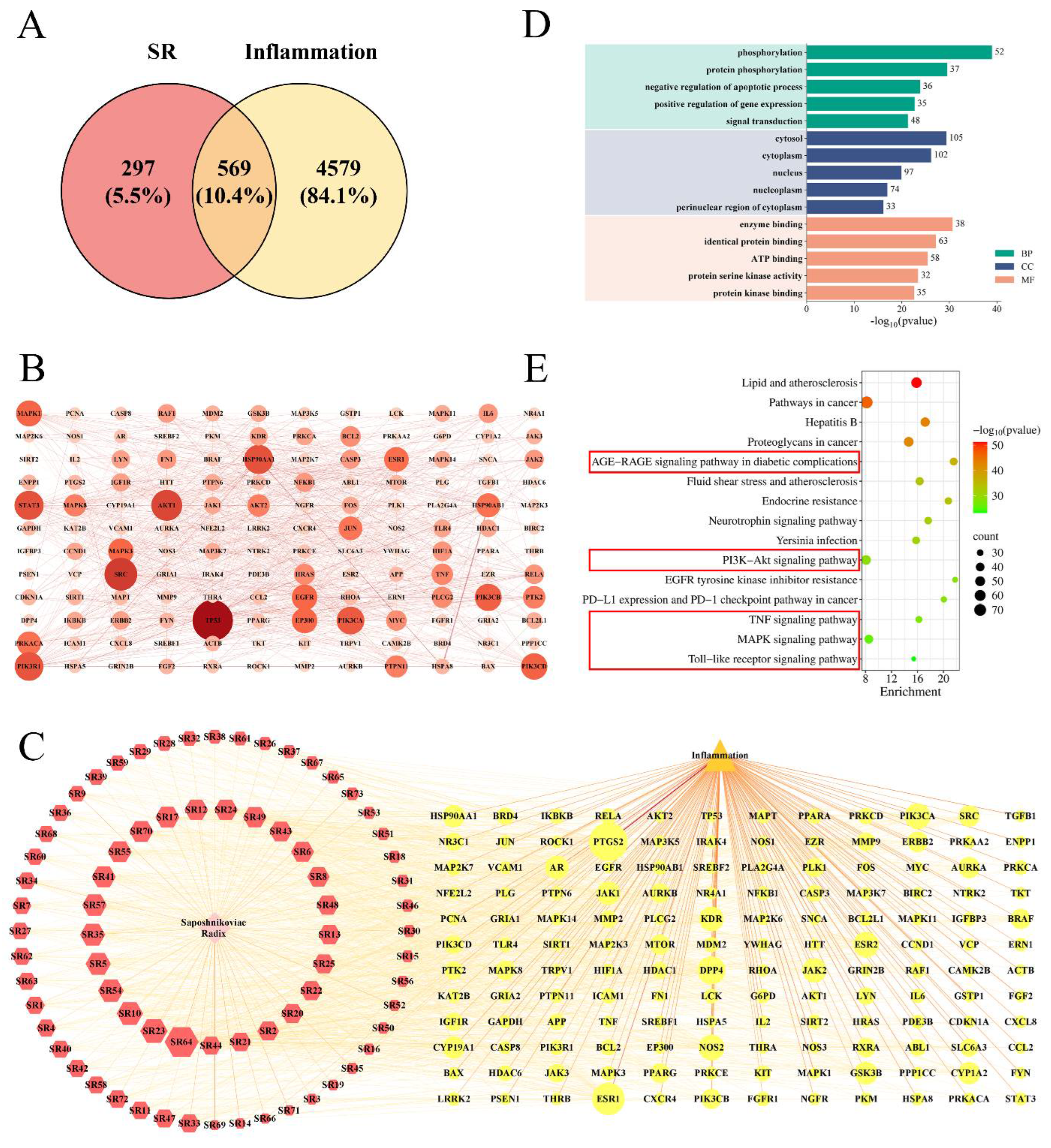
2.3.1. Network Pharmacology Analysis
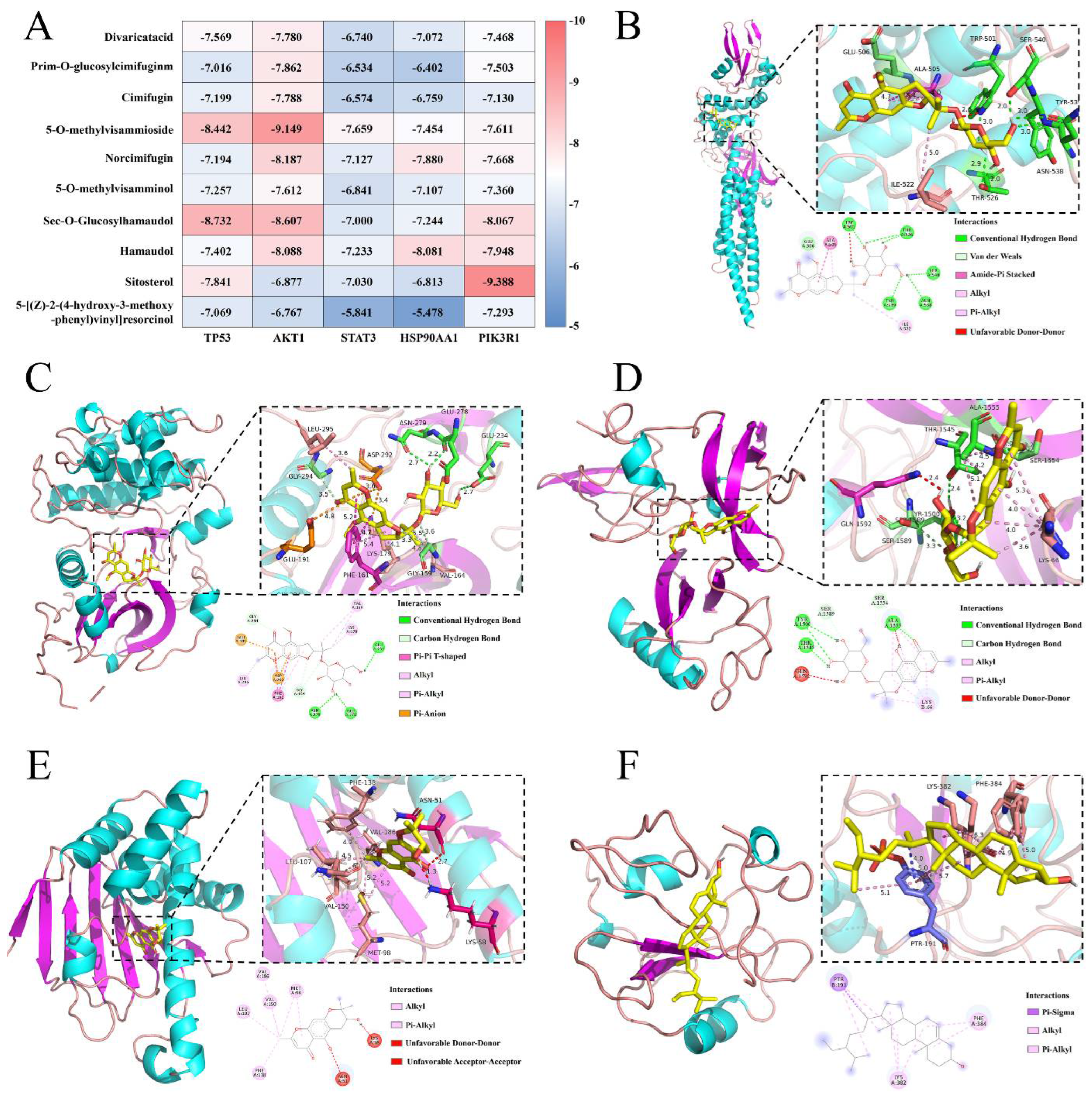
2.3.2. Molecular Docking Verification
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Sample Preparation
4.3. Systematic Chemical Constituents Analysis of Saposhnikoviae Radix
4.4. Analysis of Metabolites In Vivo of Saposhnikoviae Radix
4.5. Analysis of Exogenous Components
4.6. Analysis of Endogenous Components
4.7. LPS-Induced Inflammation Model of RAW264.7 Cells
4.8. Network Pharmacology Analysis
4.9. Molecular Docking
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIA | Adjuvant induced rheumatoid arthritis |
| BC | Betweenness centrality |
| CC | Closeness centrality |
| DL | Drug-like properties |
| DMSO | Dimethyl sulfoxide |
| GO | Gene ontology |
| KEGG | Kyoto encyclopedia of genes and genomes |
| LPS | Lipopolysaccharide |
| MSR | Model mice were given SR |
| MTT | Methyl thiazolyl tetrazolium |
| NO | Nitric oxide |
| NSR | Normal mice were given SR |
| OB | Oral bioavailability |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| PCA | Principal component analysis |
| RA | Rheumatoid arthritis |
| SR | Saposhnikoviae Radix |
| UPLC-MS/MS | Ultra-performance liquid chromatography/tandem mass spectrometry |
| VIP | variable importance in projection |
References
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jonsson, A.H.; Nathan, A.; Millard, N.; Curtis, M.; Xiao, Q.; Gutierrez-Arcelus, M.; Apruzzese, W.; Watts, G.F.M.; Weisenfeld, D.; et al. Deconstruction of rheumatoid arthritis synovium defines inflammatory subtypes. Nature 2023, 623, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Fu, X.K.; Chen, X.X.; Li, Z.; Huang, Y.H.; Liang, C. Promising Therapeutic Targets for Treatment of Rheumatoid Arthritis. Front. Immunol. 2021, 12, 686155. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, A.; Bathon, J.M.; Emery, P. Rheumatoid arthritis. Lancet 2023, 402, 2019–2033. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.J.; Du, K.Z.; Liang, C.X.; Wang, S.Q.; Boadi, E.O.; Li, J.; Pang, X.L.; He, J.; Chang, Y.X. Traditional herbal medicine: Therapeutic potential in rheumatoid arthritis. J. Ethnopharmacol. 2021, 279, 114368. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.L.; Zhang, T.W.; Liu, S.L.; Zhao, Y.; Sun, X. The prepared and characterized polysaccharide polymer in Schischk effectively controls the course of rheumatoid arthritis via TLR4/TRAF6-NF-xB/IxB-α signaling pathway. Biomed. Pharmacother. 2023, 160, 114416. [Google Scholar] [CrossRef]
- Dong, C.X.; Liu, L.; Wang, C.Y.; Fu, Z.; Zhang, Y.; Hou, X.; Peng, C.; Ran, R.X.; Yao, Z. Structural characterization of polysaccharides from and their antagonistic effects against the immunosuppression by the culture supernatants of melanoma cells on RAW264.7 macrophages. Int. J. Biol. Macromol. 2018, 113, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.W.; Zhan, Y.; Wang, Y.H.; Zhao, S.J.; Han, Z.M. Advances in Phytochemistry and Modern Pharmacology of Saposhnikovia divaricata (Turcz.) Schischk. Chin. J. Integr. Med. 2023, 29, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, P.; Jiang, Y.-K.; Pan, J.; Wu, J.-T.; Li, X.-M.; Guan, W.; Min, X.-Y.; Wang, Y.-X.; Kuang, H.-X.; et al. New chromones from the roots of Saposhnikovia divaricata (Turcz.) Schischk with anti-inflammatory activity. Bioorganic Chem. 2023, 134, 106447. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Xu, Y.; Sun, B.; Shang, Y.; Yu, Z.; Hu, Y.; Bao, H.; Yue, H. Study on HPLC fingerprint of Saposhnikovia divaricata lipophilic components and anti-aging effects. Sci. Rep. 2024, 14, 25766. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Zhou, H.; Li, Q.; Zeng, N.; Wang, Q. Extraction, purification, structural characteristics and biological properties of the polysaccharides from Radix Saposhnikoviae: A review. J. Ethnopharmacol. 2024, 318, 116956. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.T.; Sun, M.; Li, J.; Zhang, S.F.; Tu, G.Z.; Liu, K.C.; Xia, Q.; Jiang, Y.Y.; Liu, B. Structure characterization and immunomodulatory activity of a polysaccharide from Saposhnikoviae Radix. Int. J. Biol. Macromol. 