The Role of PAR2 in MASLD Progression and HCC Development
Abstract
1. Introduction
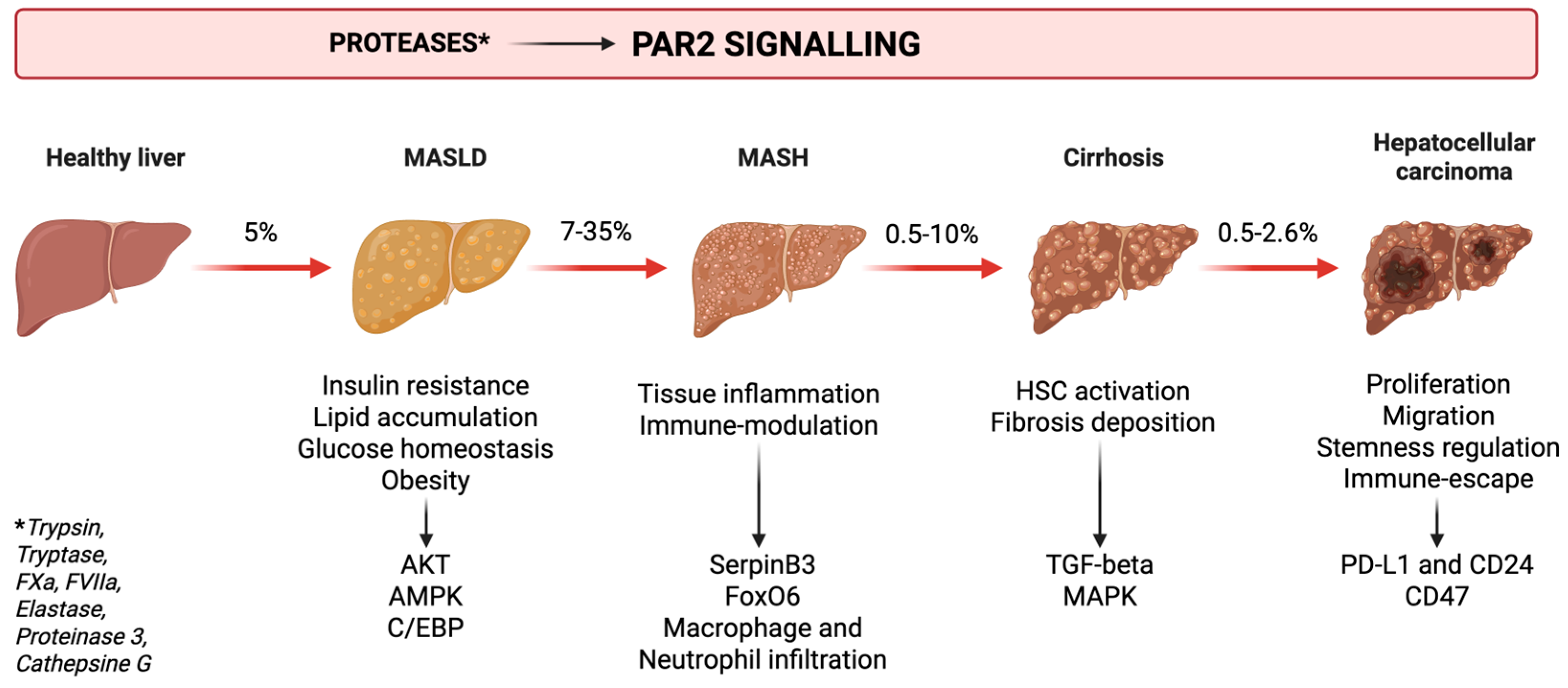
2. Metabolic Dysfunction—Associated Liver Disease and Liver Cancer
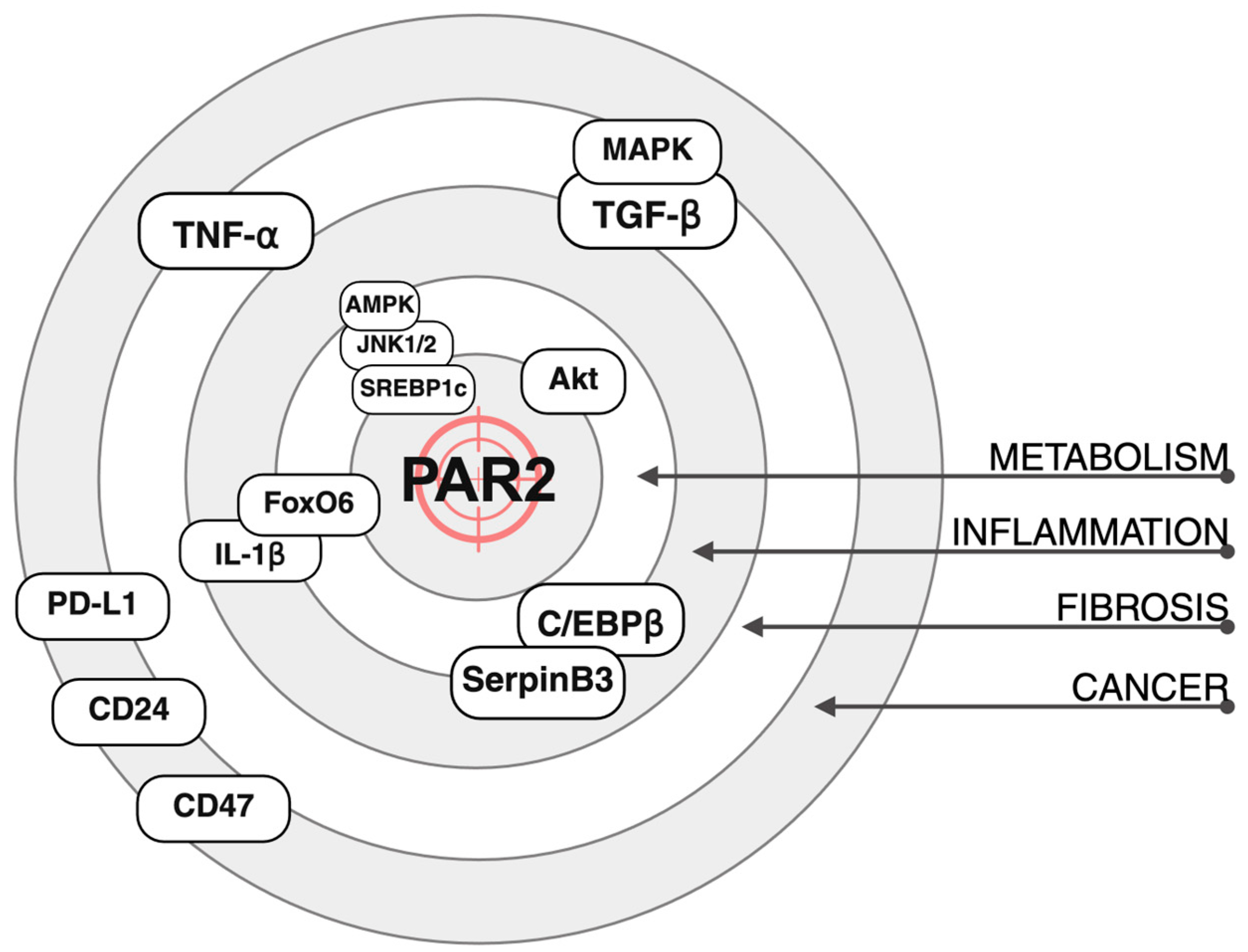
3. PAR2 and Metabolism
3.1. Glucose Metabolism and Insulin Resistance
3.2. Lipid Metabolism and Obesity
3.3. Liver Steatosis and Pathology
4. PAR2 and Inflammation
4.1. Lung and Airways
4.2. Sepsis and Infection
4.3. Neuroinflammation
4.4. Gut and Microbiome
4.5. Kidney and Vascular System
4.6. PAR2 Dual Role in Inflammation
5. PAR2 and Fibrosis
5.1. Liver Fibrosis
5.2. Other Organs Fibrosis
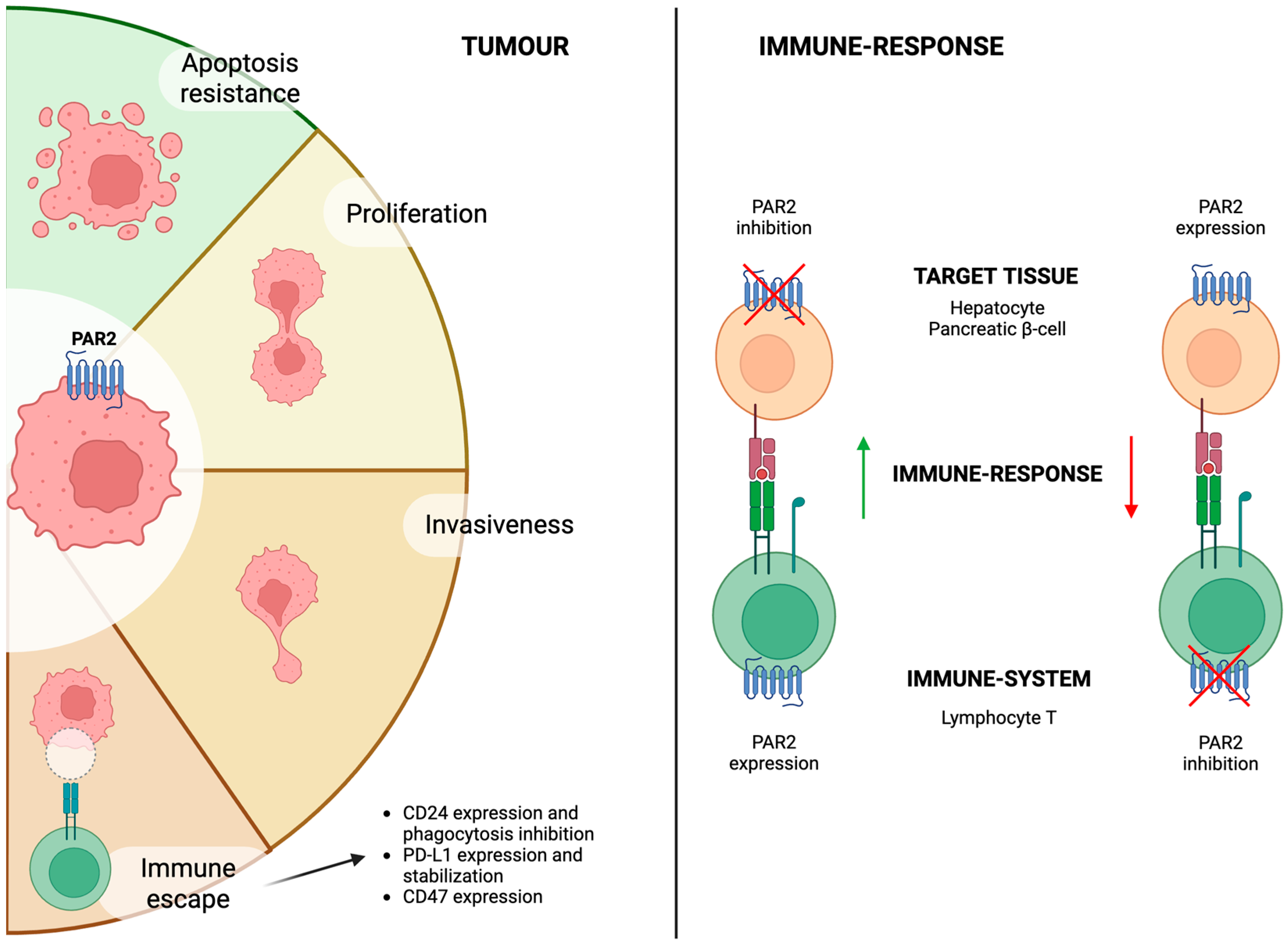
6. PAR2 and Cancer
6.1. Cancer Progression and Growth
6.2. Immune Surveillance and Immunotherapy
7. Discussion and Future Perspectives
7.1. PAR2 as a Therapeutic Target in Liver Disease Progression
7.2. PAR2 as a Therapeutic Target in HCC
7.3. PAR2 as a Prognostic Marker in HCC
8. Conclusions
9. Patents
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1-PPA | 1-Piperidie Propionic Acid |
| Akt | Protein Kinase B |
| AMPK | AMP-Activated Protein Kinase |
| AOM | Azoxymethane |
| BMI | Body Mass Index |
| C/EBP-β | CCAAT/Enhancer-Binding Protein β |
| CCL4 | Carbon Tetrachloride |
| CDAA | Choline-Deficient, L-Amino Acid-Defined |
| DSS | Dextran Sulfate Sodium |
| FoxO6 | Forkhead Transcription Factor 6 |
| FXa | Coagulation Factor Xa |
| GPCR | G-Protein Coupled Receptor |
| GLUT2 | Glucose Transporte Type 2 |
| HCC | Hepatocellular Carcinoma |
| HFD | High-Fat Diet |
| HSC | Hepatic Stellate Cell |
| ICI | Immune-Checkpoint Inhibitor |
| KO | Knock-Out |
| LFD | Low-Fat Diet |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-Activated Protein Kinase |
| MASH | Metabolic Dysfunction-Associated Steatohepatitis |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MCD | Methionine–Choline-Deficient |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NASH | Non-Alcoholic Steatohepatitis |
| PAR2 | Protease-Activated Receptor 2 |
| SLD | Steatotic Liver Disease |
| SREBP1c | Sterol Regulatory Element Binding Protein 1 |
| TF | Tissue Factor |
| TGF-β | Transforming Growth Factor β |
| TNBS | 2,4,6-Trinitrobenzene Sulfonic Acid |
| TNF-α | Tumour Necrosis Factor α |
| WT | Wild-Type |
References
- Villano, G.; Pontisso, P. Protease Activated Receptor 2 as a Novel Druggable Target for the Treatment of Metabolic Dysfunction-Associated Fatty Liver Disease and Cancer. Front. Immunol. 2024, 15, 1397441. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Obes. Facts 2024, 17, 374–444. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; Kendall, B.J.; El-Serag, H.B.; Thrift, A.P.; Macdonald, G.A. Hepatocellular and Extrahepatic Cancer Risk in People with Non-Alcoholic Fatty Liver Disease. Lancet Gastroenterol. Hepatol. 2024, 9, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.M.; Henry, L.; De Avila, L.; Younossi, E.; Racila, A.; Younossi, Z.M. Mortality Related to Nonalcoholic Fatty Liver Disease Is Increasing in the United States. Hepatol. Commun. 2019, 3, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Henry, L. Epidemiology of Non-Alcoholic Fatty Liver Disease and Hepatocellular Carcinoma. JHEP Rep. 2021, 3, 100305. [Google Scholar] [CrossRef] [PubMed]
