Lipopolysaccharide-Activated Macrophages Suppress Cellular Senescence and Promote Rejuvenation in Human Dermal Fibroblasts
Abstract
1. Introduction
2. Results
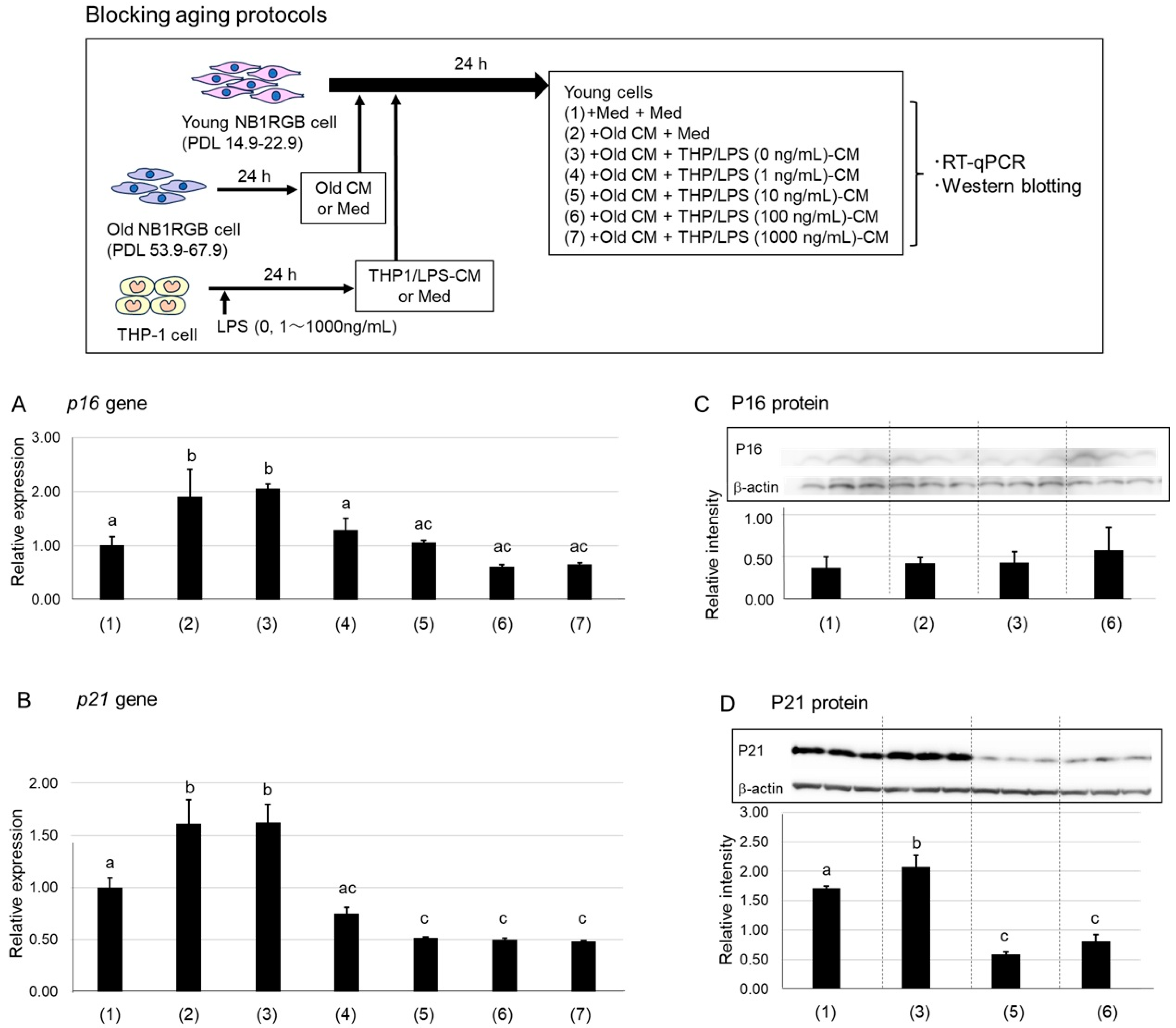
2.1. Suppression of P16 and P21 Expression by LPS-Stimulated Macrophage-Conditioned Media
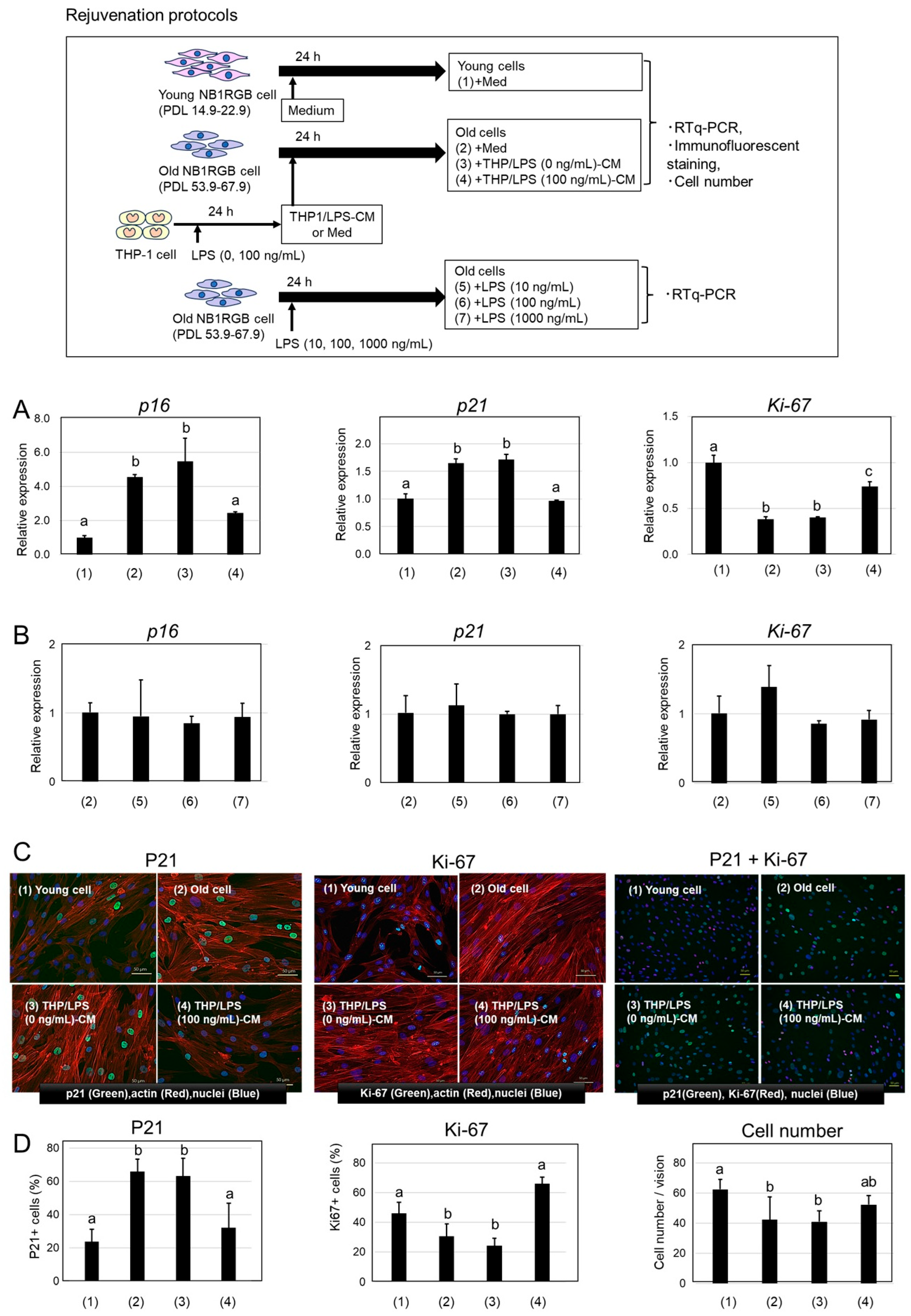
2.2. Rejuvenation of Senescent Cells by THP/LPS-Conditioned Media
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. CM Preparation
4.3. Stimulation of NB1RGB Cells with CM
4.3.1. Inhibition of Senescence Induction
- (1)
- EMEM alone
- (2)
- Old CM alone,
- (3)
- A 1:1 mixture of Old CM and THP/LPS (0 ng/mL)-CM,
- (4)
- A 1:1 mixture of Old CM and THP/LPS (1 ng/mL)-CM,
- (5)
- A 1:1 mixture of Old CM and THP/LPS (10 ng/mL)-CM,
- (6)
- A 1:1 mixture of Old CM and THP/LPS (100 ng/mL)-CM,
- (7)
- A 1:1 mixture of Old CM and THP/LPS (1000 ng/mL)-CM.