2023, 233, 123502. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Niu, Y.; Zheng, J.; Liu, H.L.; Jiang, G.R.; Chen, J.H.; Hong, M. Radix Saposhnikovia extract suppresses mouse allergic contact dermatitis by regulating dendritic-cell-activated Th1 cells. Phytomedicine 2015, 22, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, C.-C.; Wang, W.-L.; Xu, J.-P.; Wang, J.; Zhang, C.-H.; Li, M.-H. Saposhnikovia divaricata—An Ethnopharmacological, Phytochemical and Pharmacological Review. Chin. J. Integr. Med. 2020, 26, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, F.; Sun, Y.; Wang, M.; Bi, Y.; Jang, P.; Wang, S.; Guan, W.; Yan, J.; Zhang, L.; et al. Chromone components of Saposhnikovia divaricate attenuate rheumatoid arthritis development by inhibiting the inflammatory response. J. Ethnopharmacol. 2025, 337, 118912. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.Y.; Liu, C.F.; Zhang, C.; Zhao, J.; Wang, J.Z.; Wan, H.Y.; Zhu, H.W.; Zhang, P.; Chen, W.H.; Xiao, Y.Q.; et al. The suppressive effects of (Fangfeng) chromone extract on rheumatoid arthritis via inhibition of nuclear factor-κB and mitogen activated proteinkinases activation on collagen-induced arthritis model. J. Ethnopharmacol. 2013, 148, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhong, S.X.; Tan, H.S.; Fu, Y.F.; Lai, J.Y.; Liu, L.J.; Weng, J.L.; Chen, H.W.; He, S.H. Study on the mechanism of action ofand its key phytochemical on rheumatoid arthritis based on network pharmacology and bioinformatics. J. Ethnopharmacol. 2024, 322, 117586. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.M.; Cao, X.L.; Zhong, L.; Dong, Y.X.; Xu, Z.Y.; Rong, Y.C.; Xu, H.L.; Wang, Z.Y.; Yang, H.; Yin, R.; et al. Targeting phenylpyruvate restrains excessive NLRP3 inflammasome activation and pathological inflammation in diabetic wound healing. Cell Rep. Med. 2023, 4, 101129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Z.; Chen, S.Y.; Guo, Y.; He, F.; Fu, J.; Ren, W.K. Phenylalanine diminishes M1 macrophage inflammation. Sci. China Life Sci. 2023, 66, 2862–2876. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.L.; Zhong, S.F.; Cheng, K.B.; Zhang, L.S.; Bai, J.W.; Cao, Z.; Wang, S.; Chen, W.; Cheng, S.P.; Ma, L.Y.; et al. Vitamin B6 regulates IL-33 homeostasis to alleviate type 2 inflammation. Cell Mol. Immunol. 2023, 20, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Kotschenreuther, K.; Yan, S.; Kofler, D.M. Migration and homeostasis of regulatory T cells in rheumatoid arthritis. Front. Immunol. 2022, 13, 947636. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kwon, E.J.; Lee, J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, Y.; Wang, H.; Yin, A.; Hu, J.; Yuan, T.; Zhou, S.; Xu, W.; Wei, P.; Yin, S.; et al. Gut commensal Parabacteroides distasonis alleviates inflammatory arthritis. Gut 2023, 72, 1664–1677. [Google Scholar] [CrossRef] [PubMed]
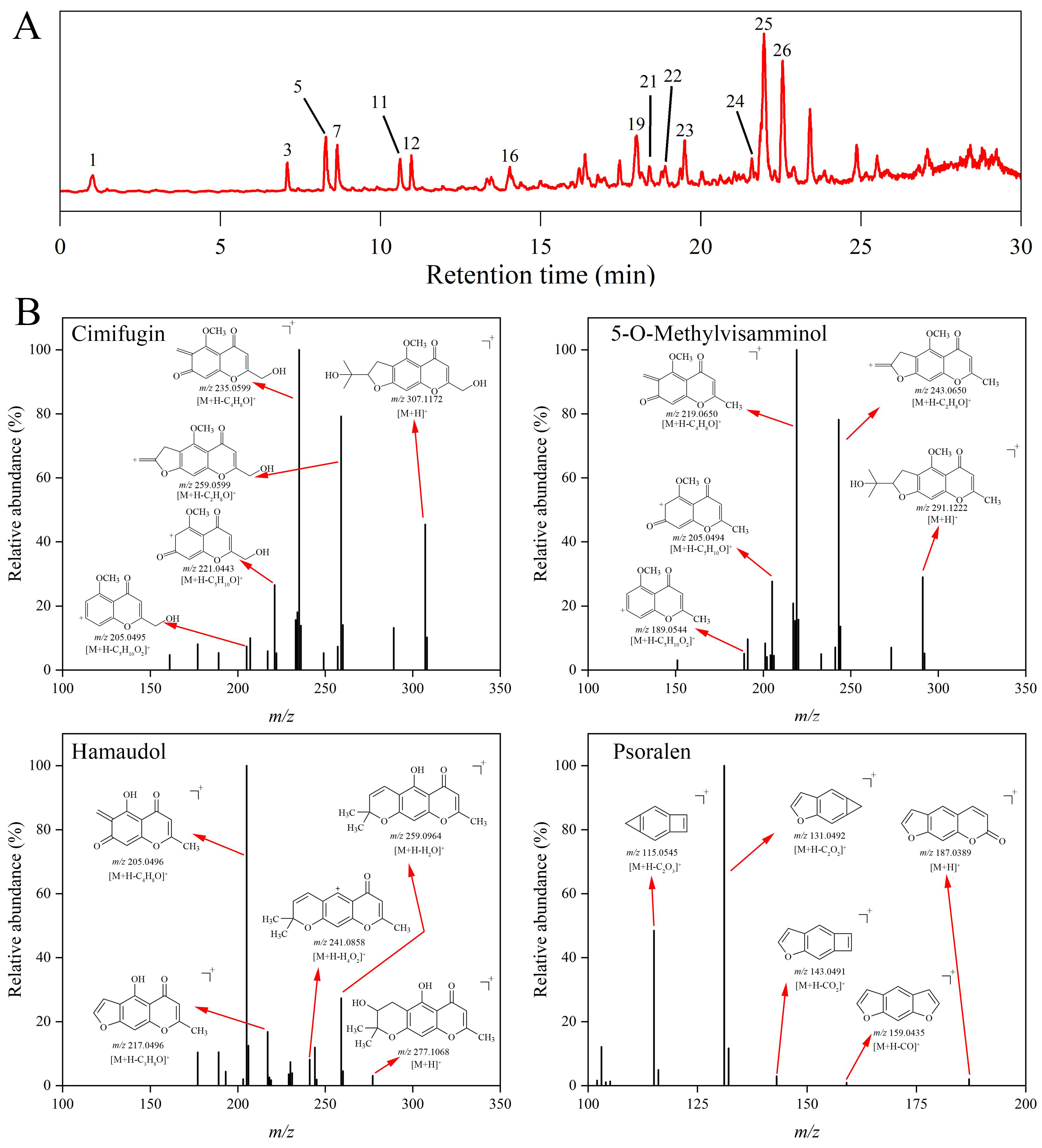
| NO | tR (min) | Compound | Formula | Identity | Theoretical (m/z) | Experimental (m/z) | Δppm | Fragment Ion (m/z) | Classification |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.03 | Adenosine | C10H13N5O4 | [M+H]+ | 268.1040 | 268.1038 | −0.75 | 136.0618 [M+H-C5H8O4]+ | Other |
| 2 | 6.96 | Divaricatacid | C16H16O7 | [M+H]+ | 321.0968 | 321.0966 | −0.62 | 303.0854 [M+H-H2O]+ 277.1068 [M+H-CO2]+ 273.0391 [M+H-C2H8O]+ 249.0390 [M+H-C3H5O]+ 235.0235 [M+H-C4H7O]+ | Chromone |
| 3 | 7.09 | Prim-O-glucosylcimifugin | C22H28O11 | [M+H]+ | 469.1704 | 469.1700 | −0.85 | 307.1173 [M+H-C6H10O5]+ 289.1068 [M+H-C6H12O6]+ | Chromone |
| 4 | 8.05 | Scopoletin | C10H8O4 | [M+H]+ | 193.0495 | 193.0495 | 0.00 | 178.0258 [M+H-CH3]+ 150.0314 [M+H-C2H3O]+ 133.0285 [M+H-C2H4O2]+ 122.0364 [M+H-C3H3O2]+ | Coumarin |
| 5 | 8.29 | Cimifugin | C16H18O6 | [M+H]+ | 307.1176 | 307.1172 | −1.30 | 289.1070 [M+H-H2O]+ 274.0825 [M+H-CH5O]+ 259.0599 [M+H-C2H8O]+ 247.0597 [M+H-C3H8O]+ 235.0599 [M+H-C4H8O]+ 221.0443 [M+H-C5H10O]+ 205.0495 [M+H-C5H10O2]+ | Chromone |