- Piscaglia, F.; Svegliati-Baroni, G.; Barchetti, A.; Pecorelli, A.; Marinelli, S.; Tiribelli, C.; Bellentani, S.; on behalf of the HCC-NAFLD Italian Study Group. Clinical Patterns of Hepatocellular Carcinoma in Nonalcoholic Fatty Liver Disease: A Multicenter Prospective Study. Hepatology 2016, 63, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Gong, G.; Ben, Q.; Qiu, W.; Chen, Y.; Li, G.; Wang, L. Increased Risk of Hepatocellular Carcinoma in Patients with Diabetes Mellitus: A Systematic Review and Meta-analysis of Cohort Studies. Int. J. Cancer 2012, 130, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current Concepts and Future Challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Dong, B. Molecular Mechanisms in MASLD/MASH-Related HCC. Hepatology 2024. [Google Scholar] [CrossRef] [PubMed]
- Shearer, A.M.; Wang, Y.; Fletcher, E.K.; Rana, R.; Michael, E.S.; Nguyen, N.; Abdelmalek, M.F.; Covic, L.; Kuliopulos, A. PAR2 Promotes Impaired Glucose Uptake and Insulin Resistance in NAFLD through GLUT2 and Akt Interference. Hepatology 2022, 76, 1778–1793. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, B.; Lee, J.; Kim, M.E.; Lee, J.S.; Chung, J.H.; Yu, B.P.; Dong, H.H.; Chung, H.Y. FoxO6-Mediated IL-1β Induces Hepatic Insulin Resistance and Age-Related Inflammation via the TF/PAR2 Pathway in Aging and Diabetic Mice. Redox Biol. 2019, 24, 101184. [Google Scholar] [CrossRef] [PubMed]
- Rana, R.; Shearer, A.M.; Fletcher, E.K.; Nguyen, N.; Guha, S.; Cox, D.H.; Abdelmalek, M.; Wang, Y.; Baleja, J.D.; Covic, L.; et al. PAR2 Controls Cholesterol Homeostasis and Lipid Metabolism in Nonalcoholic Fatty Liver Disease. Mol. Metab. 2019, 29, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Kim, D.H.; Park, Y.J.; Ha, S.; Choi, Y.J.; Yu, H.S.; Chung, K.W.; Chung, H.Y. PAR2 Promotes High-Fat Diet-Induced Hepatic Steatosis by Inhibiting AMPK-Mediated Autophagy. J. Nutr. Biochem. 2021, 95, 108769. [Google Scholar] [CrossRef] [PubMed]
- Badeanlou, L.; Furlan-Freguia, C.; Yang, G.; Ruf, W.; Samad, F. Tissue Factor–Protease-Activated Receptor 2 Signaling Promotes Diet-Induced Obesity and Adipose Inflammation. Nat. Med. 2011, 17, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chakrabarty, S.; Bui, Q.; Ruf, W.; Samad, F. Hematopoietic Tissue Factor–Protease-Activated Receptor 2 Signaling Promotes Hepatic Inflammation and Contributes to Pathways of Gluconeogenesis and Steatosis in Obese Mice. Am. J. Pathol. 2015, 185, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Villano, G.; Novo, E.; Turato, C.; Quarta, S.; Ruvoletto, M.; Biasiolo, A.; Protopapa, F.; Chinellato, M.; Martini, A.; Trevellin, E.; et al. The Protease Activated Receptor 2—CCAAT/Enhancer-Binding Protein Beta—SerpinB3 Axis Inhibition as a Novel Strategy for the Treatment of Non-Alcoholic Steatohepatitis. Mol. Metab. 2024, 81, 101889. [Google Scholar] [CrossRef] [PubMed]
- Novo, E.; Cappon, A.; Villano, G.; Quarta, S.; Cannito, S.; Bocca, C.; Turato, C.; Guido, M.; Maggiora, M.; Protopapa, F.; et al. SerpinB3 as a Pro-Inflammatory Mediator in the Progression of Experimental Non-Alcoholic Fatty Liver Disease. Front. Immunol. 2022, 13, 910526. [Google Scholar] [CrossRef] [PubMed]
- Schiff, H.V.; Rivas, C.M.; Pederson, W.P.; Sandoval, E.; Gillman, S.; Prisco, J.; Kume, M.; Dussor, G.; Vagner, J.; Ledford, J.G.; et al. β-Arrestin-biased Proteinase-activated Receptor-2 Antagonist C781 Limits Allergen-induced Airway Hyperresponsiveness and Inflammation. Br. J. Pharmacol. 2023, 180, 667–680. [Google Scholar] [CrossRef] [PubMed]