4.3.2. Rejuvenation of Senescent Cells
- (1)
- EMEM alone,
- (2)
- EMEM medium alone,
- (3)
- THP/LPS (0 ng/mL)-CM,
- (4)
- THP/LPS (100 ng/mL)-CM,
- (5)
- LPS (10 ng/mL) alone,
- (6)
- LPS (100 ng/mL) alone,
- (7)
- LPS (1000 ng/mL) alone.
4.4. RNA Extraction and RT-qPCR
4.5. Western Blotting
4.6. Immunofluorescence Staining
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DNA | deoxyribonucleic acid |
| CM | conditioned medium |
| PCR | polymerase chain reaction |
| PD-L1 | programmed death ligand 1 |
| RNA | ribonucleic acid |
References
- Coluzzi, E.; Colamartino, M.; Cozzi, R.; Leone, S.; Meneghini, C.; O’Callaghan, N.; Sgura, A. Oxidative Stress Induces Persistent Telomeric DNA Damage Responsible for Nuclear Morphology Change in Mammalian Cells. PLoS ONE 2014, 9, e110963. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef] [PubMed]
- Feldser, D.M.; Greider, C.W. Short telomeres limit tumor progression in vivo by inducing senescence. Cancer Cell 2007, 11, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Sanders, Y.Y.; Liu, H.; Zhang, X.; Hecker, L.; Bernard, K.; Desai, L.; Liu, G.; Thannickal, V.J. Histone modifications in senescence-associated resistance to apoptosis by oxidative stress. Redox. Biol. 2013, 1, 8–16. [Google Scholar] [CrossRef]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Acosta, J.C.; O’Loghlen, A.; Banito, A.; Guijarro, M.V.; Augert, A.; Raguz, S.; Fumagalli, M.; Costa, M.D.; Brown, C.; Popov, N.; et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 2008, 133, 1006–1018. [Google Scholar] [CrossRef]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef]
- Hitomi, K.; Okada, R.; Loo, T.M.; Miyata, K.; Nakamura, A.J.; Takahashi, A. DNA Damage Regulates Senescence-Associated Extracellular Vesicle Release via the Ceramide Pathway to Prevent Excessive Inflammatory Responses. Int. J. Mol. Sci. 2020, 21, 3720. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Soto-Gamez, A.; Quax, W.J.; Demaria, M. Regulation of Survival Networks in Senescent Cells: From Mechanisms to Interventions. J. Mol. Biol. 2019, 431, 2629–2643. [Google Scholar] [CrossRef]
- Prata, L.G.P.L.; Ovsyannikova, I.G.; Tchkonia, T.; Kirkland, J.L. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin. Immunol. 2018, 40, 101275. [Google Scholar] [CrossRef]
- Crunkhorn, S. Fighting ageing with immune checkpoint blockade. Nat. Rev. Drug. Discov. 2023, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.W.; Johmura, Y.; Suzuki, N.; Omori, S.; Migita, T.; Yamaguchi, K.; Hatakeyama, S.; Yamazaki, S.; Shimizu, E.; Imoto, S.; et al. Blocking PD-L1-PD-1 improves senescence surveillance and ageing phenotypes. Nature 2022, 611, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Onorati, A.; Havas, A.P.; Lin, B.; Rajagopal, J.; Sen, P.; Adams, P.D.; Dou, Z. Upregulation of PD-L1 in Senescence and Aging. Mol. Cell. Biol. 2022, 42, e0017122. [Google Scholar] [CrossRef] [PubMed]
- Moss, C.E.; Phipps, H.; Wilson, H.L.; Kiss-Toth, E. Markers of the ageing macrophage: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1222308. [Google Scholar] [CrossRef]
- Blériot, C.; Chakarov, S.; Ginhoux, F. Determinants of Resident Tissue Macrophage Identity and Function. Immunity 2020, 52, 957–970. [Google Scholar] [CrossRef]