| 6 | 8.33 | Fraxidin | C11H10O5 | [M+H]+ | 223.0600 | 223.0597 | −1.34 | 207.0286 [M+H-CH4]+ 190.0259 [M+H-CH5O]+ 179.0337 [M+H-C2H4O]+ 162.0311 [M+H-C2H5O2]+ 134.0363 [M+H-C3H5O3]+ | Coumarin |
| 7 | 8.65 | 5-O-Methylvisammioside | C22H28O10 | [M+H]+ | 453.1755 | 453.1748 | −1.54 | 291.1224 [M+H-C6H10O5]+ 273.1118 [M+H-C6H12O6]+ | Chromone |
| 8 | 8.90 | Isofraxidin | C11H10O5 | [M+H]+ | 223.0600 | 223.0600 | 0.00 | 207.0287 [M+H-CH4]+ 190.0258 [M+H-CH5O]+ 179.0337 [M+H-C2H4O]+ 162.0311 [M+H-C2H5O2]+ 134.0362 [M+H-C3H5O3]+ | Coumarin |
| 9 | 9.93 | Khellactone | C14H14O5 | [M+H]+ | 263.0914 | 263.0912 | −0.76 | 203.0702 [M+H-C2H4O2]+ 175.0389 [M+H-C3H8O2]+ | Coumarin |
| 10 | 10.53 | Norcimifugin | C15H16O6 | [M+H]+ | 293.1019 | 293.1017 | −0.68 | 275.0910 [M+H-H2O]+ 260.0677 [M+H-CH5O]+ 245.0450 [M+H-C2H8O]+ 233.0444 [M+H-C3H8O]+ 221.0443 [M+H-C4H8O]+ | Chromone |
| 11 | 10.61 | 5-O-Methylvisamminol | C16H18O5 | [M+H]+ | 291.1227 | 291.1222 | −1.72 | 273.1119 [M+H-H2O]+ 258.0884 [M+H-CH5O]+ 243.0650 [M+H-C2H8O]+ 231.0653 [M+H-C3H8O]+ 219.0650 [M+H-C4H8O]+ 205.0494 [M+H-C5H10O]+ 189.0544 [M+H-C5H10O2]+ | Chromone |
| 12 | 10.96 | Sec-O-Glucosylhamaudol | C21H26O10 | [M+H]+ | 439.1598 | 439.1592 | −1.37 | 277.1068 [M+H-C6H10O5]+ 259.0962 [M+H-C6H12O6]+ 241.0859 [M+H-C6H14O7]+ 217.0493 [M+H-C9H18O6]+ 205.0495 [M+H-C10H18O6]+ | Chromone |
| 13 | 11.21 | Clemiscosin A | C20H18O8 | [M+H]+ | 387.1074 | 387.1069 | −1.29 | 207.0286 [M+H-C4H8O]+ 179.0337 [M+H-C5H8O2]+ | Coumarin |
| 14 | 12.41 | Psoralen | C11H6O3 | [M+H]+ | 187.0389 | 187.0389 | 0.00 | 159.0435 [M+H-CO]+ 143.0491 [M+H-CO2]+ 131.0492 [M+H-C2O2]+ 115.0545 [M+H-C2O3]+ | Coumarin |
| 15 | 12.97 | Bergapten | C12H8O4 | [M+H]+ | 217.0495 | 217.0494 | −0.46 | 202.0260 [M+H-CH3]+ 174.0311 [M+H-C2H3O]+ | Coumarin |
| 16 | 14.04 | Hamaudol | C15H16O5 | [M+H]+ | 277.1070 | 277.1067 | −1.08 | 259.0964 [M+H-H2O]+ 241.0858 [M+H-H4O2]+ 217.0496 [M+H-C3H8O]+ 205.0496 [M+H-C4H8O]+ | Chromone |
| 17 | 14.11 | Divaricatol | C17H18O7 | [M+H]+ | 335.1125 | 335.1119 | −1.79 | 275.0912 [M+H-C2H3O2]+ 257.0805 [M+H-C2H5O3]+ 247.0965 [M+H-C3H3O3]+ 233.0444 [M+H-C5H9O2]+ 221.0443 [M+H-C6H9O2]+ 205.0495 [M+H-C6H9O3]+ | Chromone |