- De Matos, N.A.; Lima, O.C.O.; Da Silva, J.F.; Piñeros, A.R.; Tavares, J.C.; Lemos, V.S.; Alves-Filho, J.C.; Klein, A. Blockade of Protease-Activated Receptor 2 Attenuates Allergen-Mediated Acute Lung Inflammation and Leukocyte Recruitment in Mice. J. Biosci. 2022, 47, 2. [Google Scholar] [CrossRef]
- Luisetto, R.; Scarpa, M.; Villano, G.; Martini, A.; Quarta, S.; Ruvoletto, M.; Guerra, P.; Scarpa, M.; Chinellato, M.; Biasiolo, A.; et al. 1-Piperidine Propionic Acid Protects from Septic Shock Through Protease Receptor 2 Inhibition. IJMS 2024, 25, 11662. [Google Scholar] [CrossRef] [PubMed]
- Jesmin, S.; Gando, S.; Zaedi, S.; Sakuraya, F. Chronological Expression of PAR Isoforms in Acute Liver Injury and Its Amelioration by PAR2 Blockade in a Rat Model of Sepsis. Thromb. Haemost. 2006, 96, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Francis, N.; Sanaei, R.; Ayodele, B.A.; O’Brien-Simpson, N.M.; Fairlie, D.P.; Wijeyewickrema, L.C.; Pike, R.N.; Mackie, E.J.; Pagel, C.N. Effect of a Protease-activated Receptor-2 Antagonist (GB88) on Inflammation-related Loss of Alveolar Bone in Periodontal Disease. J. Periodontal Res. 2023, 58, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Ocak, U.; Eser Ocak, P.; Huang, L.; Xu, W.; Zuo, Y.; Li, P.; Gamdzyk, M.; Zuo, G.; Mo, J.; Zhang, G.; et al. Inhibition of Mast Cell Tryptase Attenuates Neuroinflammation via PAR-2/P38/NFκB Pathway Following Asphyxial Cardiac Arrest in Rats. J. Neuroinflamm. 2020, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Santiago, A.; Hann, A.; Constante, M.; Rahmani, S.; Libertucci, J.; Jackson, K.; Rueda, G.; Rossi, L.; Ramachandran, R.; Ruf, W.; et al. Crohn’s Disease Proteolytic Microbiota Enhances Inflammation through PAR2 Pathway in Gnotobiotic Mice. Gut Microbes 2023, 15, 2205425. [Google Scholar] [CrossRef] [PubMed]
- Rondeau, L.E.; Da Luz, B.B.; Santiago, A.; Bermudez-Brito, M.; Hann, A.; De Palma, G.; Jury, J.; Wang, X.; Verdu, E.F.; Galipeau, H.J.; et al. Proteolytic Bacteria Expansion during Colitis Amplifies Inflammation through Cleavage of the External Domain of PAR2. Gut Microbes 2024, 16, 2387857. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Wang, C.; Wu, T.; Wang, W.; Yang, Y.; Dai, Y. PAR2 Deficiency Enhances Myeloid Cell-Mediated Immunosuppression and Promotes Colitis-Associated Tumorigenesis. Cancer Lett. 2020, 469, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Yang, Y.; Kim, B.M.; Kim, J.; Son, M.; Kim, D.; Yu, H.S.; Im, D.; Chung, H.Y.; Chung, K.W. Activation of PAR2 Promotes High-Fat Diet-Induced Renal Injury by Inducing Oxidative Stress and Inflammation. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2022, 1868, 166474. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Kim, H.W.; Kim, K.M.; Kim, B.M.; Kim, J.; Son, M.; Kim, D.; Kim, M.; Yoo, J.; Yu, H.S.; et al. PAR2-mediated Cellular Senescence Promotes Inflammation and Fibrosis in Aging and Chronic Kidney Disease. Aging Cell 2024, 23, e14184. [Google Scholar] [CrossRef] [PubMed]
- Reches, G.; Blondheim Shraga, N.R.; Carrette, F.; Malka, A.; Saleev, N.; Gubbay, Y.; Ertracht, O.; Haviv, I.; Bradley, L.M.; Levine, F.; et al. Resolving the Conflicts around Par2 Opposing Roles in Regeneration by Comparing Immune-Mediated and Toxic-Induced Injuries. Inflamm. Regen. 2022, 42, 52. [Google Scholar] [CrossRef] [PubMed]