- Ogata, Y.; Yamada, T.; Hasegawa, S.; Sanada, A.; Iwata, Y.; Arima, M.; Nakata, S.; Sugiura, K.; Akamatsu, H. SASP-induced macrophage dysfunction may contribute to accelerated senescent fibroblast accumulation in the dermis. Exp. Dermatol. 2021, 30, 84–91. [Google Scholar] [CrossRef]
- Das, A.S.; Mishra, R.; Bhattacharya, S. Age-related blunting of the phagocyte arsenal and its art of killing. Curr. Mol. Biol. Rep. 2020, 6, 126–138. [Google Scholar] [CrossRef]
- Kohchi, C.; Inagawa, H.; Nishizawa, T.; Yamaguchi, T.; Nagai, S.; Soma, G.I. Applications of lipopolysaccharide derived from Pantoea agglomerans (IP-PA1) for health care based on macrophage network theory. J. Biosci. Bioeng. 2006, 102, 485–496. [Google Scholar] [CrossRef]
- Soma, G.I.; Oda, M.; Tjhin, V.T.; Kohchi, C.; Inagawa, H. Oral and transdermal administration of lipopolysaccharide safely enhances self-healing ability through the macrophage network. Front. Immunol. 2025, 16, 1563484. [Google Scholar] [CrossRef]
- Nelson, G.; Wordsworth, J.; Wang, C.; Jurk, D.; Lawless, C.; Martin-Ruiz, C.; Zglinicki, T. A senescent cell bystander effect: Senescence-induced senescence. Aging Cell 2012, 11, 345–349. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Luo, J.; Bao, T.; Wang, S.; Wu, X. Molecular mechanisms of aging and anti-aging strategies. Cell Commun. Signal. 2024, 22, 285. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Tanabe, Y.; Kitahara-Tanabe, N.; Mizuno, D.; Soma, G.I. Enhanced production of tumour necrosis factor alpha (TNF-alpha) by its precursor on the cell surface of primed THP-1 cells. Cytokine 1994, 6, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Inagawa, H. Utility of In Vitro Cellular Models of Low-Dose Lipopolysaccharide in Elucidating the Mechanisms of Anti-Inflammatory and Wound-Healing-Promoting Effects of Lipopolysaccharide Administration In Vivo. Int. J. Mol. Sci. 2023, 24, 14387. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Suzuki, H. TGF-β Signaling in Cellular Senescence and Aging-Related Pathology. Int. J. Mol. Sci. 2019, 20, 5002. [Google Scholar] [CrossRef]
- Krishnan, M.; Kopperuncholan, N.; Kalyan, C.C.; Steven, A.G.; Muralidhar, H.P.; Srinivas, M.T.; Akhil, M. Smad7 interrupts TGF-β signaling in intestinal macrophages and promotes inflammatory activation of these cells during necrotizing enterocolitis. Pediatr. Res. 2016, 79, 951–961. [Google Scholar] [CrossRef]
- Zhao, Z.; Jingfei, Y.; Dongmei, W.; Xun, H.; Yushuang, W.; Xinmeng, L.; Qiang, L.; Yifu, Q. Type 2 cytokine signaling in macrophages protects from cellular senescence and organismal aging. Immunity 2024, 12, 513–527. [Google Scholar] [CrossRef]
- Mizobushi, H. Oral route lipopolysaccharide as a potential dementia preventive agent inducing neuroprotective microglia. Front. Immunol. 2023, 14, 1110583. [Google Scholar] [CrossRef]
- Mizobuchi, H.; Soma, G.I. Low-dose lipopolysaccharide as an immune regulator for homeostasis maintenance in the central nervous system through transformation to neuroprotective microglia. Neu. Reg. Res. 2021, 16, 1928–1934. [Google Scholar] [CrossRef]
- Zou, M.L.; Chen, Z.H.; Teng, Y.Y.; Liu, S.Y.; Jia, Y.; Zhang, K.W.; Sun, Z.L.; Wu, J.; Yuan, Z.D.; Feng, Y.; et al. The Smad Dependent TGF-β and BMP Signaling Pathway in Bone Remodeling and Therapies. Front. Mol. Biosci. 2021, 8, 593310. [Google Scholar] [CrossRef] [PubMed]