| 18 | 17.31 | 5-methoxy-7-(3,3-dimethylallyloxy)-coumarin | C15H16O4 | [M+H]+ | 261.1121 | 261.1120 | −0.38 | 205.0495 [M+H-C4H8]+ 177.0546 [M+H-C5H8O]+ | Coumarin |
| 19 | 18.02 | 3′-O-acetylhamaudol | C17H18O6 | [M+H]+ | 319.1176 | 319.1172 | −1.25 | 259.0964 [M+H-C2H3O2]+ 231.1014 [M+H-C3H3O3]+ 217.0496 [M+H-C5H9O2]+ 205.0497 [M+H-C6H9O2]+ | Chromone |
| 20 | 18.15 | Imperatorin | C16H14O4 | [M+H]+ | 271.0964 | 271.0961 | −1.11 | 203.0339 [M+H-C5H8]+ 175.0390 [M+H-C6H8O]+ 147.0441 [M+H-C7H8O2]+ | Coumarin |
| 21 | 18.40 | Ledebouriellol | C20H22O7 | [M+H]+ | 375.1438 | 375.1435 | −0.80 | 275.0912 [M+H-C5H8O2]+ 233.0444 [M+H-C7H14O]+ 221.0443 [M+H-C8H14O]+ | Chromone |
| 22 | 18.89 | Phellopterin | C17H16O5 | [M+H]+ | 301.1070 | 301.1067 | −1.00 | 233.0443 [M+H-C5H8]+ 218.0209 [M+H-C6H11]+ | Coumarin |
| 23 | 19.36 | Deltoin | C19H20O5 | [M+H]+ | 329.1383 | 329.1378 | −1.52 | 229.0858 [M+H-C5H8O2]+ 214.0621 [M+H-C6H11O2]+ | Coumarin |
| 24 | 21.59 | Osthenol | C14H14O3 | [M+H]+ | 231.1015 | 231.1012 | −1.30 | 163.0389 [M+H-C5H8]+ 135.0439 [M+H-C6H8O]+ 107.0495 [M+H-C7H8O2]+ | Coumarin |
| 25 | 21.97 | 7-Hydroxycoumarine | C9H6O3 | [M+H]+ | 163.0389 | 163.0385 | −2.45 | 135.0442 [M+H-CO]+ 119.0496 [M+H-CO2]+ 107.0495 [M+H-C2O2]+ | Coumarin |
| 26 | 22.55 | 3′-O-angeloylhamaudol | C20H22O6 | [M+H]+ | 359.1489 | 359.1481 | −2.23 | 259.0963 [M+H-C5H8O2]+ 241.0856 [M+H-C5H10O3]+ 231.1013 [M+H-C6H8O3]+ 217.0494 [M+H-C8H14O2]+ 205.0495 [M+H-C9H14O]+ | Chromone |
| NO | tR (min) | Formula | Theoretical (m/z) | Experimental (m/z) | Δppm | Parent Compound | Biotransformation | Fragment Ion (m/z) | VIP |
|---|---|---|---|---|---|---|---|---|---|
| M1 | 6.07 | C16H18O7 | 323.1125 | 323.1122 | −0.93 | Cimifugin | Oxidation | 305.1019 [M+H-H2O]+ 259.0604 [M+H-C2H8O2]+ 247.0602 [M+H-C3H8O2]+ 235.0600 [M+H-C4H8O2]+ 221.0442 [M+H-C5H10O2]+ | 1.48 |
| M2 | 6.45 | C16H18O7 | 323.1125 | 323.1124 | −0.31 | Cimifugin | Oxidation | 305.0998 [M+H-H2O]+ 259.0617 [M+H-C2H8O2]+ 247.0601 [M+H-C3H8O2]+ 235.0599 [M+H-C4H8O2]+ 232.0364 [M+H-C4H11O2]+ 221.0452 [M+H-C5H10O2]+ | 1.48 |