- Reches, G.; Khoon, L.; Ghanayiem, N.; Malka, A.; Piran, R. Controlling Autoimmune Diabetes Onset by Targeting Protease-Activated Receptor 2. Biomed. Pharmacother. 2024, 175, 116622. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Yu, S.-J.; Tsai, J.-J.; Yu, C.-H.; Liao, E.-C. Antagonism of Protease Activated Receptor-2 by GB88 Reduces Inflammation Triggered by Protease Allergen Tyr-P3. Front. Immunol. 2021, 12, 557433. [Google Scholar] [CrossRef] [PubMed]
- Rivas, C.M.; Schiff, H.V.; Moutal, A.; Khanna, R.; Kiela, P.R.; Dussor, G.; Price, T.J.; Vagner, J.; DeFea, K.A.; Boitano, S. Alternaria Alternata-Induced Airway Epithelial Signaling and Inflammatory Responses via Protease-Activated Receptor-2 Expression. Biochem. Biophys. Res. Commun. 2022, 591, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.-Y.; Zheng-Gérard, C.; Huang, K.-Y.; Chang, Y.-C.; Chen, Y.-W.; I, K.-Y.; Lo, Y.-L.; Chiang, N.-Y.; Chen, H.-Y.; Stacey, M.; et al. GPR97 Triggers Inflammatory Processes in Human Neutrophils via a Macromolecular Complex Upstream of PAR2 Activation. Nat. Commun. 2022, 13, 6385. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Rojas, I.G.; Edgerton, M. Candida Albicans Sap6 Initiates Oral Mucosal Inflammation via the Protease Activated Receptor PAR2. Front. Immunol. 2022, 13, 912748. [Google Scholar] [CrossRef] [PubMed]
- Quarta, S.; Sandre, M.; Ruvoletto, M.; Campagnolo, M.; Emmi, A.; Biasiolo, A.; Pontisso, P.; Antonini, A. Inhibition of Protease-Activated Receptor-2 Activation in Parkinson’s Disease Using 1-Piperidin Propionic Acid. Biomedicines 2024, 12, 1623. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Hegron, A.; Peach, C.J.; Teng, S.; Tonello, R.; Retamal, J.S.; Klein-Cloud, R.; Bok, D.; Jensen, D.D.; Gottesman-Katz, L.; et al. Mice Expressing Fluorescent PAR2 Reveal That Endocytosis Mediates Colonic Inflammation and Pain. Proc. Natl. Acad. Sci. USA 2022, 119, e2112059119. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gai, J.; Shi, S.; Zhao, J.; Bai, X.; Liu, B.; Li, X. Protease-Activated Receptor 2 (PAR-2) Antagonist AZ3451 Mitigates Oxidized Low-Density Lipoprotein (Ox-LDL)-Induced Damage and Endothelial Inflammation. Chem. Res. Toxicol. 2021, 34, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wei, M.; Liu, G.; Song, C.; Yang, M.; Cao, Z.; Zheng, M. Silencing Protease-Activated Receptor 2 Alleviates Ox-LDL-Induced Lipid Accumulation, Inflammation, and Apoptosis via Activation of Wnt/β-Catenin Signaling. Gen. Physiol. Biophys. 2020, 39, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Shearer, A.M.; Rana, R.; Austin, K.; Baleja, J.D.; Nguyen, N.; Bohm, A.; Covic, L.; Kuliopulos, A. Targeting Liver Fibrosis with a Cell-Penetrating Protease-Activated Receptor-2 (PAR2) Pepducin. J. Biol. Chem. 2016, 291, 23188–23198. [Google Scholar] [CrossRef] [PubMed]
- Knight, V.; Tchongue, J.; Lourensz, D.; Tipping, P.; Sievert, W. Protease-Activated Receptor 2 Promotes Experimental Liver Fibrosis in Mice and Activates Human Hepatic Stellate Cells. Hepatology 2012, 55, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Knight, V.; Lourensz, D.; Tchongue, J.; Correia, J.; Tipping, P.; Sievert, W. Cytoplasmic Domain of Tissue Factor Promotes Liver Fibrosis in Mice. World J. Gastroenterol. 2017, 23, 5692. [Google Scholar] [CrossRef] [PubMed]