- Horiba, S.; Kami, R.; Tsutsui, T.; Hosoi, J. IL-34 Downregulation—Associated M1/M2 Macrophage Imbalance Is Related to Inflammaging in Sun-Exposed Human Skin. JID Innov. 2022, 2, 100112. [Google Scholar] [CrossRef] [PubMed]
- Guiteras, R.; Flaquer, M.; Cruzado, J.M. Macrophage in chronic kidney disease. Clin. Kidney J. 2016, 9, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kohchi, C.; Inagawa, H.; Ikemoto, T.; Hara-Chikuma, M. Effect of topical application of lipopolysaccharide on contact hypersensitivity. Biochem. Biophys. Res. Commun. 2022, 586, 100–106. [Google Scholar] [CrossRef]
- Suzuki, K.; Susaki, E.A.; Nagaoka, I. Lipopolysaccharides and Cellular Senescence: Involvement in Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 11148. [Google Scholar] [CrossRef]
- Skrzypczak-Wiercioch, A.; Sałat, K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. Molecules 2022, 27, 5481. [Google Scholar] [CrossRef]
- Dutkiewicz, J.; Mackiewicz, B.; Lemieszek, M.K.; Golec, M.; Milanowski, J. Pantoea agglomerans: A mysterious bacterium of evil and good. Part IV. Beneficial effects. Ann. Agric. Environ. Med. 2016, 23, 206–222. [Google Scholar] [CrossRef]
- Lorenzi, A.S.; Bonatelli, M.L.; Chia, M.A.; Peressim, L.; Quecine, M.C. Opposite Sides of Pantoea agglomerans and Its Associated Commercial Outlook. Microorganisms 2022, 10, 2072. [Google Scholar] [CrossRef]
- Paterson, N.; Lämmermann, T. Macrophage network dynamics depend on haptokinesis for optimal local surveillance. eLife 2022, 28, e75354. [Google Scholar] [CrossRef]
| Gene | Sequences | Gene Bank ID | |
|---|---|---|---|
| p16 | F | GAG CAG CAT GGA GCC TTC | NM_000077 |
| R | CCT CCG ACC GTA ACT ATT CG | ||
| p21 | F | GGA CAG CAG AGG AAG AC | NM_000389 |
| R | GGC GTT TGG AGT GGT AGA AA | ||
| Ki-67 | F | TCC CGC CTG TTT TCT TTC TGA C | NM_002417 |
| R | CTC TCC AAG GAT GAT GAT GCT TTA C | ||
| GAPDH | F | CGA GAT CCC TCC AAA ATC AA | NM_002046 |
| R | GGT GCT AAG CAG TTG GTG GT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inagawa, H.; Kohchi, C.; Uehiro, M.; Soma, G.-I. Lipopolysaccharide-Activated Macrophages Suppress Cellular Senescence and Promote Rejuvenation in Human Dermal Fibroblasts. Int. J. Mol. Sci. 2025, 26, 7061. https://doi.org/10.3390/ijms26157061
Inagawa H, Kohchi C, Uehiro M, Soma G-I. Lipopolysaccharide-Activated Macrophages Suppress Cellular Senescence and Promote Rejuvenation in Human Dermal Fibroblasts. International Journal of Molecular Sciences. 2025; 26(15):7061. https://doi.org/10.3390/ijms26157061
Chicago/Turabian StyleInagawa, Hiroyuki, Chie Kohchi, Miyuki Uehiro, and Gen-Ichiro Soma. 2025. "Lipopolysaccharide-Activated Macrophages Suppress Cellular Senescence and Promote Rejuvenation in Human Dermal Fibroblasts" International Journal of Molecular Sciences 26, no. 15: 7061. https://doi.org/10.3390/ijms26157061
APA StyleInagawa, H., Kohchi, C., Uehiro, M., & Soma, G.-I. (2025). Lipopolysaccharide-Activated Macrophages Suppress Cellular Senescence and Promote Rejuvenation in Human Dermal Fibroblasts. International Journal of Molecular Sciences, 26(15), 7061. https://doi.org/10.3390/ijms26157061