| M3 | 6.96 | C16H16O7 | 321.0968 | 321.0966 | −0.62 | Divaricatacid | Parent | 303.0862 [M+H-H2O]+ 273.0391 [M+H-C2H8O]+ 249.0392 [M+H-C3H5O]+ 235.0236 [M+H-C4H7O]+ | 1.45 |
| M4 | 7.09 | C22H28O11 | 469.1704 | 469.1702 | −0.43 | Prim-O-glucosylcimifugin | Parent | 307.1175 [M+H-C6H10O5]+ 289.1076 [M+H-C6H12O6]+ | 1.42 |
| M5 | 7.19 | C22H26O12 | 483.1497 | 483.1493 | −0.83 | Cimifugin | Glucuronide conjugation | 307.1174 [M+H-C6H8O6]+ 289.1059 [M+H-C6H10O7]+ | 1.52 |
| M6 | 7.27 | C16H18O7 | 323.1125 | 323.1124 | −0.31 | Cimifugin | Oxidation | 305.1014 [M+H-H2O]+ 275.0543 [M+H-C2H8O]+ 247.0602 [M+H-C3H8O2]+ 232.0365 [M+H-C4H11O2]+ | 1.48 |
| M7 | 8.29 | C16H18O6 | 307.1176 | 307.1173 | −0.98 | Cimifugin | Parent | 289.1067 [M+H-H2O]+ 274.0819 [M+H-CH5O]+ 259.0600 [M+H-C2H8O]+ 247.0599 [M+H-C3H8O]+ 235.0600 [M+H-C4H8O]+ 221.0444 [M+H-C5H10O]+ 205.0494 [M+H-C5H10O2]+ | 1.57 |
| M8 | 8.36 | C16H16O6 | 305.1017 | 305.1020 | 0.98 | Cimifugin | Desaturation | 289.1065 [M+H-O]+ 274.0829 [M+H-CH3O]+ 259.0598 [M+H-C2H6O]+ 247.0596 [M+H-C3H6O]+ 235.0598 [M+H-C4H6O]+ 221.0442 [M+H-C5H8O]+ 205.0493 [M+H-C5H8O2]+ | 1.55 |
| M9 | 8.65 | C22H28O10 | 453.1755 | 453.1754 | −0.22 | 5-O-Methylvisammioside | Parent | 291.1225 [M+H-C6H10O5]+ 273.1121 [M+H-C6H12O6]+ | 1.48 |
| M10 | 10.53 | C15H16O6 | 293.1019 | 293.1017 | −0.68 | Norcimifugin | Parent | 275.0911 [M+H-H2O]+ 260.0692 [M+H-CH5O]+ 233.0447 [M+H-C3H8O]+ 221.0444 [M+H-C4H8O]+ | 1.26 |
| M11 | 10.61 | C16H18O5 | 291.1227 | 291.1225 | −0.69 | 5-O-Methylvisamminol | Parent | 273.1123 [M+H-H2O]+ 243.0649 [M+H-C2H8O]+ 219.0650 [M+H-C4H8O]+ 205.0491 [M+H-C5H10O]+ | 1.34 |
| M12 | 10.96 | C21H26O10 | 439.1598 | 439.1595 | −0.68 | Sec-O-Glucosylhamaudol | Parent | 277.1069 [M+H-C6H10O5]+ 259.0965 [M+H-C6H12O6]+ 217.0494 [M+H-C9H18O6]+ 205.0497 [M+H-C10H18O6]+ | 1.42 |
| M13 | 12.21 | C14H14O5 | 263.0914 | 263.0912 | −0.76 | Cimifugin | Loss of CH2O + Demethylation | 163.039 [M+H-C5H8O2]+ 135.0437 [M+H-C6H8O3]+ 119.0495 [M+H-C6H8O4]+ 107.0497 [M+H-C7H8O4]+ | 1.42 |