- Tisch, L.J.; Bartone, R.D.; Antoniak, S.; Bonner, J.C. Protease-Activated Receptor-2 (PAR2) Mutation Attenuates Airway Fibrosis in Mice during the Exacerbation of House Dust Mite-induced Allergic Lung Disease by Multi-walled Carbon Nanotubes. Respir. Res. 2025, 26, 90. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Chung, K.W.; Lee, J.; Chung, H.Y.; Moon, H.R. Renal Tubular PAR2 Promotes Interstitial Fibrosis by Increasing Inflammatory Responses and EMT Process. Arch. Pharm. Res. 2022, 45, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, S.; Oe, Y.; Sekimoto, A.; Sato, E.; Hashizume, Y.; Yamakage, S.; Kumakura, S.; Sato, H.; Ito, S.; Takahashi, N. Dual Blockade of Protease-Activated Receptor 1 and 2 Additively Ameliorates Diabetic Kidney Disease. Am. J. Physiol.-Ren. Physiol. 2020, 318, F1067–F1073. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, M.-Q.; Yu, T.-Y.; Yin, Y.-Y.; Liu, Y.; Wang, X.-D.; He, Z.-G.; Yin, L.; Chen, C.-Q.; Li, J.-Y. Mast Cell Tryptase Promotes Inflammatory Bowel Disease–Induced Intestinal Fibrosis. Inflamm. Bowel Dis. 2021, 27, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, L.; Wang, L.; Wang, Q.; Yu, L.; Zhang, S. Naringin Alleviates Intestinal Fibrosis by Inhibiting ER Stress–Induced PAR2 Activation. Inflamm. Bowel Dis. 2024, 30, 1946–1956. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Fontenot, L.; Chupina Estrada, A.; Nelson, B.; Wang, J.; Shih, D.Q.; Ho, W.; Mattai, S.A.; Rieder, F.; Jensen, D.D.; et al. Elafin Reverses Intestinal Fibrosis by Inhibiting Cathepsin S-Mediated Protease-Activated Receptor 2. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 841–876. [Google Scholar] [CrossRef] [PubMed]
- Gaça, M.D.A.; Zhou, X.; Benyon, R.C. Regulation of Hepatic Stellate Cell Proliferation and Collagen Synthesis by Proteinase-Activated Receptors. J. Hepatol. 2002, 36, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, W.; Shen, F.; Feng, Z.; Zhu, G.; Cao, J.; Xu, B. Protease-Activated Receptor-2 Modulates Hepatic Stellate Cell Collagen Release and Apoptotic Status. Arch. Biochem. Biophys. 2014, 545, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.; Martini, A.; Pontisso, P.; Angeli, P. Novel Molecular Targets for Immune Surveillance of Hepatocellular Carcinoma. Cancers 2023, 15, 3629. [Google Scholar] [CrossRef] [PubMed]
- Mußbach, F.; Ungefroren, H.; Günther, B.; Katenkamp, K.; Henklein, P.; Westermann, M.; Settmacher, U.; Lenk, L.; Sebens, S.; Müller, J.P.; et al. Proteinase-Activated Receptor 2 (PAR2) in Hepatic Stellate Cells—Evidence for a Role in Hepatocellular Carcinoma Growth in Vivo. Mol. Cancer 2016, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, P.-B.; Yao, Y.-F.; Xiu, A.-Y.; Peng, Z.; Bai, Y.-H.; Gao, Y.-J. Proteinase-Activated Receptor 2 Promotes Tumor Cell Proliferation and Metastasis by Inducing Epithelial-Mesenchymal Transition and Predicts Poor Prognosis in Hepatocellular Carcinoma. World J. Gastroenterol. 2018, 24, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.-W.; Cheung, V.C.-H.; Lu, P.; Lau, E.Y.T.; Ma, S.; Tang, K.H.; Tong, M.; Lo, J.; Ng, I.O.L. Blockade of Cd47-Mediated Cathepsin S/Protease-Activated Receptor 2 Signaling Provides a Therapeutic Target for Hepatocellular Carcinoma. Hepatology 2014, 60, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, J.; Ma, Y.; Wang, B.; Wang, Y.; Yuan, J.; Zhang, F.; Zhao, X.; Chen, K.; Zhang, X.; et al. Neutrophil Extracellular Traps Impede Cancer Metastatic Seeding via Protease-Activated Receptor 2-Mediated Downregulation of Phagocytic Checkpoint CD24. J. Immunother. Cancer 2025, 13, e010813. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Bhoumick, A.; Paul, S.; Chatterjee, A.; Mandal, S.; Basu, A.; Mukhopadhyay, S.; Das, K.; Sen, P. FVIIa-PAR2 Signaling Facilitates Immune Escape by Reducing Phagocytic Potential of Macrophages in Breast Cancer. J. Thromb. Haemost. 2025, 23, 903–920. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, L.; Wang, B.; Hu, J.; Yu, Y.; Gu, B.; Xiang, L.; Li, X.; Li, H.; Zhang, T.; et al. FXa-Mediated PAR-2 Promotes the Efficacy of Immunotherapy for Hepatocellular Carcinoma through Immune Escape and Anoikis Resistance by Inducing PD-L1 Transcription. J. Immunother. Cancer 2024, 12, e009565. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Das, K.; Ghosh, A.; Chatterjee, A.; Bhoumick, A.; Basu, A.; Sen, P. Coagulation Factor VIIa Enhances Programmed Death-Ligand 1 Expression and Its Stability in Breast Cancer Cells to Promote Breast Cancer Immune Evasion. J. Thromb. Haemost. 2023, 21, 3522–3538. [Google Scholar] [CrossRef] [PubMed]
- Bareche, Y.; Kelly, D.; Abbas-Aghababazadeh, F.; Nakano, M.; Esfahani, P.N.; Tkachuk, D.; Mohammad, H.; Samstein, R.; Lee, C.-H.; Morris, L.G.T.; et al. Leveraging Big Data of Immune Checkpoint Blockade Response Identifies Novel Potential Targets. Ann. Oncol. 2022, 33, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Park, S.-Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Pontisso, P.; Quarta, S.; Caberlotto, C.; Beneduce, L.; Marino, M.; Bernardinello, E.; Tono, N.; Fassina, G.; Cavalletto, L.; Gatta, A.; et al. Progressive increase of SCCA-IgM immune complexes in cirrhotic patients is associated with development of hepatocellular carcinoma. Int. J. Cancer 2006, 119, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Gil-Gómez, A.; Rojas, Á.; Liu, C.-H.; Gallego-Duran, R.; Muñoz-Hernandez, R.; Fassina, G.; Pontisso, P.; Ampuero, J.; Romero-Gómez, M. Combination of Squamous Cell Carcinoma Antigen Immunocomplex and Alpha-Fetoprotein in Mid- and Long-Term Prediction of Hepatocellular Carcinoma among Cirrhotic Patients. World J. Gastroenterol. 2021, 27, 8343–8356. [Google Scholar] [CrossRef] [PubMed]
- Cagnin, M.; Biasiolo, A.; Martini, A.; Ruvoletto, M.; Quarta, S.; Fasolato, S.; Angeli, P.; Fassiona, G.; Pontisso, P. Serum squamous cell carcinoma antigen-immunoglobulin M complex levels predict survival in patients with cirrhosis. Sci. Rep. 2019, 9, 20126. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Turato, C.; Cannito, S.; Quarta, S.; Biasiolo, A.; Ruvoletto, M.; Novo, E.; Marafatto, F.; Guerra, P.; Tonon, M.; et al. The Polymorphic Variant of SerpinB3 (SerpinB3-PD) Is Associated with Faster Cirrhosis Decompensation. Aliment. Pharmacol. Ther. 2024, 59, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Correnti, M.; Cappon, A.; Pastore, M.; Piombanti, B.; Lori, G.; Oliveira, D.V.P.N.; Munoz-Garridos, P.; Lewinska, M.; Andersen, J.B.; Coulouran, C.; et al. The protease-inhibitor SerpinB3 as a critical modulator of the stem-like subset in human cholangiocarcinoma. Liver Int. 2022, 42, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Prasai, K.; Zemla, T.J.; Ahmed, F.Y.; Elnagar, M.B.; Giama, N.H.; Guzzardo, V.; Biasiolo, A.; Fassan, M.; Yin, J.; et al. SerpinB3/4 Expression Is Associated with Poor Prognosis in Patients with Cholangiocarcinoma. Cancers 2024, 16, 225. [Google Scholar] [CrossRef] [PubMed]