| M14 | 14.04 | C15H16O5 | 277.1070 | 277.1068 | −0.72 | Hamaudol | Parent | 259.0974 [M+H-H2O]+ 241.0866 [M+H-H4O2]+ 217.0498 [M+H-C3H8O]+ 205.0496 [M+H-C4H8O]+ | 1.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, W.; Lin, X.; Pi, W.; He, F.; Wu, H.; Wang, X.; Chen, J.; Cheng, X.; Wang, P.; Wei, F. Anti-Inflammatory Pharmacological Mechanism Mediated by the Conversion of Glycosides to Aglycones in Fangfeng (Saposhnikoviae Radix) in Rheumatoid Arthritis Models Based on Serum Metabolomics, Network Pharmacology, and Molecular Docking. Int. J. Mol. Sci. 2025, 26, 7088. https://doi.org/10.3390/ijms26157088
Jing W, Lin X, Pi W, He F, Wu H, Wang X, Chen J, Cheng X, Wang P, Wei F. Anti-Inflammatory Pharmacological Mechanism Mediated by the Conversion of Glycosides to Aglycones in Fangfeng (Saposhnikoviae Radix) in Rheumatoid Arthritis Models Based on Serum Metabolomics, Network Pharmacology, and Molecular Docking. International Journal of Molecular Sciences. 2025; 26(15):7088. https://doi.org/10.3390/ijms26157088
Chicago/Turabian StyleJing, Wenguang, Xiaoyu Lin, Wenmin Pi, Fangliang He, Haonan Wu, Xianrui Wang, Jia Chen, Xianlong Cheng, Penglong Wang, and Feng Wei. 2025. "Anti-Inflammatory Pharmacological Mechanism Mediated by the Conversion of Glycosides to Aglycones in Fangfeng (Saposhnikoviae Radix) in Rheumatoid Arthritis Models Based on Serum Metabolomics, Network Pharmacology, and Molecular Docking" International Journal of Molecular Sciences 26, no. 15: 7088. https://doi.org/10.3390/ijms26157088
APA StyleJing, W., Lin, X., Pi, W., He, F., Wu, H., Wang, X., Chen, J., Cheng, X., Wang, P., & Wei, F. (2025). Anti-Inflammatory Pharmacological Mechanism Mediated by the Conversion of Glycosides to Aglycones in Fangfeng (Saposhnikoviae Radix) in Rheumatoid Arthritis Models Based on Serum Metabolomics, Network Pharmacology, and Molecular Docking. International Journal of Molecular Sciences, 26(15), 7088. https://doi.org/10.3390/ijms26157088