- Pozzan, C.; Cardin, R.; Piciocchi, M.; Cazzagon, M.; Maddalo, G.; Vanin, V.; Giacomin, A.; Pontisso, P.; Cillo, U.; Farinati, F. Diagnostic and prognostic role of SCCA-IgM serum levels in hepatocellular carcinoma (HCC). J. Gastroenterol. Hepatol. 2014, 8, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.; Ruvoletto, M.; Quarta, S.; Boninsegna, G.; Biasiolo, A.; Cagnin, S.; Angeli, P.; Pontisso, P.; Martini, A. The Impact of serpinB3-PD Polymorphism on the Prognosis of Patients with Hepatocellular Carcinoma. Transl. Oncol. 2025, 57, 102413. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-C.; Lin, C.-C.; Chen, D.-W.; Liu, Y.-W.; Wu, Y.-J.; Yen, Y.-H.; Huang, P.-Y.; Yao, C.-C.; Chuang, C.-H.; Hsiao, C.-C. The Role of Protease-Activated Receptor 2 in Hepatocellular Carcinoma after Hepatectomy. Medicina 2021, 57, 574. [Google Scholar] [CrossRef] [PubMed]
| Reference | Mouse Strain | Diet/Model | PAR2 Inhibition | Outcome |
|---|---|---|---|---|
| METABOLISM | ||||
| Shearer et al., 2022 [11] | C57BL/6J C57BL/6 Db/Db | HFD, HFD + streptozotocin | Whole-body KO PZ-235 (PAR2-inhibitor) | Reduced hepatic steatosis. Increased glucose metabolism. |
| Kim et al., 2019 [12] | C57BL/6J | Standard diet | Whole-body KO | Reduced hepatic inflammation and |
| Rana et al., 2019 [13] | C57BL/6J | HFD | Whole-body KO | hepatic steatosis. Increased lipid metabolism. |
| Kim et al., 2021 [14] | C57BL/6J | HFD | Whole-body KO | Reduced hepatic steatosis. |
| Badeanlou et al., 2011 [15] | C57BL/6J | HFD | Whole-body KO Bone-marrow chimeras | Reduced obesity development and adipose tissue inflammation, hepatic steatosis and inflammation. |
| Wang et al., 2015 [16] | C57BL/6J | HFD | Whole-body KO Bone-marrow chimeras | Reduced insulin resistance and obesity development. |
| Villano et al., 2024 [17] | C57BL/6J, BALB/c | MCD and CDAA | 1-PPA (PAR2-inhibitor) | Reduced hepatic steatosis, inflammation and fibrosis. |
| Reference | Mouse/Rat Strain | Diet/Model | PAR2 Inhibition | Measured Outcomes |
|---|---|---|---|---|
| INFLAMMATION | ||||
| Schiff et al., 2023 [19] | BALB/c | Allergen sensitisation and challenge | C781 (PAR2 inhibitor) | Reduced airway hyperresponsiveness. |
| De Matos et al., 2022 [20] | BALB/c | Allergic-ovalbumin lung inflammation | ENMD1068 (PAR2 inhibitor) | Reduced airway inflammation. |
| Luisetto et al., 2024 [21] | C57BL/6J | LPS injection | 1-PPA (PAR2 inhibitor) | Reduced organ damage and mortality. Improved cardiac function. |
| Jesmin et al., 2006 [22] | Wistar rats | LPS injection | PAR2 blocking peptide (PAR2 BP) | Increased tissue healing and decreased fibrosis deposition. |
| Francis et al., 2023 [23] | BALB/c | P. gingivalis-induced periodontitis | GB88 (PAR2 inhibitor) | Reduced bone loss and inflammation. |
| Ocak et al., 2020 [24] | Sprague–Dawley rats | Asphyxia-induced cardiac arrest | APC366 (mast cell tryptase inhibitor) | Reduced neurological deficit and neuroinflammation. |
| Santiago et al., 2023 [25] | C57BL/6N, Nod2−/− | Crohn’s disease colonising microbiota | R38E-PAR2 (PAR2 cleavage-resistant mouse) | Reduced colitis development and severity. |
| Rondeau et al., 2024 [26] | C57BL/6N | Proteolytic bacteria colonisation | R38E-PAR2 (PAR2 cleavage-resistant mouse) | Reduced colitis development and severity. |
| Ke et al., 2020 [27] | C57BL/6 | AOM/DSS colitis-associated cancer | Whole-body KO | Increased cancer development. |
| Ha et al., 2022 [28] | C57BL/6J | HFD | Whole-body KO | Improved kidney function. |
| Ha et al., 2024 [29] | C57BL/6J | Renal fibrosis (adenine diet or cisplatin injection) | Whole-body KO | Reduced renal fibrosis and inflammation. |
| Reches et al., 2022 [30] | C57BL/6J | Concanavalin A CCl4 | Whole-body KO Bone-marrow chimeras | Reduced hepatic damage. |
| Reches et al., 2024 [31] | C57BL/6J/NOD | Autoimmune diabetes | Tissue-specific PAR2 deletion | Reduced pancreatic damage and T1D onset. |
| Reference | Mouse Strain | Diet/Model | PAR2 Inhibition | Measured Outcomes |
|---|---|---|---|---|
| FIBROSIS | ||||
| Shearer et al., 2016 [40] | C57BL/6 | MCD CCl4 | PZ-235 (PAR2 inhibitor) | Reduced liver fibrosis. |
| Knight et al., 2012 [41] | C57BL/6 | CCl4 | Whole-body KO | Reduced liver fibrosis. |
| Knight et al., 2017 [42] | C57BL/6 | CCl4 | Whole-body KO | Reduced liver fibrosis. |
| Tisch et al., 2025 [43] | C57BL/6 | HDM | Whole-body KO | Reduced lung fibrosis. |
| Ha et al., 2022 [44] | Wild-type mice | Adenine diet | Whole-body KO | Reduced kidney fibrosis. |
| Mitsui et al., 2020 [45] | Akita mice (eNOS-/-) | Diabetic kidney disease | FSLLRY-NH2 (PAR2 inhibitor) | Reduced kidney fibrosis. |
| Liu et al., 2021 [46] | C57BL/6 | DSS-induced colitis model | ENMD-1068 (PAR2 inhibitor) | Reduced intestinal fibrosis. |
| Liu et al., 2024 [47] | C57BL/6 | TNBS-induced colitis | Whole-body KO | Reduced intestinal fibrosis. |
| Xie et al., 2022 [48] | SAMP1/YitFc, 129Sv/J, CD-1 | Salmonella-infected mice and TNBS | Elafin (Cathepsin-S inhibitor) | Reduced intestinal fibrosis. |
| Reference | Mouse Strain | Diet/Model | PAR2 Inhibition | Measured Outcomes |
|---|---|---|---|---|
| CANCER | ||||
| Mußbach et al., 2016 [52] | SCID | Xenotransplantation (HSC and LX-2 cells) | shRNA silencing | Reduced tumour growth and angiogenesis. |
| Sun et al., 2018 [53] | BALB/c nude | Xenotransplantation (HepG2 and SMMC-7721) | shRNA silencing | Reduced tumour growth and metastasis. |
| Lee et al., 2014 [54] | BALB/c nude | Xenograft (PCL/PRF/5 cells) | shRNA silencing CD47 | Reduced chemoresistance and tumour growth. |
| Liu et al., 2025 [55] | BALB/c nude | LPS injection Xenograft (HT-29 cells) | shRNA silencing, GB88 (PAR2 inhibitor) | Enhanced phagocytosis (downregulation of CD24). |
| Ghosh et al., 2025 [56] | BALB/c | Allograft model (4T1 cells) | shRNA silencing | Enhanced phagocytosis (downregulation of CD47). |
| Li et al., 2024 [57] | NOD/SCID, C57BL/6 | Xenograft (MHCC97-H and Hepal-6 cells)) | FXa inhibition | Reduced immune-escape (downregulation of PD-L1). |
| Paul et al., 2023 [58] | BALB/c | Allograft model (4T1 cells) | shRNA silencing | Reduced immune-escape (downregulation of PD-L1). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra, P.; Pontisso, P.; Martini, A. The Role of PAR2 in MASLD Progression and HCC Development. Int. J. Mol. Sci. 2025, 26, 7076. https://doi.org/10.3390/ijms26157076
Guerra P, Pontisso P, Martini A. The Role of PAR2 in MASLD Progression and HCC Development. International Journal of Molecular Sciences. 2025; 26(15):7076. https://doi.org/10.3390/ijms26157076
Chicago/Turabian StyleGuerra, Pietro, Patrizia Pontisso, and Andrea Martini. 2025. "The Role of PAR2 in MASLD Progression and HCC Development" International Journal of Molecular Sciences 26, no. 15: 7076. https://doi.org/10.3390/ijms26157076
APA StyleGuerra, P., Pontisso, P., & Martini, A. (2025). The Role of PAR2 in MASLD Progression and HCC Development. International Journal of Molecular Sciences, 26(15), 7076. https://doi.org/10.3390/ijms26157076







